Abstract
Introduction
Gastric bypass remains the mainstay of surgical therapy for obesity. Abdominal pain after gastric bypass is common, and accounts for up to half of all postoperative complaints and emergency room visits. This manuscript reviews the most important causes of abdominal pain specific to gastric bypass and discusses management considerations.
Data Sources
The current surgical literature was reviewed using PubMed, with a focus on abdominal pain after gastric bypass and the known pathologies that underlie its pathogenesis.
Conclusions
The differential diagnosis for abdominal pain after gastric bypass is large and includes benign and life-threatening entities. Its diverse causes require a broad evaluation that should be directed by history and clinical presentation. In the absence of a clear diagnosis, the threshold for surgical exploration in patients with abdominal pain after gastric bypass should be low.
Keywords: Obesity, internal hernia, laparoscopy, abdominal pain, gastric bypass
Introduction, Epidemiology
The number of gastric bypass operations performed annually in the United States has increased dramatically over the past decade and is approaching 200,000 (1). While the benefits of gastric bypass are clear, this explosion in popularity is associated with a finite morbidity. Abdominal pain is one of the most common and vexing problems after gastric bypass. Anywhere from 15-30% of patients will visit the emergency room or require admission within three years of gastric bypass, and abdominal pain is the primary complaint in over half of these cases (2-4). Clinical presentation is highly variable and evaluation may be complicated by the fact that obese patients feel abdominal symptoms more intensely than lean subjects (5). The differential diagnosis of abdominal pain after gastric bypass is diverse and presents diagnostic and therapeutic challenges. Nonetheless, the importance of a thorough understanding of these diverse etiologies is underscored by a single center report documenting a decrease in readmissions after gastric bypass over years with alterations in operative technique based on observation of causes of readmission (4). An understanding of the pathogenesis of abdominal pain after gastric bypass can thus impact favorably on outcomes. This review focuses on causes of abdominal pain specific to gastric bypass (Table 1), but one must of course be alert to other unrelated disease processes, such as appendicitis, mesenteric ischemia, and other “non-bariatric” entities.
Table 1.
Causes of abdominal pain after gastric bypass
| Behavioral, dietary disorders | Functional disorders | Biliary disorders | Pouch, remnant stomach disorders | Small intestine disorders | Other |
|---|---|---|---|---|---|
| Overeating, rapid eating | Constipation, diarrhea, flatus | Cholelithiasis: colic, cholecystitis | Ulcer disease | Abdominal wall hernias: ventral, trocar | Omental infarction |
| Food intolerance | Irritable bowel syndrome | Choledocholithiasis: cholangitis, pancreatitis | Gastrogastric fistula | Adhesions | SMA Syndrome |
| Micronutrient deficiencies | Esophageal motility disorders | Sphincter of Oddi Dysfunction | GERD | Internal hernia | Bezoar |
| Micronutrient supplementati on | Dumping Syndrome | Hiatus hernia | Intussusception | ||
| Gastrojejunosto my stenosis | Jejeunojeunosoto my stenosis |
Behavioral and nutritional disorders
Maladaptive eating behavior is a common cause of abdominal pain in the early post-operative period. Gastric bypass alters satiety and patients may not perceive fullness until pouch distension to the point of pain. Such pain is typically epigastric and postprandial, and a careful dietary history usually reveals a correlation with rapid eating. Modifying behavior to eat slowly and use defined portion sizes provides relief. Such problems rarely persist beyond six months after surgery, after which most patients have learned appropriate eating behaviors.
Specific foods may be prone to transient impaction and cause pain. This is most common within six months of surgery and the frequency of such intolerances may increase with banded gastric bypass (6). Such food intolerances are highly individualistic, making it difficult to generalize regarding specific foods. Nonetheless, hygroscopic foods such as rice, pasta, and bread, or fibrous foods such as meats and some vegetables and fruits, are common culprits and should be eaten in small quantities or avoided altogether. Other considerations should include gluten allergies, lactose intolerance, and other similar “non-bariatric” nutritional causes of pain. Finally, iron and B-12 deficiencies have been implicated as causes of abdominal pain although mechanisms of action are unknown (7). These observations underscore the importance of a thorough dietary history and diagnostic laboratory evaluation to identify food allergies and micronutrient deficiencies.
Bacterial overgrowth in the defunctionalized stomach or small intestine may cause abdominal pain and other non-specific gastrointestinal symptoms (8). Recent studies implicate intestinal bacterial overgrowth after gastric bypass with increased inflammation (9). Furthermore, manipulation of gut flora with probiotics improved weight loss after gastric bypass in a single study (10). These data aside, however, the mechanisms linking gut flora to clinical disease are poorly understood and a causal relationship between bacterial overgrowth and clinically significant gastrointestinal pathology after gastric bypass remains uncertain (11). Bacterial overgrowth is therefore in many cases a diagnosis of exclusion and treatment is often empiric. Nonetheless, hydrogen breath tests have been employed for diagnosis and empiric antibiotic therapy improves symptoms in anecdotal reports (12). Empiric surgical resection of defunctionalized components of the gastrointestinal tract at risk for overgrowth, most commonly the remnant stomach or the anti-peristaltic (“candy-cane”) segment of the Roux limb adjacent to the gastrojejunostomy (13), has been suggested. In the absence of clear pathology within the putative pathogenic segment (i.e. dilation, distal obstruction, fistula, ulceration, gastritis), however, the benefits of such interventions are unproven.
Functional disorders
Functional gastrointestinal motility disorders may cause abdominal pain after gastric bypass. Obesity is an independent risk factor for multiple motility and other functional disorders of the gastrointestinal tract (14, 15) and surgery may exacerbate pre-existing subclinical disease. Such problems are likely more common than realized, often represent diagnoses of exclusion, and are particularly difficult to manage, as disease pathogenesis and diagnostic and treatment algorithms are poorly defined.
Constipation is common in the early post-operative period and may be associated with abdominal pain that is typically localized to the lower abdomen and diffuse and crampy in nature. Constipation may result from dehydration in the early post-operative period, and laxatives and increased water intake provide simple solutions. Gastric bypass less commonly causes diarrhea and/or increased flatus, which may be associated with abdominal pain (16). These symptoms are likely the result of mild malabsorption and loss of the remnant stomach as a storage depot for swallowed air. Judicious use of fiber supplements and anti-diarrheal medication may alleviate diarrhea, while simethicone-based medications may provide relief from excessive flatus. These problems, like constipation, usually improve with time.
More complex functional disorders are not as easily treated. Obesity is a risk factor for irritable bowel syndrome (17). Patients with chronic constipation or a history of irritable bowel syndrome should be warned that these symptoms may worsen after gastric bypass, although virtually no data exists documenting the longitudinal effects of gastric bypass on irritable bowel syndrome.
Esophageal motility disorders are also increased in prevalence in obesity (15, 18) and may cause of pain after gastric bypass that is typically substernal in location. Esophageal manometry aids in diagnosis, although findings are often non-specific. Of interest, many obese subjects demonstrate high amplitude peristaltic contractions, suggesting a nutcracker esophagus-like syndrome (18), a condition typically associated with pain. Dysphagia may be present as well. Generally, exacerbation of esophageal motility disorders is thought to be more common after gastric banding, which presents a fixed outlet obstruction to the esophagus, unlike gastric bypass, in which tissues distal to the esophagus are relatively more distensible (19). Banded gastric bypass may present similar problems, although dysphagia is more commonly reported than pain (6). As with irritable bowel syndrome, patients with esophageal motility disorders should be carefully counseled regarding possible exacerbation after bariatric surgery. Given the poorly understood pathogenesis of disease and the limited efficacy of surgical therapy for such disorders, we prefer conservative management, including dietary modification and calcium channel blockers if indicated by manometry.
Dumping syndrome is characterized by diverse symptoms including postprandial nausea, dizziness, flushing, and tachycardia. Pain is generally not a dominant symptom, but may be associated with bloating, cramping, and diarrhea that accompany dumping episodes. Symptoms are often precipitated by foods high in refined sugar, but may be caused by other foods as well. The pathogenesis of dumping syndrome is not fully understood and a complete discussion is beyond the scope of this review. Nonetheless, recent data suggest that dumping may be associated with elevated circulating levels of GLP-1 and other gut hormones (20). Other reports implicate an exaggerated post-prandial insulin response and hyperinsulinemic hypoglycemia, possibly due to islet hyperplasia (21), although this latter entity remains rare and poorly understood. Most cases of dumping syndrome are mild and respond to dietary modification (22). Calcium channel antagonists or acarbose may provide relief (23, 24). Debilitating dumping syndrome refractory to conservative management is rare. Revisional surgery has been proposed in such cases, including procedures directed towards delaying pouch emptying and restoring food restriction, such as pouch reduction and anastomotic revision with or without placement of a perianastomic ring or band (25). Endoscopic plication of the gastrojejeunal anastomosis has been also been studied, but efficacy has not yet been clearly demonstrated (26). Partial pancreatectomy for hyperinsulinemic hypoglycemia has been used as a treatment of last resort, with variable efficacy (25).
Biliary disease
Biliary colic is a well-known cause of abdominal pain after gastric bypass. The extreme weight loss after bariatric surgery may contribute to increased bile lithogenicity and is a widely accepted risk factor for cholelithiasis. While the prevalence of cholelithiasis after bariatric surgery may exceed 40% in some series (27-30), the addition of ursodiol prophylaxis in the modern era reduces these prevalence rates to less than 3% (27, 31). The role of concomitant prophylactic cholecystectomy during gastric bypass therefore continues to be debated. Most surgeons do not perform prophylactic cholecystectomy, although as many as 30% do (32). While proponents argue that cholecystectomy is safe (30, 33), most data demonstrate it to be unnecessary from risk-benefit and cost perspectives (29, 34, 35). We believe that cholecystectomy is indicated in bariatric surgery patients only in the context of symptomatic cholelithiasis, just as in non-bariatric patients. In support of this approach, most studies demonstrate a low incidence of progression of cholelithiasis to clinical disease (28). Furthermore, just as in other patients, biliary colic is a herald symptom in the majority of patients after gastric bypass. In addition, surgically induced weight loss likely improves the safety of delayed cholecystectomy if it becomes necessary. Finally, the advent of ursodiol further supports expectant management. Six months of post-operative ursoidol therapy is a commonly used regimen supported by a multi-society consensus statement (36).
Choledocholithiasis and its sequelae, including cholangitis and gallstone pancreatitis, are uncommon causes of abdominal pain after gastric bypass. The availability of specialist expertise determines the preferred therapeutic intervention, and Roux limb anatomy has led to the development of a variety of approaches to access the biliary tree. These include laparoscopic common bile duct exploration, percutaneous transhepatic common bile duct instrumentation, per-oral endoscopic retrograde cholangiopancreatography (ERCP) using specialized endoscopes, percutaneous or laparoscopic transgastric ERCP, and transenteric ERCP (37-39). Once access is achieved, stone extraction and other procedures are performed. Such techniques, while effective, require significant expertise, leaving laparoscopic or open common bile duct exploration as the most reasonable strategy for patients requiring acute intervention.
Sphincter of Oddi dysfunction is a rare cause of abdominal pain after gastric bypass. Secretin stimulated magnetic resonance cholangiopancreatography is used for diagnosis, and surgical transduodenal pancreatic sphincteroplasty is appropriate therapy (40).
Pouch-related disease
A number of problems can affect the gastric pouch after gastric bypass and cause abdominal pain, include ulcer disease, fistula, reflux disease and stenosis.
Ulcer disease
Ulcer disease within the gastric pouch or at the gastrojejunal anastomosis may arise at any point after gastric bypass and occurs in 2-15% of patients (41, 42). Gastrogastric fistula, retained pouch parietal cells, and excessive tension on the anastomosis have been implicated as causes. Gastrogastric fistula was more common in the era of undivided gastric bypass, with reported rates of up to 50% (43, 44). The advent of linear cutting staplers led to universal adoption of divided gastric bypass which greatly reduced the incidence of fistula to current rates of 0-6% (45, 46). Gastrogastric fistulae in the modern era are often the result of a pouch that is incompletely divided from the remnant stomach at initial operation. Gastrogastric fistula causes ulcer disease due to acid from the remnant stomach traversing the fistula into the gastric pouch. Upper gastrointestinal series is the diagnostic study of choice. Visualization of small fistulae by endoscopy may be difficult, and therefore represents a secondary diagnostic test. Fistulae are more often small and a cause of ulcer disease rather than large and a cause of weight regain, and may go unrecognized for long periods in patients with refractory ulcer disease. Recent studies of endoscopic fibrin glue injection demonstrate promise, but surgical revision is usually required (43).
Pouch anatomy has evolved over the past decades. Early gastric pouches were horizontal and fundus-based, allowing for use of the pliable tissues of the fundus to create the anastomosis. The increased compliance of the fundus led to pouches that were large and poorly motile, impairing emptying and contributing to poor weight loss. This led to a transition to “lesser curve-based” pouches, introduced in the early 1980s, in which a long, narrow pouch is created along the lesser curve of the stomach (47). This change in pouch morphology reduced pouch dilation, but led to an increase in anastomotic ulcer disease due to an increase in parietal cells located on the distal lesser curvature that are retained in the gastric pouch. (48). Creation of even smaller, “shorter” (in the cephalad-caudad direction) lesser curve based pouches thus may reduce ulcer disease by eliminating retained pouch parietal cells along the lesser curve (49, 50).
Ulcer disease may occur at any time after gastric bypass, and rarely presents with perforation (51). Pain is the most common presenting symptom, which is generally epigastric, burning in nature, and often immediately post-prandial secondary to passing food irritating the ulcer. Upper endosocopy provides a definitive diagnosis. Ulcer disease usually responds to medical therapy (41). Persistent ulcer disease in the absence of gastrogastric fistula that is non-responsive to medical therapy may be the result of excessive chronic tension on the anastomosis, or a large retained pouch parietal cell mass. Revisional surgery may be necessary in such cases, and consists of resection of the distal ulcerated pouch and revision of the gastrojejunal anastomosis. Lengthening maneuvers may be applied to the Roux limb to reduce tension as necessary, such as lengthening the mesenteric split or conversion to a retrocolic position. Such revisional operations are associated with significant morbidity and should be performed by surgeons with appropriate expertise (52).
Less common causes of ulcer disease include NSAID use, H. pylori infection, and foreign material such as staples or suture. While most clinicians recommend preoperative screening and treatment for H. pylori infection (53, 54), the efficacy of this practice in reducing clinical ulcer disease and its complications is questionable (53, 55). The use of non-absorbable sutures in creating gastrointestinal anastomosis is to be avoided, as this has been associated with an increased risk of anastomotic ulcer (56, 42). Finally, ulcer disease may also affect the remnant stomach or duodenum. Double balloon or shape lock enteroscopy has been used to study the duodenum and remnant stomach (57, 58). Such techniques are not widely performed, however, and medical treatment of suspected ulcer disease of the remnant stomach is therefore often empiric.
Gastroesophageal reflux disease and hiatus hernia
Obesity is an independent risk factor for gastroesophageal reflux disease (GERD). Fortunately, gastric bypass achieves remission of pre-existing GERD in over 85% of patients due to diversion of gastric contents from the gastroesophageal junction (59, 60). Persistent GERD after gastric bypass is generally not as severe as preexisting disease, and is most often attributable to either retained pouch parietal cells or fistula as discussed above. Bile reflux has also been implicated as a cause of GERD after gastric bypass (61). Hiatal hernia contributes to GERD in obese patients before and after gastric bypass. The prevalence of hiatus hernia is increased in obesity, and this anatomic defect may be under-recognized and under-treated during primary gastric bypass. As with primary GERD, medical therapy provides relief in the majority of patients. Elective repair of hiatal hernia has been shown to provide relief of abdominal pain and other GERD symptoms after gastric bypass in small series (62).
Stenosis
Anastomotic stenosis most often presents within three months of surgery with incidences ranging from 3-20%, most often between 5-10% (63, 64). Stenosis is characterized by dysphagia and is not a common cause of pain per se, but may accompany ulcer disease, anastomotic leak, or other pouch pathologies that are associated with pain. Isolated stenosis is rarely a cause of significant abdominal pain and if pain is a dominant symptom, other pathology should be sought. Stenosis appears to be more common after circular stapled gastrojenunal anastomosis (64). UGI series may be equivocal, and endoscopy is the diagnostic test of choice. Endoscopic balloon dilation provides effective therapy in most patients. Rare patients refractory to dilation may require surgical revision.
Small bowel-related disease
Small bowel-related complications that cause abdominal pain after gastric bypass include ventral and trocar site hernias, adhesive disease, internal hernias, and rarely, intussusception.
Incisional hernia, adhesions
While large ventral (incisional) hernias have been eliminated by laparoscopic bariatric surgery, trocar site hernias should always be considered in the patient with abdominal pain after gastric bypass. Trocar site hernias occur at an incidence of 0-1% after gastric bypass (65, 66), although this is likely an underreported entity. CT scan may be necessary to establish the diagnosis of a trocar site hernia in the patient with a thick subcutaneous abdominal wall fat pad. The Roux limb in an antecolic reconstruction lies directly under the abdominal wall and is particularly prone to incarceration in trocar hernias, and a Richter’s configuration may complicate diagnosis. Treatment is generally though a laparoscopic approach. For prevention, we utilize radially dilating trocars and have a low threshold for primary fascial closure of trocar sites.
Adhesive small bowel obstruction is uncommon in the era of laparoscopy, with an incidence of 0.2-1%, but should always be considered as a cause of abdominal pain after gastric bypass (67, 68). The jejunojeunostomy represents a common focus of adhesion formation. Adhesive small bowel obstruction after laparoscopic gastric bypass can often be managed with a laparoscopic approach.
Internal hernia
Internal hernia is an important cause of abdominal pain after gastric bypass with an incidence ranging from 1-9% (69-76). While risk factors are not well-defined, internal hernia is thought to occur most commonly within two to three years after primary gastric bypass, often in the context of significant weight loss. Other reports suggest that pregnancy may predispose to internal hernia, presumably secondary to alterations in intra-abdominal anatomy from the expanding uterus (77).
Types of internal hernia
The majority of Roux limbs in gastric bypass are positioned antecolic, while a minority are retrocolic, retrogastric, and even fewer are retrocolic, antegastric. These various anatomies are associated with different types of internal hernias (Figure 1). Virtually any segment of small intestine can incarcerate in any type of internal hernia, although the biliary limb is often involved, and longer biliary limb lengths may be associated with an increased risk of herniation (70). A mesenteric hernia is created by division of the jejunal mesentery at the jejunojenostomy and is present in all types of reconstruction. A Petersen’s hernia, in an antecolic reconstruction, is bounded by the Roux limb and its mesentery anteriorly/ventrally and the tranverse colon and its mesentery posteriorly/dorsally. In a retrocolic configuration, Petersen’s defect lies between the Roux limb and the colonic mesentery inferior (caudad) to the entry of the Roux limb through the mesocolic defect. A mesocolic hernia traverses the defect in the transverse colon mesentery in a retrocolic reconstruction. In series of retrocolic bypasses, mesocolic hernias comprise the majority of internal hernias (69, 74), for which reason antegastric reconstruction is believed to lead to fewer hernias overall and is the dominant approach (78). Finally, an uncommon site of internal herniation is between the limbs of jejunum distal to the end of the staple line and proximal to the distal stay suture used to create the side-to-side jejunojejunostomy (79). Prevention consists of ensuring that the staple line fully traverses the stay sutures.
Figure 1. Gastric bypass internal hernia anatomy.
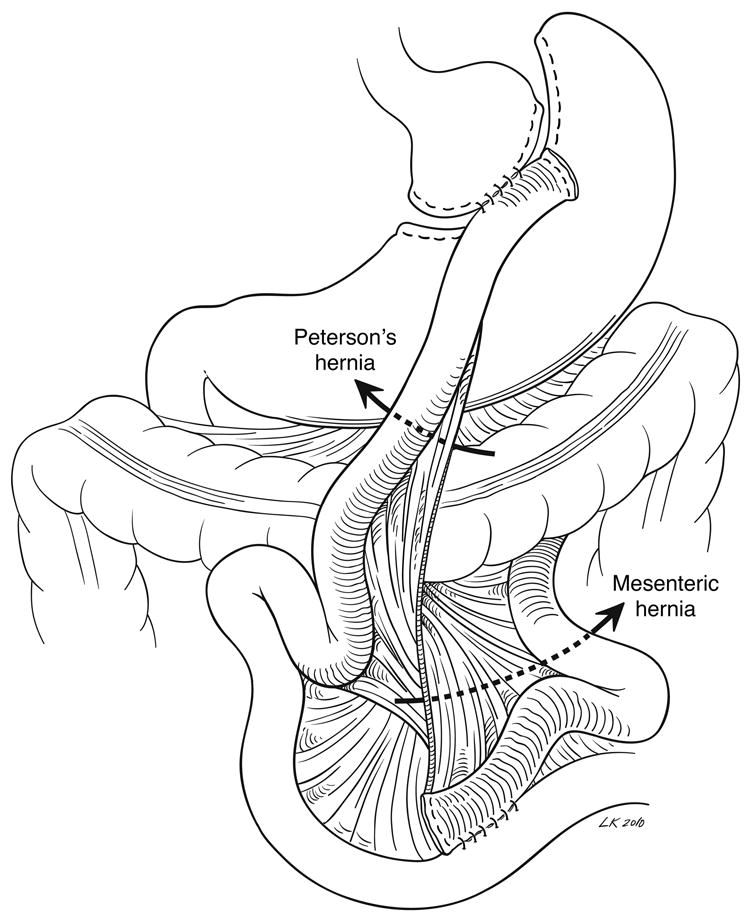
An antecolic Roux limb configuration is shown, with arrows indicating mesenteric and Petersen’s hernias.
Routine closure of internal hernia defects
Much debate surrounds the issue of prophylactic closure of internal hernia defects at the time of primary gastric bypass. The efficacy of such closures is questionable: suture closure of mesenteric fat is tenuous and does not heal, but rather scars or adheses; laparoscopy is associated with fewer adhesions, which may predispose to failure of these closures. Furthermore, with weight loss, previously closed defects may open due to fat loss within the mesentery. While some advocate for routine closure (78), the literature as a whole does not clearly demonstrate a lower incidence of internal hernia with this practice. Series with no closure and others with routine closure report similar incidences ranging from 0.2-9% (72-76). Despite this debate, routine closure is straightforward and associated with low morbidity, and for these reasons, we advocate this practice.
Presentation, diagnosis, treatment of internal hernia
Internal hernia is typically associated with diffuse, episodic, severe abdominal pain which lasts hours and may or may not be postprandial. Pain may continue for months in those who do not seek treatment, but the risk of incarceration is always present. In the case of biliopancreatic limb obstruction, obstipation may not be present, further confusing diagnosis. CT signs of internal hernia have variable diagnostic predictive values, and signs of bowel obstruction may not be present until strangulation is imminent. The “mesenteric swirl sign” has a sensitivity ranging from 78%-100% and specificity 80-90% (80-82) (Figure 2). Other CT findings are less specific and demonstrate poor interobserver agreement. The high frequency of negative imaging may be due to the fact that scans may not be obtained during an episode of incarceration or that incarceration of a short segment of biliary limb may not cause recognizable small bowel dilation. For these reasons, severe abdominal pain in a patient with prior gastric bypass mandates surgical exploration unless a clear alternative diagnosis is established. While the specific technical aspects of surgical management of internal hernia are beyond the scope of this review, reduction of hernias and repair of defects with non-absorbable suture can usually be accomplished through a laparoscopic approach. Incarceration, which in many cases is transient, may not be found at exploration, but closure of defects nonetheless achieves good results with relief of pain in the majority of patients. For example, Gandhi et al. studied over 700 gastric bypass patients over a five year period, 27 (3.8%) of whom developed post-operative small bowel obstruction. All of these patients had a preceding history of abdominal pain, bloating, and nausea. During the final year of the study, elective laparoscopic exploration with closure of defects was offered to 9 patients with similar symptoms, which were subsequently relieved in all patients (83). Agaba et al. studied 1500 patients of whom 75 were suspected of having internal hernia. Exploratory laparoscopy revealed internal hernia in 98% of those with diagnostic CT findings and in 69% of those with a non-diagnostic CT. Of note, laparoscopy revealed a treatable alternative diagnosis in all of the latter patients (84). Finally, in a series of 13 patients who underwent exploratory laparoscopy for pain after gastric bypass, 11 had a treatable cause of pain including internal hernia in four (85). These data support a low threshold for exploration for unexplained abdominal pain after gastric bypass.
Figure 2. Mesenteric swirl sign.
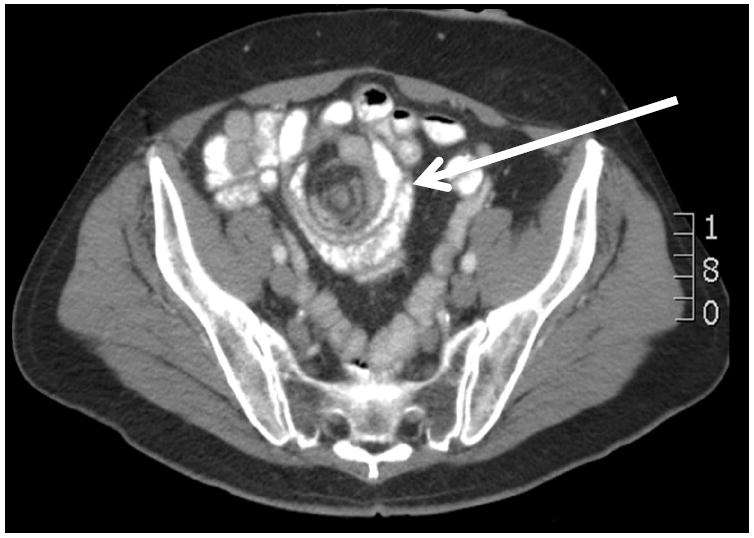
arrow indicates swirl sign
Intussusception
Intussusception is a rare cause of abdominal pain after gastric bypass, occurring with an incidence of approximately 0.1% (86). Intussusception may occur months or years after gastric bypass and is associated with nausea, vomiting, abdominal pain, and bowel obstruction (87). Like internal hernia, intussusception may be transient and chronic. Intussusception is often retrograde with the jejunojejunostomy acting as a lead point and progressing proximally along either the alimentary or biliopancreatic limb. Antegrade intussusception has also been reported (88). Altered migratory motor complexes due to ectopic myoelectric pacemakers in the Roux limb have been implicated as a possible cause (89). Plain abdominal films, gastrointestinal contrast studies, and abdominal ultrasound are unreliable diagnostic tools. CT scan may reveal a “target sign” or dilated excluded stomach, but these findings are relatively insensitive (90). A high level of clinical suspicion in the context of indications for operation, including obstruction or severe pain, should prompt exploration. Strangulation mandates resection. In the absence of strangulation, resection or reduction and plication are accepted approaches with most surgeons favoring the former (87).
Less common culprits
Stenosis of the jejeunojejunostomy may cause abdominal pain, and occurs with an incidence of approximately 0.5% (91). The use of longer (60mm) linear staplers may minimize this complication. While early jejeunojejunostomy stenosis may be due to edema and can often be managed expectantly (92), surgical anastomotic revision may be required in the late post-operative period. Endoscopic dilation using double balloon enteroscopy represents an alternative to surgical revision. Omental torsion and/or infarction is a rarely reported cause of abdominal pain after gastric bypass, usually resulting from the omental division performed in antecolic reconstruction (93, 94). Prevention consists of careful medial division of the omentum and resection of devascularized omentum as necessary. Superior mesenteric artery syndrome has been rarely reported after gastric bypass and may respond to laparoscopic duodenojejunostomy (95). Bezoars of the pouch or elsewhere are a rare cause of abdominal pain and obstruction after gastric bypass (96, 97).
General diagnostic approach, conclusions
Given the broad differential diagnosis in the patient with abdominal pain after gastric bypass, diagnostic algorithms must be flexible and guided by clinical history and physical exam. A careful dietary and food history along with serum chemistries and vitamin levels may reveal behavioral or nutritional causes of pain which are often easily treated. Most patients will require EGD and CT scan, however, which are good initial tests that provide a diagnosis in many cases. If EGD and CT are non-diagnostic, UGI series, ultrasound, and esophageal manometry may be indicated depending on the clinical presentation. More sophisticated endoscopic and laparoscopic-assisted interventions to study the biliary tree or remnant stomach should be considered in patients suspected of having disease in these organ systems. Exploratory laparoscopy should be considered in the face of a negative diagnostic evaluation.
Abdominal pain after gastric bypass is an important public health problem that presents significant diagnostic and therapeutic challenges. Its diverse causes require a broad evaluation that should be directed by history and clinical presentation. In the absence of a clear diagnosis, the threshold for surgical exploration should be low. Finally, an understanding of the pathogenesis of abdominal pain as it relates to operative technique at primary gastric bypass will guide modifications of operative technique and reduce overall postoperative morbidity.
Acknowledgments
Dr O’Rourke is supported by an American Surgical Association Foundation Fellowship Award and National Institutes of Health grant K08 DK074397. We thank Lynn Kitagawa for the creation of Figure 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. JAMA. 2005;294:1909–1917. doi: 10.1001/jama.294.15.1909. [DOI] [PubMed] [Google Scholar]
- 2.Cho M, Kaidar-Person O, Szomstein S, Rosenthal RJ. Emergency room visits after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Surg Obes Relat Dis. 2008;4(2):104–9. doi: 10.1016/j.soard.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Saunders J, Ballantyne GH, Belsley S, Stephens DJ, Trivedi A, Ewing DR, Iannace VA, Capella RF, Wasileweski A, Moran S, Schmidt HJ. One-year readmission rates at a high volume bariatric surgery center: laparoscopic adjustable gastric banding, laparoscopic gastric bypass, and vertical banded gastroplasty-Roux-en-Y gastric bypass. Obes Surg. 2008;18(10):1233–40. doi: 10.1007/s11695-008-9517-8. [DOI] [PubMed] [Google Scholar]
- 4.Kellogg TA, Swan T, Leslie DA, Buchwald H, Ikramuddin S. Patterns of readmission and reoperation within 90 days after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2009;5(4):416–23. doi: 10.1016/j.soard.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Foster A, Richards WO, McDowell J, Laws HL. Clements RH. Gastrointestinal symptoms are more intense in morbidly obese patients. Surg Endosc. 2003;17(11):1766–8. doi: 10.1007/s00464-002-8701-5. [DOI] [PubMed] [Google Scholar]
- 6.Arasaki CH, Del Grande JC, Yanagita ET, Alves AK, Oliveira DR. Incidence of regurgitation after the banded gastric bypass. Obes Surg. 2005;15(10):1408–17. doi: 10.1381/096089205774859209. [DOI] [PubMed] [Google Scholar]
- 7.Walker SP, Wein P, Ihle BU. Severe folate deficiency masquerading as the syndrome of hemolysis, elevated liver enzymes, and low platelets. Obstet Gynecol. 1997;90(4 Pt 2):655–7. doi: 10.1016/s0029-7844(97)00209-3. [DOI] [PubMed] [Google Scholar]
- 8.Decker GA, DiBaise JK, Leighton JA, Swain JM, Crowell MD. Nausea, bloating and abdominal pain in the Roux-en-Y gastric bypass patient: more questions than answers. Obes Surg. 2007;17(11):1529–33. doi: 10.1007/s11695-008-9416-z. [DOI] [PubMed] [Google Scholar]
- 9.Faintuch J, Ishida RK, Jacabi M, Ribeiro AS, Kuga R, Sakai P, Barbeiro HV, Barbeiro DF, Soriano FG, Cecconello I. Increased gastric cytokine production after Roux-en-Y gastric bypass for morbid obesity. Arch Surg. 2007;142(10):962–8. doi: 10.1001/archsurg.142.10.962. [DOI] [PubMed] [Google Scholar]
- 10.Woodard GA, Encarnacion B, Downey JR, Peraza J, Chong K, Hernandez-Boussard T, Morton JM. Probiotics improve outcomes after Roux-en-Y gastric bypass surgery: a prospective randomized trial. J Gastrointest Surg. 2009;13(7):1198–204. doi: 10.1007/s11605-009-0891-x. [DOI] [PubMed] [Google Scholar]
- 11.Ishida RK, Faintuch J, Paula AM, Risttori CA, Silva SN, Gomes ES, Mattar R, Kuga R, Ribeiro AS, Sakai P, Barbeiro HV, Barbeiro DF, Soriano FG, Cecconello I. Microbial flora of the stomach after gastric bypass for morbid obesity. Obes Surg. 2007;17(6):752–8. doi: 10.1007/s11695-007-9139-6. [DOI] [PubMed] [Google Scholar]
- 12.Machado JD, Campos CS, Lopes Dah Silva C, Marques Suen VM, Barbosa Nonino-Borges C, Dos Santos JE, Ceneviva R, Marchini JS. Intestinal bacterial overgrowth after Roux-en-Y gastric bypass. Obes Surg. 2008;18(1):139–43. doi: 10.1007/s11695-007-9365-y. [DOI] [PubMed] [Google Scholar]
- 13.Dallal RM, Cottam D. Candy cane” Roux syndrome--a possible complication after gastric bypass surgery. Surg Obes Relat Dis. 2007;3(3):408–10. doi: 10.1016/j.soard.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Teitelbaum JE, Sinha P, Micale M, Yeung S, Jaeger J. Obesity is related to multiple functional abdominal diseases. J Pediatr. 2009;154(3):444–6. doi: 10.1016/j.jpeds.2008.09.053. [DOI] [PubMed] [Google Scholar]
- 15.Suter M, Dorta G, Giusti V, Calmes JM. Gastro-esophageal reflux and esophageal motility disorders in morbidly obese patients. Obes Surg. 2004;14(7):959–66. doi: 10.1381/0960892041719581. [DOI] [PubMed] [Google Scholar]
- 16.Potoczna N, Harfmann S, Steffen R, Briggs R, Bieri N, Horber FF. Bowel habits after bariatric surgery. Obes Surg. 2008;18(10):1287–96. doi: 10.1007/s11695-008-9456-4. [DOI] [PubMed] [Google Scholar]
- 17.Svedberg P, Johansson S, Wallander MA, Hamelin B, Pedersen NL. Extra-intestinal manifestations associated with irritable bowel syndrome: a twin study. Aliment Pharmacol Ther. 2002;16(5):975–83. doi: 10.1046/j.1365-2036.2002.01254.x. [DOI] [PubMed] [Google Scholar]
- 18.Hong D, Khajanchee YS, Pereira N, Lockhart B, Patterson EJ, Swanstrom LL. Manometric abnormalities and gastroesophageal reflux disease in the morbidly obese. Obes Surg. 2004;14(6):744–9. doi: 10.1381/0960892041590854. [DOI] [PubMed] [Google Scholar]
- 19.Merrouche M, Sabaté JM, Jouet P, Harnois F, Scaringi S, Coffin B, Msika S. Gastroesophageal reflux and esophageal motility disorders in morbidly obese patients before and after bariatric surgery. Obes Surg. 2007;17(7):894–900. doi: 10.1007/s11695-007-9166-3. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto H, Mori T, Tsuchihashi H, Akabori H, Naito H, Tani T. A possible role of GLP-1 in the pathophysiology of early dumping syndrome. Dig Dis Sci. 2005;50(12):2263–7. doi: 10.1007/s10620-005-3046-2. [DOI] [PubMed] [Google Scholar]
- 21.Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353(3):249–54. doi: 10.1056/NEJMoa043690. [DOI] [PubMed] [Google Scholar]
- 22.Kellogg TA, Bantle JP, Leslie DB, Redmond JB, Slusarek B, Swan T, Buchwald H, Ikramuddin S. Postgastric bypass hyperinsulinemic hypoglycemia syndrome: characterization and response to a modified diet. Surg Obes Relat Dis. 2008;4(4):492–9. doi: 10.1016/j.soard.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Moreira RO, Moreira RB, Machado NA, Gonçalves TB, Coutinho WF. Post-prandial hypoglycemia after bariatric surgery: pharmacological treatment with verapamil and acarbose. Obes Surg. 2008;18(12):1618–21. doi: 10.1007/s11695-008-9569-9. [DOI] [PubMed] [Google Scholar]
- 24.Guseva N, Phillips D, Mordes JP. Successful treatment of persistent hyperinsulinemic hypoglycemia with nifedipine in an adult patient. Endocr Pract. 2010;16(1):107–11. doi: 10.4158/EP09110.CRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Z’graggen K, Guweidhi A, Steffen R, Potoczna N, Biral R, Walther F, Komminoth P, Horber F. Severe recurrent hypoglycemia after gastric bypass surgery. Obes Surg. 2008;18(8):981–8. doi: 10.1007/s11695-008-9480-4. [DOI] [PubMed] [Google Scholar]
- 26.Mikami D, Needleman B, Narula V, Durant J, Melvin WS. Natural orifice surgery: initial US experience utilizing the StomaphyX device to reduce gastric pouches after Roux-en-Y gastric bypass. Surg Endosc. 2010;24(1):223–8. doi: 10.1007/s00464-009-0640-y. [DOI] [PubMed] [Google Scholar]
- 27.Wudel LJ, Jr, Wright JK, Debelak JP, Allos TM, Shyr Y, Chapman WC. Prevention of gallstone formation in morbidly obese patients undergoing rapid weight loss: results of a randomized controlled pilot study. J Surg Res. 2002;102(1):50–6. doi: 10.1006/jsre.2001.6322. [DOI] [PubMed] [Google Scholar]
- 28.Swartz DE, Felix EL. Elective cholecystectomy after Roux-en-Y gastric bypass: why should asymptomatic gallstones be treated differently in morbidly obese patients? Surg Obes Relat Dis. 2005;1:555–560. doi: 10.1016/j.soard.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Portenier DD, Grant JP, Blackwood HS, Pryor A, McMahon RL, DeMaria E. Expectant management of the asymptomatic gallbladder at Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2007;3(4):476–9. doi: 10.1016/j.soard.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Tucker ON, Fajnwaks P, Szomstein S, Rosenthal RJ. Is concomitant cholecystectomy necessary in obese patients undergoing laparoscopic gastric bypass surgery? Surg Endosc. 2008;22(11):2450–4. doi: 10.1007/s00464-008-9769-3. [DOI] [PubMed] [Google Scholar]
- 31.Miller K, Hell E, Lang B, Lengauer E. Gallstone formation prophylaxis after gastric restrictive procedures for weight loss: a randomized double-blind placebo-controlled trial. Ann Surg. 2003;238:697–702. doi: 10.1097/01.sla.0000094305.77843.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mason EE, Renquist KE. Gallbladder management in obesity surgery. Obes Surg. 2002;12(2):222–9. doi: 10.1381/096089202762552395. [DOI] [PubMed] [Google Scholar]
- 33.Nougou A, Suter M. Almost routine prophylactic cholecystectomy during laparoscopic gastric bypass is safe. Obes Surg. 2008;18(5):535–9. doi: 10.1007/s11695-007-9368-8. [DOI] [PubMed] [Google Scholar]
- 34.Patel KR, White SC, Tejirian T, Han SH, Russell D, Vira D, Liao L, Patel KB, Gracia C, Haigh P, Dutson E, Mehran A. Gallbladder management during laparoscopic Roux-en-Y gastric bypass surgery: routine preoperative screening for gallstones and postoperative prophylactic medical treatment are not necessary. Am Surg. 2006;72(10):857–61. [PubMed] [Google Scholar]
- 35.Fuller W, Rasmussen JJ, Ghosh J, Ali MR. Is routine cholecystectomy indicated for asymptomatic cholelithiasis in patients undergoing gastric bypass? Obes Surg. 2007;17(6):747–51. doi: 10.1007/s11695-007-9138-7. [DOI] [PubMed] [Google Scholar]
- 36.Mechanick JI, Kushner RF, Sugerman HJ, et al. American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Obesity. 2009;17(Suppl 1):S1–70. v. doi: 10.1038/oby.2009.28. [DOI] [PubMed] [Google Scholar]
- 37.Paganini AM, Guerrieri M, Sarnari J, et al. Thirteen years’ experience with laparoscopic transcystic common bile duct exploration for stones. Effectiveness and long-term results. Surg Endosc. 2007;21:34–40. doi: 10.1007/s00464-005-0286-3. [DOI] [PubMed] [Google Scholar]
- 38.Ahmed AR, Husain S, Saad N, et al. Accessing the common bile duct after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2007;3:640–643. doi: 10.1016/j.soard.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Peeters G, Himpens J. Laparoscopically assisted transgastric endoscopy in Roux-en-Y gastric bypass: a modification of the technique. Endoscopy. 2009;41(Suppl 2):E190. doi: 10.1055/s-0029-1214772. [DOI] [PubMed] [Google Scholar]
- 40.Morgan KA, Glenn JB, Byrne TK, Adams DB. Sphincter of Oddi dysfunction after Rouxen-Y gastric bypass. Surg Obes Relat Dis. 2009;5(5):571–5. doi: 10.1016/j.soard.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 41.Rasmussen JJ, Fuller W, Ali MR. Marginal ulceration after laparoscopic gastric bypass: an analysis of predisposing factors in 260 patients. Surg Endosc. 2007;21(7):1090–4. doi: 10.1007/s00464-007-9285-x. [DOI] [PubMed] [Google Scholar]
- 42.Vasquez JC, Wayne Overby D, Farrell TM. Fewer gastrojejunostomy strictures and marginal ulcers with absorbable suture. Surg Endosc. 2009;23(9):2011–5. doi: 10.1007/s00464-008-0220-6. [DOI] [PubMed] [Google Scholar]
- 43.Stanczyk M, Deveney CW, Traxler SA, McConnell DB, Jobe BA, O’Rourke RW. Gastrogastric Fistula in the Era of Divided Roux-en-Y Gastric Bypass: Strategies for Prevention, Diagnosis, and Management. Obes Surg. 2006;16(3):359–364. doi: 10.1381/096089206776116426. [DOI] [PubMed] [Google Scholar]
- 44.Podnos YD, Jimenez JC, Wilson SE, et al. Complications after laparoscopic gastric bypass: a review of 3464 cases. Arch Surg. 2003;138:957–61. doi: 10.1001/archsurg.138.9.957. [DOI] [PubMed] [Google Scholar]
- 45.Capella JF, Capella RF. Gastro-gastric fistulas and marginal ulcers in gastric bypass procedures for weight reduction. Obes Surg. 1999;9:22–7. doi: 10.1381/096089299765553674. [DOI] [PubMed] [Google Scholar]
- 46.Carrodeguas L, Szomstein S, Soto F, et al. Management of gastrogastric fistula after divided Roux-en-Y gastric bypass surgery for morbid obesity: analysis of 1292 consecutive patients and review of literature. Surg Obes Relat Dis. 2005;1:467–74. doi: 10.1016/j.soard.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 47.Torres JC, Oca CF, Garrison RN. Gastric bypass: Roux-en-Y gastrojejunostomy from the lesser curvature. South Med J. 1983;76:1217–1221. [PubMed] [Google Scholar]
- 48.Siilin H, Wanders A, Gustavsson S, Sundbom M. The proximal gastric pouch invariably contains acid-producing parietal cells in Roux-en-Y gastric bypass. Obes Surg. 2005;15:771–777. doi: 10.1381/0960892054222849. [DOI] [PubMed] [Google Scholar]
- 49.Behrns KE, Smith CD, Kelly KA, Sarr MG. Reoperative bariatric surgery. Lessons learned to improve patient selection and results. Ann Surg. 1993;218:646–653. doi: 10.1097/00000658-199321850-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sapala JA, Wood MH, Sapala MA, et al. The micropouch gastric bypass: technical considerations in primary and revisionary operations. Obes Surg. 2001;11:3–17. doi: 10.1381/096089201321454042. [DOI] [PubMed] [Google Scholar]
- 51.Chin EH, Hazzan D, Sarpel U, Herron DM. Multimedia article. Laparoscopic repair of a perforated marginal ulcer 2 years after gastric bypass. Surg Endosc. 2007;21(11):2110. doi: 10.1007/s00464-007-9486-3. [DOI] [PubMed] [Google Scholar]
- 52.Coakley BA, Deveney CW, Spight DH, Jobe BA, Thompson SK, Le D, Wolfe BM, McConnell DB, O’Rourke RW. Revisional surgery for failed restrictive bariatric operations. Surg Obes Relat Dis. 2008;4(5):581–6. doi: 10.1016/j.soard.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 53.Hartin CW, Jr, Remine DS, Lucktong TA. Preoperative bariatric screening and treatment of Helicobacter pylori. Surg Endosc. 2009 May 15; doi: 10.1007/s00464-009-0449-8. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 54.de Moura Almeida A, Cotrim HP, Santos AS, Bitencourt AG, Barbosa DB, Lobo AP, Rios A, Alves E. Preoperative upper gastrointestinal endoscopy in obese patients undergoing bariatric surgery: is it necessary? Surg Obes Relat Dis. 2008;4(2):144–9. doi: 10.1016/j.soard.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 55.Papasavas PK, Gagné DJ, Donnelly PE, Salgado J, Urbandt JE, Burton KK, Caushaj PF. Prevalence of Helicobacter pylori infection and value of preoperative testing and treatment in patients undergoing laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2008;4(3):383–8. doi: 10.1016/j.soard.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 56.Sacks BC, Mattar SG, Qureshi FG, Eid GM, Collins JL, Barinas-Mitchell EJ, Schauer PR, Ramanathan RC. Incidence of marginal ulcers and the use of absorbable anastomotic sutures in laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2006;2(1):11–6. doi: 10.1016/j.soard.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 57.Pai RD, Carr-Locke DL, Thompson CC. Endoscopic evaluation of the defunctionalized stomach by using ShapeLock technology (with video) Gastrointest Endosc. 2007;66(3):578–81. doi: 10.1016/j.gie.2007.02.062. [DOI] [PubMed] [Google Scholar]
- 58.Baron TH. Double-balloon enteroscopy to facilitate retrograde PEG placement as access for therapeutic ERCP in patients with long-limb gastric bypass. Gastrointest Endosc. 2006;64:973–974. doi: 10.1016/j.gie.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 59.Madalosso CA, Gurski RR, Callegari-Jacques SM, Navarini D, Thiesen V, Fornari F. The impact of gastric bypass on gastroesophageal reflux disease in patients with morbid obesity: a prospective study based on the Montreal Consensus. Ann Surg. 2010 Feb;251(2):244–8. doi: 10.1097/SLA.0b013e3181bdff20. [DOI] [PubMed] [Google Scholar]
- 60.De Groot NL, Burgerhart JS, Van De Meeberg PC, de Vries DR, Smout AJ, Siersema PD. Systematic review: the effects of conservative and surgical treatment for obesity on gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2009;30(11-12):1091–102. doi: 10.1111/j.1365-2036.2009.04146.x. [DOI] [PubMed] [Google Scholar]
- 61.Swartz DE, Mobley E, Felix EL. Bile reflux after Roux-en-Y gastric bypass: an unrecognized cause of postoperative pain. Surg Obes Relat Dis. 2009;5:27–30. doi: 10.1016/j.soard.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 62.Flanagin BA, Mitchell MT, Thistlethwaite WA, Alverdy JC. Diagnosis and treatment of atypical presentations of hiatal hernia following bariatric surgery. Obes Surg. 2010;20(3):386–92. doi: 10.1007/s11695-009-0013-6. [DOI] [PubMed] [Google Scholar]
- 63.Mathew A, Veliuona MA, DePalma FJ, Cooney RN. Gastrojejunal stricture after gastric bypass and efficacy of endoscopic intervention. Dig Dis Sci. 2009;54(9):1971–8. doi: 10.1007/s10620-008-0581-7. [DOI] [PubMed] [Google Scholar]
- 64.Suggs WJ, Kouli W, Lupovici M, Chau WY, Brolin RE. Complications at gastrojejunostomy after laparoscopic Roux-en-Y gastric bypass: comparison between 21- and 25-mm circular staplers. Surg Obes Relat Dis. 2007;3(5):508–14. doi: 10.1016/j.soard.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 65.Chiu CC, Lee WJ, Wang W, Wei PL, Huang MT. Prevention of trocar-wound hernia in laparoscopic bariatric operations. Obes Surg. 2006;16(7):913–8. doi: 10.1381/096089206777822269. [DOI] [PubMed] [Google Scholar]
- 66.Johnson WH, Fecher AM, McMahon RL, Grant JP, Pryor AD. VersaStep trocar hernia rate in unclosed fascial defects in bariatric patients. Surg Endosc. 2006;20(10):1584–6. doi: 10.1007/s00464-005-0747-8. [DOI] [PubMed] [Google Scholar]
- 67.Husain S, Ahmed AR, Johnson J, Boss T, O’Malley W. Small-bowel obstruction after laparoscopic Roux-en-Y gastric bypass: etiology, diagnosis, and management. Arch Surg. 2007;142(10):988–93. doi: 10.1001/archsurg.142.10.988. [DOI] [PubMed] [Google Scholar]
- 68.Gunabushanam G, Shankar S, Czerniach DR, Kelly JJ, Perugini RA. Small-bowel obstruction after laparoscopic Roux-en-Y gastric bypass surgery. J Comput Assist Tomogr. 2009;33(3):369–75. doi: 10.1097/RCT.0b013e31818803ac. [DOI] [PubMed] [Google Scholar]
- 69.Higa KD, Ho T, Boone KB. Internal hernias after laparoscopic Roux-en-Y gastric bypass: incidence, treatment and prevention. Obes Surg. 2003;13:350–354. doi: 10.1381/096089203765887642. [DOI] [PubMed] [Google Scholar]
- 70.Bauman RW, Pirrello JR. Internal hernia at Petersen’s space after laparoscopic Rouxen-Y gastric bypass: 6.2% incidence without closure--a single surgeon series of 1047 cases. Surg Obes Relat Dis. 2009;5:565–570. doi: 10.1016/j.soard.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 71.Schweitzer MA, DeMaria EJ, Broderick TJ, Sugerman HJ. Laparoscopic closure of mesenteric defects after Roux-en-Y gastric bypass. J Laparoendosc Adv Surg Tech A. 2000;10:173–175. doi: 10.1089/lap.2000.10.173. [DOI] [PubMed] [Google Scholar]
- 72.Paroz A, Calmes JM, Giusti V, Suter M. Internal hernia after laparoscopic Roux-en-Y gastric bypass for morbid obesity: a continuous challenge in bariatric surgery. Obes Surg. 2006;16:1482–1487. doi: 10.1381/096089206778870102. [DOI] [PubMed] [Google Scholar]
- 73.Garza E, Jr, Kuhn J, Arnold D, et al. Internal hernias after laparoscopic Roux-en-Y gastric bypass. Am J Surg. 2004;188:796–800. doi: 10.1016/j.amjsurg.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 74.Champion JK, Williams M. Small bowel obstruction and internal hernias after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2003;13:596–600. doi: 10.1381/096089203322190808. [DOI] [PubMed] [Google Scholar]
- 75.Carmody B, DeMaria EJ, Jamal M, et al. Internal hernia after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2005;1:543–548. doi: 10.1016/j.soard.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 76.Cho M, Pinto D, Carrodeguas L, et al. Frequency and management of internal hernias after laparoscopic antecolic antegastric Roux-en-Y gastric bypass without division of the small bowel mesentery or closure of mesenteric defects: review of 1400 consecutive cases. Surg Obes Relat Dis. 2006;2:87–91. doi: 10.1016/j.soard.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 77.Torres-Villalobos GM, Kellogg TA, Leslie DB, Antanavicius G, Andrade RS, Slusarek B, Prosen TL, Ikramuddin S. Small bowel obstruction and internal hernias during pregnancy after gastric bypass surgery. Obes Surg. 2009;19(7):944–50. doi: 10.1007/s11695-008-9681-x. [DOI] [PubMed] [Google Scholar]
- 78.Steele KE, Prokopowicz GP, Magnuson T, et al. Laparoscopic antecolic Roux-en-Y gastric bypass with closure of internal defects leads to fewer internal hernias than the retrocolic approach. Surg Endosc. 2008;22:2056–2061. doi: 10.1007/s00464-008-9749-7. [DOI] [PubMed] [Google Scholar]
- 79.Paroz A, Calmes JM, Romy S, et al. A new type of internal hernia after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2009;19:527–530. doi: 10.1007/s11695-008-9770-x. [DOI] [PubMed] [Google Scholar]
- 80.Patel RY, Baer JW, Texeira J, Frager D, Cooke K. Internal hernia complications of gastric bypass surgery in the acute setting: spectrum of imaging findings. Emerg Radiol. 2009;16(4):283–9. doi: 10.1007/s10140-008-0781-7. [DOI] [PubMed] [Google Scholar]
- 81.Iannuccilli JD, Grand D, Murphy BL, et al. Sensitivity and specificity of eight CT signs in the preoperative diagnosis of internal mesenteric hernia following Roux-en-Y gastric bypass surgery. Clin Radiol. 2009;64:373–380. doi: 10.1016/j.crad.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 82.Lockhart ME, Tessler FN, Canon CL, et al. Internal hernia after gastric bypass: sensitivity and specificity of seven CT signs with surgical correlation and controls. AJR Am J Roentgenol. 2007;188:745–750. doi: 10.2214/AJR.06.0541. [DOI] [PubMed] [Google Scholar]
- 83.Gandhi AD, Patel RA, Brolin RE. Elective laparoscopy for herald symptoms of mesenteric/internal hernia after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2009;5(2):144–9. doi: 10.1016/j.soard.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 84.Agaba EA, Gentles CV, Shamseddeen H, Sasthakonar V, Kandel A, Gadelata D, Gellman L. Retrospective analysis of abdominal pain in postoperative laparoscopic Roux-en-Y gastric bypass patients: is a simple algorithm the answer? Surg Obes Relat Dis. 2008;4(5):587–93. doi: 10.1016/j.soard.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 85.Pitt T, Brethauer S, Sherman V, Udomsawaengsup S, Metz M, Chikunguwo S, Chand B, Schauer P. Diagnostic laparoscopy for chronic abdominal pain after gastric bypass. Surg Obes Relat Dis. 2008 May-Jun;4(3):394–8. doi: 10.1016/j.soard.2007.12.011. discussion 398. Epub 2008 Apr 14. [DOI] [PubMed] [Google Scholar]
- 86.Gunabushanam G, Shankar S, Czerniach DR, Kelly JJ, Perugini RA. Small-bowel obstruction after laparoscopic Roux-en-Y gastric bypass surgery. J Comput Assist Tomogr. 2009;33(3):369–75. doi: 10.1097/RCT.0b013e31818803ac. [DOI] [PubMed] [Google Scholar]
- 87.Coster DD, Sundberg SM, Kermode DS, et al. Small bowel obstruction due to antegrade and retrograde intussusception after gastric bypass: three case reports in two patients, literature review, and recommendations for diagnosis and treatment. Surg Obes Relat Dis. 2008;4:69–72. doi: 10.1016/j.soard.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 88.Zainabadi K, Ramanathan R. Intussusception after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2007;17:1619–1623. doi: 10.1007/s11695-007-9291-z. [DOI] [PubMed] [Google Scholar]
- 89.Hocking MP, McCoy DM, Vogel SB, et al. Antiperistaltic and isoperistaltic intussusception associated with abnormal motility after Roux-en-Y gastric bypass: a case report. Surgery. 1991;110:109–112. [PubMed] [Google Scholar]
- 90.Srikanth MS, Keskey T, Fox SR, et al. Computed tomography patterns in small bowel obstruction after open distal gastric bypass. Obes Surg. 2004;14:811–822. doi: 10.1381/0960892041590971. [DOI] [PubMed] [Google Scholar]
- 91.Cho M, Carrodeguas L, Pinto D, Lascano C, Soto F, Whipple O, Gordon R, Simpfendorfer C, Gonzalvo JP, Szomstein S, Rosenthal RJ. Diagnosis and management of partial small bowel obstruction after laparoscopic antecolic antegastric Roux-en-Y gastric bypass for morbid obesity. J Am Coll Surg. 2006;202(2):262–8. doi: 10.1016/j.jamcollsurg.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 92.Lewis CE, Jensen C, Tejirian T, Dutson E, Mehran A. Early jejunojejunostomy obstruction after laparoscopic gastric bypass: case series and treatment algorithm. Surg Obes Relat Dis. 2009;5(2):203–7. doi: 10.1016/j.soard.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 93.Bestman TJ, Valk JW, Gypen B, Declercq S, Hendrickx L. An unusual complication after Roux-en-Y gastric bypass: torsion and infarction of the divided omentum. Obes Surg. 2009;19(12):1731–3. doi: 10.1007/s11695-008-9716-3. [DOI] [PubMed] [Google Scholar]
- 94.Dallal RM, Bailey LA. Omental infarction: a cause of acute abdominal pain after antecolic gastric bypass. Surg Obes Relat Dis. 2006;2(4):451–4. doi: 10.1016/j.soard.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 95.Goitein D, Gagné DJ, Papasavas PK, Dallal R, Quebbemann B, Eichinger JK, Johnston D, Caushaj PF. Superior mesenteric artery syndrome after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Obes Surg. 2004;14(7):1008–11. doi: 10.1381/0960892041719626. [DOI] [PubMed] [Google Scholar]
- 96.Pratt JS, Van Noord M, Christison-Lagay E. The tethered bezoar as a delayed complication of laparoscopic Roux-en-Y gastric bypass: a case report. J Gastrointest Surg. 2007;11(5):690–2. doi: 10.1007/s11605-007-0098-y. [DOI] [PubMed] [Google Scholar]
- 97.Steele K, Schweitzer M, Lidor A, Magnuson T. Unusual case of gastric bezoar causing obstruction after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2006;2(5):536–7. doi: 10.1016/j.soard.2006.05.008. [DOI] [PubMed] [Google Scholar]


