Abstract
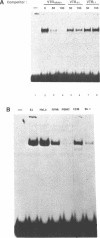
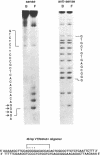
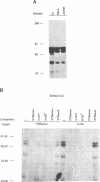
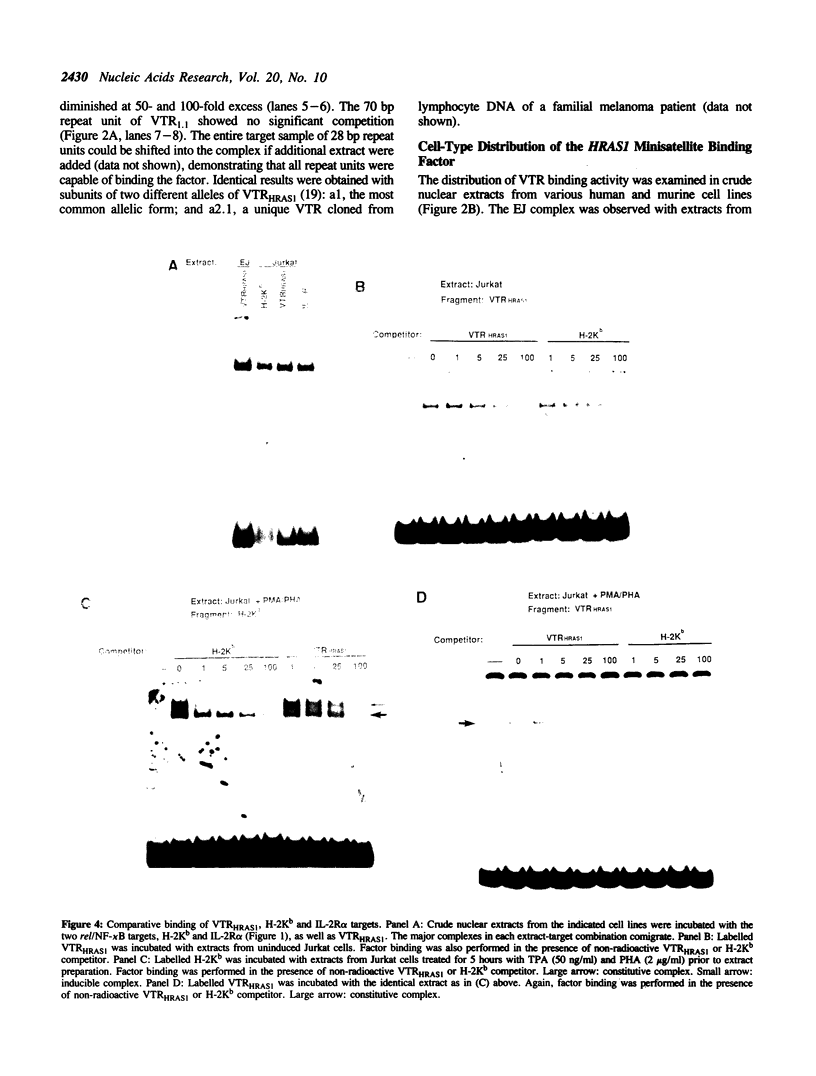
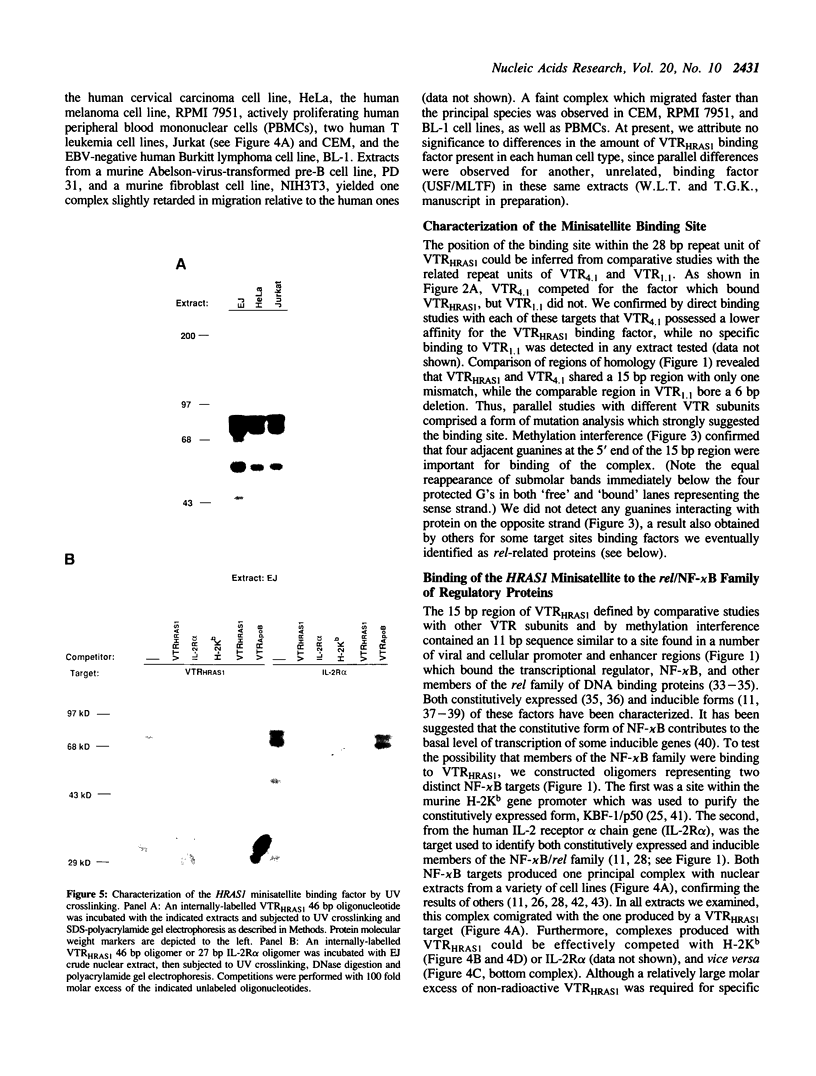
The 28 base pair repeat unit of a minisatellite 1000 bp downstream from the human HRAS1 gene (VTRHRAS1) bound four proteins (p45, p50, p72 and p85) in nuclear extracts from a variety of human cell lines which were indistinguishable from several members of the rel/NF-kappa B family of transcriptional regulatory factors. VTRHRAS1 bound the constitutively expressed, but not the inducible, forms of these proteins. Analysis of partially purified binding factors from different cell lines demonstrated qualitative differences in the p50 subunit; phosphocellulose fractionation also revealed considerable heterogeneity in the p72 and p85 subunits. These results suggest the possibility that the HRAS1 minisatellite, in serving as a tandem array of rel/NF-kappa B binding sites, may function in the transcriptional regulation of HRAS1 and nearby genes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin A. S., Jr, LeClair K. P., Singh H., Sharp P. A. A large protein containing zinc finger domains binds to related sequence elements in the enhancers of the class I major histocompatibility complex and kappa immunoglobulin genes. Mol Cell Biol. 1990 Apr;10(4):1406–1414. doi: 10.1128/mcb.10.4.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin A. S., Jr, Sharp P. A. Binding of a nuclear factor to a regulatory sequence in the promoter of the mouse H-2Kb class I major histocompatibility gene. Mol Cell Biol. 1987 Jan;7(1):305–313. doi: 10.1128/mcb.7.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin A. S., Jr, Sharp P. A. Two transcription factors, NF-kappa B and H2TF1, interact with a single regulatory sequence in the class I major histocompatibility complex promoter. Proc Natl Acad Sci U S A. 1988 Feb;85(3):723–727. doi: 10.1073/pnas.85.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhnlein E., Lowenthal J. W., Siekevitz M., Ballard D. W., Franza B. R., Greene W. C. The same inducible nuclear proteins regulates mitogen activation of both the interleukin-2 receptor-alpha gene and type 1 HIV. Cell. 1988 Jun 3;53(5):827–836. doi: 10.1016/0092-8674(88)90099-2. [DOI] [PubMed] [Google Scholar]
- Capon D. J., Seeburg P. H., McGrath J. P., Hayflick J. S., Edman U., Levinson A. D., Goeddel D. V. Activation of Ki-ras2 gene in human colon and lung carcinomas by two different point mutations. Nature. 1983 Aug 11;304(5926):507–513. doi: 10.1038/304507a0. [DOI] [PubMed] [Google Scholar]
- Cohen J. B., Walter M. V., Levinson A. D. A repetitive sequence element 3' of the human c-Ha-ras1 gene has enhancer activity. J Cell Physiol Suppl. 1987;Suppl 5:75–81. doi: 10.1002/jcp.1041330415. [DOI] [PubMed] [Google Scholar]
- Colb M., Yang-Feng T., Francke U., Mermer B., Parkinson D. R., Krontiris T. G. A variable tandem repeat locus mapped to chromosome band 10q26 is amplified and rearranged in leukocyte DNAs of two cancer patients. Nucleic Acids Res. 1986 Oct 24;14(20):7929–7937. doi: 10.1093/nar/14.20.7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collick A., Jeffreys A. J. Detection of a novel minisatellite-specific DNA-binding protein. Nucleic Acids Res. 1990 Feb 11;18(3):625–629. doi: 10.1093/nar/18.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Gifford A. M., Riviere L. R., Tempst P., Nolan G. P., Baltimore D. Cloning of the p50 DNA binding subunit of NF-kappa B: homology to rel and dorsal. Cell. 1990 Sep 7;62(5):1019–1029. doi: 10.1016/0092-8674(90)90276-k. [DOI] [PubMed] [Google Scholar]
- Herr W., Clarke J. The SV40 enhancer is composed of multiple functional elements that can compensate for one another. Cell. 1986 May 9;45(3):461–470. doi: 10.1016/0092-8674(86)90332-6. [DOI] [PubMed] [Google Scholar]
- Hoyos B., Ballard D. W., Böhnlein E., Siekevitz M., Greene W. C. Kappa B-specific DNA binding proteins: role in the regulation of human interleukin-2 gene expression. Science. 1989 Apr 28;244(4903):457–460. doi: 10.1126/science.2497518. [DOI] [PubMed] [Google Scholar]
- Israël A., Kimura A., Kieran M., Yano O., Kanellopoulos J., Le Bail O., Kourilsky P. A common positive trans-acting factor binds to enhancer sequences in the promoters of mouse H-2 and beta 2-microglobulin genes. Proc Natl Acad Sci U S A. 1987 May;84(9):2653–2657. doi: 10.1073/pnas.84.9.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7;314(6006):67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Kanno M., Fromental C., Staub A., Ruffenach F., Davidson I., Chambon P. The SV40 TC-II(kappa B) and the related H-2Kb enhansons exhibit different cell type specific and inducible proto-enhancer activities, but the SV40 core sequence and the AP-2 binding site have no enhanson properties. EMBO J. 1989 Dec 20;8(13):4205–4214. doi: 10.1002/j.1460-2075.1989.tb08606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasperczyk A., DiMartino N. A., Krontiris T. G. Minisatellite allele diversification: the origin of rare alleles at the HRAS1 locus. Am J Hum Genet. 1990 Nov;47(5):854–859. [PMC free article] [PubMed] [Google Scholar]
- Kasperczyk A., Mermer B. A., Parkinson D. R., Lonergan J. A., Krontiris T. G. Allele-specific deletion in exon I of the HRAS1 gene. Am J Hum Genet. 1989 Nov;45(5):689–696. [PMC free article] [PubMed] [Google Scholar]
- Kawakami K., Scheidereit C., Roeder R. G. Identification and purification of a human immunoglobulin-enhancer-binding protein (NF-kappa B) that activates transcription from a human immunodeficiency virus type 1 promoter in vitro. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4700–4704. doi: 10.1073/pnas.85.13.4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieran M., Blank V., Logeat F., Vandekerckhove J., Lottspeich F., Le Bail O., Urban M. B., Kourilsky P., Baeuerle P. A., Israël A. The DNA binding subunit of NF-kappa B is identical to factor KBF1 and homologous to the rel oncogene product. Cell. 1990 Sep 7;62(5):1007–1018. doi: 10.1016/0092-8674(90)90275-j. [DOI] [PubMed] [Google Scholar]
- Kochel T., Mushinski J. F., Rice N. R. The v-rel and c-rel proteins exist in high molecular weight complexes in avian and murine cells. Oncogene. 1991 Apr;6(4):615–626. [PubMed] [Google Scholar]
- Krontiris T. G., DiMartino N. A., Colb M., Mitcheson H. D., Parkinson D. R. Human restriction fragment length polymorphisms and cancer risk assessment. J Cell Biochem. 1986;30(4):319–329. doi: 10.1002/jcb.240300405. [DOI] [PubMed] [Google Scholar]
- Krontiris T. G., DiMartino N. A., Colb M., Parkinson D. R. Unique allelic restriction fragments of the human Ha-ras locus in leukocyte and tumour DNAs of cancer patients. 1985 Jan 31-Feb 6Nature. 313(6001):369–374. doi: 10.1038/313369a0. [DOI] [PubMed] [Google Scholar]
- Krowczynska A. M., Rudders R. A., Krontiris T. G. The human minisatellite consensus at breakpoints of oncogene translocations. Nucleic Acids Res. 1990 Mar 11;18(5):1121–1127. doi: 10.1093/nar/18.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenthal J. W., Ballard D. W., Bogerd H., Böhnlein E., Greene W. C. Tumor necrosis factor-alpha activation of the IL-2 receptor-alpha gene involves the induction of kappa B-specific DNA binding proteins. J Immunol. 1989 May 1;142(9):3121–3128. [PubMed] [Google Scholar]
- Macchi M., Bornert J. M., Davidson I., Kanno M., Rosales R., Vigneron M., Xiao J. H., Fromental C., Chambon P. The SV40 TC-II(kappa B) enhanson binds ubiquitous and cell type specifically inducible nuclear proteins from lymphoid and non-lymphoid cell lines. EMBO J. 1989 Dec 20;8(13):4215–4227. doi: 10.1002/j.1460-2075.1989.tb08607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitor J. A., Walker W. H., Doerre S., Ballard D. W., Greene W. C. NF-kappa B: a family of inducible and differentially expressed enhancer-binding proteins in human T cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10028–10032. doi: 10.1073/pnas.87.24.10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Leppert M., O'Connell P., Wolff R., Holm T., Culver M., Martin C., Fujimoto E., Hoff M., Kumlin E. Variable number of tandem repeat (VNTR) markers for human gene mapping. Science. 1987 Mar 27;235(4796):1616–1622. doi: 10.1126/science.3029872. [DOI] [PubMed] [Google Scholar]
- Ondek B., Shepard A., Herr W. Discrete elements within the SV40 enhancer region display different cell-specific enhancer activities. EMBO J. 1987 Apr;6(4):1017–1025. doi: 10.1002/j.1460-2075.1987.tb04854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn L., Kunkel S., Nabel G. J. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattner A., Korner M., Rosen H., Baeuerle P. A., Citri Y. Nuclear factor kappa B activates proenkephalin transcription in T lymphocytes. Mol Cell Biol. 1991 Feb;11(2):1017–1022. doi: 10.1128/mcb.11.2.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins D. R., Milman G., Hayward S. D., Hayward G. S. Sequence-specific DNA binding of the Epstein-Barr virus nuclear antigen (EBNA-1) to clustered sites in the plasmid maintenance region. Cell. 1985 Oct;42(3):859–868. doi: 10.1016/0092-8674(85)90282-x. [DOI] [PubMed] [Google Scholar]
- Reisman D., Sugden B. trans activation of an Epstein-Barr viral transcriptional enhancer by the Epstein-Barr viral nuclear antigen 1. Mol Cell Biol. 1986 Nov;6(11):3838–3846. doi: 10.1128/mcb.6.11.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman D., Yates J., Sugden B. A putative origin of replication of plasmids derived from Epstein-Barr virus is composed of two cis-acting components. Mol Cell Biol. 1985 Aug;5(8):1822–1832. doi: 10.1128/mcb.5.8.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels M., Fire A., Sharp P. A. Separation and characterization of factors mediating accurate transcription by RNA polymerase II. J Biol Chem. 1982 Dec 10;257(23):14419–14427. [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell. 1985 Nov;43(1):165–175. doi: 10.1016/0092-8674(85)90021-2. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986 Dec 26;47(6):921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Silva A. J., Johnson J. P., White R. L. Characterization of a highly polymorphic region 5' to JH in the human immunoglobulin heavy chain. Nucleic Acids Res. 1987 May 11;15(9):3845–3857. doi: 10.1093/nar/15.9.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spandidos D. A., Holmes L. Transcriptional enhancer activity in the variable tandem repeat DNA sequence downstream of the human Ha-ras 1 gene. FEBS Lett. 1987 Jun 22;218(1):41–46. doi: 10.1016/0014-5793(87)81014-1. [DOI] [PubMed] [Google Scholar]
- Strauss F., Varshavsky A. A protein binds to a satellite DNA repeat at three specific sites that would be brought into mutual proximity by DNA folding in the nucleosome. Cell. 1984 Jul;37(3):889–901. doi: 10.1016/0092-8674(84)90424-0. [DOI] [PubMed] [Google Scholar]
- Urban M. B., Baeuerle P. A. The 65-kD subunit of NF-kappa B is a receptor for I kappa B and a modulator of DNA-binding specificity. Genes Dev. 1990 Nov;4(11):1975–1984. doi: 10.1101/gad.4.11.1975. [DOI] [PubMed] [Google Scholar]
- Urban M. B., Schreck R., Baeuerle P. A. NF-kappa B contacts DNA by a heterodimer of the p50 and p65 subunit. EMBO J. 1991 Jul;10(7):1817–1825. doi: 10.1002/j.1460-2075.1991.tb07707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J., Warren N., Reisman D., Sugden B. A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3806–3810. doi: 10.1073/pnas.81.12.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenke M., Grundström T., Matthes H., Wintzerith M., Schatz C., Wildeman A., Chambon P. Multiple sequence motifs are involved in SV40 enhancer function. EMBO J. 1986 Feb;5(2):387–397. doi: 10.1002/j.1460-2075.1986.tb04224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]