Abstract
Stress exacerbates both experimental and clinical pain, most well-characterized in irritable bowel and fibromyalgia syndromes. Since it has been hypothesized that cytokines play an etiopathogenic role in fibromyalgia and other chronic widespread pain conditions, we investigated the relationship between stress and cytokines in a model of stress-induced chronic somatic pain. A series of experiments were performed to evaluate the impact of stress on the hyperalgesia-induced by endotoxin (lipopolysaccharide, LPS) and the role of two proinflammatory cytokines, interleukin-6 (IL-6) and tumor necrosis α (TNFα). Fourteen days after exposure to a 4-day protocol of unpredictable sound stress, the ability of systemic LPS (100 μg/kg, i.p) to elicit cytokine-mediated mechanical hyperalgesia was measured in gastrocnemius muscle. LPS-induced hyperalgesia was significantly greater in stressed rats, but when rats were treated intrathecally with antisense oligodeoxynucleotide (ODN), to decrease either the gp130 subunit of the IL-6 receptor or the TNFα receptor, in nociceptors, skeletal muscle hyperalgesia in sound stressed, but not control, rats was prevented. These data suggest that chronic stress alters signaling in the primary afferent nociceptor for the hyperalgesia induced by endogenously produced pro-inflammatory cytokines.
Keywords: Stress, Interleukin-6, Tumor Necrosis Factor α, skeletal muscle pain, hyperalgesia, endotoxin
INTRODUCTION
Skeletal muscle pain is a common symptom in chronic widespread pain syndromes, such as fibromyalgia (Wolfe et al., 2010), as well as being an early symptom in viral (Etaouil et al., 1997) and bacterial infection (Tofte and Williams, 1982; Harats et al., 1986). While mechanisms underlying skeletal muscle pain have not been fully elucidated, we have observed that inflammatory cytokines, which sensitize high threshold mechano-sensitive skeletal muscle afferents (Diehl et al., 1993), appear to be involved in this type of skeletal muscle pain (Dina et al., 2008a; Dina et al., 2010). Stress markedly exacerbates skeletal muscle pain in patients with chronic widespread pain syndromes (Sternbach, 1986; Lundeen et al., 1987; Lundberg et al., 1999; Kopec and Sayre, 2005), and enhances pronociceptive effects of cytokines (Khasar et al., 2008; Khasar et al., 2009). To investigate the relationship between stress and cytokine hyperalgesia, we determined the role of two endogenously produced proinflammatory cytokines, interleukin-6 (IL-6) and tumor necrosis α (TNFα) in the enhanced skeletal muscle hyperalgesia induced by unpredictable sound stress, a model of chronic widespread pain (Khasar et al., 2005; Khasar et al., 2008; Dina et al., 2009; Khasar et al., 2009; Ferrari et al., 2010). We have used this model in rats to investigate mechanisms that contribute to widespread hyperalgesia that may potentially lead to a better understanding of the mechanisms that underlie chronic clinical pain conditions, such as fibromyalgia, that are also characterized by generalized pain and hyperalgesia.
Endotoxin (lipopolysaccharide; LPS), the glycolipid component of Gram-negative bacterial cell walls (Westphal, 1975), produces several symptoms that include skeletal muscle pain in humans (Elin et al., 1981) and widespread hyperalgesia in animals (Watkins et al., 1994; Kehl et al., 2004). We evaluated the effect of LPS administration on skeletal muscle nociceptive thresholds in rats exposed to non-habituating stress (Strausbaugh et al., 2003), which activates the sympathoadrenal stress axis, to release epinephrine, and the hypothalamic pituitary adrenal (HPA) axis, to release corticosterone. While plasma levels of corticosterone return to physiological levels, 24 hours post-stress, epinephrine is still markedly elevated 14 days later (Khasar et al., 2008).
MATERIALS AND METHODS
Animals
Experiments were performed on adult male Sprague Dawley rats (250–300 g; Charles River, Hollister, CA). Animals were housed, in pairs, in acrylic cages (30 × 75 × 20 cm) in the Laboratory Animal Resource Center of the University of California, San Francisco, under a 12-hour light/dark cycle. Animal care and use conformed to NIH guidelines. Experimental protocols were approved by the University of California, San Francisco Institutional Animal Care and Use Committee. Concerted effort was made to reduce the suffering and number of animals used; animals were housed with appropriate environmental enrichment as provided by the Laboratory Animal Resource Center “Environmental Enrichment Program”, and previous studies were referred to, to establish appropriate drug doses to avoid unnecessary repetition of animal experiments.
Mechanical threshold in the gastrocnemius muscle
Mechanical nociceptive threshold was quantified using a Chatillon digital force transducer (model DFI2, Amtek Inc., Largo, FL). Rats were lightly restrained in a cylindrical acrylic holder that allows for easy access to the hind limb, and a 6 mm diameter probe attached to the force transducer applied to the gastrocnemius muscle to deliver an increasing compression force. The nociceptive threshold was defined as the force, in Newtons, at which the rat withdrew its hind leg. Baseline withdrawal threshold was defined as the mean of 2 readings taken at 5-min intervals. Each hind limb is treated as an independent measure and each experiment performed on a separate group of rats. All behavioral testing was done between 10 am and 4 pm.
Stress
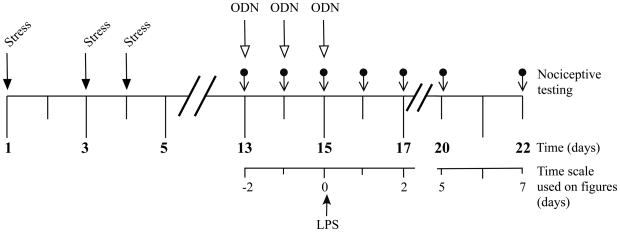
Exposure to sound stress occurred on days 1, 3 and 4 as described previously (Strausbaugh et al., 2003; Khasar et al., 2009). Animals were placed 3 per cage and the cage, in turn, placed 25 cm from a speaker that emitted 4 pure tones (5, 11, 15 and 19 kHz), whose amplitudes varied through time independently from 20–110 dB sound pressure level at random times each minute, and whose duration was either 5 or 10 s. Sham stressed animals were placed in the sound chamber for 30 minutes, but without exposure to the sound stimulus. Following sound or sham sound stress, rats were returned to their home cages in the animal care facility. Rats were used for nociceptive studies, 14 days after the last sound stress exposure. A time-line for stress exposure, drug administration and nociceptive testing is shown in Figure 1.
Figure 1.
Time line for stress exposure, administration of antisense and LPS and nociceptive testing
Antisense oligodeoxynucleotides
To attenuate the expression of TNFα receptor type-1, the antisense oligodeoxynucleotide (ODN) sequence 5′-ACACGGTGTTCTGTTTCTCC-3′ directed against a unique sequence of rat TNFα receptor type-1 was used. The mismatch ODN sequence, 5′-ACCCGTTGTTCGGTTGCTCC-3′, with four bases mismatched (denoted by bold face). We have previously shown that this ODN antisense against TNFα receptor type-1, at a dose of 40 μg, decreases TNFα receptor type-1 protein in dorsal root ganglia (Parada et al., 2003a).
To determine the contribution of IL-6, its effect on sensory neurons was disrupted by intrathecal administration of ODN antisense to the signal transducing molecule, glycoprotein 130 (gp130), a subunit of the IL-6 receptor signaling complex necessary for IL-6 receptor function (Muller-Newen, 2003). The 19-mer AS- and mismatch ODN for gp130 were purchased from Invitrogen (San Francisco, CA). The dose of ODN (40 μg) was based on prior dose–response studies (Summer et al., 2006). The antisense ODN sequence, 5′-TCC TTC CCA CCT TCT TCT G-3′, was directed against a unique sequence of rat gp130. The corresponding GenBank accession number and ODN position within the cDNA sequence are M92340 and 1834–1852, respectively (Wang et al., 1992). The mismatch ODN sequence, 5′-TAC TAC TCA CAT TCA TCA G-3′, corresponds to the gp130 subunit antisense sequence with 6 mismatched bases (denoted by bold).
The method for intrathecal ODN injection has been described previously (Khasar et al., 1996; Aley and Levine, 1997; Khasar et al., 1998; Alessandri-Haber et al., 2003; Parada et al., 2003a; Parada et al., 2003b; Alessandri-Haber et al., 2004; Dina et al., 2004). Before ODN injections, rats were briefly anesthetized with 3% isoflurane and a 30-gauge hypodermic needle inserted into the subarachnoid space, at the midline, between the L4 and L5 vertebrae. ODN (40 μg/10 μl) was slowly injected, over ~15 s. This procedure was repeated daily so that ODN was administered on 3 consecutive days. Control animals received injections of mismatch ODN.
Administration of LPS
LPS (100 μg/kg) was administered intraperitoneally (i.p.) on day 15 after stress and after the third daily administration of antisense or mismatch ODN.
Statistical analysis
Data are presented as mean ± SEM and analyzed using 2-way repeated measures analysis of variance (ANOVA), or Student’s t-test, as appropriate. Where the overall ANOVA showed significant differences between groups, Scheffe’s post hoc test was used to determine the pairs of groups that were different. The accepted level of significance was P < 0.05. The P-values for main effects are from an ANOVA, and all subsequent P-values are from Scheffe’s post hoc tests, unless otherwise stated.
RESULTS
Effect of treatment with antisense ODN against IL-6 receptor subunit gp130 on LPS-induced hyperalgesia
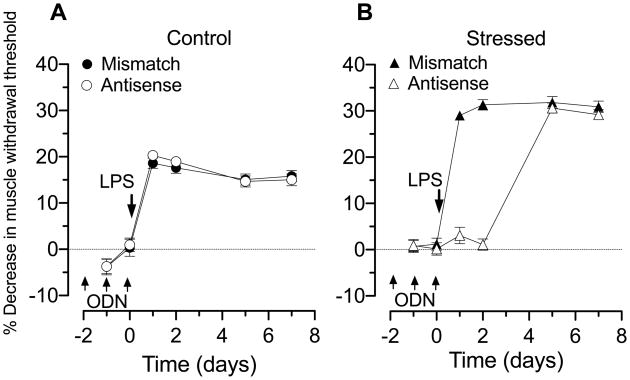
The administration of LPS (100 μg/kg, i.p.), in non-stressed rats treated with mismatch ODN, produced a decrease in mechanical nociceptive threshold in the gastrocnemius muscle when evaluated 1 day after LPS administration, which persisted for the 7-d period post-administration (open circles, n = 12, Figure 2A). The magnitude of LPS-induced decrease mechanical nociceptive threshold in skeletal muscle was not significantly different in rats treated with antisense ODN, to decrease IL-6 receptor subunit gp130 in nociceptors (filled circles, n = 12, Figure 2A). In a separate group of rats, skeletal muscle mechanical nociceptive threshold was tested after exposure to intermittent sound stress exposure; administration of LPS in this stress rats, treated with mismatch ODN (filled triangles, n = 12, Figure 2B), produced a maximal decrease in mechanical nociceptive threshold that was significantly greater than that produced in non-stressed mismatch-treated control rats (filled circles, n = 12, Figure 2A; 31.31±1.13% vs. 20.29±0.70%, P<0.05). In the stress group, treatment with antisense ODN to decrease IL-6 receptor expression in nociceptors (open triangles, n = 12), completely abolished LPS-induced skeletal muscle hyperalgesia for 2 d after ODN administration. Hyperalgesia returned by the 4th day after the last administration of ODN (Figure 2B), at a time when antisense effects would have reversed (Parada et al., 2003b), indicating that IL-6 was present at a concentration capable of producing the enhanced hyperalgesia.
Figure 2. LPS-induced muscle hyperalgesia: role of IL-6.
A. Administration of LPS (100 μg/kg i.p.), in control (mismatch ODN, filled circles n=12, baseline nociceptive threshold: 3.12±0.04 N, data point not shown) decreased nociceptive threshold ~20% beginning one day after administration. This hyperalgesia remained, undiminished, for the 7-day post-administration testing period. In rats treated with antisense ODN to gp130 (open circles, n=12, baseline nociceptive threshold: 3.13±0.03 N), to decrease expression of the gp130 subunit of the IL-6 receptor in primary afferent neurons, the magnitude and duration of LPS-induced hyperalgesia was not different from mismatch control rats.
B. Fifteen days after the last exposure to sound stress, administration of LPS in rats treated with mismatch ODN (filled triangles n=12; baseline nociceptive threshold: 3.17±0.05 N, data point not shown) decreased nociceptive threshold ~30%, beginning one day after administration; this decrease in nociceptive threshold was significantly greater than in non-stressed rats receiving mismatched ODN (P<0.05). In stressed rats treated with antisense ODN to gp130 (open triangles, n=12; baseline nociceptive threshold: 3.14±0.03 N), LPS-induced hyperalgesia was prevented during ODN administration and 1 day post administration. 4 d post ODN administration, at a time when antisense effects would have reversed, the magnitude of LPS-induced hyperalgesia was not different from stressed rats receiving mismatch ODN.
Effect of treatment with antisense ODN against TNFα receptor type-1 on LPS-induced hyperalgesia
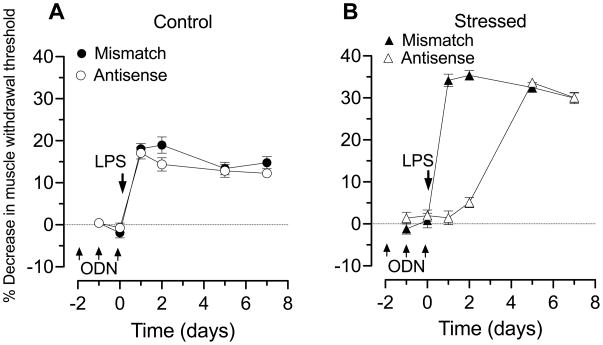
Administration of LPS (100 μg/kg, i.p.) in non-stressed rats produced a similar decrease in mechanical nociceptive threshold in the gastrocnemius in rats treated with mismatch ODN (filled circles, n = 12, Figure 3A) and antisense ODN to decrease expression of TNFα receptors in nociceptors (open circles, n = 12, Figure 3A). In contrast, in sound stress-exposed rats, LPS-induced hyperalgesia (which was significantly 35.37±1.13% vs. 18.08±1.26%, P<0.05) was completely prevented 1 and 2 days after cessation of antisense ODN administration to decrease TNFα receptor expression (Figure 3B). Hyperalgesia returned by the 4th day after the last ODN administration, at a time when antisense effects would have reversed (Parada et al., 2003b), again indicating that TNFα was present, at a concentration capable of producing the enhanced hyperalgesia.
Figure 3. LPS-induced muscle hyperalgesia: role of TNFα.
A. Administration of LPS (100 μg/kg i.p.), in control (mismatch ODN, filled circles n=12; baseline nociceptive threshold: 3.09±0.04 N, data point not shown) decreased nociceptive threshold ~20% beginning one day after administration. This hyperalgesia remained undiminished for the 7-day post-administration testing period. In rats treated with ODN antisense to the TNFα receptor (open circles, n=12; baseline nociceptive threshold: 3.12±0.03 N), to decrease expression of this receptor in primary afferent neurons, the magnitude and duration of LPS-induced hyperalgesia was not different from control rats.
Fifteen days after sound stress, administration of LPS in rats treated with mismatch ODN (filled triangles n=12; baseline nociceptive threshold: 3.12±0.04 N, data point not shown) decreased nociceptive threshold ~30% beginning one day after administration; this decrease in nociceptive threshold was significantly greater than in non-stressed rats receiving mismatched ODN (P<0.05). In stressed rats treated with antisense ODN to TNFα receptor (open triangles, n=12; baseline nociceptive threshold: 3.15±0.04 N), LPS-induced hyperalgesia was prevented during ODN administration and 1 day post administration. 4 d post ODN administration, at a time when antisense effects would have reversed, the magnitude LPS-induced hyperalgesia was not different from stressed rats that had received mismatch ODN.
DISCUSSION
In this study, we have demonstrated stress-induced long-lasting enhancement of LPS-induced mechanical hyperalgesia in skeletal muscle and mediation of this enhancement by a mechanism, different from that mediating hyperalgesia in the non-stressed control rats. Treatment with antisense ODN to decrease expression of IL-6 and TNFα receptors on primary afferent neurons (Parada et al., 2003b; Summer et al., 2008), completely blocked LPS-induced hyperalgesia in stressed rats, but did not attenuate it in non-stressed rats.
While we have previously shown that intramuscular administration of IL-6 (Dina et al., 2008a) and TNFα (Dina et al., 2010) induce skeletal muscle hyperalgesia mediated by their cognate receptors on nociceptors, the LPS hyperalgesia that we observed in control animals is not mediated by LPS-induced TNFα or IL-6 production. Thus, while LPS (1 mg/kg, i.p) in rats increases skeletal muscle TNFα (~2-fold), it returns to baseline 8 h after LPS, and did not change skeletal muscle IL-6 levels (Lang et al., 2003), although plasma IL-6 has been shown to be increased ~275-fold after LPS (100 μg/kg i.p.) (Harre et al., 2003).
Similar to our observation in skeletal muscle, enhanced visceral nociception following exposure to repeated stress has been reported (Bradesi et al., 2005; Bradesi et al., 2006). There is evidence that cytokines contribute to this increased nociception, with stress producing an increase in colonic expression of cytokines (IL-1b and IFN-gamma) and antagonists to neurokinin 1 (Bradesi et al., 2006), corticotropin-releasing factor type 1 (Larauche et al., 2009), vasopressin 3 (Bradesi et al., 2009) blocking the hyperalgesic response, and a correlation between production of inflammatory cytokines and magnitude of visceral hyperalgesia (Adam et al., 2006). In addition to a role for peripheral cytokines in stress-induced visceral hyperalgesia, it has also been suggested that stress-induced central sensitization contributes to the enhanced visceral nociception (Bradesi et al., 2005), and it is possible that central sensitization contributes to the stress-induced skeletal muscle hyperalgesia we observed in the current study.
An intriguing finding in the current study is that in the stressed, but not in the non-stressed rat, LPS-induced hyperalgesia is completely abolished when either IL-6 or TNFα receptors on peripheral nociceptors are down-regulated by ODN administration. Of note, we have previously shown that after exposure to the same sound stress protocol, cytokine hyperalgesia in skeletal muscle is enhanced and markedly prolonged (Khasar et al., 2009), and that a switch in second messenger signaling pathway mediates this enhancement and prolongation (Khasar et al., 2008). Taken together, this supports the suggestion that a change in nociceptor function may mediate the enhanced cytokine hyperalgesia. Thus, in the setting of chronic stress there is an emergence of cytokine dependence for LPS-induced hyperalgesia. While the greater LPS-induced hyperalgesia in stress-exposed rats may be at least in part dependent on the increased production of IL-6 and TNFα seen in rats previously exposed to a stressor (Johnson et al., 2002), this enhancement of cytokine level is of shorter duration than that seen in the present study. While the mechanistic basis for this ‘switch’ to cytokine dependence in LPS-induced skeletal muscle hyperalgesia is unknown, this might represent a plastic change in the primary afferent neuron that is related to the development of chronic widespread pain states observed following exposure to chronic stress (Buskila et al., 2009; Haviland et al., 2010). Indeed, our previous findings suggest that the sound stress paradigm we employ is a model for chronic widespread pain (Khasar et al., 2009) and that a phenomenon of “hyperalgesic priming” induced by this stress paradigm, is dependent on the epsilon isoform of protein kinase C (PKCε) in the primary afferent nociceptor. Hyperalgesic priming that is induced by exposure to the inflammogen, carrageenan, is present days or weeks after the initial inflammatory response abates; subsequent cytokine administration produces an enhanced hyperalgesic response in skeletal muscle that is dependent on PKCε (Dina et al., 2008b). Similarly, even following the sound stress cytokine–induced skeletal muscle hyperalgesia is enhanced for weeks after exposure to stress and is PKCε–dependent (Khasar et al., 2008). How TNFα and IL-6 receptor signaling is altered by stress such that now endogenously produced TNFα and IL-6 now contribute to LPS-induced hyperalgesia remains to be determined.
In conclusion, we present evidence that exposure to stress both enhances LPS-induced hyperalgesia and changes the underlying mechanisms. These findings may have clinical implications with regard to understanding the development of chronic widespread pain syndromes, such as fibromyalgia, in individuals exposed to stress, and could potentially provide information for the development of novel treatments of such chronic pain conditions.
Acknowledgments
This research was supported by grants from NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam B, Liebregts T, Gschossmann JM, Krippner C, Scholl F, Ruwe M, Holtmann G. Severity of mucosal inflammation as a predictor for alterations of visceral sensory function in a rat model. Pain. 2006;123:179–186. doi: 10.1016/j.pain.2006.02.029. [DOI] [PubMed] [Google Scholar]
- Alessandri-Haber N, Dina OA, Yeh JJ, Parada CA, Reichling DB, Levine JD. Transient receptor potential vanilloid 4 is essential in chemotherapy-induced neuropathic pain in the rat. J Neurosci. 2004;24:4444–4452. doi: 10.1523/JNEUROSCI.0242-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB, Levine JD. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron. 2003;39:497–511. doi: 10.1016/s0896-6273(03)00462-8. [DOI] [PubMed] [Google Scholar]
- Aley KO, Levine JD. Multiple receptors involved in peripheral alpha 2, mu, and A1 antinociception, tolerance, and withdrawal. J Neurosci. 1997;17:735–44. doi: 10.1523/JNEUROSCI.17-02-00735.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradesi S, Kokkotou E, Simeonidis S, Patierno S, Ennes HS, Mittal Y, McRoberts JA, Ohning G, McLean P, Marvizon JC, Sternini C, Pothoulakis C, Mayer EA. The role of neurokinin 1 receptors in the maintenance of visceral hyperalgesia induced by repeated stress in rats. Gastroenterology. 2006;130:1729–1742. doi: 10.1053/j.gastro.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Bradesi S, Schwetz I, Ennes HS, Lamy CM, Ohning G, Fanselow M, Pothoulakis C, McRoberts JA, Mayer EA. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2005;289:G42–53. doi: 10.1152/ajpgi.00500.2004. [DOI] [PubMed] [Google Scholar]
- Buskila D, Ablin JN, Ben-Zion I, Muntanu D, Shalev A, Sarzi-Puttini P, Cohen H. A painful train of events: increased prevalence of fibromyalgia in survivors of a major train crash. Clin Exp Rheumatol. 2009;27:S79–85. [PubMed] [Google Scholar]
- Diehl B, Hoheisel U, Mense S. The influence of mechanical stimuli and of acetylsalicylic acid on the discharges of slowly conducting afferent units from normal and inflamed muscle in the rat. Exp Brain Res. 1993;92:431–440. doi: 10.1007/BF00229031. [DOI] [PubMed] [Google Scholar]
- Dina OA, Green PG, Levine JD. Role of interleukin-6 in chronic muscle hyperalgesic priming. Neuroscience. 2008a;152:521–525. doi: 10.1016/j.neuroscience.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Joseph EK, Levine JD, Green PG. Mechanisms mediating vibration-induced chronic musculoskeletal pain analyzed in the rat. J Pain. 2010;11:369–377. doi: 10.1016/j.jpain.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Khasar SG, Gear RW, Levine JD. Activation of Gi induces mechanical hyperalgesia poststress or inflammation. Neuroscience. 2009;160:501–507. doi: 10.1016/j.neuroscience.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Levine JD, Green PG. Muscle inflammation induces a protein kinase cepsilon-dependent chronic-latent muscle pain. J Pain. 2008b;9:457–462. doi: 10.1016/j.jpain.2008.01.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Parada CA, Yeh J, Chen X, McCarter GC, Levine JD. Integrin signaling in inflammatory and neuropathic pain in the rat. Eur J Neurosci. 2004;19:634–642. doi: 10.1111/j.1460-9568.2004.03169.x. [DOI] [PubMed] [Google Scholar]
- Elin RJ, Wolff SM, McAdam KP, Chedid L, Audibert F, Bernard C, Oberling F. Properties of reference Escherichia coli endotoxin and its phthalylated derivative in humans. J Infect Dis. 1981;144:329–336. doi: 10.1093/infdis/144.4.329. [DOI] [PubMed] [Google Scholar]
- Etaouil N, Benyahya E, Janani S, el Fatimi A, Bennis R, Mkinsi O. Diffuse arthralgia and myalgia as the first manifestation of benign myeloradiculopathy due to cytomegalovirus infection in an immunocompetent patient. Rev Rhum Engl Ed. 1997;64:57–58. [PubMed] [Google Scholar]
- Ferrari LF, Gear RW, Levine JD. Attenuation of activity in an endogenous analgesia circuit by ongoing pain in the rat. J Neurosci. 2010;30:13699–13706. doi: 10.1523/JNEUROSCI.2867-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harats N, Gur H, Rubinow A. Acute poststreptococcal polymyalgia. Ann Rheum Dis. 1986;45:47–49. doi: 10.1136/ard.45.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harre EM, Roth J, Gerstberger R, Hubschle T. Interleukin-6 mediates lipopolysaccharide-induced nuclear STAT3 translocation in astrocytes of rat sensory circumventricular organs. Brain Res. 2003;980:151–155. doi: 10.1016/s0006-8993(03)02923-8. [DOI] [PubMed] [Google Scholar]
- Haviland MG, Morton KR, Oda K, Fraser GE. Traumatic experiences, major life stressors, and self-reporting a physician-given fibromyalgia diagnosis. Psychiatry Res. 2010;177:335–341. doi: 10.1016/j.psychres.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Deak T, Stark M, Watkins LR, Maier SF. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav Immun. 2002;16:461–476. doi: 10.1006/brbi.2001.0638. [DOI] [PubMed] [Google Scholar]
- Kehl LJ, Kovacs KJ, Larson AA. Tolerance develops to the effect of lipopolysaccharides on movement-evoked hyperalgesia when administered chronically by a systemic but not an intrathecal route. Pain. 2004;111:104–115. doi: 10.1016/j.pain.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Gold MS, Dastmalchi S, Levine JD. Selective attenuation of mu-opioid receptor-mediated effects in rat sensory neurons by intrathecal administration of antisense oligodeoxynucleotides. Neurosci Lett. 1996;218:17–20. doi: 10.1016/0304-3940(96)13111-6. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Gold MS, Levine JD. A tetrodotoxin-resistant sodium current mediates inflammatory pain in the rat. Neurosci Lett. 1998;256:17–20. doi: 10.1016/s0304-3940(98)00738-1. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Burkham J, Dina OA, Brown AS, Bogen O, Alessandri-Haber N, Green PG, Reichling DB, Levine JD. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. J Neurosci. 2008;28:5721–5730. doi: 10.1523/JNEUROSCI.0256-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasar SG, Dina OA, Green PG, Levine JD. Sound stress-induced long-term enhancement of mechanical hyperalgesia in rats is maintained by sympathoadrenal catecholamines. J Pain. 2009;10:1073–1077. doi: 10.1016/j.jpain.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasar SG, Green PG, Levine JD. Repeated sound stress enhances inflammatory pain in the rat. Pain. 2005;116:79–86. doi: 10.1016/j.pain.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Kopec JA, Sayre EC. Stressful experiences in childhood and chronic back pain in the general population. Clin J Pain. 2005;21:478–483. doi: 10.1097/01.ajp.0000139909.97211.e1. [DOI] [PubMed] [Google Scholar]
- Lang CH, Silvis C, Deshpande N, Nystrom G, Frost RA. Endotoxin stimulates in vivo expression of inflammatory cytokines tumor necrosis factor alpha, interleukin-1beta, -6, and high-mobility-group protein-1 in skeletal muscle. Shock. 2003;19:538–546. doi: 10.1097/01.shk.0000055237.25446.80. [DOI] [PubMed] [Google Scholar]
- Larauche M, Gourcerol G, Wang L, Pambukchian K, Brunnhuber S, Adelson DW, Rivier J, Million M, Tache Y. Cortagine, a CRF1 agonist, induces stresslike alterations of colonic function and visceral hypersensitivity in rodents primarily through peripheral pathways. Am J Physiol Gastrointest Liver Physiol. 2009;297:G215–27. doi: 10.1152/ajpgi.00072.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg U, Dohns IE, Melin B, Sandsjo L, Palmerud G, Kadefors R, Ekstrom M, Parr D. Psychophysiological stress responses, muscle tension, and neck and shoulder pain among supermarket cashiers. J Occup Health Psychol. 1999;4:245–255. doi: 10.1037//1076-8998.4.3.245. [DOI] [PubMed] [Google Scholar]
- Lundeen TF, Sturdevant JR, George JM. Stress as a factor in muscle and temporomandibular joint pain. J Oral Rehabil. 1987;14:447–456. doi: 10.1111/j.1365-2842.1987.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Muller-Newen G. The cytokine receptor gp130: faithfully promiscuous. Sci STKE. 2003;2003:PE40. doi: 10.1126/stke.2003.201.pe40. [DOI] [PubMed] [Google Scholar]
- Parada CA, Yeh JJ, Joseph EK, Levine JD. Tumor necrosis factor receptor type-1 in sensory neurons contributes to induction of chronic enhancement of inflammatory hyperalgesia in rat. Eur J Neurosci. 2003a;17:1847–1852. doi: 10.1046/j.1460-9568.2003.02626.x. [DOI] [PubMed] [Google Scholar]
- Parada CA, Yeh JJ, Reichling DB, Levine JD. Transient attenuation of protein kinase Cepsilon can terminate a chronic hyperalgesic state in the rat. Neuroscience. 2003b;120:219–226. doi: 10.1016/s0306-4522(03)00267-7. [DOI] [PubMed] [Google Scholar]
- Sternbach RA. Pain and ‘hassles’ in the United States: findings of the Nuprin pain report. Pain. 1986;27:69–80. doi: 10.1016/0304-3959(86)90224-1. [DOI] [PubMed] [Google Scholar]
- Strausbaugh HJ, Green PG, Dallman MF, Levine JD. Repeated, non-habituating stress suppresses inflammatory plasma extravasation by a novel, sympathoadrenal dependent mechanism. Eur J Neurosci. 2003;17:805–812. doi: 10.1046/j.1460-9568.2003.02493.x. [DOI] [PubMed] [Google Scholar]
- Summer GJ, Puntillo KA, Miaskowski C, Dina OA, Green PG, Levine JD. TrkA and PKC-epsilon in thermal burn-induced mechanical hyperalgesia in the rat. J Pain. 2006;7:884–891. doi: 10.1016/j.jpain.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Summer GJ, Romero-Sandoval EA, Bogen O, Dina OA, Khasar SG, Levine JD. Proinflammatory cytokines mediating burn-injury pain. Pain. 2008;135:98–107. doi: 10.1016/j.pain.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Tofte RW, Williams DN. Clinical and laboratory manifestations of toxic shock syndrome. Ann Intern Med. 1982;96:843–847. doi: 10.7326/0003-4819-96-6-843. [DOI] [PubMed] [Google Scholar]
- Wang Y, Nesbitt JE, Fuentes NL, Fuller GM. Molecular cloning and characterization of the rat liver IL-6 signal transducing molecule, gp130. Genomics. 1992;14:666–672. doi: 10.1016/s0888-7543(05)80166-1. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Wiertelak EP, Goehler LE, Smith KP, Martin D, Maier SF. Characterization of cytokine-induced hyperalgesia. Brain Res. 1994;654:15–26. doi: 10.1016/0006-8993(94)91566-0. [DOI] [PubMed] [Google Scholar]
- Westphal O. Bacterial endotoxins. The second Carl Prausnitz Memorial Lecture. Int Arch Allergy Appl Immunol. 1975;49:1–43. [PubMed] [Google Scholar]
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, Yunus MB. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62:600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]