Abstract
Itch is thought to be signaled by pruritogen-responsive neurons in the superficial spinal dorsal horn. Many neurons here express the substance P NK-1 receptor. We investigated whether neurotoxic destruction of spinal NK-1-expressing neurons affected itch-related scratching behavior. Rats received intracisternal substance P conjugated to saporin (SP-SAP), or saporin (SAP) only (controls), and were subsequently tested for scratching behavior elicited by intradermal 5-hydroxytryptamine. SAP controls exhibited dose-related hindlimb scratching, which was significantly attenuated in SP-SAP-treated rats. There was a virtual absence of NK-1 immunoreactive neurons in superficial laminae of the upper cervical and medullary dorsal horn in SP-SAP-treated rats. These results indicate that superficial dorsal horn neurons expressing NK-1 receptors play a key role in spinal itch transmission.
Keywords: itch, scratching, serotonin, substance P-saporin, superficial dorsal horn
Introduction
Recent evidence supports a role for gastrin-releasing peptide (GRP), a mammalian homolog of the neuropeptide bombesin, in spinal itch transmission [1,2]. Neurotoxic destruction of superficial dorsal horn neurons that express the GRP receptor (GRPR) markedly diminished scratching behavior elicited by a variety of pruritogens [1], and mutant mice lacking GRPR exhibited reduced scratching [2]. In addition to GRP, substance P is a candidate neuro-peptide potentially involved in spinal itch transmission. The superficial dorsal horn is richly innervated by substance P-containing primary afferent terminals, and many neurons in superficial laminae of the spinal and trigeminal dorsal horn express the substance P NK-1 receptor [3]. Neuro-toxic destruction of superficial NK-1-expressing spinal neurons, using substance P conjugated to saporin (SP-SAP), reduced hyperalgesia and nocifensive behavioral responses to intense noxious stimuli [4], supporting a role for substance P in pain [5]. We postulate that substance P may also be involved in signaling itch. Although we previously reported that mutant mice lacking substance P exhibited normal scratching behavior in response to intradermal injection of the pruritogen 5-hydroxytryptamine (5-HT) [6], developmental compensation may have helped to maintain normal itch transmission in these animals. For this reason, we wished to reassess a potential role for NK-1-expressing spinal neurons in itch.
Itch is associated with scratching, which is used as a behavioral measure of itch in rodents. In rats, 5-HT is the only mediator that reliably elicits scratching in a manner that is reduced by μ-opioid antagonists, supporting the notion that 5-HT is more pruritic than noxious in rodents [7,8]. 5-HT activates neurons in the superficial dorsal horn [7,8], some of which are thought to transmit itch-related signals rostrally through the spinothalamic tract [9,10]. In this study, we investigated if neurotoxic destruction of NK-1-expressing neurons in the upper cervical and medullary dorsal horn of adult rats affected 5-HT-evoked scratching behavior. This region includes C2–C5 dermatomes on the rostral back [11] where 5-HT was injected intradermally.
Methods
Adult male Sprague–Dawley rats were used under a protocol approved by the UC Davis Institutional Animal Care and Use Committee. Rats were housed singly in a vivarium maintained at 21±1°C on a 12: 12-h light cycle with ad libitum access to food and water.
Intracisternal microinjections of SAP or SP-SAP were made as described previously [12]. In brief, rats were anesthetized with 5% halothane and the head was positioned in a stereotaxic frame with atraumatic ear bars to ventro-flex the neck. The atlanto-occipital membrane was exposed by midline incision. SP-SAP (Advanced Targeting Systems, San Diego, California, USA; 2.27 μM, dissolved in sterile isotonic saline, n=11 rats) or saporin (SAP) only (Advanced Targeting Systems, 2.27 μM, dissolved in sterile isotonic saline, n=7), was microinjected in a volume of 20 μl through a 30.5-g hypodermic needle inserted into the cisterna magna. The needle was left in place for 20 min postinjection and then withdrawn. Cyanoacrylate was topically applied to seal the needle puncture. The incision was sutured closed and antibiotic administered. All rats recovered without incident.
After a minimum 2-week recovery period, rats were tested for scratching behavior as described previously [7]. Fur on the nape of the neck was shaved, and rats were habituated to the recording arena. For testing, the rat received a 10 μl intradermal injection in the nape of the neck of either vehicle (sterile 0.9% saline) or 5-HT at a concentration of either 0.5% (23.5mM), 1% (47 mM), or 2% (94mM) all dissolved in 0.9% sterile saline. The rat was then immediately placed in the recording chamber and videotaped from above for 46 min, with investigators leaving the room during taping. Videotapes were subsequently reviewed by the investigators blinded to treatment. We counted the number and duration of individual scratch bouts, defined as a series of one or more hindpaw scratching movements directed to the injection site and ending when the rat either licked its hind paw or placed it on the floor. For graphical purposes, the number and cumulative duration of scratch bouts was plotted at 2-min intervals over the 46-min recording period. Comparisons of numbers and cumulative duration of scratch bouts between SAP and SP-SAP treatment groups were made using analysis of variance, followed by post hoc Least Significant Difference tests, with significance occurring at a P value of less than 0.05.
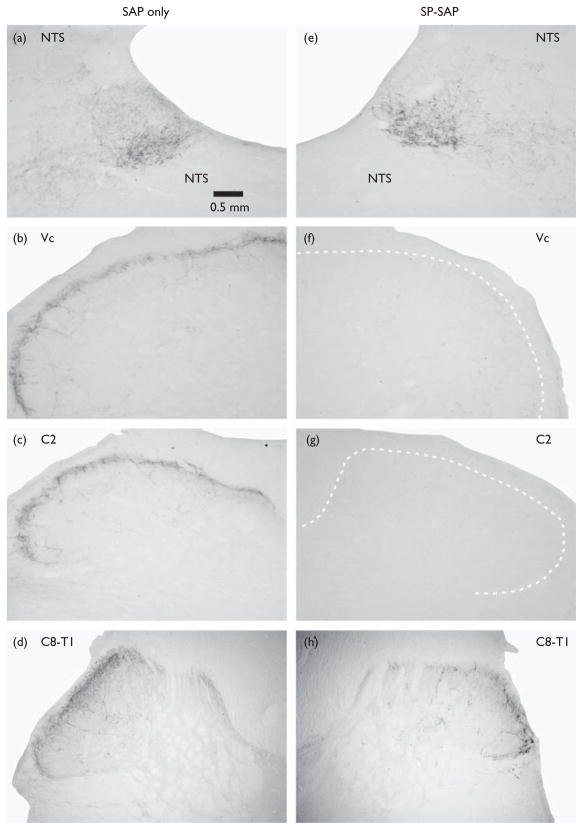
After completing behavioral testing, rats were deeply anesthetized with sodium pentobarbital (65 mg/kg intraperitonealy) and perfused transcardially with buffered saline followed by 4% paraformaldehyde. The procedure for NK-1 receptor staining was the same as described previously [12]. Fifty-micrometer sections of cervical cord and brainstem were incubated in a primary antibody directed against the NK-1 receptor (Bioscience Research Reagents, Temecula, California, USA; 1: 10 000), followed by incubation in a secondary (goat anti-rabbit) antibody, avidin–biotin complex reaction (Vectastain, Vector Laboratories, Burlingame, California, USA), and diaminobenzidine reaction to stain immunoreactive neurons. Sections were examined under the light microscope (Nikon E400, Technical Instruments, San Francisco, California, USA) and imaged on a computer using a CCD camera (Micro- Publisher 5.0, QImaging, Surry British Columbia, Canada). SP-SAP treatment resulted in a virtually complete elimination of immunostaining in the superficial layers of the medullary and cervical dorsal horns in all 11 animals (Fig. 1), whereas SAP resulted in a normal, dense pattern of immunoreactivity in these areas; hence, no quantification of the degree of staining was undertaken.
Fig. 1.
NK-1 receptor immunoreactivity in the superficial medullary and cervical spinal dorsal horn. Left column (a–d) shows sections from a rat pretreated with saporin (SAP), at the levels of (a) nucleus of solitary tract (NTS), (b) trigeminal subnucleus caudalis (Vc) in caudal medulla, (c) second cervical (C2) segment, and (d) lower cervical-upper thoracic (C8-T1) segment. Note extensive NK-1 immunoreactivity in superficial laminae of the medullary and spinal dorsal horns at all levels, as well as in NTS. This pattern of NK-1 staining was very similar in all 7 animals receiving intracisternal SAP. Calibration bar applies to all photomicrographs. Right column (e–h) similarly shows NK-1 immunoreactivity in an animal pretreated with substance P conjugated to saporin (SP-SAP) at the levels of (e) NTS, (f) Vc, (g) C2, and (h) C8-T1. All 11 animals receiving intracisternal SP-SAP exhibited a virtual absence of NK-1 immunoreactivity in the superficial dorsal horn down to mid-cervical levels, with heavy NK-1 staining in NTS comparable to that observed in SAP-treated animals. Three animals exhibited some degree of NK-1 immunoreactivity at lower cervical and upper thoracic levels (h).
Results
Figure 1b–d shows examples of the normal pattern of NK- 1 immunostaining in the superficial laminae of the medullary, upper cervical (C2) and lower cervical-upper thoracic (C8-T1) dorsal horns after SAP treatment. There was also dense staining in the nucleus of solitary tract (Fig. 1a). After SP-SAP treatment, there was a complete absence of NK-1 staining in upper cervical and medullary dorsal horns (Fig. 1f and g). All 11 of the SP-SAP-treated animals showed a virtual absence of NK-1 staining in these areas. The effective spread of intracisternally administered SP-SAP was, however, limited. NK-1 staining further rostrally in nucleus of solitary tract was comparable to that observed in SAP-treated animals (Fig. 1e). Further caudally, some degree of NK-1 staining started to appear at upper thoracic levels in three animals treated with SP-SAP (Fig. 1h).
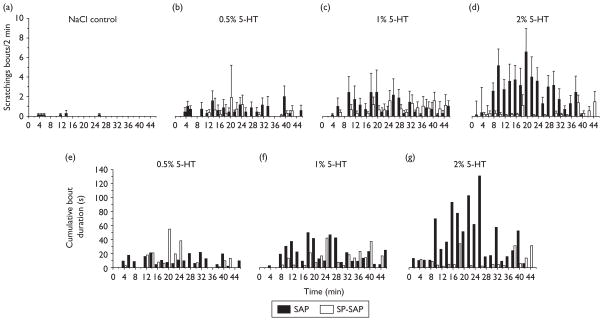
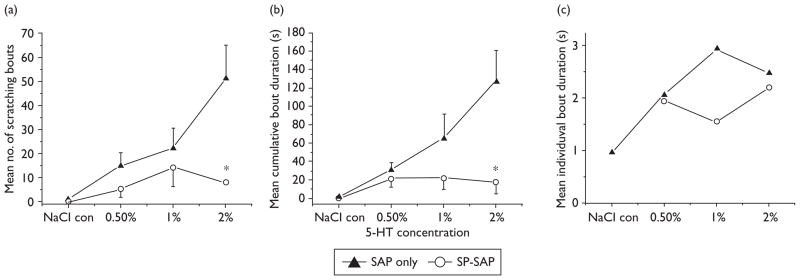
5-HT-evoked scratching behavior was significantly attenuated in SP-SAP-treated rats. Figure 2a–d plots mean numbers of scratch bouts/2 min for SP-SAP (open bars) and SAP (filled bars)-treated rats. There was a dose-dependent increase in scratch bouts in the SAP group, whereas the SP-SAP group did not exhibit a dose-related increase in scratch bouts. Figure 3a plots total mean counts of scratch bouts versus 5-HT concentration, showing a significant reduction in scratching elicited by the highest 5-HT concentrations. Figure 2e–g plots cumulative durations of scratch bouts per 2 min-interval for SP-SAP and SAP groups, and Fig. 3b shows that the mean cumulative duration of all scratch bouts over the 46-min recording period was significantly reduced in the SP-SAP group. Scratch bout durations ranged from lesser than 1 to greater than 5 s and averaged approximately 1– 3 s with no significant difference between treatment groups or 5-HT concentrations (Fig. 3c).
Fig. 2.
Reduced 5-hydroxytryptamine (5-HT)-evoked scratching in rats receiving substance P conjugated to saporin (SP-SAP). (a–d) Bar graphs show, from left to right, the mean number of scratching bouts/2 min intervals evoked by intradermal injection of NaCl (a, vehicle control) and 5-HT at concentrations of (b) 0.5% (23.5 mM), (c) 1% (47 mM), and (d) 2% (94 mM). Filled bars: SAP; open bars, SP-SAP. Error bars: SEM. (e–g) Bar graphs plot total cumulative duration of 5-HT-evoked scratching bouts (recorded at 2-min intervals).
Fig. 3.
Summary of scratching data. (a) Graph plots mean number of scratching bouts versus 5-hydroxytryptamine (5-HT) concentration to show significant reduction in substance P conjugated to saporin (SP-SAP)-treated rats. Error bars: SEM. *Overall significant between-group difference (analysis of variance, P<0.01) with the 2% treatment group significantly different from vehicle and 0.5% (least significant difference; P<0.05). (b) Graph of mean cumulative duration of scratching bouts. *Significant (analysis of variance, P<0.01) between-group difference. (c) The mean individual bout duration did not significantly vary between groups across the range of 5-HT concentrations tested.
Discussion
The present data support a role for NK-1 receptor-expressing superficial dorsal horn neurons in the spinal transmission of itch signals. The results are discussed in terms of possible roles for substance P in addition to other candidates such as GRP as central pruritic neuropeptide transmitters.
As noted in the Introduction, recent studies have implicated GRP as a spinal neuropeptide transmitter in itch. Knockout mice lacking GRPR [2], and mice pre-treated with bombesin-SAP to ablate superficial GRPR-expressing dorsal horn neurons [1], exhibited reduced pruritogen-evoked scratching behavior with no change in nocifensive behavioral responses. In the latter study, bombesin- SAP treatment was reported to not significantly reduce the level of NK-1 receptor staining in the superficial spinal dorsal horn. If it is, however, assumed that itch-signaling neurons project in the spinothalamic tract, and that the vast majority of superficial spinothalamic tract neurons express NK-1 [13], then one would expect that neurotoxic ablation of itch-signaling spinothalamic neurons would reduce dorsal horn NK-1 staining. Possibly, such neurons represent a small subpopulation of spinothalamic tract neurons, so that their loss might not produce a detectable reduction in overall NK-1 staining in the dorsal horn. Our present results support a role for superficial dorsal horn NK-1 receptor-expressing neurons in 5-HT-evoked itch in rats. It is conceivable that substance P may be importantly involved in signaling itch in this species. It is also conceivable that some superficial neurons coexpress NK-1 and GRPR, with both substance P and GRP being candidate itch-signaling neuropeptides. Such neurons would be destroyed by internalizing either substance P or bombesin conjugated to saporin, resulting in a significant attenuation of pruritogen-evoked scratching behavior. Double-labeling of superficial dorsal horn neurons for both NK-1 and GRPR would be consistent with this possibility.
The present data suggest that substance P may play a role as a spinal neuropeptide transmitter for itch. Although many previous preclinical animal studies showed that antagonizing the NK-1 receptor attenuated nociceptive transmission, this approach has not translated effectively to the treatment of clinical pain [5]. The present results suggest that NK-1 receptor antagonism may prove useful in treating certain types of itch that are resistant to antihistamines. Earlier studies have reported that intradermal or subcutaneous injection of NK-1 antagonists (spantide; L-688 169; L-733 060) attenuated scratching behavior elicited by intradermal substance P in normal mice [14] and allergen-evoked scratching in sensitized mice [15], supporting a role for peripheral NK-1 receptors in certain types of itch. Systemically administered NK-1 antagonists significantly attenuated scratching behavior elicited by intradermal substance P [16] or trypsin [17]. Finally, systemic but not local administration of an NK-1 antagonist (SR140333) attenuated scratching elicited by intradermal injection of spider venom [18], suggesting a central site of action to reduce itch signaling. Additional studies are needed to investigate the potential anti-pruritic value of systemic, and particularly spinal, administration of NK-1 receptor antagonists.
Recent studies suggest that separate primary afferent fibers and central pathways may signal histaminergic and nonhistaminergic types of itch. Intradermal insertion of spicules from the bean pods of the tropical legume cowhage elicits itch sensation that is not attenuated by antihistamines [19]. The active chemical in cowhage, mucunain, contains proteases that act at protease-activated receptor (PAR) subtypes PAR-2 and PAR-4 [20]. A sub-population of mechanically insensitive C-fiber afferents responds to intradermally applied histamine in a manner paralleling concomitant itch sensation [21]; such fibers are generally unresponsive to cowhage [22]. Conversely, polymodal C-fiber nociceptors respond to cowhage, with some also giving smaller responses to histamine [22,23]. Subpopulations of primate spinothalamic tract neurons responded to either intradermal histamine or cowhage, but not both, with all neurons additionally responding to capsaicin [10]. These results suggest the existence of segregated neural pathways for histaminergic and nonhistaminergic itch, and it will be interesting to determine the potential roles of GRPR-expressing and NK-1-expressing spinal neurons in these pathways. In mice, the large majority of superficial dorsal horn neurons identified using a pruritogen search strategy responded to both histamine and a PAR-2 agonist, as well as to the algogens capsaicin and allyl isothiocyanate [24]. Moreover, a recent human psychophysical study reported that focal skin application of histamine, cowhage or capsaicin each elicited simultaneous itch and nociceptive (stinging and/or burning) sensations [25]. These observations suggest that pruritogen-responsive primary afferents and substance P-containing polymodal nociceptors may converge onto spinal neurons involved in signaling itch, a scenario that is consistent with the present observation that loss of NK-1-expressing spinal neurons significantly attenuates itch-related scratching behavior.
In conclusion, the present results indicate that superficial dorsal horn neurons that express the substance P NK-1 receptor are important for itch-related scratching behavior in the rat.
Acknowledgments
The authors thank Dr Tasuku Akiyama for his helpful comments. Grant support: National Institute of Health Grants DE-013685 and AR-057194.
References
- 1.Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science. 2009;325:1531–1534. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- 3.Brown JL, Liu H, Maggio JE, Vigna SR, Mantyh PW, Basbaum AI. Morphological characterization of substance P receptor-immunoreactive neurons in the rat spinal cord and trigeminal nucleus caudalis. J Comp Neurol. 1995;356:327–344. doi: 10.1002/cne.903560302. [DOI] [PubMed] [Google Scholar]
- 4.Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, et al. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science. 1997;278:275–279. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- 5.Hill R. NK1 (substance P) receptor antagonists – why are they not analgesic in humans? Trends Pharmacol Sci. 2000;21:244–246. doi: 10.1016/s0165-6147(00)01502-9. [DOI] [PubMed] [Google Scholar]
- 6.Cuellar JM, Jinks SL, Simons CT, Carstens E. Deletion of the preprotachykinin A gene in mice does not reduce scratching behavior elicited by intradermal serotonin. Neurosci Lett. 2003;339:72–76. doi: 10.1016/s0304-3940(02)01458-1. [DOI] [PubMed] [Google Scholar]
- 7.Jinks SL, Carstens E. Responses of superficial dorsal horn neurons to intradermal serotonin and other irritants: comparison with scratching behavior. J Neurophysiol. 2002;87:1280–1289. doi: 10.1152/jn.00431.2001. [DOI] [PubMed] [Google Scholar]
- 8.Nojima H, Simons CT, Cuellar JM, Carstens MI, Moore JA, Carstens E. Opioid modulation of scratching and spinal c-fos expression evoked by intradermal serotonin. J Neurosci. 2003;23:10784–10790. doi: 10.1523/JNEUROSCI.23-34-10784.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrew D, Craig AD. Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nat Neurosci. 2001;4:72–77. doi: 10.1038/82924. [DOI] [PubMed] [Google Scholar]
- 10.Davidson S, Zhang X, Yoon CH, Khasabov SG, Simone DA, Giesler GJ., Jr The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27:10007–10014. doi: 10.1523/JNEUROSCI.2862-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi Y, Nakajima Y. Dermatomes in the rat limbs as determined by antidromic stimulation of sensory C-fibers in spinal nerves. Pain. 1996;67:197–202. doi: 10.1016/0304-3959(96)03116-8. [DOI] [PubMed] [Google Scholar]
- 12.Simons CT, Gogineni AG, Iodi Carstens M, Carstens E. Reduced aversion to oral capsaicin following neurotoxic destruction of superficial medullary neurons expressing NK-1 receptors. Brain Res. 2002;945:139–143. doi: 10.1016/s0006-8993(02)02913-x. [DOI] [PubMed] [Google Scholar]
- 13.Al-Khater KM, Kerr R, Todd AJ. A quantitative study of spinothalamic neurons in laminae I, III, and IV in lumbar and cervical segments of the rat spinal cord. J Comp Neurol. 2008;511:1–18. doi: 10.1002/cne.21811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andoh T, Nagasawa T, Satoh M, Kuraishi Y. Substance P induction of itch-associated response mediated by cutaneous NK1 tachykinin receptors in mice. J Pharmacol Exp Ther. 1998;286:1140–1145. [PubMed] [Google Scholar]
- 15.Ueda Y, Inoue T, Rahman MA, Yatsuzuka R, Jiang S, Kamei C. A new chronic itch model accompanied by skin lesions in hairless mice. Int Immunopharmacol. 2006;6:1609–1615. doi: 10.1016/j.intimp.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Ohmura T, Hayashi T, Satoh Y, Konomi A, Jung B, Satoh H. Involvement of substance P in scratching behaviour in an atopic dermatitis model. Eur J Pharmacol. 2004;491:191–194. doi: 10.1016/j.ejphar.2004.03.047. [DOI] [PubMed] [Google Scholar]
- 17.Costa R, Marotta DM, Manjavachi MN, Fernandes ES, Lima-Garcia JF, Paszcuk AF, et al. Evidence for the role of neurogenic inflammation components in trypsin-elicited scratching behaviour in mice. Br J Pharmacol. 2008;154:1094–1103. doi: 10.1038/bjp.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costa SK, Starr A, Hyslop S, Gilmore D, Brain SD. How important are NK1 receptors for influencing microvascular inflammation and itch in the skin? Studies using Phoneutria nigriventer venom. Vascul Pharmacol. 2006;45:209–214. doi: 10.1016/j.vph.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 19.Johanek LM, Meyer RA, Hartke T, Hobelmann JG, Maine DN, LaMotte RH, Ringkamp M. Psychophysical and physiological evidence for parallel afferent pathways mediating the sensation of itch. J Neurosci. 2007;27:7490–2497. doi: 10.1523/JNEUROSCI.1249-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reddy VB, Iuga AO, Shimada SG, LaMotte RH, Lerner EA. Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J Neurosci. 2008;28:4331–4335. doi: 10.1523/JNEUROSCI.0716-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjörk HE. Specific C-receptors for itch in human skin. J Neurosci. 1997;17:8003–8008. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Namer B, Carr R, Johanek LM, Schmelz M, Handwerker HO, Ringkamp M. Separate peripheral pathways for pruritus in man. J Neurophysiol. 2008;100:2062–2069. doi: 10.1152/jn.90482.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johanek LM, Meyer RA, Friedman RM, Greenquist KW, Shim B, Borzan J, et al. A role for polymodal C-fiber afferents in nonhistaminergic itch. J Neurosci. 2008;28:7659–7669. doi: 10.1523/JNEUROSCI.1760-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akiyama T, Carstens MI, Carstens E. Excitation of mouse superficial dorsal horn neurons by histamine and/or PAR-2 agonist: potential role in itch. J Neurophysiol. 2009;102:2176–2183. doi: 10.1152/jn.00463.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sikand P, Shimada SG, Green BG, LaMotte RH. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain. 2009;144:66–75. doi: 10.1016/j.pain.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]