Abstract
We examined the temporal relationships between smoking frequency and craving and withdrawal. 351 heavy smokers (≥15 cigarettes per day) used ecological momentary assessment and electronic diaries to track smoking, craving, negative affect, arousal, restlessness, and attention disturbance in real time over 16 days. The waking day was divided into 8 2-hour “bins” during which cigarette counts and mean levels of craving and withdrawal were computed. Cross-sectional analyses showed no association between restlessness and smoking, and arousal and smoking, but craving (b=0.65, p<0.01) was positively associated, and negative affect (b=-0.20, p<0.01), and attention disturbance (b=-0.24, p<0.01) were inversely associated with smoking. In prospective lagged analyses, higher craving predicted more subsequent smoking and higher smoking predicted lower craving (p's < 0.01). Higher restlessness also predicted more subsequent smoking and higher smoking predicted lower restlessness (p's < 0.01). Higher negative affect did not predict later smoking, but more smoking preceded lower negative affect (p<0.01). Neither attention disturbance nor arousal predicted, or were predicted by variations in smoking. In short, smoking exhibits time-lagged, reciprocal relationships with craving and restlessness, and a one-way predictive relationship with negative affect. Temporal patterns of craving and restlessness may aid in the design of smoking cessation interventions.
Keywords: Smoking, Withdrawal, Craving, Negative Affect, Cycle, Lag
1. Introduction
Previous studies have demonstrated systematic, temporal variations in cigarette consumption during the waking day. In addition to evidence from a series of early laboratory studies (Ray et al., 1982; Nellis et al., 1982), we (Chandra et al., 2007) recently demonstrated circadian variations in smoking behavior with Ecological Momentary Assessments (EMA) -- smoking data collected in real-time and in real-world settings (Stone and Shiffman, 1994; Shiffman et al, 2008). Our work identified several distinct temporal patterns of smoking related to stable, participant characteristics (e.g., demographics, history of depression) and that also prospectively predicted relapse risk. Several dynamic factors have been hypothesized to maintain systematic patterns in daily smoking (e.g., pharmacokinetics and pharmacodynamics, environmental smoking restrictions, exposure to smoking cues; Gries, Benowitz, and Verotta, 1996; Emurian et al., 1982; Pederson et al., 1993; Chandra et al., 2007). Few of these potential influences however, have been systematically studied. One should expect patterns of smoking to relate dynamically to complementary patterns of craving and withdrawal symptoms. That is, periods of the day when craving is high should be followed by periods of increased smoking. Conversely, periods of increased smoking should be followed by periods of decreased craving, with similar patterns for nicotine withdrawal symptoms. In this study, we explore whether patterns of smoking behavior are related to circadian patterns of nicotine craving and nicotine withdrawal symptoms including negative affect, restlessness, arousal and attention disturbance. Specifically, we examine these lead and lag relationships between smoking and patterns of craving and withdrawal symptoms.
Studies have identified systematic daily patterns in craving that might influence or maintain patterns of daily smoking. During ad lib smoking, craving is typically highest in the morning, lowest midday, and in some studies, elevated again in the evening hours (e.g., Perkins et al., 2009; Dunbar et al., 2010). Craving has also been shown to be reliably generated by smoking deprivation (Giomeni et al., 2002; Jarvik et al., 2000; Schuh and Stitzer, 1995; Teneggi et al., 2002), and smoking reliably relieves craving in the laboratory (e.g., Donny et al., 2007). Despite these craving-smoking links (Shiffman et al, 2002), particularly in the context of relapse (e.g., Shiffman et al, 1996; Zhou et al., 2009), some researchers have questioned the importance of craving for understanding smoking patterns, for example, because the reciprocal relationship (i.e., craving predicting or leading to smoking) is unreliable (Tiffany, 1990). Indeed, smoking can occur in the absence of craving (Shiffman et al., 2002; Dunbar et al., 2010). Conflicting findings about the importance of craving in maintaining smoking behavior make it important to understand how craving and smoking relate to each other during ad lib smoking.
A potential, related influence on smoking patterns is symptoms of nicotine withdrawal. Nicotine withdrawal is marked primarily by negative affect (or NA; APA, 2000), including tension or anxiety, irritability, and depressed affect. Like craving, previous studies have also demonstrated systematic, daily patterns in withdrawal symptoms that might influence or maintain patterns in daily smoking (e.g., Shiffman, 1979; Shiffman et al., 1996; Teneggi et al., 2002; Perkins et al., 2009). Studies of negative affect have generally shown daily cycles, with the most negative affect in the morning and improvement in mood throughout the day (although other patterns have been reported; Rusting and Larsen, 1998). Some studies of negative affect in smokers have even shown that smokers report a pattern of repetitive fluctuations in affect related to smoking, such that negative affect increases in periods between cigarettes (O'Neill and Parrott, 1992; Parrott et al., 1995) suggesting systematic, lagged co-variation between smoking and negative affect, consistent with the nicotine regulation model of smoking.
Yet, analyses of real-time data have generally not shown smoking occasions to be associated with negative affect states at the time of smoking (Carter et al., 2008; Shiffman et al., 2002; Shiffman et al., 2004b; see also Kassel et al., 2003 for a review). This may be because such instantaneous measures, right at the time of smoking are muddied by smokers' affective reactions in anticipation of enjoying a cigarette. Indeed, nicotine withdrawal symptoms and craving are usually assessed during abstinence, and contemporary models of smoking suggest that more subtle withdrawal reactions occur during the natural ebb and flow of ad lib smoking, and help maintain smoking, especially in an era of smoking restrictions. Research that addresses the relationship between negative affect and smoking in participants' natural environments (that include time spent in environments where smoking is both restricted and permitted) is needed to resolve these conflicting findings. Restlessness is also a nicotine withdrawal symptom (APA, 2000), but seems to represent a distinct phenomenon (Shiffman et al, 1996, 2002) that is uniquely related to ad lib smoking, even when NA has been accounted for (Shiffman et al., 2002). Accordingly, we analyzed both NA and restlessness as separate symptoms of nicotine withdrawal.
The overall aim of this study was to characterize the dynamic relationship between craving, symptoms of nicotine withdrawal, and smoking, in temporal patterns seen during ad lib smoking. To do this, we analyzed EMA data on craving, nicotine withdrawal symptoms (negative affect, restlessness, arousal and attention disturbance), and smoking, taken in real-time and in participants' natural environments, with a novel analytic model in which we examined both contemporaneous and prospective relationships among these variables over successive 2-hour time-blocks during smokers' waking day. We conceptualized 2-h time blocks as a “middle-ground” between other common units of analysis, such as entire days, which may blur temporal dynamics, and momentary assessments, which may focus too narrowly on the moment the smoker lights up. In addition to approximating the elimination half-life of nicotine (Le Houezec, 2003), 2-h intervals represented an even division of a typical, 16-h waking day that were able to reveal meaningful temporal variations in cigarette consumption (Chandra et al., 2007). We first analyzed data cross-sectionally (i.e., assessing how smoking, craving and nicotine withdrawal symptoms correlate within each time block), but recognize that such cross-sectional analyses do not establish temporal priority and thus can confound cause and effect. Accordingly, we also analyzed the data prospectively, adopting the approach of Granger's Analysis of Precedence (Granger, 1969), which examines, e.g., how craving at time 1 predicts smoking at time 2, while controlling for smoking at time 1. While the results of such analyses are not regarded as firm proof of causality, Granger analyses are regarded as strong indicators of potentially causal relationships (Hacker and Hatemi-J, 2006). We hypothesized reciprocal relationships between craving, nicotine withdrawal symptoms and smoking such that increased craving and withdrawal symptoms would drive later increased smoking, and increased smoking would drive later decreased craving and withdrawal symptoms.
2. Methods
2.1 Participants
Participants in the study were 412 adult men and women between the ages of 21 and 65 (mean age 39.3±9) recruited through local media advertisements for a research-based smoking cessation program (Table 1). Inclusion criteria were: smoking rate ≥ 15 cigarettes per day for ≥ 5 years; self-reported good health; and high motivation and confidence to quit smoking (defined as a total of ≥ 150 on the sum of two 100 point scales). Exclusion criteria were: regular use of non-cigarette forms of tobacco; weight < 110 lbs or 50 kg; specific medical counter-indications to nicotine patch use (e.g., uncontrolled hypertension; allergy to adhesives); serious medical illness; history of recent alcohol/drug abuse or mental illness; current participation in a smoking cessation clinic or study; recent (in the last 30 days) participation in a clinical trial for smoking cessation; or use of bupropion hydrochloride within the past two months. Women who were pregnant, planning to become pregnant, or breast-feeding were also excluded. Eligible smokers who also passed a medical screening, and who signed an informed consent form were enrolled. Overall, study participants smoked an average of 24±9 cigarettes per day and had smoked on average for 22±10 years. Data were collected between 1995 and 2000 in Pittsburgh, Pennsylvania. During this time, state law prohibited smoking in government and private work sites, restaurants, and other work sites (Shelton et al., 1995). The study was approved by the University of Pittsburgh Institutional Review Board. Clinical outcomes and other findings from the cessation phase of this study have been reported elsewhere (Shiffman et al., 2006; Ferguson et al, 2009; Chandra et al., 2007). After data cleaning and processing, described in Section 2.6 below, data for 351 subjects were used in the analysis.
Table 1. Participant Characteristics*.
| Baseline Characteristics | |
|---|---|
| N | 351 |
| Demographics | |
| Age | 39.5 (9.5) |
| Female (%) | 51.3 |
| Ethnicity (% Caucasian) | 84.9 |
| Education (% with some college) | 64.4 |
| Income (% households > $30,000) | 57.2 |
| Smoking Characteristics | |
| Cigarettes per day | 24.1 (9.2) |
| Years smoked | 22.0 (9.7) |
| Breath CO (ppm) | 37.69 (15.12) |
| Salivary cotinine (ng/mL) | 314.36 (157.09)** |
| FTND | 5.9 (2.0)† |
| FTND Range | [1,10] |
| NDSS total NDSS Range |
-0.01 (0.9) [-2.007,2.521] |
Means, with standard deviations in parentheses, unless otherwise indicated
n=314
n=304
2.2 Procedure
Upon entering the study, participants completed a battery of questionnaires (described below) and were instructed in how to use the Electronic Diary (ED). Participants were instructed to continue smoking ad libitum, without changing their smoking frequency or pattern. The EMA protocol followed the methods described for a prior study (Shiffman et al., 2002). Participants were directed to use the ED for 16 days to record each cigarette smoked before smoking it. Both debriefing and quantitative analysis indicated that recording each cigarette before smoking it did not affect the number of cigarettes smoked (Shiffman et al, 2002). Also, the number of cigarettes recorded before smoking was generally slightly less than that later recalled, suggesting that it was unlikely that cigarettes were foregone as a result of the recording process. Finally, in a study of identical design (Shiffman, 2009), analysis of biochemical measures, including CO and cotinine, validated recording of cigarettes. Changes in CO were strongly correlated with changes in cigarette entries, and CO levels correlated particularly with recent cigarette entries, indicating timely recording of cigarettes. A random selection of cigarette entries (approximately 5/day) was followed by detailed assessments. Each evening, ED asked subjects how many cigarettes they had smoked without recording them. In earlier studies, Shiffman (Shiffman et al., 2002b; Shiffman, 2009) validated self-monitoring of smoking using biochemical measures as well as time-line follow-back self-report measures. Although participants were preparing to quit smoking, smoking rates were almost completely unchanged from the first day of observation until the Target Quit Day (TQD) (overall reduction in daily smoking = 0.07 cigarettes per day; Dunbar et al., 2007).
In addition to recording cigarettes, participants were also prompted audibly by the ED 4-5 times daily to complete an assessment while they were not smoking. These assessments were identical to the ones made after cigarette entries. The timing of the prompts was random and evenly spread throughout the waking day, with the constraint that no prompts were issued for 10 minutes after a cigarette entry. Prompting covered all waking hours. Compliance was high: subjects responded to prompts within the allotted 2 minutes 90% of the time. To allow for periods where prompting would be inappropriate (e.g., business meetings, naps), ED also incorporated features to allow subjects appropriate time out from being beeped, as well as an alarm clock so subjects could suppress beeping while they slept, and so that sleep and wake times could be recorded.
Visits were scheduled on days 2, 7, and 14 to ensure participant compliance; behavioral treatment was also provided at these occasions. A target quit date was scheduled on the 17th day of the study, at which point participants were instructed to stop smoking and were randomized to active or placebo nicotine patches (see Shiffman et al, 2006). Analyses for this study use only the data from the baseline period.
2.3 Baseline Measures
Participants completed measures of demographic variables, including their age, years of education, gender, annual income, and ethnicity (Table 1). They also completed measures of nicotine dependence, including daily smoking rate, CO levels, salivary cotinine, the Fagerström Test of Nicotine Dependence (FTND; Heatherton et al., 1991), and the Nicotine Dependence Syndrome Scale (NDSS; Shiffman et al., 2004b) (Table 1).
2.4 Momentary measures
Smoking, Craving, Withdrawal Symptoms, and Smoking Restrictions Assessments of momentary measures were made on EDs. Smoking was assessed by tallying the number of cigarettes recorded on ED. Craving and restlessness were single items on 11-point Likert scales (e.g., Craving? 0 = no craving, 10 = maximum craving). Measures of negative affect, attention disturbance, and arousal were composites of several adjectives (Shiffman et al., 1996) measured individually with 11-pt Likert scales. Previous analyses (Shiffman et al, 1996) suggested that withdrawal-related affect is indistinguishable from negative affect from exogenous sources, so we used a scale assessing NA broadly. This is also consistent with the hypothesis that NA, even if unrelated to withdrawal, motivates smoking (Piasecki et al., 1997). ED also assessed smoking restrictions by asking whether participants were in an environment where smoking was permitted, discouraged, or forbidden. We have demonstrated that smoking restrictions suppress smoking (Shiffman et al., 2002; Chandra et al., 2007).
2.5 ED System
ED system hardware consisted of a Palm Pilot Professional palmtop computer (Version 2.0, 3Com Corporation; 7.9 × 12.2 1.3 cm (3.1 × 4.8 0.5 inches), 161.6 g (5.7 oz)), with a touchscreen LCD that was used to present questions and solicit responses. Data from the ED were uploaded to a PC at each study visit. Software for the study was developed specifically for this purpose (invivodata, Pittsburgh, PA), and resembled software used in previously-published studies (Shiffman et al., 1996; 2002).
2.6 Data Processing
The data for this analysis consisted of time-tagged records of cigarettes smoked (one cigarette per entry), and assessments administered by ED. In order to obtain a sufficiently large dataset that fully represented subjects' experiences during each interval, we used all data collected during each time block, both those from cigarette smoking events and those from randomly prompted non-smoking occasions. The raw data set covered a total of 5,186 subject-days collected from 412 subjects for an average of 13 days per subject. Our analysis focused on weekdays (Monday-Thursday), because weekends appeared to demonstrate different temporal patterns (see Scharf et al., 2007). For each subject, we eliminated the first two days of data, in which participants were becoming accustomed to using the EDs. We also excluded data from the ninth day of observation, on which subjects were instructed to abstain from smoking for half the day (Shiffman et al, 2006). We eliminated all days (n=185 days) in which subjects' end-of-day reports indicated that they had failed to enter more than five cigarettes in real time, and all days (n=318 days) for which the EDs had suffered a technical malfunction.
In constructing the dataset, we followed the procedures employed by Chandra et al (2007). As the absence of smoking during sleep created a strong cyclical pattern that was not of interest, we removed sleep time from the dataset. In addition, including waking days of excessive length would have blurred the definition of a time bin, which was measured as 1/8 of a waking day. Therefore, to standardize the size of each time bin to approximately two hours, we eliminated days (n=34 out of 2233 days, or 1.5% of all days, results are robust to inclusion of these observations) on which the duration was more than two SDs away from the mean duration for that individual subject. We also eliminated days in which subjects were not awake and in-protocol for at least 12 hours. In our sample, the mean duration of the waking day across subjects and days was almost exactly 16 hours. Participants (n=59) who did not have at least 3 days of useable data after all of the above adjustments were excluded from the analyses, as were participants (n=2) whose data were highly atypical and appeared not to reflect true smoking behavior. The final data set covered a total of 2186 subject-days from 351 subjects for an average of 6.23 days per subject.
Each day was divided into eight segments, each representing two hours on average (Mean (in minutes) = 120.22, SD = 10.99). The number of cigarettes smoked, mean craving level, and mean levels of withdrawal symptoms (negative affect, arousal, restlessness, and attention disturbance) during each time block, on each day were computed.1 The time at which a subject woke was set as “zero hour” (Morgan et al., 1985). The mean time at which subjects woke up in the morning was approximately 7am, and the mean time at which they went to sleep was approximately 11pm. Accordingly, the 8 time blocks can be interpreted as being two hours long, and beginning with the 7am-9am block and ending with the 9pm-11pm block.
2.7 Cyclical Adjustment of Circadian Data
As in Chandra et al. (2007), we used a variant of a statistical algorithm for seasonal adjustment (SPSS, 1994) to decompose the series for smoking, craving, negative affect, arousal, attention disturbance, and restlessness in three steps. (1) For each subject, for each variable, we first computed a moving average as the mean of two consecutive moving averages, each with a (moving) window of seven two-hour time blocks. The total number of means computed was equal to eight times the number of days for which data were available, or one for each block of time. The central observation of each of the two moving averages was given twice the weight of the three observations on each side of the central observation. For observations occurring at either end of the time series, a slightly different set of weights was applied. This is the trend component of the series. (2) We then subtracted the moving average from the original series of two-hourly observations to obtain a trend-adjusted series so that cyclical variations around a (moving) mean of the variable of interest could be examined. (3) The daily mean was then subtracted from the preliminary cyclical components obtained in step (2) above. These cyclical components were normalized by the daily mean to produce percentage deviations from the daily mean. The final values represent cyclical deviations, measured as a percentage of the de-trended daily mean, henceforth “adjusted variable frequency.”2 For each subject, the cyclical deviations obtained from the above decomposition of the data were then averaged by time block to obtain the eight observations for each variable used for the analysis. The entire algorithm was implemented using SAS software (SAS Institute, Inc., 2010). Figures A1-A3 in the online supplementary materials show the data for one subject at three stages of the adjustment process.
In other words, the data analyzed consisted of deviations from the daily mean, which were de-trended and adjusted for any one-time irregularities. Importantly, the data were averaged across days, resulting in 8 observations per subject, representing the averaged cyclical deviation for each block across all days for that subject. That is, the data capture the variations across time blocks that represent consistent temporal patterns, with day-to-day variability removed. Unless otherwise specified, all results below are derived from this analysis of adjusted variable frequencies.
2.8 Analyses of Temporal Precedence
We conducted prospective lagged analyses to establish temporal precedence; i.e., whether changes in craving, negative affect, restlessness, arousal, or attention disturbance predict changes in smoking a few hours later, and whether changes in smoking predict changes in the above variables a few hours later. Tests of Granger Precedence (Granger, 1969) were conducted by running two sets of regressions. For example, in the first set, smoking at time t was regressed on smoking at time t-1. In the second set, data on craving and withdrawal at time t-1 was added to the equation and the results of the two regressions were compared. In order to compare the robustness of the results to variations across subjects, comparable models were estimated which treated subject variations as fixed effects (hence the estimators are “within-subjects” estimates) and as random effects. The results were robust across specifications; we report results from the random effects models.
3. Results
3.1 Temporal Patterns of Smoking and Symptoms
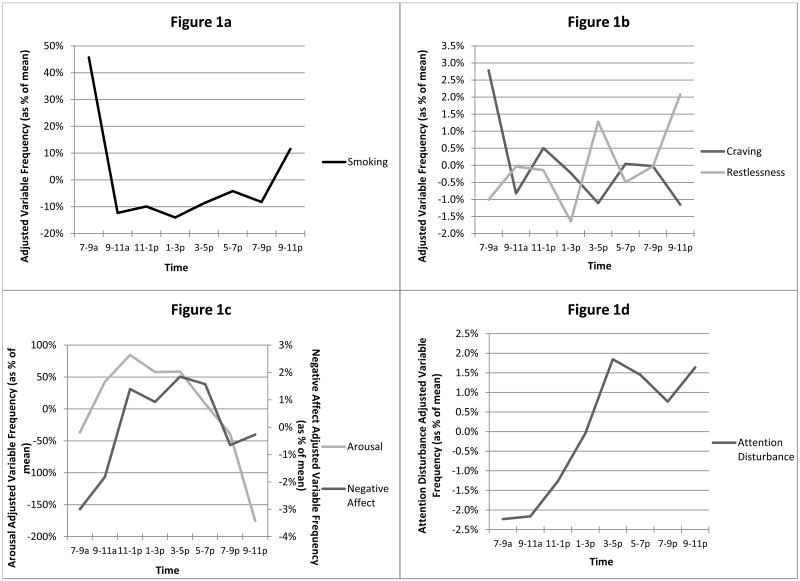
As has been demonstrated in an earlier study, the data suggest strong evidence of circadian patterns in smoking (Figure 1a; see also Chandra et al., 2007). Aggregate smoking frequency for the whole sample was approximately 45% greater (M=1.09 cigarettes) than average in the first 2h of the day, relatively stable just below mean levels throughout the later morning and afternoon (between -4 and -14%; 0.13 - 0.25 cigarettes), and reaching a secondary, smaller peak in the evening when smoking was ∼12% (M=0.28 cigarettes) above the mean.
Figure 1.
Daily patterns of (a) smoking, (b) craving and restlessness, (c) negative affect and arousal, and (d) attention disturbance. Times on the X-axis represent approximate times for each of 8 2-hour bins.
Analyses also suggest systematic, circadian patterns of craving and withdrawal symptoms (negative affect, restlessness, arousal, and attention disturbance). Figures 1a-d show the patterns for the adjusted variable frequencies. Data showed that negative affect was lowest in the morning (3% below mean), highest during the day (2% above mean), and that it fell off toward the evening (0.7% below mean). Arousal was low in the morning (35% below mean), peaked in the afternoon (70% above mean), and dropped for the rest of the day, reached its low point at night (175% below mean). Craving was highest early in the morning (2.8% above mean) and declined for the rest of the day (lowest 1.2% below mean, 9-11pm time block). Patterns of restlessness were distinct from negative affect and craving: restlessness was low in the morning (1.0% below mean), and peaked during mid-afternoon and late evening (1.3% and 2.1% above mean).
3.2 Cross-sectional Associations
Prior to examining the lagged prospective associations, we examined contemporaneous associations between smoking and craving and withdrawal symptoms (Table 2). To do this, we pooled all of the observations and computed bivariate linear regression coefficients from random effects (for subject-level heterogeneity) models of the association between smoking and each of craving, restlessness, negative affect, arousal, and attention disturbance. As a robustness check, we controlled for smoking restrictions in a parallel set of models. Craving was positively associated with same time-period smoking (b=0.86, p<0.0001). Negative affect (b=-0.20, p=0.0002) and attention disturbance (b=-0.22, p<0.0001) were negatively associated with smoking. Arousal and restlessness were not associated with contemporaneous smoking.
Table 2. Associations between Smoking, and Craving, Restlessness, Arousal, Attention Disturbance, and Negative Affect (Mood).
| Smoking | Smoking | |
|---|---|---|
| No Control | Controlled for Restrictions | |
| Craving | +0.86*** | +0.65*** |
| 0.0001 | 0.0001 | |
| Restlessness | -0.01 | -0.05 |
| 0.64 | 0.11 | |
| Negative Affect | -0.20*** | -0.20*** |
| 0.0002 | 0.0001 | |
| Arousal | -0.001 | -0.0002 |
| 0.68 | 0.75 | |
| Attention Disturbance | -0.22*** | -0.24*** |
| 0.0001 | 0.0001 | |
| Number of observations | 2808 | 2800 |
Significant at the 1% level
p-values for null of β=0 in italics
3.3 Lagged Prospective Associations
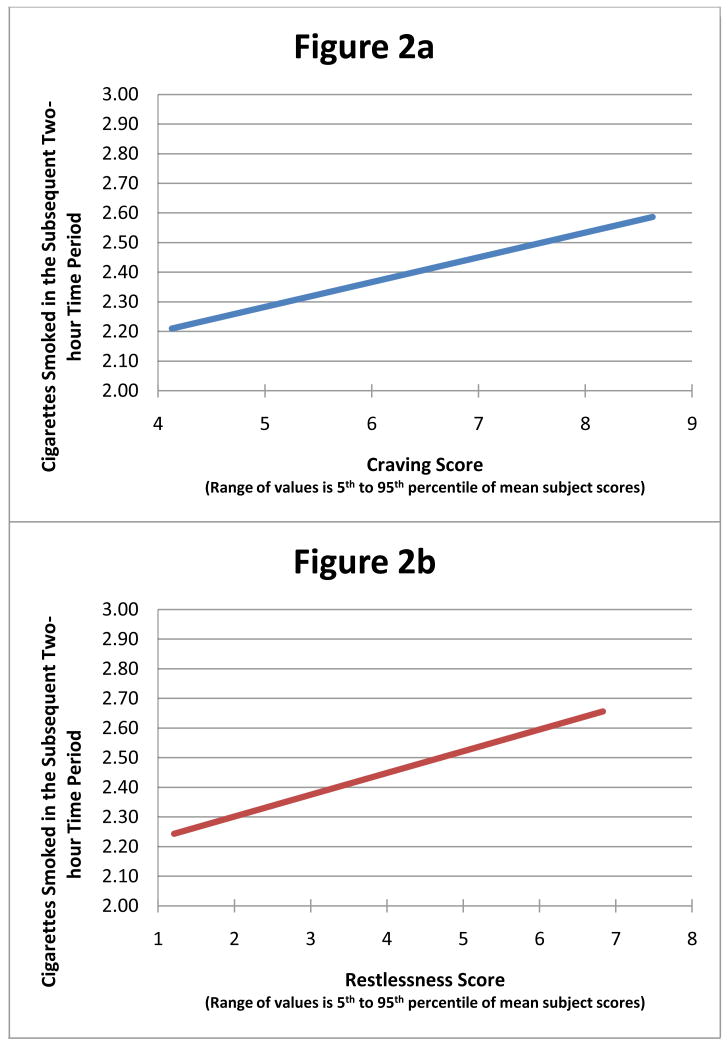
We next examined the lagged, prospective associations between smoking and craving, and smoking with nicotine withdrawal symptoms. These analyses are summarized in Table 3. Results showed that craving temporally preceded smoking (b=0.23, F(1,2103) = 9.64, p=0.0019, see Table 3), such that a higher level of craving in a 2-hour time-block is associated with a higher level of smoking two hours later. For every 100% increase in craving during a 2-h block, cigarette smoking was 23% higher in the succeeding 2-hour period. This translates into an increase of approximately 1/10 of a cigarette per 2-hour interval for a one-point increase in the craving score, or 3.2 cigarettes per day for a four-point increase in the craving score (Figure 2a). Conversely, more smoking was associated with lower craving two hours later (b=-0.02, F(1,2103) = 12.78, p=0.0004), such that each cigarette smoked reduced the subsequent craving score by 2%. Like craving, higher restlessness also temporally preceded more smoking (b=0.11, F(1,2103) = 10.24, p=0.0009; Table 3) two hours later, and more smoking was associated with lower subsequent restlessness (b=-0.03, F(1,2103) = 7.60, p=0.0059) two hours later. These results, translated into the relationship between actual cigarettes smoked and the restlessness score are presented in Figure 2b.
Table 3. Results for Granger Precedence Tests.
| Model: Subjects Modeled as Random Effects | Variable | |||||
|---|---|---|---|---|---|---|
| Withdrawal | ||||||
| Craving | Restlessness | Negative Affect (Mood) | Arousal | Attention Disturbance | ||
| Smoking preceded (Granger-caused) by | Coefficient | +0.23*** | +0.11*** | -0.03 | +0.0000 | +0.06 |
| p-value | 0.0019 | 0.0009 | 0.5084 | 0.9392 | 0.1583 | |
| Smoking precedes (Granger-causes) | Coefficient | -0.02*** | -0.03*** | -0.03*** | +0.89 | -0.01 |
| p-value | 0.0004 | 0.0059 | 0.0017 | 0.1184 | 0.1073 | |
Significant at the 1% level
p-values for F(1,2103) in italics
Figure 2.
Relationships between (a) craving and (b) restlessness and subsequent smoking, based on models described in the text. The range on the X-axis represents the 5th to 95th percentile of observed values.
In contrast to craving and restlessness, negative affect showed a one-way (but not reciprocal) relationship with smoking: Variations in antecedent negative affect were not associated with subsequent smoking, however more smoking was associated with lower subsequent negative affect (b=-0.03, p=0.0017) two hours later (Table 3). Attention disturbance and arousal were not associated with prior or future smoking. As a robustness check, we controlled for smoking restrictions in a parallel set of models, which produced results that were similar to those reported in Table 3.
4. Discussion
The primary aim of this study was to assess the dynamic relationship between craving, nicotine withdrawal symptoms, and cigarette consumption within typical daily patterns of smoking, craving, and nicotine withdrawal symptoms. We used Granger's precedence test, based on controlled, lagged prospective associations. This is the first study to use EMA data to examine how systematic, within-day temporal patterns in craving and nicotine withdrawal symptoms relate to temporal variations in smoking. A major finding is that craving and restlessness prospectively predict subsequent smoking in the subsequent 2-hour time block, and greater cigarette consumption predicts subsequent diminution of craving and restlessness in the subsequent block. Second, while negative affect does not predict future smoking, smoking does diminish subsequent negative affect. Third, such relationships did not hold for arousal or attention disturbance. And fourth, there are notable differences between contemporaneous associations (Table 2) and intertemporal relationships between smoking, craving and symptoms of nicotine withdrawal (Table 3, Figures 2a-b). Specifically, restlessness shows no contemporaneous association with smoking even though the intertemporal relationship is strong and reciprocal. Negative affect showed an inverse contemporaneous association with smoking, but no prospective association with subsequent smoking. Craving, on the other hand, shows a strong and positive contemporaneous association with smoking in addition to the reciprocal intertemporal relationship. And higher attention disturbance is contemporaneously associated but shows no intertemporal relationship with smoking. In other words, smokers' “standing” circadian patterns of smoking and nicotine withdrawal symptoms can in part be explained by the reciprocal relationships between cigarette consumption and antecedent and subsequent symptoms of nicotine withdrawal.
Patterns
Our analysis also characterized temporal patterns of smoking, craving, and nicotine withdrawal symptoms. As previously demonstrated (Chandra et al., 2007), smoking varied systematically by time of day. The pattern of smoking that we found was similar to work by Mead and Wald (1977) in which smoking was heaviest in non-work hours, including first thing in the morning, and in the later evening. The most striking aspect of this pattern is the significant elevation in cigarette consumption during the first two hours of the day. This is likely due to smokers' drive to replenish nicotine after most of it has been cleared overnight, and reflects the degree of nicotine dependence in the sample.
The pattern of craving that we found also resembled those reported in prior studies (e.g., Perkins et al., 2009; Dunbar et al., 2010). Specifically, craving was highest early in the morning. The morning peak in craving may also reflect the effect of low nicotine levels after overnight deprivation and clearance of most nicotine. These observations of early smoking and craving are related to the role of time to first cigarette as a powerful predictor of smoking cessation outcomes (TTURC, 2007).
The pattern of negative affect was somewhat dissimilar to earlier reports of affect patterns throughout the day, in which negative affect is highest in the morning and lower in the afternoon (Rusting and Larsen, 1998; Perkins et al., 2009). In this sample, negative affect was highest in the middle of the day, suggesting that smokers' mood symptoms may be additionally affected by unique factors such as nicotine withdrawal in the middle of the day due to smoking restrictions at work. Restlessness, whose temporal patterning has not been previously studied, increased throughout the day, with the exclusion of low levels at lunch time (1pm). One possibility is that patterns of restlessness are closely associated with the demands (or anticipation of demands) of the typical work day. Attention disturbance also showed a steadily increasing pattern throughout the day.
Relationship to Smoking
A potentially important finding was that our two indicators of mood-related withdrawal symptoms (negative affect and restlessness) did not show the same relationship with smoking. Instead, restlessness showed a reciprocal pattern similar to craving. The fact that patterns of negative affect and restlessness were discrepant is consistent with research by Shiffman et al (Shiffman et al, 1996, 2002) who found that restlessness was psychometrically distinct from other mood-related symptoms of nicotine withdrawal. We have previously suggested that restlessness was related to activation of motivational systems without the ability to attach the motivational drive to a specific target that can then be sought and consumed (Shiffman et al, 1996, 2002), which may explain the resemblance of its temporal patterning to that of craving. Other ways in which restlessness may be distinct from other withdrawal symptoms require further investigation.
Our analyses showed that although negative affect, a DSM-IV nicotine withdrawal symptom (APA, 2000) varied systematically by time of day, these variations do not predict temporal patterns of smoking. This is consistent with findings from previous EMA studies that showed that negative affect seemed to exercise no immediate effect on smoking (see Kassel et al., 2003). Although the notion that negative affect precipitates smoking behavior is a widely-held belief among smokers and theorists (reviewed in Kassel et al., 2003), a mounting body of empirical studies suggests that negative affect is not a reliable antecedent of ad libitum smoking. It is important to recognize that negative affect can vary for reasons other than nicotine withdrawal (indeed, negative affect seemed to rise during the work-day, likely reflecting the stress of work), which may limit its relationship to smoking. However, theorists have proposed that even non-withdrawal negative affect due to life stress prompts smoking (Piasecki et al., 1997), so our finding that negative affect is unrelated to subsequent smoking challenges those theories. While the findings of this paper support earlier findings that negative affect, on average and in aggregate, is not predictive of smoking (Shiffman et al, 2002, Shiffman et al, 2004a), they are not inconsistent with conclusions that negative affect may prompt or suppress smoking for specific individuals or groups of subjects (Shiffman et al., 2007).
We did find improvement in negative affect after periods of heavier smoking. This finding is similar to Carter et al (2008), who reported that mood improved immediately after smoking. Taking into account Carter's findings and ours, one might conclude that smoking leads to an immediate improvement in mood, and that the effect is lasting. Smoking more in a two-hour period may improve negative affect in the subsequent two hours. Or, conversely, not smoking, or smoking less, in an interval, may foreshadow withdrawal-related negative affect in the subsequent 2-hour interval (Parrott 1995). Additional studies measuring smoking and negative affect over larger time intervals could help shed light on how and why smoking affects subsequent mood.
In contrast to negative affect, restlessness did show a reciprocal relationship to smoking behavior, with increased restlessness predicting subsequent cigarette consumption, and with greater prior consumption predicting decreased subsequent restlessness. As we have previously speculated (Shiffman et al., 1996; 2002), restlessness may reflect motivation to smoke or a global activation of motivational “go” systems that mobilize behavior towards a goal.
The motivational relevance of craving to smoking (and drug use generally) has been questioned (Tiffany, 1990). In a prior study, we demonstrated a contemporaneous or immediate association between craving and smoking in real-world EMA data (Shiffman et al., 2002), but such cross-sectional data could be confounded by smokers' awareness that they are smoking. The present analysis demonstrated a prospective relationship between craving and subsequent smoking over periods of two to four hours. Moreover, smokers' daily patterns also showed the expected reciprocal relationship in which greater smoking is associated with subsequent decreases in craving. This reciprocal relationship is consistent with the nicotine regulation model of smoking, which suggests that smoking aims to keep nicotine levels within limits to avoid large increases in craving (and restlessness), and with the notion that that craving may provide a subject read-out (albeit an imperfect one) of nicotine “need.”
A common aspect of all the relationships observed here is that they are relatively small in absolute magnitude, seemingly affecting craving, nicotine withdrawal symptoms, or smoking rate to a small degree. However, it must be remembered that the associations are corrected for prior values, e.g., for the number of cigarettes smoked in the prior period, which makes the deviations all the more important. Also, these data represent patterns averaged over multiple days, so they do not reflect all of the variations in craving, nicotine withdrawal symptoms, or smoking, over time, but only those that are common across days. Finally, even small changes across a two-hour lag may have great cumulative effect over multiple two-hour time blocks, days and weeks of smoking.
4.1 Study Limitations
Perhaps the biggest limitation of this analysis was its use of relatively arbitrary intervals for summarizing smoking and other variables. The choice of the intervals of approximately two hours for the main analyses was based primarily on the density of the data (cigarette recordings and random assessments). However, this interval does correspond approximately to the half-life of nicotine (Le Houzec, 2003) so it may be a suitable interval for examining the effects of smoking. In any case, we also examined intervals of roughly 90 minutes and 160 minutes, and found similar results. Moreover, the use of aggregate intervals may have some advantages over the momentary assessments used in some prior studies (Shiffman et al., 2002; Carter et al., 2008; Whalen et al., 2001) by allowing prospective analyses and eliminating the potential bias when subjects report their craving and nicotine withdrawal symptoms when they already know they are about to smoke. To the extent that smoking restrictions may confound momentary associations (because smokers cannot necessarily smoke at the moment they want to), aggregate data may also provide a clearer read on the relationship between smoking and subjective states.
A further limitation of this study was that the sample of participants was selected based criteria that may have influenced smoking patterns. Participants were seeking smoking cessation treatment, and were screened to be heavy smokers who were very nicotine dependent and likely had failed in unaided quitting. The limited range of smoking, dependence, and demographic characteristics may have limited the range of smoking patterns that were detected, and may have limited our ability to distinguish the groups based on these characteristics. We also only studied smoking during weekdays, as we did not have an adequate sample of weekend days to allow a robust separate analysis of weekend patterns. Analysis of weekend smoking may be informative since it is likely less subject to restrictions, and may be more influenced by social setting and by alcohol consumption. Preliminary analyses of weekday – weekend patterns of smoking suggest that such differences exist (Scharf et al., 2007).
Similarly, the baseline period from which pattern data were derived immediately preceded a quit attempt for all participants. Although smokers were directed to continue smoking as usual, they may have adjusted their smoking patterns as the quit day approached, an effect that might have been facilitated by the self-monitoring imposed by the study. Analyses show that smokers essentially did not change their overall daily smoking rate and to the extent that various features of the patterns detected are consistent with the findings of earlier studies, we believe that the findings of this study are valid.
A final limitation relates to the nature of the EMA data collection process. The smoking data collected using event recording via EMA may not have perfectly represented smoking patterns. In end-of-day assessments on the ED, smokers sometimes admitted to having failed to record some smoking. While we dropped data for those days on which subjects indicated that they had not recorded five or more cigarettes smoked, it was not possible to directly verify that cigarette entries were made in a timely way, though some evidence suggests that they were (Shiffman, 2009). Similarly, it should be noted that our data are drawn from moments just as smoking is being initiated, and moments during which smoking is not taking place. Therefore, for a variable like craving, we have no measures of what happens to craving during the act of smoking itself and cannot therefore distinguish between a situation in which craving falls during smoking and one in which it falls after smoking. In any case, EMA data represent the best method currently in use to capture events in real time.
4.2 Study Strengths
To our knowledge, this is the first study to formally investigate daily cycles in smoking as they relate to nicotine withdrawal symptoms and craving using EMA. Real-time data collection and electronic time-tagging of events helps eliminate some of the problems associated with other forms of data collection on smoking activity, such as retrospective recall, faked diaries, or diaries filled in after-the-fact (Stone et al., 2003). Other strengths of this design included the length of the observation period, with participants carrying EDs for over 2 weeks, and naturalistic data collection, which eliminates situational effects on smoking that occur when smoking is monitored in a laboratory. The size of the dataset enabled us to overcome the small sample-size problem of several earlier studies. This is also the first study (to our knowledge) to use Granger precedence analysis to empirically test the temporal precedence hypotheses inherent in many contemporary models of smoking and nicotine dependence. Results of this analysis suggest the utility of Granger analysis for investigating other sequential, process hypotheses about factors that increase or decrease the likelihood of smoking.
5. Conclusions
Our analyses confirmed the expected reciprocal relationship between craving and smoking, and between restlessness and smoking, and thereby help strengthen dependence-based models of smoking (e.g., Benowitz, 2008). The fact that we observed only a one-way relationship between negative affect and smoking is more problematic for these models. It could be that natural variations in negative affect mask its association with smoking, or that smokers manage their smoking in anticipation of overall mood and withdrawal changes, and thereby dampen this association (Baker et al, 2004). However, these findings extend, in a new sample, with new analytic methods, the puzzling but consistent findings from multiple prior studies (Shiffman et al., 2002; Shiffman et al., 1996; Carter et al., 2008; Whalen et al, 2001; Piasecki et al, 1997) showing no or at best weak associations between negative affect and smoking. Despite smokers' propensity to report that they smoke more when they are distressed, the relationship between negative affect and smoking must be seriously questioned.
Supplementary Material
Footnotes
Figures illustrating data for one subject at three stages of the adjustment process can be found as supplementary material by accessing the online version of this paper at http://dx.doi.org and entering doi:…
Parallel analyses were conducted on days divided into six and ten (rather than eight) intervals. The results for the six- and ten-interval analyses broadly paralleled those for the eight-interval analysis. Therefore, we present the eight-interval analyses, which can be neatly interpreted as consisting of two-hour intervals in typical sixteen-hour day.
In addition to this, the trend and irregular components were computed as follows: the cyclical component was subtracted from the original series to give the composite of the trend and irregular components of the data. The final trend component was computed using a weighted moving average of the trend-irregular composite, with adjustments for terms at temporal extremes. Finally, the irregular component was computed by subtracting the final trend component from the trend-irregular composite.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Health Disorders. Fourth. Washington, DC: 2000. Text Revision. [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addition. Clin Pharmacol Ther. 2008;83:531–541. doi: 10.1038/clpt.2008.3. [DOI] [PubMed] [Google Scholar]
- Carter BL, Lam CY, Robinson JD, Paris MM, Waters AJ, Wetter DW, Cinciripini PM. Real-time craving and mood assessments before and after smoking. Nicotine Tob Res. 2008;10:1165–1169. doi: 10.1080/14622200802163084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Shiffman S, Scharf DM, Dang Q, Shadel WG. Daily smoking patterns, their determinants, and implications for quitting. Exp Clin Psychopharmacol. 2007;15:67–80. doi: 10.1037/1064-1297.15.1.67. [DOI] [PubMed] [Google Scholar]
- Donny EC, Houtsmuller E, Stitzer ML. Smoking in the absence of nicotine: behavioral, subjective and physiological effects over 11 days. Addiction. 2007;102:324–334. doi: 10.1111/j.1360-0443.2006.01670.x. [DOI] [PubMed] [Google Scholar]
- Dunbar M, Scharf DM, Kircher T, Shiffman S. Craving trajectories approaching target day are related to achievement of initial (24h) abstinence. Poster presented at the Society for Research on Nicotine and Tobacco; Austin, Texas. 2007. [Google Scholar]
- Dunbar MS, Schard DM, Kirchner T, Shiffman S. Do smokers crave cigarettes in some smoking situations more than others? Situation correlates of craving when smoking. Nicotine Tob Res. 2010;12:226–234. doi: 10.1093/ntr/ntp198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emurian HH, Nellis MJ, Brady JV, Ray RL. Event time-series relationship between cigarette smoking and coffee drinking. Addict Behav. 1982;7:441–444. doi: 10.1016/0306-4603(82)90016-8. [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Gitchell JG, Shiffman S, Sembower MA. Prediction of abstinence at 10 weeks based on smoking status at 2 weeks during a quit attempt: secondary analysis of two parallel, 10-week, randomized, double-blind placebo-controlled clinical trials of 21-mg nicotine patch in adult smokers. Clin Pharmacol Ther. 2009;31:1957–1965. doi: 10.1016/j.clinthera.2009.08.029. [DOI] [PubMed] [Google Scholar]
- Granger CWJ. Investigating causal relations by econometric models and cross-spectral methods. Econometrica. 1969;37:424–438. [Google Scholar]
- Gries J, Benowitz N, Verotta D. Chronopharmacokinetics of nicotine. Clin Pharmacol Ther. 1996;60:385–395. doi: 10.1016/S0009-9236(96)90195-2. [DOI] [PubMed] [Google Scholar]
- Hacker RS, Hatemi-J A. Test for causality between integrated variables using asymptotic and bootstrap distributions: theory and application. Appl Econ. 2006;38:1489–1500. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Jarvik ME, Madsen DC, Olmstead RE, Iwamoto-Schapp PN, Elins JL, Benowitz NL. Nicotine blood levels and subjective craving for cigarettes. Pharmacol Biochem Behav. 2000;66:553–558. doi: 10.1016/s0091-3057(00)00261-6. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Le Houezac J. Role of nicotine pharmacokinetics in nicotine addiction and nicotine replacement therapy: a review. Int J Tuberc Lung Dis. 2003;7:811–819. [PubMed] [Google Scholar]
- Morgan SF, Gust SW, Pickens RW, Champagne SE, Hughes JR. Temporal patterns of smoking topography in the natural environment. Int J Addict. 1985;20:613–621. doi: 10.3109/10826088509044940. [DOI] [PubMed] [Google Scholar]
- O'Neill ST, Parrott AC. Stress and arousal in sedative and stimulant cigarette smokers. Psychopharmacology. 1992;107:422–446. doi: 10.1007/BF02245173. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Stress modulation over the day in cigarette smokers. Addiction. 1995;90:233–244. doi: 10.1046/j.1360-0443.1995.9022339.x. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Friski J, Fonte C, Scott J, Lerman C. Severity of tobacco abstinence symptoms varies by time of day. Nicotine Tob Res. 2009;11:84–91. doi: 10.1093/ntr/ntn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Kenford SL, Smith SS, Fiore MC, Baker TB. Listening to nicotine: negative affect and the smoking withdrawal conundrum. Psychol Sci. 1997;8:184–189. [Google Scholar]
- Ray RL, Emurian HH, Brady JV, Nellis MJ. On the regularity of smoking. Addict Behav. 1982;7:261–270. doi: 10.1016/0306-4603(82)90053-3. [DOI] [PubMed] [Google Scholar]
- Rusting CL, Larsen RJ. Diurnal patterns of unpleasant mood: associations with neuroticism, depression, and anxiety. J Pers. 1998;66:85–103. doi: 10.1111/1467-6494.00004. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc . SAS® Software, version 9.2. Sas Institute, Inc.; Cary, NC: 2010. [Google Scholar]
- Scharf D, Chandra S, Shiffman S. Temporal Patterns of Craving and Smoking: Differences between Weekdays and Weekends?. Poster presented at the Society for Research on Nicotine and Tobacco; Austin, Texas. 2007. [Google Scholar]
- Schuh KJ, Stitzer ML. Desire to smoke during spaced smoking intervals. Psychopharmacology. 1995;120:289–295. doi: 10.1007/BF02311176. [DOI] [PubMed] [Google Scholar]
- Shelton DM, Alciati MH, Chang MM, Fishman JA, Fues LA, Michaels J, Bazile JR, Bridgers CJ, Rosenthal LJ, Kutty L, Eriksen PM. State laws on tobacco control – United States. Morb Mortal Wkly Rep. 1995;44:1–28. [PubMed] [Google Scholar]
- Shiffman S. The Tobacco Withdrawal Syndrome. In: Krasnegor NM, editor. Cigarette Smoking as a Dependence Process. National Institute on Drug Abuse, United States Department of Health, Education, and Welfare; Rockville, Maryland: 1979. pp. 158–184. Monograph 23. [Google Scholar]
- Shiffman S, Paty JA, Ginys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. J Consult Clin Psychol. 1996a;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Hickox M, Paty JA, Gnys M, Kassel JD, Richards TJ. Progression from a smoking lapse to relapse: prediction from abstinence violation effects, nicotine dependence, and lapse characteristics. J Consult Clin Psychol. 1996b;64:993–1002. doi: 10.1037//0022-006x.64.5.993. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Gwaltney CJ, Balabanis MH, Liu KS, Paty JA, Kassel JD, Hickox M, Gnys M. Immediate antecedents of cigarette smoking: an analysis from ecological momentary assessment. J Abnorm Psychol. 2002;111:531–545. doi: 10.1037//0021-843x.111.4.531. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gwaltney CJ, Dang Q. Immediate antecedents of cigarette smoking: An analysis of unrestricted smoking patterns. J Abnorm Psychol. 2004a;113:166–171. doi: 10.1037/0021-843X.113.1.166. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters A, Hickox M. The nicotine dependence syndrome scale: a multidimensional measure of nicotine dependence. Nicotine Tob Res. 2004b;6:327–348. doi: 10.1080/1462220042000202481. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Scharf DM, Shadel WG, Gwaltney CJ, Dang Q, Paton SM, Clark DB. Analyzing milestones in smoking cessation: illustration in a nicotine patch trial in adult smokers. J Consult Clin Psychol. 2006;74:276–285. doi: 10.1037/0022-006X.74.2.276. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Balabanis MH, Gwaltney CJ, Paty JA, Gnys M, Kassel JD, Hickcox M, Paton SM. Prediction of lapse from associations between smoking and situational antecedents assessed by ecological momentary assessment. Drug Alcohol Depend. 2007;91:159–168. doi: 10.1016/j.drugalcdep.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- Shiffman S. How many cigarettes did you smoke? Assessing cigarette consumption by global report, Time-Line Follow-Back, and ecological momentary assessment. Health Psychol. 2009;28:519–526. doi: 10.1037/a0015197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AA, Shiffman S. Ecological Momentary Assessment (EMA) in behavioral medicine. Ann Behav Med. 1994;16:199–202. [Google Scholar]
- Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufford MR. Patient compliance with paper and electronic diaries. Control Clin Trials. 2003;24:182–199. doi: 10.1016/s0197-2456(02)00320-3. [DOI] [PubMed] [Google Scholar]
- Teneggi V, Tiffany ST, Squassanta L, Milleri S, Ziviani L, Bye A. Smokers deprived of cigarettes for 72h: effect of nicotine patches on craving and withdrawal. Psychopharmacology. 2002;16:177–187. doi: 10.1007/s00213-002-1176-1. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Transdisciplinary Tobacco Use Research Center (TTURC) Tobacco Dependence. Baker TB, Piper ME, McCarthy DE, Bolt DM, Smith SS, Kim SY, Colby S, Conti D, Giovino GA, Hatsukami D, Hyland A, Krishnan-Sarin S, Niaura R, Perkins KA, Toll BA. Time to first cigarette in the morning as an index of ability to quit smoking: implications for nicotine dependence. Nicotine Tob Res. 2008;9 4:S555–S570. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen CK, Jamner LD, Henker B, Delfino RJ. Smoking and moods in adolescents with depressive and aggressive dispositions: evidence from surveys and electronic diaries. Health Psychol. 2001;20:99–111. [PubMed] [Google Scholar]
- Zhou X. Attempts to quit smoking and relapse: factors associated with success or failure from the ATTEMPT cohort study. Addict Behav. 2008;34:265–373. doi: 10.1016/j.addbeh.2008.11.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.