Abstract
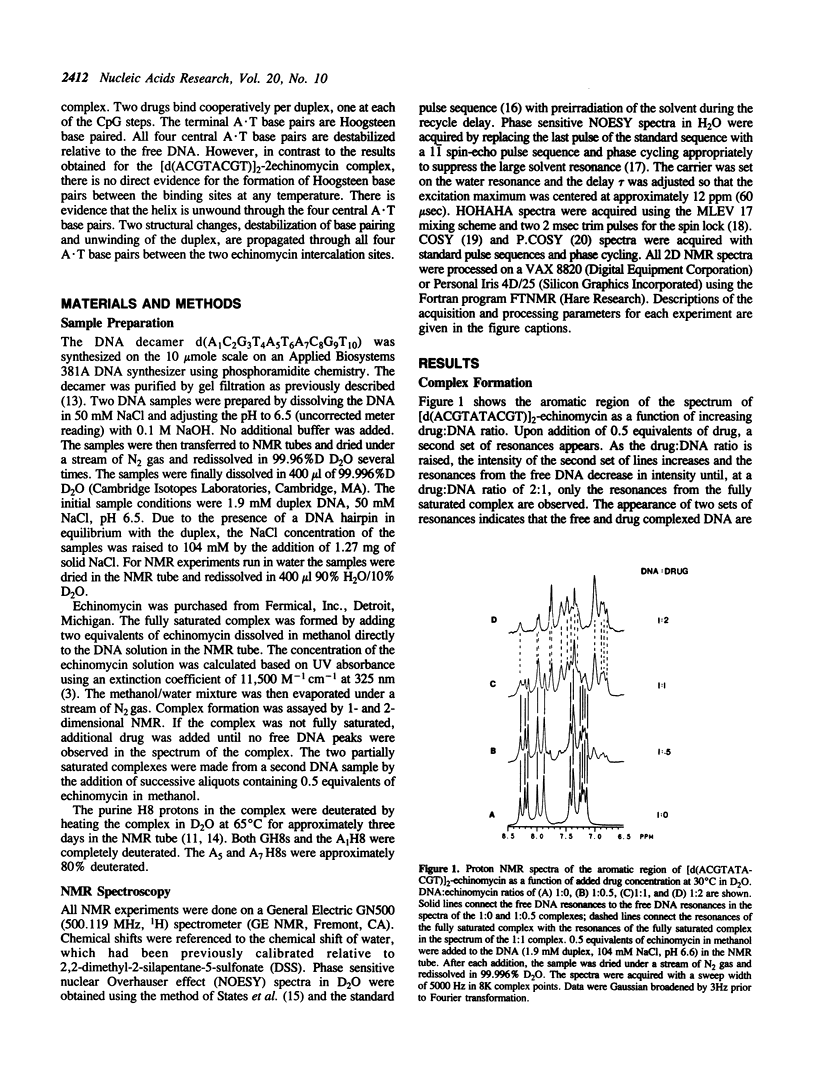
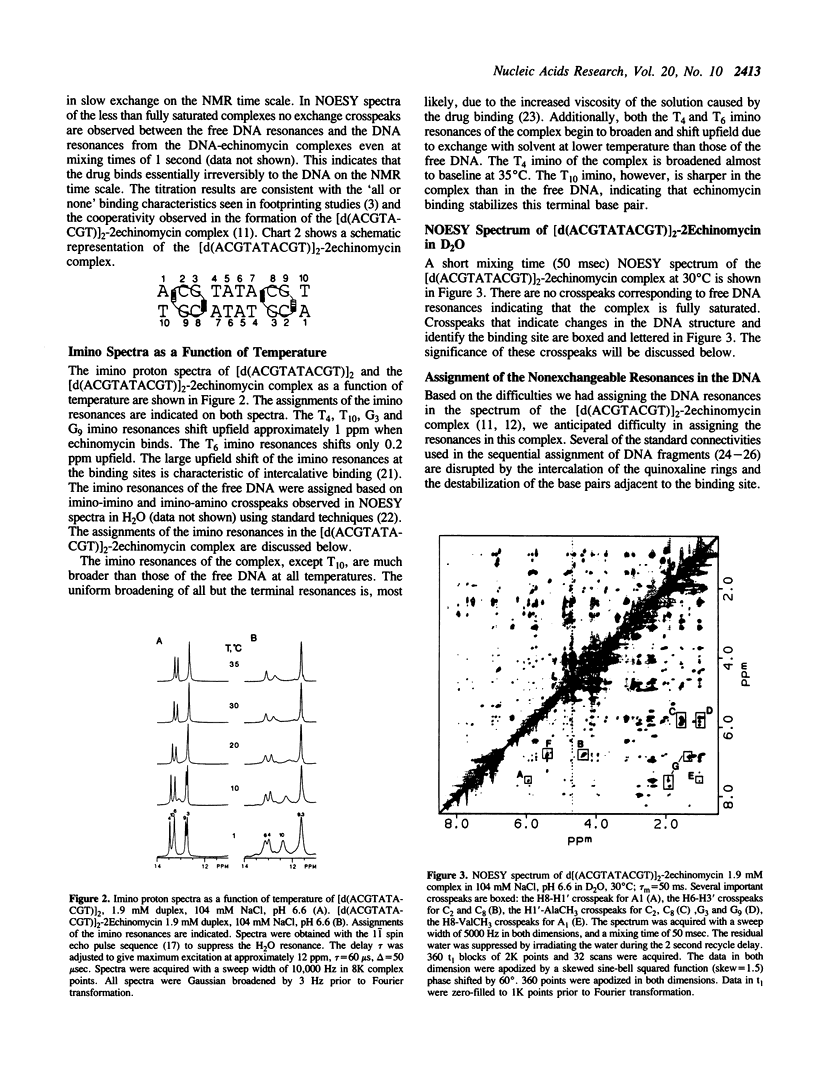
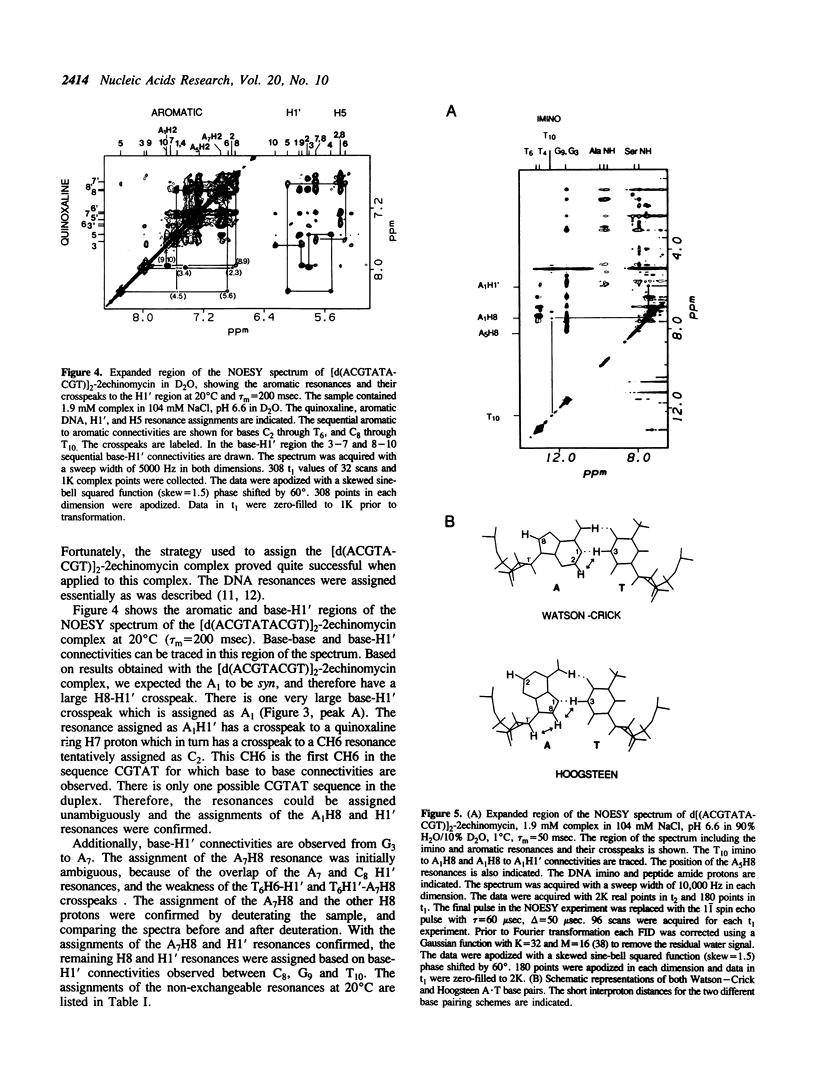
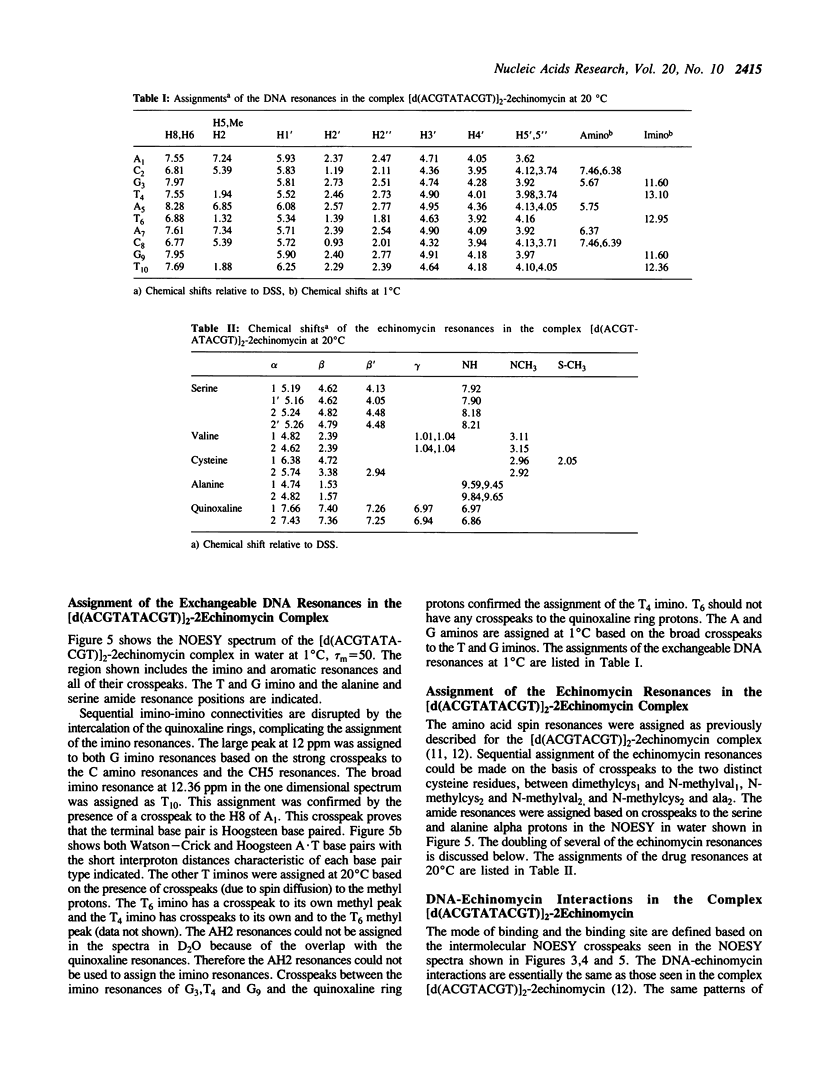
The interactions of echinomycin and the DNA decamer [d(ACGTATACGT)]2 were studied by proton NMR. Echinomycin binds cooperatively as a bisintercalator at the CpG steps. The terminal A.T base pairs are Hoogsteen base paired, but none of the four central A.T base pairs are Hoogsteen base paired. However, binding of the drug induces unwinding of the DNA which is propagated to the central ApT step. All four central A.T base pairs are destabilized relative to those in the free DNA. Furthermore, based on these and other results from our laboratory, we conclude that the formation of stable Hoogsteen base pairs may not be the relevant structural change in vivo. The structural changes propagated between adjacent ACGT binding sites are the unwinding of the duplex and destabilization of the base pairing between binding sites.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addess K. J., Gilbert D. E., Olsen R. K., Feigon J. Proton NMR studies of [N-MeCys3,N-MeCys7]TANDEM binding to DNA oligonucleotides: sequence-specific binding at the TpA site. Biochemistry. 1992 Jan 21;31(2):339–350. doi: 10.1021/bi00117a005. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Travers A. A. DNA structural variations in the E. coli tyrT promoter. Cell. 1984 Jun;37(2):491–502. doi: 10.1016/0092-8674(84)90379-9. [DOI] [PubMed] [Google Scholar]
- Feigon J., Denny W. A., Leupin W., Kearns D. R. Interactions of antitumor drugs with natural DNA: 1H NMR study of binding mode and kinetics. J Med Chem. 1984 Apr;27(4):450–465. doi: 10.1021/jm00370a007. [DOI] [PubMed] [Google Scholar]
- Feigon J., Leupin W., Denny W. A., Kearns D. R. Two-dimensional proton nuclear magnetic resonance investigation of the synthetic deoxyribonucleic acid decamer d(ATATCGATAT)2. Biochemistry. 1983 Dec 6;22(25):5943–5951. doi: 10.1021/bi00294a038. [DOI] [PubMed] [Google Scholar]
- Fox K. R., Kentebe E. Echinomycin binding to the sequence CG(AT)nCG alters the structure of the central AT region. Nucleic Acids Res. 1990 Apr 25;18(8):1957–1963. doi: 10.1093/nar/18.8.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratini A. V., Kopka M. L., Drew H. R., Dickerson R. E. Reversible bending and helix geometry in a B-DNA dodecamer: CGCGAATTBrCGCG. J Biol Chem. 1982 Dec 25;257(24):14686–14707. [PubMed] [Google Scholar]
- Gao X. L., Patel D. J. Antitumour drug-DNA interactions: NMR studies of echinomycin and chromomycin complexes. Q Rev Biophys. 1989 May;22(2):93–138. doi: 10.1017/s0033583500003814. [DOI] [PubMed] [Google Scholar]
- Gao X. L., Patel D. J. NMR studies of echinomycin bisintercalation complexes with d(A1-C2-G3-T4) and d(T1-C2-G3-A4) duplexes in aqueous solution: sequence-dependent formation of Hoogsteen A1.T4 and Watson--Crick T1.A4 base pairs flanking the bisintercalation site. Biochemistry. 1988 Mar 8;27(5):1744–1751. doi: 10.1021/bi00405a054. [DOI] [PubMed] [Google Scholar]
- Gilbert D. E., Feigon J. The DNA sequence at echinomycin binding sites determines the structural changes induced by drug binding: NMR studies of echinomycin binding to [d(ACGTACGT)]2 and [d(TCGATCGA)]2. Biochemistry. 1991 Mar 5;30(9):2483–2494. doi: 10.1021/bi00223a027. [DOI] [PubMed] [Google Scholar]
- Gilbert D. E., van der Marel G. A., van Boom J. H., Feigon J. Unstable Hoogsteen base pairs adjacent to echinomycin binding sites within a DNA duplex. Proc Natl Acad Sci U S A. 1989 May;86(9):3006–3010. doi: 10.1073/pnas.86.9.3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare D. R., Wemmer D. E., Chou S. H., Drobny G., Reid B. R. Assignment of the non-exchangeable proton resonances of d(C-G-C-G-A-A-T-T-C-G-C-G) using two-dimensional nuclear magnetic resonance methods. J Mol Biol. 1983 Dec 15;171(3):319–336. doi: 10.1016/0022-2836(83)90096-7. [DOI] [PubMed] [Google Scholar]
- Kintanar A., Klevit R. E., Reid B. R. Two-dimensional NMR investigation of a bent DNA fragment: assignment of the proton resonances and preliminary structure analysis. Nucleic Acids Res. 1987 Jul 24;15(14):5845–5862. doi: 10.1093/nar/15.14.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn J. G., Von Hoff D. D., Hersh M., Melink T., Clark G. M., Weiss G. R., Coltman C. A. Phase I trial of echinomycin (NSC 526417), a bifunctional intercalating agent, administered by 24-hour continuous infusion. Eur J Cancer Clin Oncol. 1989 May;25(5):797–803. doi: 10.1016/0277-5379(89)90123-5. [DOI] [PubMed] [Google Scholar]
- Kumar A., Ernst R. R., Wüthrich K. A two-dimensional nuclear Overhauser enhancement (2D NOE) experiment for the elucidation of complete proton-proton cross-relaxation networks in biological macromolecules. Biochem Biophys Res Commun. 1980 Jul 16;95(1):1–6. doi: 10.1016/0006-291x(80)90695-6. [DOI] [PubMed] [Google Scholar]
- Leroy J. L., Gao X. L., Misra V., Guéron M., Patel D. J. Proton exchange in DNA-luzopeptin and DNA-echinomycin bisintercalation complexes: rates and processes of base-pair opening. Biochemistry. 1992 Feb 11;31(5):1407–1415. doi: 10.1021/bi00120a017. [DOI] [PubMed] [Google Scholar]
- Low C. M., Drew H. R., Waring M. J. Sequence-specific binding of echinomycin to DNA: evidence for conformational changes affecting flanking sequences. Nucleic Acids Res. 1984 Jun 25;12(12):4865–4879. doi: 10.1093/nar/12.12.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean M. J., Seela F., Waring M. J. Echinomycin-induced hypersensitivity to osmium tetroxide of DNA fragments incapable of forming Hoogsteen base pairs. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9687–9691. doi: 10.1073/pnas.86.24.9687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendel D., Dervan P. B. Hoogsteen base pairs proximal and distal to echinomycin binding sites on DNA. Proc Natl Acad Sci U S A. 1987 Feb;84(4):910–914. doi: 10.1073/pnas.84.4.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle M. S., Wakelin L. P. Conformation and dynamics of the deoxyribose rings of a (nogalamycin)2-d (5'-GCATGC)2 complex studied in solution by 1H-n.m.r. spectroscopy. Biochem J. 1990 Jul 15;269(2):341–346. doi: 10.1042/bj2690341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle M. S., Wickham G. Hoogsteen versus Watson-Crick A-T basepairing in DNA complexes of a new group of 'quinomycin-like' antibiotics. FEBS Lett. 1990 Oct 15;272(1-2):171–174. doi: 10.1016/0014-5793(90)80476-y. [DOI] [PubMed] [Google Scholar]
- Taylor E. R., Olson W. K. Theoretical studies of nucleic acid interactions. I. Estimates of conformational mobility in intercalated chains. Biopolymers. 1983 Dec;22(12):2667–2702. doi: 10.1002/bip.360221213. [DOI] [PubMed] [Google Scholar]
- Ughetto G., Wang A. H., Quigley G. J., van der Marel G. A., van Boom J. H., Rich A. A comparison of the structure of echinomycin and triostin A complexed to a DNA fragment. Nucleic Acids Res. 1985 Apr 11;13(7):2305–2323. doi: 10.1093/nar/13.7.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke M. M., Dervan P. B. Echinomycin binding sites on DNA. Science. 1984 Sep 14;225(4667):1122–1127. doi: 10.1126/science.6089341. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Ughetto G., Quigley G. J., Hakoshima T., van der Marel G. A., van Boom J. H., Rich A. The molecular structure of a DNA-triostin A complex. Science. 1984 Sep 14;225(4667):1115–1121. doi: 10.1126/science.6474168. [DOI] [PubMed] [Google Scholar]
- Waring M. J., Wakelin L. P. Echinomycin: a bifunctional intercalating antibiotic. Nature. 1974 Dec 20;252(5485):653–657. doi: 10.1038/252653a0. [DOI] [PubMed] [Google Scholar]



