Abstract
Objective
To understand the influence of cancer-related infertility on women’s long-term distress and quality of life. Women diagnosed at age 40 or less with breast cancer, Hodgkin disease (HD), non-Hodgkin lymphoma (NHL), were interviewed an average of 10 years later. We predicted that women whose desire for a child at diagnosis remained unfulfilled would be significantly more distressed.
Methods
Participants completed a semi-structured phone interview, including the SF-12®, Brief Symptom Inventory-18, Impact of Events Scale (IES), Reproductive Concerns Scale (RCS), brief measures of marital satisfaction or comfort with dating, sexual satisfaction, and menopause symptoms.
Results
Of 455 women contacted by phone, 240 (53%) participated. Seventy-seven women had wanted a child at diagnosis but did not conceive subsequently (38 remaining childless and 39 with secondary infertility). Even controlling for other psychosocial and health factors, this group had higher distress about infertility (RCS) (P < 0.001), had more intrusive thoughts about infertility, and used more avoidance strategies when reminded of infertility (IES) (P < 0.001). Childless women were the most distressed. Women with adopted or stepchildren were intermediate, and those with at least one biological child were least distressed. Infertility-related distress did not differ significantly by cancer site.
Conclusions
Even at long-term follow-up, distress about interrupted childbearing persists, particularly in childless women. Social parenthood buffers distress somewhat, but not completely. Not only is it important to offer fertility preservation before cancer treatment, but interventions should be developed for survivors to alleviate unresolved grief about cancer-related infertility.
Keywords: Cancer, infertility, survivorship, childbearing, distress, parenting
Introduction
Infertility related to cancer treatment has received increasing attention in the past ten years. More women are delaying pregnancy until their thirties, only to have cancer interrupt their life plans [1]. By age 39, one in 51 women will be diagnosed with an invasive cancer [2].
Treatment for the cancers most common in premenopausal women often decreases fertility or leads to permanent ovarian failure. For example, after adjuvant chemotherapy for breast cancer with current regimens, only 10% to 20% of women under age 35 experience permanent amenorrhea. However, the risk of ovarian failure increases tenfold for women treated in their late thirties, and up to 90% of women aged 40 and older are left with permanent ovarian failure [3, 4].
Even in young women who continue or resume menstruation after chemotherapy, diagnostic tests reveal that ovarian reserve is diminished. Ultrasound imaging of the ovaries to measure volume and antral follicle counts as well as testing levels of anti-Müllerian hormone are more sensitive measures of potential fertility than amenorrhea [5,6]. These tests indicate reduced numbers of primordial follicles not only in breast cancer survivors, but also in women treated for Hodgkin disease [7] or pediatric malignancies [8–10]. Although many of these women can become pregnant after cancer treatment, they will probably experience permanent ovarian failure at an age far younger than normal.
Most women treated for cervical cancer can no longer carry a pregnancy because they have had radical hysterectomy or a large dose of pelvic radiotherapy. Only those with very early stage disease are eligible for the fertility-sparing surgical options of conization or trachelectomy [11]. Irradiation of the uterus [12] can also cause pregnancy complications including miscarriage, prematurity, and low birth weights [13]. Occult damage to heart or lung function after cancer treatment can manifest as acute illness during the physiological stress of pregnancy [14]. Furthermore, some women forego pregnancy because of fears about triggering a cancer recurrence or having a child with birth defects [15], even though pregnancy is not associated with recurrence even after breast cancer [16] and children born to cancer survivors have no excess rate of malformations [17].
With the increasing success of assisted reproductive technology [18], fertility preservation before cancer treatment has become an option. Several professional organizations have published ethical and practice guidelines [13,19, 20]. Most women can delay cancer treatment for several days to surgically harvest ovarian tissue [21], or up to two to three weeks in order to undergo a cycle of ovarian stimulation to collect oocytes that can be cryopreserved in the unfertilized state [22] or used to create embryos for freezing [23,24]. Unfortunately, only a few live births have occurred from auto-transplanted ovarian tissue [21]. The chance of a conception and live birth from stored oocytes or embryos is far more reasonable, at least for women under age 35. However, insurance coverage for fertility preservation is not mandated nationally or in any state [25,26], making it unaffordable for the majority of eligible women. Even if women wish to consider fertility preservation, many do not receive timely information. Two recent surveys of oncologists concur that less than half routinely refer young female patients for fertility preservation [27,28]. These barriers undoubtedly contribute to a 20% reduction in achieving a first pregnancy in women who survive childhood cancer [29] and a 50% decrease in women diagnosed as young adults [30,31], even though marriage rates in female survivors are only slightly less than in peers [32].
Young cancer survivors are often encouraged to consider adoption, yet they face discrimination from international and domestic agencies, as well as from birth mothers choosing a family for independent adoptions [33]. Only a minority of cancer survivors consider using oocyte donors or gestational carriers to become parents [15]. High out-of-pocket costs and legal restrictions may also limit third-party reproduction.
Cancer-related infertility is likely to be distressing, since most women in the United States want to have children. In 2002, the National Survey of Family Growth interviewed over 7,500 women aged 15 to 44 [34]. Forty-two percent said they would be bothered a great deal and 29% somewhat, if they never had a child. In a survey of young cancer survivors, 75% of those without children wanted to have one in the future [15].
Although considerable research has documented women’s high distress during infertility treatment [35], few studies have assessed the long-term impact of unresolved infertility. Research in women whose infertility was unrelated to cancer suggests lingering distress when women remain childless. In a qualitative study of 14 women 20 years after unsuccessful infertility treatment, marital relationships, sexuality, and self-esteem remained problematic. Emotional distress was exacerbated when peers began to have grandchildren [36]. Three to 15 years after in vitro fertilization (IVF), women who remained childless had lower well-being and life satisfaction scores than those who had a child [37–40]. Women who adopted a child had intermediate scores [40].
A recent analysis of responses of 2,894 women from the population-based National Survey of Fertility Barriers confirms that women who are involuntarily childless have higher rates of depression and lower life satisfaction than women who have not experienced infertility [41]. Women who had lost a pregnancy and were still childless had particularly poor mental health, even at an average follow-up of 7 years, suggesting that involuntary childlessness is a traumatic event. It is possible that women who suffer the dual traumas of a cancer diagnosis and infertility would also have more severe symptoms.
Aims and Hypotheses
Since having cancer at a young age is emotionally distressing in itself [42], it is unclear how much cancer-related infertility influences quality of life in long-term cancer survivors. To answer this question, we conducted a cross-sectional survey of younger women diagnosed with cancer, unselected for their desire for children at diagnosis, and now 5 to 10 years post-treatment. Our hypotheses were as follows:
Even with other medical and demographic factors taken into account, women who wanted a child at cancer diagnosis and were unable to have one would have significantly higher general and infertility-specific distress, and poorer health-related quality of life, than women who did not want a child at diagnosis or women who had a child after cancer.
Within the group of women whose childbearing was interrupted, those childless at follow-up would be the most distressed.
Within the group of women who wanted a child at diagnosis, those who had a biological child after cancer would be less distressed than women who became social parents through adoption or step-parenting.
Because of their unique concerns about the impact of pregnancy or hormonal treatments for infertility on cancer recurrence, survivors of breast cancer would be more distressed about childbearing issues than women with other types of cancer.
Methods
Design of the Study
The University of Texas M. D. Anderson tumor registry provided contact information for 1,852 eligible survivors diagnosed from age 14 to 40 between 1992 to 1997 with invasive cervical cancer, breast cancer, Hodgkin disease, or non-Hodgkin lymphoma. We chose these malignancies because they commonly affect younger women, have reasonable prognoses, and many standard treatments impair fertility. Some women had a recurrence or second primary in the interim, but we only contacted women currently free of disease. The protocol was approved by our Institutional Review Board.
A letter was mailed describing the study. Participation entailed completing a 60-minute, semi-structured phone interview including several standardized questionnaires. A modest payment of $20 to compensate for time and trouble was offered. Women who wanted to opt out could call a toll-free number or return a postage-paid card. After three weeks, research staff began contacting remaining women by phone to describe the study in detail. Women interested in participating were mailed informed consent forms and a postage-paid return envelope. When signed forms were received, the interview time was scheduled.
Assessment
Interviewers were trained and supervised by the primary investigator. Responses were coded directly into a computerized database using the Nova Research Questionnaire Data System, (QDS) version 2. Women were identified only with a study number. The list pairing study numbers with names was kept confidential and destroyed after completion of data collection and cleaning.
The phone interview assessed demographic and medical history items. Standardized questionnaires included the SF-12®, a brief measure of health-related quality of life yielding a Physical Health Component Score (PCS) and Mental Health Component Score (MCS) [15, 43]. The Brief Symptom Inventory 18 (BSI-18), with oncology-based norms, assessed general emotional distress in the past week [44].
Infertility-specific post-traumatic symptoms were measured with the Impact of Event Scale (IES) [45,46]. Scores on the avoidance and intrusion subscales indicate an oscillating cycle of intrusive imagery and emotions after a stressful event, followed by attempts to avoid painful thoughts or feelings. The definition of the stressor was: “In the past week you have thought about the impact of cancer on your ability to conceive or carry a pregnancy.”
The Reproductive Concerns Scale is a unidimensional, 14-item inventory measuring specific distress about infertility in female cancer survivors with an internal consistency of 0.91 [47]. Sample items include: “I feel I have lost control over my reproductive future” and “I feel sad about my inability to have children.” The response format is a 5-point Likert scale ranging from 0 = “not at all” to 4 = “very much.”
Most young women with cancer-related infertility also have premature ovarian failure. Menopausal symptoms could account for increased distress or poorer quality of life. Therefore, we included a 7-item symptom scale derived from the Breast Cancer Prevention Trial (BCPT) Symptom Checklist. Women rated each symptom on a 5-point scale from “not at all” to “extremely” bothersome over the past 4 weeks [48–50].
Women who were married or in a serious relationship of ≥ 6 months completed an abbreviated, 7-item form of the Dyadic Adjustment Scale (A-DAS) [51]. Single women completed the 5-item dating subscale of the Cancer Rehabilitation Evaluation System (CARES)[52] instead. The subscale has an internal consistency score of 0.90 and measures comfort in dating despite cancer concerns. Sexual satisfaction was assessed by the 3-item sexual satisfaction subscale of the Female Sexual Function Index (FSFI) [53].
Interview questions had multiple-choice responses, using a 5-point Likert-scale format and included the following: How much did you want to have a child in the future at the time your cancer was diagnosed? (from “I definitely wanted to have a child in the future” to “ I definitely did not want to have a child in the future}; How much has your experience of cancer affected your wish to have children in the future? (from “It greatly increased my wish to have children” to “It greatly decreased my wish to have children”); and the following questions each with a 5-point scale from “I have not worried at all” to “This worry is a major life stress for me”: How much have you worried that your cancer treatment would interfere with your fertility (ability to conceive and carry a baby to term)? How much have you worried that becoming pregnant could trigger a new episode of cancer for you? How much have you worried that your cancer treatment could cause health problems for any children born afterwards? How much have you worried that your children would have an increased risk for cancer in their lifetimes?[15]. Women were also asked: What is your current view of your own fertility? (4-point scale from “I think I am more fertile than most women of my age” to “I think I am infertile (could not become pregnant”). Finally, history of fertility preservation (listing each major option), infertility treatment after cancer, and attempts to adopt were documented.
Data Management and Statistical Analysis
Statistical analyses were conducted using SPSS (Version 15). Descriptive statistics (e.g., frequencies, mean, range, standard deviation, skewness, and kurtosis) and 95% confidence intervals (CIs) were obtained where appropriate. Scoring guidelines for each questionnaire were followed and item analyses were conducted on composite score instruments. Internal consistency was estimated using Cronbach’s α. Distributional characteristics of the variables were examined using chi-square tests, boxplots, histograms, and the Kolmogorov-Smirnov test of normality. Regression analyses examined the amount of variance significantly associated with interrupted childbearing after controlling for demographic and medical factors in these outcomes: IES scores and RCS.
Results
Response Rate
Of the 1,852 women in the database prepared by the UTMDACC Department of Medical Informatics, 93 (4.8%) turned out to be deceased. Another 307 letters (15.8%) were undeliverable and no updated address could be found for the patient. This left a potential sample of 1,452 women. Two hundred and fifteen (15.4%) declined to participate with an “opt out” postcard or telephone call. Of the remaining 1,237 survivors, our research staff members were only able to reach 455 by phone (37%). The others either had no valid phone number, or never answered a call or returned phone messages. We left a maximum of five phone messages for a woman, calling at different times of day and week. Of the women successfully contacted by phone, 215 (47%) declined participation and 240 (53%) completed interviews.
The 240 study participants were compared with all others (N = 1,612). Participants and non-responders did not differ significantly in age or percent non-Caucasian. However, participants had a longer follow-up time since cancer diagnosis (mean 9.9 ± 1.9 vs. 9.6 ± 1.8 years, P = 0.013) and were less likely to be Hispanic (7.1% of participants vs. 12.1% of non-responders, P = 0.021).
Demographic and Medical Characteristics of Participants
Table 1 lists demographic and medical characteristics of the 240 participants. Participation rates for those successfully contacted by phone differed by cancer site (65.0% for HD/NHL, 49.4% for breast, and 37.8% for cervical, P < 0.001). Among African-American respondents, breast cancer was the most likely diagnosis ((χ2 [8] = 19.57, P = .01), whereas Hispanic women were most likely to have HD/NHL (χ2 [2] = 7.47, p = .02). There were no significant differences in education or income between women with different cancer sites, however. Of the 47 women who were not married at the time of the survey, 12 women (26%) were in a committed relationship, yielding a total of 205 partnered women (85%) in the sample.
Table 1.
Demographic and Medical Characteristics of Participants (N = 240)
| Variable | Range | Mean ± SD |
|---|---|---|
| Current Age in Years | 24 – 53 | 43.7 ± 6.2 |
| Years since Diagnosis | 6 – 15 | 9.9 ± 1.9 |
| Variable | Value | N | % |
|---|---|---|---|
| Ethnicity | Caucasian | 195 | 81.7 |
| African-American | 11 | 4.6 | |
| Hispanic | 17 | 7.1 | |
| Other | 16 | 6.7 | |
| Marital Status | Married | 193 | 80.4 |
| Widowed | 4 | 1.7 | |
| Divorced/Separated | 19 | 7.9 | |
| Never Married | 24 | 10.0 | |
| Education | ≤ High School | 39 | 16.3 |
| Some College | 68 | 28.3 | |
| Bachelor’s Degree | 79 | 33.3 | |
| Postgraduate Degree | 53 | 22.1 | |
| Income | < $25,000 | 19 | 7.9 |
| $26,000–$50,000 | 34 | 14.2 | |
| $51,000 – 75,000 | 50 | 20.8 | |
| $76,000 – 100,000 | 41 | 17.1 | |
| > $100,000 | 83 | 35.0 | |
| Unknown | 12 | 5.0 | |
| Type of Cancer | HD/NHL | 89 | 37.1 |
| Breast Cancer | 130 | 54.6 | |
| Cervical Cancer | 20 | 8.3 | |
| Self-Reported Treatment | Surgery | 225 | 93.8 |
| Chemotherapy | 185 | 77.1 | |
| Radiation Therapy | 146 | 61.3 | |
| Cancer Recurrence | 74 | 31.2 | |
| New Primary (other than basal cell) | 27 | 11.1 |
Psychosocial Adjustment of Participants
Table 2 compares participants’ scores with normative data on questionnaires. Long-term survivors reported fewer symptoms on the IES related to interrupted childbearing than a normative sample instructed to recall an “upsetting event.” Twenty percent of long-term survivors had GSI scores in the distressed range on the BSI-18 vs. 25% in the normative sample of female oncology patients in active treatment [47]. Single long-term survivors in the current sample had more dating concerns (CARES – Dating Subscale) than the normative group [52]. Rates of menopausal symptoms on the BCPT were also higher than norms [50], but only for menopausal women(N = 133, mean 5.11 ± 4.96), not for premenopausal participants (N = 91, mean 1.90 ± 2.79, t[222] = 5.60, P < 0.001). Questionnaire scores did not differ significantly by cancer site.
Table 2.
Psychosocial Questionnaire Scores for Survivors Compared with Norms
| Questionnaire | Long-Term Survivors | Normative Samples | |||
|---|---|---|---|---|---|
| N | Mean | SD | Mean | SD | |
| Impact of Event Scale | 229 | 4.86 | 11.71 | 8.1 | 12.3 |
| SF-12® Physical Health | 235 | 47.67 | 12.13 | 50.0 | 10.0 |
| SF-12® Mental Health | 235 | 49.41 | 9.78 | 50.0 | 10.0 |
| BSI Global Severity * | 239 | 47.33 | 10.56 | 50.0 | 10.0 |
| Reproductive Concerns* | 173 | 9.16 | 9.91 | 9.76 | 11.6 |
| CARES-Dating* | 31 | 7.61 | 5.40 | 1.4 | 1.3 |
| A-DAS | 201 | 24.75 | 4.96 | 25.6 | 4.8 |
| FSFI Sexual Satisfaction | 192 | 11.10 | 4.10 | 12.8 | 3.0 |
| BCPT Menopause Symptoms | 224 | 3.81 | 4.49 | 1.4 | 0.6 |
Scores normed on oncology sample
Parental Status at Diagnosis and at Time of Survey
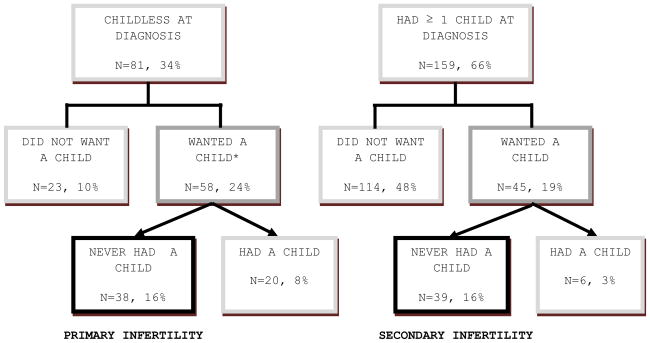
Figure 1 illustrates the parental status of women at diagnosis and currently. At diagnosis, the 66% of women with at least one biological child were significantly older than childless women (35.62 ± 4.17 years vs. 30.09 ± 7.30, t[238] = 7.48, P < 0.001). Twenty-four of 31 women who gave birth during or after cancer treatment had probably or definitely wanted a child at diagnosis. The experience of cancer had not influenced the desire for a child in 68% of women, but increased it for 15% and decreased it for 17%.
Figure 1.
Biological Parenthood at Diagnosis and Follow-Up
*Wanted a child at diagnosis was defined as responding “definitely” or “probably” on the multiple-choice question
Only 10 women had attempted fertility preservation, which is not surprising given the era of their cancer diagnoses. Ovarian tissue cryopreservation and ovarian stimulation to bank embryos were only inaugurated in the late 1990s [54,55]. Five women took hormones to induce temporary menopause during chemotherapy. Three had ovarian transposition prior to pelvic irradiation. Two opted for a less toxic chemotherapy regimen. Thirty-two women (13%) had a fertility evaluation after cancer and 16 (7%) had infertility treatment. Of 31 women who had a child during or after cancer treatment, 5 conceived via infertility treatment and 26 without medical intervention.
Parental Status and Psychosocial Adjustment
Table 3 compares psychosocial adjustment of the 77 women in Figure 1 who were unable to have a desired biological child after cancer to the rest of the sample. These women reported significantly more infertility-related traumatic symptoms (e.g., both intrusion and avoidance scales on the IES), greater distress about infertility (RCS), less sexual satisfaction (FSFI subscale), and poorer mental health (SF-12® MCS) than their peers. Women whose childbearing had been interrupted worried more that cancer treatment had interfered with fertility (χ2(4) = 76.28, P < 0.001), that pregnancy could trigger a recurrence (χ2(4) = 16.23, P < 0.01), and that their cancer treatment could harm their children’s health (χ2(4) = 26.07, P < 0.001). Each of these issues was assessed with a multiple-choice question.
Table 3.
Psychosocial Outcomes by Unfulfilled Desire for a Biological Child at Cancer Diagnosis
| Unfulfilled Desire | Had Biological Children if Desired | |||||
|---|---|---|---|---|---|---|
| Questionnaire | N | Mean (SD) | N | Mean (SD) | t | P |
| IES Intrusion | 77 | 5.51 (7.75) | 152 | 0.49 (2.66) | 7.19 | < 0.001 |
| IES Avoidance | 77 | 6.52 (9.12) | 152 | 0.74 (3.64) | 6.82 | < 0.001 |
| IES Total | 77 | 12.03 (16.00) | 152 | 1.24 (6.23) | 7.30 | < 0.001 |
| Reproductive Concerns | 55 | 18.11 (11.04) | 118 | 4.99 (5.73) | 10.29 | < 0.001 |
| A-DAS | 58 | 24.59 (4.13) | 143 | 24.82 (5.27) | −0.30 | 0.77 |
| CARES Dating | 17 | 8.29 (6.03) | 14 | 6.79 (4.61) | 1.42 | 0.24 |
| FSFI Sex Satisfaction | 56 | 10.21 (4.56) | 136 | 11.46 (3.86) | 6.10 | 0.01 |
| SF-12® PCS | 77 | 48.31 (13.44) | 158 | 47.35 (11.47) | .57 | 0.57 |
| SF-12® MCS | 77 | 46.93 (11.34) | 158 | 50.61 (8.71) | −2.75 | < 0.01 |
| BSI-18 GSI T-score | 77 | 47.67 (11.51) | 161 | 47.17 (10.09) | 0.35 | 0.73 |
Within the group of 77 women who wanted a child at diagnosis, those remaining childless (primary infertility, N = 38) were significantly more distressed on the RCS than those who had ≥ one child at diagnosis but were unable to have another, desired child (secondary infertility, N = 39) (mean 18.11 ± 11.04 vs. 7.85 ± 8.16, F = 37.22, P < 0.001). A similar difference between primary and secondary infertile groups was seen on the IES (total: mean 12.03 ± 16.00 vs. 2.25 ± 7.54, F = 17.76, P < 0.001).
As noted in the Methods section, women were asked to rate their current fertility compared to peers. Those who viewed themselves as infertile (N=135) had significantly lower sexual satisfaction (P < 0.001), relationship satisfaction (P = 0.002) and confidence about dating (P = 0.052) compared to other women in the study.
The Impact of Social Parenting
We define social parenting as raising children not genetically related to the mother. Thirteen women tried to adopt a child after their cancer and nine were ultimately successful. Adoption sources were: birthmother (three), public agency (four), private agency (one), and international agency (one). Three additional women had already adopted before cancer, six raised informally adopted children, and 29 had stepchildren. Table 4 compares psychosocial adjustment in women currently not raising any children with those who are raising only biological children, only “social” children or both. These categories do not correspond exactly to those in Table 3. Distress about infertility (RCS) is greatest in childless women, but is also elevated in those only raising social children. IES total and subscale scores follow the same pattern. The groups do not differ significantly in general distress levels (BSI-18) or mental health (SF-12® MCS).
Table 4.
Impact of Current Social vs. Biological Parenthood vs. Childlessness on Psychosocial Adjustment
| Biological Only (N=158) | Combined Biological and Social* (N=26) | Social Only* (N=19) | Childless (N=37) | |||
|---|---|---|---|---|---|---|
| Measure | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | F | P |
| RCS | 6.64 (7.65) | 9.60 (11.02) | 14.77 (9.07) | 18.43 (12.91) | 12.85 | <0.001 |
| IES Total | 2.94 (9.20) | 3.96 (10.74) | 4.71 (8.36) | 13.67 (18.00) | 9.07 | <0.001 |
| SF-12® MCS | 50.32 (9.36) | 47.95 (9.94) | 48.87 (10.35) | 46.57 (10.87) | 1.72 | 0.17 |
| BSI-18, GSI T-Score | 46.34 (10.57) | 50.38 (11.16) | 49.53 (8.29) | 48.32 (10.85) | 1.59 | 0.19 |
Social parenthood is defined as raising a stepchild (29 women), adopted child (12 women), informally adopted child (6 women), or child conceived with donor oocytes (0 women).
Contribution of Interrupted Childbearing to Infertility-related Distress and Symptoms of Trauma
Factors such as younger age, lower socioeconomic status, a cancer recurrence or second primary, type of cancer treatment received, or more severe menopause symptoms could account for some of the variance in psychosocial adjustment associated in long-term survivors. Our major hypothesis was that interrupted childbearing would predict outcomes even with such factors controlled. Table 5 shows results of regression analyses. Unfulfilled desire for a child still accounted for significant variation in intrusive thoughts and avoidance (IES) and in emotional distress about infertility (RCS). However, unfulfilled desire for a child was not significantly associated with mental health when other factors were taken into account (SF-12® MCS; F[131] = 0.910, P = 0.342).
Table 5.
Regression Analyses: Interrupted Childbearing and Psychosocial Adjustment
| Impact of Event Scale | Reproductive Concerns Scale | |||
|---|---|---|---|---|
| Model 1 β | Model 2 β | Model 1 β | Model 2 β | |
| Age | −0.105 | −0.053 | −0.173 | −0.156 |
| Cancer recurrence | −0.152 | −0.065 | −0.189 | 0.008 |
| Second primary | −0.029 | 0.019 | −0.031 | 0.028 |
| Income | −0.058 | −0.049 | −0.122 | −0.023 |
| Education | 0.155 | 0.106 | .0310 | 0.165 |
| Menopause symptoms | 0.109 | 0.032 | 0.127 | 0.035 |
| Surgery | −0.032 | −0.112 | 0.036 | −0.096 |
| Radiation | 0.024 | −0.104 | 0.000 | −0.072 |
| Chemotherapy | 0.007 | −0.036 | −0.085 | −0.089 |
| Had desired children after cancer | - | −0.380*** | - | −0.582*** |
| R2 (ΔR2) | 0.069 | 0.190 (.121) | 0.179 | 0.436 (.257) |
| Significance of F change | .386 | <.001 | .025 | <.001 |
Note.
P < 0.001
Discussion
Our primary hypothesis was partially supported. At an average of ten years after their illness, women who reported that cancer interrupted desired childbearing had significantly more distress about cancer-related infertility, intrusive thoughts, and use of avoidance in dealing with this painful issue, even when other medical and demographic factors were taken into account, including age, cancer recurrence, second primary tumors, income, education, type of cancer treatment, and menopause symptoms.
An unfulfilled desire for a child was not associated with a higher level of general emotional distress, however, as measured by the BSI-18. Women whose childbearing had been interrupted by cancer did have lower Mental Health Component scores on the SF-12 than their peers, but this relationship was no longer significant after controlling for medical and demographic factors. In general, women in our sample had good overall psychological adjustment. The mean SF-12 scores were very close to norms for community dwelling women on both mental and physical health. Only 20% of women had clinically significant levels of distress on the BSI-18, similar to rates of 18% to 22% in larger samples of survivors of pediatric and adult cancers [56]. Thus the distress appears to be limited to the fertility issue.
Women who viewed themselves as infertile reported lower sexual and relationship satisfaction than women who believed they had normal fertility. Although cancer treatment and premature ovarian failure undoubtedly contributed to sexual problems in this sample, women treated for infertility also have excess rates of sexual dysfunction and relationship stress [57].
Since we interviewed our cohort, two cross-sectional surveys of the impact of infertility on younger adult female cancer survivors [58,59] and one prospective survey of survivors of hematopoietic cell transplant [60] have been published. The samples in these studies were small (N’s ranged from 70 to 131). One focused only on transplant survivors [60] and one on breast cancer survivors [59]. However, their findings concur with ours. A woman’s unfulfilled desire for a child at the time of cancer diagnosis, whether assessed prospectively or retrospectively, remains strong and influences her quality of life even 5 to 10 years after cancer treatment. Women who remain childless are the most distressed group [58–60]. A strength of our study was having a large enough sample to compare women who had completed their families before cancer diagnosis to those who still wanted children.
Most surveys, including ours, have asked retrospectively about desire for a child at cancer diagnosis. It is possible that women currently distressed about fertility are over-reporting their past desire for a child. However, the agreement between the results of the prospective and retrospective studies of cancer survivors, and the very similar outcomes in a large, population-based study of women with unresolved infertility [41]do not suggest a large impact of recall bias. Of course prospective research would be desirable in the future.
None of the studies has recruited an appropriate comparison group to look at the emotional impact of infertility after cancer treatment compared to infertility from other causes. Hammond and colleagues had transplant survivors bring a sibling or friend as control [60]. The groups showed equal rates of distress about infertility. Rates of infertility in the control group were much higher than would be expected, however, undoubtedly due to recruitment bias. Carter et al. compared their cancer survivors to 50 infertile women on a waiting list to receive oocyte donation [58]. The groups had similar levels of depression and distress about infertility, which is not surprising. Women who pursue assisted reproductive technology include less than 2% of infertile women in the United States and are thus likely to be more distressed than women who do not seek help [61]. The question of dual trauma remains unresolved.
Our study was also able to compare women who became social versus biological parents. As in the literature on women seeking assisted reproductive technology [37,38,40], social parenting does not resolve distress completely. However, we combined step-parents with adoptive parents. Mothers who create a family after infertility appear to value their children more than women who conceived without a problem [62,63]. This held true in a comprehensive, longitudinal study whether children were conceived with IVF, with donor sperm or oocytes, or adopted, although with adoption there was slightly less warmth between mother and child at adolescence [62]. Similar, longitudinal research is needed on parenting and family relationships for cancer survivors who adopt or conceive using third-party reproduction.
We had hypothesized that women with breast cancer would be more distressed about cancer-related infertility than women with HD/NHL or cervical cancer, because of lingering uncertainty about whether risk of recurrence could be increased by fertility treatment [64] or pregnancy [65]. No differences in psychosocial adjustment were observed according to cancer site, however, nor did distress over interrupted childbearing interact with cancer site.
A major limitation of this survey is the difficulty we had in contacting the majority of women listed in our tumor registry. The women with cervical cancer were the most difficult to find, which is consistent with the lower socioeconomic status and greater geographic mobility of this group [66]. Another major problem was women who did not answer their phone or return messages. Response rates for telephone surveys have dropped precipitously in the last ten years with the advent of call screening, women working outside of the home, and more households using cell phones rather than landlines [67]. Our phone contact rate of 37% is not unusually low. On the other hand, 53% of women contacted did participate in the study, a much higher response rate than we have achieved in postal surveys [15,43]. Our participants also were similar to the original sample in age and race, though we seem to have missed some Hispanic women.
These data certainly highlight the importance of fertility preservation for young women facing cancer treatment. Perhaps the most important task for oncology professionals is to lobby for insurance coverage for these procedures so that women can afford them [25,26]. However, we already have many thousands of younger women in the United States who have unresolved grief about interrupted childbearing. We are developing an evidence-based cognitive-behavioral intervention to provide women with social support [40], help them find meaning in their experience of cancer and infertility [68], and make informed decisions about whether to live without children or to pursue infertility treatment, adoption, or third party reproduction. Given the difficulty of building groups of younger female survivors outside of large urban areas, we hope to compare an internet [69] or phone-based format to a more traditional, in-person group.
Acknowledgments
We gratefully acknowledge funding from the National Cancer Institute from grant R21CA106958 (Canada, PI) that supported this research. Data were presented in part at the 4th Biennial Cancer Survivorship Research Conference, Atlanta, GA, June, 2008. We also thank the tumor registry of the UT M. D. Anderson Cancer Center for providing information for eligible participants.
References
- 1.Nabukera S, Wingate MS, Alexander GR, Salihu HM. First-time births among women 30 years and older in the United States; patterns and risk of adverse outcomes. J Reprod Med. 2006;51:676–82. [PubMed] [Google Scholar]
- 2.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, et al., editors. SEER Cancer Statistics Review, 1975–2007. National Cancer Institute; Bethesda, MD: 2010. http://seer.cancer.gov/csr/1975_2007/, based on November 2009 SEER data submission, posted to the SEER web site. [Google Scholar]
- 3.Abusief ME, Missmer SA, Ginsburg ES, Weeks JC, Partridge AH. The effects of paclitaxel, dose density, and trastuzumab on treatment-related amenorrhea in premenopausal women with breast cancer. Cancer. 2010;116:791–8. doi: 10.1002/cncr.24835. [DOI] [PubMed] [Google Scholar]
- 4.Swain SM, Land SR, Ritter MW, Costantino JP, Cecchini RS, Mamounas EP, et al. Amenorrhea in premenopausal women on the doxorubicin-and-cyclophosphamide-followed-by-docetaxel arm of NSABP B-30 trial. Breast Cancer Res Treat. 2009;113:315–30. doi: 10.1007/s10549-008-9937-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Partridge AH, Ruddy KJ, Gelber S, Schapira L, Abusief M, Meyer M, Ginsburg E. Ovarian reserve in women who remain premenopausal after chemotherapy for early stage breast cancer. Fertil Steril. 2010;94:638–44. doi: 10.1016/j.fertnstert.2009.03.045. [DOI] [PubMed] [Google Scholar]
- 6.Su HI, Sammel MD, Green J, Velders L, Stankiewicz C, Matro J, et al. Antimullerian hormone and inhibin B are hormone measures of ovarian function in late reproductive-aged breast cancer survivors. Cancer. 2010;116:592–9. doi: 10.1002/cncr.24746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giuseppe L, Attilio G, Edoardo DN, Loredana G, Cristina L, Vincenzo L. Ovarian function after cancer treatment in young women affected by Hodgkin disease (HD) Hematology. 2007;12:141–7. doi: 10.1080/10245330600954072. [DOI] [PubMed] [Google Scholar]
- 8.Larsen EC, Muller J, Rechnitzer C, Schmiegelow K, Nyboe Andersen A. Diminished ovarian reserve in female childhood cancer survivors with regular menstrual cycles and basal FSH <10 IU/l. Hum Reprod. 2003;18:417–22. doi: 10.1093/humrep/deg073. [DOI] [PubMed] [Google Scholar]
- 9.Rendtorff R, Hohmann C, Reinmuth S, Müller A, Dittrich R, Beyer M, et al. Hormone and sperm analyses after chemo- and radiotherapy in childhood and adolescence. Klin Padiatr. 2010;222:145–9. doi: 10.1055/s-0030-1249658. [DOI] [PubMed] [Google Scholar]
- 10.van Beek RD, van den Heuvel-Eibrink MM, Laven JS, de Jong FH, Themmen AP, Hakvoort-Cammel FG, et al. Anti-Mullerian hormone is a sensitive serum marker for gonadal function in women treated for Hodgkin’s lymphoma during childhood. J Clin Endocrinol Metab. 2007;92:3869–74. doi: 10.1210/jc.2006-2374. [DOI] [PubMed] [Google Scholar]
- 11.Gien LT, Covens A. Fertility-sparing options for early stage cervical cancer. Gynecol Oncol. 2010;117:350–7. doi: 10.1016/j.ygyno.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 12.Green DM, Lange JM, Peabody EM, Grigorieva NN, Peterson SM, Kalapurakal JA, et al. Pregnancy outcome after treatment for Wilms tumor: a report from the National Wilms Tumor Long-Term Follow-Up Study. J Clin Oncol. 2010;28:2824–30. doi: 10.1200/JCO.2009.27.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SJ, Schover LR, Partridge A, Patrizio P, Wallace WH, Hagerty K, et al. American Society of Clinical Oncology Recommendations on Fertility Preservation in People Treated for Cancer. J Clin Oncol. 2006;24:2917–31. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 14.van Dalen EC, van der Pal HJ, van den Bos C, Kok WE, Caron HN, Kremer LC. Clinical heart failure during pregnancy and delivery in a cohort of female childhood cancer survivors treated with anthracyclines. Eur J Cancer. 2006;42:2549–53. doi: 10.1016/j.ejca.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Schover LR, Rybicki LA, Martin BA, Bringelsen KA. Having children after cancer: a pilot survey of survivors’ attitudes and experiences. Cancer. 1999;86:697–709. doi: 10.1002/(sici)1097-0142(19990815)86:4<697::aid-cncr20>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 16.de Bree E, Makrigiannakis A, Askoxylakis J, Melissas J, Tsiftsis DD. Pregnancy after breast cancer. A comprehensive review. J Surg Oncol. 2010;101:534–42. doi: 10.1002/jso.21514. [DOI] [PubMed] [Google Scholar]
- 17.Mueller BA, Chow EJ, Kamineni A, Daling JR, Fraser A, Wiggins CL, et al. Pregnancy outcomes in female childhood and adolescent cancer survivors: a linked cancer-birth registry analysis. Pediatr Adolesc Med. 2009;163:879–86. doi: 10.1001/archpediatrics.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moragianni VA, Penzias AS. Cumulative live-birth rates after assisted reproductive technology. Curr Opin Obstet Gynecol. 2010;22:189–92. doi: 10.1097/GCO.0b013e328338493f. [DOI] [PubMed] [Google Scholar]
- 19.Ethics Committee of the American Society for Reproductive Medicine: Fertility preservation and reproduction in cancer patients. Fertil Steril. 2005;83:1622–1628. doi: 10.1016/j.fertnstert.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Fallat ME, Hutter J. American Academy of Pediatrics Committee on Bioethics; American Academy of Pediatrics Section on Hematology/Oncology; American Academy of Pediatrics Section on Surgery: Preservation of fertility in pediatric and adolescent patients with cancer. Pediatrics. 2008;121:e1461–9. doi: 10.1542/peds.2008-0593. [DOI] [PubMed] [Google Scholar]
- 21.von Wolff M, Donnez J, Hovatta O, Keros V, Maltaris T, Montag M, et al. Cryopreservation and autotransplantation of human ovarian tissue prior to cytotoxic therapy–a technique in its infancy but already successful in fertility preservation. Eur J Cancer. 2009;45:1547–53. doi: 10.1016/j.ejca.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 22.Grifo JA, Noyes N. Delivery rate using cryopreserved oocytes is comparable to conventional in vitro fertilization using fresh oocytes: potential fertility preservation for female cancer patients. Fertil Steril. 2010;93:391–6. doi: 10.1016/j.fertnstert.2009.02.067. [DOI] [PubMed] [Google Scholar]
- 23.Klock SC, Zhang JX, Kazer RR. Fertility preservation for female cancer patients: early clinical experience. Fertil Steril. 2010;94:149–55. doi: 10.1016/j.fertnstert.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 24.Robertson AD, Missmer SA, Ginsburg ES. Embryo yield after in vitro fertilization in women undergoing embryo banking for fertility preservation before chemotherapy. Fertil Steril. 2010 Jun 8; doi: 10.1016/j.fertnstert.2010.04.028. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 25.Quinn GP, Vadaparampil ST, Lowry KM, Eidson S, Knapp C, Bukulmez O. State laws and regulations addressing third-party reimbursement for infertility treatment implications for cancer survivors. Fertil Steril. 2010 Jun 23; doi: 10.1016/j.fertnstert.2010.05.017. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 26.Campo-Engelstein L. Consistency in insurance coverage for iatrogenic conditions resulting from cancer treatment including fertility preservation. J Clin Oncol. 2010;28:1284–6. doi: 10.1200/JCO.2009.25.6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quinn GP, Vadaparampil ST, Lee J-H, Jacobsen PB, Bepler G, Lancaster J, et al. Physician referral for fertility preservation in oncology patients: A national study of practice behaviors. J Clin Oncol. 2009;27:5952–7. doi: 10.1200/JCO.2009.23.0250. [DOI] [PubMed] [Google Scholar]
- 28.Forman EJ, Anders CK, Behera MA. A nationwide survey of oncologists regarding treatment-related infertility and fertility preservation in female cancer patients. Fertil Steril. 2009 Nov 26; doi: 10.1016/j.fertnstert.2009.10.008. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Green DM, Kawashima T, Stovall M, Leisenring W, Sklar CA, Mertens AC, et al. Fertility of female survivors or childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006;27:2677–85. doi: 10.1200/JCO.2008.20.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madanat LM, Nalila N, Dyba T, Hakulinen T, Sankila R, Boice JD, Jr, et al. Probability of parenthood after early onset cancer: a population-based study. Int J Cancer. 2008;123:2891–8. doi: 10.1002/ijc.23842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cvancarova M, Samuelsen SO, Magelssen H, Fosså SD. Reproduction rates after cancer treatment: experience from the Norwegian Radium Hospital. J Clin Oncol. 2008;27:334–43. doi: 10.1200/JCO.2007.15.3130. [DOI] [PubMed] [Google Scholar]
- 32.Syse A. Does cancer affect marriage rates? J Cancer Surviv. 2008;2:205–14. doi: 10.1007/s11764-008-0062-1. [DOI] [PubMed] [Google Scholar]
- 33.Rosen A. Third-party reproduction and adoption in cancer patients. J Natl Cancer Inst Monogr. 2005;34:91–3. doi: 10.1093/jncimonographs/lgi021. [DOI] [PubMed] [Google Scholar]
- 34.Martinez GM, Chandra A, Abma JC, Jones J, Mosher WD. Fertility, contraception, and fatherhood: Data on men and women from Cycle 6 (2002) of the National Survey of Family Growth. National Center for Health Statistics. Vital Health Stat. 2006;23:1–142. [PubMed] [Google Scholar]
- 35.Cousineau TM, Domar AD. Psychological impact of infertility. Best Practice Res Clin Obstet Gynaecol. 2007;21:293–308. doi: 10.1016/j.bpobgyn.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Wirtberg I, Möller A, Hogstrüm L, Tronstad SE, Lalos A. Life 20 years after unsuccessful infertility treatment. Human Reprod. 2007;22:598–604. doi: 10.1093/humrep/del401. [DOI] [PubMed] [Google Scholar]
- 37.Leiblum SR, Aviv A, Hamer R. Life after infertility treatment: a long-term investigation of marital and sexual function. Human Reprod. 1998;13:3569–74. doi: 10.1093/humrep/13.12.3569. [DOI] [PubMed] [Google Scholar]
- 38.Johansson M, Adolfsson A, Berg M, Francis J, Hogström L, Janson PO, et al. Quality of life for couples 4–5. 5 years after unsuccessful IVF treatment. Acta Obstet Gynecol Scand. 2009;88:291–300. doi: 10.1080/00016340802705956. [DOI] [PubMed] [Google Scholar]
- 39.Van Balen F, Tribos-Kemper TC. Long-term infertile couples: a study of their well-being. J Psychosom Obstet Gynaecol. 1993;14(suppl):53–60. [PubMed] [Google Scholar]
- 40.Daniluk JC, Tench E. Long-term adjustment of infertile couples following unsuccessful medical intervention. J Counseling Devel. 2007;85:89–100. [Google Scholar]
- 41.Schwerdtfeger KL, Shreffler KM. Trauma of pregnancy loss and infertility among mothers and involuntarily childless women in the United States. J Loss and Trauma. 2010;14:211–27. doi: 10.1080/15325020802537468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ganz PA, Greendale GA, Petersen L, Kahn B, Bower JE. Breast cancer in young women: reproductive and late effects of treatment. J Clin Oncol. 2003;21:4184–93. doi: 10.1200/JCO.2003.04.196. [DOI] [PubMed] [Google Scholar]
- 43.Schover LR, Brey K, Lichtin A, Lipshultz LI, Jeha S. Knowledge and experience regarding cancer, infertility, and sperm banking in younger male survivors. J Clin Oncol. 2002;20:1880–1889. doi: 10.1200/JCO.2002.07.175. [DOI] [PubMed] [Google Scholar]
- 44.Derogatis LR. Brief Symptom Inventory 18: Administration, scoring, and procedures manual. N Minneapolis, MN: CS Pearson; 2001. BSI 18. [Google Scholar]
- 45.Sundin EC, Horowitz MJ. Impact of Event Scale: Psychometric properties. Brit J Psychiatry. 2002;180:205–9. doi: 10.1192/bjp.180.3.205. [DOI] [PubMed] [Google Scholar]
- 46.Thewes B, Meiser B, Hickie IB. Psychometric properties of the Impact of Event Scale among women at increased risk for hereditary breast cancer. Psyco-oncology. 2001;10:459–68. doi: 10.1002/pon.533. [DOI] [PubMed] [Google Scholar]
- 47.Wenzel L, Dogan-Ates A, Habbal R, Berkowitz R, Goldstein DP, Kluhsman BC, et al. Defining and measuring reproductive concerns of female cancer survivors. J Natl Cancer Instit Monogr. 2005;34:94–8. doi: 10.1093/jncimonographs/lgi017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ganz PA, Rowland JH, Desmond K, Meyerowitz BE, Wyatt GE. Life after breast cancer: Understanding women’s health-related quality of life and sexual functioning. J Clin Oncol. 1998;16:501–14. doi: 10.1200/JCO.1998.16.2.501. [DOI] [PubMed] [Google Scholar]
- 49.Day R, Ganz PA, Costantino JP, Cronin WM, Wickerham DL, Fisher B. Health-related quality of life and tamoxifen in breast cancer prevention: a report from the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Clin Oncol. 1999;17:2659–69. doi: 10.1200/JCO.1999.17.9.2659. [DOI] [PubMed] [Google Scholar]
- 50.Ganz PA, Greendale GA, Petersen L, Zibecchi L, Kahn B, Belin TR. Managing menopausal symptoms in breast cancer survivors: results of a randomized controlled trial. J Natl Cancer Inst. 2000;92:1054–64. doi: 10.1093/jnci/92.13.1054. [DOI] [PubMed] [Google Scholar]
- 51.Carey MP, Spector IP, Lantinga LJ, Krauss DJ. Reliability of the Dyadic Adjustment Scale. Psychol Assess. 1993;5:238–40. [Google Scholar]
- 52.Schag CAC, Heinrich RL, Aadland RL, Ganz PA. Assessing problems of cancer patients: Psychometric properties of the Cancer Inventory of Problem Situations. Health Psychol. 1990;9:83–102. doi: 10.1037//0278-6133.9.1.83. [DOI] [PubMed] [Google Scholar]
- 53.Meston CM. Validation of the Female Sexual Function Index (FSFI) in women with female orgasmic disorder and female hypoactive sexual desire disorder. J Sex Marital Ther. 2003;29:39–46. doi: 10.1080/713847100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oktay K, Newton H, Aubard Y, Salha O, Gosden RG. Cryopreservation of immature human oocytes and ovarian tissue: an emerging technology? Fertil Steril. 1998;69:1–7. doi: 10.1016/s0015-0282(97)00207-0. [DOI] [PubMed] [Google Scholar]
- 55.Brown JR, Modell E, Obasaju M, King YK. Natural cycle in-vitro fertilization with embryo cryopreservation prior to chemotherapy for carcinoma of the breast. Hum Reprod. 1996;11:197–9. doi: 10.1093/oxfordjournals.humrep.a019017. [DOI] [PubMed] [Google Scholar]
- 56.Recklitis CJ, Parsons SK, Shih M, Mertens A, Robison LL. Factor structure of the Brief Symptom Inventory 18 in adult survivors of childhood cancer: results from the Childhood Cancer Survivor Study. Psychol Assess. 2006;18:22–32. doi: 10.1037/1040-3590.18.1.22. [DOI] [PubMed] [Google Scholar]
- 57.Millheiser LS, Helmer AE, Quintero RB, Westphal LM, Milki AA, Lathi RB. Is infertility a risk factor for female sexual dysfunction? A case-control study. Fertil Steril. 2010 Feb 24; doi: 10.1016/j.fertnstert.2010.01.037. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 58.Carter J, Raviv L, Applegarth L, Ford JS, Josephs L, Grill E, et al. A cross-sectional study of the psychosexual impact of cancer-related infertility in women: third party reproductive assistance. J Cancer Surviv. 2010 April 7; doi: 10.1007/s11764-010-0121-2. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gorman JR, Malcarne VL, Roesch SC, Madlensky L, Pierce JP. Depressive symptoms among young breast cancer survivors: the importance of reproductive concerns. Breast Cancer Res Treat. 2010 Feb 4; doi: 10.1007/s10549-010-0768-4. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hammond C, Abrams JR, Syrjala KL. Fertility and risk factors for elevated infertility concern in 10-year hematopoietic cell transplant survivors and case-matched controls. J Clin Oncol. 2007;25:3511–17. doi: 10.1200/JCO.2007.10.8993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chandra A, Stephen EH. Infertility service use among U.S. women: 1995 and 2002. Fertil Steril. 2010;93:725–36. doi: 10.1016/j.fertnstert.2008.10.049. [DOI] [PubMed] [Google Scholar]
- 62.Owen L, Golombok S. Families created by assisted reproduction: parent-child relationships in late adolescence. J Adolesc. 2009;32:835–48. doi: 10.1016/j.adolescence.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 63.Murray C, MacCallum F, Golombok S. Egg donation parents and their children: follow-up at age 12 years. Fertil Steril. 2006;85:610–8. doi: 10.1016/j.fertnstert.2005.08.051. [DOI] [PubMed] [Google Scholar]
- 64.Azim AA, Costantini-Ferrando M, Oktay K. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: a prospective controlled study. J Clin Oncol. 2008;26:2630–5. doi: 10.1200/JCO.2007.14.8700. [DOI] [PubMed] [Google Scholar]
- 65.Hickey M, Peate M, Saunders CM, Friedlander M. Breast cancer in young women and its impact on reproductive function. Hum Reprod Update. 2009;15:323–39. doi: 10.1093/humupd/dmn064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benard VB, Johnson CJ, Thompson TD, Roland KB, Lai SM, Cokkinides V, et al. Examining the association between socioeconomic status and potential human papillomavirus-associated cancers. Cancer. 2008;113 (10 Suppl):2910–8. doi: 10.1002/cncr.23742. [DOI] [PubMed] [Google Scholar]
- 67.Kempf AM, Remington PL. New challenges for telephone survey research in the twenty-first century. Annu Rev Public Health. 2007;28:113–26. doi: 10.1146/annurev.publhealth.28.021406.144059. [DOI] [PubMed] [Google Scholar]
- 68.Peterson BD, Pirritano M, Christensen U, Boivin J, Block J, Schmidt L. The longitudinal impact of partner coping in couples following 5 years of unsuccessful fertility treatments. Human Reprod. 2009;24:1656–64. doi: 10.1093/humrep/dep061. [DOI] [PubMed] [Google Scholar]
- 69.Cousineau TM, Green TC, Corsini E, Seibring A, Showstack MT, Applegarth L, et al. Online psychoeducational support for infertile women: a randomized controlled trial. Hum Reprod. 2008;23:554–66. doi: 10.1093/humrep/dem306. [DOI] [PMC free article] [PubMed] [Google Scholar]