Abstract
Regulatory T cells contain a mix of CD4 and CD8 T cell subsets that can suppress immune activation and at the same time suppress immune responses, thereby contributing to disease progression. Recent studies have shown that an increased prevalence of CD8+FoxP3+ T regulatory cells was associated with immune suppression and diminished viral control in simian immunodeficiency virus (SIV)-infected rhesus macaques. Preventing an increase in the prevalence of CD8 T regulatory subsets is likely to lead to a better long-term outcome. Here we show that short-term antiretroviral therapy initiated within 1 week after SIV infection was associated with lower viral set point and immune activation after withdrawal of therapy as compared to untreated animals. Early short-term treated controller animals were found to have better SIV-specific immune responses and a significantly lower prevalence of immunosuppressive CD8+FoxP3+ T cells. Lower levels of CD8+FoxP3+ T cells coincided with preservation of CD4+FoxP3+ T cells at homeostatic levels, and significantly correlated with lower immune activation, suggesting a role for viral infection-driven immune activation in the expansion of CD8+FoxP3+ T cells. Interestingly, initiation of continuous therapy later in infection did not reduce the increased prevalence of CD8+FoxP3+ T cells to homeostatic levels. Taken together, our results suggest that early antiretroviral therapy preserves the integrity of the immune system leading to a lower viral set point in controller animals, and prevents alterations in the homeostatic balance between CD4+ and CD8+ T regulatory cells that could aid in better long-term outcome.
Introduction
Human immunodeficiency virus (HIV) infection is characterized by chronic immune activation and loss of viral control. Numerous mechanisms have been proposed for this phenomenon such as active viral replication, dysfunctional immune responses, and the dysregulation of the regulatory T cell network.
T regulatory cells are a heterogeneous mix of CD4 and CD8 T cell subsets. Most of the CD4 T regulatory cells are considered natural T regulatory cells, whereas CD8 T regulatory cells are induced during specific disease states.1,2 Though multiple subsets of CD4 and CD8 T regulatory cells have been described, most T regulatory cells have been found to express Forkhead transcription factor (FoxP3), which has been shown to be essential for its regulatory function.3,4
Regulatory T cells are thought to play conflicting roles in HIV infection. On the one hand, they potentially suppress immune activation, yet at the same time they have been shown to suppress immune responses.5–13 Higher frequencies of CD4+ T regulatory cells have been positively associated with lower viremia and higher CD4 T cell counts in HIV-infected patients,14 whereas others have reported a loss of CD4+ T regulatory cells during HIV and SIV infections.5,13,15–18 Unlike CD4+ T regulatory cells, studies have shown that CD8+ T regulatory cells increase during HIV and simian immunodeficiency virus (SIV) infections12,19–21 and to suppress immune responses that correlated with diminished viral control12. The above studies suggest that HIV/SIV infections are characterized by changes in T regulatory subsets, and these changes likely have an effect on the course of viral infection.
It is not clear if antiretroviral therapy (ART) corrects the changes in CD4 and CD8 T cell subsets of regulatory cells that are altered by HIV and SIV infections, and whether it has an effect on outcome. Previous studies22–25 have shown that ART initiated early during infection protects CD4 T cells in the periphery, but little is known about the effect of early ART on T regulatory cell subsets. Baker et al.26 showed that CD4+ T regulatory cells were significantly decreased in untreated HIV-infected individuals, whereas they remained unchanged compared to pretreatment levels in subjects undergoing ART. On the other hand, Gaardbo et al.27 showed that initiating highly active antiretroviral therapy (HAART) had a minor effect on the level of T regulatory cells in HIV-infected subjects, and Kolte et al.28 reported that T regulatory cells were elevated in HIV-infected patients who were under HAART for over 5 years. The effect of ART on CD8 T regulatory cells during HIV and SIV infections is not known.
The primary objective of our study was to evaluate if SIV infection alters the dynamics of regulatory T cell subsets, and if these alterations could be prevented with antiretroviral therapy. To address this question we evaluated the prevalence of CD4+ and CD8+FoxP3+ T cell subsets in peripheral blood and mesenteric lymph nodes (LN) of SIV-infected rhesus macaques and compared them to previously reported22 animals that received a short course of ART or animals that were on continuous ART. As early viral infection has been associated with acute immune activation, we evaluated the expression of Ki-67 on CD8 T cells and correlated them with CD4+ and CD8+FoxP3+ T cells to determine if changes in T regulatory cell subsets were associated with immune activation. Finally, we evaluated SIV-specific T and B cell responses to determine if early ART was associated with better immune responses.
Materials and Methods
Animals and infection
Colony-bred healthy rhesus macaques (Macaca mulatta) of Indian origin housed at Advanced Bioscience Laboratories Inc. (ABL), Kensington, MD were used in this study. Animals were housed in accordance with American Association for Accreditation of Laboratory Animal Care guidelines and were seronegative for SIV, simian retrovirus, and simian T cell leukemia virus type-1. All animal care and procedures were reviewed and approved by the Institutional Animal Care and Use Committee. All the animals were infected intravenously with 100 animal infectious doses of uncloned pathogenic SIVmac251 that were titrated in Indian origin rhesus macaques. The animals were divided into three groups: Untreated (UT; n = 3), early Short-term ART (ST; n = 3), and Late-continuous ART (LT; n = 3). The ST group of animals was treated with PMPA (tenofovir) and FTC (emtricitabine) daily for a short period of 8 weeks, starting at 1-week pi as previously reported,22 after which ART was discontinued, whereas the LT group of animals received continuous PMPA and FTC starting at week 13 pi until sacrifice. Both the drugs were obtained from Gilead Sciences, Inc. (Foster City, CA) and administered at 20(ST)–30(LT) mg/kg BW/day. The UT groups of animals served as SIV-infected untreated controls.
One of the short-term treated animals was sacrificed at week 32 pi for collection of tissues and was found to be healthy without any gross abnormalities on pathological examination. All the other animals were sacrificed at various time points post-1-year pi. Peripheral blood and plasma samples collected at various time points and mesenteric lymph nodes (LN) and peripheral blood collected were used for analyses. Additionally, blood samples were obtained from healthy uninfected animals (UI; n = 4) for comparison.
Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation, whereas mesenteric LN was processed as previously described.29 Plasma viral loads30 and cell-associated viral loads29 were determined by real-time reverse transcriptase polymerase chain reaction (RT-PCR) assay as previously described. The limit of detection for plasma viral loads was 30 copies/ml of plasma and 1 copy/cell for cell-associated viral loads.
Antibodies and flow cytometry
All antibodies used in this study were titrated using rhesus macaque PBMCs. Isolated cells were used for ex vivo analysis of SIV-env, and gag + pol specific responses, and to evaluate the expression of FoxP3 and Ki-67 in CD4+ and CD8+ T cell subsets.
SIV-specific responses were determined using mesenteric LN cells in an in vitro stimulation assay as per protocols described previously. Briefly, cells were stimulated with pools of overlapping SIVmac239-env and gag + pol peptides (2 μg/ml)29 in the presence of anti-CD28 (clone CD28.2), anti-CD49d (clone 9F10), and Brefeldin A (Golgiplug; BD Biosciences, San Diego, CA). SIVmac239-env (catalog #6883), gag (catalog #6204), and pol (catalog #6443) overlapping peptides were obtained from the NIH AIDS Reference Reagent Program, Division of AIDS, NIAID, NIH. Samples stimulated with anti-CD28 and anti-CD49d, Golgiplug, and DMSO alone served as background controls. After harvesting, cells were labeled with antihuman CD8 (clone RPA-T8)-Alexa-700, CD4 (clone M-T477)-APC, and the amine reactive dye ViViD31 (Invitrogen, Carlsbad, CA) to exclude dead cells from analysis. After washing, cells were fixed and permeabilized using the BD Biosciences fixation and permeabilization kit, and labeled intracellularly with CD3 (clone SP34.2)-Cy7APC, interleukin (IL)-2 (clone MQ1-17H12)-PE, interferon (IFN)-γ (clone 4SB3)-FITC, and tumor necrosis factor (TNF)-α (clone MAB11)-Cy7PE.
To evaluate FoxP3 expression, cells were labeled with anti-CD4-APC and anti-CD8- Alexa-700 antibodies as above, fixed and permeabilized using the eBiosciences fixation and permeabilization kit (eBiosciences; San Diego, CA), and labeled with antihuman CD3-Cy7APC and FoxP3 (clone 206D)-PE. In addition, cells were labeled with CD25 (clone 4E3)-FITC (Miltenyi Biotech, Inc., Auburn, CA) to evaluate if FoxP3-expressing T cells also expressed CD25. Ki-67 expression was evaluated by labeling cells with anti-CD4-APC and CD8-Alexa-700 antibodies as above, fixed, permeabilized, and labeled with antihuman CD3-Cy7-APC and Ki-67 (clone B56)-FITC.
For determining cell-associated viral loads, memory CD4 T cells were sorted using a panel of CD3-Cy-7APC, CD4-APC, CD8-Alexa700, CD95 (clone DX2)-FITC, and CD28-Cy-5PE (BD Biosciences). Memory CD4 T cells were discriminated based on the expression of CD28 and CD95 as described previously.29–32
Labeled cells were fixed with 0.5% paraformaldehyde and analyzed using a modified Becton Dickinson Aria Sorter. Approximately one million total events were collected for analysis.
Genotyping for rhesus macaque MHC class I alleles
Genotyping for MHC class I alleles was performed by ABL, Inc. and at the Laboratory of Molecular Microbiology, NIAID (Bethesda, MD). Genomic DNA was extracted from frozen PBMCs using the QIAamp DNA blood kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Concentration and quality of the recovered genomic DNA were determined using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Inc., Wilmington DE) and an MHC-A2 locus site-specific primer PCR assay (SSP-PCR) was performed to assess for the absence of amplification inhibitors.33 Macaques carrying the Mamu-B*00801 allele (formerly Mamu-B*08) in their genome were identified by using the SSP-PCR assay described by Loffredo et al.34 Genotyping for Mamu-B*0290101 (Mamu-B*29) and Mamu-B*07301 was performed as described previously.35 Genotyping for Mamu-A1*00101 (Mamu-A*01), Mamu-A1*00201 (Mamu-A*02), Mamu-A1*00801 (Mamu-A*08), Mamu-A1*01101 (Mamu-A*11), Mamu-B*00101 (Mamu-B*01), Mamu-B*00301 (Mamu-B*03), Mamu-B*00401 (Mamu-B*04), Mamu-B*0701 (Mamu-B*07), and Mamu-B*01701 (Mamu-B*17) was performed as described previously.36
Antibody-dependent cellular cytotoxicity (ADCC) assay
The rapid fluorometric ADCC (RF-ADCC) assay was performed as previously described.37–42 Briefly, H9 cells infected with SIVmac251 were used as targets. The target cells were dually labeled with the cytoplasmic dyes PKH-26 (Sigma Aldrich) and CFSE (Molecular Probes), a dye that is rapidly lost when cell membranes are damaged. Labeled targets were resuspended in RPMI 1640 medium containing 10% fetal calf serum (R-10) and allowed to react with heat-inactivated (56°C, 30 min) serially diluted plasma in a 96-well microtiter plate for 10 min at room temperature.
Human PBMCs used as effector cells were added at a 50:1 effector/target ratio. The reaction mixture was incubated for 4 h at 37°C in 5% CO2, after which the cells were fixed with 3.7% paraformaldehyde for flow cytometry. Controls included unstained and single-stained target cells. Nongated events (n = 25,000 in each duplicate well) were acquired within 18 h using a FACSCalibur instrument (BD Biosciences). Acquisition was done using CellQuest software and data analysis was performed with WinMDI 2.9. The percent ADCC cell killing is reported as the percentage of membrane-labeled target cells having lost the viability dye, i.e., the percentage of CFSE-negative cells within the PKH-26 high gate. ADCC titers were defined as the reciprocal dilution at which the percent ADCC killing was greater than the mean percent killing of the negative control samples plus three standard deviations.
Virus neutralization assay
Neutralizing antibody responses against primary isolates of SIVmac251 were measured as previously described43 with brief modifications. Neutralization was measured using SIVmac251.30 Env pseudoviruses (Courtesy of James Whitney, Michael Seaman, and Norman Letvin) to infect TZM-bl cells as described previously.44,45 Briefly, 40 μl of virus was incubated for 30 min at 37°C with 10 μl of serially diluted test antibody in duplicate wells of a 96-well flat-bottom culture plate. To keep assay conditions constant, sham medium alone controls were used in place of antibody in specified control wells.
The virus input was set at a multiplicity of infection of ∼0.1, which generally results in 100,000 to 400,000 relative light units in the luciferase assay. Neutralization curves were fit by nonlinear regression using a four-parameter hill slope equation programmed into JMP statistical software (JMP 5.1; SAS Institute Inc., Cary, NC). The 50% and 80% inhibitory concentrations (IC50 and IC80) were reported as the concentration of antibody required to inhibit infection by 50%, and 80%, respectively. Note that neutralizing antibody responses were evaluated only in untreated and short-term-treated animals.
Data analysis
Flow cytometric data were analyzed using FlowJo version 8.6 (Tree Star, Inc., Ashland, OR). SIV-specific CD4 and CD8 T cell responses were determined by excluding dead cells, and are reported as background (costimulatory antibodies alone) subtracted data. Due to the small sample size per group, statistical analysis was performed using nonparametric tests, with GraphPad Prism Version 4.0 software (GraphPad Prism Software, Inc., San Diego, CA). Linear regression analysis was performed to determine line of fit, and correlations were derived using Pearson's rank test. The p < 0.5 was considered significant. Error bars represent standard error.
Results
Short-term treatment lowered viral infection and preserved CD4 T cells after removal of ART
Previous studies29 had shown that there were few CD4 T cells that were infected prior to 1 week pi. We hypothesized that initiation of ART at 1 week pi would lower viral loads by reducing the pool of infected CD4 T cells. To address this question, we evaluated the plasma and memory CD4 T cell-associated viral loads in samples that were collected during the late stages of disease from short-term treated animals and compared them to untreated and late-continuous treated animals.
Plasma viral loads (Fig. 1a) ranged from ∼6–7 logs/ml of plasma in untreated animals. The short-term treated animals had plasma viral loads of ∼2–4 logs/ml of plasma, whereas plasma viral loads were below the levels of detection in the late-continuous treated group of animals. Based on previous reports,23 the three short-term treated animals that had lower viral loads were deemed controllers (ST-controller).
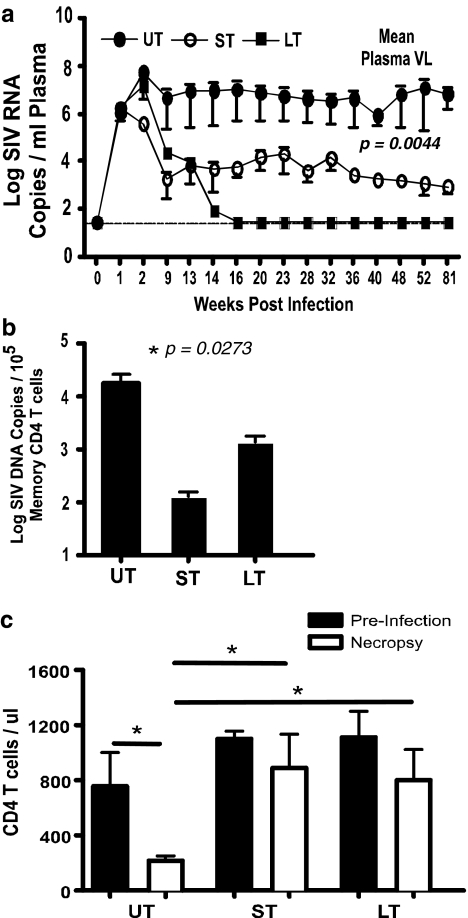
FIG. 1.
Early antiretroviral therapy (ART) is associated with significantly lower viral rebound in short-term controller animals. (a) Plasma viral loads at various time points, (b) cell-associated viral loads, and (c) CD4 T cell counts in untreated (UT), short-term, treated controller (ST), and late-continuous treated (LT) animals.
The mean plasma viral loads were significantly different (p = 0.0044) between the three groups of animals as determined by one-way ANOVA, suggesting that early ART significantly lowered the viral set point in controller animals after withdrawal of therapy.
To determine if the lower plasma viral loads in short-term treated controller animals were due to the lower level of infection in memory CD4 T cells, we quantified the level of SIV-gag copies in memory CD4 T cells from peripheral blood at 9 and 13 weeks pi and during late stage of disease using a highly quantitative qPCR assay29 for SIV-gag. We had previously shown that an infected memory CD4 T cell carried approximately two copies of SIV-gag/cell.29
The mean cell-associated viral loads at week 9 pi, the time point when ART was stopped in short-term treated controller animals, was ∼896 copies/105 memory CD4 T cells, whereas it was ∼25,171 copies and ∼5786 copies in the untreated and late-continuous treated animals, respectively. At 13 weeks pi, the mean cell-associated viral load was ∼890 copies/105 memory CD4 T cells in short-term treated controller animals, whereas it was ∼28,006 copies and ∼5914 copies in the untreated and late-continuous treated animals, respectively. The cell-associated viral loads (Fig. 1b) in short-term treated controller animals were significantly less than those of the untreated and other animals. Interestingly, the late-continuous treated group of animals was found to harbor significantly higher cell-associated virus as compared to the short-term treated controller animals, suggesting that by the time ART was initiated at 13 weeks pi, a significantly higher pool of latently infected cells had been established within the CD4 T cell compartment. On the other hand, early ART restricted the pool of infected cells in short-term treated controller animals, which remained low until sacrifice.
Lower levels of infection were associated with a significant preservation of CD4 T cells (Fig. 1c) in short-term treated controller animals, presumably due to the lower destruction of the memory CD4 T cell compartment associated with acute infection as previously reported.22 Like the short-term treated controller animals, the absolute number of CD4 T cells in the late-continuous group of animals did not differ significantly from preinfection levels. In contrast to the short-term treated controller and late-continuous treated animals, there was a significant loss of CD4 T cells in untreated animals; two out of the three animals had absolute CD4 T cell counts that were <200 cells/μl, indicative of simian AIDS.
Early ART preserves the homeostatic balance between CD4 and CD8+FoxP3+ T cell subsets
Recent studies9,12,18,20 have suggested that perturbations in prevalence of CD4 and CD8 T regulatory cells likely plays a role in SIV pathogenesis. We hypothesized that early ART, by containing early viral replication and CD4 T cell destruction associated with acute infection, will prevent any major perturbations in regulatory T cell subsets. To address this question, we evaluated the effect of SIV infection and ART on the proportion and absolute numbers of CD4 and CD8+FoxP3+ T subsets in peripheral blood and mesenteric LN in short-term treated controller animals and compared them to other groups. The gating strategy used is shown in Fig. 2a. As shown previously,9,12 most FoxP3+ T cells were found to express CD25 (Fig. 2b) with >90% of CD4+ and CD8+FoxP3 T cells expressing CD25 (n = 4; data not shown).
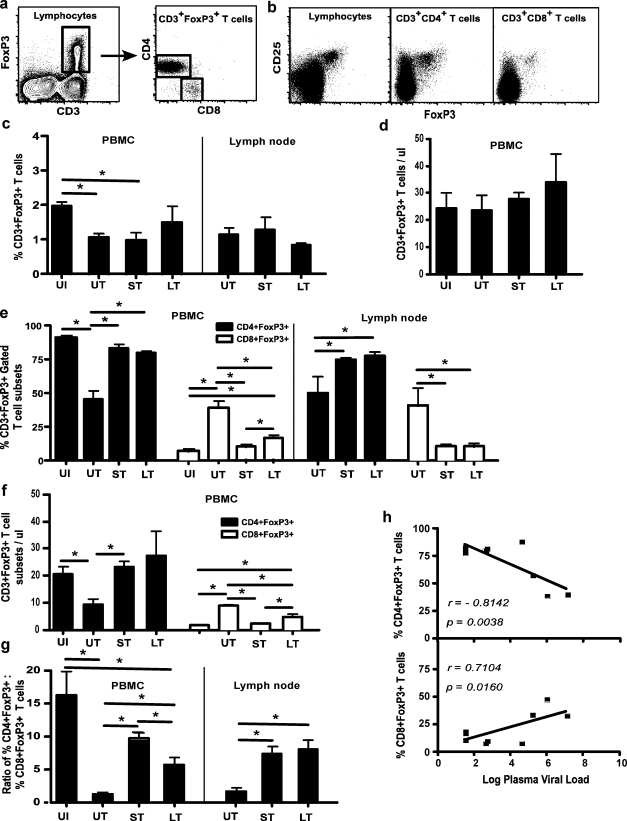
FIG. 2.
Early ART prevents the induction of higher numbers of CD8+FoxP3+ T cells. (a) Gating strategy used to determine the prevalence of CD4 and CD8+FoxP3+ T cell subsets of CD3+FoxP3+ T cells and (b) FoxP3+ lymphocytes; CD3+CD4+ and CD3+CD8+ T cells coexpress CD25. (c) Proportions and (d) absolute numbers of CD3+FoxP3+ T cells in uninfected (UI), untreated (UT), short-term treated controller (ST), and late-continuous treated (LT) animals and (e) proportions and (f) absolute numbers of CD4 and CD8+FoxP3+ T cell subsets in UI, UT, ST, and LT animals. (g) Ratio of CD4+FoxP3+:CD8+FoxP3+ T cells in PBMCs and mesenteric LN from UI, UT, ST, and LT animals and (h) correlation of CD4+ and CD8+FoxP3+ T cells with plasma viral loads (n = 9). Note that proportions and absolute numbers in PBMCs are shown, whereas only proportions for mesenteric lymph node (LN) are shown in (c–f).
We first evaluated the proportions of total CD3+FoxP3+ T cells in peripheral blood and mesenteric LN. Approximately 2% of peripheral blood CD3+ T cells from healthy animals expressed FoxP3. There was a significant decrease in the proportions of CD3+FoxP3+ T cells in untreated and short-term treated controller animals (Fig. 2c) as compared to uninfected animals. There was, however, no significant difference in the absolute numbers of peripheral blood CD3+FoxP3+ T cells between groups (Fig. 2d). Mesenteric LN was found to harbor similar proportions of CD3+FoxP3+ T cells as in peripheral blood, which did not differ significantly between the groups (Fig. 2c). Since we did not have mesenteric LN from uninfected animals, we restricted our analysis to SIV-infected untreated and treated animals.
Next we investigated if SIV infection or treatment was associated with alterations in the proportions of CD4 and CD8+FoxP3+ T cell subsets. Our results showed that a majority (>95%) of peripheral blood CD3+FoxP3+ T cells in uninfected healthy animals were CD4+FoxP3+ T cell subsets, with the rest being CD8+FoxP3+ T cells (Fig. 2e). SIV infection was associated with a significant decrease in the frequency (Fig. 2e) and absolute numbers (Fig. 2f) of peripheral blood CD4+FoxP3+ T cells in untreated animals as compared to uninfected animals. In contrast, CD4+FoxP3+ T cells in both short-term and late-continuous treated animals did not differ significantly from those of uninfected healthy animals, suggesting that ART is associated with preservation of CD4+FoxP3+ T cells. As seen in peripheral blood, ART was associated with significantly higher proportions of CD4+FoxP3+ T cells in the mesenteric LN from both short-term and late-continuous treated animals (Fig. 2e).
The decrease in CD4+FoxP3+ T cells in untreated animals was accompanied by a significant increase in frequency (Fig. 2e) and absolute numbers (Fig. 2f) of CD8+FoxP3+ T cells in peripheral blood. In contrast, both short-term and late-continuous treated animals had significantly lower proportions and absolute numbers of CD8+FoxP3+ T cells as compared to untreated animals, whereas they did not differ from that seen in uninfected healthy animals. Interestingly, however, late-continuous treated animals had significantly higher proportions and absolute numbers of CD8+FoxP3+ T cells than the short-term treated controller and uninfected animals. This trend was evident even during the early phase of infection (Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/aid).
In line with the above reported changes, the ratio of CD4+FoxP3+:CD8+FoxP3+ T cells (Fig. 2g) was significantly decreased in untreated animals, whereas there was no significant difference between uninfected animals and the short-term treated controller group of animals. Late-continuous treated animals had a significantly lower CD4+FoxP3+:CD8+FoxP3+ T cell ratio than uninfected and short-term treated controller animals indicative of the higher proportions of CD8+FoxP3+ T cells.
Similar to peripheral blood, mesenteric LN from untreated animals had significantly higher proportions of CD8+FoxP3+ T cells (Fig. 2e). Unlike blood, however, there was no significant difference in the proportions of CD8+FoxP3+ T cells between short-term and late-continuous treated animals.
The higher proportions of CD4+FoxP3+ T cells in peripheral blood was found to have a significantly high negative correlation (r = −0.8142; p = 0.0038) with plasma viral loads (Fig. 2h), suggesting that lower viral loads are associated with higher levels CD4+FoxP3+ T cells. On the other hand, CD8+FoxP3+ T cells had a significantly positive correlation with plasma viral loads (r = 0.7104; p = 0.0160).
Higher proportions of CD4+FoxP3+ T cells negatively correlate with immune activation
Next we evaluated if the lower viral loads seen in short-term controller animals after withdrawal of therapy was likely due to lower levels of immune activation. Numerous studies46 have shown that immune activation plays a major role in HIV disease progression.
To determine if short-term ART reduced immune activation, we measured Ki-67 expression on CD8 T cells in peripheral blood from short-term treated controller animals, and compared this to untreated and late-continuous treated animals. Due to the unavailability of mesenteric LN samples, we restricted our analysis to peripheral blood.
Our results showed that ART was associated with a significant decrease in Ki-67 expression on CD8 T cells in both the short-term controller and late-continuous group of animals as compared to the untreated animals (Fig. 3a).
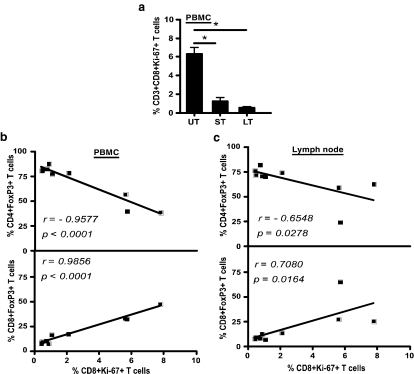
FIG. 3.
Early ART suppressed immune activation after removal of therapy. (a) Expression of Ki-67 on peripheral blood CD3+CD8+ T cells at time of sacrifice in untreated (UT), short-term treated controller (ST), and late-continuous treated (LT) animals. (b) Correlation of CD4+FoxP3+ T cells and CD8+FoxP3+ T cells from PBMCs and (c) mesenteric LN of UT, ST, and LT animals with peripheral blood CD3+CD8+Ki67+ T cells (n = 9).
Previous studies9 have shown that CD4+FoxP3+ T regulatory cells contribute to lower immune activation in SIV-infected rhesus macaques. To determine if the low level of immune activation seen was associated with higher proportions of CD4+FoxP3+ T cells, we correlated CD8+Ki-67+ T cells with CD4 and CD8+FoxP3+ T cells from peripheral blood (Fig. 3b) and mesenteric LN (Fig. 3c). We found a significantly high negative correlation between CD8+Ki-67+ T cells and CD4+FoxP3+ T cells in peripheral blood (r =−0.9577; p < 0.0001) and mesenteric LN (r = −0.6548; p =0.0278). On the other hand, there was a significantly high positive correlation between CD8+Ki-67+ T cells and CD8+FoxP3+ T cells in peripheral blood (r = 0.9856; p < 0.0001) and mesenteric LN (r = 0.7080; p = 0.0164), indicating that the higher proportions of CD8+FoxP3+ T cells seen in untreated animals were associated with higher levels of immune activation seen in those animals.
Early treatment is associated with better SIV-specific immune responses
Recent studies12 have shown that CD8+FoxP3+ T cells suppressed immune responses in SIV-infected rhesus macaques. To determine if the significantly lower proportion of CD8+FoxP3+ T cells in short-term treated controller animals was associated with better immune responses, we evaluated SIV-specific T and B cell responses in short-term treated controller animals, and compared them to untreated and late-continuous treated animals.
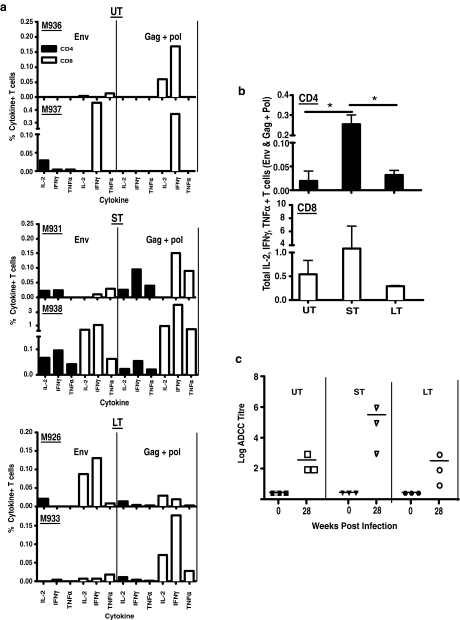
SIV-env and gag + pol specific IL-2, IFN-γ, and TNF-α responses in CD4 and CD8 T cells were determined in mesenteric LN using flow cytometry. Since limited PBMCs were available, we used mesenteric LN from two animals in each group for analyses. Representative dotplots showing the gating strategy from one of the short-term treated controller animals are shown in Fig. 4.
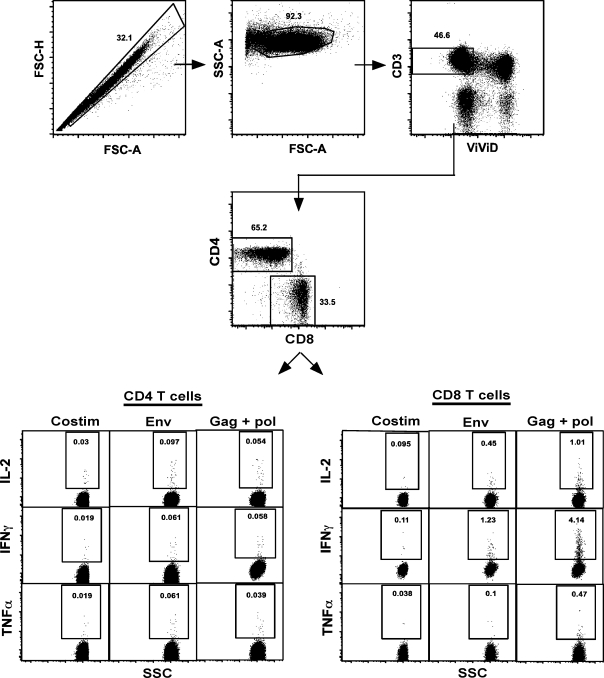
FIG. 4.
Gating strategy used to evaluate SIV-specific CD4 and CD8 T cell responses. Mesenteric LN were stimulated with SIV-env or gag + pol in a 4-h stimulation assay, and stained with a panel of antibodies described in Materials and Methods. Dead cells were excluded from analysis, and IL-2, IFN-γ, and TNF-α production was determined.
Both envelope and gag + pol responses were detectable in all of the animals (Fig. 5a). However, there were differences in the functionality and magnitude of responses. Untreated animals had predominantly CD8 T cell responses that were largely monofunctional and characterized by IFN-γ production. Responses were marginally better in late-continuous treated animals with evidence of IL-2-specific responses. On the other hand, short-term treated controller animals displayed detectable CD4 responses against both envelope and gag + pol peptides, which were characterized by the production of all three cytokines. The total SIV-specific CD4 T cell responses were significantly better in short-term treated controller animals as compared to the untreated and late-continuous animals (Fig. 5b).
FIG. 5.
Animals treated at 1-week pi generate polyfunctional CD4 and CD8 responses. (a) SIV-env and gag + pol-specific CD4 and CD8 T cell responses in mesenteric LN collected from untreated (UT; n = 2), short-term treated controller (ST; n = 2), and late-continuous treated (LT; n = 2) animals. (b) Total SIV-env and gag + pol specific CD4 and CD8 T cell responses in mesenteric LN from two UT, ST, and LT animals. All the three SIV-specific cytokines responses against SIV-env, and gag + pol shown in (a) for each animal were added to derive the total response. (c) ADCC titers in plasma of UT, ST, LT, animals at time 0 and week 28 postinfection. Note that only background subtracted data are shown.
Like CD4 T cell responses, CD8 T cell responses in short-term treated controller animals were better than either late-continuous treated or untreated animals, though these differences were not significant (Fig. 5b). The low level of responses in the late-continuous treated group of animals, even though they experienced significant repopulation of CD4 T cells, is likely due to the low antigenic load in these animals after complete viral suppression with ART. Previous studies47–50 have shown that ART started during primary HIV infection preserves HIV-specific CD4 T cell responses leading to lower viral rebound when therapy is interrupted.
As seen with T cells responses, two out of the three short-term treated controller animals were found to have higher ADCC titers (Fig. 5c) as compared to either untreated, short-term treated noncontroller, and the late-continuous group of animals, though this difference was not statistically significant. Interestingly, the short-term treated animal (M934) that had a low ADCC titer displayed high levels of neutralizing antibody responses against primary SIVmac251 isolates (Table 1).
Table 1.
Neutralizing Antibody Responses Against Primary Isolates of SIVmac251
| Animal | Group | Day 0 | Week 32 (ID50) | Week 32 (ID80) |
|---|---|---|---|---|
| M637 | UT | <5 | 185 | <5 |
| M936 | UT | <5 | 269 | <5 |
| M937 | UT | 8 | 2602 | 20 |
| M931 | ST-c | <5 | 313 | <5 |
| M934 | ST-c | <5 | 1162 | 14 |
| M938 | ST-c | <5 | <10 | <5 |
UT, untreated; ST-c, short-term treated controller.
Previous studies23 have suggested that protective MHC class I alleles provide a basis for the lower viral loads in early short-term treated controller animals. To determine whether specific protective MHC class I alleles contributed to the lower viral loads in short-term treated controller animals, we genotyped all our animals for known protective Mamu-A and Mamu-B MHC class I alleles (Table 2). Surprisingly, we observed no specific association between the expression of protective MHC class I alleles and lower viral loads seen in short-term treated controller animals.
Table 2.
MHC Class I Alleles Expressed by Rhesus Macaques Used in This Study
| Animal | Group | A*01 | A*02 | A*08 | A*11 | B*01 | B*03 | B*04 | B*08 | B*17 | B*0290101 | B*07 | B*07301 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M637 | UT | − | + | − | − | − | + | − | − | − | − | − | − |
| M936 | UT | − | + | − | − | + | − | − | + | − | − | − | − |
| M937 | UT | − | − | + | − | + | − | − | − | + | − | − | − |
| M931 | ST-c | − | + | + | − | + | − | − | − | − | − | − | − |
| M934 | ST-c | − | + | − | − | + | − | − | − | − | − | − | − |
| M938 | ST-c | − | − | − | − | − | − | − | − | − | − | − | − |
| M926 | LT | − | − | − | − | − | − | − | − | − | + | − | − |
| M932 | LT | − | + | − | − | − | + | − | − | − | − | − | − |
| M933 | LT | − | − | − | − | − | − | − | − | − | + | − | − |
UT, untreated; ST-c, short-term treated controller; LT, late continuous treated.
Discussion
Acute infection is characterized by a massive loss of CD4 T cells, immune activation, and a significant amplification of viral infection.22,29,51–57 T regulatory cells are thought to play a major role in maintaining a state of homeostatic balance with changes in their prevalence likely contributing to disease pathogenesis.
Our results suggest that changes in prevalence of CD4 and CD8 T regulatory T cells occurs very early during the course of infection. Lending support to this argument is the significantly higher proportions and absolute numbers of CD8 T regulatory cells in the untreated and late-continuous treated group of animals. Though ART lowered proportions and absolute numbers of CD8 T regulatory cells in the late-continuous treated group of animals as compared to untreated animals, their prevalence was significantly higher than short-term treated controller animals. On the other hand, the prevalence of CD8 T regulatory cells in short-term treated controller animals did not differ from that of healthy animals. The persistence of CD8 T regulatory cells in the late-continuous treated group of animals suggests that by the time ART was initiated at 13 weeks pi, significant numbers of CD8 T regulatory cells were already induced.
Studies12,20 have shown that CD8+FoxP3+ T cells are highly immunosuppressive and correlate with diminished viral control in SIV-infected rhesus macaques. In line with these reports, untreated animals were found to display SIV-specific immune responses that were largely monofunctional, and predominantly CD8 T cell mediated. In contrast to untreated animals, short-term treated controller animals had significantly higher level of polyfunctional SIV-specific CD4 and CD8 T cell responses. Likewise, the short-term treated controller animals had better overall B cell responses as compared to the other groups.
Better immune responses alongside a normal prevalence of CD4 T regulatory cells posed a conundrum as CD4 T regulatory cells have been shown to suppress HIV-specific immune responses.58,59 Interestingly, however, Hartigan-O'Connor et al.9 have shown that CD4 T regulatory cells from rhesus macaques fail to suppress SIV-specific T cell responses, whereas others14 have shown that HIV+ elite controllers had low HIV-specific T cell activation with normal T regulatory cell levels yet maintain strong, polyfunctional T cell responses. It is important to point out that the proportions and absolute numbers of CD4 T regulatory cells in short-term controller animals, though significantly higher than untreated animals, were similar to the levels seen in healthy uninfected animals. Both the proportions and absolute numbers of total CD3+FoxP3+ T cells did not differ between the groups.
Unlike the untreated animals, short-term treated controller animals had significantly lower levels of immune activation that correlated with higher prevalence of CD4 T regulatory cells, suggesting a role for these cells in suppressing immune activation as shown by Hartigan-O'Connor et al.9 Numerous studies46 have shown that immune activation plays a major role in increased viral replication and disease progression. It is interesting to note that a decrease in the prevalence of CD4 T regulatory cells in untreated animals is accompanied by increased immune activation. Taken together, these results suggest that lower immune activation along with better immune responses likely contribute to the lower viral rebound in short-term treated controller animals.
It is difficult to determine why CD4+FoxP3+ T cells were less immunosuppressive than CD8+FoxP3+ T cells at this point. It is possible that the differences may be attributable to the way these two subsets mediate their action; CD4+FoxP3+ T cells likely suppress immune activation by secreting suppressive cytokines such as TGF-β into the local microenvironment.60 On the other hand, CD8+FoxP3+ T cells likely suppress immune responses by directly suppressing or killing SIV-specific CD4 T cells. In line with this argument, untreated animals had significantly lower levels of SIV-specific CD4 T cell responses as compared to short-term treated controller animals.
Previous studies61 have shown that HIV-specific CD8 T cell dysfunction was associated with the loss of HIV-specific CD4 T cells, and functional CD8 T cell responses could be restored with autologous HIV-specific CD4 T cells. On the other hand, Cantor et al.62 showed that certain subpopulations of CD8 T cells specifically inhibited CD4 T helper responses, whereas Noble et al.63 showed that this inhibition was mediated by the recognition of Qa-1, a nonclassical MHC class 1 molecule, on target CD4 T cells by CD8 T regulatory cells. In other disease states such as experimental autoimmune encephalomyelitis, CD8 T regulatory cells have been shown to directly lyse encephalitogenic CD4 T cells in both human and mouse models.64,65 Others12 have demonstrated that CD8+FoxP3+ T cells suppressed SIV-specific immune responses that correlated with diminished viral control in SIV-infected rhesus macaques. Garba et al.66 showed that HIV antigens could induce TGF-β-producing CD8+ T regulatory cells that can suppress HIV-specific immune responses, whereas Estes et al.67,68 demonstrated increased levels of TGF-β during SIV infection.
The mechanisms for the decrease in CD4+FoxP3+ T cells in untreated animals are not yet clear. It is possible that peripheral CD4+FoxP3+ T cells redistribute to secondary lymphoid organs such as the mucosa, or these cells may be infected and destroyed during SIV infection. Studies have shown that SIV and HIV infections are associated with an increase in CD4+FoxP3+ T cells in the mucosa.6,69,70 On the other hand, Moreno-Fernandez et al.71 showed that human regulatory T cells are targets for HIV infection, whereas Allers et al.72 showed that mucosal T regulatory cells are less susceptible to productive SIV infection, and are selectively spared from SIV-mediated death. Though the exact mechanisms for the loss of CD4+FoxP3+ T cells is not clear from our study, our results suggest that their loss correlated with increased immune activation and higher viral loads. In line with this argument, Baker et al.26 found that loss of CD4+ T regulatory cells was associated with persistent viremia in HIV-infected patients.
The lower viral rebound seen in the short-term treated controller animals could be due to other factors such as the lower pool of infected memory CD4 T cells. Though a smaller pool of infected cells is likely to contribute to the lower viral rebound, recent studies suggest that other factors may play a role in the differential viral rebound seen in short-term treated controller vs. noncontroller animals. Kubo et al.23 showed that when ART was initiated at 48 h pi, and infection was completely suppressed to levels below detection, removal of ART after 4 weeks was associated with a lower viral rebound in three short-term controller rhesus macaques (∼2–3 logs/ml of plasma) and higher viral loads in three noncontroller animals (∼5–6 logs/ml of plasma). Interestingly, all six animals had very few infected memory CD4 T cells at the time of initiation of ART and during 4 weeks of ART, ∼10−1 copies of SIV/105 memory CD4 T cells, suggesting that both groups of animals had equally low pools of latently infected cells. Yet the removal of ART was associated with a differential rebound in viremia. Though, Kubo et al.23 found an association between lower viral loads and expression of protective MHC class I alleles such as Mamu-B*2901 and B*17, we did not find any specific association in our study (Table 2).
In conclusion, our results demonstrate that suppression of viral replication early during the course of infection significantly influences the course of disease and viral set point. Lower viral replication preserved the CD4 T helper compartment, decreased immune activation, and prevented the emergence of immunosuppressive CD8+FoxP3+ T cells in controller animals, thereby contributing to better immune responses that likely play a role in the lower viral rebound seen in short-term controller animals. Taken together, our findings suggest that early therapy can lead to better long-term outcome and viral control in HIV infection.
Supplementary Material
Acknowledgments
We thank Sandra Bixler, Andrew Moore, and Muhamuda Kader at Uniformed Services University for assistance in processing some of the samples and assays; Jeffrey Lifson and Michael Piatak for assistance with plasma viral load assays; Malcolm Martin at the Laboratory of Molecular Microbiology for some uninfected samples; Karen Wolcott and Kateryna Lund at the Biomedical Instrumentation Center; and Dr. Deborah Weiss and Jim Treece at ABL, Inc., Rockville, MD for expert assistance with the animals. The studies described here were supported by Grants AI07812 from National Institutes of Allergy and Infectious diseases (NIAID) and DE018339 and DE019397 from the National Institute of Dental and Craniofacial Research (NIDCR) awarded to J.J.M. This research was supported in part by the Intramural Research Program of the NIH–NIAID and NCI. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIAID, NIDCR, NCI, or the National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Mills KH. Regulatory T cells: Friend or foe in immunity to infection? Nat Rev Immunol. 2004;4(11):841–855. doi: 10.1038/nri1485. [DOI] [PubMed] [Google Scholar]
- 2.Morgan ME. van Bilsen JH. Bakker AM, et al. Expression of FOXP3 mRNA is not confined to CD4+CD25+ T regulatory cells in humans. Hum Immunol. 2005;66(1):13–20. doi: 10.1016/j.humimm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 3.Bacchetta R. Passerini L. Gambineri E, et al. Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J Clin Invest. 2006;116(6):1713–1722. doi: 10.1172/JCI25112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elrefaei M. Ventura FL. Baker CA. Clark R. Bangsberg DR. Cao H. HIV-specific IL-10-positive CD8+ T cells suppress cytolysis and IL-2 production by CD8+ T cells. J Immunol. 2007;178(5):3265–3271. doi: 10.4049/jimmunol.178.5.3265. [DOI] [PubMed] [Google Scholar]
- 5.Andersson J. Boasso A. Nilsson J, et al. The prevalence of regulatory T cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J Immunol. 2005;174(6):3143–3147. doi: 10.4049/jimmunol.174.6.3143. [DOI] [PubMed] [Google Scholar]
- 6.Boasso A. Vaccari M. Hryniewicz A, et al. Regulatory T-cell markers, indoleamine 2,3-dioxygenase, and virus levels in spleen and gut during progressive simian immunodeficiency virus infection. J Virol. 2007;81(21):11593–11603. doi: 10.1128/JVI.00760-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cecchinato V. Tryniszewska E. Ma ZM, et al. Immune activation driven by CTLA-4 blockade augments viral replication at mucosal sites in simian immunodeficiency virus infection. J Immunol. 2008;180(8):5439–5447. doi: 10.4049/jimmunol.180.8.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunham RM. Cervasi B. Brenchley JM, et al. CD127 and CD25 expression defines CD4+ T cell subsets that are differentially depleted during HIV infection. J Immunol. 2008;180(8):5582–5592. doi: 10.4049/jimmunol.180.8.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartigan-O'Connor DJ. Abel K. McCune JM. Suppression of SIV-specific CD4+ T cells by infant but not adult macaque regulatory T cells: Implications for SIV disease progression. J Exp Med. 2007;204(11):2679–2692. doi: 10.1084/jem.20071068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinter AL. Hennessey M. Bell A, et al. CD25(+)CD4(+) regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4(+) and CD8(+) HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J Exp Med. 2004;200(3):331–343. doi: 10.1084/jem.20032069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macatangay BJ. Szajnik ME. Whiteside TL. Riddler SA. Rinaldo CR. Regulatory T cell suppression of Gag-specific CD8 T cell polyfunctional response after therapeutic vaccination of HIV-1-infected patients on ART. PLoS One. 2010;5(3):e9852. doi: 10.1371/journal.pone.0009852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nigam P. Velu V. Kannanganat S, et al. Expansion of FOXP3+ CD8 T cells with suppressive potential in colorectal mucosa following a pathogenic simian immunodeficiency virus infection correlates with diminished antiviral T cell response and viral control. J Immunol. 2010;184(4):1690–1701. doi: 10.4049/jimmunol.0902955. [DOI] [PubMed] [Google Scholar]
- 13.Nilsson J. Boasso A. Velilla PA, et al. HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood. 2006;108(12):3808–3817. doi: 10.1182/blood-2006-05-021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oswald-Richter K. Grill SM. Shariat N, et al. HIV infection of naturally occurring and genetically reprogrammed human regulatory T-cells. PLoS Biol. 2004;2(7):E198. doi: 10.1371/journal.pbio.0020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aandahl EM. Michaelsson J. Moretto WJ. Hecht FM. Nixon DF. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J Virol. 2004;78(5):2454–2459. doi: 10.1128/JVI.78.5.2454-2459.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chase AJ. Sedaghat AR. German JR, et al. Severe depletion of CD4+ CD25+ regulatory T cells from the intestinal lamina propria but not peripheral blood or lymph nodes during acute simian immunodeficiency virus infection. J Virol. 2007;81(23):12748–12757. doi: 10.1128/JVI.00841-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chase AJ. Yang HC. Zhang H. Blankson JN. Siliciano RF. Preservation of FoxP3+ regulatory T cells in the peripheral blood of human immunodeficiency virus type 1-infected elite suppressors correlates with low CD4+ T-cell activation. J Virol. 2008;82(17):8307–8315. doi: 10.1128/JVI.00520-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pereira LE. Villinger F. Onlamoon N, et al. Simian immunodeficiency virus (SIV) infection influences the level and function of regulatory T cells in SIV-infected rhesus macaques but not SIV-infected sooty mangabeys. J Virol. 2007;81(9):4445–4456. doi: 10.1128/JVI.00026-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao W. Jamieson BD. Hultin LE. Hultin PM. Detels R. Regulatory T cell expansion and immune activation during untreated HIV type 1 infection are associated with disease progression. AIDS Res Hum Retroviruses. 2009;25(2):183–191. doi: 10.1089/aid.2008.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karlsson I. Malleret B. Brochard P, et al. FoxP3+ CD25+ CD8+ T-cell induction during primary simian immunodeficiency virus infection in cynomolgus macaques correlates with low CD4+ T-cell activation and high viral load. J Virol. 2007;81(24):13444–13455. doi: 10.1128/JVI.01466-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim A. French MA. Price P. CD4+ and CD8+ T cells expressing FoxP3 in HIV-infected patients are phenotypically distinct and influenced by disease severity and antiretroviral therapy. J Acquir Immune Defic Syndr. 2009;51(3):248–257. doi: 10.1097/QAI.0b013e3181a74fad. [DOI] [PubMed] [Google Scholar]
- 22.Kader M. Hassan WM. Eberly M, et al. Antiretroviral therapy prior to acute viral replication preserves CD4 T cells in the periphery but not in rectal mucosa during acute simian immunodeficiency virus infection. J Virol. 2008;82(22):11467–11471. doi: 10.1128/JVI.01143-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubo M. Nishimura Y. Shingai M, et al. Initiation of antiretroviral therapy 48 hours after infection with simian immunodeficiency virus potently suppresses acute-phase viremia and blocks the massive loss of memory CD4+ T cells but fails to prevent disease. J Virol. 2009;83(14):7099–7108. doi: 10.1128/JVI.02522-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lifson JD. Piatak M., Jr. Cline AN, et al. Transient early post-inoculation anti-retroviral treatment facilitates controlled infection with sparing of CD4+ T cells in gut-associated lymphoid tissues in SIVmac239-infected rhesus macaques, but not resistance to rechallenge. J Med Primatol. 2003;32(4–5):201–210. doi: 10.1034/j.1600-0684.2003.00026.x. [DOI] [PubMed] [Google Scholar]
- 25.Verhoeven D. Sankaran S. Silvey M. Dandekar S. Antiviral therapy during primary SIV infection fails to prevent acute CD4+ T-cell loss in gut mucosa but enhances their rapid restoration through central memory T-cells. J Virol. 2008;82(8):4016–4027. doi: 10.1128/JVI.02164-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker CA. Clark R. Ventura F, et al. Peripheral CD4 loss of regulatory T cells is associated with persistent viraemia in chronic HIV infection. Clin Exp Immunol. 2007;147(3):533–539. doi: 10.1111/j.1365-2249.2006.03319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaardbo JC. Nielsen SD. Vedel SJ, et al. Regulatory T cells in human immunodeficiency virus-infected patients are elevated and independent of immunological and virological status, as well as initiation of highly active anti-retroviral therapy. Clin Exp Immunol. 2008;154(1):80–86. doi: 10.1111/j.1365-2249.2008.03725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolte L. Gaardbo JC. Skogstrand K. Ryder LP. Ersboll AK. Nielsen SD. Increased levels of regulatory T cells (Tregs) in human immunodeficiency virus-infected patients after 5 years of highly active anti-retroviral therapy may be due to increased thymic production of naive Tregs. Clin Exp Immunol. 2009;155(1):44–52. doi: 10.1111/j.1365-2249.2008.03803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mattapallil JJ. Douek DC. Hill B. Nishimura Y. Martin M. Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434(7037):1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 30.Endo Y. Igarashi T. Nishimura Y, et al. Short- and long-term clinical outcomes in rhesus monkeys inoculated with a highly pathogenic chimeric simian/human immunodeficiency virus. J Virol. 2000;74(15):6935–6945. doi: 10.1128/jvi.74.15.6935-6945.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perfetto SP. Chattopadhyay PK. Lamoreaux L, et al. Amine reactive dyes: An effective tool to discriminate live and dead cells in polychromatic flow cytometry. J Immunol Methods. 2006;313(1–2):199–208. doi: 10.1016/j.jim.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Pitcher CJ. Hagen SI. Walker JM, et al. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;168(1):29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 33.Lafont BA. McGraw CM. Stukes SA, et al. The locus encoding an oligomorphic family of MHC-A alleles (Mane-A*06/Mamu-A*05) is present at high frequency in several macaque species. Immunogenetics. 2007;59(3):211–223. doi: 10.1007/s00251-007-0190-1. [DOI] [PubMed] [Google Scholar]
- 34.Loffredo JT. Maxwell J. Qi Y, et al. Mamu-B*08-positive macaques control simian immunodeficiency virus replication. J Virol. 2007;81(16):8827–8832. doi: 10.1128/JVI.00895-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ourmanov I. Kuwata T. Goeken R, et al. Improved survival in rhesus macaques immunized with modified vaccinia virus Ankara recombinants expressing simian immunodeficiency virus envelope correlates with reduction in memory CD4+ T-cell loss and higher titers of neutralizing antibody. J Virol. 2009;83(11):5388–5400. doi: 10.1128/JVI.02598-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaizu M. Borchardt GJ. Glidden CE, et al. Molecular typing of major histocompatibility complex class I alleles in the Indian rhesus macaque which restrict SIV CD8+ T cell epitopes. Immunogenetics. 2007;59(9):693–703. doi: 10.1007/s00251-007-0233-7. [DOI] [PubMed] [Google Scholar]
- 37.Florese RH. Demberg T. Xiao P, et al. Contribution of nonneutralizing vaccine-elicited antibody activities to improved protective efficacy in rhesus macaques immunized with Tat/Env compared with multigenic vaccines. J Immunol. 2009;182(6):3718–3727. doi: 10.4049/jimmunol.0803115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Florese RH. Van Rompay KK. Aldrich K, et al. Evaluation of passively transferred, nonneutralizing antibody-dependent cellular cytotoxicity-mediating IgG in protection of neonatal rhesus macaques against oral SIVmac251 challenge. J Immunol. 2006;177(6):4028–4036. doi: 10.4049/jimmunol.177.6.4028. [DOI] [PubMed] [Google Scholar]
- 39.Gomez-Roman VR. Florese RH. Patterson LJ, et al. A simplified method for the rapid fluorometric assessment of antibody-dependent cell-mediated cytotoxicity. J Immunol Methods. 2006;308(1–2):53–67. doi: 10.1016/j.jim.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 40.Gomez-Roman VR. Florese RH. Peng B, et al. An adenovirus-based HIV subtype B prime/boost vaccine regimen elicits antibodies mediating broad antibody-dependent cellular cytotoxicity against non-subtype B HIV strains. J Acquir Immune Defic Syndr. 2006;43(3):270–277. doi: 10.1097/01.qai.0000230318.40170.60. [DOI] [PubMed] [Google Scholar]
- 41.Hidajat R. Xiao P. Zhou Q, et al. Correlation of vaccine-elicited systemic and mucosal nonneutralizing antibody activities with reduced acute viremia following intrarectal simian immunodeficiency virus SIVmac251 challenge of rhesus macaques. J Virol. 2009;83(2):791–801. doi: 10.1128/JVI.01672-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiao P. Zhao J. Patterson LJ, et al. Multiple vaccine-elicited nonneutralizing antienvelope antibody activities contribute to protective efficacy by reducing both acute and chronic viremia following simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J Virol. 2010;84(14):7161–7173. doi: 10.1128/JVI.00410-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu X. Zhou T. O'Dell S. Wyatt RT. Kwong PD. Mascola JR. Mechanism of human immunodeficiency virus type 1 resistance to monoclonal antibody B12 that effectively targets the site of CD4 attachment. J Virol. 2009;83(21):10892–10907. doi: 10.1128/JVI.01142-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li M. Gao F. Mascola JR, et al. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2005;79(16):10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shu Y. Winfrey S. Yang ZY, et al. Efficient protein boosting after plasmid DNA or recombinant adenovirus immunization with HIV-1 vaccine constructs. Vaccine. 2007;25(8):1398–1408. doi: 10.1016/j.vaccine.2006.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Douek DC. Picker LJ. Koup RA. T cell dynamics in HIV-1 infection. Annu Rev Immunol. 2003;21:265–304. doi: 10.1146/annurev.immunol.21.120601.141053. [DOI] [PubMed] [Google Scholar]
- 47.Malhotra U. Holte S. Dutta S, et al. Role for HLA class II molecules in HIV-1 suppression and cellular immunity following antiretroviral treatment. J Clin Invest. 2001;107(4):505–517. doi: 10.1172/JCI11275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oxenius A. Price DA. Easterbrook PJ, et al. Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc Natl Acad Sci USA. 2000;97(7):3382–3387. doi: 10.1073/pnas.97.7.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenberg ES. Altfeld M. Poon SH, et al. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407(6803):523–526. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- 50.Rosenberg ES. Billingsley JM. Caliendo AM, et al. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278(5342):1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 51.Brenchley JM. Schacker TW. Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200(6):749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eberly MD. Kader M. Hassan W, et al. Increased IL-15 production is associated with higher susceptibility of memory CD4 T cells to simian immunodeficiency virus during acute infection. J Immunol. 2009;182(3):1439–1448. doi: 10.4049/jimmunol.182.3.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guadalupe M. Reay E. Sankaran S, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77(21):11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kader M. Wang X. Piatak M, Jr., et al. a4+b7hi CD4+ memory T cells harbor most Th–17 cells and are preferentially infected during acute SIV infection. Mucosal Immunol. 2009. [DOI] [PMC free article] [PubMed]
- 55.Li Q. Duan L. Estes JD, et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434(7037):1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 56.Mehandru S. Poles MA. Tenner-Racz K, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200(6):761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Veazey RS. DeMaria M. Chalifoux LV, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280(5362):427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 58.Kinter A. McNally J. Riggin L. Jackson R. Roby G. Fauci AS. Suppression of HIV-specific T cell activity by lymph node CD25+ regulatory T cells from HIV-infected individuals. Proc Natl Acad Sci USA. 2007;104(9):3390–3395. doi: 10.1073/pnas.0611423104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kinter AL. Horak R. Sion M, et al. CD25+ regulatory T cells isolated from HIV-infected individuals suppress the cytolytic and nonlytic antiviral activity of HIV-specific CD8+ T cells in vitro. AIDS Res Hum Retroviruses. 2007;23(3):438–450. doi: 10.1089/aid.2006.0162. [DOI] [PubMed] [Google Scholar]
- 60.Tang Q. Bluestone JA. The Foxp3+ regulatory T cell: A jack of all trades, master of regulation. Nat Immunol. 2008;9(3):239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lichterfeld M. Kaufmann DE. Yu XG, et al. Loss of HIV-1-specific CD8+ T cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1-specific CD4+ T cells. J Exp Med. 2004;200(6):701–712. doi: 10.1084/jem.20041270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cantor H. Shen FW. Boyse EA. Separation of helper T cells from suppressor T cells expressing different Ly components. II. Activation by antigen: After immunization, antigen-specific suppressor and helper activities are mediated by distinct T-cell subclasses. J Exp Med. 1976;143(6):1391–1340. doi: 10.1084/jem.143.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Noble A. Zhao ZS. Cantor H. Suppression of immune responses by CD8 cells. II. Qa-1 on activated B cells stimulates CD8 cell suppression of T helper 2 responses. J Immunol. 1998;160(2):566–571. [PubMed] [Google Scholar]
- 64.Koh DR. Fung-Leung WP. Ho A. Gray D. Acha-Orbea H. Mak TW. Less mortality but more relapses in experimental allergic encephalomyelitis in CD8-/- mice. Science. 1992;256(5060):1210–1213. doi: 10.1126/science.256.5060.1210. [DOI] [PubMed] [Google Scholar]
- 65.Penninger JM. Neu N. Timms E, et al. The induction of experimental autoimmune myocarditis in mice lacking CD4 or CD8 molecules [corrected] J Exp Med. 1993;178(5):1837–1842. doi: 10.1084/jem.178.5.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garba ML. Pilcher CD. Bingham AL. Eron J. Frelinger JA. HIV antigens can induce TGF-beta(1)-producing immunoregulatory CD8+ T cells. J Immunol. 2002;168(5):2247–2254. doi: 10.4049/jimmunol.168.5.2247. [DOI] [PubMed] [Google Scholar]
- 67.Estes JD. Li Q. Reynolds MR, et al. Premature induction of an immunosuppressive regulatory T cell response during acute simian immunodeficiency virus infection. J Infect Dis. 2006;193(5):703–712. doi: 10.1086/500368. [DOI] [PubMed] [Google Scholar]
- 68.Estes JD. Wietgrefe S. Schacker T, et al. Simian immunodeficiency virus-induced lymphatic tissue fibrosis is mediated by transforming growth factor beta 1-positive regulatory T cells and begins in early infection. J Infect Dis. 2007;195(4):551–561. doi: 10.1086/510852. [DOI] [PubMed] [Google Scholar]
- 69.Epple HJ. Loddenkemper C. Kunkel D, et al. Mucosal but not peripheral FOXP3+ regulatory T cells are highly increased in untreated HIV infection and normalize after suppressive HAART. Blood. 2006;108(9):3072–3078. doi: 10.1182/blood-2006-04-016923. [DOI] [PubMed] [Google Scholar]
- 70.Favre D. Lederer S. Kanwar B, et al. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009;5(2):e1000295. doi: 10.1371/journal.ppat.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moreno-Fernandez ME. Zapata W. Blackard JT. Franchini G. Chougnet CA. Human regulatory T cells are targets for human immunodeficiency virus (HIV) infection, and their susceptibility differs depending on the HIV type 1 strain. J Virol. 2009;83(24):12925–12933. doi: 10.1128/JVI.01352-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Allers K. Loddenkemper C. Hofmann J, et al. Gut mucosal FOXP3+ regulatory CD4+ T cells and nonregulatory CD4+ T cells are differentially affected by simian immunodeficiency virus infection in rhesus macaques. J Virol. 2010;84(7):3259–3269. doi: 10.1128/JVI.01715-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.