Abstract
Purpose
This multicenter phase 2 study assessed the tolerability and efficacy of motesanib, an oral inhibitor of Kit, platelet-derived growth factor receptor (PDGFR), and vascular endothelial growth factor receptors (VEGFR), in patients with imatinib-resistant gastrointestinal stromal tumors (GIST).
Methods
Patients with advanced GIST who failed imatinib mesylate after ≥8 weeks of treatment with ≥600 mg daily received motesanib 125 mg orally once daily continuously for 48 weeks or until unacceptable toxicity or disease progression occurred. The primary endpoint was confirmed objective tumor response per RECIST and independent review. Secondary endpoints included progression-free survival (PFS), time to progression (TTP); objective response by 18FDG-PET and by changes in tumor size and/or density (Choi criteria); pharmacokinetics and safety.
Results
In the patients evaluable for response (N = 102), the objective response rate was 3%; 59% of patients achieved stable disease, with 14% achieving durable stable disease ≥24 weeks; 38% had disease progression. Higher objective response rates were observed per 18FDG-PET (N = 91) (30%) and Choi criteria (41%). The median PFS was 16 weeks (95% CI = 14–24 weeks); the median TTP was 17 weeks (95% CI = 15–24 weeks). The most common motesanib treatment-related grade 3 adverse events included hypertension (23%), fatigue (9%), and diarrhea (5%). Motesanib did not accumulate with daily dosing.
Conclusions
In this study of patients with imatinib-resistant GIST, motesanib treatment resulted in acceptable tolerability and modest tumor control as evident in the proportion of patients who achieved stable disease and durable stable disease.
Keywords: Angiogenesis, GIST, Imatinib, Kit receptor, Motesanib, VEGF receptor
Introduction
Until the success of imatinib mesylate, patients with metastatic gastrointestinal stromal tumors (GIST) had a dismal prognosis since no systemic therapy provided clinical benefit. Surgery is the initial therapy for patients with primary resectable GIST; however, approximately half of all patients eventually develop metastatic disease [1]. Mutually exclusive gain-of-function mutations in the KIT proto-oncogene (occurring in 85–90% of GISTs) or in the platelet-derived growth factor receptor α gene (PDGFRA; about 5% of tumors) play a fundamental role in the development of GISTs [2]. Imatinib, a selective inhibitor of Kit and PDGFR kinase activity, has been shown to substantially improve clinical outcomes in patients with advanced disease [3, 4]. However, approximately 14% of treated patients exhibit primary imatinib resistance, which is associated with shortened survival, and many if not all, develop secondary resistance after an initial response [3, 4]. Sunitinib malate, a multikinase inhibitor, has recently been demonstrated to be effective as second-line therapy in imatinib-resistant GIST [5]. The duration of secondary disease control with sunitinib is much shorter though than the initial control with imatinib, despite considerable toxicity, indicating that alternative therapies are needed [5].
Motesanib is a highly selective, oral inhibitor of VEGF receptors (VEGFR) 1, 2, and 3; and PDGFR (IC50, 84 nmol/L). In addition, motesanib potently inhibits wildtype Kit in vitro (IC50, 8 nmol/L) [6]. Motesanib has also been shown to inhibit autophosphorylation of a number of clinically relevant primary Kit mutants with greater potency than imatinib and has demonstrated activity against some imatinib-resistant mutants (e.g., Y823D and D816H) [7], suggesting that it may have antitumor activity in imatinib-resistant GIST. In preclinical studies, motesanib induced significant tumor regression in xenograft models of human breast carcinoma [8], nonsmall cell lung cancer [9], medullary thyroid cancer [10], and epidermoid and colon carcinoma [6, 11]. When tumor blood vessel density was investigated the results showed that motesanib treatment selectively targeted neovascularization indicating that the observed antitumor effect was mediated at least in part by inhibition of angiogenesis. Motesanib did not inhibit the proliferation of tumor cells in vitro [6, 8–10]. In the phase 1 first-in-human study in solid malignancies, a motesanib dose of 125 mg once daily was established as the maximum tolerated dose. In that study, three patients with thyroid cancer and one patient with leiomyosarcoma achieved a partial response [12].
The aim of this study was to evaluate the tolerability and efficacy of motesanib, using different methods for tumor response measurement including Response Evaluation Criteria in Solid Tumors (RECIST), Choi criteria [13, 14] and 18fluorodeoxyglucose positron emission tomography (18FDG-PET) [15, 16], in patients with advanced GIST who developed progressive disease (PD) or relapsed while receiving imatinib therapy.
Methods
Eligibility criteria
Key inclusion criteria were age ≥18 years; histologically confirmed GIST (expressing CD117); disease progression per RECIST during previous treatment with imatinib mesylate ≥600 mg daily for ≥8 weeks, as per two independently assessed prestudy computerized tomography (CT) scans; imatinib treatment terminated at least 7 days before study day 1; presence of at least one measurable (per RECIST) and progressing tumor lesion not previously treated with radiotherapy or embolization and evaluable by CT scan or magnetic resonance imaging (MRI); Karnofsky performance status ≥60; and adequate hepatic, renal, and cardiac function. Key exclusion criteria were prior malignancy (other than GIST, in situ cervical cancer, or basal cell cancer of the skin) unless treated with curative intent and without evidence of disease for ≥3 years; uncontrolled hypertension (systolic > 145 mmHg or diastolic > 85 mmHg), or history of arterial or deep vein thrombosis (including pulmonary embolus) within 1 year of study day 1; and prior treatment with VEGF or KIT (except imatinib) inhibitors. The study was approved by the institutional review board of each participating institution, and all patients provided written informed consent before any study-related procedures were performed.
Study design and treatment
This was a phase 2, multicenter, open-label, single-arm study. The primary endpoint was objective response (partial response [PR] or complete response [CR]) per RECIST, as determined by central independent review. Secondary endpoints prospectively included the duration of response, progression-free survival (PFS), time to disease progression (TTP), time to response, and overall survival; objective response by 18FDG-PET at week 8 and by changes in target tumor size and/or density (Hounsfield units) at week 8 (Choi response criteria) [13, 14]; pharmacokinetic (PK) parameters of motesanib, and adverse events.
After a ≥7-day washout of imatinib prior to study day 1, motesanib 125 mg (Amgen Inc., Thousand Oaks, CA, USA) was self-administered orally once daily without interruption until disease progression or intolerance occurred, for a maximum of 48 weeks. Patients deriving clinical benefit at 48 weeks could enroll in an extension study.
Adverse events were recorded from day 1 through to 30 days after the last dose of motesanib and were graded based on the Common Terminology Criteria for Adverse Events (CTCAE) Version 3.0.
Tumor response
Tumor measurements were performed within 21 days of study day 1 (baseline) and after 8, 16, 24, 32, 40, and 48 weeks (±4 days) of treatment using nonenhanced and contrast-enhanced CT. Measurable target lesions were evaluated for response using RECIST. Objective responses were confirmed by a second CT scan ≥4 weeks later. 18FDG-PET scans and tumor size and density measurements per CT were obtained at baseline and after 8 weeks. All tumor responses were reviewed retrospectively by an independent central panel.
Objective response by 18FDG-PET at week 8 was defined as a >25% decrease in the average standardized uptake value (SUV) in all RECIST target lesions compared with the average baseline SUV of all RECIST target lesions [17]. Objective response by Choi response criteria [14] was defined as ≥10% decrease in the sum of longest diameters (SLD) and/or ≥15% decrease in the average target tumor density using the RECIST target lesions on CT scans at week 8, compared with baseline.
Pharmacokinetics
Blood samples for intensive PK were collected by design from up to 20% of patients on study days 1 and 28 at predose, and 0.25, 0.5, 1, 2, 4, 6, 8, and 24 h after motesanib diphosphate administration from patient. Trough PK blood samples were collected at scheduled visits every 2 weeks (±4 days) for the first 16 weeks and then every 4 weeks (±4 days). Motesanib concentrations in the plasma were analyzed at using a validated liquid chromatography assay coupled with tandem mass spectrometry (CEDRA Corporation, Austin, TX). The lower limit of quantification was 0.2 ng/mL. Pharmacokinetic parameters were estimated from plasma concentration–time data using standard noncompartmental methods (WinNonlin Professional version 4.1e, Pharsight Corp., Mountain View, CA).
Statistical analysis
The planned sample size was 100, which would produce a 2-sided 95% confidence interval (CI) of 5–18% for the objective response rate if a point estimate of 10% was observed. Primary and secondary efficacy analyses were performed on the prospectively defined per-protocol analysis set (all patients from the full analysis set who had prestudy disease progression per RECIST and according to independent review). Additional sensitivity analyses were conducted on the full analysis set (all patients who received ≥1 dose of motesanib). Efficacy analysis per 18FDG-PET or Choi response criteria was also performed on the evaluable patient population (all patients with a baseline and week-8 18FDG-PET or CT scan, respectively). Objective response by 18FDG-PET and Choi criteria assessed at week 8 were correlated with PFS using RECIST to evaluate progression (Cox proportional hazards model) to assess the predictive value of early changes in tumor density and/or size. Reported P-values were not adjusted for multiple comparisons. Duration of response was the time from first confirmed objective response to the first PD assessment per RECIST or death if due to disease progression; PFS was the time from study day 1 to the date of confirmed disease progression or death regardless of cause; TTP was the time from study day 1 to the date when physical or radiological evidence of disease progression is determined, or death if due to progression; and overall survival was the time from study day 1 to the date of death due to any reason.
Results
Patient characteristics and disposition
The study enrolled 138 patients with advanced imatinib-resistant GIST from 29 centers in 7 countries (October 2004 through July 2005). In order to ensure that at least 100 enrolled patients would be considered evaluable, overenrollment of 20% was targeted. At the time of enrollment closure, 20 more evaluable patients had been enrolled and an additional 18 patients who were in screening at that time were also evaluable and enrolled.
Baseline demographics and clinical characteristics are summarized in Table 1. All patients had received imatinib at a dose of at least 600 mg/day for ≥8 weeks (78% received a daily dose of 800 mg); median duration of imatinib therapy was 32.5 months. Thirty-eight percent of patients had an objective response to imatinib therapy per RECIST, and 28% had SD. All patients received ≥1 dose of motesanib and were evaluated for safety and tolerability; median duration of therapy was 16 weeks (range 0.3–52), with 15 patients (9%) completing the scheduled 48-week treatment period. Reasons for early therapy discontinuation were as follows: disease progression (n = 79), adverse events (n = 22), withdrawal of consent (n = 6), death (n = 3), and other (n = 4). Based on the full analysis set, 77 patients died; for the remaining patients, median follow-up was 12.8 months (minimum, 4.5; maximum, 23.4).
Table 1.
Baseline demographics and clinical characteristics
| All patients (N = 138) | |
|---|---|
| Male/female, n (%) | 84/54 (61/39) |
| Age years) | |
| Median (range) | 61 (25, 90) |
| ≥65 years, n (%) | 54 (39) |
| Karnofsky performance status, n (%) | |
| 100 | 33 (24) |
| 90 | 63 (46) |
| 80 | 22 (16) |
| 70 | 14 (10) |
| 60 | 6 (4) |
| Number of sites of disease, n (%) | |
| 1 | 51 (37) |
| 2 | 55 (40) |
| 3 | 27 (20) |
| ≥4 | 5 (4) |
| Imatinib resistance, n (%)a | |
| Primary | 47 (34) |
| Secondary | 91 (66) |
| Prior chemotherapy, n (%) | |
| None | 117 (85) |
| 1 Regimen | 11 (8) |
| ≥2 Regimens | 10 (7) |
| Duration of first imatinib therapy, n (%) | |
| ≤6 months | 34 (25) |
| >6 months | 104 (75) |
| Total duration of imatinib therapy (months) | |
| Median (range) | 33 (4, 57) |
| Best response to initial imatinib, n (%) | |
| Complete response | 4 (3) |
| Partial response | 48 (35) |
| Stable disease | 39 (28) |
| Progressive disease | 47 (34) |
| Highest imatinib dose administered (mg), n (%) | |
| 600 | 21 (15) |
| 700 | 1 (1) |
| 800 | 108 (78) |
| 1,000 | 4 (3) |
| 1,100 | 1 (1) |
| 1,200 | 3 (2) |
aPrimary resistance: failure to achieve stable disease in response to imatinib or development of disease progression within 6 months of an initial clinical response to imatinib. Secondary resistance: development of one or more sites of disease progression after more than 6 months of clinical response to imatinib
Efficacy
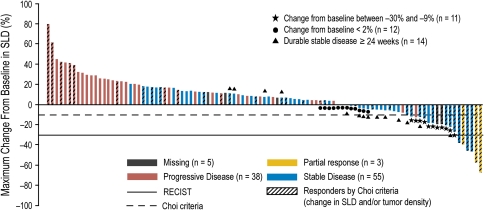
The response to therapy is summarized in Table 2. The change from baseline in tumor measurement for individual patients from the per-protocol analysis set is shown in Fig. 1.
Table 2.
Tumor response to treatment with motesanib
| Criteria and response | All patients (N = 138) | Per-protocol analysis seta (N = 120) | RECIST/Choi evaluable patients (N = 102) | 18FDG-PET evaluable patients (N = 91) |
|---|---|---|---|---|
| RECIST | ||||
| Confirmed RECIST response, n (%) | 3 (2) | 3 (3) | 3 (3) | 3 (3) |
| 95% CI | 0.5–6.2 | 0.5–7.1 | 0.6–8.4 | 0.7–9.3 |
| Confirmed partial response, n (%) | 3 (2) | 3 (3) | 3 (3) | 3 (3) |
| Stable disease, n (%) | 70 (51) | 60 (50) | 60 (59) | 55 (60) |
| Durable stable disease ≥24 weeks, n (%) | 19 (14) | 14 (12) | 14 (14) | 14 (15) |
| Progressive disease, n (%) | 43 (31) | 39 (33) | 39 (38) | 33 (36) |
| Not assessed, n (%)b | 22 (16) | 18 (15) | 0 (0) | 0 (0) |
| 18FDG-PET at week 8c | ||||
| Objective response, n (%) | 28 (20) | 27 (23) | – | 27 (30) |
| 95% CI | 13.9–28.0 | 15.4–31.0 | 20.5–40.2 | |
| Nonresponse, n (%) | 110 (80) | 93 (78) | – | 64 (70) |
| 95% CI | 72.0–86.1 | 69.0–84.6 | 59.8–79.5 | |
| Choi criteria at week 8d | ||||
| Objective response, n (%) | 45 (33) | 42 (35) | 42 (41) | – |
| 95% CI | 24.9–41.1 | 26.5–44.2 | 31.5–51.4 | |
| Nonresponse, n (%) | 93 (67) | 78 (65) | 60 (59) | – |
| 95% CI | 58.9–75.1 | 55.8–73.5 | 48.6–68.5 | |
aAll patients who received at least one dose of motesanib and who had prestudy disease progression per RECIST as assessed by independent review
bEnded motesanib treatment prior to the first scheduled assessment of response
cDefined as >25% decrease in average standardized uptake value (SUVmax) in all RECIST target lesions compared with the average SUVmax in all RECIST target lesions at baseline (measured by independent reviewer)
dDefined as ≥10% decrease in the sum of the longest diameter of the target lesions (identified by RECIST) and/or ≥15% decrease in the average target tumor density using the RECIST target lesions compared with the average baseline density based on CT scans
Fig. 1.
Maximum percent change from baseline in sum of longest diameters (SLD) of target lesions per RECIST and independent review in patients evaluable for response by RECIST or Choi criteria. Not shown: n = 1 progressive disease (new lesion; SLD unavailable). Not included: 18 patients (from the per-protocol set) without tumor response information (SLD measured prior to week 8). Changes in SLD > 30% shown as stable disease represent unconfirmed PRs
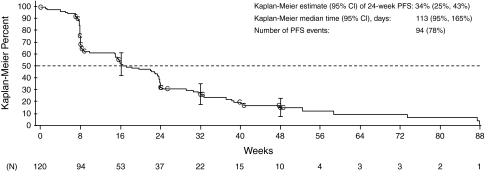
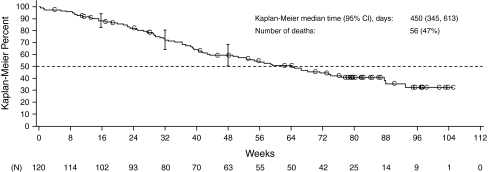
The time to RECIST response per independent review was 7, 8, and 16 weeks for the three responders, with the duration of response of 16, 40 (patient completed the 48-week treatment without observed disease progression), and 16 weeks, respectively. The patient who achieved the duration of response of 40 weeks continued motesanib in an extension study. As of January 13, 2009, the patient has not progressed per investigator assessment (censored duration of response from the initial response: 177 weeks). Median PFS time was 16 weeks (95% CI = 14–24), and 34% of patients were progression-free at 24 weeks (Fig. 2). Median TTP was 17 weeks (95% CI = 15–24). At the time of data cutoff, 67 patients (56%; based on the per-protocol analysis set) had died. The median overall survival for the per-protocol population was 64 weeks (95% CI = 49–88) (Fig. 3).
Fig. 2.
Kaplan–Meier estimate of progression-free survival (PFS) time during treatment with motesanib (analysis includes all patients in the full analysis set who had prestudy disease progression per RECIST and independent review; N = 120). Vertical bars represent 95% CIs of the Kaplan–Meier estimates at week 16, 32, and 48. C censored observation
Fig. 3.
Kaplan–Meier estimate of overall survival time during treatment with motesanib (analysis includes all patients in the full analysis set who had prestudy disease progression per RECIST and independent review; N = 120). Vertical bars represent 95% CIs of the Kaplan–Meier estimates at week 16, 32, and 48. C censored observation
The objective response rate was 30% (95% CI = 20.5–40.2) using 18FDG-PET at week 8 and the EORTC PET response definition for the evaluable subset (N = 91). Using Choi response criteria, the objective response rate at week 8 was 41% (95% CI = 31.5–51.4) in the evaluable population (N = 102) (Table 2). Best tumor response per Choi criteria is shown in Fig. 1. The estimated median TTP for evaluable Choi responders was 22 weeks (95% CI = 16–24), compared with 15 weeks (95% CI = 8–22) for nonresponders (P = 0.35). Using Cox proportional hazard modeling in evaluable patients, none of the three tumor response assessment methods were predictive of PFS (RECIST, P = 0.31; 18FDG-PET, P = 0.17; and Choi criteria, P = 0.34).
Safety
Nearly, all patients (92%) experienced at least one treatment-related adverse event; 52% of these events were grade 1 or 2 and reversible. Thirty-seven patients (27%) discontinued treatment due to an adverse event. The three most common related grade 3 events were hypertension (23%), fatigue (9%), and diarrhea (5%) (Table 3). There were nine related grade 4 events, including one event of hypertension (reversible posterior leucoencephalopathy with sudden blindness syndrome; others were acute cardiac failure, enterocutaneous fistula, subarachnoid hemorrhage, pulmonary embolism, pulmonary edema, respiratory failure, and peripheral ischemia; n = 1 each). One fatal grade 5 event due to myocardial infarction occurring on study day 2 was considered by the investigator to be possibly related to treatment; of note, this patient had a number of pre-existing and concurrent comorbidities. Several related events of specific clinical interest occurred, including thromboembolic events, hemorrhage, anemia, and cardiac failure (Table 3). There was one event each of cholecystitis (grade 1) and pancreatitis (grade 3) considered unrelated by the investigator. Twenty-two deaths were recorded on study: disease progression (n = 12), cardiac disorders (n = 4), aspiration pneumonia (n = 2), deterioration of general condition (n = 1), intestinal obstruction (n = 2), and sepsis (n = 1).
Table 3.
Treatment-related adverse events occurring in ≥5% of patients and treatment-related adverse events of specific interest
| All patients (N = 138) | Grade 3 | Grade 4 | |
|---|---|---|---|
| Treatment-related adverse events, n (%) | |||
| Diarrhea | 67 (49) | 7 (5) | 0 |
| Hypertension | 65 (47) | 32 (23) | 1 (1)a |
| Fatigue | 41 (30) | 12 (9) | 0 |
| Headache | 34 (25) | 4 (3) | 0 |
| Nausea | 28 (20) | 3 (2) | 0 |
| Anorexia | 22 (16) | 5 (4) | 0 |
| Dysphonia | 16 (12) | 0 | 0 |
| Vomiting | 15 (11) | 2 (1) | 0 |
| Asthenia | 14 (10) | 2 (1) | 0 |
| Weight decreased | 13 (9) | 4 (3) | 0 |
| Flatulence | 11 (8) | 0 | 0 |
| Dehydration | 8 (6) | 5 (4) | 0 |
| Dizziness | 8 (6) | 1 (1) | 0 |
| Abdominal distension | 7 (5) | 2 (1) | 0 |
| Abdominal pain upper | 7 (5) | 1 (1) | 0 |
| Treatment-related adverse events of specific interest, n (%) | |||
| Thromboembolic eventsb | 7 (5) | 1 (1) | 3 (2) |
| Hemorrhage | 2 (1) | 1 (1) | 1 (1) |
| Impaired wound healing | 1 (1) | 1 (1) | 0 |
| Cardiac failureb | 2 (1) | 1 (1) | 1 (1) |
| Anemia | 2 (1) | 0 | 0 |
| Thrombocytopenia | 1 (1) | 0 | 0 |
| Hypothyroidismc | 1 (1) | 1 (1) | 0 |
aReversible posterior leucoencephalopathy syndrome
bTwo patients experienced both a thromboembolic event and a cardiac disorder: myocardial infarction/acute cardiac failure and grade 4 coronary artery arteriosclerosis; grade 4 cardiac failure and grade 2 ischemic stroke. One grade 5 event of myocardial infarction occurred
cPatients were not monitored with serial thyroid-stimulating hormone levels during the study
Pharmacokinetics
Twenty-six patients had assessable intensive PK results. Motesanib was rapidly absorbed after a single dose of 125 mg on day 1 with a median (range) t max of 0.58 (0.25–4.0) h, and after daily administration for 28 days (t max 1.0 [0.42–4.0] h). Compared with day 1, mean values for C max, AUC0–24, and trough concentrations at 24 h after dosing (C24) on day 28 were slightly lower, indicating that motesanib did not accumulate after daily administration (mean ratios of day 28 to day 1 in the same patients: 0.89 [n = 27], 0.84 [n = 19], and 0.92 [n = 19], respectively). Mean C24 values suggest that a dose of 125 mg (or higher) would provide continuous coverage above the concentration that inhibits 50% of proliferation in human umbilical vein endothelial cells in vitro (data on file; Amgen Inc., Thousand Oaks, CA).
The day-1 PK parameters of motesanib were similar in patients with partial or full gastrectomy (n = 8) and in patients without gastrectomy (n = 19). However, in the gastrectomy cohort, the mean (SD) day 28 values for AUC0–24 (2.09 [1.48] vs. 2.53 [2.44] μg h/mL) and C24 (6.67 [3.64] vs. 21.8 [37.3] ng/mL) were slightly lower and, based on the mean concentration–time profile, there was a slightly faster elimination in the terminal phase (mean [SD] t 1/2,z 4.42 [0.95] h vs. 5.45 [1.87] h). Substantially, shorter t 1/2,z values (>50% decrease) on day 28 were observed in only four out of eight patients in the gastrectomy group.
Discussion
Treatment with motesanib, an investigational inhibitor of VEGF receptors, PDGFR and Kit, resulted in clinical benefit, acceptable tolerability, and a PK profile similar to what has been observed in a previous monotherapy study [12], indicating no accumulation with repeat daily dosing. While the number of patients in the gastrectomy subgroup was small, the data showed that motesanib PK profiles were similar in patients with and without gastrectomy.
Currently, sunitinib is the only approved multitargeted kinase inhibitor in this disease setting. Although comparing study results outside of a head-to-head setting is difficult, a few points of comparison between the large randomized, controlled study of sunitinib [5] and the study described herein are worth mentioning. Both studies focused on patients with imatinib-resistant GIST. In the sunitinib study, which enrolled patients who had failed any dose of prior imatinib therapy or were imatinib intolerant, the RECIST response rate was relatively low (7%), but 58% of patients achieved SD and 24.2% achieved clinical benefit (objective response plus durable SD [≥22 weeks]); the median PFS and TTP was 24.1 and 27.3 weeks, respectively [5]. In comparison, in the study described here, the RECIST response rate (based on patients evaluable for response, N = 102) was only 3%; however, 59% of patients achieved SD, including 14% with durable SD ≥ 24 weeks, and 17% achieved clinical benefit (i.e., confirmed CR or PR or durable SD ≥ 24 weeks), indicating antitumor activity after failure of high-dose imatinib. The median PFS and TTP was only 16 and 17 weeks, respectively.
Since the availability of targeted therapies for patients with GIST, increasing concern has been raised over the suitability of RECIST to estimate the proportion of patients likely to benefit from therapy, particularly in the early phase of treatment, as targeted therapies may be more likely to induce more modest, yet sustained, reductions in tumor volume or progression arrest, which can be clinically meaningful [18]. Several reports have shown that 18FDG-PET not only is highly sensitive in detecting early response to imatinib therapy but also predicts the long-term response of GISTs to imatinib treatment [15, 16]. In our study, the tumor response rate per 18FDG-PET at week 8 was 30% in the evaluable population, compared with a 3% response rate per RECIST. Choi response criteria, which were only recently validated in a single-center study in patients with GIST treated with imatinib as initial tyrosine kinase inhibitor therapy, are based on a combination of tumor size and density measurements by CT [13] and have been shown by Choi et al. [14] and Benjamin et al. [19] to be more sensitive in identifying 18FDG-PET responders than RECIST. Furthermore, the same investigators reported that Choi response criteria have been shown to correlate significantly with TTP and disease-specific survival, compared with RECIST [19]. In the evaluable population of our study, 41% had a tumor response at week 8 based on Choi criteria, but the difference in TTP between Choi criteria responders and nonresponders was not significant (P = 0.35). However, it is noteworthy that only criteria of RECIST determined progression and not the progression criteria of Choi. Importantly, as can be seen in Fig. 1, 11 (26%) of the Choi responders (N = 42) were RECIST progressors at initial assessment. Comparison of response with survival was not planned in the protocol.
Another limitation of the study is the fact that it was not powered for the exploratory endpoints discussed above. Therefore, definitive conclusions about the comparison of tumor response using RECIST, 18FDG-PET, or Choi response criteria in this population cannot be reached. However, the results contribute to the ongoing discussion about the insensitivity of RECIST in determining tumor response to targeted therapies in patients with GIST [14, 19]. Future, adequately powered studies may provide more insight.
Most motesanib-related adverse events were mild and reversible, and consistent with those reported earlier in monotherapy settings [12, 20]. Hypertension, a known adverse event of VEGFR inhibitors [21], occurred most frequently (including one grade 4 event) but was generally manageable with antihypertensive therapy. Other related events, in particular hemorrhage and thromboembolic events, have been reported previously for other VEGFR inhibitors, indicating a class effect [21]. Three patients (2%) developed hypothyroidism; one of these events was considered related to treatment. Hypothyroidism has recently been described as a complication associated with sunitinib and imatinib treatment [5, 22–25]. Close monitoring of thyroid-stimulating hormone levels in patients receiving multikinase inhibitors is probably warranted.
In conclusion, in this study of patients with imatinib-resistant GIST, treatment with motesanib resulted in clinical benefit with acceptable tolerability. Although antitumor activity was evident based on the proportion of patients who achieved PR and durable SD and toxicity was relatively mild, thus suggesting that further study of motesanib in patients with imatinib-resistant GIST is warranted, the insufficient overall efficacy does not support further development of motesanib in GIST.
Acknowledgments
This study was funded by Amgen Inc. The authors would like to acknowledge the contributions of Isabelle Ray-Coquard, MD, and would like to thank Beate D Quednau, PhD (Amgen Inc.) for providing an initial draft of the manuscript and for general assistance in writing and editing of the manuscript.
Disclosures
None for the following authors: Robert S. Benjamin, Patrick Schoeffski, Joerg Thomas Hartmann, Justus Duyster, Peter Reichardt, Laurence Baker.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Clinical trial registration: www.ClinicalTrials.gov Identifier: NCT00254267.
References
- 1.DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51–58. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342–4349. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- 3.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 4.Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364:1127–1134. doi: 10.1016/S0140-6736(04)17098-0. [DOI] [PubMed] [Google Scholar]
- 5.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 6.Polverino A, Coxon A, Starnes C, et al. AMG 706, an oral, multikinase inhibitor that selectively targets vascular endothelial growth factor, platelet-derived growth factor, and kit receptors, potently inhibits angiogenesis and induces regression in tumor xenografts. Cancer Res. 2006;66:8715–8721. doi: 10.1158/0008-5472.CAN-05-4665. [DOI] [PubMed] [Google Scholar]
- 7.Caenepeel S, Renshaw-Gegg L, Baher A, et al. Motesanib inhibits kit mutations associated with gastrointestinal stromal tumors. J Exp Clin Cancer Res. 2010;29:96. doi: 10.1186/1756-9966-29-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coxon A, Bush T, Saffran D, et al. Broad antitumor activity in breast cancer xenografts by motesanib, a highly selective, oral inhibitor of vascular endothelial growth factor, platelet-derived growth factor, and kit receptors. Clin Cancer Res. 2009;15:110–118. doi: 10.1158/1078-0432.CCR-08-1155. [DOI] [PubMed] [Google Scholar]
- 9.Ziegler B, Kaufman S, Xu M et al (2010) Antitumor activity of motesanib alone and in combination with chemotherapy in xenograft models of human non-small cell lung cancer [abstract]. In: Proceedings of the 101st annual meeting of the American association for cancer research, 2010 Apr 17–21. AACR 2010. Abstract No 1380, Washington, DC. Available at http://aacrmeetingabstracts.org/
- 10.Motesanib diphosphate (AMG 706) inhibits the growth of medullary thyroid carcinoma in a nude mouse model [abstract]. In: Proceedings of the 98th annual meeting of the American association for cancer research, 2007 Apr 14–18. AACR 2007. Abstract No LB-283, Los Angeles, CA. Available at http://www.aacrmeetingabstracts.org/archive/2007.dtl
- 11.Starnes CO, Coxon A, Scully S et al (2006) Antitumor activity of AMG 706 alone and in combination with chemotherapy against established human tumor xenograft models in nude mice [abstract]. Proc Am Assoc Cancer Res 47:889; Abstract No 3780. Available at http://www.aacrmeetingabstracts.org/archive/2006.dtl
- 12.Rosen LS, Kurzrock R, Mulay M, et al. Safety, pharmacokinetics, and efficacy of AMG 706, an oral multikinase inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2007;25:2369–2376. doi: 10.1200/JCO.2006.07.8170. [DOI] [PubMed] [Google Scholar]
- 13.Choi H, Charnsangavej C, de Castro Faria S, et al. CT evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: a quantitative analysis correlated with FDG PET findings. AJR Am J Roentgenol. 2004;183:1619–1628. doi: 10.2214/ajr.183.6.01831619. [DOI] [PubMed] [Google Scholar]
- 14.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 15.Stroobants S, Goeminne J, Seegers M, et al. 18FDG-Positron emission tomography for the early prediction of response in advanced soft tissue sarcoma treated with imatinib mesylate (Glivec) Eur J Cancer. 2003;39:2012–2020. doi: 10.1016/S0959-8049(03)00073-X. [DOI] [PubMed] [Google Scholar]
- 16.Antoch G, Kanja J, Bauer S, et al. Comparison of PET, CT, and dual-modality PET/CT imaging for monitoring of imatinib (STI571) therapy in patients with gastrointestinal stromal tumors. J Nucl Med. 2004;45:357–365. [PubMed] [Google Scholar]
- 17.Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–1782. doi: 10.1016/S0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 18.Ratain MJ, Eckhardt SG. Phase II studies of modern drugs directed against new targets: if you are fazed, too, then resist RECIST. J Clin Oncol. 2004;22:4442–4445. doi: 10.1200/JCO.2004.07.960. [DOI] [PubMed] [Google Scholar]
- 19.Benjamin RS, Choi H, Macapinlac HA, et al. We should desist using RECIST, at least in GIST. J Clin Oncol. 2007;25:1760–1764. doi: 10.1200/JCO.2006.07.3411. [DOI] [PubMed] [Google Scholar]
- 20.Sherman SI, Wirth LJ, Droz J-P, et al. Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med. 2008;359:31–42. doi: 10.1056/NEJMoa075853. [DOI] [PubMed] [Google Scholar]
- 21.Eskens FA, Verweij J. The clinical toxicity profile of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) targeting angiogenesis inhibitors; a review. Eur J Cancer. 2006;42:3127–3139. doi: 10.1016/j.ejca.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Desai J, Yassa L, Marqusee E, et al. Hypothyroidism after sunitinib treatment for patients with gastrointestinal stromal tumors. Ann Intern Med. 2006;145:660–664. doi: 10.7326/0003-4819-145-9-200611070-00008. [DOI] [PubMed] [Google Scholar]
- 23.Rini BI, Tamaskar I, Shaheen P, et al. Hypothyroidism in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2007;99:81–83. doi: 10.1093/jnci/djk008. [DOI] [PubMed] [Google Scholar]
- 24.de Groot JW, Zonnenberg BA, Plukker JT, et al. Imatinib induces hypothyroidism in patients receiving levothyroxine. Clin Pharmacol Ther. 2005;78:433–438. doi: 10.1016/j.clpt.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Wong E, Rosen LS, Mulay M, et al. Sunitinib induces hypothyroidism in advanced cancer patients and may inhibit thyroid peroxidase activity. Thyroid. 2007;17:351–355. doi: 10.1089/thy.2006.0308. [DOI] [PubMed] [Google Scholar]