Abstract
Matrix metalloproteinases (MMPs) are a family of proteinases known to play a role in cell migration. In the present study, we evaluated the role of MMP-2 on tropism of human cord blood derived stem cells (hUCBSCs) in a human medulloblastoma tumor model. Consequences of MMP-2 inhibition on stem cell tropism towards medulloblastoma were studied in terms of stem cell migration by using cell culture inserts, transwell chamber assay, western blotting for MMP-2 and migratory molecules, and immunohistochemistry. Conditioned medium from Daoy/D283 cells infected with adenoviral vector encoding MMP-2 siRNA (Ad-MMP-2 si) reduced stem cell migration as compared to conditioned medium from mock and scrambled vector (Ad-SV) infected cells. In addition, MMP-2 inhibition in the tumor cells decreased the expression of SDF1 in the tumor conditioned medium, which results in impaired SDF1/CXCR4 signaling leading to decreased stem cell tropism towards the tumor cells. We further show that MMP-2 inhibition in the tumor cells repressed stem cell tropism towards medulloblastoma tumors in vivo. In summary, we conclude that hUCBSCs can integrate into human medulloblastoma after local delivery and that MMP-2 expression by the tumor cells mediates this response through the SDF1/CXCR4 axis.
Keywords: MMP-2, Medulloblastoma, Human umbilical cord blood stem cells, migration, tropism, SDF1, CXCR4
Introduction
Many in vivo and in vitro studies have demonstrated that human stem cells (SCs) as attractive vehicles to deliver the desired gene product, making them a very promising agent in brain tumor therapy. Recently, neural stem/progenitor cells (NSCs), engineered to express or carry to therapeutic agents, have been recognized for their ability to migrate throughout the central nervous system.1,2 Cytolytic viruses and genes coding for anti-tumor cytokines, pro-drug converting enzymes and various neurotrophic factors have all been engineered into engraftable NSCs for delivery to tumors.3 However, their clinical application is limited by ethical and logistic problems such as their isolation and immunologic compatibility in allogenic transplantation. Human cord blood is an alternative source of adult stem cells. Several studies indicate that human umbilical cord blood derived stem cells (hUCBSCs) are similar to stem cells from bone marrow with respect to cell characteristics and multilineage differentiation potential.4,5 hUCBSCs have the capacity to differentiate into several mesodermal tissues (bone, cartilage, tendon, muscle and adipose), endodermal tissue (hepatocyte), and ectodermal tissue and were proven to be more advantageous in cell procurement, storage, and transplantation than bone marrow-derived SCs.6 Recently, hUCBSCs were shown to display tropism for human glioma, and that the treatment of trimeric form of tumor necrosis factor-related apoptosis-inducing ligand (stTRAIL)-secreting hUCBSCs have significant anti-tumor effects compared with adenoviral TRAIL gene therapy.7 However, tumor-specific migratory properties require further elucidation in relation to their potential use in therapeutic applications.
Different factors that guide the motility of stem cells have been identified including soluble factors, cell adhesion molecules, and extracellular matrix components. Migration through the ECM is facilitated by ECM-degrading enzymes such as the matrix metalloproteases (MMPs), which free bound chemokines and allow movement along the chemokine gradient within the local tissue.8 Although this process represents a likely paradigm for stem cell migration, the molecular mediators and chemotactic signals that guide stem cells to appropriate microenvironments are yet to be fully identified. MMPs are a family of enzymes that collectively degrade all the components of the ECM.9 MMPs participate in a host of important physiological processes, including CNS development, embryological remodeling, wound healing, and angiogenesis, and their role in cancer cell metastasis has been studied extensively.10,11 MMPs were shown to be responsible for the proteolytic processing of extracellular matrix structural proteins, which regulate endothelial cell migration.12,13 A neutralizing antibody that blocked MMP-2 impaired transendothelial migration in vitro.14
The aim of this study was to investigate the role of MMP-2 on migratory behaviour of hUCBSCs in an experimental intracranial medulloblastoma tumor model. Although pharmacological MMP inhibitors have been used widely to study the roles of MMPs in cellular functions, these inhibitors lack specificity, which confounds data interpretation. We therefore used small interfering RNA (siRNA) that specifically knock down endogenous MMP-2 expression. We further analyzed the signaling mechanism involved in tumor cell-induced tropism of stem cell towards medulloblastoma tumors in vitro and show that MMP-2 inhibition in the tumor cells suppressed SDF1/CXCR4 pathway-mediated stem cell migration towards the tumor cells.
Results
Medulloblastoma tumor cells enhance human umbilical cord blood stem cell (hUCBSC) migration
Mesenchymal stem cells isolated from umbilical cord blood were positive for CD133, CD44 and STRO-1, displayed multilineage differentiation ability and demonstrated tropism towards glioma.15 In the present study, we first determined the ability of these mesenchymal stem cells from human umbilical cord blood (hUCB) to migrate towards tumor cells and conditioned medium using tissue culture inserts and transwell assays, respectively. We plated 5 × 103 and 1 × 104 cells of Daoy and D283 (more number of D283 were plated as this cell line is semi adherent; about 50%) respectively and allowed CD133 positive cells (hUCBSCs) to migrate towards the tumor cells or conditioned medium. Daoy and D283 tumor cells significantly stimulated the directional migration of hUCBSCs (Fig. 1A). Tumor conditioned medium from Daoy and D283 cells enhanced the migration of stem cells compared to serum free medium or conditioned medium from fibroblast cells (Fig. 1 B; P < 0.05 vs serum free medium, P < 0.05 vs tumor cell conditioned medium).
Figure 1. Medulloblastoma tumor cells enhance human umbilical cord blood stem cell (hUCBSCs) migration.
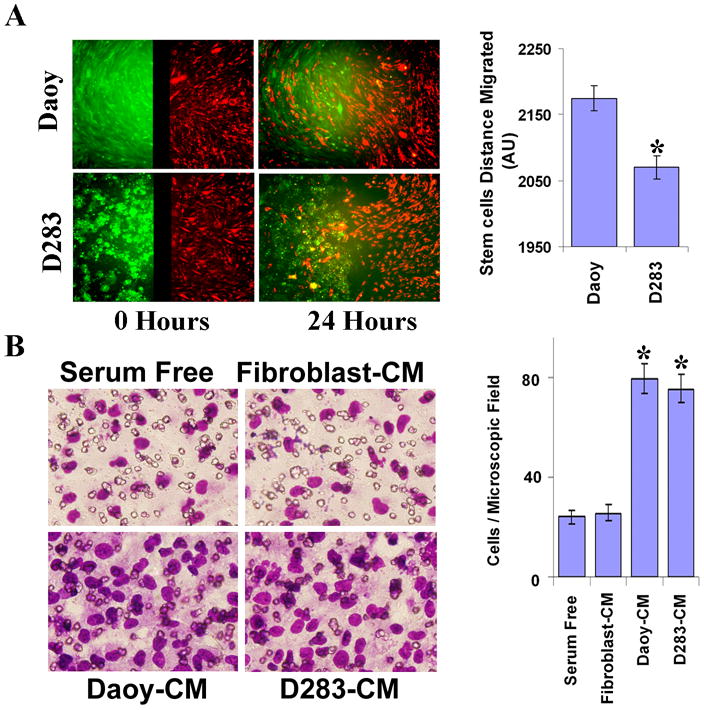
(A) Qtracker-525-Green (Molecular Probes, Carlsbard, CA) labeled medulloblastoma cells (Daoy and D283 cells) were seeded into one well and Qtracker-655-Red (Molecular Probes, Carlsbard, CA) labeled hUCBSCs seeded into another well of cell culture insert placed in 8-well chamber slides. After 16 h of culture, the culture-inserts were removed and incubated for further 24 h. Images were captured at 0 h and 24 h of incubation using a fluorescence microscope. Cell migration was quantified and was shown for 24 h time period as bar graph. Columns: mean of triplicate experiments; bars: SD; *p<0.05, significant difference from 0 h migration. (B) hUCBSCs in serum free medium were added into top chamber of transwells and conditioned medium from Medulloblastoma cells (Daoy-CM and D283-CM) or Fibroblast conditioned medium (Fibroblast-CM) was added into bottom chamber of transwells and cultured for 16 h as described in Materials and Methods. hUCBSCs that are migrated (lower side of the membrane) towards tumor cell conditioned medium (Daoy-CM/D283-CM) were stained with HEMA-3 staining kit and photographed under a light microscope at 20× magnification. Percentages of migrated cells were quantified by counting five fields in each condition. Results are representative of three independent experiments. Columns, mean of three experiments; bars, SD.
Stem cell migration is inhibited by the inhibition of MMP-2
Factors released from tumor cells may serve as a potential chemo-attractant involved in the tropism of stem cells towards tumor cells. As medulloblastoma tissue samples and cell lines expressed MMP-2,16 therefore, we investigated the effect of MMP-2 inhibition in the tumor cells on stem cell migration. To examine the role of MMP-2 in hUCBSC migration towards medulloblastoma cells, we used an adenovirus expressing siRNA against MMP-2 (Ad-MMP-2 si) to specifically inhibit MMP-2. We have previously demonstrated that Ad- MMP-2 si specifically inhibited MMP-2 mRNA and protein levels without inducing the interferon pathway and with out effecting other MMP's.17 We first confirmed that this Ad-MMP-2 si could indeed reduce the protein expression levels of endogenous MMP-2 compared with the scrambled vector (Ad-SV) and control (mock) transfected cells. As figure 2A indicated, Daoy and D283 medulloblastoma cells infected with 50 MOI of Ad-MMP-2 si decreased the expression of MMP-2 activity, protein and mRNA levels by 50 % compared to mock and Ad-SV.
Figure 2. Ad-MMP-2 si-infection inhibits MMP-2 expression in medulloblastoma cells and decreases human umbilical cord blood stem cell (hUCBSCs) migration towards tumor cells.
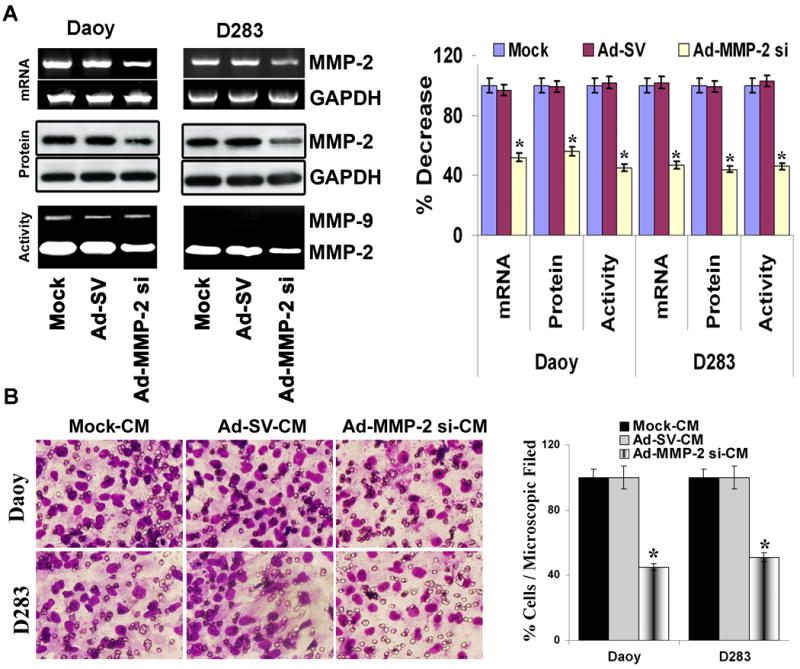
Medulloblastoma cells (Daoy and D283 cells) were treated with mock (PBS Control), 50 MOI of Ad-SV or Ad-MMP-2 si for 24 h, and conditioned medium and cells were collected. (A) Top panel: Total RNA was extracted and semi-quantitative RT-PCR was performed for MMP-2; GAPDH was served as a control for RNA quality. Middle panel: MMP-2 levels were determined in the total cell lysates by western blot analysis using anti-MMP-2 antibody. GAPDH served as a loading control. Bottom panel: MMP-2 gelatinolytic activity was determined in the conditioned medium by gelatin zymography. Band intensities were quantified by densitometric analysis using ImageJ software (National Institutes of Health). The levels of MMP-2 mRNA, protein and gelatinolytic activity were normalized to levels in mock-transfected cells. Columns: mean of triplicate experiments; bars: SD; *p<0.05, significant difference from mock transfected control cells. (B) hUCBSCs migration towards tumor cell conditioned medium from medulloblastoma cells infected with Ad-MMP-2 si was studied in a transwell migration assay. Conditioned medium collected from mock, Ad-SV and Ad-MMP-2 si-infected tumor cells were designated as mock-CM, Ad-SV-CM and Ad-MMP-2 si-CM, respectively. The cells that had migrated to the lower side of the membrane were photographed under a light microscope at 20× magnification. Percentages of migrated cells were quantified by counting five fields in each condition. Results are representative of three independent experiments. Columns, mean of three experiments; bars, SD.
For preparation of conditioned medium, cell number was corrected to account for 15-20 % growth inhibition observed with Ad-MMP-2 si-infection (data not shown). The conditioned medium was collected and used to study the migration of hUCBSCs. There was a significant reduction in stem cell migration in response to tumor conditioned medium from medulloblastoma cells infected with Ad-MMP-2 si (Fig. 2B). Tumor conditioned medium from medulloblastoma cells infected with 50 MOI of Ad-MMP-2 si decreased the stem cells migration by 40-50% (P < 0.05 vs mock) compared to the migration of stem cells in the conditioned medium from Ad-SV-infected tumor cells. The migration of stem cells in conditioned medium from tumor cells infected with Ad-SV was not significantly different from the conditioned medium from mock infected cells, suggesting that the adenovirus itself had no significant negative effect on our in vitro migration assay. To further determine the role of MMP-2 in stem cell migration, migration was determined after exogenous addition of recombinant human MMP-2 (rhMMP-2) to tumor conditioned medium. Figure 4C indicates that exogenous addition of rhMMP-2 protein to the conditioned medium of Ad-MMP-2 si-infected tumor cells did not alter the migratory ability of stem cells, suggesting the involvement of other factors regulated by MMP-2 expression in tumor cell initiated migration of stem cells. Further, to ensure that the effect of MMP-2 inhibition in stem cell migration was not due to their effects on cell survival, we performed MTT assay. There was no change in the hUCBSCs growth in the cells grown on Ad-MMP-2 si-CM compared to controls up to a 48 h time period (data not shown). Together these data indicate that MMP-2 induced factors from medulloblastoma cells are required for their migratory response of hUCBSCs towards medulloblastoma tumor cells.
Figure 4. Addition of rhMMP-2 to the tumor cells rescues SDF1 mediated hUCBSCs migration towards tumor cells.
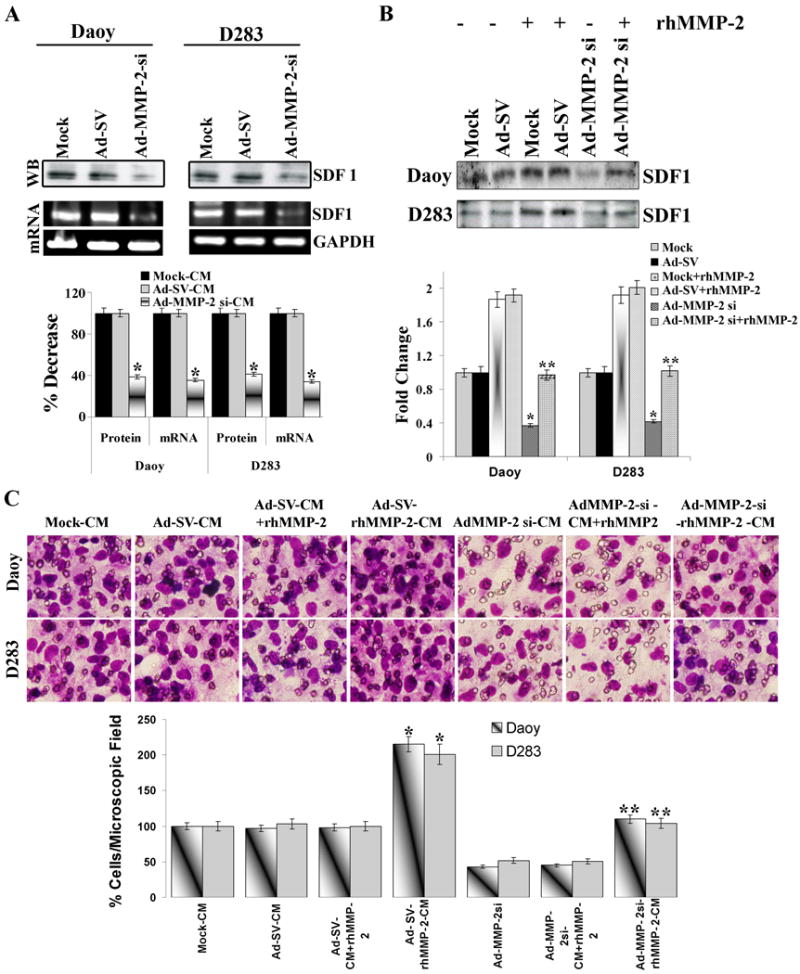
Medulloblastoma cells (Daoy and D283 cells) were infected with mock (PBS Control), 50 MOI of Ad-SV or Ad-MMP-2 si for 24 h, and the conditioned medium was prepared as described in Materials and Methods. (A) Top: Tumor cell conditioned medium was used for western blot analysis for SDF1. Bottom: Total RNA was extracted and RT-PCR was performed for SDF1 as described in Materials and Methods; GAPDH was served as a control for RNA quality. Band intensities were quantified by densitometric analysis using ImageJ software (National Institutes of Health). The levels of SDF1 protein and mRNA were normalized to levels in mock-transfected cells. Columns: mean of triplicate experiments; bars: SD; *p<0.05, significant difference from mock transfected control cells. (B) Medulloblastoma cells (Daoy and D283 cells) were infected with mock (PBS Control), 50 MOI of Ad-SV or Ad-MMP-2 si for 12 h, and the recombinant MMP-2 was then added to the cells and allowed to grow for a further 24 h. Conditioned medium was collected and used for western blot analysis for SDF1. Band intensities were quantified by densitometric analysis using ImageJ software (National Institutes of Health). The levels of SDF1 protein were normalized to levels in mock-transfected cells. Columns: mean of triplicate experiments; bars: SD; *p<0.05, significant difference from mock-CM; **p<0.05, significant difference from Ad-MMP-2 si-CM (C) Medulloblastoma cells (Daoy and D283 cells) were infected with mock (PBS Control), 50 MOI of Ad-SV or Ad-MMP-2 si for 24 h, the conditioned medium was then collected and rhMMP-2 was added (Ad-MMP-2 si-CM+rhMMP2) or Medulloblastoma cells (Daoy and D283 cells) were infected with mock (PBS Control), 50 MOI of Ad-SV or Ad-MMP-2 si, after 12h incubation recombinant MMP-2 was then added to the cells and allowed to grow for further 24 h, and conditioned medium was collected (Ad-MMP-2 si-rhMMP-2-CM). hUCBSCs migration was performed in the presence of these tumor cell conditioned media using transwell migration assay. The cells that were migrated to the lower side of the membrane were stained and photographed under a light microscope at 20× magnification. Percentages of migrated cells were quantified by counting five fields in each condition. Results are representative of three independent experiments. Columns, mean of three experiments; bars, SD. *p<0.05, significant difference from mock-CM; **p<0.05, significant difference from Ad-MMP-2 si-CM.
MMP-2 inhibition in medulloblastoma cell alters Cytokine profile
We hypothesized that the reason for the decreased stem cell migration towards MMP-2 suppressed tumor cells is due to the alterations in the expression of cytokines involved in migration. To test this possibility, antibody arrays associated with migration against several cytokines was employed. These antibody arrays were incubated with conditioned media from Daoy and D283 tumor cells infected with Ad-SV and Ad-MMP-2 si. High levels of IL-6, IL8, MCP1, SDF1, VEGF and RANTES expression was observed in the conditioned medium from control Daoy and D283 cells. The levels of several cytokines were decreased from medium collected from Ad-MMP-2 si infected medulloblastoma cells relative to control treated cells. Fig 3 indicates that SDF1 and VEGF were decreased in both the cell lines by 70-75 % and 55-65% respectively. We therefore evaluated the role of SDF1 and VEGF on the migratory capacity of stem cells towards tumor conditioned media. We first evaluated the role of SDF1/ CXCR4 axis on the tumor-tropic component of the hUCBSCs towards medulloblastoma cells. To further confirm the antibody array, we performed western blotting using tumor cell conditioned medium and RT PCR using total RNA for SDF1 in Daoy and D283 tumor cells infected with mock, Ad-SV and Ad-MMP-2 si. The expression of SDF1 protein and mRNA were suppressed by about 60% in the Ad-MMP-2 si-infected cells compared to mock and Ad-SV controls (Fig. 4A). We next determined the role of SDF1 in stem cell migration with tumor conditioned media from MMP-2 inhibited tumor cells, and demonstrated that supplementing MMP-2 in Ad-MMP-2 si-infected cells before preparation of conditioned medium restored SDF1 levels in tumor cells (Fig. 4B). Accordingly, we also observed that stem cell migration towards the tumor conditioned medium from AdMMP-2-si infected tumor cells supplemented with MMP-2 was significantly higher (p< 0.05) compared to conditioned medium from Ad-MMP2-si alone infected cells (Fig. 4C). These findings suggest that tumor cell-expressed SDF1 plays a chemotactic role in the migration of human stem cells towards the tumor cells.
Figure 3. MMP-2 inhibition in medulloblastoma cells alters Cytokine profile.
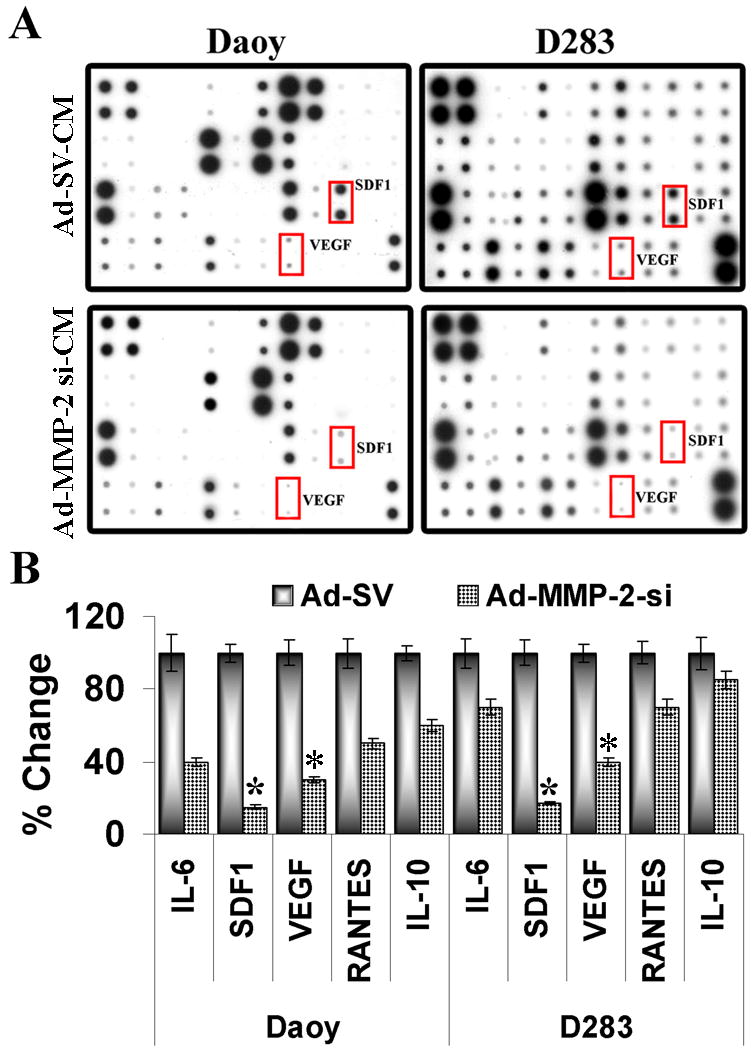
(A) Human cytokine antibody array (Ray Biotech, Inc) was used to measure the secretion of 42 Cytokines into tumor conditioned medium from mock, Ad-SV or Ad-MMP-2 si treated Daoy/ D283 cells. Shown in rectangle enclosed boxes are the factors that are highly downregulated with MMP-2 inhibition in Daoy/D283 cells. (B) The dot intensities of different cytokines were quantified by densitometry using ImageJ software (National Institutes of Health) and normalized with the intensity of internal positive controls for comparison. The experiments were performed independently at least three times with similar results. Columns: mean of triplicate experiments; bars: SD; *p<0.01, and **p<0.05, significant difference from mock transfected control cells.
hUCBSCs migration towards tumor conditioned media in vitro can be inhibited by blocking hUCBSCs surface SDF1 receptor CXCR4 (C-X-C motif receptor 4). To examine hUCBSC response to SDF1 in the tumor cells, we performed immunocytochemical analysis for its receptor CXCR4 in hUCBSC cultured in the presence of tumor conditioned medium of Ad-MMP-2 si-infected Daoy and D283 medulloblastoma cells. Figure 5A demonstrates that tumor conditioned medium from mock and Ad-SV-infected cells induced CXCR4 expression compared to the stem cell culture (or serum-free) medium. In contrast, CXCR4 expression was decreased in hUCBSC cultured with conditioned medium from Ad-MMP-2 si-infected medulloblastoma cells. Neutralization of endogenous hUCBSC-associated CXCR4 with anti-CXCR4 resulted in decreased migration (Fig. 5B) demonstrating an essential role of SDF1/CXCR4 interaction in hUCBSC migration towards the tumor cells.
Figure 5. SDF1/CXCR4 mediated pathway is affected in hUCBSCs cultured with tumor cell conditioned medium from MMP-2 inhibited tumor cells.
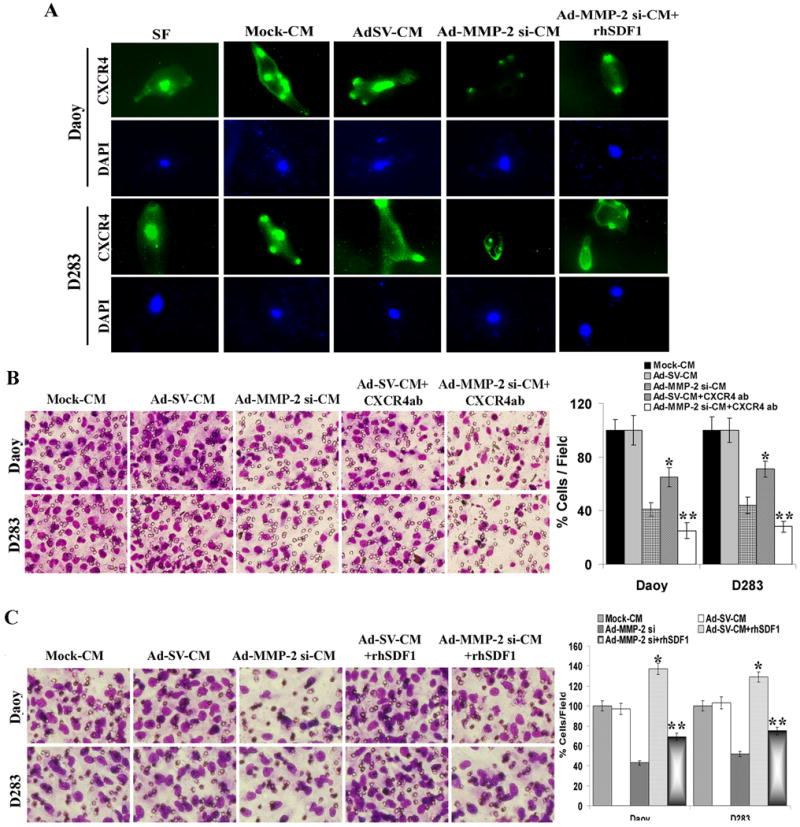
(A) Medulloblastoma cells (Daoy and D283 cells) were infected with mock (PBS Control), 50 MOI of Ad-SV or Ad-MMP-2 si and conditioned medium was collected. hUCBSCs were grown in the presence of serum-free medium or tumor cell conditioned medium in the presence/absence of human recombinant SDF1 (rhSDF1) for 36 h, and immunocytochemical analysis for CXCR4 (green) using CXCR4 specific antibody. Nucleus was counter stained with DAPI and photographed using fluorescence microscope. (B) hUCBSCs migration was performed in the presence of tumor cell conditioned medium with or without rhSDF1 using transwell migration assay. The cells that were migrated to the lower side of the membrane were photographed under a light microscope at 20× magnification. Percentages of migrated cells were quantified by counting five fields in each condition. Results are representative of three independent experiments. Columns, mean of three experiments; bars, SD. *p<0.05, significant difference from mock-CM; **p<0.05, significant difference from Ad-MMP-2 si-CM. (C) hUCBSC migration was performed in the presence of tumor conditioned medium with or without CXCR4 antibody using transwell migration assay. The cells that were migrated to the lower side of the membrane were photographed under a light microscope at 20× magnification. Percentages of migrated cells were quantified by counting five fields in each condition. Results are representative of three independent experiments. Columns, mean of three experiments; bars, SD. *p<0.05, significant difference from mock-CM; **p<0.05, significant difference from Ad-MMP-2 si-CM.
Recombinant human SDF1 (rhSDF1) but not rhVEGF reverts hUCBSCs migration in Ad-MMP-2 si-infected tumor cell conditioned medium
Densitometric analysis of chemokine array showed that SDF1 and VEGF were decreased in both the cell lines (Daoy and D283) by 70-75 % and 55-65% respectively. We therefore tested the ability of hUCBSCs migration towards tumor cell conditioned medium in the presence of SDF1 and VEGF. Addition of recombinant human SDF1 increased stem cell migration towards tumor cell conditioned medium when compared to Ad-MMP-2 si alone (Fig.5C). Addition of rhVEGF to the Ad-MMP-2 si inhibited tumor cell conditioned medium did not alter the stem cell migration towards tumor cell conditioned medium when compared to Ad-MMP-2 si alone (Supplementary figure 1). hUCBSCs showed increased CXCR4 expression when cultured in the presence of rhSDF1 when compared to Ad-MMP-2 si-CM alone (Fig. 5A).
Migratory capacity of hUCBSCs in vivo
Next, we investigated whether MMP-2 expression in the tumor cells could influence hUCBSCs migration towards intracranial medulloblastoma tumors in vivo. Daoy cells stably expressing luciferase gene were stereotactically implanted as described previously.18 The mice were separated into three groups and treated with PBS, 5 × 107 Ad-SV or of Ad-MMP-2 si. Quantum dots labeled hUCBSCs cells were injected into the contralateral hemisphere of mice with comparable tumor sizes in all three treatments as determined by in vivo imaging (Fig. 6A). The tumor sections from mice that received PBS and Ad-SV displayed very high expression of MMP-2 and SDF1. In contrast, very littlie immunoreactivity for MMP-2 and SDF1 were observed in the tumor sections of mice that received Ad-MMP-2 si-treatment (Fig. 6B). Transplanted hUCBSCs, enriched for CD133, were seen migrating across and populating in to the medulloblastoma tumors in the tumor sections of mice that received PBS and Ad-SV-treatments. In contrast, in the tumors from mice that received Ad-MMP-2 si in which MMP-2 expression was inhibited, the migratory capacity of hUCB SCs was suppressed and stem cells remained within the injection site in the normal brain (Fig. 6C and D).
Figure 6. MMP-2 inhibition in medulloblastoma tumor decreases stem cell tropism in vivo.
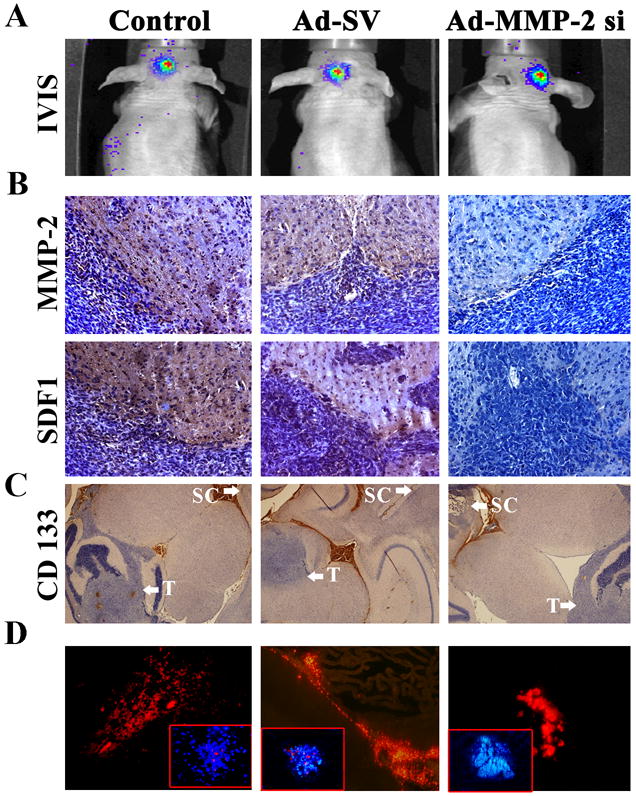
Brain tumor sections from mice that received mock, Ad-SV and Ad-MMP-2 si treatments were analyzed. (A) IVIS Imaging of mice two weeks after Daoy cell implantation. (B) Three days after Ad- MMP-2 si injection, mice were sacrificed and brains were collected and fixed in 10% burrered formalin and Tissue sections (5 mm thick) were subjected to immunostaining with antibodies for either for MMP-2, and SDF1 (C) Images showing the Injection site of hUCBSCs (CD133 staining) at the frontal lobe and tumor site at the cerebellum. Arrows showing Tumor cell implantation site (T, near cerebellum) and Stem cell implantation site (SC, in the frontal lobe) (D) Five days after stem cell implantation, quantum dot labeled hUCBSCs (red) are seen migrating towards tumor site. Inset shows Quantum dot labeled hUCBSCs in the tumor bed (DAPI stain)
Discussion
Previous studies have explored the migratory capacity and therapeutic potential of human stem cells in experimental medulloblastoma and demonstrated their therapeutic efficacy against human medulloblastoma.19 Understanding of the molecular events that regulate stem cell tropism towards tumors is needed to ensure their safety and to maximize therapeutic efficacy. Our in vitro and in vivo studies clearly show for the first time the ability of human umbilical cord blood stem cells (hUCBSCs) to migrate towards medulloblastoma tumors. The migration of hUCBSCs was significantly enhanced in the presence of conditioned medium from medulloblastoma tumor cells compared to that of the control serum-free medium. In contrast, the migration of stem cells was decreased in response to the conditioned medium of medulloblastoma cells infected with Ad-MMP-2 si (MMP-2 expression was decreased by 50-60% compared to controls as determined by western blotting). To better define the crosstalk between medulloblastoma and hUCBSCs and the role of MMP-2 expression in the tumor cells, we investigated the signals that have been shown to modulate the medulloblastoma tumor tropism of hUCBSCs. Supplementing the conditioned medium from MMP-2 inhibited cells with recombinant MMP-2 did not alter the stem cell migration, indicating that MMP-2 is not directly involved in stem cell migration towards the tumor cells. The chemokines, which are small secreted molecules, act through their receptors, which belong to the superfamily of G protein-coupled receptors (GPCRs).20 SDF1 stimulates chemotaxis, survival and proliferation in glioblastoma multiforme and medulloblastoma primary cell cultures and xenografted tumors.21 Our data indicate that only SDF1 and VEGF were decreased in both the cell lines by 70-75 % and 55-65% respectively. Based on the established role of SDF1 and its receptor CXCR4 in governing neuronal and glial precursor migration within the developing brain,22,23 we next investigated the role of MMP-2 in SDF1/CXCR4 mediated stem cell tropism towards the tumor cells. A major function of SDF1/CXCR4 signaling is to regulate the movement of cells, as SDF1 attracts CXCR4-expressing cells. Previous studies demonstrate that stromal cell-derived factor 1 (SDF1) and its receptor, CXCR4, regulate neural progenitor cell motility24 and it is possible that SDF1 also contributes to medulloblastoma tumor cell induced stem cell migration. Agents such as stem cell factor1, monocyte chemo attractant protein-1, and stromal cell-derived factor-1 are potent chemotactic molecules originally identified as inducers of hematopoietic cell migration20,25 and recently shown to stimulate NSC migration.24,26 Our results indicate that signaling from the SDF1/CXCR4 is important for the activation of stem cell migration towards tumor cells. MMP-2 inhibition significantly inhibited cytokine expression; of note, SDF1 expression was inhibited by more than 80% in the MMP-2 inhibited medulloblastoma cells. Addition of SDF1 to conditioned medium from MMP-2 inhibited tumor cells induced stem cell migration, suggesting that MMP-2 inhibition in tumor cells suppressed SDF1 mediated migration of stem cell towards the tumor cells.
We next elucidated the molecular mechanism by which hUCBSCs migrate in response to tumor cells. Here, we show that cultured hUCBSCs express the CXCR4 receptor for SDF1 in response to tumor cell conditioned medium. In contrast, CXCR4 expression was suppressed in the stem cells grown in the presence of conditioned medium from AdMMP-2-si infected cells, indicating that the SDF1/CXCR4 axis was involved in regulating the migratory behaviour of hUCBSCs. The expression of functional CXCR4 has been observed on the surface of embryonic stem cells,27 and several tissue committed stem/progenitor cells, such as HSC,28 and neural stem cells.22 Wynn et al29 reported that a small proportion of mesenchymal stem cells expressed CXCR4, which contributed to their migration in vitro. Similarly, Sordi et al. found that CXCR4 expressed on MSC was capable of promoting migration to pancreatic islets.30 In addition, we show that blocking CXCR4 receptors using function-blocking antibodies in stem cells grown in the conditioned medium supplemented with MMP-2 suppressed the migration of hUCBSCs towards meduloblastoma-conditioned media in vitro, confirming the relevance of this pathway in hUCBSCs migration towards tumor conditioned medium. Further, stem cells cultured with conditioned medium from MMP-2 inhibited tumor cells, supplemented with SDF1, induced the expression of CXCR4, and restored the migration of these cells towards the tumor cells. Further, the addition of anti-CXCR4 antibody to Ad-SV treated medulloblastoma conditioned medium decreased decreased the migration of stem cell towards the tumor cells. Taken together, these studies indicate that MMP-2 inhibition in the tumor cells inhibits the SDF1/CXCR4 mediated signaling in the stem cells, thereby decreasing their tropism towards the tumor cells.
In conclusion, we demonstrated that MMP-2 inhibition suppressed SDF1-promoted hUCBSCs stem cells migration towards the tumor cells by blocking the SDF1/ CXCR4 mediated signaling. Finally, this experimental model suggests a need for caution when stem cells are chosen as a chemotherapeutic target and matrix metalloproteinase inhibitors are used against cancer cells since MMP-2 depletion can reduce the ability of stem cells to migrate towards the tumor cells.
Materials and Methods
Cell cultures
We used the Daoy (ATCC #HTB 186), D283 (ATCC #HTB 185) and Fibroblast cell lines. Cells were cultured in Advanced-MEM (for Daoy), Advanced-MEM (Zn option medium without phenol red; for D283) or DMEM 1× medium (for Fibroblasts). Media were supplemented with 5% fetal bovine serum, 2 mM/L L-glutamine, 2 mM/L sodium pyruvate, 100 units/mL penicillin, and 100 μg/mL streptomycin. All cells were maintained in a humidified atmosphere containing 5% CO2 at 37°C.
Human umbilical cord blood stem cells (hUCBSCs) isolation and culture
Human umbilical cord blood was collected from full-term normal deliveries with informed consent according to a protocol approved by the Institutional Review Board. The cell fraction was separated on Ficoll-PaqueOˆ Plus (GE Healthcare, Piscataway, NJ, USA) by density centrifugation. CD133+ cell fraction was isolated using CD133-conjugated super paramagnetic micro-beads and MiniMACS columns (Miltenyi Biotech, Auburn, CA, USA; Bergisch Gladbal, Germany) according to manufacturer's instructions. Briefly, the cells collected from density centrifugation were incubated with CD133 antibody conjugated-micro beads for 30 min at 4°C. Cells were washed in Dulbecco's phosphate-buffered saline without sodium bicarbonate (PBS, Sigma Aldrich, St. Louis, MO, USA) supplemented with 2mmol/l EDTA and 0.5% fetal calf serum (FCS) (Invitrogen, Carlsbad, CA, USA). Cells were passed through a MiniMACS column retained in a magnetic field, and the column was washed with PBS to remove unbound cells. Releasing the magnetic field and flushing cells from the column recovered CD133+ cells. The cells obtained by the column were plated in knockout DMEM basal medium supplemented with 10% FBS, 10% knockout serum, 100 units/ml penicillin, and 100 μg/mL streptomycin and cultured until they reach confluence. The purity of the positively selected CD133 population was evaluated by a FACS Calibur flow cytometer (Becton Dickinson Bioscience, San Jose, CA, USA). To further determine the presence of other stem cell marker proteins CD44 and STRO-1, the cells were fixed in 10% cold methanol/buffered formaldehyde followed by incubation with 1% BSA in PBS for 1 hour at room temperature. These cells were used to perform either immunohistochemistry or FACS analysis as described earlier.15
Antibodies and reagents
Antibodies against MMP-2 and GAPDH were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against SDF1 and CXCR4 were obtained from Novus Biologicals (Littleton, CO, USA). Recombinant human MMP-2 (R&D Systems, Minneapolis, MN, USA), recombinant human SDF1 and recombinant VEGF (ProSpec-Tany TechnoGene Ltd., Rehovot, Israel) were used in this study. Transwell inserts were obtained from VWR International (West Chester, PA, USA), cell culture inserts were obtained from ibidi GmbH (Martinsried, Germany). All other reagents were of analytical reagent grade or better.
Adenoviral siRNA constructs and infection
The adenoviral siRNA for MMP-2 (AdMMP-2 si) and scrambled vector (Ad-SV) were constructed and amplified as described by us previously.31 Viral titers were quantified as pfu/mL following infection of 293 cells. Titers obtained for the viruses used in this work are 7.6 × 1011 pfu/mL (Ad-SV), and 5.0 × 1011 (Ad-MMP-2 si). The amount of infective adenoviral vector per cell in culture media was expressed as multiplicity of infection (MOI). Virus constructs were diluted in serum-free culture media to the desired concentration, added to cells and incubated at 37°C for 1 hour. The necessary amount of complete medium was then added and cells were incubated for the desired time periods. We have clearly demonstrated the specificity of Ad-MMP-2 si construct in our earlier published results17 and also showed that the virus does not activate components of the interferon system17
Preparation of tumor conditioned media (CM)
1.5 × 106 Daoy or D283 cells were seeded in 100mm Petri dishes and incubated for overnight. Cells were infected with mock (PBS), 50 MOI of either adenovirus carrying a scrambled sequence (Ad-SV) or, adenovirus carrying siRNA against MMP-2 (Ad-MMP-2 si) (1.8 × 106 cells for Ad-MMP-2 si infection, cell number was corrected to account for 15-20% growth inhibition) and incubated for a further 24 h. The medium was replaced with serum-free DMEM/F-12 50/50 medium and incubated for 16 h. Conditioned media collected from mock, Ad-SV and Ad-MMP-2 si-infected cells were designated as mock-CM, Ad-SV-CM and Ad-MMP-2 si-CM, respectively. All the experiments were performed in the presence of serum-free media as a control.
Migration assay
Tumor cell-induced hUCBSCs migration was measured by using the Culture-Inserts (ibidi GmbH, Martinsried, Germany) as described previously with minor modifications.32 The Culture-Inserts containing two wells were placed in to 8-well chamber slides and 0.5 or 1×104 Qtracker-525-Green (Molecular Probes, Carlsbard, CA) labeled medulloblastoma cells (Daoy or D283 cells) were seeded into one well (one side of the insert), and 1×104 Qtracker-655-Red (Molecular Probes, Carlsbard, CA) labeled hUCBSCs were seeded into another well (other side of the insert). The cells were allowed to attach on to the plates for 16 h, and the Culture-Inserts were removed to create 500 μm cell-free gap between two different cell types. The cells were then allowed to migrate for a further 24 h. Images (20× magnification) were captured at 0 h and 24 h of incubation using a fluorescence microscope.
Transwell Chamber Migration Assay
A cell culture insert system along with a companion tissue culture plate with 12 wells (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) was used for the migration assay. hUCBSCs (2×105 cells) were added into top wells and the conditioned medium from Daoy/D283 cells infected with mock, Ad-SV or Ad-MMP-2 si was added into the bottom chamber and incubated for 16 h. Viable hUCBSCs (1×106) for each treatment conditioned medium were allowed to invade through polycarbonate filters (12-μm pore size). The migrating cells on the reverse side of the filter were stained photographed and counted. Five different fields per filter were analyzed, and all experiments were done in triplicate
Human Cytokines Antibody Array
Human cytokine antibody arrays (Ray Biotech, Inc) were used according to the manufacturer's instructions. Briefly, cells were infected with mock (PBS), 50 MOI of either Ad-SV or Ad-MMP-2 si and prepared conditioned medium as described above. Cytokine array Membranes were incubated in equal quantities of conditioned media either from Ad-SV or Ad-MMP-2 si treated medulloblastoma cells for 1hr. After washing with PBS, membranes were incubated in biotin labeled primary antibodys follwed by 1,000 fold diluted HRP-conjugated streptavidin was added and developed. After developing, films were scanned and the images processed and quantified using ImageJ software (National Institutes of Health). Signal intensity was normalized to internal positive controls for comparison.
Western blotting
Western blot analysis was performed as described previously.17,18 Briefly, Daoy or D283 cells were cultured and infected with mock, 50 MOI of Ad-SV or Ad-MMP-2 si and incubated for 48 h at 37°C. Cell lysates were prepared in radioimmunoprecipitation assay (RIPA) buffer with protease inhibitors and protein concentrations were measured using bicinchoninic acid protein assay regents (Pierce, Rockford, IL, USA). Equal amounts of protein was resolved on SDS-PAGE gel and transferred onto PVDF membrane. Next, the blot was blocked and probed overnight with different primary antibodies at 4°C, followed by HRP conjugated secondary antibodies for 1 h and signals were detected by using ECL reagent.
Gelatin zymography
The tumor-conditioned medium was prepared as described above and equal amounts of proteins were used to determine MMP-2 activity. Gelatin zymography was performed as described previously.33 Briefly, medulloblastoma cells (Daoy and D283) were grown in six-well tissue culture plates and infected with mock (PBS), 50 multiplicities of infection (MOI) of Ad-SV, or 50 MOI of Ad-MMP-2 si. After a 24-h incubation period, cells were washed with PBS and cultured overnight in serum-free DMEM/F-12 medium. The total protein concentration of the conditioned media was estimated using bicinchoninic acid (BCA) reagent (Pierce). Equal amounts of protein from various treatments were used to determine gelatinase activity.
RT-PCR
Daoy or D283 cells were cultured and infected with mock, 50 MOI of Ad-SV or Ad-MMP-2 and incubated for 36 h at 37°C. Total RNA was extracted from cells as described by Chomczynski and Sacchi.34 The RNA was then treated with DNase I (Ambion, Austin, TX) for 30 min at 37°C. PCR was performed as described previsouly.33 The expected PCR products were resolved on 2% agarose gels and visualized using ethidium bromide staining. The primers used in this study are: MMP-2 primers, sense 5′-GTGCTGAAGGACACACTAAAGAAGA-3′ and antisense 5′-TTGCCATCCTTCTCAAAGTTGTAGG-3′, and SDF1 primers sense 5′-CGCCCATTGGAGACATAAAA-3′ and antisense 5′- antisense 5′-ATCTGAAGGGCACAGTTTGG-3′, GAPDH primers sense 5′-TGAAGGTCGGAGTCAACGGATTTGGT-3′ and antisense 5′-CATGTGGGCCATGA GGTCCACCAC-3′. To normalize for the amount of input RNA, RT-PCR was performed with primers for the constitutively expressed GAPDH gene.
In vivo migration assay
All animal experiments were carried out following approval by the Institutional Animal Care and Use Committee (IACUC) on a project-specific basis in accordance with Public Health Service Policy on Humane Care and Use of Laboratory Animals (PHS Policy) and meet the standards required by the UKCCCR guidelines.35 Animals were housed in pathogen-free conditions with a light/dark cycle of 12/12 h and fed with rodent chow and water ad libitum. Daoy cells (1×105), stably transfected with plasmid containing luciferase (luc) gene, were stereotactically implanted as described previously.18,36 To establish intracerebellar xenograft models, 6- to 8-week-old mice were anesthetized with isoflurane inhalation (2.5% in 100% oxygen); after which, a small skin incision (5 mm) was made and a burr hole (0.7 mm in diameter) created with microsurgical drill. Tumor cells were suspended in 5 μL of culture medium and injected slowly through the burr hole into the right cerebellar hemisphere using a 10-μL, 26-gauge Hamilton syringe needle that was inserted perpendicular to the cranial surface. Tumor growth was monitored in mice by using an in vivo imaging system (IVIS, Xenogen). Fifteen days after tumor cell implantation, the animals were randomized into three groups. Tumors were treated with a single dose of mock, 5 × 107 PFU Ad-SV or Ad-MMP-2 si intracranially at the tumor site as described previously.33 One set of animal were sacrificed and the mice brains were collected and fixed. To asses the migratory ability of human umbilical cord blood stem cells (hUCBSCs) in vivo, three days after the treatment with Ad- MMP-2 si, Quantam dots labeled(Molecular Probes, Eugene, OR, USA) CD133 enriched hUCBSCs (2.0 × 105 in 6 μL PBS) were injected in the frontal lobe of the brain. Animals were sacrificed 5 days after stem cell implantation. Mice brains were fixed in 10% buffered formalin and embedded in paraffin. Tissue sections (5 mm thick) were obtained from the paraffin blocks and used for immunohistochemical analysis.
Immunocyto- and Immunohisto-chemical analysis
Stem cells (5×103) were plated in chamber slides and cultured on mock-CM, Ad-SV-CM or Ad-MMP-2 si-CM for 48h. Cells were washed with PBS and fixed for 30 min in cold methanol and permeabilized in 0.1% Triton-X100 in PBS. Non-specific binding was blocked by 3% BSA in PBS for 1h, followed by incubation with 1:100 dilution of anti-CXCR4. The excess antibody was removed by washing at least three times with PBS and then incubated with FITC/ Texas Red - conjugated secondary antibody for 1h. The cells were washed with PBS and mounted with 4′, 6-diamidino-2-phenylindole (DAPI) staining solution. For immunohistochemical analysis, tissue sections (4-5 μm-thick) were deparaffinized in xylene, rehydrated in graded ethanol solutions, washed with PBS and permeabilized in 0.1% Triton X-100 and incubated overnight with primary antibodies for MMP-2, CD133 and SDF1. After primary antibody, slides were incubated with HRP-conjugated secondary antibody for 1h followed by 3,3-diaminobenzidine (DAB peroxidase substrate) solution. The slides were counterstained with hematoxylin and mounted. The bright field images were captured with an Olympus BX 60 research microscope attached with CCD camera.
Statistical methods
All of the values were calculated as mean ± SD or were expressed as percentage of control ± SD. Significant differences between assessment of in vitro migration, cell viability, and tumor volume were determined using the Mann-Whitney U test. P < 0.05 was considered significant.
Acknowledgments
We acknowledge Shellee Abraham for manuscript preparation, Sushma Jasti and Diana Meister for manuscript review.
This research was supported by National Cancer Institute Grant CA132853 (to S.L.). Contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of NIH.
Abbreviations used
- MMP-2
Matrix metalloproteinases 2
- ECM
extracellular matrix
- hUCBSCs
Human umbilical cord blood stem cells
- PBS
phosphate-buffered saline
- H&E
haematoxylin & eosin
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- RIPA
Radioimmunoprecipitation assay
- SV
Scrambled vector
- Ad
Adenoviral
- siRNA
small interfering RNA
- FITC
Fluorescein isothiocyanate
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Supplemental information is available at Gene Therapy's website.
Reference List
- 1.Benedetti S, Pirola B, Pollo B, Magrassi L, Bruzzone MG, Rigamonti D, et al. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med. 2000;6:447–450. doi: 10.1038/74710. [DOI] [PubMed] [Google Scholar]
- 2.Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yip S, Aboody KS, Burns M, Imitola J, Boockvar JA, Allport J, et al. Neural stem cell biology may be well suited for improving brain tumor therapies. Cancer J. 2003;9:189–204. doi: 10.1097/00130404-200305000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Lee KH, Choi EY, Hyun MS, Kim JR. Involvement of MAPK pathway in hypoxia-induced up-regulation of urokinase plasminogen activator receptor in a human prostatic cancer cell line, PC3MLN4. Exp Mol Med. 2004;36:57–64. doi: 10.1038/emm.2004.8. [DOI] [PubMed] [Google Scholar]
- 5.Gang EJ, Hong SH, Jeong JA, Hwang SH, Kim SW, Yang IH, et al. In vitro mesengenic potential of human umbilical cord blood-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2004;321:102–108. doi: 10.1016/j.bbrc.2004.06.111. [DOI] [PubMed] [Google Scholar]
- 6.Yang SE, Ha CW, Jung M, Jin HJ, Lee M, Song H, et al. Mesenchymal stem/progenitor cells developed in cultures from UC blood. Cytotherapy. 2004;6:476–486. doi: 10.1080/14653240410005041. [DOI] [PubMed] [Google Scholar]
- 7.Kim SM, Lim JY, Park SI, Jeong CH, Oh JH, Jeong M, et al. Gene therapy using TRAIL-secreting human umbilical cord blood-derived mesenchymal stem cells against intracranial glioma. Cancer Res. 2008;68:9614–9623. doi: 10.1158/0008-5472.CAN-08-0451. [DOI] [PubMed] [Google Scholar]
- 8.Kollar K, Cook MM, Atkinson K, Brooke G. Molecular mechanisms involved in mesenchymal stem cell migration to the site of acute myocardial infarction. Int J Cell Biol. 2009 doi: 10.1155/2009/904682. E-pub: 2009:904682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol. 2003;200:448–464. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- 10.Chang C, Werb Z. The many faces of metalloproteases: cell growth, invasion, angiogenesis and metastasis. Trends Cell Biol. 2001;11:S37–S43. doi: 10.1016/s0962-8924(01)02122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mannello F, Tonti GA, Bagnara GP, Papa S. Role and function of matrix metalloproteinases in the differentiation and biological characterization of mesenchymal stem cells. Stem Cells. 2006;24:475–481. doi: 10.1634/stemcells.2005-0333. [DOI] [PubMed] [Google Scholar]
- 12.Karagiannis ED, Popel AS. Distinct modes of collagen type I proteolysis by matrix metalloproteinase (MMP) 2 and membrane type I MMP during the migration of a tip endothelial cell: insights from a computational model. J Theor Biol. 2006;238:124–145. doi: 10.1016/j.jtbi.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 13.Segarra M, Vilardell C, Matsumoto K, Esparza J, Lozano E, Serra-Pages C, et al. Dual function of focal adhesion kinase in regulating integrin-induced MMP-2 and MMP-9 release by human T lymphoid cells. FASEB J. 2005;19:1875–1877. doi: 10.1096/fj.04-3574fje. [DOI] [PubMed] [Google Scholar]
- 14.De Becker A, Van Hummelen P, Bakkus M, Vande Broek I, De Wever J, De Waele M, et al. Migration of culture-expanded human mesenchymal stem cells through bone marrow endothelium is regulated by matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-3. Haematologica. 2007;92:440–449. doi: 10.3324/haematol.10475. [DOI] [PubMed] [Google Scholar]
- 15.Gondi CS, Veeravalli KK, Gorantla B, Dinh DH, Fassett D, Klopfenstein JD, et al. Human umbilical cord blood stem cells show PDGF-D-dependent glioma cell tropism in vitro and in vivo. Neuro Oncol. 2010;12:453–465. doi: 10.1093/neuonc/nop049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozen O, Krebs B, Hemmerlein B, Pekrun A, Kretzschmar H, Herms J. Expression of matrix metalloproteinases and their inhibitors in medulloblastomas and their prognostic relevance. Clin Cancer Res. 2004;10:4746–4753. doi: 10.1158/1078-0432.CCR-0625-03. [DOI] [PubMed] [Google Scholar]
- 17.Chetty C, Bhoopathi P, Lakka SS, Rao JS. MMP-2 siRNA induced Fas/CD95-mediated extrinsic II apoptotic pathway in the A549 lung adenocarcinoma cell line. Oncogene. 2007;26:7675–7683. doi: 10.1038/sj.onc.1210584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhoopathi P, Chetty C, Kunigal S, Vanamala SK, Rao JS, Lakka SS. Blockade of tumor growth due to matrix metalloproteinase-9 inhibition is mediated by sequential activation of beta1-integrin, ERK, and NF-kappaB. J Biol Chem. 2008;283:1545–1552. doi: 10.1074/jbc.M707931200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Kim SK, Kim SU, Park IH, Bang JH, Aboody KS, Wang KC, et al. Human neural stem cells target experimental intracranial medulloblastoma and deliver a therapeutic gene leading to tumor regression. Clin Cancer Res. 2006;12:5550–5556. doi: 10.1158/1078-0432.CCR-05-2508. [DOI] [PubMed] [Google Scholar]
- 20.Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska-Wieczorek A, et al. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23:879–894. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- 21.Rubin JB, Kung AL, Klein RS, Chan JA, Sun Y, Schmidt K, et al. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci USA. 2003;100:13513–13518. doi: 10.1073/pnas.2235846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lazarini F, Tham TN, Casanova P, renzana-Seisdedos F, Dubois-Dalcq M. Role of the alpha-chemokine stromal cell-derived factor (SDF-1) in the developing and mature central nervous system. Glia. 2003;42:139–148. doi: 10.1002/glia.10139. [DOI] [PubMed] [Google Scholar]
- 23.Reiss K, Mentlein R, Sievers J, Hartmann D. Stromal cell-derived factor 1 is secreted by meningeal cells and acts as chemotactic factor on neuronal stem cells of the cerebellar external granular layer. Neuroscience. 2002;115:295–305. doi: 10.1016/s0306-4522(02)00307-x. [DOI] [PubMed] [Google Scholar]
- 24.Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci USA. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dao MA, Creer MH, Nolta JA, Verfaillie CM. Biology of umbilical cord blood progenitors in bone marrow niches. Blood. 2007;110:74–81. doi: 10.1182/blood-2006-08-034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erlandsson A, Larsson J, Forsberg-Nilsson K. Stem cell factor is a chemoattractant and a survival factor for CNS stem cells. Exp Cell Res. 2004;301:201–210. doi: 10.1016/j.yexcr.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Guo Y, Hangoc G, Bian H, Pelus LM, Broxmeyer HE. SDF-1/CXCL12 enhances survival and chemotaxis of murine embryonic stem cells and production of primitive and definitive hematopoietic progenitor cells. Stem Cells. 2005;23:1324–1332. doi: 10.1634/stemcells.2005-0085. [DOI] [PubMed] [Google Scholar]
- 28.Rosu-Myles M, Gallacher L, Murdoch B, Hess DA, Keeney M, Kelvin D, et al. The human hematopoietic stem cell compartment is heterogeneous for CXCR4 expression. Proc Natl Acad Sci USA. 2000;97:14626–14631. doi: 10.1073/pnas.97.26.14626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wynn RF, Hart CA, Corradi-Perini C, O'Neill L, Evans CA, Wraith JE, et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643–2645. doi: 10.1182/blood-2004-02-0526. [DOI] [PubMed] [Google Scholar]
- 30.Sordi V, Malosio ML, Marchesi F, Mercalli A, Melzi R, Giordano T, et al. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 2005;106:419–427. doi: 10.1182/blood-2004-09-3507. [DOI] [PubMed] [Google Scholar]
- 31.Chetty C, Bhoopathi P, Joseph P, Chittivelu S, Rao JS, Lakka SS. Adenovirus-mediated siRNA against MMP-2 suppresses tumor growth and lung metastasis in mice. Mol Cancer Ther. 2006;5:2289–2299. doi: 10.1158/1535-7163.MCT-06-0169. [DOI] [PubMed] [Google Scholar]
- 32.Lee IO, Kim JH, Choi YJ, Pillinger MH, Kim SY, Blaser MJ, et al. Helicobacter pylori CagA phosphorylation status determines the gp130-activated SHP2/ERK and JAK/STAT signal transduction pathways in gastric epithelial cells. J Biol Chem. 2010;285:16042–16050. doi: 10.1074/jbc.M110.111054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhoopathi P, Chetty C, Gujrati M, Dinh DH, Rao JS, Lakka SS. The role of MMP-9 in the anti-angiogenic effect of secreted protein acidic and rich in cysteine. Br J Cancer. 2010;102:530–540. doi: 10.1038/sj.bjc.6605538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 35.Workman P, Balmain A, Hickman JA, McNally NJ, Rohas AM, Mitchison NA, et al. UKCCCR guidelines for the welfare of animals in experimental neoplasia. Lab Anim. 1988;22:195–201. doi: 10.1258/002367788780746467. [DOI] [PubMed] [Google Scholar]
- 36.Shu Q, Antalffy B, Su JM, Adesina A, Ou CN, Pietsch T, et al. Valproic Acid prolongs survival time of severe combined immunodeficient mice bearing intracerebellar orthotopic medulloblastoma xenografts. Clin Cancer Res. 2006;12:4687–4694. doi: 10.1158/1078-0432.CCR-05-2849. [DOI] [PubMed] [Google Scholar]


