Abstract
During the last five years there has been enormous progress in developing a deeper understanding of the molecular mechanisms that control the self-renewal and pluripotency of embryonic stem cells (ESC). Early progress resulted from studying individual transcription factors and signaling pathways. Unexpectedly, these studies demonstrated that small changes in the levels of master regulators, such as Oct4 and Sox2, promote the differentiation of ESC. More recently, impressive progress has been made using technologies that provide a global view of the signaling pathways and the gene regulatory networks that control the fate of ESC. This review provides an overview of the progress made using several different high-throughput technologies and focuses on proteomic studies, which provide the first glimpse of the protein-protein interaction networks used by ESC. The latter studies indicate that transcription factors required for the self-renewal of ESC are part of a large, highly integrated protein-protein interaction landscape, which helps explain why the levels of master regulators need to be regulated precisely in ESC.
Keywords: Embryonic stem cells, iPS cells, Sox2, Oct4, Nanog, systems biology, proteomics
Embryonic stem cells (ESC) have the ability to self-renew indefinitely and, under appropriate conditions, differentiate into cells derived from each of the three embryonic germ layers (Yu and Thomson, 2008). These properties make ESC an attractive model system for studying normal developmental processes. ESC have also generated considerable excitement due to their enormous potential in regenerative medicine. Equally exciting, the discovery of induced pluripotent stem (iPS) cells has brought the prospect of patient-specific cell replacement therapy closer to reality (Takahashi and Yamanaka, 2006). However, a comprehensive understanding of the biology of ESC and iPS cells is needed before these cells can be used most effectively in regenerative medicine.
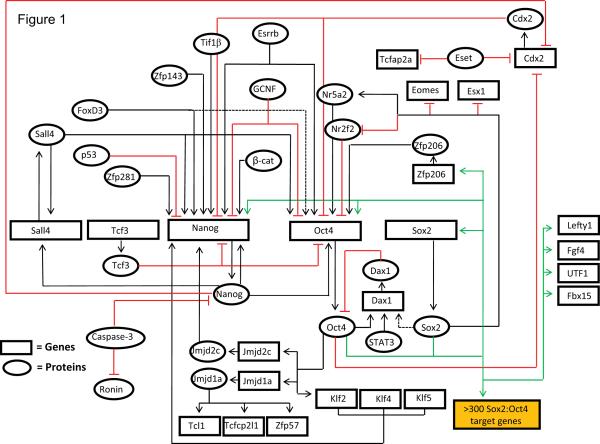
The behavior of pluripotent stem cells is controlled by a complex array of signaling pathways and gene regulatory networks. Over the past decade, study of the transcription regulatory networks in ESC has generated an extensive, yet incomplete, understanding of the mechanisms that regulate the fate of ESC (Figure 1). These studies, which involved transcription-based assays and genome-wide transcription factor binding analyses, argue that Sox2, Oct4, and Nanog function as master regulators that control the expression of several thousand genes in ESC (Boyer, et al., 2005, Chen, et al., 2008b, Chakravarthy, et al., 2008, Kim, et al., 2008, Rizzino, 2009). The pivotal roles played by Sox2, Oct4, and Nanog in the establishment and maintenance of undifferentiated ESC is reinforced by the discovery that exogenous expression of the transcription factors, Sox2, Oct4, Klf4, and c-Myc or Sox2, Oct4, Nanog and the RNA binding protein Lin28 are capable of reprogramming mouse and human somatic cells into iPS cells (Takahashi and Yamanaka, 2006, Yu, et al., 2007, Cox and Rizzino, 2010).
Figure 1. Transcription regulatory networks in ESC.
Our understanding of the gene regulatory networks that control the fate of ESC is far from complete. Over the past decade, important progress has been made in defining the regulation of a large number of genes that are critical for the maintenance of ESC. This figure compiles many of the regulatory loops known to control the expression of a subset of genes required for the maintenance of ESC. Clearly, it is incomplete. The purpose of this figure is to help illustrate that the expression of these genes is controlled by a highly integrated network of both positive and negative feedback and feedforward gene regulatory loops. Solid arrows: direct binding to the gene and/or activation of transcription. Dashed arrows: evidence of binding to the gene and/or activation of transcription. Red lines with a vertical end: inhibition of transcription or protein activity. Green arrows: target genes regulated, at least in part, by Sox2 and Oct4.
This figure was compiled by drawing on a large source of published data, which involve findings for both mouse and human cells. Key studies used to generate this figure are cited below. Genome-wide transcription factor binding analyses and transcription-based assays have identified >300 Sox2:Oct4 target genes in ESC (Boyer, et al., 2005, Chen, et al., 2008b, Chakravarthy, et al., 2008, Kim, et al., 2008). Identification of target genes of Nanog in ESC has demonstrated that it co-occupies many Sox2:Oct4 target genes (Boyer, et al., 2005, Chen, et al., 2008b). Several positive and negative regulatory mechanisms exist to maintain the precise levels of these master regulators in ESC. Expression of Sox2 is positively regulated by Sox2:Oct4 heterodimer (Chew, et al., 2005). Other regulatory mechanisms that control the expression of Sox2 have not been elucidated. Oct4 expression is controlled by multiple positive and negative mechanisms. Similar to Sox2, expression of Oct4 is controlled by Sox2:Oct4 heterodimer (Chew, et al., 2005). Additionally, Zfp206, Nr5a2, Esrrb, FoxD3, Sall4, and Nanog activate the transcription of Oct4 (Zhang, et al., 2006, Masui, et al., 2007, Zhang, et al., 2008, Yu, et al., 2009, Pan, et al., 2006); whereas, Nr2f2, Cdx2, GCNF, and Tcf3 inhibit the transcription of Oct4 (Masui, et al., 2007, Tam, et al., 2008, Niwa, et al., 2005, Gu, et al., 2005). Expression of Nanog is also controlled by multiple positive and negative regulatory signal inputs (Chen, et al., 2008a, Wang, et al., 2008b, Seki, et al., 2010, Pan, et al., 2006, Gu, et al., 2005, Takao, et al., 2007, Lin, et al., 2005, van den Berg, et al., 2008, Chen, et al., 2009). In addition to transcriptional activation by the Sox2:Oct4 heterodimer, Nanog autoregulates the expression of its own gene (Rodda, et al., 2005, Wu, et al., 2006). Oct4 also regulates the expression of proteins, such as Klf2 and Jmjd2c, which in turn transcriptionally activate the expression of Nanog (Hall, et al., 2009, Loh, et al., 2007, Jiang, et al., 2008). Sox2, Oct4, and Nanog maintain ESC identity by regulating the expression of several critical proteins (Sun et al., 2008). Sox2, through its opposing roles on the expression of Nr5a2 and Nr2f2, helps maintain the expression of Oct4 (Masui, et al., 2007). Oct4 and Nanog inhibit the expression of Cdx2, and hence the trophectoderm differentiation of ESC (Niwa, et al., 2005, Chen, et al., 2009). Recent genome-wide studies have suggested that several pluripotency factors, including Sall4 and Tcf3, are an integral part of the core ESC transcription regulatory circuit (Yang, et al., 2008, Cole, et al., 2008). In this regard, Nanog and Sall4 interact with one another and autoregulate the expression of their own genes (Wu, et al., 2006). Other mechanisms that modulate the binding of master regulators to their target genes, and alter the stability of master regulators, also significantly influence the functioning of the above described transcription regulatory network in ESC (Dejosez, et al., 2008, Fujita, et al., 2008, Sun, et al., 2009).
In the 1990s, efforts to understand the mechanisms that control ESC focused primarily on the expression and function of individual genes. During the last five years, with the advent of new technologies, research has shifted from studying individual molecules to using approaches that provide a more global understanding of key regulatory processes in ESC. In this review, we summarize some of the insights gained by application of systems biology to the study of ESC. We provide an overview of salient developments encompassing different aspects of ESC, and we discuss in more detail the progress made using unbiased proteomic screens to elucidate the protein-protein interaction landscape of pluripotency-associated factors in ESC. The latter studies lead to the conclusion that transcription factors required for the self-renewal of ESC are part of a large, highly integrated protein-protein interaction landscape. In addition, we discuss recent progress made in defining the surprising changes that occur rapidly in the phosphoproteome when ESC undergo differentiation. Not discussed in this review are recent developments in our understanding of signaling pathways in ESC or changes in the cell cycle that accompany the differentiation of ESC. Interested readers are directed to excellent reviews on these topics (Dalton, 2009, Wray, et al., 2010).
Systems biology: a powerful approach for understanding the self-renewal and pluripotency of ESC
Genome-wide transcription factor binding analysis in ESC
Transcriptome analysis was one of the earliest systems biological approaches applied to understanding the genetic programs that control the fate of ESC. For example, several groups used microarray analysis to define the RNA expression profiles of ESC and their differentiated cells (Kelly and Rizzino, 2000, Ramalho-Santos, et al., 2002, Ivanova, et al., 2002). Athough transcriptome analysis provided a foundation for understanding the genetic programs in ESC, it left many questions unanswered. More recently, transcriptome analysis in combination with other analytical tools, such as genome-wide transcription factor binding analysis, and genome-wide epigenetic profiling (e.g. histone modifications and DNA methylation), have proven to be extremely useful in delineating the transcription regulatory networks in ESC (Boyer, et al., 2005, Chen, et al., 2008b, Kim, et al., 2008, Fouse, et al., 2008, Hawkins, et al., 2010, Marson, et al., 2008). The pioneering work of Boyer et al. defined, on a global scale, a key transcription regulatory network in ESC (Boyer, et al., 2005). This study used ChIP-chip (chromatin immunoprecipitation followed by hybridization to a DNA microarray) to identify the genome-wide target genes of the transcription factors Sox2, Oct4, and Nanog. Remarkably, this study demonstrated that the regulatory regions of over 300 genes are co-occupied by all three master regulators. More recently, the target genes of nearly 30 transcription factors, including both transcriptional activators and repressors, have been identified in ESC by ChIP-chip and ChIP-seq (chromatin immunoprecipitation followed by sequencing of the enriched DNA fragments) (Chen, et al., 2008b, Kim, et al., 2008). Together, these studies demonstrated that there is considerable overlap in the target genes of Sox2, Oct4, Nanog, and other essential transcription factors. Moreover, these studies demonstrated that miRNAs are an integral part of the core transcription networks anchored by Sox2, Oct4, and Nanog in ESC (Marson, et al., 2008). Interestingly, the finding that there is a large overlap in the target genes of Sox2, Oct4 and Nanog raises the question: Do Sox2, Oct4, and Nanog function together as part of large protein complexes? As discussed later in this review, identification of the proteins that associate with Oct4, Nanog, and Sox2 argues that, at the very least, these core pluripotency factors interact with many of the same proteins in ESC (Wang, et al., 2006, Liang, et al., 2008, Pardo, et al., 2010, van den Berg, et al., 2010, Mallanna, et al., 2010).
Epigenetic regulation of gene expression in ESC
Epigenetic processes are major players in the regulation of gene expression (Minard, et al., 2009). Recent genome-wide profiling of epigenetic modifications (histone methylation, histone acetylation, DNA methylation, etc.), and identification of genome-wide targets of chromatin-modifying proteins (e.g. PcG proteins, Swi/Snf proteins) have provided valuable insights into mechanisms involved in regulating gene expression in ESC (Fouse, et al., 2008, Hawkins, et al., 2010, Boyer, et al., 2006, Bernstein, et al., 2006, Lee, et al., 2006, Ho, et al., 2009). For example, target genes of Sox2, Oct4, and Nanog that are not expressed in ESC are co-occupied by PcG proteins and harbor both activating and repressive histone modifications. These genes, referred to as bivalent genes, are believed to be poised for rapid activation when ESC are induced to differentiate into specific cell types (Boyer, et al., 2006, Bernstein, et al., 2006, Lee, et al., 2006). Recent work on the bivalent gene Sox21, which must remain silent in ESC (Mallanna, et al., 2010), has provided mechanistic details surrounding the regulation of bivalent genes (Chakravarthy, et al., 2010). These studies have shown that activation of the Sox21 gene, when ESC begin to differentiate, involves the displacement of multiple repressors complexes that act redundantly to ensure that the expression of this gene is blocked in ESC.
Roles of non coding RNAs in ESC
In addition to the seminal studies described above, which provided a better understanding of the roles played by transcription factors and chromatin modifying machinery, significant progress has been made recently in determining the roles played by non-coding RNAs in ESC, in particular miRNAs and long non-coding RNAs (lncRNAs). The earliest efforts in this regard involved identification of miRNAs expressed in ESC (Houbaviy, et al., 2003). Since then, several independent studies have identified miRNAs that are specifically expressed in ESC [reviewed in Mallanna and Rizzino (2010)]. Additionally, using ESC with defective miRNA maturation (specifically DGCR8 null ESC), the function of individual ESC-specific miRNAs have been evaluated for their ability to regulate ESC self-renewal and pluripotency (Wang, et al., 2008a). Importantly, miRNAs have also been demonstrated to regulate reprogramming of somatic cells into iPS cells, and more than a dozen miRNAs are predicted to influence somatic cell reprogramming (Mallanna and Rizzino, 2010). Studies that focus on the roles played by lncRNAs in regulating ESC have barely begun, yet it is already evident that lncRNAs play important roles in maintaining the ESC identity. For example, a positive feedback regulatory loop has recently been identified in ESC between Oct4 and ESC-specific lncRNA (Sheik Mohamed, et al., 2010).
Global knockdown and overexpression studies
In addition to the systems biological approaches described above, genome-wide loss of function and gain of function studies have been employed to identify novel regulators that control the fate of ESC. For loss of function studies, shRNA/siRNA-mediated genome-wide knockdown analyses were utilized by several groups. These studies identified a significant number of proteins required by ESC, including Esrrb, Tbx3, Tcl1, Tip60-p400, Cnot3, Trim28, and Paf1C (Ivanova, et al., 2006, Fazzio, et al., 2008, Hu, et al., 2009, Ding, et al., 2009). Forward genetic screens have also been utilized successfully to identify proteins involved in the regulation of ESC. Nanog was identified as a pluripotency factor using a forward genetic/gain of function screen (Chambers, et al., 2003). More recently, gain of function analysis using cDNA clones has identified both promoters and inhibitors of ESC pluripotency (Abujarour, et al., 2010).
Elucidation of protein-protein interaction networks in ESC
Differential gene expression is central to both generating and maintaining the self-renewal capacity and pluripotency of ESC. The inventory of transcription factors responsible for regulating gene expression in ESC, as well as the inventory of their target genes, is expanding rapidly; however, our understanding of the molecular mechanisms used by these transcription factors remains superficial. Given that transcription factors do not function in isolation, but work as part of large protein complexes, determining the composition of these complexes is fundamental to our efforts to understand the mechanisms used by transcription factors to regulate gene expression.
Protein-protein interaction networks of core pluripotency factors Oct4, Nanog, and Sox2
Over the last five years significant progress has been made towards identification of protein-protein interaction networks in ESC. Wang et al. (2006) identified Nanog-associated proteins using ESC that express dual epitope-tagged Nanog, which was expressed at 20% of endogenous Nanog levels. Nanog protein complexes were immunoprecipitated by single-step as well as tandem affinity purification, and the Nanog-associated proteins were identified by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Additionally, this study assessed the importance of Nanog-associated proteins in regulating the ESC phenotype, and demonstrated that shRNA-mediated knockdown of Dax1, Sall4, Nac1, or Zfp281 results in loss of pluripotency and derepression of lineage-specific markers. Importantly, they also performed proteomic analyses of select Nanog-associated proteins, Oct4, Dax1, Nac1 and Zfp281. From the combined proteomics data, a mini-interactome of partner proteins was generated that consisted of six transcription factors (Wang, et al., 2006). This mini-interactome was found to be enriched for proteins that are essential for ESC as well as early development. This study also noted that >50% of the genes coding for proteins in the interactome are bound by Oct4, and/or Nanog, which suggests that a large percentage of these proteins are regulated by Oct4 and/or Nanog. Subsequently, Liang et al. (2008) immunoprecipitated endogenous Nanog and Oct4 from ESC, and identified Nanog- and Oct4-associated proteins by LC-MS/MS. Interestingly, Liang et al. (2008) reported that Nanog and Oct4 interact with only select members of the NuRD and Sin3A repressor complex, and they referred to these Nanog and Oct4-associated deacetylase complexes as NODE complexes. Specifically, they argued that NODE complexes lack Mbd3 (NuRD component) and Rbbp7 (NuRD and Sin3A components), but contain the deacetylase activity. Both Wang et al. (2006) and Liang et al. (2008) demonstrated that the size distributions of Nanog- and Oct4-containing protein complexes overlap.
More recently, Van den Berg et al. (2010) used LC-MS/MS to identify Oct4-associated proteins in mouse ESC. Specifically, they used ZHBTc4 ESC, which allows for expression of Flag epitope-tagged Oct4 at physiological levels. Additionally, they identified proteins that associate with endogenous Oct4 by immunoprecipitating untagged endogenous Oct4. Together, over 50 Oct4-associated proteins were identified, of which a significant number of proteins have already been shown to control the self-renewal and pluripotency of ESC. They also noted that over 25% of the genes encoding Oct4-associated proteins are target genes of Oct4. This finding argues that Oct4 and its associated proteins are interconnected at two critical levels - at the protein-protein interaction level and at the level of their transcriptional expression.
Importantly, the study by Van den Berg et al. (2010) differs in several respects from the study by Liang and coworkers (2008). In addition to the differences between the lists of Oct4-associated proteins identified in the two studies, Van den Berg et al. (2010) demonstrated that Oct4 binds all major components of the classical NuRD repressor complex (van den Berg, et al., 2010). It is possible that immunoprecipitation of endogenous Oct4 and Nanog by Liang et al. (2008) was less efficient in enriching partner proteins present at sub-stoichiometric levels. Alternatively, wash conditions and/or antibodies used by Liang et al. (2008) failed to immunoprecipitate weakly interacting partner proteins. Further study will be needed to resolve this issue.
Pardo et al. (2010) also performed proteomic analysis of Oct4 complexes. For this purpose they used ESC that express Flag-Oct4 from the Hprt locus at ~30% of endogenous Oct4 protein levels. Oct4 protein complexes were analyzed by LC-MS/MS, and 92 Oct4-associated proteins were identified by one-step purification. They also identified Oct4-associated proteins by immunoprecipitating untagged endogenous Oct4, and determined that 46 proteins overlap with the one-step Flag-Oct4 proteomics data set. Examination of genome-wide transcription factor binding data indicates that ~50% of the genes encoding Oct4-associated proteins are targets of at least one key ESC transcription factor, and 20 out of 92 genes encoding Oct4-associated proteins are targets of at least three key ESC transcription factors. Furthermore, about one third of the Oct4-associated proteins identified in this study are expressed at significantly higher levels in ESC than in their differentiated counterparts. Thus, as ESC begin to differentiate, the Oct4-interactome is expected to change significantly, because Oct4 generally turns off more slowly than some of its partner proteins, such as Sox2 and Nanog (Boer, et al., 2006).
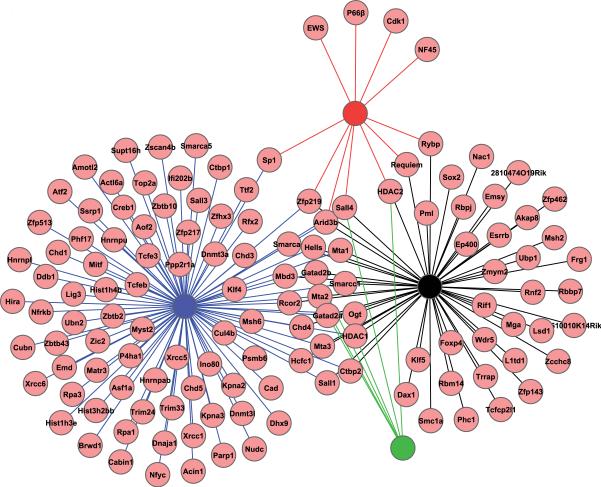
Among the protein interactome data available for different pluripotency factors in ESC, the Oct4 interactome dataset provides the most comprehensive coverage because four independent studies were performed by different investigators (Wang, et al., 2006, Liang, et al., 2008, Pardo, et al., 2010, van den Berg, et al., 2010). Together, these studies have identified 131 Oct4-interacting proteins. Integration of the four published Oct4 interactomes identified 23 proteins as common Oct4-interacting proteins in two or more independent studies (Figure 2). However, the four Oct4 proteomic studies each identified Oct4-associated proteins that were not detected by the other studies (Wang, et al., 2006, Liang, et al., 2008, Pardo, et al., 2010, van den Berg, et al., 2010). In fact, the largest Oct4 proteomic studies identified a total of 92 proteins (Pardo, et al., 2010), only 20 of which were identified in other studies (Wang, et al., 2006, Liang, et al., 2008, van den Berg, et al., 2010). This demonstrates an important variability in proteomic studies, which is probably due to the different immunoprecipitation strategies, different wash conditions, and the different mass spectrometry platforms employed. Thus, one can expect that the depth of these interactomes will increase by employing different strategies for the identification of proteins that associate with pluripotency factors in ESC. Finally, apart from the protein interactomes of transcription factors discussed above, interacting partners of several other proteins have been identified in ESC, including Ronin and interacting partners of proteins that make up Paf1 and BAF complexes in ESC (Ho, et al., 2009, Ding, et al., 2009, Dejosez, et al., 2008).
Figure 2. Integration of Oct4 interactomes identified in ESC.
Oct4-interacting proteins identified in four independent studies are integrated to determine the degree of overlap in the four Oct4 interactomes. Oct4-interacting proteins identified by Pardo et al. (2010), Van den Berg et al. (2010), Wang et al. (2006) and Liang et al. (2008) are indicated in blue, black, red, and green lines, respectively.
Protein-protein interaction landscape of ESC
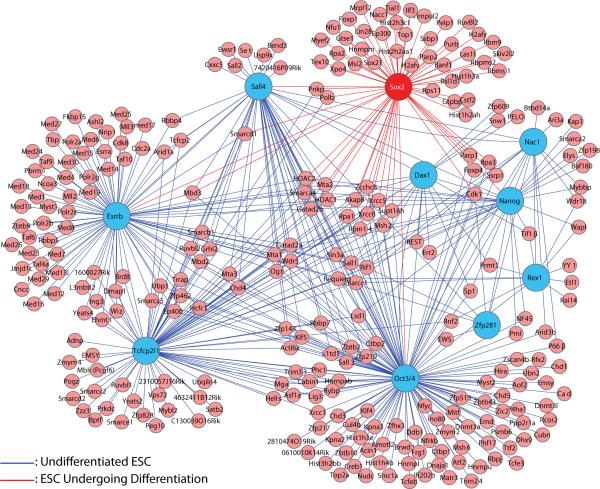
To assess the overall interrelationships that exist between the different transcription factor networks that control the fate of ESC, we integrated the published proteomic data for nine transcription factors (Wang, et al., 2006, Liang, et al., 2008, Pardo, et al., 2010, van den Berg, et al., 2010). Using Cytoscape (http://www.cytoscape.org/), we generated a virtual protein-protein interaction landscape for ESC that depicts the extent to which individual proteins interact with other proteins within the network. This analysis suggests that the nine transcription factors and their associated proteins form a highly interconnected protein-protein interaction landscape (Figure 3).
Figure 3. Protein-protein interaction landscape of essential transcription factors in ESC.
Protein interactomes of ten different transcription factors are integrated to generate a virtual protein-protein interaction landscape in ESC. Interacting partners of Oct4, Nanog, Sall4, Esrrb, Zfp281, Tcfcp2l1, Rex1, Dax1, and Nac1 identified in undifferentiated ESC (blue filled circles) (Wang, et al., 2006, Liang, et al., 2008, Pardo, et al., 2010, van den Berg, et al., 2010, Mallanna, et al., 2010) were integrated with the partner proteins of Sox2 identified during the early stages of ESC differentiation (red filled circle) (Mallanna, et al., 2010).
Identification of proteins that interact with multiple transcription factors in ESC has at least two important implications. First, proteins that interact with multiple partners are themselves likely to have important regulatory functions in ESC. In this regard, several proteins that interact with multiple transcription factors in ESC, such as Brg-1, Zfp143, Zfp281, TIF1β, have been shown to influence the behavior of ESC (Ho, et al., 2009, Chen, et al., 2008a, Wang, et al., 2008b, Seki, et al., 2010) (Figure 3). Accordingly, we predict that other proteins that are part of this protein-protein interaction landscape and which interact with three or more pluripotency factors will also be found to control the fate of ESC. Second, and equally important, the high degree of integration of this protein interaction landscape helps explain why a change as small as 2-fold in the expression levels of master regulators, such Oct4 and Sox2 (Niwa, et al., 2000, Kopp, et al., 2008), disrupts the self-renewal of ESC and triggers differentiation. More specifically, a small change in the level of one of these transcription factors is expected to be strongly amplified due to its interactions with multiple proteins within the protein-protein interaction landscape. This raises an important question: How do ESC control the expression of these master regulators within narrow limits? The available data argues that ESC use multiple mechanisms to carefully control the expression of master regulators, including regulation at the transcription level, at the post-transcription level (e.g. by miRNAs) and by post-translational modifications, which control the function, subcellular localization and/or stability of these proteins (Mallanna and Rizzino, 2010, Tsuruzoe, et al., 2006, Baltus, et al., 2009, Jeong, et al., 2010).
Protein-protein interaction networks in ESC undergoing differentiation
In addition to establishing the protein-protein interaction networks in ESC, it is important to identify proteins that associate with pluripotency factors during ESC differentiation. Thus far, this has only been done for Sox2. A recent proteomic screen has identified proteins that associate with Sox2 during the early stages of mouse ESC differentiation (Mallanna, et al., 2010). In this study, ESC were induced to differentiate for 24 hours by elevating the levels of Sox2 ~2-fold. In this system, Flag epitope-tagged Sox2 can be expressed from an inducible promoter. Using Multidimensional Protein Identification Technology (MudPIT), epitope-tagged Sox2 was found to associate with >60 nuclear proteins, including known pluripotency regulators, such as Sall4, Lin28, Brg-1 and Nanog (Yu, et al., 2007, Ho, et al., 2009, Chambers, et al., 2003, Zhang, et al., 2006, Yang, et al., 2008). As one might expect, Sox2-protein complexes in ESC undergoing differentiation vary in size from small to high molecular weight complexes (>880 kDa) (Cox, et al., 2010), which overlap with the size distributions of Nanog-, and Oct4-containing protein complexes (Wang, et al., 2006, Liang, et al., 2008). Interestingly, 25 Sox2-associated proteins identified in ESC undergoing differentiation are part of the protein-protein interaction landscape described above for undifferentiated ESC (Figure 3). Thus, it will be important to determine which of these proteins associate with Sox2 in ESC and to determine the extent to which the Sox2-interactome changes when ESC begin to differentiate. Studies are underway in this laboratory to describe the Sox2-interactome in undifferentiated ESC. Finally, ~75% of the genes that code for Sox2-associated proteins are bound by one or more core pluripotency factors, including Sox2 (Mallanna, et al., 2010). Thus, like Oct4, Sox2 and its associated proteins are interrelated at two important levels - at the protein-protein interaction level and at the level of their transcriptional expression.
Phosphoproteomic analysis in ESC and their differentiated cells
Phosphoproteome dynamics during the differentiation of ESC
Post-translational modifications of proteins, in particular protein phosphorylation, play critical roles in regulating protein function. Indeed, several recent studies have directly implicated differential protein phosphorylation in the control of ESC. For example, TIF1β interacts with Oct4 in a phosphorylation-dependent manner to control the fate of ESC (Seki, et al., 2010). Additionally, the interaction of Pin1 with Nanog, which helps to stabilize Nanog, is also phosphorylation-dependent (Moretto-Zita, et al., 2010). Importantly, significant progress has been also been made in defining the global phosphorylation status of proteins in ESC (Swaney, et al., 2009, Van Hoof, et al., 2009, Brill, et al., 2009).
Swaney et al. (2009) identified 10,844 nonredundant phosphorylation sites in human ESC, including phosphorylation sites in the pluripotency factors Oct4 and Sox2. In addition, van Hoof et al. (2009) used SILAC-based quantitative MS to perform phosphoproteome analysis of human ESC and human ESC induced to differentiate by BMP. This study identified 5,222 proteins in human ESC, out of which 1,399 (27%) were phosphorylated on one or more residues. Included in their phosphoproteome inventory are well established regulators of ESC, such as Sox2, Lin28, and UTF1. Remarkably, they determined that the phosphorylation status of ~50% of the phosphopeptides changed within one hour after induction of differentiation, and correlated this with a significant increase in kinase activity. Additionally, kinase-substrate analysis in ESC identified CDK1/2 as a major kinase predicted to phosphorylate ~1200 of the peptides identified in this study.
In another phosphoproteomic analysis of human ESC and their differentiated cells, Brill et al. (2009) identified 1,602 phosphoproteins. In this study, ESC were induced to differentiate by retinoic acid instead of BMP. Like the findings of van Hoof et al. (2010), phosphorylation events changed dramatically when human ESC were induced to differentiate. Among the 1,602 proteins identified, 389 proteins exhibited greater phosphorylation in undifferentiated human ESC and 540 proteins exhibited greater phosphorylation in the differentiated cells. Importantly, the studies of Brill et al. (2009) and van Hoof et al. (2010) demonstrated that phosphoproteome analysis can help identify signaling pathways that promote the differentiation of ESC to different cell lineages.
More recently, Li et al. (2010) examined the phosphoproteome of mouse ESC and identified 1,642 phosphoproteins. Importantly, this study demonstrated that a significant number of proteins (22 out of 52 proteins) that regulate the fate of ESC are phosphorylated. Furthermore, by comparing the phosphoproteome data of mouse and human ESC, they demonstrated that the phosphorylation status of over 1,000 proteins is conserved between mouse and human ESC. More studies are needed to understand the effects of phosphorylation on the function of these proteins in ESC.
Conclusions
The study of ESC has witnessed enormous progress in the past five years owing to the application of systems biology. Systems biology provides a bird's eye view of the complex biological processes that regulate ESC identity, and helps discern trends and patterns that distinguish ESC from their differentiated counterparts. Additionally, systems biology provides an excellent starting point to study the roles of individual molecules in the regulation of the self-renewal and pluripotency of ESC. Currently, a wide array of high-throughput data is available for ESC, including, but not limited to, whole cell transcriptome and miRNA analyses, genome-wide transcription factor binding data, genome-wide epigenetic modification profiles, protein-protein interaction networksand, phosphoproteome dynamics. Moving forward, integration of different high-throughput data generated in ESC is needed to determine how distinct biological processes converge to regulate the self-renewal capacity and pluripotency of ESC.
Currently, there is no shortage of important questions to address. In the area of proteomics alone, there are numerous questions. For example how similar are the protein-protein interaction landscapes of mouse ESC and human ESC? How similar are the protein-protein interaction landscapes of ESC and iPS cells? What are the interactomes of pluripotency transcription factors, in particular Sox2 and Oct4, during different stages of somatic cell reprogramming? How quickly and to what extent does the protein-protein interaction landscape of ESC change when ESC differentiate? Last, but not least, what is the post-translational modification status of proteins in the protein-protein interaction landscapes of ESC and their differentiated cells? Answering these questions will provide further insights into the interrelationships that exist between key regulatory factors responsible for the metastable state in ESC, which determines whether these cells self-renew or undergo differentiation. In turn, answering these questions will be extremely useful to the field of regenerative medicine.
Acknowledgments
Heather Rizzino is thanked for editorial assistance.
Contract grant sponsor: NIH
Contract grant number: GM 080751
Contract grant sponsor: Nebraska Department of Health
Contract grant number: Stem Cell-2009-01
Literature Cited
- Abujarour R, Efe J, Ding S. Genome-wide gain-of-function screen identifies novel regulators of pluripotency. Stem Cells. 2010;28:1487–1497. doi: 10.1002/stem.472. [DOI] [PubMed] [Google Scholar]
- Baltus GA, Kowalski MP, Zhai H, Tutter AV, Quinn D, Wall D, Kadam S. Acetylation of sox2 induces its nuclear export in embryonic stem cells. Stem Cells. 2009;27:2175–2184. doi: 10.1002/stem.168. [DOI] [PubMed] [Google Scholar]
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, Jaenisch R, Wagschal A, Feil R, Schreiber SL, Lander ES. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- Boer B, Bernadt CT, Desler M, Wilder PJ, Kopp JL, Rizzino A. Differential activity of the FGF-4 enhancer in F9 and P19 embryonal carcinoma cells. J Cell Physiol. 2006;208:97–108. doi: 10.1002/jcp.20635. [DOI] [PubMed] [Google Scholar]
- Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, Levine SS, Wernig M, Tajonar A, Ray MK, Bell GW, Otte AP, Vidal M, Gifford DK, Young RA, Jaenisch R. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349–353. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill LM, Xiong W, Lee KB, Ficarro SB, Crain A, Xu Y, Terskikh A, Snyder EY, Ding S. Phosphoproteomic analysis of human embryonic stem cells. Cell Stem Cell. 2009;5:204–213. doi: 10.1016/j.stem.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy H, Ormsbee BD, Mallanna SK, Rizzino A. Rapid activation of the bivalent gene Sox21 requires displacement of multiple layers of gene-silencing machinery. FASEB J. 2011;25:206–218. doi: 10.1096/fj.10-166926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy H, Boer B, Desler M, Mallanna SK, McKeithan TW, Rizzino A. Identification of DPPA4 and other genes as putative Sox2 : Oct-3/4 target genes using a combination of in silico analysis and transcription-based assays. J Cell Physiol. 2008;216:651–662. doi: 10.1002/jcp.21440. [DOI] [PubMed] [Google Scholar]
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- Chen L, Yabuuchi A, Eminli S, Takeuchi A, Lu CW, Hochedlinger K, Daley GQ. Cross-regulation of the Nanog and Cdx2 promoters. Cell Res. 2009;19:1052–1061. doi: 10.1038/cr.2009.79. [DOI] [PubMed] [Google Scholar]
- Chen X, Fang F, Liou YC, Ng HH. Zfp143 regulates Nanog through modulation of Oct4 binding. Stem Cells. 2008a;26:2759–2767. doi: 10.1634/stemcells.2008-0398. [DOI] [PubMed] [Google Scholar]
- Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, Wong E, Orlov YL, Zhang W, Jiang J, Loh YH, Yeo HC, Yeo ZX, Narang V, Govindarajan KR, Leong B, Shahab A, Ruan Y, Bourque G, Sung WK, Clarke ND, Wei CL, Ng HH. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008b;133:1106–1117. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- Chew JL, Loh YH, Zhang W, Chen X, Tam WL, Yeap LS, Li P, Ang YS, Lim B, Robson P, Ng HH. Reciprocal transcriptional regulation of Pou5f1 and Sox2 via the Oct4/Sox2 complex in embryonic stem cells. Mol Cell Biol. 2005;25:6031–6046. doi: 10.1128/MCB.25.14.6031-6046.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MF, Johnstone SE, Newman JJ, Kagey MH, Young RA. Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes Dev. 2008;22:746–755. doi: 10.1101/gad.1642408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JL, Mallanna SK, Luo X, Rizzino A. Sox2 uses multiple domains to associate with proteins present in Sox2-protein complexes. PLoS One. 2010;5:e15486. doi: 10.1371/journal.pone.0015486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JL, Rizzino A. Induced pluripotent stem cells: what lies beyond the paradigm shift. Exp Biol Med (Maywood) 2010;235:148–158. doi: 10.1258/ebm.2009.009267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton S. Exposing hidden dimensions of embryonic stem cell cycle control. Cell Stem Cell. 2009;4:9–10. doi: 10.1016/j.stem.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejosez M, Krumenacker JS, Zitur LJ, Passeri M, Chu LF, Songyang Z, Thomson JA, Zwaka TP. Ronin is essential for embryogenesis and the pluripotency of mouse embryonic stem cells. Cell. 2008;133:1162–1174. doi: 10.1016/j.cell.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Paszkowski-Rogacz M, Nitzsche A, Slabicki MM, Heninger AK, de Vries I, Kittler R, Junqueira M, Shevchenko A, Schulz H, Hubner N, Doss MX, Sachinidis A, Hescheler J, Iacone R, Anastassiadis K, Stewart AF, Pisabarro MT, Caldarelli A, Poser I, Theis M, Buchholz F. A genome-scale RNAi screen for Oct4 modulators defines a role of the Paf1 complex for embryonic stem cell identity. Cell Stem Cell. 2009;4:403–415. doi: 10.1016/j.stem.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Fazzio TG, Huff JT, Panning B. An RNAi screen of chromatin proteins identifies Tip60-p400 as a regulator of embryonic stem cell identity. Cell. 2008;134:162–174. doi: 10.1016/j.cell.2008.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouse SD, Shen Y, Pellegrini M, Cole S, Meissner A, Van Neste L, Jaenisch R, Fan G. Promoter CpG methylation contributes to ES cell gene regulation in parallel with Oct4/Nanog, PcG complex, and histone H3 K4/K27 trimethylation. Cell Stem Cell. 2008;2:160–169. doi: 10.1016/j.stem.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita J, Crane AM, Souza MK, Dejosez M, Kyba M, Flavell RA, Thomson JA, Zwaka TP. Caspase activity mediates the differentiation of embryonic stem cells. Cell Stem Cell. 2008;2:595–601. doi: 10.1016/j.stem.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu P, LeMenuet D, Chung AC, Mancini M, Wheeler DA, Cooney AJ. Orphan nuclear receptor GCNF is required for the repression of pluripotency genes during retinoic acid-induced embryonic stem cell differentiation. Mol Cell Biol. 2005;25:8507–8519. doi: 10.1128/MCB.25.19.8507-8519.2005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Hall J, Guo G, Wray J, Eyres I, Nichols J, Grotewold L, Morfopoulou S, Humphreys P, Mansfield W, Walker R, Tomlinson S, Smith A. Oct4 and LIF/Stat3 additively induce Kruppel factors to sustain embryonic stem cell self-renewal. Cell Stem Cell. 2009;5:597–609. doi: 10.1016/j.stem.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Hawkins RD, Hon GC, Lee LK, Ngo Q, Lister R, Pelizzola M, Edsall LE, Kuan S, Luu Y, Klugman S, Antosiewicz-Bourget J, Ye Z, Espinoza C, Agarwahl S, Shen L, Ruotti V, Wang W, Stewart R, Thomson JA, Ecker JR, Ren B. Distinct epigenomic landscapes of pluripotent and lineage-committed human cells. Cell Stem Cell. 2010;6:479–491. doi: 10.1016/j.stem.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L, Ronan JL, Wu J, Staahl BT, Chen L, Kuo A, Lessard J, Nesvizhskii AI, Ranish J, Crabtree GR. An embryonic stem cell chromatin remodeling complex, esBAF, is essential for embryonic stem cell self-renewal and pluripotency. Proc Natl Acad Sci U S A. 2009;106:5181–5186. doi: 10.1073/pnas.0812889106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houbaviy HB, Murray MF, Sharp PA. Embryonic stem cell-specific MicroRNAs. Dev Cell. 2003;5:351–358. doi: 10.1016/s1534-5807(03)00227-2. [DOI] [PubMed] [Google Scholar]
- Hu G, Kim J, Xu Q, Leng Y, Orkin SH, Elledge SJ. A genome-wide RNAi screen identifies a new transcriptional module required for self-renewal. Genes Dev. 2009;23:837–848. doi: 10.1101/gad.1769609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova N, Dobrin R, Lu R, Kotenko I, Levorse J, DeCoste C, Schafer X, Lun Y, Lemischka IR. Dissecting self-renewal in stem cells with RNA interference. Nature. 2006;442:533–538. doi: 10.1038/nature04915. [DOI] [PubMed] [Google Scholar]
- Ivanova NB, Dimos JT, Schaniel C, Hackney JA, Moore KA, Lemischka IR. A stem cell molecular signature. Science. 2002;298:601–604. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- Jeong CH, Cho YY, Kim MO, Kim SH, Cho EJ, Lee SY, Jeon YJ, Lee KY, Yao K, Keum YS, Bode AM, Dong Z. Phosphorylation of Sox2 cooperates in reprogramming to pluripotent stem cells. Stem Cells. 2010;28:2141–2150. doi: 10.1002/stem.540. [DOI] [PubMed] [Google Scholar]
- Jiang J, Chan YS, Loh YH, Cai J, Tong GQ, Lim CA, Robson P, Zhong S, Ng HH. A core Klf circuitry regulates self-renewal of embryonic stem cells. Nat Cell Biol. 2008;10:353–360. doi: 10.1038/ncb1698. [DOI] [PubMed] [Google Scholar]
- Kelly DL, Rizzino A. DNA microarray analyses of genes regulated during the differentiation of embryonic stem cells. Mol Reprod Dev. 2000;56:113–123. doi: 10.1002/(SICI)1098-2795(200006)56:2<113::AID-MRD1>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Kim J, Chu J, Shen X, Wang J, Orkin SH. An extended transcriptional network for pluripotency of embryonic stem cells. Cell. 2008;132:1049–1061. doi: 10.1016/j.cell.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp JL, Ormsbee BD, Desler M, Rizzino A. Small increases in the level of Sox2 trigger the differentiation of mouse embryonic stem cells. Stem Cells. 2008;26:903–911. doi: 10.1634/stemcells.2007-0951. [DOI] [PubMed] [Google Scholar]
- Lee TI, Jenner RG, Boyer LA, Guenther MG, Levine SS, Kumar RM, Chevalier B, Johnstone SE, Cole MF, Isono K, Koseki H, Fuchikami T, Abe K, Murray HL, Zucker JP, Yuan B, Bell GW, Herbolsheimer E, Hannett NM, Sun K, Odom DT, Otte AP, Volkert TL, Bartel DP, Melton DA, Gifford DK, Jaenisch R, Young RA. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell. 2006;125:301–313. doi: 10.1016/j.cell.2006.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QR, Xing XB, Chen TT, Li RX, Dai J, Sheng QH, Xin SM, Zhu LL, Jin Y, Pei G, Kang JH, Li YX, Zeng R. Large-scale phosphoproteome profiles comprehensive features of mouse embryonic stem cells. Mol Cell Proteomics. 2010 doi: 10.1074/mcp.M110.001750. ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Wan M, Zhang Y, Gu P, Xin H, Jung SY, Qin J, Wong J, Cooney AJ, Liu D, Songyang Z. Nanog and Oct4 associate with unique transcriptional repression complexes in embryonic stem cells. Nat Cell Biol. 2008;10:731–739. doi: 10.1038/ncb1736. [DOI] [PubMed] [Google Scholar]
- Lin T, Chao C, Saito S, Mazur SJ, Murphy ME, Appella E, Xu Y. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat Cell Biol. 2005;7:165–171. doi: 10.1038/ncb1211. [DOI] [PubMed] [Google Scholar]
- Loh YH, Zhang W, Chen X, George J, Ng HH. Jmjd1a and Jmjd2c histone H3 Lys 9 demethylases regulate self-renewal in embryonic stem cells. Genes Dev. 2007;21:2545–2557. doi: 10.1101/gad.1588207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallanna SK, Ormsbee BD, Iacovino M, Gilmore JM, Cox JL, Kyba M, Washburn MP, Rizzino A. Proteomic analysis of Sox2-associated proteins during early stages of mouse embryonic stem cell differentiation identifies Sox21 as a novel regulator of stem cell fate. Stem Cells. 2010;28:1715–1727. doi: 10.1002/stem.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallanna SK, Rizzino A. Emerging roles of microRNAs in the control of embryonic stem cells and the generation of induced pluripotent stem cells. Dev Biol. 2010;344:16–25. doi: 10.1016/j.ydbio.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marson A, Levine SS, Cole MF, Frampton GM, Brambrink T, Johnstone S, Guenther MG, Johnston WK, Wernig M, Newman J, Calabrese JM, Dennis LM, Volkert TL, Gupta S, Love J, Hannett N, Sharp PA, Bartel DP, Jaenisch R, Young RA. Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell. 2008;134:521–533. doi: 10.1016/j.cell.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui S, Nakatake Y, Toyooka Y, Shimosato D, Yagi R, Takahashi K, Okochi H, Okuda A, Matoba R, Sharov AA, Ko MS, Niwa H. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat Cell Biol. 2007;9:625–635. doi: 10.1038/ncb1589. [DOI] [PubMed] [Google Scholar]
- Minard ME, Jain AK, Barton MC. Analysis of epigenetic alterations to chromatin during development. Genesis. 2009;47:559–572. doi: 10.1002/dvg.20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretto-Zita M, Jin H, Shen Z, Zhao T, Briggs SP, Xu Y. Phosphorylation stabilizes Nanog by promoting its interaction with Pin1. Proc Natl Acad Sci U S A. 2010;107:13312–13317. doi: 10.1073/pnas.1005847107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H, Toyooka Y, Shimosato D, Strumpf D, Takahashi K, Yagi R, Rossant J. Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell. 2005;123:917–929. doi: 10.1016/j.cell.2005.08.040. [DOI] [PubMed] [Google Scholar]
- Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- Pan G, Li J, Zhou Y, Zheng H, Pei D. A negative feedback loop of transcription factors that controls stem cell pluripotency and self-renewal. FASEB J. 2006;20:1730–1732. doi: 10.1096/fj.05-5543fje. [DOI] [PubMed] [Google Scholar]
- Pardo M, Lang B, Yu L, Prosser H, Bradley A, Babu MM, Choudhary J. An expanded Oct4 interaction network: implications for stem cell biology, development, and disease. Cell Stem Cell. 2010;6:382–395. doi: 10.1016/j.stem.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA. “Stemness”: transcriptional profiling of embryonic and adult stem cells. Science. 2002;298:597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- Rizzino A. Sox2 and Oct-3/4: a versatile pair of master regulators that orchestrate the self-renewal and pluripotency of embryonic stem cells. Wiley Interdiscip Rev Syst Biol Med. 2009;1:228–236. doi: 10.1002/wsbm.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodda DJ, Chew JL, Lim LH, Loh YH, Wang B, Ng HH, Robson P. Transcriptional regulation of Nanog by Oct4 and Sox2. J Biol Chem. 2005;280:24731–24737. doi: 10.1074/jbc.M502573200. [DOI] [PubMed] [Google Scholar]
- Seki Y, Kurisaki A, Watanabe-Susaki K, Nakajima Y, Nakanishi M, Arai Y, Shiota K, Sugino H, Asashima M. TIF1beta regulates the pluripotency of embryonic stem cells in a phosphorylation-dependent manner. Proc Natl Acad Sci U S A. 2010;107:10926–10931. doi: 10.1073/pnas.0907601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheik Mohamed J, Gaughwin PM, Lim B, Robson P, Lipovich L. Conserved long noncoding RNAs transcriptionally regulated by Oct4 and Nanog modulate pluripotency in mouse embryonic stem cells. RNA. 2010;16:324–337. doi: 10.1261/rna.1441510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Nakatake Y, Akagi T, Ura H, Matsuda T, Nishiyama A, Koide H, Ko MS, Niwa H, Yokota T. Dax1 binds to Oct3/4 and inhibits its transcriptional activity in embryonic stem cells. Mol Cell Biol. 2009;29:4574–4583. doi: 10.1128/MCB.01863-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Nakatake Y, Ura H, Akagi T, Niwa H, Koide H, Ko MS, Niwa H, Yokota T. Stem cell-specific expression of Dax1 is conferred by STAT3 and Oct3/4 in embryonic stem cells. Biochem Biophys Res Commun. 2008;372:91–96. doi: 10.1016/j.bbrc.2008.04.154. [DOI] [PubMed] [Google Scholar]
- Swaney DL, Wenger CD, Thomson JA, Coon JJ. Human embryonic stem cell phosphoproteome revealed by electron transfer dissociation tandem mass spectrometry. Proc Natl Acad Sci U S A. 2009;106:995–1000. doi: 10.1073/pnas.0811964106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takao Y, Yokota T, Koide H. Beta-catenin up-regulates Nanog expression through interaction with Oct-3/4 in embryonic stem cells. Biochem Biophys Res Commun. 2007;353:699–705. doi: 10.1016/j.bbrc.2006.12.072. [DOI] [PubMed] [Google Scholar]
- Tam WL, Lim CY, Han J, Zhang J, Ang YS, Ng HH, Yang H, Lim B. T-cell factor 3 regulates embryonic stem cell pluripotency and self-renewal by the transcriptional control of multiple lineage pathways. Stem Cells. 2008;26:2019–2031. doi: 10.1634/stemcells.2007-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruzoe S, Ishihara K, Uchimura Y, Watanabe S, Sekita Y, Aoto T, Saitoh H, Yuasa Y, Niwa H, Kawasuji M, Baba H, Nakao M. Inhibition of DNA binding of Sox2 by the SUMO conjugation. Biochem Biophys Res Commun. 2006;351:920–926. doi: 10.1016/j.bbrc.2006.10.130. [DOI] [PubMed] [Google Scholar]
- van den Berg DL, Snoek T, Mullin NP, Yates A, Bezstarosti K, Demmers J, Chambers I, Poot RA. An Oct4-centered protein interaction network in embryonic stem cells. Cell Stem Cell. 2010;6:369–381. doi: 10.1016/j.stem.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg DL, Zhang W, Yates A, Engelen E, Takacs K, Bezstarosti K, Demmers J, Chambers I, Poot RA. Estrogen-related receptor beta interacts with Oct4 to positively regulate Nanog gene expression. Mol Cell Biol. 2008;28:5986–5995. doi: 10.1128/MCB.00301-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoof D, Munoz J, Braam SR, Pinkse MW, Linding R, Heck AJ, Mummery CL, Krijgsveld J. Phosphorylation dynamics during early differentiation of human embryonic stem cells. Cell Stem Cell. 2009;5:214–226. doi: 10.1016/j.stem.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Wang J, Rao S, Chu J, Shen X, Levasseur DN, Theunissen TW, Orkin SH. A protein interaction network for pluripotency of embryonic stem cells. Nature. 2006;444:364–368. doi: 10.1038/nature05284. [DOI] [PubMed] [Google Scholar]
- Wang Y, Baskerville S, Shenoy A, Babiarz JE, Baehner L, Blelloch R. Embryonic stem cell-specific microRNAs regulate the G1-S transition and promote rapid proliferation. Nat Genet. 2008a;40:1478–1483. doi: 10.1038/ng.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZX, Teh CH, Chan CM, Chu C, Rossbach M, Kunarso G, Allapitchay TB, Wong KY, Stanton LW. The transcription factor Zfp281 controls embryonic stem cell pluripotency by direct activation and repression of target genes. Stem Cells. 2008b;26:2791–2799. doi: 10.1634/stemcells.2008-0443. [DOI] [PubMed] [Google Scholar]
- Wray J, Kalkan T, Smith AG. The ground state of pluripotency. Biochem Soc Trans. 2010;38:1027–1032. doi: 10.1042/BST0381027. [DOI] [PubMed] [Google Scholar]
- Wu Q, Chen X, Zhang J, Loh YH, Low TY, Zhang W, Zhang W, Sze SK, Lim B, Ng HH. Sall4 interacts with Nanog and co-occupies Nanog genomic sites in embryonic stem cells. J Biol Chem. 2006;281:24090–24094. doi: 10.1074/jbc.C600122200. [DOI] [PubMed] [Google Scholar]
- Yang J, Chai L, Fowles TC, Alipio Z, Xu D, Fink LM, Ward DC, Ma Y. Genomewide analysis reveals Sall4 to be a major regulator of pluripotency in murine-embryonic stem cells. Proc Natl Acad Sci U S A. 2008;105:19756–19761. doi: 10.1073/pnas.0809321105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HB, Kunarso G, Hong FH, Stanton LW. Zfp206, Oct4, and Sox2 are integrated components of a transcriptional regulatory network in embryonic stem cells. J Biol Chem. 2009;284:31327–31335. doi: 10.1074/jbc.M109.016162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Thomson JA. Pluripotent stem cell lines. Genes Dev. 2008;22:1987–1997. doi: 10.1101/gad.1689808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zhang J, Tam WL, Tong GQ, Wu Q, Chan HY, Soh BS, Lou Y, Yang J, Ma Y, Chai L, Ng HH, Lufkin T, Robson P, Lim B. Sall4 modulates embryonic stem cell pluripotency and early embryonic development by the transcriptional regulation of Pou5f1. Nat Cell Biol. 2006;8:1114–1123. doi: 10.1038/ncb1481. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang J, Wang T, Esteban MA, Pei D. Esrrb activates Oct4 transcription and sustains self-renewal and pluripotency in embryonic stem cells. J Biol Chem. 2008;283:35825–35833. doi: 10.1074/jbc.M803481200. [DOI] [PubMed] [Google Scholar]