Abstract
The cells responsible for the second phase decay of HIV-1 viremia following the initiation of antiretroviral therapy have yet to be identified. A dynamic model that considers where drugs act in the virus life cycle places constraints on candidate cell types. In this regard, the rapid drop in viremia in patients starting regimens containing the integrase inhibitor raltegravir is of particular interest. We show here that the time delay between reverse transcription and integration is short in differentiated macrophages, making these cells poor candidates for the second phase compartment under the assumptions of standard models of viral dynamics.
Pioneering studies of viral dynamics1–5 established that the decay in plasma HIV-1 levels following initiation of treatment depends on the turnover rate of productively infected cells. The classic model assumes that antiretroviral drugs completely stop new infection, revealing the decay rates of previously infected cells. The rapid initial decay in viremia reflects the short half-life (1 day) of productively infected CD4+ T lymphoblasts, the primary target cells for HIV-1. When most of these cells have died, a second slower phase becomes apparent, reflecting virus production by another as yet unidentified population of infected cells with a half-life of ∼14 days.
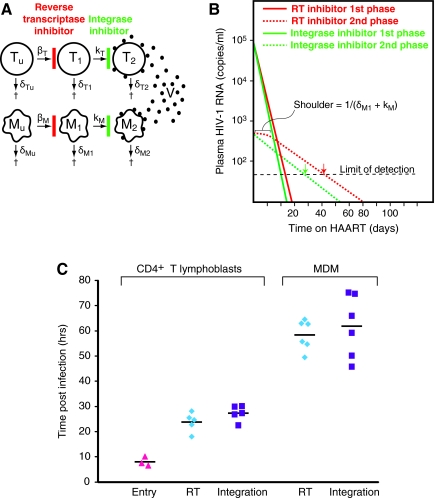
Differences in decay rates of viremia in patients starting different regimens can provide insight into the nature of the second phase compartment. A general model developed by Sedaghat et al. describes how decay dynamics are influenced by the stage in the virus life cycle at which an antiviral drug acts, termed a stage effect (Fig. 1A).6 Assuming full efficacy, drugs acting later after virus entry can produce a more rapid decay rate in viremia than drugs acting earlier in the life cycle. This effect is observed if the sum of the early stage infected cell death rate and the rate of conversion of early stage infected cells to late stage, productively infected cells is less than the decay rate of productively infected cells. If not, decay dynamics may still be altered by the fact that for drugs acting earlier in the life cycle there is a shoulder period before decay begins (Fig. 1B), reflecting the decay of early stage infected cells, either through death or progression to late stages of infection.6,7 The length of this shoulder can be estimated as 1/(δM1 + kM), where δM1 is the decay rate of the early stage cells and kM is the rate of conversion to late stage cells.
FIG. 1.
(A) A model of HIV-1 dynamics reflecting the action of RT inhibitors and integrase inhibitors. The model is based on the work of Sedaghat et al.6 and envisions first (T) and second (M) phase compartments, each consisting of uninfected cells (Tu, Mu), early stage infected cells (T1, M1), and late stage infected cells (T2, M2). For this analysis, we consider early stage infected cells to be cells in which the virus has completed reverse transcription but not integration. The conversion of uninfected cells to early stage infected cells occurs at a rate that depends on the concentrations of uninfected cells and free virus and the rate constants βT and βM and is blocked by RT inhibitors. Late stage cells are cells in which integration has occurred. The conversion of early to late stage infected cells is governed by the rate constants kT and kM and is blocked by integrase inhibitors. Each cell population decays at a characteristic rate (δ). The accumulation of a large number of M1 cells can in principle explain the more rapid decay observed in patients on RAL. (B) Decay dynamics are predicted by the model shown in (A) in patients on RT inhibitor-based (red lines) or integrase inhibitor-based (green lines) regimens. First phase decay (solid lines) and second phase decay (dotted lines) are shown for each regimen. In patients on RT inhibitor-based regimens, there is a shoulder representing the time it takes for M1 cells to convert to M2 cells. The length of this shoulder is given by 1/(δM1 + kM). Note that this shoulder can be obscured by first phase viremia. The time required for viremia to fall below the limit of detection for each regimen is shown with a colored arrow. (C) Experimental measurements of the time required for completion of the fusion, reverse transcription, and integrase reactions in primary CD4+ T lymphoblasts and MDM. CD4+ T lymphoblasts were generated by culturing peripheral blood mononuclear cells (PBMCs) from multiple healthy blood donors for 72 h in the presence of phytohemagglutinin, followed by negative selection to isolate CD4+ cells. To prepare MDM, monocytes isolated from PBMCs by positive selection with CD14+ microbeads (Miltenyi Biotec) were cultured in the presence of 1 ng/ml M-CSF (R&D Systems) for 7 days. Cells were infected with GFP-expressing HIV-1 pseudoviruses capable of a single round of viral replication as previously described.10,11,19 Maximally inhibitory concentrations of the antiretroviral drugs enfuvirtide, EFV, and RAL were added at various times after infection to block fusion, reverse transcription, and integration, respectively. For MDM infections, the virus was pseudotyped vesicular stomatitis virus G protein instead of an X4 HIV-1 envelope, and thus enfurvitide could not be used. The progressive loss of inhibition with later addition of drug allowed calculation of the weighted average time for completion of each process.
This model directly reflects the stepwise nature of viral replication, and as long as a fundamental assumption of complete inhibition is met, it can be used to evaluate candidate second phase cell types in situations in which decay dynamics differ with different drug regimens. In treatment-naive patients, viremia falls below the limit of detection more quickly in the patients starting regimens including the integrase inhibitor raltegravir (RAL) than in patients starting comparable regimens including the reverse transcriptase (RT) inhibitor efavirenz (EFV) in place of RAL.8 Murray and colleagues9 suggested that the rapid drop in viremia in patients on RAL is due to a smaller second phase compartment in the presence of RAL rather than a change in the first or second phase decay rates. In this situation, the rapid first phase decay continues for longer before virus production from the second phase compartment becomes quantitatively dominant. The end result is that viremia falls below the limit of detection more quickly in patients on RAL.
As is illustrated in Fig. 1B, the lack of a shoulder effect could contribute to lower apparent second phase viremia in patients starting RAL. According to the model described in Fig. 1A, second phase viremia on RAL is suppressed by a factor of δM2/(δM1 + kM) relative to second phase viremia on EFV, where δM2 is the decay rate of productively infected second phase cells. The derivation of this effect involves considering the limit of the ratio between the model predictions of second phase viremia on RAL and second phase viremia on EFV as time increases.6,7 Essentially the reduced second phase viremia on RAL reflects the fact that second phase decay at rate δM2 begins as soon as an RAL-based regimen is started while decay is delayed by the shoulder period equal to 1/(δM1 + kM) in patients starting an EFV-based regimen. By this analysis, the observed 70% reduction in second phase viremia9 can be completely explained by a stage effect if the transition from early to late stages of infection in second phase cells (kM) is slow (t1/2 >6 days), so that infected cells can accumulate in an EFV-resistant, RAL-susceptible state (M1 cells, Fig. 1A).
Based on susceptibility to infection, turnover rate, and resistance to HIV-1 cytopathic effects, the macrophage represents a reasonable candidate for the second phase compartment. To determine whether terminally differentiated macrophages meet the kinetic criteria described above, we measured kM in monocyte-derived macrophages (MDM) using time of addition experiments.10 The completion of reverse transcription was slower in MDM than in CD4+ T lymphoblasts (Fig. 1C). However, the interval between completion of reverse transcription, measured by loss of EFV-mediated inhibition, and completion of integration, measured by loss of RAL-mediated inhibition, was short in both cell types (∼0.14 days). If this interval is an accurate reflection of the transition from M1 to M2 cells, then kM for macrophages is approximately 7.14 day–1. Thus for reasonable values of δM1 (0.69–0.05 day–1),7,10 the shoulder is short (3.1–3.3 h), and the reduction in second phase viremia on RAL due to the stage effect is small (0.6–0.7%).
If the fundamental assumption of viral dynamics models is correct (complete or near complete inhibition of ongoing replication) and if macrophages derived from monocytes by in vitro differentiation accurately model infection in vivo, then differentiated macrophages do not meet the kinetic criteria for the second phase compartment. It is possible that the fundamental assumption is not satisfied and that the virus continues to replicate in patients on HAART. However, this assumption is strongly supported by recent pharmacodynamic11 and treatment intensification studies,12 and thus alternatives for the second phase compartment should be considered. The second phase may reflect infection of monocytes, which differentiate into macrophages after leaving the circulation. Integration is delayed in monocytes, occurring days not hours after integration.13 In resting CD4+ T cells, integration may be delayed by a block in nuclear import.9,10,14–18 Both cell types thus represent candidates for the second phase compartment. Interestingly, the length of the shoulder (Fig. 1B) is also influenced by the decay of early stage infected cells (δM1). In in vitro studies,10 the decay rate of unintegrated viral genomes in resting CD4+ T cells is too rapid (t1/2 = 1 day) to be consistent with the observed second phase decay. However, in vivo studies of the loss of recoverable virus from resting CD4+ T cells following initiation of therapy suggest a slower decay.17
These results highlight the importance of definitively identifying the cells responsible for the second phase decay. While macrophages have often been considered as the source, the measured kinetics of replication in differentiated macrophages are not consistent with predictions from standard models of viral dynamics, and thus other cell types should be considered.
Acknowledgments
This work was supported by the Doris Duke Charitable Foundation and by NIH Grants AI51178 and AI081600. A.S. was supported by NIH Grants T32 AI07247 and T32 AI007291.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Wei X. Ghosh SK. Taylor ME. Johnson VA. Emini EA. Deutsch P, et al. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 2.Ho DD. Neumann AU. Perelson AS. Chen W. Leonard JM. Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 3.Perelson AS. Neumann AU. Markowitz M. Leonard JM. Ho DD. HIV-1 dynamics in vivo: Virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 4.Perelson AS. Essunger P. Cao Y. Vesanen M. Hurley A. Saksela K, et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 5.Wodarz D. Nowak MA. Mathematical models of HIV pathogenesis and treatment. Bioessays. 2002;24:1178–1187. doi: 10.1002/bies.10196. [DOI] [PubMed] [Google Scholar]
- 6.Sedaghat AR. Dinoso JB. Shen L. Wilke CO. Siliciano RF. Decay dynamics of HIV-1 depend on the inhibited stages of the viral life cycle. Proc Natl Acad Sci USA. 2008;105:4832–4837. doi: 10.1073/pnas.0711372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sedaghat AR. Siliciano RF. Wilke CO. Constraints on the dominant mechanism for HIV viral dynamics in patients on raltegravir. Antiviral Ther. 2009;14:263–271. [PMC free article] [PubMed] [Google Scholar]
- 8.Markowitz M. Nguyen BY. Gotuzzo E. Mendo F. Ratanasuwan W. Kovacs C, et al. Rapid and durable antiretroviral effect of the HIV-1 integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection: Results of a 48-week controlled study. J Acquir Immune Defic Syndr. 2007;46:125–133. doi: 10.1097/QAI.0b013e318157131c. [DOI] [PubMed] [Google Scholar]
- 9.Murray JM. Emery S. Kelleher AD. Law M. Chen J. Hazuda DJ, et al. Antiretroviral therapy with the integrase inhibitor raltegravir alters decay kinetics of HIV, significantly reducing the second phase. AIDS. 2007;21:2315–2321. doi: 10.1097/QAD.0b013e3282f12377. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Y. Zhang H. Siliciano JD. Siliciano RF. Kinetics of human immunodeficiency virus type 1 decay following entry into resting CD4+ T cells. J Virol. 2005;79:2199–2210. doi: 10.1128/JVI.79.4.2199-2210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen L. Peterson S. Sedaghat AR. McMahon MA. Callender M. Zhang H, et al. Dose-response curve slope sets class-specific limits on inhibitory potential of anti-HIV drugs. Nat Med. 2008;14:762–766. doi: 10.1038/nm1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinoso JB. Kim SY. Wiegand AM. Palmer SE. Gange SJ. Cranmer L, et al. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc Natl Acad Sci USA. 2009;106:9403–9408. doi: 10.1073/pnas.0903107106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arfi V. Riviere L. Jarrosson-Wuilleme L. Goujon C. Rigal D. Darlix JL, et al. Characterization of the early steps of infection of primary blood monocytes by human immunodeficiency virus type 1. J Virol. 2008;82:6557–6565. doi: 10.1128/JVI.02321-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zack JA. Arrigo SJ. Weitsman SR. Go AS. Haislip A. Chen IS. HIV-1 entry into quiescent primary lymphocytes: Molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 15.Bukrinsky MI. Stanwick TL. Dempsey MP. Stevenson M. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science. 1991;254:423–427. doi: 10.1126/science.1925601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spina CA. Guatelli JC. Richman DD. Establishment of a stable, inducible form of human immunodeficiency virus type 1 DNA in quiescent CD4 lymphocytes in vitro. J Virol. 1995;69:2977–2988. doi: 10.1128/jvi.69.5.2977-2988.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blankson JN. Finzi D. Pierson TC. Sabundayo BP. Chadwick K. Margolick JB, et al. Biphasic decay of latently infected CD4+ T cells in acute human immunodeficiency virus type 1 infection. J Infect Dis. 2000;182:1636–1642. doi: 10.1086/317615. [DOI] [PubMed] [Google Scholar]
- 18.Bukrinsky MI. Sharova N. Dempsey MP. Stanwick TL. Bukrinskaya AG. Haggerty S, et al. Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc Natl Acad Sci USA. 1992;89:6580–6584. doi: 10.1073/pnas.89.14.6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang H. Zhou Y. Alcock C. Kiefer T. Monie D. Siliciano J, et al. Novel single-cell-level phenotypic assay for residual drug susceptibility and reduced replication capacity of drug-resistant human immunodeficiency virus type 1. J Virol. 2004;78:1718–1729. doi: 10.1128/JVI.78.4.1718-1729.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]