Abstract
Objectives
We assessed the influence of tumor size and surgical approach on the use of lymphadenectomy and adrenalectomy with radical nephrectomy.
Methods
We evaluated patients with renal cell carcinoma (RCC) enrolled in the US Kidney Cancer Study, a case–control study in the metropolitan areas of Detroit and Chicago from 2002–2007. We identified patients who underwent open (ORN) or laparoscopic radical nephrectomy (LRN). We used medical records and SEER data to determine the proportion of patients who underwent lymphadenectomy or adrenalectomy. Bivariate analyses were performed to evaluate associations between tumor size, surgical approach, and receipt of lymphadenectomy or adrenalectomy.
Results
We identified 730 patients who underwent ORN (427, 58%) or LRN (303, 42%) for RCC from 2002–2007. Among this group, 11% and 24% underwent lymphadenectomy or adrenalectomy, respectively. Lymphadenectomy was more common among patients treated from an open surgical approach (14.1% ORN vs 5.9% LRN, p<0.01); this difference was most pronounced for cases with tumors between 4–7 cm (15.9% vs 2.9%, p=0.01). Patients treated with ORN were also more likely to undergo adrenalectomy, with the greatest discrepancy among cases with tumors ≤ 4 cm (21.7% vs. 11.4%, p<0.01).
Conclusions
Among patients undergoing radical nephrectomy for RCC, the use of lymphadenectomy and adrenalectomy is relatively uncommon and varies by tumor size and surgical approach. With an increasing number of patients with small tumors, the diffusion of laparoscopy, and the emergence of clinical trials evaluating systemic adjuvant therapies, our findings highlight important considerations for optimizing surgical management of patients with RCC.
Keywords: lymph node excision, adrenalectomy, carcinoma, renal cell, laparoscopy, neoplasm staging
Introduction
Recent trends in surgical management of patients with renal cell carcinoma (RCC) have been influenced by a host of evolving issues. First, the rising incidence of RCC has coincided with increasing rates of surgical therapy, particularly among patients with small, early-stage tumors [1,2] Second, the emergence and refinement of laparoscopic urology has prompted a shift from open to minimally-invasive surgical treatments for many patients undergoing radical nephrectomy. Third, the development and approval of effective systemic targeted therapy has raised the prospect for potential adjuvant treatments for patients treated with radical nephrectomy for high-risk RCC.
Of direct relevance to this evolving therapeutic landscape is the still poorly-defined role of lymphadenectomy and adrenalectomy as adjuncts to kidney removal during radical nephrectomy. Although retrospective studies have suggested longer survival associated with lymphadenectomy for select patients with high-risk RCC, a large clinical trial noted no similar benefit.[3–6] Likewise, while adrenal involvement provides powerful prognostic information, this finding is exceptionally rare with negative radiographic imaging, and there is no known survival benefit from adrenalectomy, especially for patients with small renal tumors.[7–12] As such, there is no standard approach to management of the adrenal gland and regional lymph nodes in patients undergoing radical nephrectomy. Although European Association of Urology guidelines describe indications for adrenalectomy and lymphadenectomy, there is no such discussion in the recently-released American Urological Association Guidelines for management of patients with small renal masses.[13,14]
Given this uncertainty, there is likely to be significant variation in surgeons’ use of lymphadenectomy and adrenalectomy. However, the implications of such variation will depend on the clinical context. For instance, this variation might be beneficial if these procedures are common among patients with higher-risk tumors and rare in other clinical settings. Conversely, this variation might be detrimental if, for instance, the use of lymphadenectomy and adrenalectomy differs systematically based on surgical approach. In this context, we used data from the National Cancer Institute’s (NCI’s) United States Kidney Cancer Study (KCS) to evaluate the utilization of lymphadenectomy and adrenalectomy among contemporary patients undergoing radical nephrectomy for renal cell carcinoma. We also specifically assessed the influence of tumor size and surgical approach on the use of these adjuncts to total nephrectomy. By providing a better understanding of current practice patterns with respect to lymphadenectomy and adrenalectomy, these data may ultimately prove useful to efforts aimed at optimizing the contemporary surgical management of patients with RCC.
Material and Methods
Analytic cohort
Our analysis includes RCC patients from the NCI’s U.S. KCS, a population-based case–control study conducted in the metropolitan areas of Detroit, Michigan (Wayne, Oakland, and Macomb Counties) and Chicago, Illinois (Cook County) from 2002 through 2007. Eligible cases for this study included resident Caucasian and African-American men and women aged 20 to 79 years, newly diagnosed with histologically confirmed carcinoma of the renal parenchyma (ICD-02-C64.9) from February 1, 2002 through January 31, 2007 in Detroit, or January 1, 2003 through December 31, 2003 in Chicago. The KCS oversampled African-Americans, as the study’s primary focus was racial disparities in the diagnosis and treatment of RCC. In Detroit, potential cases were identified through the Metropolitan Detroit Cancer Surveillance System, an NCI Surveillance, Epidemiology, and End Results (SEER) program member. In Chicago, investigators identified potential cases from pathology reports issued at local hospitals. Additional details regarding case recruitment are summarized elsewhere. [15] The study was approved by the Institutional Review Boards at all participating institutions.
During the study interval, KCS investigators identified 1,918 men and women diagnosed with RCC in the two study regions. Because our analysis relied in part on SEER data that were available only for the Detroit study area, we limited our study to the 1,603 patients diagnosed in Detroit. Among this sample, 1,018 and 951 patients consented to the interview or the interview and medical record review, respectively. From the latter group, we identified all patients who underwent open (ORN) or laparoscopic radical nephrectomy (LRN).
Study variables
For each case in the analytic cohort, KCS personnel ascertained demographic and clinical characteristics from the following sources: 1) medical record review; 2) participant interview; and 3) SEER tumor registry data (Detroit cases only). Patient demographics (e.g., age, sex), medical history (including presenting signs and symptoms and comorbidities), clinical and pathological characteristics of the kidney cancer, and treatment(s) received (including adrenalectomy) were abstracted from the medical record. We used routinely-collected SEER data to identify patients who received lymphadenectomy, defined by presence of lymph nodes in the surgical specimen (as documented in the pathology report). For patients who had lymphadenectomy performed, we also used SEER data to determine the number of nodes that were examined and/or positive for malignancy.
Statistical analysis
As our initial analytic step, we determined the proportions of patients who underwent lymphadenectomy and/or adrenalectomy at the time of radical nephrectomy. We then performed bivariate analyses to evaluate for an association between patient-and cancer-specific variables and receipt of lymphadenectomy or adrenalectomy. After stratifying patients by tumor size (≤ 4 cm vs 4–7 cm vs > 7 cm), we compared the proportions of patients receiving lymphadenectomy or adrenalectomy according to surgical approach (open vs laparoscopic). Finally, for patients undergoing lymphadenectomy, we compared the number of lymph nodes removed for those treated with laparoscopic versus open surgery. We used chi-squared tests for all tests of statistical significance except for comparisons of lymph node counts, where we used Fisher’s exact test to account for small cell size. All statistical testing was two-sided, carried out using computerized software (SAS v9.1, SAS Institute, Cary, NC) and performed at the 5% significance level.
Results
Table 1 summarizes demographic and cancer-specific factors for our analytic cohort of 730 patients who underwent radical nephrectomy for RCC from 2002 through 2007. Compared to the remaining cases accrued in Detroit, patients treated with radical nephrectomy were more likely to be white (74% vs 69%, p=0.03), female (44% vs 39%, p=0.05), and to have localized tumors (78% vs 70%, p<0.01). The operations for patients in the analytic cohort were performed by 121 surgeons practicing at 32 different hospitals. Although the annual proportion of patients undergoing LRN increased significantly from 23% to 48% between 2002 and 2007, ORN was performed more frequently (427/730, 58%) over the entire study interval. A majority of patients had low-grade (56%), organ-confined tumors (78%) with clear cell histology (75%). Patients with more advanced tumors (based on size, stage, and grade) were significantly more likely to receive ORN (all p<0.01).
Table 1.
Patient and tumor characteristics stratified by surgical approach.
| Total (n=730) | ORN (n=427) | LRN (n=303) | ||
|---|---|---|---|---|
| N | N (%)* | N (%)* | p** | |
| PATIENT CHARACTERISTICS | ||||
| Age at diagnosis (years) | ||||
| 20 – 44 | 90 | 47(52) | 43(48) | >0.20 |
| 45 – 54 | 170 | 109(64) | 61(36) | |
| 55 – 64 | 224 | 127(57) | 97(43) | |
| 64 – 74 | 183 | 109(60) | 74(40) | |
| 75 – 79 | 63 | 35(56) | 28(44) | |
| Year of diagnosis | ||||
| 2002 | 130 | 100 (77) | 30 (23) | <0.001 |
| 2003 | 147 | 94 (64) | 53 (36) | |
| 2004 | 186 | 99 (53) | 87 (47) | |
| 2005 | 145 | 70 (48) | 75 (52) | |
| 2006 & 2007 | 122 | 64 (52) | 58 (48) | |
| Sex | ||||
| Male | 414 | 250(61) | 163(39) | >0.20 |
| Female | 316 | 177(56) | 140(44) | |
| Race | ||||
| White | 543 | 323(59) | 220(41) | >0.20 |
| Black | 187 | 104(56) | 84(44) | |
| Education | ||||
| <= 11 years | 113 | 70(62) | 43(38) | >0.20 |
| High school grad or equivalent | 262 | 155(59) | 107(41) | |
| 1–3 years college | 196 | 114(58) | 82(42) | |
| 4+ years college | 159 | 88 (55) | 71(45) | |
| Body Mass Index (BMI)a | ||||
| <25 | 138 | 78(57) | 60(43) | >0.20 |
| 25 – <30 | 253 | 144(57) | 109(43) | |
| 30 – <35 | 184 | 110(60) | 74(40) | |
| 35+ | 149 | 90(60) | 59(40) | |
| TUMOR CHARACTERISTICS | ||||
| Presence of renal cystsb | ||||
| No | 482 | 292(61) | 190(39) | 0.08 |
| Yes | 238 | 128(54) | 110(46) | |
| Tumor size (largest tumor)c | ||||
| < 4 cm | 310 | 152(49) | 158(51) | <0.001 |
| 4 – 7 cm | 228 | 126(55) | 102(45) | |
| > 7 cm | 183 | 142(78) | 41(22) | |
| SEER Staged | ||||
| Local | 571 | 313(55) | 258(45) | <0.001 |
| Regional | 125 | 88(70) | 37(30) | |
| Distant | 33 | 25(76) | 8(24) | |
| Histology | ||||
| Clear cell | 549 | 325(59) | 224(41) | 0.17 |
| Papillary | 81 | 38(47) | 43(53) | |
| Chromophobe | 46 | 31(67) | 15(33) | |
| Cystic RCC | 37 | 22(60) | 15(40) | |
| Other histologye | 17 | 11(65) | 6(35) | |
| Tumor gradef | ||||
| Well-differentiated | 67 | 40(60) | 27(40) | >0.20 |
| Moderately-differentiated | 239 | 122(51) | 117(49) | |
| Poorly-differentiated | 150 | 85(57) | 65(43) | |
| Undifferentiated | 47 | 31(66) | 16(34) | |
| Fuhrman Grade (FG)g | ||||
| FG 1 | 77 | 46(60) | 31(40) | 0.01 |
| FG 2 | 329 | 171(52) | 158(48) | |
| FG 3 | 188 | 116(62) | 72(38) | |
| FG 4 | 48 | 36(75) | 12(25) | |
Row percentages;
p-value evaluating for association between patient/tumor characteristics and surgical approach (ORN vs LRN);
6 cases missing/unknown;
10 cases missing/unknown;
9 cases missing/unknown;
1 case missing/unknown;
other histologies include acidophil carcinoma, sarcomatoid renal cell carcinoma, collecting duct carcinoma, and granular cell carcinoma;
227 cases missing/unknown;
88 cases missing/unknown
Table 2 presents characteristics of the analytic cohort according to receipt of lymphadenectomy or adrenalectomy. Overall, lymphadenectomy and adrenalectomy were performed in 11% and 24% of patients undergoing radical nephrectomy, respectively. The use of lymphadenectomy decreased with age (p=0.04), and adrenalectomy was performed more frequently among male (vs female) patients (p<0.01). Lymphadenectomy was performed more frequently among patients with larger, higher-stage, and higher-grade tumors (all p<0.01). Adrenalectomy was also performed more frequently for patients with larger or higher-stage cancers (all p< 0.01), but its use did not vary by tumor grade. While the proportion of patients undergoing lymphadenectomy remained stable overall, utilization of adrenalectomy increased significantly during the study interval (p<0.01). Among patients who underwent ORN, utilization of lymphadenectomy (p=0.01) and adrenalectomy (p<0.01) generally increased from 2002 through 2007 (Table 3).
Table 2.
Patient and tumor characteristics stratified by receipt of lymphadenectomy or adrenalectomy.
| Total | Lymphadenectomy | Adrenalectomy | |||||
|---|---|---|---|---|---|---|---|
| N | No (%)* | Yes (%)* | p** | No (%)* | Yes (%)* | p** | |
| TOTAL | 730 | 652 (89) | 78 (11) | 556 (76) | 174 (24) | ||
| PATIENT CHARACTERISTICS | |||||||
| Age at diagnosis (years) | |||||||
| 20 – 44 | 90 | 73 (81) | 17 (19) | 0.04 | 69 (77) | 21 (23) | >0.20 |
| 45 – 54 | 168 | 148 (88) | 20 (12) | 129 (77) | 39 (23) | ||
| 55 – 64 | 226 | 204 (90) | 22 (10) | 170 (75) | 56 (25) | ||
| 64 – 74 | 184 | 168 (91) | 16 (9) | 142 (77) | 42 (23) | ||
| 75 – 79 | 62 | 59 (95) | 3 (5) | 46 (74) | 16 (26) | ||
| Sex | |||||||
| Male | 414 | 374 (90) | 40 (10) | >0.20 | 297 (72) | 117 (28) | <0.01 |
| Female | 316 | 278 (88) | 38 (12) | 259 (82) | 57 (18) | ||
| Race | |||||||
| White | 543 | 488 (90) | 55 (10) | >0.20 | 410 (76) | 133 (24) | >0.20 |
| Black | 187 | 164 (88) | 23 (12) | 146 (78) | 41 (22) | ||
| Education | |||||||
| <= 11 years high school | 113 | 99 (88) | 14 (12) | >0.20 | 94 (83) | 19 (17) | 0.15 |
| High school grad or equivalent | 262 | 235 (90) | 27 (10) | 203 (77) | 59 (23) | ||
| 1–3 years college | 196 | 177 (90) | 19 (10) | 143 (73) | 53 (27) | ||
| 4 years college or more | 159 | 141 (89) | 18 (11) | 116 (73) | 43 (27) | ||
| Body Mass Index (BMI)a | |||||||
| <25 | 138 | 118 (86) | 20 (14) | >0.20 | 113 (82) | 25 (18) | >0.20 |
| 25 – <30 | 253 | 227 (90) | 26 (10) | 195 (77) | 58 (23) | ||
| 30 – <35 | 184 | 166 (90) | 18 (10) | 133 (72) | 51 (28) | ||
| 35+ | 149 | 135 (91) | 14 (9) | 110 (74) | 39 (26) | ||
| Smoking status | |||||||
| Never smoker | 260 | 227 (87) | 33 (13) | 0.20 | 192 (74) | 68 (26) | >0.20 |
| Occasional | 33 | 30 (91) | 3 (9) | 23 (70) | 10 (30) | ||
| Former smoker | 255 | 236 (93) | 19 (7) | 195 (76) | 60 (24) | ||
| Current smoker | 182 | 159 (87) | 23 (13) | 146 (80) | 36 (20) | ||
| TUMOR CHARACTERISTICS | |||||||
| Presence of renal cystsb | |||||||
| No | 482 | 433 (90) | 49 (10) | >0.20 | 369 (77) | 113 (23) | >0.20 |
| Yes | 238 | 209 (88) | 29 (12) | 177 (74) | 61 (26) | ||
| Tumor size (largest tumor)c | |||||||
| < 4 cm | 310 | 298 (96) | 12 (4) | <0.01 | 259 (84) | 51 (16) | <0.01 |
| 4 – 7 cm | 228 | 205 (90) | 23 (10) | 178 (78) | 50 (22) | ||
| > 7 cm | 183 | 140 (77) | 43 (23) | 114 (62) | 69 (38) | ||
| SEER Staged | |||||||
| Local | 571 | 530 (93) | 41 (7) | <0.01 | 453 (79) | 118 (21) | <0.01 |
| Regional | 125 | 97 (78) | 28 (22) | 80 (64) | 45 (36) | ||
| Distant | 33 | 24 (73) | 9 (27) | 23 (70) | 10 (30) | ||
| Histology | |||||||
| Clear cell | 549 | 492 (90) | 57 (10) | 0.01 | 409 (74) | 140 (26) | >0.20 |
| Papillary | 81 | 73 (90) | 8 (10) | 65 (80) | 16 (20) | ||
| Chromophobe | 46 | 44 (96) | 2 (4) | 37 (80) | 9 (20) | ||
| Cystic RCC | 37 | 32 (86) | 5 (14) | 32 (86) | 5 (14) | ||
| Other histologye | 17 | 11 (65) | 6 (35) | 13 (76) | 4 (24) | ||
| Tumor gradef | |||||||
| Well-differentiated | 67 | 65 (97) | 2 (3) | 0.06 | 54 (81) | 13 (19) | >0.20 |
| Moderately-differentiated | 239 | 215 (90) | 24 (10) | 182 (76) | 57 (24) | ||
| Poorly-differentiated | 150 | 130 (87) | 20 (13) | 106 (71) | 44 (29) | ||
| Undifferentiated | 47 | 39 (83) | 8 (17) | 32 (68) | 15 (32) | ||
| Fuhrman Grade (FG)g | |||||||
| FG 1 | 77 | 73 (95) | 4 (5) | <0.01 | 63 (82) | 14 (18) | 0.06 |
| FG 2 | 329 | 300 (91) | 29 (9) | 257 (78) | 72 (22) | ||
| FG 3 | 188 | 167 (89) | 21 (11) | 131 (70) | 57 (30) | ||
| FG 4 | 48 | 35 (73) | 13 (27) | 33 (69) | 15 (31) | ||
Row percentages;
p-value evaluating for association between patient/tumor characteristics and surgical approach (ORN vs LRN);
6 cases missing/unknown;
10 cases missing/unknown;
9 cases missing/unknown;
1 case missing/unknown;
other histologies include acidophil carcinoma, sarcomatoid renal cell carcinoma, collecting duct carcinoma, and granular cell carcinoma;
227 cases missing/unknown;
88 cases missing/unknown
Table 3.
Temporal trends in receipt of lymphadenectomy or adrenalectomy stratified by surgical approach.
| Total (n=730) | ||||||
|---|---|---|---|---|---|---|
| Lymphadenectomy | Adrenalectomy | |||||
| No (%)* | Yes (%)* | p** | No (%)* | Yes (%)* | p** | |
| TOTAL | 652 (89) | 78 (11) | 556 (76) | 174 (24) | ||
| Year of Diagnosis | ||||||
| 2002 | 123 (91) | 12 (9) | >0.20 | 113 (87) | 17 (13) | <0.01 |
| 2003 | 135 (88) | 18(12) | 120 (82) | 27 (18) | ||
| 2004 | 159 (90) | 19 (10) | 133 (71) | 53 (29) | ||
| 2005 | 124 (88) | 17 (12) | 103 (71) | 42 (29) | ||
| 2006/2007 | 111 (90) | 12 (10) | 87 (71) | 35 (29) | ||
|
ORN (n = 427) | ||||||
| Lymphadenectomy | Adrenalectomy | |||||
| No (%)* | Yes (%)* | p** | No (%)* | Yes (%)* | p** | |
| TOTAL | 367 (86) | 60 (14) | 307 (72) | 120 (28) | ||
| Year of Diagnosis | ||||||
| 2002 | 93 (93) | 7 (7) | 0.01 | 83 (83) | 17 (17) | <0.01 |
| 2003 | 84 (89) | 10 (11) | 73 (78) | 21 (22) | ||
| 2004 | 78 (79) | 21 (21) | 64 (65) | 35 (35) | ||
| 2005 | 55 (79) | 15 (21) | 45 (64) | 25 (36) | ||
| 2006/2007 | 57 (89) | 7 (11) | 42 (66) | 22 (34) | ||
|
LRN (n= 303) | ||||||
| Lymphadenectomy | Adrenalectomy | |||||
| No (%)* | Yes (%)* | p** | No (%)* | Yes (%)* | p** | |
| TOTAL | 285 (94) | 18 (6) | 249 (82) | 54 (18) | ||
| Year of Diagnosis | ||||||
| 2002 | 30 (100) | 0 (0) | >0.20 | 30 (100) | 0 (0) | 0.03 |
| 2003 | 51 (96) | 2 (4) | 47 (89) | 6 (11) | ||
| 2004 | 81 (93) | 6 (7) | 69 (79) | 18 (21) | ||
| 2005 | 69 (92) | 6 (8) | 58 (77) | 17 (23) | ||
| 2006/2007 | 54 (93) | 4 (7) | 45 (78) | 13 (22) | ||
Row percentages;
p-value evaluating for association between year of diagnosis and performance of lymphadenectomy or adrenalectomy
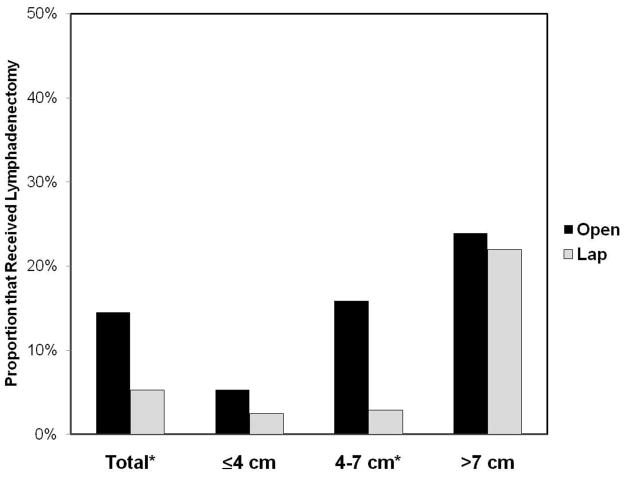
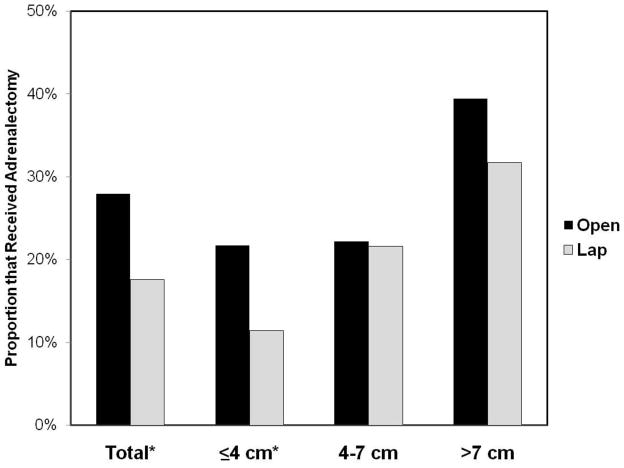
Figures 1 and 2 compare the receipt of lymphadenectomy and adrenalectomy, respectively, for patients undergoing ORN versus LRN, stratified by tumor size. For the entire cohort, lymphadenectomy was performed more frequently for patients who underwent ORN vs LRN (14.1% vs 5.9%, p<0.01); this difference was most pronounced among patients with tumors between 4–7 cm (15.9% ORN vs 2.9% LRN, p=0.01). Likewise, patients treated with ORN were more likely to receive adrenalectomy (27.9% ORN vs 17.6% LRN, p<0.01), with the greatest discrepancy among cases with tumors ≤ 4 cm (21.7% ORN vs. 11.4% LRN, p<0.01) (Figure 2).
FIGURE 1.
Use of Lymphadenectomy Stratified by Tumor Size.
*p<0.05 for comparison between open and laparoscopic radical nephrectomy
FIGURE 2.
Use of Adrenalectomy Stratified by Tumor Size.
*p<0.05 for comparison between open and laparoscopic radical nephrectomy
Table 4 describes the frequency of node-positive disease and number of nodes removed among those who underwent lymphadenectomy. Among this group, 41% had only one node examined, and only 17% had >6 nodes examined (representing 2% of the entire analytic cohort). Although lymphadenectomy was rare, its yield was considerable, with 23% of patients having lymph node-positive RCC. Neither the extent of lymphadenectomy (i.e., the number of nodes examined) nor the proportion of cases with lymph node involvement differed significantly based on surgical approach (all p>0.20, Table 4).
Table 4.
Lymph nodes examined for patients with lymphadenectomy by surgical approach
| Total (N=78) | ORN (N=62) | LRN (N=16) | |||
|---|---|---|---|---|---|
| N (%)* | N (%)* | N (%)* | p** | ||
| Number of Nodes Examined^ | |||||
| 1 | 32 (45) | 26 (45) | 6 (46) | >0.20 | |
| 2 to 5 | 26 (37) | 21 (36) | 5 (39) | ||
| >6 | 13 (18) | 11 (19) | 2 (15) | ||
| Frequency of node-positive disease by tumor size | p*** | ||||
| < 4 cm | Positive | 1 (8) | 1 (12) | 0 (0) | >0.20 |
| Negative | 11 (92) | 7 (88) | 4 (100) | ||
| 4–7 cm | Positive | 5 (22) | 4 (20) | 1 (33) | >0.20 |
| Negative | 18 (78) | 16 (80) | 2 (67) | ||
| > 7 cm | Positive | 12 (28) | 11 (32) | 1 (11) | >0.20 |
| Negative | 31 (72) | 23 (68) | 8 (89) | ||
Column percentages;
p-value evaluating for association between of number of nodes examined by surgical approach (ORN vs LRN);
Not reported in 7 cases;
p-value evaluating for association between node-positive disease and surgical approach (ORN vs LRN), stratified by tumor size
Comment
In this contemporary, multi-institutional sample of patients undergoing radical nephrectomy as primary treatment for RCC, we observed that lymphadenectomy and ipsilateral adrenalectomy are performed in 11% and 24% of cases, respectively. Utilization of lymphadenectomy and adrenalectomy was more common among patients with higher-risk tumors (e.g., large size, high-grade), suggesting that urologists (perhaps appropriately) perform a more aggressive surgical excision for patients with RCC who are at heightened risk for regional lymph node and/or adrenal gland involvement.
In addition to cancer severity, type of surgical approach was also associated with whether patients received these adjuncts to radical nephrectomy. Overall, patients who underwent laparoscopic surgery were less likely to undergo concurrent lymphadenectomy, with this difference being most pronounced and statistically significant for tumors from 4–7 cm (i.e., T1b tumors). This finding most likely reflects idiosyncratic surgical practice patterns, rather than specific clinical or oncologic factors. For instance, surgeons who primarily treat T1b tumors with open radical nephrectomy may also perform lymphadenectomy based on historical practice patterns, whereas surgeons favoring laparoscopy may use lymphadenectomy only for larger tumors, based on risk stratification. Conversely, the difference could reflect a reluctance to perform lymphadenectomy among laparoscopic surgeons who treat mainly smaller tumors and who may have technical concerns about laparoscopically excising nodal tissue adjacent to the great vessels. As with other solid tumors, [16] progression from speculation to substantive answers to this question could come from planned empirical analyses of the relationship between surgeon characteristics (e.g., volume of laparoscopic procedures) and performance of lymphadenectomy.
Although it is not immediately evident why utilization of lymphadenectomy differs based on surgical approach, several considerations underscore the potential implications of these findings. First, identification of patients for ongoing trials of adjuvant RCC therapies is facilitated by performance of a regional lymph node dissection. For instance, the ASSURE study, a randomized trial evaluating survival benefits from adjuvant sunitinib or sorafenib, uses lymph node involvement as an inclusion criterion, regardless of primary tumor characteristics.[17] Accordingly, as the proportion of laparoscopic radical nephrectomies continues to increase, one potential concern is that infrequent performance of concurrent lymph node dissection may result in missed opportunities for clinical trial participation and, in turn, for understanding the potential benefits of adjuvant RCC therapies, particularly among patients with T1b tumors. As such, if any adjuvant RCC therapies prove beneficial with respect to survival after surgical therapy, then current surgical practice styles (for both open and laparoscopic radical nephrectomy) will require modification to nearly always include removal of regional lymph nodes to maximize opportunities to enroll patients into active clinical trials, regardless of tumor size.
Second, there are some authorities who contend that patients with higher-risk RCC may derive a survival benefit from extended lymphadenectomy at the time of nephrectomy.[18] If this (widely-debated) hypothesis is true, our finding that only 11% of patients received lymphadenectomy (including only 22% of those with tumors > 7 cm) highlights an immediate opportunity to improve the quality of treatment for this group of patients. Moreover, if the purported benefits of lymphadenectomy are real, the fact that roughly one of every two patients had only one lymph node removed underscores the need to optimize the extent of lymphadenectomy, regardless of surgical approach.
Urologists’ utilization of adrenalectomy also differed by both tumor size and surgical approach. Because the risk of metastatic involvement is minimal, there is general agreement that routine adrenalectomy is unwarranted among patients undergoing radical nephrectomy as treatment for small RCCs.[7,9,10] Accordingly, the observation that adrenalectomy was performed for 16% of KCS patients with tumors ≤ 4 cm highlights another opportunity to improve surgical quality. That is, optimal treatment of RCC would reduce the utilization of unnecessary ipsilateral adrenalectomy among patients with the smallest kidney tumors. Directly relevant to this concern is the fact that for patients with T1a tumors, adrenalectomy is twice as common among patients undergoing open versus laparoscopic surgical excision. This discrepancy may be explained by unmeasured differences in patient or tumor characteristics (e.g., polarity of tumor); however, it seems equally plausible that—as with lymphadenectomy—surgeons who perform open radical nephrectomy for smaller tumors are also utilizing the traditional technique of concurrent removal of the adrenal gland. If this hypothesis is accurate, then continued dissemination of laparoscopy may have the unanticipated benefit of decreasing the prevalence of avoidable adrenalectomies. More broadly speaking, the best strategy for reaching this optimal situation will likely involve continuing efforts to expand the utilization of nephron-sparing surgery, as this would yield the synchronous benefit of increasing the number of patients who also appropriately get “adrenal-sparing” surgical therapy.
Our study has several limitations. First, like any non-randomized observational study, observed differences based on tumor size and/or surgical approach might be explained by unmeasured confounding variables. Second, preoperative suspicion for nodal or adrenal involvement with RCC is an important factor in the clinical decision to perform lymphadenectomy or adrenalectomy, and we were unable to assess this with the data from the KCS. Third, despite the population-based nature of the overall KCS sample, the analytic cohort was drawn from only one metropolitan area and our findings might be influenced by idiosyncratic local practice patterns at the surgeon and hospital level. Fourth, because our sample included only those patients who consented to the interview and medical record review, and non-responders in epidemiological studies often have more severe disease, it is possible that we underestimated the true prevalence of lymphadenectomy and adrenalectomy. Furthermore, although we describe the overall frequency of node-positive disease, grouping by surgical approach created small sample sizes. As a result, it is difficult to draw definitive conclusions about the association between use of laparoscopy and yield of lymph node-positive disease. Finally, because we defined lymphadenectomy by the presence of at least one node in the surgical specimen, we may have either underestimated surgeons’ intent to perform a lymph node dissection (i.e., some surgeons may have removed what is anticipated to be regional nodal tissue, but the pathologists identified no actual lymph nodes in the specimen). However, it can be argued that the presence of nodal tissue is what is truly clinically relevant. Moreover, any potential underestimation would be countered by the possibility of incidental lymph node removal along with the primary specimen(s). Given that a notable proportion of lymphadenectomy specimens had one lymph node, it may be more likely we have overestimated the true incidence of lymphadenectomy among our analytic cohort. These limitations notwithstanding, our findings provide useful estimates of the contemporary utilization of lymphadenectomy and adrenalectomy.
Conclusions
In this contemporary sample of patients undergoing radical nephrectomy for RCC, we observed that the use of lymphadenectomy and adrenalectomy was uncommon and varied by both tumor size and surgical approach. In particular, lymphadenectomy was performed less frequently among patients undergoing laparoscopic radical nephrectomy for T1b tumors, whereas adrenalectomy was more common among patients undergoing open radical nephrectomy for smaller RCCs. In an era characterized by an increasing number of patients with small tumors, the progressive diffusion of minimally-invasive surgical techniques, and the emergence of potential systemic adjuvant therapies, our collective findings highlight several important considerations for urologists interested in optimizing the surgical management of patients with RCC.
Acknowledgments
This research was supported by the National Institutes of Health Intramural Research Program (NIH-N02-CP-11004) and Training in Clinical Investigation in Urology grant (NIH-T32-DK007782); and the Edwin Beer Research Fellowship in Urology and Urology-Related Fields from the New York Academy of Medicine to D.C.M.
References
- 1.Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331–1334. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 2.Kane CJ, Mallin K, Ritchey J, Cooperberg MR, Carroll PR. Renal cell cancer stage migration: analysis of the National Cancer Data Base. Cancer. 2008;113:78–83. doi: 10.1002/cncr.23518. [DOI] [PubMed] [Google Scholar]
- 3.Giberti C, Oneto F, Martorana G, Rovida S, Carmignani G. Radical nephrectomy for renal cell carcinoma: long-term results and prognostic factors on a series of 328 cases. Eur Urol. 1997;31:40–48. doi: 10.1159/000474416. [DOI] [PubMed] [Google Scholar]
- 4.Herrlinger A, Schrott KM, Schott G, Sigel A. What are the benefits of extended dissection of the regional renal lymph nodes in the therapy of renal cell carcinoma. J Urol. 1991;146:1224–1227. doi: 10.1016/s0022-5347(17)38052-7. [DOI] [PubMed] [Google Scholar]
- 5.Peters PC, Brown GL. The role of lymphadenectomy in the management of renal cell carcinoma. Urol Clin North Am. 1980;7:705–709. [PubMed] [Google Scholar]
- 6.Blom JHM, van Poppel H, Maréchal JM, et al. Radical nephrectomy with and without lymph-node dissection: final results of European Organization for Research and Treatment of Cancer (EORTC) randomized phase 3 trial 30881. Eur Urol. 2009;55:28–34. doi: 10.1016/j.eururo.2008.09.052. [DOI] [PubMed] [Google Scholar]
- 7.Paul R, Mordhorst J, Busch R, Leyh H, Hartung R. Adrenal sparing surgery during radical nephrectomy in patients with renal cell cancer: a new algorithm. J Urol. 2001;166:59–62. [PubMed] [Google Scholar]
- 8.Han KR, Bui MH, Pantuck AJ, et al. TNM T3a renal cell carcinoma: adrenal gland involvement is not the same as renal fat invasion. J Urol. 2003;169:899–903. doi: 10.1097/01.ju.0000051480.62175.35. [DOI] [PubMed] [Google Scholar]
- 9.Kletscher BA, Qian J, Bostwick DG, Blute ML, Zincke H. Prospective analysis of the incidence of ipsilateral adrenal metastasis in localized renal cell carcinoma. J Urol. 1996;155:1844–1846. [PubMed] [Google Scholar]
- 10.Shalev M, Cipolla B, Guille F, Staerman F, Lobel B. Is Ipsilateral Adrenalectomy a Necessary Component of Radical Nephrectomy? J Urol. 1995;153:1415–1417. [PubMed] [Google Scholar]
- 11.Siemer S, Lehmann J, Kamradt J, et al. Adrenal metastases in 1,635 patients with renal cell carcinoma: outcome and indication for adrenalectomy. J Urol. 2004;171:2155–2159. doi: 10.1097/01.ju.0000125340.84492.a7. [DOI] [PubMed] [Google Scholar]
- 12.Tsui KH, Shvarts O, Barbaric Z, Figlin R, DeKernion JB, Belldegrun A. Is adrenalectomy a necessary component of radical nephrectomy? UCLA experience with 511 radical nephrectomies. J Urol. 2000;163:437–441. [PubMed] [Google Scholar]
- 13.Ljungberg B, Cowen N, Hanbury DC, et al. Guidelines on Renal Cell Carcinoma. Eur Urol. 2010 doi: 10.1016/j.eururo.2010.06.032. in press. [DOI] [PubMed] [Google Scholar]
- 14.Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182:1271–1279. doi: 10.1016/j.juro.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Colt JS, Wacholder S, Schwartz K, Davis F, Graubard B, Chow WC. Response rates in a case–control study: effect of disclosure of biologic sample collection in the initial contact letter. Ann Epidemiol. 2005;15:700–704. doi: 10.1016/j.annepidem.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Petrik D, McCready D, Sawka C, Goel V. Association between extent of axillary lymph node dissection and patient, tumor, surgeon, and hospital factors in patients with early breast cancer. J Surg Oncol. 2003;82:84–90. doi: 10.1002/jso.10198. [DOI] [PubMed] [Google Scholar]
- 17.National Institutes of Health. Sunitinib or sorafenib in treating patients with kidney cancer that was removed by surgery. ClinicalTrials.gov; [Accessed February 27, 2010]. Available at http://clinicaltrials.gov/ct2/show/NCT00326898. [Google Scholar]
- 18.Blute ML, Leibovich BC, Cheville JC, Lohse CM, Zincke H. A protocol for performing extended lymph node dissection using primary tumor pathological features for patients treated with radical nephrectomy for clear cell renal cell carcinoma. J Urol. 2004;172:465–469. doi: 10.1097/01.ju.0000129815.91927.85. [DOI] [PubMed] [Google Scholar]