Abstract
Objective
To test the hypothesis that a constricted life space, the extent of movement through the environment covered during daily functioning, is associated with increased risk of incident Alzheimer disease (AD), increased risk of mild cognitive impairment (MCI), and more rapid cognitive decline in older adults.
Design
Two prospective cohort studies.
Setting
Retirement communities, community-based organizations, churches, and senior subsidized housing facilities across the Chicago metropolitan area.
Participants
A total of 1,294 community-dwelling elders without baseline clinical dementia.
Main Outcome Measures
Detailed annual clinical evaluation to diagnose incident AD and MCI, and document change in cognitive function.
Results
During a mean (SD) follow-up of 4.4 (1.7) years, 180 persons developed AD. In a proportional hazards model controlling for age, sex, race, and education, a more constricted life space was associated with an increased risk of AD (hazard ratio = 1.21, confidence interval: 1.08--1.36). A person with a life space constricted to their home was almost twice as likely to develop AD than a person with the largest life space (out of town). The association did not vary along demographic lines and persisted after the addition of terms for performance-based physical function, disability, depressive symptoms, social network size, vascular disease burden, and vascular risk factors. The association remained consistent after excluding persons with MCI at baseline and who developed AD in the first 2 years of observation. A constricted life space was also associated with an increased risk of MCI (hazard ratio = 1.17, confidence interval: 1.06--1.28), and a more rapid rate of global cognitive decline (estimate: –0.012, standard error: 0.003, t[5033] = –3.58, p <0.001).
Conclusions
A constricted life space is associated with increased risk of AD, MCI, and cognitive decline among older persons.
Keywords: Alzheimer disease, cognition, dementia, epidemiology, life space, mild cognitive impairment (MCI)
Constriction of life space1--4---the extent of movement through the environment covered in daily functioning---is a consequence of aging for many older adults, often resulting in restriction in movement beyond the home.5 A smaller life space is correlated with adverse health outcomes including chronic illness,6,7 depression,7 and frailty.8 Life space has been linked to cognitive function and dementia in cross-sectional analyses1,6,8,9 and one longitudinal study suggests an association between life space and cognitive decline.10 To our knowledge, however, the relationship between life space and the risk of Alzheimer disease (AD) remains unexamined.
Establishing a relationship between life space and AD could extend recent work showing that disability and functional impairment may predict AD onset in older persons without cognitive impairment many years in advance.11 Life space is a multidimensional construct that reflects physical ability and mobility as well as the psychosocial and environmental context that shapes and modifies performance,6 and therefore may capture aspects of functioning in the “real world” that complement other commonly used measures of disability.12 A constricted life space may, therefore, represent a novel and relatively easy identifier of older persons in the community who are at risk for the development of AD. In this study, we used data from almost 1,300 community-dwelling older persons without baseline clinical dementia to test the hypothesis that a constricted life space is related to an increased risk of developing AD. In subsequent analyses, we attempted to verify that our results were not affected by confounders, diagnostic misclassification, or the inclusion of participants with preclinical dementia. We also tested whether a constricted life space was related to mild cognitive impairment (MCI), a precursor of AD, and cognitive decline. Because we were interested in examining differences across race and ethnicity in a diverse sample, we combined data from two racially dissimilar cohorts with similar recruitment and operations.
METHODS
Participants
Participants are from two ongoing cohort studies, described later, approved by the institutional review board of Rush University Medical Center. These studies have a large common core of recruitment, data collection, and operational methods, which facilitates analyses of data from the combined cohorts. Both cohorts were treated as one analytical cohort.
The Rush Memory and Aging Project13 is a longitudinal clinical-pathological study of chronic conditions of aging. Older persons are recruited from about 40 continuous care retirement communities and senior subsidized housing facilities around the Chicago metropolitan area. Study participants agree to detailed annual clinical evaluations and organ donation. Between the start of the study in 1997 and January 2010, more than 1,300 persons enrolled in the study. The life space measure was added in 2001. The Memory and Aging Project is 92% white, non-Hispanic.
The Minority Aging Research Study (MARS)14 is a study of risk factors for cognitive decline in older African Americans. Participants are recruited from community-based organizations, churches, and senior subsidized housing facilities in the same catchment area as the Rush Memory and Aging Project. Recruitment for MARS was very similar to the recruitment of African American participants in the Memory and Aging Project. Participation in MARS requires agreeing to detailed annual clinical evaluations. Between the start of the study in 2004 and January 2010, more than 350 persons enrolled. The life space measure was included at baseline.
Eligibility for these analyses included valid life space measurement, the absence of dementia at baseline, and at least one valid follow-up clinical evaluation. At the time of analyses, 1,554 persons had completed baseline clinical evaluation and life space assessment. Of those, we excluded 92 with dementia and 168 without one or more follow-up visits with valid data. This left 1,294 persons (987 from MAP, 307 from MARS) eligible for analyses.
Clinical Diagnosis of AD and MCI and Assessment of Cognitive Function
Detailed annual clinical evaluations in MAP and MARS include medical history, complete neurological examination, and a battery of cognitive tests.13,14 Clinical diagnoses were performed using a three-stage process including computer scoring of cognitive tests, clinical judgment by an experienced neuropsychologist, and diagnostic classification by an experienced clinician, as previously described.13 Diagnosis of dementia and probable AD followed the criteria of the joint working group of the National Institute of Neurologic and Communicative Disorders and Stroke and the Alzheimer Disease and Related Disorders Association.15 Diagnosis of MCI was rendered for individuals found to have cognitive impairment but who did not meet criteria for dementia.13 Persons who did not meet criteria for MCI or dementia were classified as having no cognitive impairment. A composite measure of global cognitive function and subscale measures of five specific cognitive domains (episodic memory, semantic memory, working memory, perceptual speed, and visuospatial ability) were constructed from the battery of 18 cognitive tests.14,16
Assessment of Life Space
Life space was assessed using a modified version of the Life Space Questionnaire,1 which asked participants whether they had been in six specific zones within their surrounding environment in the past week. Each zone represents a concentric enlargement of life space. We summed the “yes” responses (scored as 1, versus “no” = 0), then reverse-coded the life space score so that the reference group (score = 0) was individuals with the least constricted (i.e., largest) life space and larger scores indicate a more constricted life space. The two most constricted life space categories were combined due to small frequencies, resulting in the following life space scores: 0 (“Outside of town,” n = 867 [67.1%]), 1 (“Outside of neighborhood,” n = 253 [19.5%]), 2(“Neighborhood,” n = 70 [5.4%]), 3 (“Parking lot/yard,” n = 45 [3.5%]), 4 (“Porch/patio,” n = 31 [2.4%]), and 5 (“Bedroom/rooms in home,” n = 28 [2.2%]).
Other Covariates
Physical function was assessed via a performance-based test of gait shown to be valid and reliable for use in studies of older persons.17 A composite gait score was developed based on time and steps required to walk 8 feet and time and steps required to turn 360°.18 Disability was assessed via the Katz scale, the number of items on which a participant reported the need for assistance on six basic activities of daily living: walking across a small room, bathing, dressing, eating, transferring from a bed to a chair, and toileting.19 Depressive symptoms were assessed as the number of symptoms reported based on the 10-item version of the Center for Epidemiologic Studies Depression (CES-D) scale.20 Social network size was the number of children, family, and friends participants had seen at least once a month.21 Vascular risk factors were the sum of hypertension, diabetes mellitus, and smoking. Vascular disease burden was the sum of myocardial infarction, congestive heart failure, claudication, and stroke.13
Statistical Analysis
We first examined crude associations of life space with demographics and covariates using nonparametric tests. Next, we examined the relation of life space with incident AD using a proportional hazards model for discrete (tied) data22,23 adjusted for age, sex, education, and race (core model). Models for discrete time were used to account for “clumping” in time-to-event due to the annual schedule of evaluations. In subsequent models, we added terms for the interactions of age, sex, education, and race with life space and examined several potential confounders of the association of life space with AD. To ensure that persons with preclinical dementia did not account for the results, we conducted a number of sensitivity analyses in which we repeated this model after excluding persons with MCI at baseline, and then further excluded persons who developed AD in the first or second year of follow-up. We also sequentially excluded persons at the lowest 5th, 10th, and 15th percentile of cognitive function at baseline. We then excluded persons with MCI at baseline (leaving only participants with no cognitive impairment) to examine the association of life space and incident MCI. Finally, to ensure that diagnostic misclassification did not influence the results, we used a series of mixed-effects models adjusted for age, sex, education, and race and including terms for time and time squared and interactions between time and all demographic variables to examine the association of life space with rate of decline in global cognitive function and five cognitive domains. Model validation was performed graphically and analytically and there was no evidence of nonlinearity or nonproportionality. Programming was done in SAS version 9.2. (SAS Institute, Inc., Cary, NC).
RESULTS
Descriptive Statistics
The mean score on the life space measure was 0.61, (SD = 1.13); median (interquartile range) = 0 (0, 1). Eight percent of subjects reported that they had not been to an area beyond the yard, driveway, or parking lot of their home in the previous week (i.e., immediate home environment; life space score ≥ 3). In Spearman correlations, a more constricted life space was correlated with age (r = 0.19, n = 1,294, p <0.0001), education (r = –0.17, n = 1,294, p <0.0001), physical function (r = –0.27, n = 1,294, p <0.0001), disability (r = 0.23, n = 1,293, p <0.0001), social networks (r = –0.21, n = 1,290, p <0.0001), depressive symptoms (r = 0.14, n = 1,293, p <0.0001), and vascular disease burden (r = 0.09, n = 1,294, p = 0.002), but not with vascular risk factors (r = 0.04, n = 1,294, p = 0.19). Male participants had less constricted life spaces than females (0.48 versus 0.66, ZWilcoxon rank sum test = –3.33, p <0.0001). There was not a statistically significant difference in size of life space between black and white participants (0.48 versus. 0.66 respectively, ZMann-Whitney test = –1.76, p = 0.08).
Life Space and the Risk of AD
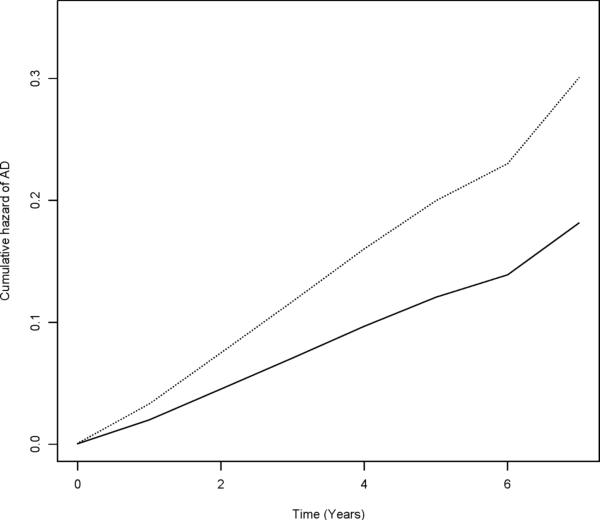
Over up to 8 years of follow-up (mean [SD] = 4.4 [1.7]), 180 persons (13.9%) developed AD. Participants who developed AD had more constricted life spaces (Table 1). In a proportional hazards model adjusted for age, sex, education, and race, a more constricted life space was associated with an increased risk of AD (Table 2, Model 1), such that the risk of AD increased by 21% for each successively smaller zone of life space. As illustrated in Figure 1, a person with a life space constricted to their immediate home environment (score = 3) was about 1.8 times more likely to develop AD than a person with the largest life space (score = 0).
Table 1.
Demographics and baseline characteristics of participants who developed AD versus those who did not
| Characteristics 1 | Did not develop AD (n=1114) | Developed AD (n=180) | F(df1,df2)2 or χ2(df) | p value |
|---|---|---|---|---|
| Age | 77.5 (7.6) | 84.1 (6.3) | 1.51 (1,1292) | <0.0001 |
| Education (years) | 14.6 (3.3) | 14.3 (3.0) | 24.50 (1,1292) | 0.219 |
| Sex (% Female) | 74.5% | 70.0% | 1.63 (1) | 0.202 |
| Race (% White, non-Hispanic) | 67.6% | 88.9% | 33.8 (1) | <0.0001 |
| MMSE | 28.2 (1.8) | 26.4 (2.8) | 128.23 (1,1289) | <0.0001 |
| Life space score | 0.55 (1.07) | 0.99 (1.36) | 24.50 (1,1292) | <0.0001 |
| Gait | 0.12 (0.80) | -0.25 (0.81) | 33.87 (1,1292) | <0.0001 |
| ADL disability | 0.16 (0.62) | 0.27 (0.74) | 5.01 (1,1291) | 0.025 |
| Depressive symptoms | 1.27 (1.70) | 1.53 (2.01) | 3.50 (1,1291) | 0.062 |
| Social network size | 6.80 (6.49) | 5.79 (4.77) | 3.98(1,1288) | 0.046 |
| Vascular risk factors | 1.24 (0.84) | 1.08 (0.77) | 6.27 (1,1292) | 0.012 |
| Vascular diseases | 0.32 (0.61) | 0.34 (0.60) | 0.21 (1,1292) | 0.645 |
Mean values and standard deviations are presented unless otherwise noted and p values are based on t-tests for continuous variables and Chi-squared tests for categorical variables
df1 = between-groups degrees of freedom, df2 = within-groups degrees of freedom
Table 2.
Relation of life space with AD; core model and sensitivity analyses
| Model | (# incident AD cases / total # subjects) | Hazard Ratio 1 | (95% CI) | χ 21df | P value |
|---|---|---|---|---|---|
| Model 1: Core model | 180 / 1,294 | 1.21 | (1.08—1.36) | 10.78 | 0.001 |
| Model 2: Excluding MCI at baseline | 65/ 930 | 1.29 | (1.08—1.54) | 8.09 | 0.004 |
| Model 3: Excluding MCI at baseline and incident AD in 1st year of follow-up | 61 / 926 | 1.29 | (1.08—1.56) | 7.53 | 0.006 |
| Model 4: Excluding MCI at baseline and incident AD in 1st or 2nd year of follow-up | 48/ 913 | 1.25 | (1.00—1.55) | 3.94 | 0.047 |
| Model 5: Excluding lowest 5% cognitive function at baseline | 139 / 1,227 | 1.28 | (1.12—1.45) | 13.80 | <0.001 |
| Model 6: Excluding lowest 10% cognitive function at baseline | 119 / 1,162 | 1.25 | (1.09—1.44) | 9.75 | 0.002 |
| Model 7: Excluding lowest 15% cognitive function at baseline | 97 / 1,098 | 1.28 | (1.09—1.49) | 9.69 | 0.002 |
Based on separate Cox proportional hazards models; all models controlled for age, sex, education, and race. Estimates are for a 1 unit change in life space score (higher score = more constricted life space).
FIGURE 1.
Cumulative Hazard of AD for Participants With Constricted (dotted line, score = 3) versus Largest (solid line, score = 0) Life Space (n = 1,294), MAP and MARS Studies.
Because the association of life space with AD may vary along demographic lines, we repeated the core model with terms for the interactions of age, sex, education, and race with life space in separate models. No interactions were found (data not shown). In stratified analyses, the estimate was higher for African Americans (HR = 1.49, 95% CI 1.06--2.08, X21 = 5.40, p = 0.020) than white participants (HR = 1.19, 95% CI 1.05--1.34, X21 = 7.39, p = 0.007). In addition, because physical function, disability, depressive symptoms, social networks, and vascular risk factors and diseases may influence both life space and AD, we repeated the core model with terms to control for these covariates, and the association of life space with AD persisted (HR = 1.15, 95% CI 1.02--1.31, X21 = 5.00, p = 0.025).
Because it is possible that the inclusion of persons with mild, undiagnosed AD could influence the findings reported above, we repeated the core analysis after excluding persons with MCI at baseline (Table 2, Model 2), and then further excluding persons who developed AD in year 1 (Table 2, Model 3) or year 2 (Table 2, Model 4) of follow-up. We also repeated the core analysis after sequentially excluding persons at the lowest 5th, 10th, and 15th percentiles of cognitive function at baseline (Table 2, Models 5--7). In each case, and with as few as 48 cases of incident AD, life space remained consistently associated with the risk of AD.
Life Space and the Risk of MCI
To further ensure that our findings were not due to the inclusion of persons with preclinical AD, we examined the relation of life space with the risk of developing MCI. In these models, 340 persons with MCI at baseline were excluded, leaving a sample of 954 persons without cognitive deficits. Over up to 8 years of follow-up (mean [SD] = 4.5 [1.6]), 390 persons (41%) developed MCI (incident dementia was treated as an event as well). These same covariates that were associated with developing AD were associated with developing MCI, the exceptions being depressive symptoms (F1, 952 = 5.48, p = 0.019) and social networks (F1, 950 = 0.03 [1, 950], p = 0.87)
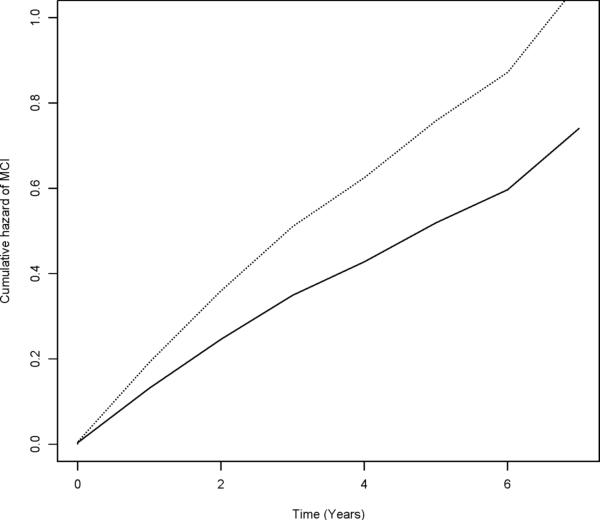
In a proportional hazards model adjusted for age, sex, education, and race, a more constricted life space was associated with an increased risk of MCI (HR = 1.17, 95% CI 1.06--1.28, X21 = 10.15, p = 0.001). As illustrated in Figure 2, a person with a life space constricted to their home environment (score = 3) was about 1.6 times more likely to develop MCI than a person with the largest life space (score = 0).
FIGURE 2.
Cumulative Hazard of MCI for Participants With Constricted (Dotted Line, score = 3) versus Largest (Solid Line, score = 0) Life Space (n = 954), MAP and MARS Studies.
Because MCI does not uniformly progress to dementia or even persist, we examined the association of life space with persistent MCI defined as having MCI on two or more consecutive examinations (or MCI followed by dementia or death), as done in previous studies.24 Of 390 persons with incident MCI, 169 had MCI or dementia at the next evaluation or died before the next evaluation. The remaining 221 were included in the reference group. In a proportional hazards model adjusted for age, sex, education, and race, a more constricted life space was associated with an increased risk of persistent MCI (HR = 1.23, 95% CI = 1.08--1.39, X21 = 10.20, p = 0.001).
Life Space and Change in Cognitive Function
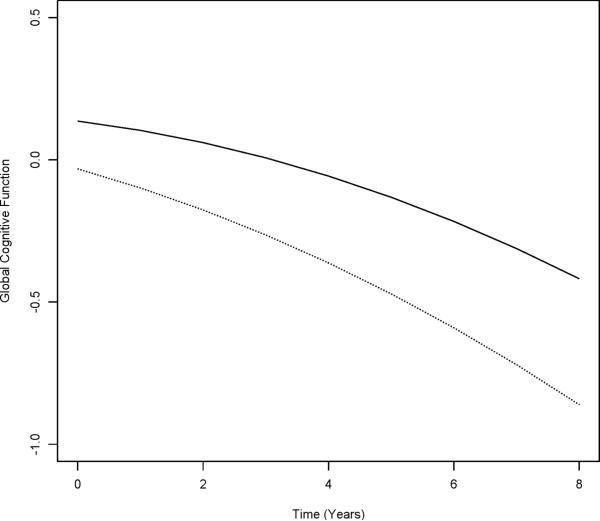
Because AD develops slowly over many years and the principal manifestation is cognitive decline, we used mixed-effects models to estimate the association of life space with the rate of change in cognition while controlling for baseline level of cognition and demographics. Life space was associated with the baseline level of global cognition (life space estimate, Table 3, column A) and rate of global cognitive decline (life space × time estimate, Table 3, column A). Figure 3 shows the estimated trajectories of cognitive decline for participants with a life space constricted to their home environment (score = 3) versus. the largest life space (score = 0). Persons with a more constricted life space started at a lower level of global cognition and declined more rapidly than those with a large life space. The difference in rate of decline for 1 zone of life space was equivalent to the decline in cognition associated with a 2.3-year increase in age (derived by dividing the life space × time estimate by the age × time estimate). In separate models, life space was associated with rate of decline in each of five cognitive abilities (Table 3, column A).
Table 3.
Relation of life space with change in cognitive function1
| A | B | ||
|---|---|---|---|
| Cognitive Domain | Model Term | Estimate (SE) among persons without dementia at baseline | Estimate (SE) among persons without MCI at baseline |
| Global cognition | Life space | -0.056 (0.012)** | -0.035(0.011)* |
| Life space × time | -0.012 (0.003)** | -0.012(0.004)** | |
| Episodic memory | Life space | -0.051 (0.016)* | -0.019(0.014) |
| Life space × time | -0.013 (0.004)* | -0.012(0.005)+ | |
| Semantic memory | Life space | -0.092 (0.016)** | -0.067(0.017)** |
| Life space × time | -0.010 (0.005)+ | -0.011(0.005)+ | |
| Working memory | Life space | -0.030 (0.017) | -0.021(0.020) |
| Life space × time | -0.008(0.004)+ | -0.006(0.005) | |
| Perceptual speed | Life space | -0.075 (0.017)** | -0.054(0.020)* |
| Life space × time | -0.016 (0.004)** | -0.017(0.005)** | |
| Visuospatial ability | Life space | -0.048 (0.017)* | -0.036(0.017)+ |
| Life space × time | -0.015 (0.004)** | -0.015(0.005)* | |
From mixed effects models including terms for age, sex, education, race, time, time-squared, and the interactions of time with age, sex, education, and race.
Estimates are for a 1 unit change in life space score (higher score = more constricted life space). p values are based on t-tests. All degrees of freedom for models in column A are over 4,800. All degrees of freedom for models in column B are over 3,600.
p<0.001
p<0.01
p<0.05
Figure 3.
Decline in Global Cognition for Participants With Constricted (Dotted Line, Score = 3) versus Largest (Solid Line, Score = 0) Life Space (n = 1,294), MAP and MARS Studies.
Finally, we repeated the mixed effects models after excluding persons with MCI at baseline to examine the relationship between life space and cognitive decline in participants without cognitive impairment at baseline. In these models, findings were similar (Table 3, column B), though working memory was not statistically significant.
DISCUSSION
We examined the association of life space with the risk of incident AD in almost 1,300 community-dwelling older persons. During up to 8 years of follow-up, a more constricted life space was associated with a substantially increased risk of AD, such that a person who had not been to an area beyond their home environment in the previous week was almost twice as likely to develop AD as a person who traveled out of town. This association remained consistent even after excluding persons who were likely to have preclinical dementia. A constricted life space was also associated with an increased risk of MCI, an early manifestation of AD. Further, life space was associated with change in cognitive function in analyses that controlled for baseline level of cognition in persons without cognitive impairment.
These findings suggest that restrictions in movement through the environment may be an indicator of a greater risk for developing AD in older adults, even in persons with no cognitive impairments. Specifically, persons who do not leave their home environment may be at high risk. Life space was originally conceived as a measure of mobility4 that represents actual functioning in the real world, reflecting not only physical capabilities but also the environment and available resources,2 and psychosocial factors that motivate or limit spatial movement.1,6 Life space may therefore measure aspects of functional status that complement commonly-used measures of disability. Correlates of life space have been identified, but only recently has life space been examined as a predictor of adverse health outcomes. Our findings greatly expand upon observed associations with cognitive function and dementia from cross-sectional analyses1,6,8,9 and one longitudinal study showing an association between life space and change in MMSE score.10
The reasons why a constricted life space is associated with an increased risk of AD are unknown. One explanation is that persons with constricted life space have early, preclinical cognitive impairments that have led to restrictions in movement beyond the home environment, and later manifest as clinical AD. However, our findings were robust to a number of sensitivity analyses designed to address this reverse causality scenario, as the association persisted after excluding persons with the lowest levels of cognitive function, persons with MCI at baseline, and persons who developed AD in the first or second year of follow-up. A related possibility is that an underlying disease process in the brain, such as subclinical AD pathology or cerebrovascular disease, may contribute to both constriction of life space and, later, cognitive impairment; that is, a constricted life space may be a prodromal feature of AD or dementia. AD pathology accumulates insidiously over many years before cognitive problems are clinically detectable,25 and AD pathology has been shown to affect motor function in older persons both with and without dementia.26 Thus, the accumulation of neuropathology may bring about changes in both motor functioning and cognition. If this is the case, our results would indicate that constriction of life space may occur years in advance of cognitive changes as detectable through standard clinical assessment, thus serving as a sentinel indicator of impending cognitive impairment. This is in line with other findings that impairments in motor functioning and mobility can manifest many years before cognitive changes.11,27
Another possibility is that affect or some other health or psychological factor may be related to the propensity to not travel beyond the home and to later cognitive impairments. We tried to account for this possibility by adjusting for perceived confounders of this relationship, but unmeasured or residual confounding may still be present. For example, controlling for depressive symptoms28 did not change the association of life space with AD, but other unmeasured psychological or affective features such as apathy were not accounted for. Furthermore, the association persisted after adjusting for vascular disease and vascular risk factors, disability, and physical functioning, but other age-related comorbidities could potentially influence this association.
Finally, life space may be an indicator of the degree of complexity in an older person's living environment,10 and environmental complexity has been theorized to protect against cognitive impairments in later life.29,30 Animal studies have provided evidence of a wide scope of neuroplastic responses to enriched environments such as neurogenesis and synaptogenesis.31 Studies in humans have shown that occupational complexity and leisure-time activities are associated with better cognitive outcomes including lowered incidence of AD.32--34 A larger life space may be an additional avenue for environmental complexity, whereas a life space restricted to the home may limit the complex experiences and demands encountered by those who travel to new and diverse places. If this were the case, life space could represent a potentially modifiable risk factor amenable to interventions designed to aid older adults in expanding their zones of life experience, such as the Experience Corps,35 but more research is necessary before recommending such interventions.
This study has several limitations. As is the case for all observational studies, we are not able to establish causality with certainty. Another limitation is that the selected nature of this volunteer cohort may be more mobile than the general older population or, alternatively, as they were recruited primarily from retirement homes and subsidized living facilities may have more restricted life spaces. These potential recruitment biases may have led to a restricted range of life space scores and limited the generalizability of findings. We do not know how the life space distribution in this study compares to the general older population, but we have previously shown that the mean social network size of our participants is comparable to other population-based studies.21 To examine differences across race, we combined two separate cohort studies. Though these studies were designed with very similar recruitment and operational methods, the differences across the cohorts could result in artifactual inferences regarding unobserved differences across race. We relied upon self-report of life space; a limitation that could be addressed in future studies with global positioning system devices that track movement through space. This study has a number of strengths including the assessment of life space in two large cohorts of racially diverse community-dwelling older adults. Cognitive outcomes were assessed annually with psychometrically sound measures for up to 8 years with high rates of follow-up participation. These findings provide initial evidence that a constricted life space may be an early indicator of increased risk of AD in older adults.
Acknowledgments
The authors thank the participants and the staff of the Rush Memory and Aging Project, the Minority Aging Research Study, and the Rush Alzheimer's Disease Center for this work, and to Sue Leurgans and Woojeong Bang for help with statistical programming. They thank Sue Leurgans for help with presentation of statistical findings as well.
Statistical analysis performed by B.D.J. with the assistance of Dr. Sue Leurgans, Senior Statistician at the Rush Alzheimer's Disease Center, and Woojeong Bang, Supervising Statistician at the Rush Alzheimer's Disease Center.
The study was supported by NIA grants R01AG17917, R01AG22018, R01AG034374, R01AG033678, and R01AG24480, the Illinois Department of Public Health, and the Robert C. Borwell Endowment Fund. The authors declare no conflict of interest. Presented as a poster at the International Conference on Alzheimer's Disease 2010, July 13, Honolulu, Hawaii.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Barnes LL, Wilson RS, Bienias JL, et al. Correlates of life space in a volunteer cohort of older adults. Exp Aging Res. 2007;33:77–-93. doi: 10.1080/03610730601006420. [DOI] [PubMed] [Google Scholar]
- 2.Stalvey BT, Owsley C, Sloane ME, et al. The Life Space Questionnaire: a measure of the extent of mobility of older adults. J Appl Gerontol. 1999;18:460–-478. [Google Scholar]
- 3.Tinetti ME, Ginter SF. The nursing home life-space diameter. A measure of extent and frequency of mobility among nursing home residents. J Am Geriatr Soc. 1990;38:1311–-1315. doi: 10.1111/j.1532-5415.1990.tb03453.x. [DOI] [PubMed] [Google Scholar]
- 4.May D, Nayak US, Isaacs B. The life-space diary: a measure of mobility in old people at home. Int Rehabil Med. 1985;7:182–-186. doi: 10.3109/03790798509165993. [DOI] [PubMed] [Google Scholar]
- 5.Cox H. The Realities of Aging. 5th Ed. Prentice Hall, Inc.; Englewood Cliffs, New Jersey: 2001. Later Life. [Google Scholar]
- 6.Murata C, Kondo T, Tamakoshi K, et al. Factors associated with life space among community-living rural elders in Japan. Public Health Nurs. 2006;23:324–-331. doi: 10.1111/j.1525-1446.2006.00568.x. [DOI] [PubMed] [Google Scholar]
- 7.Baker PS, Bodner EV, Allman RM. Measuring life-space mobility in community-dwelling older adults. J Am Geriatr Soc. 2003;51:1610–-1614. doi: 10.1046/j.1532-5415.2003.51512.x. [DOI] [PubMed] [Google Scholar]
- 8.Xue QL, Fried LP, Glass TA, et al. Life-space constriction, development of frailty, and the competing risk of mortality: the Women's Health And Aging Study I. Am J Epidemiol. 2008;167:240–-248. doi: 10.1093/aje/kwm270. [DOI] [PubMed] [Google Scholar]
- 9.Allman RM, Sawyer P, Roseman JM. The UAB Study of Aging: background and insights into life-space mobility among older Americans in rural and urban settings. Aging Health. 2006;2:417–-429. [Google Scholar]
- 10.Crowe M, Andel R, Wadley VG, et al. Life-space and cognitive decline in a community-based sample of African American and Caucasian older adults. J Gerontol A Biol Sci Med Sci. 2008;63:1241–-1245. doi: 10.1093/gerona/63.11.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Larson EB, Bowen JD, et al. Performanc e-based physical function and future dementia in older people. Arch Intern Med. 2006;166:1115–-1120. doi: 10.1001/archinte.166.10.1115. [DOI] [PubMed] [Google Scholar]
- 12.Glass TA. Conjugating the “tenses” of function: discordance among hypothetical, experimental, and enacted function in older adults. Gerontologist. 1998;38:101–-112. doi: 10.1093/geront/38.1.101. [DOI] [PubMed] [Google Scholar]
- 13.Bennett DA, Schneider JA, Buchman AS, et al. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25:163–-175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 14.Arvanitakis Z, Bennett DA, Wilson RS, et al. diabetes and cognitive systems in older black and white persons. Alzheimer Dis Assoc Disord. 2009 doi: 10.1097/WAD.0b013e3181a6bed5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–-944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 16.Wilson RS, Barnes LL, Krueger KR, et al. Early and late life cognitive activity and cognitive systems in old age. J Int Neuropsychol Soc. 2005;11:400–-407. [PubMed] [Google Scholar]
- 17.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–-561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyle PA, Wilson RS, Buchman AS, et al. Lower extremity motor function and disability in mild cognitive impairment. Exp Aging Res. 2007;33:355–-371. doi: 10.1080/03610730701319210. [DOI] [PubMed] [Google Scholar]
- 19.Katz S, Akpom CA. A measure of primary sociobiological functions. Int J Health Serv. 1976;6:493–-508. doi: 10.2190/UURL-2RYU-WRYD-EY3K. [DOI] [PubMed] [Google Scholar]
- 20.Kohout FJ, Berkman LF, Evans DA, et al. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5:179–-193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 21.Bennett DA, Schneider JA, Tang Y, et al. The effect of social networks on the relation between Alzheimer's disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol. 2006;5:406–-412. doi: 10.1016/S1474-4422(06)70417-3. [DOI] [PubMed] [Google Scholar]
- 22.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. John Wiley & Sons, Inc.; Hoboken, New Jersey: 1980. [Google Scholar]
- 23.Gail MH, Lubin JH, Rubinstein LV. Likelihood calculations for matched case-control studies and survival studies with tied death times. Biometrika. 1981;68:703–-707. [Google Scholar]
- 24.Wilson RS, Schneider JA, Arnold SE, et al. Conscientiousness and the Incidence of Alzheimer Disease and Mild Cognitive Impairment. Arch Gen Psychiatry. 2007;64:1204–-1212. doi: 10.1001/archpsyc.64.10.1204. [DOI] [PubMed] [Google Scholar]
- 25.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–-1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 26.Buchman AS, Schneider JA, Leurgans S, et al. Physical frailty in older persons is associated with Alzheimer disease pathology. Neurology. 2008;71:499–-504. doi: 10.1212/01.wnl.0000324864.81179.6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buchman AS, Boyle PA, Wilson RS, et al. Frailty is associated with incident Alzheimer's disease and cognitive decline in the elderly. Psychosom Med. 2007;69:483–-489. doi: 10.1097/psy.0b013e318068de1d. [DOI] [PubMed] [Google Scholar]
- 28.Wilson RS, Barnes LL, Mendes de Leon CF, et al. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology. 2002;59:364–-370. doi: 10.1212/wnl.59.3.364. [DOI] [PubMed] [Google Scholar]
- 29.Schooler C. Psychological effects of complex environments during the life span: A review and theory. In: Schooler C, Schaie KW, editors. Cognitive functioning and social structure over the life course. Ablex; Norwood, NJ: 1987. pp. 24–-49. [Google Scholar]
- 30.Milgram NW, Siwak-Tapp CT, Araujo J, et al. Neuroprotective effects of cognitive enrichment. Ageing Res Rev. 2006;5:354–-369. doi: 10.1016/j.arr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Diamond MC. Response of the brain to enrichment. An Acad Bras Cienc. 2001;73:211–-220. doi: 10.1590/s0001-37652001000200006. [DOI] [PubMed] [Google Scholar]
- 32.Schooler C, Mulatu MS, Oates G. The continuing effects of substantively complex work on the intellectual functioning of older workers. Psychol Aging. 1999;14:483–-506. doi: 10.1037//0882-7974.14.3.483. [DOI] [PubMed] [Google Scholar]
- 33.Andel R, Crowe M, Pedersen NL, et al. Complexity of work and risk of Alzheimer's disease: a population-based study of Swedish twins. J Gerontol B Psychol Sci Soc Sci. 2005;60:P251–-258. doi: 10.1093/geronb/60.5.p251. [DOI] [PubMed] [Google Scholar]
- 34.Scarmeas N, Levy G, Tang MX, et al. Influence of leisure activity on the incidence of Alzheimer's disease. Neurology. 2001;57:2236–-2242. doi: 10.1212/wnl.57.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fried LP, Carlson MC, Freedman M, et al. A social model for health promotion for an aging population: initial evidence on the Experience Corps model. J Urban Health. 2004;81:64–-78. doi: 10.1093/jurban/jth094. [DOI] [PMC free article] [PubMed] [Google Scholar]