Abstract
Background
Gonadotropin receptors, unlike the thyrotropin receptor (TSHR), are not cleaved into disulfide-linked A- and B-subunits, nor do they shed A-subunits. Heavily glycosylated TSHR A-subunits initiate or amplify responses leading to stimulating TSHR-autoantibodies and Graves' hyperthyroidism.
Methods
To investigate the possibility that mice immunized with luteinizing hormone receptor (LHR) would develop functional antibodies, we constructed adenoviruses expressing the rat-LH holoreceptor (LHR-Ad) and an LHR A-subunit equivalent (LHR-289-Ad). Female BALB/c mice were immunized with high doses (1011 particles) of LHR-Ad, LHR-289-Ad, or control (Con)-Ad. Sera were tested using LHR-expressing eukaryotic cells for antibody binding by flow cytometry and for bioactivity by measuring cyclic adenosine monophosphate (cAMP) stimulation.
Results
Elevated serum binding to LHR cells in some LHR-Ad and LHR-289-Ad immunized mice was not specific for LHR-expressing cells. Moreover, sera lacked bioactivity, consistent with unchanged serum estradiol and ovary histology. The difference between rat and mouse LHR-ectodomains is relatively small (3% at the amino-acid level). In contrast, despite amino-acid identity, immunization of mice with adenovirus expressing membrane-bound mouse thyroid peroxidase (TPO), but not soluble mouse TPO ectodomain, elicited strong TPO-specific antibodies.
Conclusions
Our investigations provide insight into antibody responses to self-antigens. First, antibodies are induced to large self-antigens like mouse-TPO when membrane bound. Second, lesser amino acid homology between the immunogen and mouse protein (91% vs. 97% for the human-TSHR and rat-LHR, respectively) favors antibody induction. Finally, from previous studies demonstrating the immunogenicity of the highly glycosylated human TSHR A-subunit versus our present data for the nonimmunogenic less glycosylated rat LHR, we suggest that the extent of glycosylation contributes to breaking self-tolerance.
Introduction
Humoral autoimmunity to the thyrotropin receptor (TSHR) is very common in humans. In particular, thyroid-stimulating autoantibodies (TSAb) are the direct cause of Graves' disease, one of the most common autoimmune diseases affecting humans. More rarely, TSHR-blocking autoantibodies can cause hypothyroidism [reviewed in ref. (1)]. Remarkably, despite high structural similarity between the glycoprotein hormone receptors, functionally significant (blocking or stimulating) autoantibodies to the latter receptors have been sought for many years, without success [e.g., ref. (2)] and no Graves' disease of the gonads has been reported.
A structural difference between the TSHR and the gonadotropin receptors may explain, at least in part, the basis for this dichotomy. Only the TSHR, not the luteinizing hormone/human choriogonadotropin-receptor (LHR) or follicle stimulating hormone-receptor (FSH) receptors, undergo intramolecular cleavage on the cell surface into disulfide linked A- and B-subunits [reviewed in ref. (1)]. Experimental evidence suggests that subsequent shedding of the heavily glycosylated A-subunits plays a role in initiating or amplifying the autoimmune response to the TSHR in genetically susceptible individuals. TSAb preferentially recognize the shed A-subunit relative to the TSH holoreceptor (3). Further, immunizing mice with adenovirus encoding the TSHR A-subunit induces Graves'-like hyperthyroidism more effectively than adenovirus expressing a TSHR modified so as not to cleave or shed (4).
As mentioned above, the LHR does not cleave into subunits and shed part of its ectodomain (ECD). With this background, the present study was performed to test whether immunizing mice with an adenovirus expressing that portion of the LHR ECD equivalent to the shed TSHR A-subunit would induce stimulating antibodies to the LHR. For comparison, we also immunized mice with an adenovirus expressing the LH holoreceptor. We used the rat LHR because of our previous studies with this species of receptor [e.g., refs. (5,6)], because of significant amino acid differences between the rat and mouse LHR A-subunit equivalents, and because the adjuvant properties of the adenovirus vector would contribute to breaking tolerance to a protein from a relatively close animal species. For example, immunizing mice with adenovirus expressing mouse thyroid peroxidase (TPO) readily generates TPO-specific antibodies (7). Although we did not generate LHR antibodies, our results provide insight into the requirements for breaking tolerance to self-antigens.
Materials and Methods
Construction of adenoviruses expressing the rat LHR, an ECD variant (LHR-289) and the mouse TPO ECD
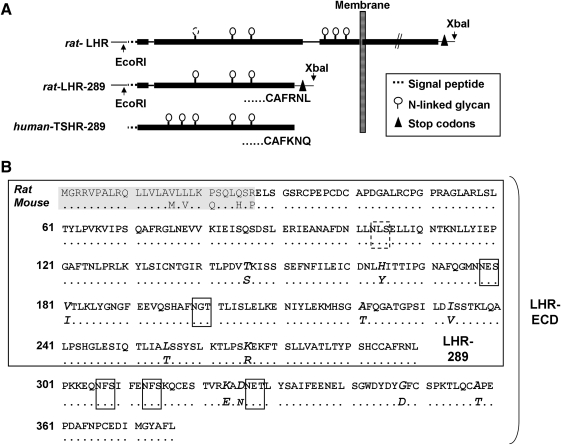
We constructed a rat LHR ECD variant equivalent to the TSHR A-subunit, (TSHR-289) based on an alignment between the human TSHR and the rat LHR (Fig. 1A). This fragment, termed “LHR-289,” contains 289 amino acids (including the signal peptide) (Fig. 1B). From the rat LHR in pSVneo-ECE (8), the polymerase chain reaction was used to amplify full length LHR and LHR-289 cDNA incorporating the 5′ restriction site EcoRI, two stop codons, and an XbaI restriction site at the 3′ end. The cDNAs for LHR and LHR-289 were digested with EcoRI (New England Biolabs, Beverly, MA), blunted with DNA polymerase Klenow fragment (USB, Cleveland, OH), digested with XbaI (New England Biolabs), and ligated into the shuttle vector pHMCMV6 (9). The correct orientation and sequences of the inserts was confirmed by restriction digests and nucleotide sequencing. Insert-positive plasmids were digested with I-Ceu I and PI-Sce I (BD Biosciences Clontech, Palo Alto, CA) and ligated into the same sites of pAdHM4CMV (10). The mouse TPO ECD, residues 1-848, was introduced into the same adenovirus vector to generate mouse-TPO-ECD-Ad using the approach previously reported for the generation of adenovirus expressing the holoenzyme (7), whose cDNA was kindly provided by Dr. S. Ohtaki (Miyazaki Medical College, Japan) (11). Adenovirus vectors were linearized with PacI (New England Biolabs) and transfected into human embryonal kidney 293 cells (HEK293; American Type Culture Collection, Manassas, VA) with Superfect (QIAGEN, Valencia, CA). Adenoviruses with cDNA inserts as well as control adenovirus lacking a cDNA insert (Con-Ad) (12) were propagated in HEK293 cells and purified by CsCl-gradient centrifugation. Viral particle concentration was determined by measuring the absorbance at 260 nm (13).
FIG. 1.
(A) Schematic representation of the rat LHR, the human-TSHR A-subunit (TSHR-289), and the rat-A-subunit LHR equivalent, LHR-289. Included are the restriction sites introduced to clone the LHR and LHR-289 into the shuttle vector, the terminal 6 amino acid residues of the TSHR- and LHR-A-subunits, as well as the 6 putative N-glycans. Because the first N-glycan is not glycosylated in the rat LHR (40), it is depicted with dashed lines (and in a dashed box in panel B). (B) Homologies between the rat- and mouse-LHR ECD and LHR-289. Identical amino acids are indicated by dots; amino acid differences in the mouse LHR are in italics. The signal peptide (first 27 amino acids) is shaded. N-linked glycan motifs are boxed. LHR, luteinizing hormone receptor; TSHR, thyrotropin receptor; ECD, ectodomain.
To confirm LHR expression by adenovirus, COS-7 cells (American Type Culture Collection) were cultured in Dulbecco's minimal essential medium + 10% fetal calf serum and infected with LHR-Ad (2 × 1010 particles per 5 × 106 cells). Highly purified human chorionic gonadotropin (hCG) (14900 IU/mg, National Hormone Distribution Program) was labeled with 125I (Perkin-Elmer, Covina, CA) to a specific activity of ∼20 μCi/μg using IodoGen (Pierce, Rockford, IL). Free iodine was separated from radiolabeled hormone using a desalting column (Bio- Spin 6; Bio-Rad, Hercules, CA). After 24 hours, the cells were lifted from their plastic supports using trypsin and then transferred to 24-well culture plates, and 125I-hCG binding was determined the following day. Cells were incubated for 3 hours with 125I-hCG (10,000 cpm) in NaCl-free buffer (containing 242 mM sucrose and 0.1% bovine serum albumin) in the absence or presence of increasing concentrations of unlabeled hCG, as previously described (8). After rinsing with ice-cold buffer, cells were solublized with 0.25 mL 1 M NaOH and radioactivity was counted. Specific binding was determined by subtracting the value for 125I-labeled hCG binding in the presence of 10−6 M unlabeled hCG. Percentage labeled hCG bound was calculated and the binding affinity (Kd) determined by Scatchard analysis (14).
Immunization of mice to induce antibodies to the LHR
Female BALB/c mice (6–7 weeks old; Jackson Laboratories, Bar Harbor, Maine) were injected intramuscularly with LHR-Ad, LHR-289-Ad, or Con-Ad [adenovirus lacking an insert (12)] on three occasions, at three weekly intervals, with a moderate or high dose [1010 or 1011 particles/injection], respectively), as described for TSHR A-subunit-Ad (15). Blood was drawn 1 week after the second injection. Four weeks after the third injection, vaginal smears were examined daily to establish when an individual animal was in metestrus or diestrous, at which time the mouse was euthanized to obtain blood and ovaries. Euthanizing mice at this stage of the estrus cycle ensured minimal levels of estradiol in control adenovirus immunized animals.
The BALB/c strain was selected for LHR immunization because numerous studies showed successful induction of antibodies to the related TSHR in this strain by conventional immunization, injecting TSHR-expressing M12 B cells, and immunization with DNA for the TSHR or its A-subunit in plasmid or adenoviral vectors [reviewed in ref. (16)]. In addition, monoclonal LHR antibodies had been isolated from BALB/c mice immunized with Freund's complete adjuvant and complexes of purified porcine LHR + hCG (17) or partially purified rat LHR (18), as well as by injecting Chinese hamster ovary (CHO) cells expressing the LHR (19).
Another issue concerns the possibility that high endogenous LH levels in female mice could bind to the LHR and possibly attenuate the antibody response. It should be appreciated that the estrus cycle in rodents is short (4–5 days) (20). Our protocol involves three immunizations at three weekly intervals, and the estrus cycles of the individual mice studied were clearly different because it was necessary to euthanize them on different days to ensure that all animals were in metestrus or diestrus (see above). Moreover, antibodies were generated by conventional immunization of a BALB/c mouse using purified LHR complexed with hCG (17) and by immunizing females of the same strain with LHR-expressing CHO cells (19). All animal studies (described above for immunization against the LHR and below for the mouse TPO ECD) were approved by the Institutional Animal Care and Use Committee and performed with the highest standards of care in a pathogen-free facility.
Immunization using the mouse TPO-ECD
Immunization with mouse-TPO-ECD-Ad was performed in female C57BL/6 mice (aged 6–7 weeks; Jackson Laboratories). As previously described for mouse-TPO-Ad and Con-Ad (7), mice were injected intramuscularly with mouse TPO-ECD-Ad (5 × 1010 particles/injection) on three occasions, at three weekly intervals and euthanized 4 weeks after the third immunization.
Eukaryotic cells stably expressing the LHR
CHO cells stably expressing the rat LHR were generated using the Flp-In system (Invitrogen, Carlsbad CA) as follows: the wild-type rat LHR cDNA in pSV2neo-ECE (8) was cut with EcoRI, blunted, then digested with XbaI, and transferred to the pcDNA5/FRT vector. This construct was co-transfected with the poG44 plasmid using FuGENE HD (Roche, Indianapolis, IN) into Flp-In CHO host cells and selected with Hygromycin-B (∼300 μg/mL) according to the protocol of the manufacturer (Invitrogen). Culture medium was F-12 supplemented with 10% fetal bovine serum, penicillin (100 U/mL), gentamycin (50 μg/mL), and fungizone (2.5 μg/mL).
LHR expression by CHO cells was confirmed by measuring the hCG-induced cyclic adenosine monophosphate (cAMP) responses as follows: LHR-CHO cells were plated in 10 cm tissue culture dishes overnight, transferred to 96-well plates, and studied approximately 24 hours later. For bioassay, the culture medium was replaced with F12 medium supplemented with 1 mM isobutyl methylxanthine, 10 mM HEPES, 0.3% bovine serum albumin, and human hCG (Sigma-Aldrich, St. Louis, MO). After 2 h at 37°C, the medium was aspirated and intracellular cAMP was extracted with 0.2 mL 95% ethanol. The extracts were evaporated to dryness and resuspended in 0.2 mL Dulbecco's phosphate-buffered saline (pH 7.5), and assayed as described previously for TSH antibody responses to the TSHR (21) using the LANCE cAMP kit according to the protocol of the manufacturer (Perkin-Elmer, Shelton, CT).
Incidentally, in our earlier studies (data not shown), we tested polyclonal and monoclonal antibodies as positive controls for LHR expression by flow cytometry. Murine hybridomas LHR McAb 29 and 1055 (American Type Tissue Culture, Manassas, VA) were cultured and immunoglobulin G (IgG) purified from supernatants using Protein G. These LHR monoclonals, generated by immunizing mice with human LH/hCG expressed in Escherichia coli, recognize amino acids 21–362 (22). However, neither monoclonal antibody was positive by flow cytometry using LHR-expressing CHO cells (determined by 125-I hCG binding). In previous studies, other monoclonals provided by Dr. E. Milgrom's group (National Institute of Health and Medical Research [INSERM], Unit 135, Hospital of Bicêtre, Le Kremlin-Bicêtre, France) and polyclonal rabbit antibodies from Dr. D.L. Segaloff (University of Iowa College of Medicine, Iowa City, IA) were likewise negative by flow cytometry. There was no response from Dr. J. Wimalesena to our request for his mouse monoclonal LHR antibodies (18).
Testing for antibody binding to the LHR and mouse TPO in sera from immunized mice
LHR-expressing CHO cells were removed from their plastic supports and incubated with sera (1:50 dilution); after washing, antibody binding was detected with goat anti-mouse-fluorescein-isothiocyanata (Caltag, Burlingame, CA). Negative controls included cells without antibody, second antibody alone, sera from Con-Ad immunized mice, and, in some experiments, untransfected CHO cells. Antibody binding to mouse TPO was studied in the same way using CHO-cells stably expressing the mouse TPO holoenzyme on their surface (7). Flow cytometry was performed (10,000 events) using a FACScan flow cytofluorimeter (Becton-Dickinson, Franklin Lakes, NJ). Data are reported as the geometric mean fluorescence (Geo Mean).
Ovary histology, estradiol, and serum bioactivity
The genitalia were removed at euthanasia and fixed in buffered formaldehyde (pH 7.4). The ovaries were embedded in paraffin wax and 16–18 serial sections for each mouse were stained with hematoxylin and eosin. Estradiol was measured by a radioimmunoassay (Diagnostic Systems Laboratories, Webster, TX) modified to use 50 μL (rather than 200 μL) of mouse serum, as described (23). Sera from mice immunized with LHR-Ad and LHR-A-subunit-Ad were tested (1:20 dilution; duplicate aliquots) for bioactivity by incubation with LHR-CHO cells and measuring cAMP production (as described above for hCG responses). In addition, their ability to block stimulation by hCG was tested by measuring cAMP responses to the same sera (1:20 dilution) in the presence of hCG (20 mU/mL).
Statistical analyses
The significance of differences between the magnitude of responses in different groups was determined by Mann–Whitney Rank Sum test or, when normally distributed, by Student's t-test. Tests were performed using SigmaStat (Jandel Scientific Software, San Rafael, CA).
Results
Recombinant LHR expression
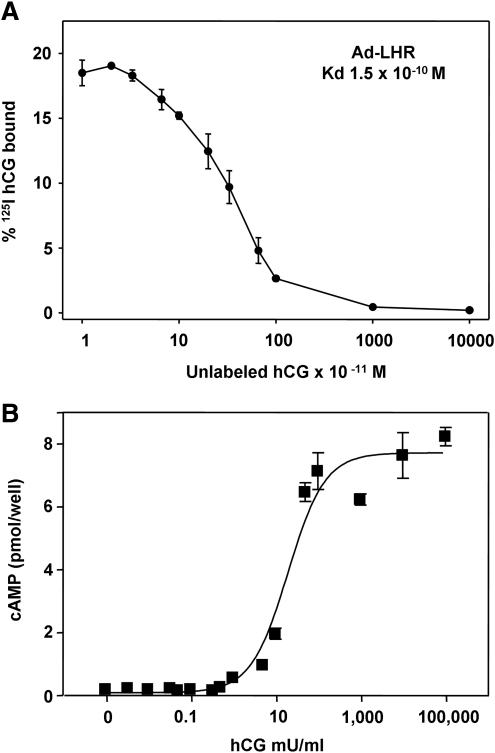
The LH holoreceptor expressing adenovirus (Ad-LHR) to be used for immunizing mice was tested by infecting COS-7 cells in monolayer culture. Two days after infection, competition by hCG for 125I-hCG binding to these cells revealed excellent expression with a high affinity interaction (1.5 × 10−10 Kd) (Fig. 2A).
FIG. 2.
Confirmation of LHR expression by the adenovirus construct and by CHO cells stably transfected with the receptor. (A) 125I-hCG binding and inhibition by unlabeled hCG. Data are the mean ± SD for duplicates. (B) cAMP response to increasing concentrations of HCG in CHO cells. CHO, Chinese hamster ovary; SD, standard deviation.
To assess induction of antibodies to the LHR expressed on the cell surface, we generated a stable LHR-expressing CHO cell line. Quantitation of antibody levels by flow cytometry, as well as potential functional activity of these antibodies, in multiple samples over time, would be optimized by using a clonal cell, which we obtained using the pcDNA5/FRT vector. The functionality of this cell line was evident by its high sensitivity to hCG stimulation of intracellular cAMP levels with an EC50 (concentration required for half-maximal stimulation) of 20 mU/mL (Fig. 2B).
Immunization with adenovirus expressing the LHR or its “A-subunit equivalent”
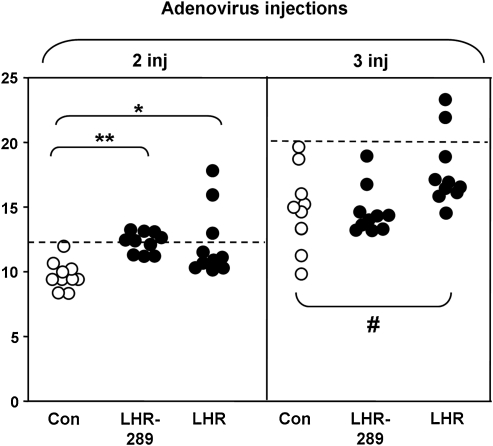
BALB/c mice were immunized three times with LHR-Ad (the LH holoreceptor), LHR-289-Ad (the A-subunit equivalent), or Con-Ad. Sera were tested by flow cytometry with CHO cells stably expressing the LHR 1 week after two injections and 4–5 weeks after the third (and final) injection. There was virtually no IgG class antibody binding to the LHR in mice injected with the lower adenovirus dose (1010 particles) (data not shown). However, LHR binding was observed with sera from some mice immunized with the higher adenovirus dose (1011 particles). Thus, after two injections, binding to LHR-CHO cells was significantly higher in sera from LHR-Ad and LHR-289-Ad mice than for sera from Con-Ad immunized mice, particularly in two LHR-Ad mice (Fig. 3, left panel). After the third high dose adenovirus injection, sera from the same LHR-Ad immunized animals remained positive compared with the cut-off for control immunized mice (Fig. 3, right panel).
FIG. 3.
Antibody binding (IgG class) to LHR-CHO cells in mice immunized with high dose adenovirus (1011 particles/injection) of Con-Ad (open circles), LHR-Ad, or LHR-2889-Ad (both closed circles). Sera were tested 1 week after two injections (2 inj) and at euthanasia 4–5 weeks after three immunizations (3 inj). Data shown are the geometric mean (Geo Mean) fluorescence values for sera from individual mice. The dashed line represents the mean + 2SD for sera from Con-Ad immunized mice (open circles). Asterisks indicate values significantly higher for mice immunized with high dose LHR-Ad or LHR-289-Ad versus Con-Ad. After two injections: *p = 0.005 (Rank sum test); **p = 0.001 (t test); after three injections: #p = 0.029 (t test). IgG, immunoglobulin G.
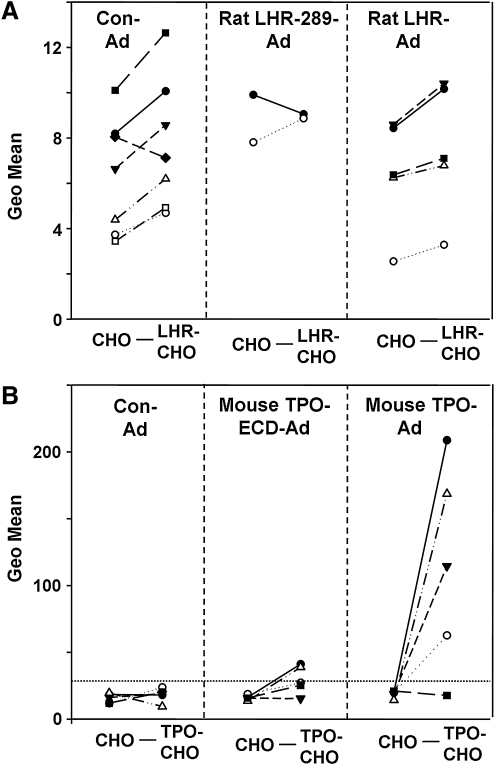
A feature of concern in interpreting the foregoing data was the increased IgG binding on flow cytometry to LHR-CHO cells by Con-Ad sera after the third versus the second injection (Fig. 3, horizontal dashed lines). For this reason, we re-assayed sera that were potentially LHR antibody positive after three immunizations with LHR-Ad and LHR-289-Ad using untransfected CHO cells as well as LHR-CHO cells. Included were sera from Con-Ad immunized mice, as well the two sera from LHR-289-Ad mice with the highest values (see Fig. 3). As would be expected, sera from LHR-Ad immunized mice (and from one LHR-289-Ad immunized mouse) produced higher fluorescence values with LHR-CHO cells than with control cells not expressing the LHR (Fig. 4A). However, the majority of sera from Con-Ad immunized mice also yielded higher fluorescence values with LHR-CHO versus nonexpressing CHO cells (Fig. 4A).
FIG. 4.
Antibodies induced to the LHR are not specific for the receptor, unlike antibodies induced to mouse TPO. (A) Flow cytometry was compared in the same serum for antibody binding (IgG class) to LHR-CHO cells versus untransfected CHO cells. Sera were tested from BALB/c mice that had received three injections of rat-LHR-Ad, rat-LHR-289-Ad, or Con-Ad. Symbols indicate values for sera from individual mice. For most sera, binding was higher to LHR-CHO than to CHO cells, particularly those from Con-Ad immunized mice. (B) Flow cytometry was compared in the same serum for antibody binding (IgG class) to mouse-TPO-CHO cells versus untransfected CHO cells. In this experiment, we re-tested sera from C57BL/6 mice immunized three times with mouse-TPO-Ad (7) and examined sera from mice immunized three times with mouse TPO-ECD-Ad (present study). The horizontal dashed line indicates the mean + 2SD for Con-Ad immunized mice. Note the difference in magnitude of the geometric means (Y axis): 0 to >200 (B) versus only 0 to 14 (A). TPO, thyroid peroxidase.
These observations demonstrate that antibody binding to LHR-CHO cells in immunized mice is not specific for the LHR. Moreover, sera from LHR-Ad or LHR-289-Ad immunized mice lacked bioactivity as determined by their inability to stimulate cAMP generation in LHR-CHO cells or to inhibit hCG stimulation of cAMP generation by these cells (data not shown). The normal histology of the ovaries and comparable estradiol levels in all three groups of mice (data not shown) were also consistent with the absence of specific antibody binding to the LHR in mice immunized with LHR-Ad or LHR-289-Ad.
Autoantibodies induced to mouse TPO
An important question raised by the above studies was why specific LHR antibodies were not induced in mice by immunization with rat LHR-ad or rat LHR-289-Ad despite nonidentity of the rat and mouse LHR amino acid sequences (Fig. 1B) and the adjuvant properties of the adenovirus vector. We had previously observed that adenovirus expressing the mouse TPO holoenzyme could break tolerance in mice as evident by the induction of TPO antibodies (7). Because of the importance of TSHR A-subunit shedding in the animal model of Graves' disease, we wished to test the effectiveness of immunization with adenovirus expressing the mouse TPO ECD. As with the LHR-Ad experiments, flow cytometry was performed testing each serum in parallel with CHO cells stably expressing mouse-TPO on the cell surface and untransfected CHO cells as controls. Only two of five mice immunized with TPO-ECD-adenovirus had marginally higher binding to mouse-TPO than to untransfected CHO cells (Fig. 4B, center panel). In contrast, consistent with our previous observations (7), four of five sera from mouse-TPO-Ad immunized mice exhibited strong fluorescence signals with mouse-TPO CHO cells (Geo means 50 to >200), far higher than the low level binding with untransfected CHO cells (Fig. 4B, right vs. left panels) and far higher than any binding observed with the LHR experiments (note the different ordinates in Fig. 4A, B).
It should be noted that antibodies specific for CHO cells were cloned from BALB/mice that developed TSHR antibodies after immunization with TSHR A-subunit (Dr. Y. Nagayama, University of Nagasaki, pers. comm.). These findings demonstrate that repeated adenovirus immunization induces IgG class antibodies that bind to proteins on untransfected CHO cells as well as antibodies to specific immunogens. However, as shown for C57/BL6 mice immunized with mouse-TPO adenovirus (Fig. 4B), recognition of CHO cell proteins is not a problem when serum antibody binding is several orders of magnitude greater than for sera from the control adenovirus immunized mice.
Discussion
Polyclonal or monoclonal antibodies (mAb) to the LHR have been generated by numerous groups [e.g., refs. (18,19,22,24,25)]. With one exception (19), LHR mAb antibodies were generated by conventional immunization with protein or peptides together with a highly potent adjuvant such as Freund's. In two of the foregoing studies, mAb were reported to activate the LHR. The mAb generated by Funaro et al. (19) had minimal activity. Functional activities of the more potent mAb reported by Bedin et al. (25) have not been confirmed by other investigators and these mAb were not used by the same laboratory in subsequent studies (26). None of the LHR antibodies made available to us by other investigators have activated the LHR, or even recognized the native LHR on the cell surface. In the light of this information, together with the proven ability to generate TSAb capable of inducing hyperthyroidism in mice, we attempted to produce “Graves' disease of the gonads” in mice by in vivo expression of the LHR or its A-subunit equivalent. Although this primary goal did not succeed, our studies provide valuable insight into antibody responses to self-antigens.
Our data demonstrate a remarkable difference between the ability of the rat-LHR and mouse-TPO to break immune tolerance to self with the generation of antigen-specific antibodies. An antigen is recognized as “nonself” if there is a significant amino acid residue or structural difference between the immunogen and the “self” protein. However, immune tolerance can be broken by including an adjuvant with a self-antigen for immunization. Freund's Complete adjuvant (frequently used in conventional immunization) is likely the most potent and naked DNA is the least potent of numerous adjuvants. Adenovirus is more powerful than naked DNA for inducing antibody responses to the human-TSHR (27).
Mouse-TPO expressed in vivo by an adenovirus vector induces strong antibody responses in mice (7). This immune response to a self-antigen demonstrates the adjuvant properties of the adenovirus vector. However, the adjuvant “kick” provided by adenovirus is less powerful in breaking tolerance with other self thyroid antigens. For example, mice immunized with the same adenovirus vector expressing the mouse-TSHR A-subunit fail to develop TSHR antibodies (28). Another example is provided by transgenic mice expressing the human TSHR A-subunit targeted to the thyroid (29). The presence of the transgene during embryogenesis makes this human antigen “self.” Consequently, unlike wild-type littermates, mice transgenic for the human-TSHR A-subunit are resistant to immunization with low dose human TSHR-A-subunit adenovirus. Breaking tolerance in mice that express low levels of the transgene requires immunization with high doses of TSHR-Ad or A-subunit-Ad. Moreover, in high level transgene expressors, tolerance is only broken by immunization with recombinant protein together with the highly potent Freund's Complete adjuvant (29,30). In the latter case, the TSHR antibodies generated are nonpathogenic.
The question then arises as to why, despite the same adenovirus adjuvant and immunization protocol, the mouse-TPO holoenzyme (self) induces a strong antibody response in mice, whereas neither rat LHR (nonself) nor mouse-TSHR A-subunit (self), administered with the same moderately potent adjuvant, is capable of inducing antibodies. In general, a number of factors may contribute to breaking tolerance to thyroid autoantigens by immunization using adenovirus, as described below.
Genetic background of the host
There is a strong genetic component to the development of thyroid autoimmunity in humans [reviewed in ref. (31)]. Different strains of mice also vary in their quantitative and qualitative immune responses to immunization to the same antigen. For example, although both BALB/c and C57/BL6 mice respond very well to human- TSHR-A-subunit adenovirus in terms of antibody generation, only the former develop thyroid-stimulating antibodies and hyperthyroidism in a large proportion of mice (32).
We cannot exclude the possibility that rat LHR-Ad immunization could have broken tolerance in a mouse strain other than BALB/c. However, this mouse strain develops antibodies to the LHR in response to conventional immunization (17,18), as well in response to LHR-expressing CHO cells (19), indicating its potential for recognizing the LHR. Moreover, independent of mouse strain, our data for the LHR and the TPO ECD, together with information from previous studies, illustrate the striking differences (outlined below) between the self-proteins against which antibody responses can, or cannot, be induced.
Amino acid homology between antigens from different species
Breaking tolerance occurs readily on immunizing mice with adenovirus expressing the human-TSHR (33) or the human-TSHR-A-subunit (4) with homologies to the mouse-TSHR of 91.9% and 90.3%, respectively. The failure to induce antibodies to the LHR could be explained by the greater homology between the rat and mouse LHR and LHR-A-subunit equivalent, 96.7% and 95.4%, respectively. Yet, to reiterate, immunization of mice with adenovirus expressing mouse-TPO (100% homology) induces a strong antibody response.
Immunogen size and membrane association
As observed in the present study, immunization with adenovirus expressing the soluble mouse-TPO ECD (848 amino acid residues) was less effective than as previously observed with the full-length (933 amino acid residue), membrane-associated mouse-TPO (7). These findings are consistent with the greater efficacy of immunization with some membrane-bound versus soluble nonself proteins [e.g., refs. (34,35)]. Indeed, immunogen size can be critical, as illustrated by the requirement in conventional immunization to couple synthetic peptides to a larger protein for conventional immunization; for example, LHR peptides coupled to the very large keyhole limpet hemocyanin molecule (36). On the other hand, the relatively small (289 amino acid residue) nonself human TSHR A-subunit is as effective as the 764 amino acid human-TSH holoreceptor for inducing TSHR antibodies and is more effective than the holoreceptor for inducing pathogenic stimulating TSHR antibodies (4).
Antigen glycosylation
Glycosylation has been shown to influence the antigenicity of infectious organisms, for example, Ebola virus (37) and simian immunodeficiency virus (38). The glycoprotein hormone receptors, including the TSHR and LHR, as well as TPO are all glycoproteins with N-linked glycans. The immunogenic human TSH holoreceptor and human TSHR A-subunit contain six and five N-linked glycans, respectively. Indeed, in the latter, the five N-linked glycans on a relatively small amino acid backbone (Fig. 1A) comprise approximately 50% of its mass (39), making it almost more a proteoglycan than a glycoprotein. The rat LHR A-subunit equivalent has three N-linked glycan motifs (Fig. 1A), of which only two contain glycan moieties (40), which could contribute to its lesser immunogenicity compared to the human TSHR A-subunit. However, mitigating against the importance of glycan, at least in the present study, is that both the TSH and LH holoreceptors have similar numbers of N-linked glycans (six and five, respectively). Moreover, despite mouse TPO eliciting a strong immune response, TPO is far less glycosylated (approximately 12% of total mass) (41) than the glycoprotein hormone receptors. In addition, unlike the TSHR, TPO does not bind to the mannose receptor (42), an important mechanism for glycosylated antigen capture by cells presenting antigen to T cells for initiating or amplifying an immune response [e.g., (43)].
Summary and Conclusions
Breaking self-tolerance is a complex process that involves interactions between central tolerance, the autoantigen, and the genetic background of the mouse (or human). From the present study, we obtained insight into the characteristics of the autoantigen that permit antibody induction by adenovirus immunization. First, antibodies can be induced to large self-antigens such as mouse TPO, particularly when membrane bound. Second, lesser amino acid homology between the immunogen and host (mouse) protein (91% for the human TSHR and 97% for the rat LHR) is likely to influence generation of an immune response, at least as determined by antibody production. Finally, the lesser N-linked glycan content of the rat LHR A-subunit equivalent compared to the human TSHR A-subunit suggests that the extent of glycosylation may contribute, at least in part, to breaking tolerance in the latter.
Acknowledgment
We thank Dr. Gregorio Chazenbalk and Dr. Pavel Pichurin for their assistance in the early stages of this study. This work was supported by National Institutes of Health Grants DK54684 (S.M.M.) and DK19289 (B.R). We are also grateful for contributions by Dr. Boris Catz, Los Angeles, CA.
Disclosure Statement
No competing financial interests exist.
References
- 1.Rapoport B. McLachlan SM. The thyrotropin receptor in Graves' disease. Thyroid. 2007;17:911–922. doi: 10.1089/thy.2007.0170. [DOI] [PubMed] [Google Scholar]
- 2.Tonacchera M. Ferrarini E. Dimida A. Agretti P. De Marco G. De Servi M. Gianetti E. Chiovato L. Pucci E. Pra CD. Betterle C. Aghini-Lombardi F. Vitti P. Pinchera A. Gonadotrophin receptor blocking antibodies measured by the use of cell lines stably expressing human gonadotrophin receptors are not detectable in women with 46,XX premature ovarian failure. Clin Endocrinol (Oxf) 2004;61:376–381. doi: 10.1111/j.1365-2265.2004.02107.x. [DOI] [PubMed] [Google Scholar]
- 3.Chazenbalk GD. Pichurin P. Chen CR. Latrofa F. Johnstone AP. McLachlan SM. Rapoport B. Thyroid-stimulating autoantibodies in Graves disease preferentially recognize the free A subunit, not the thyrotropin holoreceptor. J Clin Invest. 2002;110:209–217. doi: 10.1172/JCI15745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen C-R. Pichurin P. Nagayama Y. Latrofa F. Rapoport B. McLachlan SM. The thyrotropin receptor autoantigen in Graves' disease is the culprit as well as the victim. J Clin Invest. 2003;111:1897–1904. doi: 10.1172/JCI17069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagayama Y. Russo D. Chazenbalk GD. Wadsworth HL. Rapoport B. Extracellular domain chimeras of the TSH and LH/CG receptors reveal the mid-region (amino acids 171–260) to play a vital role in high affinity TSH binding. Biochem Biophys Res Comm. 1990;173:1150–1156. doi: 10.1016/s0006-291x(05)80906-4. [DOI] [PubMed] [Google Scholar]
- 6.Chazenbalk GD. McLachlan SM. Chen C-R. Rapoport B. Insight into thyrotropin receptor cleavage by engineering the single polypeptide chain luteinizing hormone receptor into a cleaving, two subunit receptor. Eur J Biochem. 2001;268:2261–2269. doi: 10.1046/j.1432-1327.2001.02103.x. [DOI] [PubMed] [Google Scholar]
- 7.Chen CR. Hamidi S. Braley-Mullen H. Nagayama Y. Bresee C. Aliesky HA. Rapoport B. McLachlan SM. Antibodies to thyroid peroxidase arise spontaneously with age in NOD.H-2h4 mice and appear after thyroglobulin antibodies. Endocrinology. 2010;151:4583–4593. doi: 10.1210/en.2010-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagayama Y. Wadsworth HL. Chazenbalk GD. Russo D. Seto P. Rapoport B. Thyrotropin-luteinizing hormone/chorionic gonadotropin receptor extracellular domain chimeras as probes for TSH receptor function. Proc Natl Acad Sci USA. 1991;88:902–905. doi: 10.1073/pnas.88.3.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mizuguchi H. Kay MA. Efficient construction of a recombinant adenovirus vector by an improved in vitro ligation method. Hum Gene Ther. 1998;9:2577–2583. doi: 10.1089/hum.1998.9.17-2577. [DOI] [PubMed] [Google Scholar]
- 10.Mizuguchi H. Kay MA. A simple method for constructing E1- and E1/E4-deleted recombinant adenoviral vectors. Hum Gene Ther. 1999;10:2013–2017. doi: 10.1089/10430349950017374. [DOI] [PubMed] [Google Scholar]
- 11.Kotani T. Umeki K. Yamamoto I. Takeuchi M. Takechi S. Nakayama T. Ohtaki S. Nucleotide sequence of the cDNA encoding mouse thyroid peroxidase. Gene. 1993;123:289–290. doi: 10.1016/0378-1119(93)90141-o. [DOI] [PubMed] [Google Scholar]
- 12.Chen CR. Aliesky HA. Guo J. Rapoport B. McLachlan SM. Blockade of costimulation between T cells and antigen-presenting cells: an approach to suppress murine Graves' disease induced using thyrotropin receptor-expressing adenovirus. Thyroid. 2006;16:427–434. doi: 10.1089/thy.2006.16.427. [DOI] [PubMed] [Google Scholar]
- 13.Mittereder N. March KL. Trapnell BC. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol. 1996;70:7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scatchard G. The attractions of proteins for small molecules and ions. Ann NY Acad Sci. 1949;51:660–672. [Google Scholar]
- 15.Chen C-R. Pichurin P. Chazenbalk GD. Aliesky H. Nagayama Y. McLachlan SM. Rapoport B. Low-dose immunization with adenovirus expressing the thyroid-stimulating hormone receptor A-subunit deviates the antibody response toward that of autoantibodies in human Graves' disease. Endocrinology. 2004;145:228–233. doi: 10.1210/en.2003-1134. [DOI] [PubMed] [Google Scholar]
- 16.McLachlan SM. Nagayama Y. Rapoport B. Insight into Graves' hyperthyroidism from animal models. Endocr Rev. 2005;26:800–832. doi: 10.1210/er.2004-0023. [DOI] [PubMed] [Google Scholar]
- 17.VuHai-LuuThi MT. Misrahi M. Houllier A. Jolivet A. Milgrom E. Variant forms of the pig lutropin/choriogonadotropin receptor. Biochem. 1992;31:8377–8383. doi: 10.1021/bi00150a035. [DOI] [PubMed] [Google Scholar]
- 18.Indrapichate K. Meehan D. Lane TA. Chu SY. Rao CV. Johnson D. Chen TT. Wimalasena J. Biological actions of monoclonal luteinizing hormone/human chorionic gonadotropin receptor antibodies. Biol Reprod. 1992;46:265–278. doi: 10.1095/biolreprod46.2.265. [DOI] [PubMed] [Google Scholar]
- 19.Funaro A. Sapino A. Ferranti B. Horenstein AL. Castellano I. Bagni B. Garotta G. Malavasi F. Functional, structural, and distribution analysis of the chorionic gonadotropin receptor using murine monoclonal antibodies. J Clin Endocrinol Metab. 2003;88:5537–5546. doi: 10.1210/jc.2003-030977. [DOI] [PubMed] [Google Scholar]
- 20.Turner CD. Bagnara JT. General Endocrinology. 6th. Saunders; Philadelphia: 1976. Endocrinology of the ovary. [Google Scholar]
- 21.Misharin A. Aliesky H. Nagayama Y. Rapoport B. McLachlan SM. Studies in mice deficient for the autoimmune regulator and transgenic for the thyrotropin receptor reveal a role for the autoimmune regulator in tolerance for thyroid autoantigens. Endocrinol. 2009;150:2948–2956. doi: 10.1210/en.2008-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meduri G. Charnaux N. Loosfelt H. Jolivet A. Spyratos F. Brailly S. Milgrom E. Luteinizing hormone/human chorionic gonadotropin receptors in breast cancer. Cancer Res. 1997;57:857–864. [PubMed] [Google Scholar]
- 23.Hamidi S. Aliesky H. Chen CR. Rapoport B. McLachlan SM. Variable suppression of serum thyroxine in female mice of different inbred strains by triiodothyronine administered in drinking water. Thyroid. 2010;20:1157–1162. doi: 10.1089/thy.2010.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosemblit N. Ascoli M. Segaloff DL. Characterization of an antiserum to the rat luteal luteinizing hormone/chorionic gonadotropin receptor. Endocrinology. 1988;123:2284–2290. doi: 10.1210/endo-123-5-2284. [DOI] [PubMed] [Google Scholar]
- 25.Bedin C. Antonicelli F. Jallal B. Salesse R. Bidart JM. Remy JJ. Lutropin receptor and thyrotropin receptor share a common epitope. Mol Cell Endocrinol. 1989;65:135–144. doi: 10.1016/0303-7207(89)90174-3. [DOI] [PubMed] [Google Scholar]
- 26.Remy JJ. Nespoulous C. Grosclaude J. Grebert D. Couture L. Pajot E. Salesse R. Purification and structural analysis of a soluble human chorionogonadotropin hormone-receptor complex. J Biol Chem. 2001;276:1681–1687. doi: 10.1074/jbc.M005206200. [DOI] [PubMed] [Google Scholar]
- 27.Pichurin P. Guo J. Yan X. Rapoport B. McLachlan SM. Human monoclonal autoantibodies to B-cell epitopes outside the thyroid peroxidase autoantibody immunodominant region. Thyroid. 2001;11:301–313. doi: 10.1089/10507250152039037. [DOI] [PubMed] [Google Scholar]
- 28.Nakahara M. Mitsutake N. Sakamoto H. Chen CR. Rapoport B. McLachlan SM. Nagayama Y. Enhanced response to mouse thyroid-stimulating hormone (TSH) receptor immunization in TSH receptor-knockout mice. Endocrinology. 2010;151:4047–4054. doi: 10.1210/en.2010-0315. [DOI] [PubMed] [Google Scholar]
- 29.Pichurin PN. Chen C-R. Chazenbalk GD. Aliesky H. Pham N. Rapoport B. McLachlan SM. Targeted expression of the human thyrotropin receptor A-subunit to the mouse thyroid: insight into overcoming the lack of response to A-subunit adenovirus immunization. J Immunol. 2006;176:668–676. doi: 10.4049/jimmunol.176.1.668. [DOI] [PubMed] [Google Scholar]
- 30.McLachlan SM. Nagayama Y. Pichurin PN. Mizutori Y. Chen CR. Misharin A. Aliesky HA. Rapoport B. The link between Graves' disease and Hashimoto's thyroiditis: a role for regulatory T cells. Endocrinology. 2007;148:5724–5733. doi: 10.1210/en.2007-1024. [DOI] [PubMed] [Google Scholar]
- 31.Jacobson EM. Tomer Y. The Genetic basis of thyroid autoimmunity. Thyroid. 2007;17:949–961. doi: 10.1089/thy.2007.0153. [DOI] [PubMed] [Google Scholar]
- 32.Chen CR. Aliesky H. Pichurin PN. Nagayama Y. McLachlan SM. Rapoport B. Susceptibility rather than resistance to hyperthyroidism is dominant in a thyrotropin receptor adenovirus-induced animal model of Graves' disease as revealed by BALB/c-C57BL/6 hybrid mice. Endocrinology. 2004;145:4927–4933. doi: 10.1210/en.2004-0716. [DOI] [PubMed] [Google Scholar]
- 33.Nagayama Y. Kita-Furuyama M. Ando T. Nakao K. Mizuguchi H. Hayakawa T. Eguchi K. Niwa M. A novel murine model of Graves' hyperthyroidism with intramuscular injection of adenovirus expressing the thyrotropin receptor. J Immunol. 2002;168:2789–2794. doi: 10.4049/jimmunol.168.6.2789. [DOI] [PubMed] [Google Scholar]
- 34.Unanue ER. Cerottini JC. The immunogenicity of antigen bound to the plasma membrane of macrophages. J Exp Med. 1970;131:711–725. doi: 10.1084/jem.131.4.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergers JJ. Den Otter W. De Groot JW. De Blois AW. Dullens HF. Steerenberg PA. Crommelin DJ. Reconstituted membranes of tumour cells (proteoliposomes) induce specific protection to murine lymphoma cells. Cancer Immunol Immunother. 1992;34:233–240. doi: 10.1007/BF01741791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez M. Segaloff DL. The orientation of the lutropin/choriogonadotropin receptor in rat luteal cells as revealed by site-specific antibodies. Endocrinology. 1990;127:674–681. doi: 10.1210/endo-127-2-674. [DOI] [PubMed] [Google Scholar]
- 37.Dowling W. Thompson E. Badger C. Mellquist JL. Garrison AR. Smith JM. Paragas J. Hogan RJ. Schmaljohn C. Influences of glycosylation on antigenicity, immunogenicity, and protective efficacy of ebola virus GP DNA vaccines. J Virol. 2007;81:1821–1837. doi: 10.1128/JVI.02098-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mori K. Sugimoto C. Ohgimoto S. Nakayama EE. Shioda T. Kusagawa S. Takebe Y. Kano M. Matano T. Yuasa T. Kitaguchi D. Miyazawa M. Takahashi Y. Yasunami M. Kimura A. Yamamoto N. Suzuki Y. Nagai Y. Influence of glycosylation on the efficacy of an Env-based vaccine against simian immunodeficiency virus SIVmac239 in a macaque AIDS model. J Virol. 2005;79:10386–10396. doi: 10.1128/JVI.79.16.10386-10396.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chazenbalk GD. Jaume JC. McLachlan SM. Rapoport B. Engineering the human thyrotropin receptor ectodomain from a non-secreted form to a secreted, highly immunoreactive glycoprotein that neutralizes autoantibodies in Graves' patients' sera. J Biol Chem. 1997;272:18959–18965. doi: 10.1074/jbc.272.30.18959. [DOI] [PubMed] [Google Scholar]
- 40.Zhang R. Cai H. Fatima N. Buczko E. Dufau ML. Functional glycosylation sites of the rat luteinizing hormone receptor required for ligand binding. J Biol Chem. 1995;270:21722–21728. doi: 10.1074/jbc.270.37.21722. [DOI] [PubMed] [Google Scholar]
- 41.Guo J. McLachlan SM. Hutchison JS. Rapoport B. The greater glycan content of recombinant human thyroid peroxidase of mammalian than on insect cell origin facilitates purification to homogeneity of enzymatically active protein remaining soluble at high concentration. Endocrinology. 1998;139:999–1005. doi: 10.1210/endo.139.3.5782. [DOI] [PubMed] [Google Scholar]
- 42.Chazenbalk GD. Pichurin PN. Guo J. Rapoport B. McLachlan SM. Interactions between the mannose receptor and thyroid autoimmunity. Clin Exp Immunol. 2004;139:216–224. doi: 10.1111/j.1365-2249.2004.02689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Engering AJ. Cella M. Fluitsma D. Brockhaus M. Hoefsmit EC. Lanzavecchia A. Pieters J. The mannose receptor functions as a high capacity and broad specificity antigen receptor in human dendritic cells. Eur J Immunol. 1997;27:2417–2425. doi: 10.1002/eji.1830270941. [DOI] [PubMed] [Google Scholar]