Abstract
Information regarding the remediation of event-based prospective memory (EB-PM) impairments following pediatric traumatic brain injury (TBI) is scarce. Addressing this, two levels of monetary incentives were used to improve EB-PM in children ages 7 to 16 years with orthopedic injuries (OI, n = 51), or moderate (n = 25), and severe (n = 39) TBI at approximately three months postinjury. The EB-PM task consisted of the child giving a specific verbal response to a verbal cue from the examiner while performing a battery of neuropsychological measures (ongoing task). Significant effects were found for Age-at-Test, Motivation Condition, Period, and Group. Within-group analyses indicated OI and moderate TBI groups performed significantly better under the high-versus low-incentive condition, but the severe TBI group demonstrated no significant improvement. These results indicate EB-PM can be significantly improved at three months postinjury in children with moderate, but not severe, TBI.
Keywords: Event-based prospective memory, Traumatic brain injury, Monetary Incentives, Motivation, Memory rehabilitation, Pediatrics
To date, several studies have investigated the effects of traumatic brain injury (TBI) on prospective memory (PM) in adults (Cockburn, 1995; Fortin, Godbout, & Braun, 2002; Groot, Wilson, Evans, & Watson, 2002; Hannon, Adams, Harrington, Fries-Dias, & Gipson, 1995; Henry et al., 2007; Kinsella et al., 1996; Kliegel, Eschen, & Thöne-Otto, 2004; Knight, Harnett, & Titov, 2005; Knight, Titov, & Crawford, 2006; Louda, Loseva, & Mielke, 2007; Mathias & Mansfield, 2005; Roche, Fleming, & Shum, 2002; Roche, Moody, Szabo, Fleming, & Shum, 2007; Shum, Valentine, & Cutmore, 1999); however, few experimental studies have been conducted in PM involving children with TBI. One of the earliest of these was reported by McCauley and Levin (2004) in which children with severe TBI demonstrated impaired event-based PM (EB-PM) performance during an ongoing lexical decision task (i.e, whether the word belonged to a given category or not). In this task, children were asked to press a specific key in response to a nonfocal (Hicks, Cook, & Marsh, 2005) PM cue (e.g., words presented in blue color). Even after the presentation of a reminder of the EB-PM instructions during the ongoing task (given at the same point in the task to all participants), children with severe TBI remained impaired relative to children with orthopedic injury (OI) or mild TBI. Using the same paradigm as in their previous work with typically-developing children (Heather Ward, Shum, McKinlay, Baker-Tweney, & Wallace, 2005), Ward et al. (H. Ward, Shum, McKinlay, Baker, & Wallace, 2007) used a lexical decision task (word vs. nonword) with two levels of cognitive demand for the ongoing task (shorter vs. longer letter strings). The participants were asked to press a specific key in response to a nonfocal PM cue (i.e., a single italicized letter embedded in the stimuli). They found that children with TBI performed poorly compared to a typically-developing control group and that performance was significantly worse (in adolescents) when the ongoing task had high cognitive processing demands compared to the low-demand condition.
The remediation of deficits of event-based PM (EB-PM; remembering to perform an intended action in response to a specific target event or cue (G. O. Einstein & M. A. McDaniel, 1990; Einstein & McDaniel, 1996)) in children following TBI has been rarely investigated in spite of the importance of PM abilities in daily life (Harris, 1984; John A. Meacham & Dumitru, 1976; J. A. Meacham & Leiman, 1982; Wilkins & Baddeley, 1978; Winograd, 1988). The capability to mitigate TBI-related PM deficits in adults significantly predicts the ability to live independently (Fortin, et al., 2002; Fortin, Godbout, & Braun, 2003; Thöne-Otto & Walther, 2003; Wilson, 1987). The issue of independence extends beyond adults with TBI. For instance, in an interview study involving children and adolescents with TBI (H. Ward, Shum, Dick, McKinlay, & Baker-Tweney, 2004), parents reported that they had serious concerns for their child’s safety and ability to be left unsupervised even briefly because of their substantial PM impairments. The amount of study in PM deficit remediation involving adults with acquired brain injury, TBI in particular, is lacking (Furst, 1986; Raskin & Sohlberg, 2009) leaving little guidance for rehabilitation professionals to turn to in order to design effective evidence-based interventions. The potential negative impact of impaired PM on academic performance and age-appropriate independent living are just two examples that highlight the practical importance of investigating PM and its possible remediation in children and adolescents with TBI.
In the first known study to investigate PM deficit remediation following pediatric TBI, McCauley et al. (2009) reported that in their study comparing children with uncomplicated mild or severe TBI ranging from 1 to 15 years postinjury to an orthopedic injury (OI) comparison group with similar demographic and postinjury chronicity characteristics, children in all groups significantly improved their PM performance under a high motivation (dollar-per-point incentive) compared to a low motivation condition (penny-per-point incentive). However, performance in the high motivation condition of children with severe TBI was still significantly below that of the OI and mild TBI groups’ performance in both the high- and low-motivation conditions.
In a follow-up to this retrospective study using the same experimental procedures, McCauley and colleagues (2010) investigated the effect of monetary incentives in a prospective cohort of children with moderate to severe TBI. In the subacute phase of recovery (i.e., the earliest date after emerging from posttraumatic amnesia, when considered medically stable, and could cooperate well enough to participate in a neuropsychological evaluation), children with OI or moderate TBI were able to significantly improve their EB-PM performance in the high- relative to low-motivation condition. Contrary to previous findings, however, children with severe TBI were not able to significantly improve their performance. It remains unclear as to when in the course of recovery that monetary incentives could induce significantly improved EB-PM performance in children with severe TBI.
In our line of research, a monetary incentive was specifically chosen as the extrinsic motivator in this study because providing pocket money/allowance is a common technique used by parents to reinforce the concept of earning income for work performed (Neul & Drabman, 2001) which teaches children to successfully meet responsibilities at home and in work situations as they enter adulthood (Pastore & Friedman, 1992). Among Western nations, over 90% of parents report that 6 to 7 years is an appropriate age range in which to start an allowance program (Barnet-Verzat & Wolff, 2002; Furnham, 1999; Furnham & Kirkcaldy, 2000), and adolescents frequently have had one or more formal or informal part-time jobs of one type or another. It appears reasonable that the concept of receiving money for work performed correctly might be familiar to the participants and could be reasonably introduced as a motivator in experimental tasks.
In the present study, motivation was varied using two levels of monetary incentive (i.e., dollars and pennies); participants exchanged points for units of money (1:1 ratio) based on their accurate PM performance after the EB-PM task was completed. Based on our previous work in children with chronic TBI, we had two main hypotheses: 1) children at three months following moderate to severe TBI would demonstrate significantly impaired EB-PM performance compared to children with OI, and 2) children with TBI would demonstrate significantly improved EB-PM performance under high- versus low-motivation conditions.
METHOD
Participants
Informed consent was obtained from the parent/guardian through a procedure and consent form approved by the Institutional Review Boards of Baylor College of Medicine, the University of Texas at Dallas, the University of Texas Southwestern Medical Center, and the University Miami School of Medicine. Child assent was obtained in accordance with federal regulations. Participants were prospectively recruited from American College of Surgeons Level-1 trauma centers in Houston, Dallas, and Miami as part of a longitudinal study of neurobehavioral outcome following moderate to severe TBI.
TBI severity was appraised by the lowest post-resuscitation Glasgow Coma Scale (GCS) score (Teasdale & Jennett, 1974) in the first 24 hours postinjury. Our sample included children ranging in age from 7 to 16 years: 39 children with severe TBI (post-resuscitation GCS ≤ 8), 25 children with moderate TBI [either post-resuscitation GCS 13–15 with trauma-related intracranial abnormalities on computed tomography (CT) scan of the head at hospital admission (Williams, Levin, & Eisenberg, 1990) or GCS of 9–12 irrespective of CT results], and 51 children who sustained orthopedic injuries (OI) not involving the head (e.g., broken bones, fractures) requiring emergency room treatment. The OI participants had mild to moderate injuries as defined by the Abbreviated Injury Scale (Committee on Injury Scaling, 1990). Children with OI were included to control for risk factors predisposing children to traumatic injury and to equate for other nonspecific factors associated with trauma and hospitalization. All participants were fluent in English, full-term births (i.e., ≥ 37 weeks of gestation and > 2500 g), had no preexisting major neuropsychiatric disorder (e.g., schizophrenia, bipolar disorder), and no previous hospitalization for head injury. No child in any group had a preinjury history of being retained a grade. As part of the design of the larger study, children were assessed at approximately three months postinjury.
Measures
Socioeconomic Composite Index
The SCI (Yeates et al., 1997) measures family socioeconomic status (SES) by computing Z-scores based on aggregate data of the OI and TBI groups for three variables: a) an 8-point scale coding family income, b) a 7-point scale of parent/guardian education, and c) occupational prestige rating using the Total Socioeconomic Index (TSEI; Hauser & Warren, 1999). The Z-scores for these variables were summed and standardized as a Z-score based on the aggregate sample of participants forming the SCI score. The mean TSEI for each respective group was imputed for parents/guardians who were not employed. The SCI has been shown to moderate the effects of severe TBI on long-term outcomes (Yeates, et al., 1997), but thus far has not been shown to have an effect on EB-PM performance in children with either subacute (McCauley et al., 2010) or chronic (McCauley, et al., 2009) TBI. The SCI was included in this study to determine if SES influenced EB-PM performance in children with TBI at a more recent postinjury time point.
Event-Based Prospective Memory Task
We attempted to make the EB-PM task as naturalistic as possible within the limits of a laboratory setting in order to maximize the ecological validity of the experiment. Using a casual phrase (i.e., “Let’s try something different”) that would not seem unusual to encounter during a neuropsychological evaluation seemed to be a reasonable compromise between the ‘real world’ ecological validity of a naturalistic task and the laboratory control of an experimental task (Kvavilashvili, 1992). The child was asked to repeat the instructions to ensure adequate comprehension of the gist of the task and instructions were repeated, as necessary, until the examiner was assured that the child adequately understood the nature of the task. The following are the scripts used in the administration of the ‘naturalistic’ EB-PM task.
High Motivation Condition
Participants were given the following verbatim instructions: “We will be doing several different types of tests this morning. I want you to listen carefully and every time I say ‘Let’s try something different,’ I would like you to say ‘Please give me three points.’ At the end of testing today, you’ll be able to trade those points in for dollar bills. The more points you get, the more dollar bills you’ll get. Okay, now tell me what it is that I would like you to do.”
Low Motivation Condition
Participants were given the following verbatim instructions: “We will be doing some more tests. I want you to listen carefully and every time I say ‘Let’s try something different,’ I would like you to say ‘Please give me three points.’” At the end of testing today, you’ll be able to trade those points in for pennies. The more points you get, the more pennies you’ll get. Okay, now tell me what it is that I would like you to do.”
Design and Procedure
The study used a crossover design with one of two motivation conditions presented during each of the two periods (e.g., either the first or second one-hour block) with no wash-out interval between as previously described in McCauley and colleagues (McCauley, et al., 2009). Briefly, the extrinsic motivation conditions involved the monetary units of either dollars (high) or pennies (low). A randomization table was used to vary motivation condition order across participants. While performing other tasks during the neuropsychological battery (a standard battery order for all participants was maintained to control for ongoing task difficulty), the child was asked to respond “Please give me three points” each time the examiner said “Let’s try something different.” This EB-PM cue was presented every 15–20 minutes (to accommodate the neuropsychological battery), with three PM cue presentations in each of the motivation conditions; each motivation ‘period’ required one hour. The scoring algorithm for the EB-PM task was 2 points for realizing the delayed intention (PM component) and 2 additional points for recalling the correct phrase (retrospective memory component or RM). Correct responses were awarded 4 points, and responses with incorrect RM content (e.g., “Please give me five points” or “Please give me some points”) were awarded 2 points. A maximum of 12 points was available in each motivation condition.
Data Analysis
Statistical significance was defined as α = .05 for all analyses unless otherwise specified. Planned comparisons were analyzed holding significance at α = .05 and all post-hoc comparisons were adjusted using the Bonferroni correction for multiple comparisons. All analyses were conducted with SAS software for Windows, Version 9.2. Categorical variables were analyzed with either chi-square test or Fisher’s exact test as appropriate. The data were analyzed as a cross-over design using a mixed model. Sequence (motivation condition order) and Period (time factor for repeated measures) effects were included in the model. Sequence was nested within-subject and the subject variable was treated as a random effect to account for correlation between multiple measures within the same participant. Other main effects of interest included Age-at-Test (as a continuous variable), Gender, SCI (a measure of socioeconomic status), Group, Race/Ethnicity, Time Postinjury, Motivation Condition, and 2-way interactions between Group and Age-at-Test, Sequence, Period, and Motivation Condition.
RESULTS
Sample Characteristics
The groups differed significantly by racial/ethnic composition due to the higher percentage of African Americans in the OI group and Hispanics in the moderate TBI group (Table 1). There were significant differences by Age-at-Test (F(2,112) = 6.88, p < .02) as the severe TBI group was significantly older than the OI group (p < .05 with Bonferroni correction), but no other between-group comparisons were significant. The groups also differed significantly by mechanism of injury as the OI group sustained more low-velocity injuries (e.g., sports/play) compared to the greater proportion of high-velocity injuries sustained by both TBI groups (e.g., MVA, auto-pedestrian); this difference was also significant between the moderate and severe TBI groups where high-velocity injuries were more frequent in the severe TBI group (Fisher’s exact test, p = .028). There was no significant difference between the groups for the postinjury interval of the three month assessment. The groups did not differ significantly by socioeconomic status (SCI) or gender.
Table 1.
Demographic and Injury Variables of the Sample
| Variable | OI (n=51) | Moderate TBI (n=25) | Severe TBI (n=39) | Statistical Comparison |
|---|---|---|---|---|
| Age-at-Test (years), mean (SD) | 12.3 (2.4) | 13.5 (2.8) | 14.3 (2.6) | F(2,112)=6.88, p<.002 |
| Gender (female : male) | 15 : 36 | 10 : 15 | 12 : 27 | χ2(2)=0.91, p=.63 |
| SCI, mean (SD) | 0.2 (0.85) | 0.03 (0.68) | 0.01 (0.95) | F(2,112)=0.63, p=.53 |
| Race / Ethnicity, n (%) | ||||
| African American | 17 (33.3) | 2 (8.0) | 4 (10.3) | p = .034* |
| American Indian | 0 (0) | 0 (0) | 1 (2.5) | |
| Asian | 1 (2.0) | 0 (0) | 0 (0) | |
| Biracial | 1 (2.0) | 1 (4.0) | 0 (0) | |
| Caucasian | 17 (33.3) | 9 (36.0) | 19 (48.7) | |
| Hispanic | 15 (29.4) | 13 (52.0) | 15 (38.5) | |
| Time Postinjury (days), mean (SD) | 122.7 (24.5) | 116.2 (22.9) | 132.8 (37.4) | F(2,112)=2.65, p=.08 |
| GCS (lowest in 1st 24 hours) | 15.0 (0) | 12.5 (2.3) | 4.8 (2.2) | N/A |
| Mechanism of Injury, n (%) | ||||
| MVA | 1 (2.0) | 6 (24.0) | 16 (41.0) | p < .0001* |
| MCA / Scooter / Moped | 3 (5.9) | 4 (16.0) | 3 (7.7) | |
| RV | 1 (2.0) | 3 (12.0) | 4 (10.3) | |
| Bicycle | 4 (7.8) | 1 (4.0) | 3 (7.7) | |
| Fall | 11 (21.6) | 7 (28.0) | 4 (10.3) | |
| Hit by falling object | 2 (3.9) | 0 (0) | 0 (0) | |
| Sports / Play | 25 (49.0) | 3 (12.0) | 0 (0) | |
| Hit by motor vehicle | 2 (3.9) | 1 (4.0) | 9 (23.0) | |
| Other | 2 (3.9) | 0 (0) | 0 (0) |
Fisher’s exact test
SCI = Socioeconomic Composite Index
GCS = Glasgow Coma Scale score
MVA = motor vehicle accident
MCA = motorcycle accident
RV = recreational or other off-road vehicle
Attrition Analyses
As these data were collected as part of a longitudinal study, analyses were performed to determine if significant differences existed between the sample previously reported by McCauley et al. (McCauley, et al., 2010) in the subacute phase and the current sample which could introduce biases affecting the dependent measures. The groups were not significantly different in terms of age-at-injury, age-at-test, SCI, or GCS (all p > .05) across the two testing occasions. The retention rate from the McCauley et al. (2010) study to the current study was 84% in the OI group, 89.3% in the moderate TBI group; in the severe TBI group, however, we actually had 30% more children (39 vs. 30) who were able to participate in the 3-month assessment than at the prior endpoint.
Prospective Memory Performance
The test for a carryover effect (Motivation Condition × Period) was not significant (F = 0). There were no main effects of gender or SCI (both F < 1). Because of significant age differences between the groups and the wide age range of the sample as a whole, the main effect of and interactions with Age-at-Test were explored; Age-at-Test was dichotomized based on the median age of the total sample (i.e., < 12 years versus ≥ 12 years) and included to test the Age × Group interaction. The Group × Sequence (p = .90), Group × Motivation (p = .25), and Group × Age (p = .74) interactions were not significant and all were removed from the model. The model was then re-estimated retaining the significant Group × Period Condition interaction (p = .0009; see Table 2). Examination of this interaction revealed that the OI (t111 = −4.29, p < .0001; Cohen’s d = −0.85) and severe TBI (t111 = −3.27, p = .001; Cohen’s d = −0.74) groups performed significantly better in the second versus the first Period, irrespective of Motivation Condition; however, the moderate TBI group failed to demonstrate a similar Period effect (p = .13)—this group actually did worse in the second Period compared to the first irrespective of the Motivation Condition (Cohen, 1988). There was no main effect of Sequence (F = .02), but Age-at-Test was significant (p = .03) such that PM performance improved with increasing age in all groups. Main effects for Motivation Condition and Group were found in that performance was better in the high versus low motivation condition, and the groups demonstrated significantly different EB-PM performance levels.
Table 2.
Type III Tests of Fixed Effects for the Event-Based Prospective Memory Scores
| Source | df | F | p-value |
|---|---|---|---|
| Age-at-Test | 1, 105 | 4.82 | .03 |
| SCI | 1, 105 | 1.37 | .25 |
| Gender | 1, 105 | 1.20 | .28 |
| Race/Ethnicity | 3, 105 | 0.47 | .71 |
| Sequence | 1, 105 | 0.02 | .90 |
| Condition | 1, 105 | 15.88 | .0001 |
| Group | 2, 111 | 12.39 | < .0001 |
| Period | 1, 105 | 7.89 | < .006 |
| Group × Period | 2, 111 | 7.48 | .0009 |
SCI = Socioeconomic Composite Index
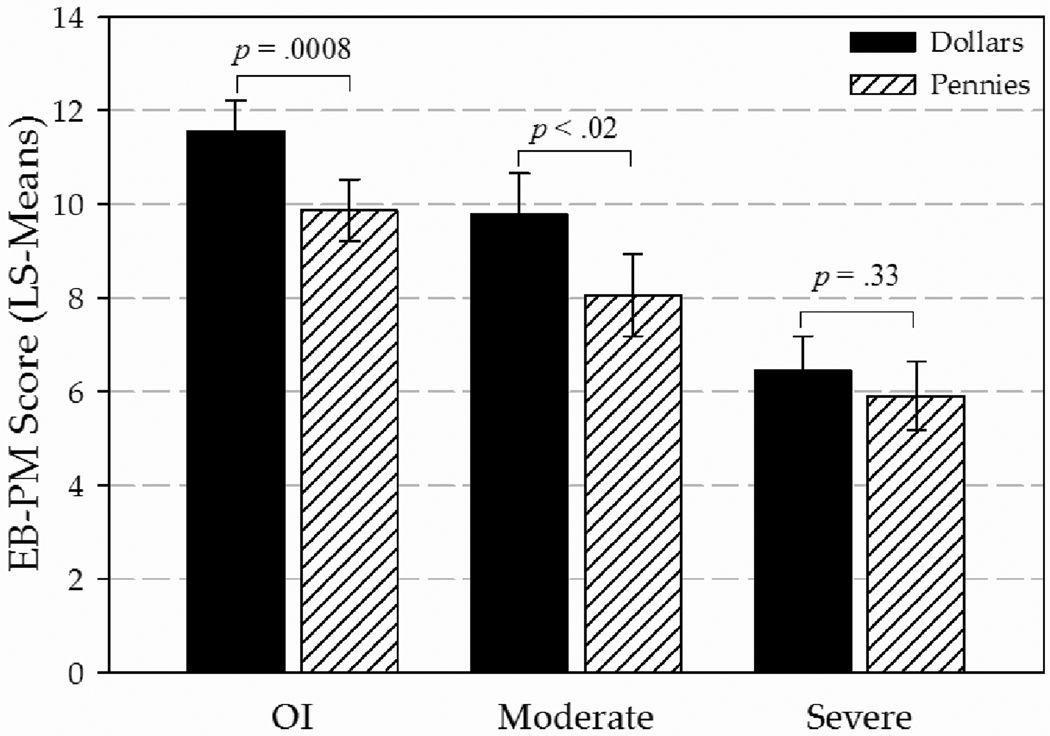
In planned comparisons to address the hypotheses, the OI group outperformed the moderate TBI group (t105 = 1.98, p = .05), and both the OI (t105 = 4.97, p < .0001) and moderate TBI (t105 = 2.57, p < .02) groups scored better than the severe TBI group. The OI and moderate TBI groups demonstrated significantly better EB-PM performance in response to the high motivation incentive (Cohen’s d effect sizes of 0.68 and 0.69, respectively, indicating generally moderate level effects of motivation through monetary incentive condition), but the severe TBI group failed to demonstrate significant improvement (Cohen’s d = 0.22; see Figure 1).
Figure 1.
EB-PM least-squares mean scores by Group and Motivation Condition depict the significant improvements demonstrated within the OI and moderate TBI groups under the High- versus Low-Motivation conditions, whereas the Severe TBI did not. Error bars represent standard errors.
Post-hoc analyses of the incentive effect specifically in the high motivation condition (with Bonferroni correction for multiple comparisons maintained at α < 0.0083) indicated no significant difference in performance of the OI group compared to moderate TBI group although a positive trend was noted (p = .08). The OI (p < .0001) and moderate TBI (p < .006) groups, however, both outperformed the severe TBI group in the high motivation condition. Post-hoc analyses in the low motivation condition again revealed a positive trend (p = .07) for better performance in the OI group compared to the moderate TBI group. Whereas the OI group outperformed the severe TBI group (t105 = 4.0, p = .0001), the performance of the moderate and severe TBI groups did not differ significantly (p = .07).
DISCUSSION
The effect of a monetary incentive (i.e., an extrinsic motivator) on EB-PM performance was investigated in children with moderate to severe TBI. Similar to our findings in children in the subacute phase of recovery, we failed to find complete support for our primary hypothesis that children with severe TBI would respond significantly to monetary incentives on an EB-PM task. However, we found partial support for this in that children with OI and moderate TBI were able to significantly improve their EB-PM performance in the face of a high monetary incentive condition. These results extend previous results of McCauley et al. (2010) by further delineating the postinjury timeframe during which this type and level of incentive fails to produce significant remedial results for children with severe TBI. The results of our previous retrospective study also have been extended by the finding of significantly improved EB-PM performance in children with moderate TBI as early as three months postinjury. Future work in the longitudinal cohort will continue to explore when and if this type of deficit-reduction strategy will prove effective with these children. Given the results in the retrospective study of McCauley et al. (2009), we anticipate that monetary incentives will improve the EB-PM performance of children with severe TBI at an endpoint beyond three months postinjury. We are continuing to collect longitudinal data in this cohort to definitively address this issue.
The question also remains open for further investigation as to how this improved EB-PM performance was achieved in the moderate TBI group. That is, was the effect the result of a generalized effect of arousal in response to reward or the initiation of compensatory PM strategies? Conversely, was the failure of the severe TBI group to improve performance due to the lack of compensatory PM strategies, a failure to appreciate the differential nature of the rewards, or a substantial failure of arousal to the reward as has been found to be the case in adults with severe TBI (Larson, Kelly, Stigge-Kaufman, Schmalfuss, & Perlstein, 2007). It should be noted that the severe TBI group performed better in the second vs. first Period suggesting that either practice with the task or being reminded of the EB-PM task itself was a benefit to them; however, it is unclear why the moderate TBI group would have actually performed more poorly in the second vs. first Period. Future studies in this area are well-advised to seriously consider the measurement of physiological and psychological arousal to further delineate these effects in EB-PM.
On the whole, these results support the idea that motivation is an important factor to consider in PM research, not only children with TBI, but also typically-developing children (Baddeley, 1990; Best, 1992; Einstein & McDaniel, 1996; Gentry & Herrmann, 1990; Winograd, 1988) or children with OI given the improved EB-PM response to a high-value incentive in the OI group of our study. Rehabilitation professionals working with children sustaining TBI should note that, unlike the significant response to incentive demonstrated in these children in the chronic postinjury phase, children with severe TBI at approximately three months postinjury may not benefit significantly in EB-PM performance from some forms of motivation enhancement.
Monetary incentives could easily be incorporated into pediatric rehabilitation programs in a manner similar to that of Furst (1986) where patients were awarded prizes based on the number of points earned in performing an intention. High priority PM behaviors could be identified for each child (e.g., remembering to bring memory notebook to the memory group sessions) and a cash-for-points system could be devised similar to an allowance scheme that the child might already be familiar with. Similarly in an academic setting, PM behaviors such as remembering to bring work to school or completing assignments on time could be rewarded with a cash-for-points system (e.g., completion of relatively long-term and higher value projects are rewarded more points than completion of daily assignments). However, further study will be needed to explore other types of extrinsic and intrinsic motivators to determine what combination of these factors and their postinjury timing could significantly improve EB-PM following pediatric severe TBI.
While it is common to find gender effects in episodic memory (e.g., relatively better recall of verbal stimuli by females and relatively better recall of visuospatial information by males), the same has phenomenon not generally been found in PM. In fact, several studies of PM in healthy adults have reported a failure to find gender effects (G. O. Einstein & M. A. McDaniel, 1990; Kidder, Park, Hertzog, & Morrell, 1997; Maujean, Shum, & McQueen, 2003). Although far less studied, no significant gender effect on PM has been reported in typically-developing children either (Kerns, 2000; Kerns & Price, 2001). Previous work in children with subacute (McCauley, et al., 2010) and chronic (McCauley, et al., 2009) TBI have failed to find a significant effect of gender, and a recent study of EB-PM in children with sickle cell disease has also failed to find a gender effect (McCauley & Pedroza, 2010). Although SES has been shown to moderate outcomes of children with TBI (Taylor, 2004; Taylor et al., 1999; Taylor et al., 2002; Yeates, et al., 1997), no significant effect of SES on EB-PM performance was found in our sample at three months postinjury and this result is similar to findings in pediatric TBI studies by McCauley and colleagues (2009; 2010) and a recent study in children with sickle cell disease (McCauley & Pedroza, 2010).
There are some limitations in this study that should be addressed. The design of the larger study precluded an assessment of retrospective memory (RM) which could have determined the extent to which poor RM abilities may have accounted for impaired EB-PM performance in these children. However, a number of studies have reported that PM and RM are not strongly associated in adults and healthy elderly (Brandimonte & Passolunghi, 1994; Driscoll, McDaniel, & Guynn, 2005; G. O. Einstein & M. A. McDaniel, 1990; Huppert & Beardsall, 1993; Kidder, et al., 1997; Kvavilashvili, 1987; Maylor, 1990; McDaniel & Einstein, 1993; Salthouse, Berish, & Siedlech, 2004), typically-developing children (Kvavilashvili, Messer, & Ebdon, 2001), or most recently, children with sickle cell disease (McCauley & Pedroza, 2010). RM and PM appear to be closely related quite early in development, but tend to dissociate rapidly; there is evidence that by the age of five years, these two memory domains are distinct (Guajardo & Best, 2000; Ruther & Best, 1993). Findings in adults with TBI have produced contradictory findings on the relation between PM and RM (Groot, et al., 2002; Henry, et al., 2007; Mathias & Mansfield, 2005) which may be explained, in part, by differing degrees of RM load in the PM tasks such that when greater demands are made on the RM component, PM and RM become more closely correlated (G.O. Einstein & M.A. McDaniel, 1990). It would be advisable to resist generalizing these contradictory results in adults with TBI to children with TBI. It remains to be seen to what degree PM functioning in children with TBI is dependent on RM and medial temporal lobe integrity, or that of other critical brain structures. Future studies would do well to include a formal assessment of RM abilities to further explore this relation. Advanced neuroimaging in children with TBI would help to more definitively elucidate the direct brain-behavior effects of TBI on EB-PM.
CONCLUSION
Our results suggest that EB-PM performance can be improved with the use of monetary incentives in children with OI and moderate TBI. However, these incentives were not effective for children with severe TBI at three months postinjury. Motivation, arousal, compensatory and other strategies to improve EB-PM in these children at this point in recovery following TBI remain to be devised, implemented, and assessed.
Acknowledgments
This work was supported by National Center for Medical Rehabilitation Research grant K23 HD-40896 (“Prospective memory in normal and head-injured children,” awarded to Stephen R McCauley) and National Institute Neurological Disorders and Stroke grant NS-21889 (“Neurobehavioral outcome of head injury in children,” awarded to Harvey S. Levin). The information in this manuscript and the manuscript itself has never been published either electronically or in print. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Medical Rehabilitation Research or the National Institutes of Health.
We thank the participants and their families for their interest and willingness to be part of this research. I would like to extend my personal appreciation to Drs. Mark A. McDaniel and Harvey S. Levin who graciously served as mentors on my K-23 mentored patient-oriented research career development award. We also acknowledge the support of the General Clinical Research Center at Texas Children’s Hospital, Houston.
Footnotes
None of the authors have any financial or other relationship(s) that could be construed as a conflict of interest with respect to the content of this manuscript.
References
- Baddeley AD. Human memory. Toronto: Allyn & Bacon; 1990. [Google Scholar]
- Barnet-Verzat C, Wolff F-C. Motives for pocket money allowance and family incentives. Journal of Economic Psychology. 2002;23(3):339–366. [Google Scholar]
- Best DL. The role of social interaction in memory improvement. In: Herrmann DJ, Searleman H, Searleman A, McEvoy C, editors. Memory improvement: Implications for memory theory. New York: Springer-Verlag; 1992. pp. 122–149. [Google Scholar]
- Brandimonte MA, Passolunghi MC. The effect of cue-familiarity, cue-distinctiveness, and retention interval on prospective remembering. Quarterly Journal of Experimental Psychology A. 1994;47(3):565–587. doi: 10.1080/14640749408401128. [DOI] [PubMed] [Google Scholar]
- Cockburn J. Task interruption in prospective memory: A frontal lobe function? Cortex. 1995;31(1):87–97. doi: 10.1016/s0010-9452(13)80107-4. [DOI] [PubMed] [Google Scholar]
- Cohen J. Power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc.; 1988. [Google Scholar]
- Committee on Injury Scaling. Abbreviated Injury Scale. Des Plaines, IL: Association for the Advancement of Automotive Medicine; 1990. [Google Scholar]
- Driscoll I, McDaniel MA, Guynn MJ. Apolipoprotein E and prospective memory in normally aging adults. Neuropsychology. 2005;19(1):28–34. doi: 10.1037/0894-4105.19.1.28. [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA. Normal aging and prospective memory. Journal of Experimental Psychology: Learning, Memory, & Cognition. 1990;16(4):717–726. doi: 10.1037//0278-7393.16.4.717. [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA. Normal aging and prospective memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1990;16(4):717–726. doi: 10.1037//0278-7393.16.4.717. [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA. Retrieval processes in prospective memory: Theoretical approaches and some new empirical findings. In: Brandimonte MA, Einstein GO, McDaniel MA, editors. Prospective memory: Theory and applications. Mahwah: Lawrence Erlbaum Associates; 1996. pp. 115–142. [Google Scholar]
- Fortin S, Godbout L, Braun C. Strategic sequence planning and prospective memory impairments in frontally lesioned head trauma patients performing activities of daily living. Brain and Cognition. 2002;48(2–3):361–365. [PubMed] [Google Scholar]
- Fortin S, Godbout L, Braun CM. Cognitive structure of executive deficits in frontally lesioned head trauma patients performing activities of daily living. Cortex. 2003;39(2):273–291. doi: 10.1016/s0010-9452(08)70109-6. [DOI] [PubMed] [Google Scholar]
- Furnham A. Economic socialization: A study of adults' perceptions and uses of allowances (pocket money) to educate children. British Journal of Developmental Psychology. 1999;17(4):585–604. [Google Scholar]
- Furnham A, Kirkcaldy B. Economic socialization: German parents' perceptions and implementation of allowances to educate children. European Psychologist. 2000;5(3):202–215. [Google Scholar]
- Furst C. The memory derby: Evaluating and remediating intention memory. Cognitive Rehabilitation. 1986;4(3):24–26. [Google Scholar]
- Gentry M, Herrmann DJ. Memory contrivances in everyday life. Personality and Social Psychology Bulletin. 1990;16(2):241–253. [Google Scholar]
- Groot YC, Wilson BA, Evans J, Watson P. Prospective memory functioning in people with and without brain injury. Journal of the International Neuropsychological Society. 2002;8(5):645–654. doi: 10.1017/s1355617702801321. [DOI] [PubMed] [Google Scholar]
- Guajardo NR, Best DL. Do preschoolers remember what to do? Incentive and external cues in prospective memory. Cognitive Development. 2000;15(1):75–97. [Google Scholar]
- Hannon R, Adams P, Harrington S, Fries-Dias C, Gipson MT. Effects of brain injury and age on prospective memory self-rating and performance. Rehabilitation Psychology. 1995;40(4):289–298. [Google Scholar]
- Harris JE. Remembering to do things: A forgotten topic. In: Gruneberg MM, Morris PE, Sykes RN, editors. Everyday memory: Actions and absent-mindedness. New York: Academic Press; 1984. pp. 71–92. [Google Scholar]
- Hauser RM, Warren JR. Socioeconomic indexes for occupations: A review, update, and critique. In: Raftery A, editor. Sociological Methodology. Vol. 27. Blackwell Publishing; 1999. pp. 177–298. [Google Scholar]
- Henry JD, Phillips LH, Crawford JR, Kliegel M, Theodorou G, Summers F. Traumatic brain injury and prospective memory: influence of task complexity. Journal of Clinical and Experimental Neuropsychology. 2007;29(5):457–466. doi: 10.1080/13803390600762717. [DOI] [PubMed] [Google Scholar]
- Hicks JL, Cook GI, Marsh RL. Detecting event-based prospective memory cues occurring within and outside the focus of attention. American Journal of Psychology. 2005;118(1):1–11. [PubMed] [Google Scholar]
- Huppert FA, Beardsall L. Prospective memory impairment as an early indicator of dementia. Journal of Clinical and Experimental Neuropsychology. 1993;15(5):805–821. doi: 10.1080/01688639308402597. [DOI] [PubMed] [Google Scholar]
- Kerns KA. The CyberCruiser: An investigation of development of prospective memory in children. Journal of the International Neuropsychological Society. 2000;6(1):62–70. doi: 10.1017/s1355617700611074. [DOI] [PubMed] [Google Scholar]
- Kerns KA, Price KJ. An investigation of prospective memory in children with ADHD. Child Neuropsychology. 2001;7(3):162–171. doi: 10.1076/chin.7.3.162.8744. [DOI] [PubMed] [Google Scholar]
- Kidder DP, Park DC, Hertzog C, Morrell RW. Prospective memory and aging: The effects of working memory and prospective memory task load. Neuropsychology, Development, and Cognition Section B Aging, Neuropsychology, and Cognition. 1997;4(2):93–112. [Google Scholar]
- Kinsella G, Murtagh D, Landry A, Homfray K, Hammond M, O'Beirne L, et al. Everyday memory following traumatic brain injury. Brain Injury. 1996;10(7):499–507. doi: 10.1080/026990596124214. [DOI] [PubMed] [Google Scholar]
- Kliegel M, Eschen A, Thöne-Otto AI. Planning and realization of complex intentions in traumatic brain injury and normal aging. Brain and Cognition. 2004;56(1):43–54. doi: 10.1016/j.bandc.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Knight RG, Harnett M, Titov N. The effects of traumatic brain injury on the predicted and actual performance of a test of prospective remembering. Brain Injury. 2005;19(1):27–38. doi: 10.1080/02699050410001720022. [DOI] [PubMed] [Google Scholar]
- Knight RG, Titov N, Crawford M. The effects of distraction on prospective remembering following traumatic brain injury assessed in a simulated naturalistic environment. Journal of the International Neuropsychological Society. 2006;12(1):8–16. doi: 10.1017/S1355617706060048. [DOI] [PubMed] [Google Scholar]
- Kvavilashvili L. Remembering intention as a distinct form of memory. British Journal of Psychology. 1987 Nov;78(4):507–518. 1987. [Google Scholar]
- Kvavilashvili L. Remembering intentions: A critical review of existing experimental paradigms. Applied Cognitive Psychology. 1992;6(6):507–524. [Google Scholar]
- Kvavilashvili L, Messer DJ, Ebdon P. Prospective memory in children: The effects of age and task interruption. Developmental Psychology. 2001;37(3):418–430. doi: 10.1037//0012-1649.37.3.418. [DOI] [PubMed] [Google Scholar]
- Larson MJ, Kelly KG, Stigge-Kaufman DA, Schmalfuss IM, Perlstein WM. Reward context sensitivity impairment following severe TBI: an event-related potential investigation. Journal of the International Neuropsychological Society. 2007;13(4):615–625. doi: 10.1017/S1355617707070762. [DOI] [PubMed] [Google Scholar]
- Louda J, Loseva D, Mielke R. Prospective memory in patients with traumatic brain injury: An overview. Zeitschrift fur Neuropsychologie. 2007;18(2):91–99. [Google Scholar]
- Mathias JL, Mansfield KM. Prospective and declarative memory problems following moderate and severe traumatic brain injury. Brain Injury. 2005;19(4):271–282. doi: 10.1080/02699050400005028. [DOI] [PubMed] [Google Scholar]
- Maujean A, Shum D, McQueen R. The effect of cognitive demand on prospective memory in individuals with traumatic brain injury. Brain Impairment. 2003;4(2):135–145. [Google Scholar]
- Maylor EA. Age and prospective memory. Quarterly Journal of Experimental Psychology A. 1990;42(3-A):471–493. doi: 10.1080/14640749008401233. [DOI] [PubMed] [Google Scholar]
- McCauley SR, Levin HS. Prospective memory in pediatric traumatic brain injury: A preliminary study. Developmental Neuropsychology. 2004;25(1–2):5–20. doi: 10.1080/87565641.2004.9651919. [DOI] [PubMed] [Google Scholar]
- McCauley SR, McDaniel MA, Pedroza C, Chapman SB, Levin HS. Incentive effects on event-based prospective memory performance in children and adolescents with traumatic brain injury. Neuropsychology. 2009;23(2):201–209. doi: 10.1037/a0014192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley SR, Pedroza C. Event-based prospective memory in children with sickle cell disease: effect of cue distinctiveness. Child Neuropsychology. 2010;16(3):293–312. doi: 10.1080/09297041003601470. [DOI] [PubMed] [Google Scholar]
- McCauley SR, Pedroza C, Chapman SB, Cook LG, Hotz G, Vasquez AC, et al. Event-based prospective memory performance during subacute recovery following moderate to severe traumatic brain injury in children: Effects of monetary incentives. Journal of the International Neuropsychological Society. 2010;16(2):335–341. doi: 10.1017/S135561770999138X. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Einstein GO. The importance of cue familiarity and cue distinctiveness in prospective memory. Memory. 1993;1(1):23–41. doi: 10.1080/09658219308258223. [DOI] [PubMed] [Google Scholar]
- Meacham JA, Dumitru J. Prospective remembering and external-retrieval cues. Washington, DC: 1976. (No. MS 1284) [Google Scholar]
- Meacham JA, Leiman B. Remembering to perform future actions. In: Neisser U, editor. Memory observed; Remembering in natural contexts. San Francisco: W.H. Freeman and Company; 1982. pp. 327–336. [Google Scholar]
- Neul SKT, Drabman RS. A practical procedure for instituting a chore and allowance program for grade school children: Specific guidelines for clinicians. Child & Family Behavior Therapy. 2001;23(4):37–45. [Google Scholar]
- Pastore DR, Friedman SB. Allowances, household chores, and curfews. In: Friedman SB, Fisher M, editors. Comprehensive adolescent health care. St. Louis, MO: Quality Medical Publishing; 1992. [Google Scholar]
- Raskin SA, Sohlberg MM. Prospective memory intervention: A review and evaluation of a pilot restorative intervention. Brain Impairment. 2009;10(1):76–86. [Google Scholar]
- Roche NL, Fleming JM, Shum DH. Self-awareness of prospective memory failure in adults with traumatic brain injury. Brain Injury. 2002;16(11):931–945. doi: 10.1080/02699050210138581. [DOI] [PubMed] [Google Scholar]
- Roche NL, Moody A, Szabo K, Fleming JM, Shum DHK. Prospective memory in adults with traumatic brain injury: An analysis of perceived reasons for remembering and forgetting. Neuropsychological Rehabilitation. 2007;17(3):314–334. doi: 10.1080/09602010600831004. [DOI] [PubMed] [Google Scholar]
- Ruther NM, Best DL. Development of prospective memory in preschoolers; Paper presented at the Meeting of the Southeastern Psychological Association.1993. [Google Scholar]
- Salthouse TA, Berish DE, Siedlech KL. Construct validity and age sensitivity of prospective memory. Memory and Cognition. 2004;32(7):1133–1148. doi: 10.3758/bf03196887. [DOI] [PubMed] [Google Scholar]
- Shum D, Valentine M, Cutmore T. Performance of individuals with severe long-term traumatic brain injury on time-, event-, and activity-based prospective memory tasks. Journal of Clinical and Experimental Neuropsychology. 1999;21(1):49–58. doi: 10.1076/jcen.21.1.49.943. [DOI] [PubMed] [Google Scholar]
- Taylor HG. Research on outcomes of pediatric traumatic brain injury: current advances and future directions. Developmental Neuropsychology. 2004;25(1–2):199–225. doi: 10.1080/87565641.2004.9651928. [DOI] [PubMed] [Google Scholar]
- Taylor HG, Yeates KO, Wade SL, Drotar D, Klein SK, Stancin T. Influences on first-year recovery from traumatic brain injury in children. Neuropsychology. 1999;13(1):76–89. doi: 10.1037//0894-4105.13.1.76. [DOI] [PubMed] [Google Scholar]
- Taylor HG, Yeates KO, Wade SL, Drotar D, Stancin T, Minich N. A prospective study of short- and long-term outcomes after traumatic brain injury in children: behavior and achievement. Neuropsychology. 2002;16(1):15–27. doi: 10.1037//0894-4105.16.1.15. [DOI] [PubMed] [Google Scholar]
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness: A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Thöne-Otto AI, Walther K. How to design an electronic memory aid for brain-injured patients: Considerations on the basis of a model of prospective memory. International Journal of Psychology. 2003;38(4):236–244. [Google Scholar]
- Ward H, Shum D, Dick B, McKinlay L, Baker-Tweney S. Interview study of the effects of paediatric traumatic brain injury on memory. Brain Injury. 2004;18(5):471–495. doi: 10.1080/02699050310001646107. [DOI] [PubMed] [Google Scholar]
- Ward H, Shum D, McKinlay L, Baker-Tweney S, Wallace G. Development of prospective memory: Tasks based on the prefrontal-lobe model. Child Neuropsychology. 2005;11(6):527–549. doi: 10.1080/09297040490920186. [DOI] [PubMed] [Google Scholar]
- Ward H, Shum D, McKinlay L, Baker S, Wallace G. Prospective memory and pediatric traumatic brain injury: effects of cognitive demand. Child Neuropsychology. 2007;13(3):219–239. doi: 10.1080/09297040600910003. [DOI] [PubMed] [Google Scholar]
- Wilkins A, Baddeley AD. Remembering to recall in everyday life: An approach to absent-mindedness. In: Gruneberg MM, Morris PE, Sykes RN, editors. Practical aspects of memory. London: Academic Press; 1978. [Google Scholar]
- Williams DH, Levin HS, Eisenberg HM. Mild head injury classification. Neurosurgery. 1990;27(3):422–428. doi: 10.1097/00006123-199009000-00014. [DOI] [PubMed] [Google Scholar]
- Wilson B. The rehabilitation of memory. New York: Guilford; 1987. [Google Scholar]
- Winograd E. Some observations on prospective remembering. In: Gruneberg MM, Morris PE, Sykes RN, editors. Practical Aspects of Memory: Current Research and Issues. Vol. 1. Oxford, England: John Wiley & Sons; 1988. pp. 348–353. (Memory in everyday life) [Google Scholar]
- Yeates KO, Taylor HG, Drotar D, Wade SL, Klein S, Stancin T, et al. Preinjury family environment as a determinant of recovery from traumatic brain injuries in school-age children. Journal of the International Neuropsychological Society. 1997;3(6):617–630. [PubMed] [Google Scholar]