Abstract
Objective
This study indirectly tested the hypothesis that individuals with autism spectrum disorders (ASDs) have impaired neural connections between the amygdala, fusiform face area, and superior temporal sulcus, key processing nodes of the “social brain.” This would be evidenced by abnormalities in the major fibre tracts known to connect these structures, including the inferior longitudinal fasciculus and inferior fronto-occipital fasciculus.
Method
Magnetic resonance diffusion tensor imaging was performed on 20 right-handed males (ASD = 10, controls = 10) with a mean age 13.5 ± 4.0 years. Subjects were group-matched according to age, full-scale IQ, handedness, and ethnicity. Fractional anisotropy was used to assess structural integrity of major fibre tracts. Voxel-wise comparison of white matter fractional anisotropy was conducted between groups using ANCOVA adjusting for age, full-scale IQ, and brain volume. Volumes of interest were identified using predetermined probability and cluster thresholds. Follow-up tractography was performed to confirm the anatomic location of all volumes of interest.
Results
All volumes of interest were regions of lower FA and were observed primarily in pericallosal regions and temporal lobes. As confirmed by tractography, affected white matter structures included the inferior longitudinal fasciculus/inferior fronto-occipital fasciculus, superior longitudinal fasciculus, and corpus callosum/cingulum. Notably, some volumes of interest were adjacent to the fusiform face area, bilaterally, corresponding to involvement of the inferior longitudinal fasciculus. The largest effect sizes were noted for volumes of interest in the right anterior radiation of the corpus callosum/cingulum and right fusiform face area (inferior longitudinal fasciculus).
Conclusions
This study provides preliminary evidence of impaired neural connectivity in the corpus callosum/cingulum and temporal lobes involving the inferior longitudinal fasciculus/inferior fronto-occipital fasciculus and superior longitudinal fasciculus in ASDs. These findings provide preliminary support for aberrant neural connectivity between the amygdala, fusiform face area, and superior temporal sulcus – temporal lobe structures critical for normal social perception and cognition.
Keywords: autism, connectivity, diffusion tensor imaging, social brain, white matter
INTRODUCTION
Autism spectrum disorders (ASDs) are neurodevelopmental disorders characterized by impairments in reciprocal social interaction and verbal/non-verbal communication, and the presence of stereotyped behaviour [1]. When Leo Kanner first described autism in 1943, he noted affected individuals’ “innate inability to form the usual, biologically-provided affective contact with other people” [2]. For over half a century, what exactly was “biologically provided” to typically developing children and missing in those with ASDs remained poorly understood. Concurrent advances in functional/anatomical imaging techniques studying brain-behaviour relationships [3] and the application of these promising technologies in the study of ASDs, has resulted in a substantial body of evidence converging on theories of aberrant brain connectivity [4–6]. It has been argued that the most adverse effects involve temporal lobe structures critical for normal social perception and cognition [7].
Until relatively recently it was impossible to know whether aberrant connectivity existed without post-mortem studies. With the advent of diffusion tensor imaging (DTI), in vivo assessment of white matter pathology became possible by measuring fractional anisotropy (FA), a measure of fibre tract coherence derived from water diffusion properties [8]. Moreover, DTI can also be used to examine the 3D structure of major axonal bundles, using a technique called tractography [9]. Use of DTI has become increasingly common in the study of neuropsychiatric disorders, including ASDs [10–12]. However, earlier studies using DTI in ASDs have limited their analyses to voxel-wise comparisons of FA. Although useful for identifying the presence of white matter pathology, this approach is very limited in its ability to identify the underlying white matter structures involved (i.e. specific fibre tracts). The latter can be addressed by taking advantage of the wealth of anatomical information available through tractography, a technique implemented in the current study.
Thus, using the combination of both voxel-wise comparisons of FA and follow-up tractography, the current DTI study was conducted to investigate the integrity of major fibre tracts between key structures critically important for normal social information processing. Based on a large body of converging evidence and a heuristic model of the pathophysiology of ASDs [7], it was hypothesized that individuals with ASD might have abnormalities in fbre tracts connecting the amygdala, fusiform face area, and superior temporal sulcus. This will be evidenced by reduced FA along the inferior longitudinal fasciculus and inferior fronto-occipital fasciculus, two major fibre tracts known to link these major nodes of the “social brain” [13,14]. Given the consistent reports of corpus callosum abnormalities in ASDs, FA reductions are also predicted along this structure.
MATERIALS AND METHODS
Participants
Participants were 20 males with a mean age 13.5 ± 4.0 years (range = 8 to 19 years): 10 with an ASD and 10 typically developing controls (TDCs). Those included in the ASD group had confirmed DSM-IV [1] diagnoses of autistic disorder (n = 4), Asperger’s syndrome (n = 4), or pervasive developmental disorder not otherwise specified (PDD-NOS, n = 2); and absence of medical and/or neurologic disease that might be associated with ASDs. Diagnosis of an ASD (autistic disorder, Asperger’s syndrome, or PDD-NOS) was based on parental information, clinical history, and expert evaluation. In addition, two standard research diagnostic instruments were used: the Autism Diagnostic Interview - Revised [15] and the Autism Diagnostic Observation Schedule [16]. The TDC group consisted of healthy volunteers recruited from the same community as participants with an ASD. A semi-structured clinical interview was conducted with TDCs (and/or their parents) to rule out any history of neurological problems, neurological insult resulting in loss of consciousness, psychiatric disorders, and history of pervasive developmental disorders in first- or second-degree relatives. An age-appropriate version of the Wechsler Intelligence Scale [17,18] was administered to assess full-scale IQ. The study was approved by the University Human Investigations Committee. After study procedures were thoroughly explained, informed consent was obtained from all subjects or from their parent/guardian as appropriate. Verbal assent was obtained from all participants.
Image acquisition
MRI was performed using a 1.5-Tesla General Electric Signa system (Milwaukee, WI, USA). Single-shot, echo-planar imaging was used to acquire diffusion-weighted data using the following parameters: echo time = 92 ms, repetition time = 11200 ms, field of view = 256 mm, matrix = 128 × 128, resolution = 2 × 2 × 4 mm, slices = 36, thickness = 4 mm, and skip = 0. Diffusion sensitizing gradients were applied along six non-collinear directions (b = 1000 s/mm2). Two reference T2-weighted (b = 0) images were obtained per run, totalling six runs per subject (average = 6). Total DTI scan time was approximately 15 min, including all runs.
In addition, high-resolution T1-weighted images were acquired for each subject: 3D spoiled gradient recalled (SPGR), sagittal acquisition, contiguous images = 124, thickness = 1.2 mm, repetition time = 24 ms, echo time = 5 ms, flip angle = 45°, matrix = 192 × 256, number of excitations = 2, field of view = 300 mm, and frequency encoding direction = superior/inferior. All T1 images included were judged as good quality by an experienced rater.
Image processing
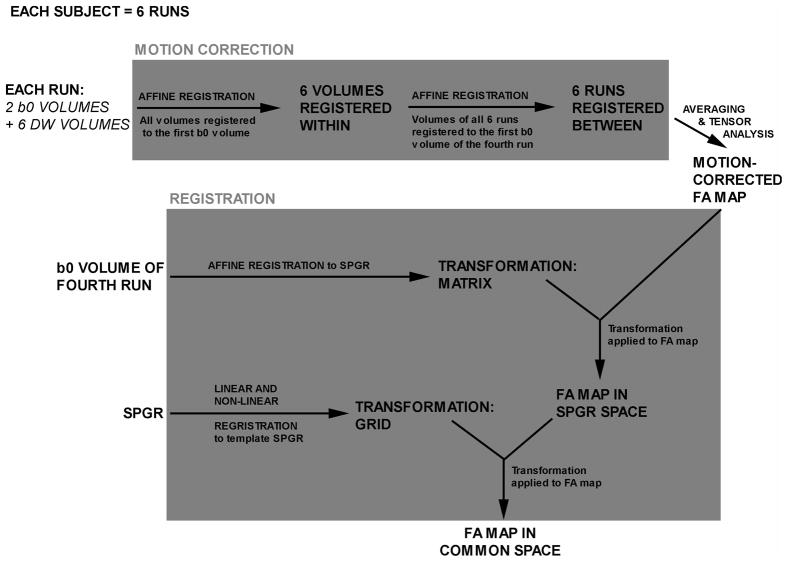
Diffusion-weighted images were processed using the BioImage Suite software platform [19] and consisted of a motion correction and averaging process followed by a registration procedure to reslice images to a common space (Figure 1). All registrations used a normalized mutual information similarity metric [20]. Nonlinear registrations used the intensity-only component of an integrated registration method [21]. Applied mathematical approaches related to the analysis of structural MRI data (neuroanatomical MRI and diffusion-weighted imaging) are discussed in detail elsewhere [22].
Figure 1.
Schematic diagram of image processing procedure: diffusion-weighted images were processed using the BioImage Suite software platform and consisted of a motion correction and averaging process followed by a registration procedure to reslice images to a common space.
All six diffusion-weighted runs for each subject underwent motion correction (Figure 1). Briefly, all volumes within each run were registered to the first b0 volume of that run. Next, all six runs of each subject were registered to the first b0 volume of the subject’s fourth run. Prior to tensor calculation and generation of FA maps, all six runs for each subject were then averaged to improve signal-to-noise ratio. The averaging procedure is equivalent to adding all runs together and dividing by the total number of runs.
Tensor analysis was performed on averaged motion-corrected runs to generate a motion-corrected FA map. These maps were subsequently resliced to a common space (a TDC subject), using transformations generated from (i) registrations of the individual b0 to SPGR images, and (ii) individual SPGR images to the common space SPGR image. To the common space image, six brain-limiting points, along with both anterior and posterior commissures were identified to place the image in standard Talairach 3D space. After fitting images to a standard 3D space, voxels representing grey matter, white matter, and cerebrospinal fluid were identified using a segmentation algorithm as described elsewhere [23].
Data analysis
The twenty FA maps were separated into ASD and TDC groups and used to create group-specific average and standard deviation (SD) FA maps. Voxel-wise, two-tailed t tests were performed at a threshold value of t = 2.878 (p < 0.005, two-tailed) to generate a t map displaying voxel clusters representing significant group differences between mean FA values. Any voxel cluster located within white matter (i.e. grey matter excluded) and surviving a threshold of 40 contiguous voxels (640 mm3) was identified and labelled as a volume of interest (VOI). For each VOI, FA means and SDs were calculated and used for other statistical analyses. Effect sizes (Cohen’s d) were calculated using means and SDs. Finally, ANCOVA of FA data was performed to adjust for (i) age and full-scale IQ, and (ii) age, full-scale IQ, and total brain volume. Covariance analyses were performed using a p-value of 0.005 (two-tailed). Again, any voxel cluster located within white matter and surviving a threshold of 40 contiguous voxels was identified and labelled as a VOI.
Anatomical locations of VOIs were first estimated with the aid of post-mortem, MRI, and DTI brain atlases [24–26]. These locations were then confirmed using tractography [27]. The fibre tracking process was initiated from a seed volume (4 × 4 × 4 mm) placed according to the Talairach coordinates of the most significant p-value corresponding to each VOI. The fibre tracking program used the Runge-Kutta method for integrating the eigenvector field, following the principal direction of diffusion. The step length was set at 0.25 with a maximum angle of 45° allowed between steps. A minimum FA of 0.1 and a maximum FA of 1.0 were used as stopping criteria. Tractography was performed individually on all ASD subjects for each VOI with the resulting tracts sorted by VOI. For illustrative purposes, the authors identified one tractography image for each unique VOI as most representative of the underlying affected fibre tract(s).
RESULTS
Subject characteristics and volumetric measurements are summarized in Table 1. There were no significant differences in age and full-scale IQ between groups; however, full-scale IQ data were not available for three subjects in the TDC group. Total brain volume and total white matter volume were significantly higher in the ASD group; however, total grey matter volume was not significantly different between groups, though there was a trend towards statistical significance. Mean white matter FA did not differ significantly between groups.
Table 1.
Subject characteristics and volumetric measurements
| ASD | TDC | Student’s t-test | ||||
|---|---|---|---|---|---|---|
| N = 10 | N = 10 | df = 18 | ||||
| Mean | SD | Mean | SD | t-statistic | p-value | |
| Clinical data† | ||||||
| Age | 13.06 | 3.85 | 13.94 | 4.23 | 0.487 | 0.632 |
| FSIQ‡ | 91.00 | 24.79 | 105.00 | 17.83 | 1.276 | 0.222 |
| Brain volume measurements | ||||||
| Total volume | 1522.51 | 96.55 | 1393.86 | 125.40 | −2.570 | 0.019 |
| Total gray matter volume | 787.28 | 71.66 | 724.88 | 72.02 | −1.942 | 0.068 |
| Total white matter volume | 476.11 | 50.07 | 423.41 | 60.22 | −2.128 | 0.047 |
ASD, autism spectrum disorder group; TDC, typically developing control group; SD, standard deviation; FSIQ, full-scale IQ; age in years, volume in ml, italicized print indicates statistical significance (p < 0.05, two-tailed).
Study was confined to right-handed Caucasian males.
FSIQ data missing from three TDC subjects; df = 15.
Results are summarized in Table 2 and Figure 2. It is notable that there were no VOIs where FA was significantly higher in the ASD group. As illustrated in Figure 3, VOIs with significantly lower FA in the ASD group were located along the following white matter tracts as identified using atlases and confirmed by tractography:
Table 2.
Summary of affected fibre tracts corresponding to volumes of interest (VOI) or regions where structural integrity is significantly compromised
| VOI LOCATION SORTED BY MAJOR CEREBRAL REGIONS | ANALYSIS USING STUDENT’S T-TEST | |||||
|---|---|---|---|---|---|---|
| Volume | Mean FA ± SD | Cohen’s d | Peak p-value | Talairach coordinates: Peak p-value | ||
| TDC | ASD | |||||
| Peri-callosal region | ||||||
| 1) Right anterior radiation of corpus callosum/cingulum | 721 | 0.35 ±0.02 | 0.27 ±0.03 | 3.37 | 0.00003 | 14, 18, 25 |
| 2) Left anterior radiation of corpus callosum/cingulum | 553 | 0.38 ±0.05 | 0.28 ±0.03 | 2.26 | 0.00009 | −14, 21, 23 |
| 3) Left anterior body of corpus callosum/cingulum | 252 | 0.36 ±0.04 | 0.28 ±0.03 | 2.43 | 0.0002 | −11, −3, 33 |
| 4) Left posterior body of corpus callosum/cingulum | 91 | 0.32 ±0.02 | 0.26 ±0.03 | 2.32 | 0.0002 | −18, −26, 30 |
| 5) Right body of corpus callosum/cingulum | 110 | 0.37 ±0.02 | 0.29 ±0.03 | 2.54 | 0.0005 | 11, −3, 29 |
| Frontal lobe | ||||||
| 6) Left superior longitudinal fasciculus | 146 | 0.31 ±0.04 | 0.22 ±0.02 | 2.60 | 0.00004 | −33, −9, 33 |
| 7) Left inferior fronto-occipital fasciculus* | 109 | 0.30 ±0.05 | 0.22 ±0.03 | 1.94 | 0.001 | −23, 18, 14 |
| Temporal lobe | ||||||
| 8) Right inferior longitudinal fasciculus/ inferior fronto-occipital fasciculus | 79 | 0.41 ±0.02 | 0.33 ±0.05 | 2.22 | 0.0007 | 35, −42, 5 |
| 9) Left inferior longitudinal fasciculus/ inferior fronto-occipital fasciculus –seeded anteriorly | 66 | 0.36 ±0.03 | 0.28 ±0.03 | 2.37 | 0.0005 | −40, −20, −7 |
| 10) Left inferior longitudinal fasciculus/ inferior fronto-occipital fasciculus –seeded posteriorly | 290 | 0.47 ±0.05 | 0.36 ±0.04 | 2.33 | 0.0002 | −34, −57, 8 |
| Temporal/Occipital lobe | ||||||
| 11) Left inferior longitudinal fasciculus –seeded left FFA | 319 | 0.25 ±0.03 | 0.16 ±0.02 | 2.90 | 0.00002 | −35, −68, −6 |
| 12) Right inferior longitudinal fasciculus –seeded from right FFA | 72 | 0.22 ±0.02 | 0.15 ±0.02 | 3.24 | 0.000008 | 35, −64, −8 |
FA, fractional anisotropy; SD, standard deviation; ASD, autism spectrum disorder group; TDC, typically developing control group; FFA, fusiform face area, volume in ml.
Loss of statistical significance after controlling for age, full-scale IQ, and total brain volume.
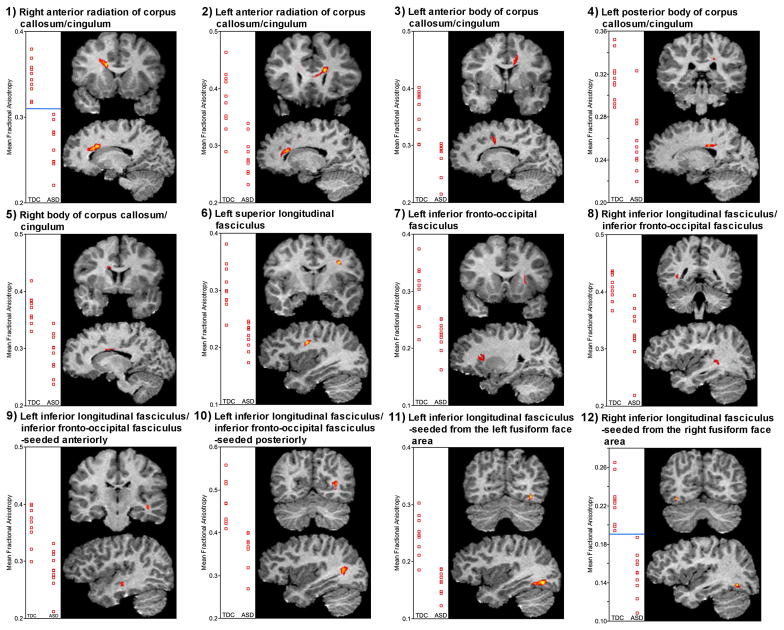
Figure 2.
Scatterplots of mean fractional anisotropy for the 12 volumes of interest and anatomical location as seen in coronal and sagittal views; TDC, typically developing control group; ASD, autism spectrum disorder group, the horizontal blue line (#1 and #12) represents complete separation of data between groups.
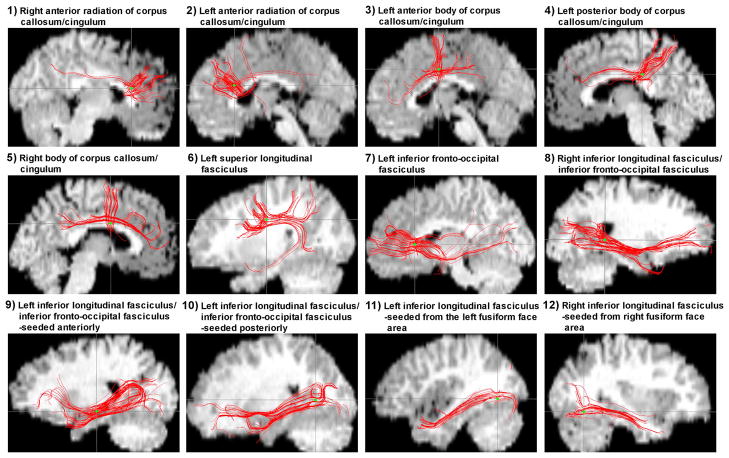
Figure 3.
Diffusion tensor tractography: seeding from Talairach coordinates of the most significant p value for each volume of interest reveals fibre bundles readily identified as major association and commissure tracts. Seed locations are identified by green dots.
Right anterior radiation of the corpus callosum/cingulum
Left anterior radiation of the corpus callosum/cingulum
Left anterior body of the corpus callosum/cingulum
Left posterior body of the corpus callosum/cingulum
Right body of the corpus callosum/cingulum
Left superior longitudinal fasciculus
Left inferior fronto-occipital fasciculus
Right inferior longitudinal fasciculus/inferior fronto-occipital fasciculus
Left inferior longitudinal fasciculus/inferior fronto-occipital fasciculus, seeded anteriorly
Left inferior longitudinal fasciculus/inferior fronto-occipital fasciculus, seeded posteriorly
Left inferior longitudinal fasciculus, seeded from the left fusiform face area
Right inferior longitudinal fasciculus, seeded from the right fusiform face area
Large effect sizes were noted across all VOI comparisons, ranging from 1.94–3.37. The largest were observed for the right anterior radiation of the corpus callosum/cingulum and right inferior longitudinal fasciculus, seeded from the right fusiform face area. This indicated a 100% non-overlap of TDC and ASD distributions for both VOIs. The peak p values for VOIs ranged from 0.001 to 0.000008, with the most significant p values observed in the right anterior radiation of the corpus callosum/cingulum and right inferior longitudinal fasciculus, seeded from the right fusiform face area. All VOIs remained statistically significant after co-varying for age and full-scale IQ, using the aforementioned thresholds. After adding total brain volume as a covariate along with age and full-scale IQ there was a loss of statistical significance in the left inferior fronto-occipital fasciculus (no. 7). VOIs where FA was lower in the ASD group were located along major white matter association and commissure tracts (Figure 2). While there was some expected individual variability, diffusion tensor tractography yielded fibre bundles easily identified as those major fibre tracts known to course through the identified VOIs (Figure 3).
DISCUSSION
The central question addressed by the present study was whether individuals with an ASD might have abnormalities in major fibre tracts connecting the amygdala, fusiform face area, and superior temporal sulcus as evidenced by reductions in FA along the inferior longitudinal fasciculus and inferior fronto-occipital fasciculus. This study provides preliminary evidence of impaired connectivity in the temporal lobe involving these structures. The inferior longitudinal fasciculus and inferior fronto-occipital fasciculus have been demonstrated to extend from the amygdala and fusiform face area [13]. As is evident in Table 2, almost half (n = 5) of the identified VOIs involve the inferior longitudinal fasciculus and/or inferior fronto-occipital fasciculus and lie within the temporal lobe or near the temporal/occipital junction, all within proximity to the fusiform face area, amygdala, or superior temporal sulcus. In fact, inferior longitudinal fasciculus involvement is not apparent in the two VOIs seeded from the fusiform face area (Figure 2) until tractography is performed (Figure 3). As expected, abnormalities of the corpus callosum are also prominent and nearly half (n = 5) of the identified VOIs involve this structure (Table 2).
In addition to involvement of the inferior longitudinal fasciculus, inferior fronto-occipital fasciculus, and corpus callosum, there appears to be involvement of the superior longitudinal fasciculus and cingulum, which were not previously predicted. However, the involvement of the superior longitudinal fasciculus is intuitive given its well-known role in language and communication [28], which are core deficits of autism [1]. Moreover, its connection to the “social brain” has been demonstrated by virtue of its bridging the frontal lobe to the superior temporal sulcus [14]. The unexpected cingulum finding does not occur in isolation. That is, VOIs are not located in the cingulum, only. Therefore, it is plausible that this structure is highlighted with tractography because of normal anatomical overlap/crossing of the cingulum with the corpus callosum which cannot be resolved using conventional tractography techniques (see further discussion in limitations section). Moreover, these unexpected findings would not necessarily contradict the hypotheses set forth, and remain consistent with the literature on the neurobiology of ASDs. Abnormal connectivity need not be limited to the inferior longitudinal fasciculus, inferior fronto-occipital fasciculus, and corpus callosum. The possibility that other white matter structures are affected is expected, as this is consistent with the widely held belief that ASDs are heterogeneous and distributed disorders [29].
The finding of reduced FA in the current study is consistent with the majority of DTI studies performed in ASDs which also report mainly reductions in FA. It is important to note that despite the between-study differences in subject characteristics, image acquisition, and processing/analysis methodology, FA is consistently reduced, although the distribution of the reduction is variable. Out of six DTI studies in ASDs which examined whole-brain FA, four reported primarily on areas of reduction [11,12,30,31], one reported areas of decrease and increase [32], and one reported increases [33]. The last of these studies is uniquely different from the others because of its focus on young children ages 1.8–3.3 years (compared to older children and adults in the other studies), which is the age range for which accelerated brain growth has been reported [5]. Five studies restricted their analysis to specific areas including the corpus callosum [10], superior temporal gyrus [34], cerebellum [35], temporal lobe [36], and frontal lobe [37]. These regional studies primarily report abnormal FA or other measures of white matter structure, suggesting some degree of impairment. It is notable, however, that the last of these studies specifically examined short-range and long-range connections and did not find evidence of excessive short-range connectivity or reduced long-range connectivity [37]. However, the focus of that study was examining frontal lobe connections which excludes other key regions thought to play vital roles in the pathobiology of autism such as the temporal lobe [7] and possibly the cerebellum [38].
It is worth emphasizing that most of the aforementioned studies identified areas where FA is decreased compared to controls. Although there is some variation in anatomic location, most studies report a reduction in FA. In addition, studies examining the corpus callosum have reported abnormalities, but again there is some variation in exact locations. To date, these abnormalities may be the most consistent DTI findings in ASDs, and support a theory of impaired neural connectivity. Moreover, the corpus callosum consists of long commissural fibres, thus lending support to the possibility of reduced long-range connectivity and impaired inter-hemispheric communication. Evidence for impaired long-range connectivity is also supported by the current study, not only through extensive corpus callosum involvement, but also through the involvement of various association fibres such as the inferior longitudinal fasciculus, inferior fronto-occipital fasciculus, and superior longitudinal fasciculus. However, another version of the disconnectivity theory asserts that there is an overabundance of short-range connections [5,39]. This might theoretically result in higher FA in white matter closer to the cortex, which has not been reported in any study to date. However, the lack of findings could be due to methodological limitations (e.g. image resolution). More specifically, the microscopic information about fibre tracts is averaged over a significantly larger voxel volume. If multiple U-fibres are overlapping with different orientations (i.e. disorganized), their contributions to the signal could be cancelled out [8].
In addition to the growing body of DTI literature examining ASDs, findings of the current study are also consistent with the larger body of behavioural, neuropathological, and neuroimaging data. Taken together, these data provide convergent support for abnormal brain connectivity in ASDs, with clear adverse effects on those temporal lobe structures critical for normal social perception and cognition [7]. Early hints to the neurobiology of ASDs were apparent in Kanner’s seminal report when he noted that some affected children had enlarged heads [2]. Although a tendency towards megencephaly was confirmed in earlier studies [40], this observation only gained considerable attention in the 1990s when it was confirmed by neuroimaging studies [41], becoming the most consistent structural MRI finding in ASDs. Later research suggested that megencephaly was driven more by an increase in white matter rather than grey matter [42]. However, it also appeared that white matter was not uniformly enlarged. More recent volumetric studies in ASDs found that temporal lobe enlargement is greater than other lobes, with temporal lobe white matter enlarged about 10% [43]. Another consistently reported structural abnormality is a smaller corpus callosum which consists of long-range fibre tracts [44]. As mentioned previously, investigators have also suggested that the white matter abnormality consists of an overabundance short-range fibres [5,39]. These latter studies are consistent with neuropathological evidence of decreased neuronal cell size in ASDs, suggesting a paucity of long-range fibres since longer fibres require larger cell bodies to support their metabolic needs [45]. Therefore, the structural data seem to suggest a paucity of long-range fibres and relative overabundance of short-range fibres, possibly affecting the temporal lobes greater than other lobes of the brain.
Other than implicating the temporal lobe, which contains key components of the “social brain” [46], the structural findings alone do little to explain the social impairment unique to ASDs. Much behavioural data suggested that individuals with ASDs have deficits in face perception [47] which has been extensively studied using functional MRI. In fact, perhaps the best replicated functional MRI finding in ASDs is hypoactivation of the fusiform face area during face perception tasks [48], which has been replicated by at least nine independent laboratories [7]. In addition to fusiform face area hypoactivation, other studies have reported hypoactivation of the amygdala [46] and superior temporal sulcus [49] in ASDs. Functional MRI has also been used to study the presence of abnormal connectivity in ASDs which was implied by structural MRI findings. Multiple studies have reported reduced synchronization and functional connectivity in ASDs [49,50]. More importantly, there appears to be reduced effective connectivity between the fusiform face area and amygdala in ASDs [51]. Therefore, even without more direct evidence of impaired connectivity from DTI, convergent evidence from behavioural, neuropathological, structural MRI, and functional MRI findings appear to suggest aberrant brain connectivity with profound effects and consequences on the temporal lobe. The white matter abnormalities reported in the current study provide more direct evidence of aberrant connectivity in ASDs. Reductions in FA along the corpus callosum are consistent with abnormal long-range connectivity. FA reductions along the inferior longitudinal fasciculus, inferior fronto-occipital fasciculus, and superior longitudinal fasciculus in ASDs support aberrant connectivity between the amygdala, fusiform face area, and superior temporal sulcus, structures critical for normal social perception and cognition. Notably, these data are consistent with one proposed model on the developmental deficits in social perception and cognition in ASDs [7]. According to this model, congenital dysfunction of the amygdala disrupts normal social learning and adversely affects fibre pathways of the occipital temporal projection system, including connections involving the amygdala, fusifiorm face area, and superior temporal sulcus.
Findings reported in this study must be interpreted in the context of several methodological limitations. The ASD sample was relatively heterogeneous, consisting of individuals with autistic disorder, Asperger ’s syndrome, and PDD-NOS. Including these individuals in one ASD group assumes that these disorders have a common neurobiological underpinning, an assertion which currently lacks strong empirical support as ASDs continue to be considered separate disorders according to DSM-IV [1]. Also, DTI remains an indirect method used to study fibre tracts. While 2D visualization (FA maps) has some validation, the 3D reconstruction of white matter tracts (tractography) requires further anatomic validation [52]. Resolution of DTI is limited such that conventional tractography techniques cannot resolve crossing/overlapping fibre tracts [8]. Furthermore, the current study uses only six gradient directions, which is the minimum number required for tensor calculation and generation of FA maps. Finally, tractography could not be performed using group-averaged data because tensors could not be properly averaged using standard registration procedures [53].
In summary, this study provides evidence of impaired neural connectivity in the corpus callosum and temporal lobes involving the inferior longitudinal fasciculus, inferior fronto-occipital fasciculus, and superior longitudinal fasciculus in ASDs. These findings partially support a theory of aberrant long-range neural connectivity between the amygdala, fusiform face area, and superior temporal sulcus; temporal lobe structures critical for normal social perception and cognition. The clinical implication of these findings is that individuals with ASDs are not, as Kanner put it, “biologically provided” the proper neural connections between those cortical nodes vital for processing social information, thus leading to the characteristic deficits that define this unique group of disorders. How such a system develops remains to be determined and continues to be an area of vigorous research.
Acknowledgments
We graciously thank Dr Robert Schultz, who kindly provided the data and computational resources necessary for our analysis and Dr Sarah Paterson, who assisted with the preparation of the manuscript. We would also like to acknowledge the help of Lawrence Win, Kenneth Rando, and Marcello DiStasio with data assembly. We gratefully acknowledge the effort and commitment of the participants and their families in this study.
We are thankful for the grant support we have received for this project from the National Institute of Child Health and Human Development (NICHD) Collaborative Programs of Excellence in Autism (CPEA) (U19 HD035482), the National Institute of Neurological Disorders and Stroke (NINDS) (R01 NS035193), and the General Clinical Research Centers (GCRC) (MO1 RR000125). This work was also supported, in part, by the American Psychiatric Institute for Research and Education/Eli Lilly and Company Psychiatric Research Fellowship and the ANA/Pfizer Fellowships in Clinical Practice from Pfizer’s Medical and Academic Partnership program.
Footnotes
This article appeared in a journal published by Informa Healthcare. The attached copy is furnished for non-commercial research and education use. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: APA; 2000. text revision. [Google Scholar]
- 2.Kanner L. Autistic disturbances of affective contact. Nerv Child. 1943;2:217–250. [PubMed] [Google Scholar]
- 3.Gothelf D, Furfaro JA, Penniman LC, Glover GH, Reiss AL. The contribution of novel brain imaging techniques to understanding the neurobiology of mental retardation and developmental disabilities. Ment Retard Dev Disabil Res Rev. 2005;11:331–339. doi: 10.1002/mrdd.20089. [DOI] [PubMed] [Google Scholar]
- 4.Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24:9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Curr Opin Neurobiol. 2005;15:225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Minshew NJ, Williams DL. The new neurobiology of autism: cortex, connectivity, and neuronal organization. Arch Neurol. 2007;64:945–950. doi: 10.1001/archneur.64.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci. 2005;23:125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51:527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- 10.Alexander AL, Lee JE, Lazar M, et al. Diffusion tensor imaging of the corpus callosum in autism. Neuroimage. 2007;34:61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 11.Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry. 2004;55:323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 12.Keller TA, Kana RK, Just MA. A developmental study of the structural integrity of white matter in autism. Neuroreport. 2007;18:23–27. doi: 10.1097/01.wnr.0000239965.21685.99. [DOI] [PubMed] [Google Scholar]
- 13.Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- 14.Catani M, Jones DK, ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- 15.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 16.Lord C, Rutter M, Goode S, et al. Autism Diagnostic Observation Schedule: a standardized observation of communicative and social behavior. J Autism Dev Disord. 1989;19:185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- 17.Wechsler D. Wechsler Intelligence Scale for Children. 3. San Antonio, TX: Psychological Corporation; 1991. [Google Scholar]
- 18.Wechsler D. Wechsler Adult Intelligence Scale. 3. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 19.Papademetris X, Jackowski M, Rajeevan N, Constable RT, Staib LH. BioImage Suite: an integrated medical image analysis suite. New Haven, CT: Section of Bioimaging Sciences, Department of Diagnostic Radiology; Yale University School of Medicine; 2006. [cited 20 July 2010]. Available from URL: http://www.bioimagesuite.org. [Google Scholar]
- 20.Studholme C, Hill DL, Hawkes DJ. Automated three-dimensional registration of magnetic resonance and positron emission tomography brain images by multiresolution optimization of voxel similarity measures. Med Phys. 1997;24:25–35. doi: 10.1118/1.598130. [DOI] [PubMed] [Google Scholar]
- 21.Papademetris X, Staib LH, Jackowski AP, Win LY, Schultz RT, Duncan JS. Integrating intensity and feature nonrigid registration. In: Barillot C, Haynor DR, Hellier P, editors. Medical image computing and computer-assisted intervention. New Haven, CT: Springer; 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duncan JS, Papademetris X, Yang J, Jackowski M, Zeng X, Staib LH. Geometric strategies for neuroanatomic analysis from MRI. Neuroimage. 2004;23:S34–S45. doi: 10.1016/j.neuroimage.2004.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsatsanis KD, Rourke BP, Klin A, Volkmar FR, Cicchetti D, Schultz RT. Reduced thalamic volume in high-functioning individuals with autism. Biol Psychiatry. 2003;53:121–129. doi: 10.1016/s0006-3223(02)01530-5. [DOI] [PubMed] [Google Scholar]
- 24.Duvernoy HM. The human brain: surface, blood supply, and three-dimensional sectional anatomy. New York, NY: Springer; 1999. [Google Scholar]
- 25.Gluhbegovic N, Williams TH. The human brain: a photographic guide. Hagerstown, MD: Harper & Row; 1980. [Google Scholar]
- 26.Mori S, Wakana S, Nagae-Poetscher LM, van Zijl PC. MRI atlas of human white matter. Amsterdam, Netherlands: Elsevier; 2005. [Google Scholar]
- 27.Jackowski M, Kao CY, Qiu M, Constable RT, Staib LH. White matter tractography by anisotropic wavefront evolution and diffusion tensor imaging. Med Image Anal. 2005;9:427–440. doi: 10.1016/j.media.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wernicke C. The aphasic symptom-complex: a psychological study on an anatomical basis. Breslau, Germany: Cohn & Weigert; 1874. [Google Scholar]
- 29.Muller RA. The study of autism as a distributed disorder. Ment Retard Dev Disabil Res Rev. 2007;13:85–95. doi: 10.1002/mrdd.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JE, Chung MK, Lazar M, et al. A study of diffusion tensor imaging by tissue-specific, smoothing-compensated voxel-based analysis. Neuroimage. 2009;44:870–883. doi: 10.1016/j.neuroimage.2008.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thakkar KN, Polli FE, Joseph RM, et al. Response monitoring, repetitive behaviour and anterior cingulate abnormalities in autism spectrum disorders (ASD) Brain. 2008;131:2464–2478. doi: 10.1093/brain/awn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ke X, Tang T, Hong S, et al. White matter impairments in autism, evidence from voxel-based morphometry and diffusion tensor imaging. Brain Res. 2009;1265:171–177. doi: 10.1016/j.brainres.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 33.Ben Bashat D, Kronfeld-Duenias V, Zachor DA, et al. Accelerated maturation of white matter in young children with autism: a high b value DWI study. Neuroimage. 2007;37:40–47. doi: 10.1016/j.neuroimage.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 34.Lee JE, Bigler ED, Alexander AL, et al. Diffusion tensor imaging of white matter in the superior temporal gyrus and temporal stem in autism. Neurosci Lett. 2007;424:127–132. doi: 10.1016/j.neulet.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 35.Catani M, Jones DK, Daly E, et al. Altered cerebellar feedback projections in Asperger syndrome. Neuroimage. 2008;41:1184–1191. doi: 10.1016/j.neuroimage.2008.03.041. [DOI] [PubMed] [Google Scholar]
- 36.Conturo TE, Williams DL, Smith CD, Gultepe E, Akbudak E, Minshew NJ. Neuronal fibre pathway abnormalities in autism: an initial MRI diffusion tensor tracking study of hippocampo-fusiform and amygdalo-fusiform pathways. J Int Neuropsychol Soc. 2008;14:933–946. doi: 10.1017/S1355617708081381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sundaram SK, Kumar A, Makki MI, Behen ME, Chugani HT, Chugani DC. Diffusion tensor imaging of frontal lobe in autism spectrum disorder. Cereb Cortex. 2008;18:2659–2665. doi: 10.1093/cercor/bhn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Courchesne E. Abnormal early brain development in autism. Mol Psychiatry. 2002;7:S21–S23. doi: 10.1038/sj.mp.4001169. [DOI] [PubMed] [Google Scholar]
- 39.Herbert MR, Ziegler DA, Makris N, et al. Localization of white matter volume increase in autism and developmental language disorder. Ann Neurol. 2004;55:530–540. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- 40.Walker HA. Incidence of minor physical anomaly in autism. J Autism Child Schizophr. 1977;7:165–176. doi: 10.1007/BF01537727. [DOI] [PubMed] [Google Scholar]
- 41.Piven J, Arndt S, Bailey J, Havercamp S, Andreasen NC, Palmer P. An MRI study of brain size in autism. Am J Psychiatry. 1995;152:1145–1149. doi: 10.1176/ajp.152.8.1145. [DOI] [PubMed] [Google Scholar]
- 42.Courchesne E, Karns CM, Davis HR, et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- 43.Schultz RT, Win L, Jackowski A, et al. Brain morphology in autism spectrum disorders: an MRI study. Paper presented at the International Meeting for Autism Research (IMFAR); Boston, MA. May 2005. [Google Scholar]
- 44.Frazier TW, Hardan AY. A meta-analysis of the corpus callosum in autism. Biol Psychiatry. 2009;66:935–641. doi: 10.1016/j.biopsych.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002;58:428–432. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- 46.Baron-Cohen S, Ring HA, Wheelwright S, et al. Social intelligence in the normal and autistic brain: an fMRI study. Eur J Neurosci. 1999;11:1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- 47.Klin A, Sparrow SS, de Bildt A, Cicchetti DV, Cohen DJ, Volkmar FR. A normed study of face recognition in autism and related disorders. J Autism Dev Disord. 1999;29:499–508. doi: 10.1023/a:1022299920240. [DOI] [PubMed] [Google Scholar]
- 48.Schultz RT, Gauthier I, Klin A, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch Gen Psychiatry. 2000;57:331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- 49.Castelli F, Frith C, Happe F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- 50.Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- 51.Schultz RT, Hunyadi E, Connors C, Pasley B. FMRI study of facial expression perception in autism: the amygdala, fusiform face area and their functional connectivity. Paper presented at the annual meeting of the Organization for Human Brain Mapping; Toronto, Canada. June 2005. [Google Scholar]
- 52.Moseley ME, Cohen Y, Kucharczyk J, et al. Diffusion-weighted MR imaging of anisotropic water diffusion in cat central nervous system. Radiology. 1990;176:439–445. doi: 10.1148/radiology.176.2.2367658. [DOI] [PubMed] [Google Scholar]
- 53.Alexander DC, Pierpaoli C, Basser PJ, Gee JC. Spatial transformations of diffusion tensor magnetic resonance images. IEEE Trans Med Imaging. 2001;20:1131–1139. doi: 10.1109/42.963816. [DOI] [PubMed] [Google Scholar]