Abstract
The cerebellum is involved in the control of motor functions with Purkinje cells serving as the only output from the cerebellum. Purkinje cells are important targets for toxic substances and are vulnerable to prenatal insults. Intrauterine infection (IUI) has been shown to selectively target the developing cerebral white matter through lesioning, necrosis and inflammatory cytokine activation. Developmental and cognitive delays have been associated with animal models of IUI. The aim of this study was to determine if IUI leads to damage to Purkinje cells in the developing cerebellum and if any damage is associated with decreases in calbindin and motor behaviors in surviving pups. Pregnant rats were injected with Escherichia coli (1 × 105 colony-forming units) or sterile saline at gestational day 17. Beginning at postnatal day (PND) 2, the pups were subjected to a series of developmental tests to examine developmental milestones. At PND 16, some pups were sacrificed and their brains extracted and processed for histology or protein studies. Hematoxylin and eosin (HE) staining was done to examine the general morphology of the Purkinje cells and to examine Purkinje cell density, area and volume. Calbindin expression was examined in the cerebellum via immunohistochemistry and Western blot techniques. The remaining rat pups were used to examine motor coordination and balance on a rotating rotarod at the prepubertal and adult ages. Prenatal E. coli injection did not significantly change birth weight or delivery time, but did delay surface righting and negative geotaxis in pups. Pups in the E. coli group also had a decrease in the number of Purkinje cells, as well as a decrease in Purkinje cell density and volume. HE staining demonstrated a change in Purkinje cell morphology. Calbindin expression was decreased in rats from the E. coli group as well. Locomotor tests indicated that while there were no significant changes in gross motor activity, motor coordination and balance was impaired in both prepubertal and adult rats from the E. coli group. In this model of IUI, we observed changes in Purkinje cell development which were associated with alterations in cerebellum-dependent motor behaviors. The decreases in calbindin and Purkinje cells were associated with developmental delays. These data further support the importance of IUI in brain development.
Key Words: Calbindin; Brain development, fetal; Motor behavior; Purkinje neurons; Escherichia coli
Introduction
Intrauterine infection (IUI) causes neurodevelopmental brain damage, often resulting in neurological disorders associated with serious motor impairment such as cerebral palsy [1,2,3]. White matter damage, astrocytosis and cytokine activation have been demonstrated in experimental models of IUI, all of which are capable of leading to delays in brain development [4,5,6]. The cerebellum is particularly vulnerable to infectious insults since it is not fully developed until after birth in both humans and rodents [7,8]. Due to the nearly 5-fold increase in growth in the cerebellum in the last trimester of pregnancy, IUI or activation of the fetal immune system could cause irreparable damage to this structure [9].
We recently reported that in a rodent model, developmental as well as cognitive deficits occur as a result of IUI [10]. Moreover, we found that the developmental delays induced by IUI were specific to cerebellar functioning. In addition, we previously reported that this same model of IUI induced white matter damage is similar to that seen in periventricular leukomalacia and is capable of inducing astrocytosis, ventriculomegaly and changes in oligodendrocyte precursors [11,12].
Our recent data and that of others suggest that the cerebellum might be more vulnerable to IUI, and thus cause motor and possibly cognitive impairments [13,14]. Purkinje cells are the major output neurons of the cerebellum and are unique in that they finish development in the last portion of pregnancy in the rat, which is different from the postnatal development of other neurons in the cerebellum such as basket, stellate and granule cells [15]. It is unclear what, if any, effect IUI has on Purkinje cell development or whether IUI leads to selective impairment of cerebellar behavior in juvenile as well as adult rats. The goal of this study was to test the hypothesis that Escherichia coli induces IUI, disturbs the development of Purkinje cells, and impairs motor coordination and balance in juvenile and adult rats.
Methods
Animals
Fourteen timed pregnant Sprague-Dawley rats (Harlan Sprague-Dawley, Indianapolis, Ind., USA) were obtained at gestational day (GD) 13 [16] and housed individually with food and water ad libitum. The colony was maintained in a 12:12-hour light/dark schedule. All animal experiments were carried out with the approval of the institutional animal care and use committee of the University of Mississippi Medical Center. Every attempt was made to minimize the number of animals used, consistent with obtaining statistically reliable results.
Prenatal Treatment
The timed pregnant Sprague-Dawley rats were randomly assigned to either saline (n = 6) or E. coli (ATCC No. 25922; Manassas, Va., USA; n = 8) treatment groups. To ensure a significant number of male pups were available for this study, an additional 2 dams were used in the E. coli group (increasing n = 8) since E. coli injection has been shown to reduce litter size [10]. On GD 17, animals were placed under general anesthesia with 5% isoflurane and injected with either 100 μl of saline or 1 × 105 colony-forming units of E. coli into the lumen of the uterine horns where the 2 horns meet [10,11,12]. The animals were allowed to deliver at term without any additional experimental manipulation. After birth the pups were weighed daily until postnatal day (PND) 16, and then were weighed once weekly. At PND 4, female pups were culled, so that only male pups remained in the study. To equalize study and litter sizes, all litters were reduced to 3–5 pups per litter, so that both treatment groups had 25 male pups.
Sensorimotor Testing
Beginning at PND 2, the pups were put through a series of sensorimotor tests specific to cerebellar development [17,18,19]. Surface righting was defined as the time in seconds required for a pup lying on its back to right itself on all 4 limbs. The pups were observed for up to 60 s. Negative geotaxis was defined as the time in seconds required for a pup placed head down on a 45-degree incline to turn 180° and begin crawling up the slope. The pups were observed for up to 60 s. Forelimb placing was defined as the ability of the pup to place forepaws on a thin rod after having the rod stroked against the dorsal surface of a forepaw. The response to forelimb placing and grasp was recorded as yes or no. Forelimb grasp was defined as the ability of the pup to grasp a thin rod for at least 1 s once it had been stroked against the dorsal surface of a forepaw. The response to forelimb placing and grasping was recorded as yes or no.
Tissue Preparation
At PND 16, male pups were selected from each litter (saline: n = 10; E. coli: n = 10) and decapitated, followed by brain removal. Since fetal position is not accounted for in this study, and to avoid choosing pups who may have been closer or further from the site of E. coli injection (see online supplementary figure 1, www.karger.com/doi/10.1159/000319506), the pups sacrificed were selected based on their weight at PND 16 and not their behavioral performance (online suppl. table 1). The posterior skull was removed, and the cerebellum dissected out, weighed and immediately snap frozen with liquid nitrogen or placed in formalin for immunohistochemistry. Five brains from each group were used for histology and immunohistochemistry and the remaining 5 for protein analysis.
Immunopreparation. The brains were placed into 4% paraformaldehyde solution for at least 24 h before being processed for paraffin embedding. They were sectioned at 20 μm, and every fourth section was stained using hematoxylin and eosin (HE). An adjacent section was immunostained with polyclonal rabbit antibodies against calbindin. For calbindin immunostaining, sections underwent antigen retrieval via pressure cooking with Trilogy (CellMarque, Rocklin, Calif., USA) for 20 min. Nonspecific binding was blocked with 0.05 M Tris, 5% dry milk (BioRad, Hercules, Calif., USA), 2% goat serum and 0.2% SDS for 1 h. The sections were incubated with the rabbit primary antibody to calbindin overnight at 4°C (1:100 rabbit anti-calbindin; Chemicon, Temecula, Calif., USA). After washing the sections with PBS + 0.01% Tween 20, they were incubated with 1:50 Rhodamine-red-conjugated goat anti-rabbit (Jackson Immunoresearch, West Grove, Pa., USA). The slides were coverslipped and allowed to dry overnight. Sections incubated without primary antibody served as a negative control.
Western Blot Analysis. Samples of previously frozen cerebellum were immediately homogenized in homogenate buffer (10 mM Tris base, 0.1 mM EDTA, pH 7.0) with a protease inhibitor (Roche, Mannheim, Germany). The total protein concentration was determined using the bicinchoninic acid protein assay (Pierce, Rockford, Ill., USA). The samples were mixed with sample buffer (125 mM Tris base, 20% glycerol, 4% SDS, 10% mercaptoethanol, 0.05% bromophenol blue, pH 6.8) and heated at 95°C for 10 min. Solubilized protein (20 μg per lane) was loaded on a 7.5% Criterion Precast Tris-HCl gel (BioRad), subjected to electrophoresis and transferred to nitrocellulose membranes (Hybond ECL; Amersham Biosciences, Piscataway, N.J., USA). Nitrocellulose blots were blocked in 5% nonfat milk and TBS (20 mM Tris base and 0.5 M NaCl, pH 7.5) for 1 h and then incubated (overnight at 4°C) with 1:5,000 anti-calbindin (Chemicon). The membranes were then washed in TBS buffer and incubated with 1:5,000 goat anti-rabbit IgG horseradish peroxidase (Chemicon) for 1 h. After incubation, the blots were washed and developed using enhanced chemiluminescence detection (ECL; Perkin-Elmer Life Sciences Inc., Boston, Mass., USA) and immediately exposed to film (Hyperfilm-ECL; Amersham Biosciences). To control for accuracy of loading and efficiency of transfer, the data were normalized to mouse anti-actin protein (Chemicon) detected on the same blots.
Stereological Procedures
Design-based stereology was performed to count Purkinje cells in each lobule of the vermis in HE-stained sections, using Stereoinvestigator software (Microbrightfield, Williston, Vt., USA) and a stereological computer microscopy system. The Purkinje cell layer was identified at ×2 magnification on live microscopic images displayed on a monitor. Purkinje cells with a visible nucleolus were counted using a ×40 oil-immersion objective lens (1.0 numerical aperture) and with the optical fractionator method. Briefly, counting frames (75 × 100 μm) were created by the software and placed at the intersections of a grid (150 × 200 μm) that was randomly placed over a section. Purkinje cells were marked if they were positive and in focus within the counting frame. This process was repeated in all of the counting frames in the region of interest. The mean cellular volume and area of Purkinje cells were also estimated using the nucleator method [17]. Three sections containing lobules of the vermis were analyzed per animal. All Purkinje cell values (packing density, sectional area, volume) were averaged to obtain 1 value per animal.
Locomotor Activity
To determine if IUI affected overall locomotor activity, the total distance traveled by rats was examined at 2 different time points in the remaining 15 rats per group. On the day of behavioral testing, the rats were brought to a sound-attenuated testing room to acclimate for 1 h before each test. The animals were tested at 2 different time points, PND 30 and 60. All testing was done at the same time of day for each time point. The rats were placed individually into a monitoring system (Opto-Varimex-Minor System; Columbus Instruments, Columbus, Ohio, USA) under low lights for 30 min to test gross motor function. A computer system recorded horizontal and vertical activity as determined by the breaking of infrared detectors and sensors. Total distance traveled was the main outcome.
Rotarod
Motor coordination and balance was tested using a rotarod (Economy Rotamex; Columbus Instruments) [18]. Animals underwent rotarod testing between PND 30–32 and PND 60–62. The animals were placed on a rotating rotarod for a period of 3 min at 15 rpm, followed by 3 min at 30 rpm. This test was again repeated on the following 2 days. The first 30 s at each speed was used as an acclimation period and falls were not recorded. Any falls after that were recorded and the animal was placed back on the rotarod. Latency to fall off was the main outcome.
Statistical Analysis
Birth weight, cerebellar weight, Purkinje cell counts and data from the Western blot were analyzed using a two-tailed Student t test. Immunoreactive bands were analyzed using MCID Elite 7.0 (Imaging Research, St. Catherines, Ont., Canada). Sensorimotor and rotarod data were analyzed using a mixed model which allowed us to incorporate within-pup correlations and also to adjust for dams and litters using variance components models. A repeated measures ANOVA incorporating random effects for dams and litters with postnatal age serving as the repeated measure was used to analyze behavioral data. Behavior-corrected t tests were used for post hoc analysis. The data are represented as means ± SD. p < 0.05 was considered significant.
Results
Prenatal E. coli injection did not significantly decrease pup birth weight (saline 5.63 ± 0.091 g vs. E. coli 5.49 ± 0.13 g; p = 0.315). There was no significant difference in day of delivery between the groups, with both groups delivering on GD 21 (saline 21 ± 0 days vs. E. coli 20.75 ± 0.21 days; p = 0.215). There was no fetal mortality during the course of the study or pup rejection by the dam. When E. coli is injected into the lumen of the uterine horns at this dose (1 × 105 colony-forming units), litter size is reduced and fetal brain inflammation is increased in the long term [10], while inflammatory markers in maternal serum decrease to levels comparable to saline control dams within 48 h of delivery (unpublished data).
Prenatal E. coli Injection Delays Sensorimotor Skills
Surface Righting. E. coli infection did not cause a significant delay in the time to complete surface righting (p = 0.118) (fig. 1a). There was a statistically significant difference in behavioral performance over the course of the experiment (p < 0.001).
Fig. 1.
Effects of IUI on sensorimotor development. Data are represented as average time taken to complete the task ± SEM. At each time point, saline n = 25 and E. coli n = 25. * Significantly different from corresponding saline group as indicated by mixedmodel repeated measures ANOVA and post hoc Bonferroni-corrected t tests (p < 0.05). a E. coli-induced IUI did not significantly increase the time taken to complete surface righting compared with the saline group. b Animals in the E. coli group took significantly more time to complete negative geotaxis on PND 6–11 compared with the saline group.
Negative Geotaxis. E. coli infection significantly increased the time taken to turn 180° and traverse an incline on PND 6–11 (p = 0.001) (fig. 1b).
Forelimb Placing and Grasp. Fine motor skills were tested via forepaw placing on a wire rod and forelimb grasp on that same wire rod and was tested on PND 2–16. Prenatal E. coli injection significantly delayed forepaw placement on PND 2 compared to pups in the saline group (p = 0.003; data not shown) as well as delaying forepaw grasp on PND 6–8 compared to pups in the saline group (p < 0.001; data not shown). On average, animals in both groups began forelimb placing on PND 3. Animals in the saline group began forelimb grasp on PND 9 and animals in the E. coli group on PND 8 (p = 0.234; data not shown).
Purkinje Cell Packing Density Is Significantly Decreased after Prenatal E. coli Injection
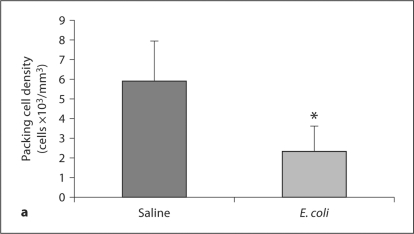
Juvenile Animals. Prenatal E. coli injection did not significantly change cerebellar weight in comparison to the saline group (saline 0.129 ± 0.009 g vs. E. coli 0.117 ± 0.007 g; p = 0.310). To determine if Purkinje cell deficits occur as a result of IUI, we counted Purkinje cells at PND 16 in the lobules of the vermis. HE staining demonstrated that Purkinje cells from animals in the IUI group appeared to be smaller when compared to Purkinje cells from animals in the saline group (fig. 2). After stereological analysis, IUI was found to significantly decrease the packing density of Purkinje cells in comparison to controls (saline 5.91 ± 2.04 cells × 103/mm3 vs. E. coli 2.32 ± 1.30 cells × 103/mm3; p = 0.018) (fig. 3a). IUI also significantly decreased the sectional area (saline 270.02 ± 30.16 mm2 vs. E. coli 211.96 ± 35.13 mm2; p = 0.009) and the nucleator-estimated volume (saline 3,544.1 ± 591.02 μm3 vs. E. coli 2,753.9 ± 514.39 μm3; p = 0.005) of the Purkinje cell bodies (fig. 3b). There were no significant differences in the packing density of Purkinje cells that appeared atrophied or irregular between the groups (saline 1.46 ± 0.396 cells × 103/mm3 vs. E. coli 1.79 ± 0.435 cells × 103/mm3; p = 0.208).
Fig. 2.
Photomicrographs of coronal sections of juvenile rat brains. Cerebellum from male rats sacrificed at PND 16. Intrauterine E. coli injection decreased Purkinje cells in the Purkinje cell layer (b, d) compared to the saline group (a, c). Notice at high-power magnification (×400) that, relative to saline (a, c), the E. coli group has numerous spaces between stained Purkinje cells (b, d). Arrows: Purkinje cells. White arrow: abnormally shaped Purkinje cell that appears to be atrophied. ML = Molecular layer; PCL = Purkinje cell layer; GCL = granule cell layer. HE. Scale bar = 100 μm.
Fig. 3.
Purkinje cells are decreased in response to intrauterine E. coli injection. Purkinje cell packing density (a) and nucleatorestimated volume (b) were significantly decreased in the vermis of the cerebellum in animals from the E. coli group. Each brain was sectioned, and every 4th section (for a total of 3 sections per brain) analyzed. Data from all 3 sections were averaged to equal 1 value per animal. Data are reported as average cell count or volume per treatment group ± SEM. N = 5 subjects per treatment group for each time point. * Significantly different from Purkinje cells in corresponding saline group as indicated by Student's t test and post hoc Bonferroni-corrected t tests (p < 0.05).
Adult Animals. To determine if Purkinje cell deficits remained throughout adulthood as a result of IUI, we counted Purkinje cells at PND 65 in the lobules of the vermis. Prenatal E. coli injection did not significantly change cerebellar weight in comparison to the saline group (saline 0.270 ± 0.047 g vs. E. coli 0.268 ± 0.045 g; p = 0.925). IUI significantly decreased the packing density of Purkinje cells in comparison to controls (saline 3.11 ± 1.49 cells × 103/mm3 vs. E. coli 1.33 ± 1.17 cells × 103/mm3; p = 0.0001) (fig. 4). There were no significant differences in the sectional area (saline 272.30 ± 67.06 mm2 vs. E. coli 287.16 ± 66.11 mm2; p = 0.464) and the nucleator-estimated volume (saline 3,640.05 ± 1,393.4 μm3 vs. E. coli 3,791.94 ± 971.60 μm3; p = 0.687) of the Purkinje cell bodies. There were also no significant differences in the packing density of Purkinje cells that appeared atrophied or irregular between the groups (saline 1.75 ± 1.78 cells × 103/mm3 vs. E. coli 1.68 ± 1.22 cells × 103/mm3; p = 0.931). These results indicate that prenatal E. coli injection directly affects Purkinje cell development in the cerebellum.
Fig. 4.
Purkinje cells are decreased in response to intrauterine E. coli injection at PND 65. Purkinje packing cell density was significantly decreased in the vermis of the cerebellum in animals from the E. coli group. Data are reported as average packing cell density per treatment group ± SD. N = 5 subjects per treatment group for each time point. * Significantly different from Purkinje cells in corresponding saline group as indicated by Student's t test and post hoc Bonferroni-corrected t tests (p < 0.05).
Calbindin Expression Is Decreased after Prenatal E. coli Injection
Calbindin D28k (calbindin) is a calcium-binding protein expressed only in Purkinje cells in the cerebellum. To determine if IUI decreased calbindin expression, we examined calbindin immunoreactivity and protein levels in the cerebellum.
Immunohistochemistry. Visual inspection of Purkinje cells in the vermis indicated that IUI decreased calbindin immunoexpression since there was a decrease in calbindin reactivity in Purkinje cells in the cerebellum at PND 16 (fig. 5).
Fig. 5.
Calbindin immunoreactivity is decreased in the vermis of the cerebellum. Intrauterine E. coli injection decreased calbindin staining (b) in comparison to the saline group (a). × 200.
Western Blot Analysis. Immunoreactive bands corresponding to molecular masses of 28 kDa were revealed for calbindin (fig. 6a). Calbindin expression in animals of the IUI group was significantly lower than that in the saline group (saline 1.827 ± 0.702 vs. E. coli 1.097 ± 0.34; p < 0.05) (fig. 6b).
Fig. 6.
Calbindin was decreased after E. coli injection in the cerebellum. Calbindin was significantly decreased in the PND 16 cerebellum in the E. coli group when compared to the saline group. Data are reported as average calbindin concentrations ± SEM. N = 5 subjects per treatment group. * Significantly different from immunoblots in the corresponding saline group as indicated by Student's t tests and post hoc Bonferroni-corrected t tests (p < 0.05).
Motor Coordination and Balance
General locomotor abilities as tested via the locomotor chamber did not significantly differ between saline and IUI animals at either PND 30 (saline 4,739 ± 1,138.1 cm vs. E. coli 4,088 ± 905.7 cm; p = 0.094) or PND 60 (saline 3,899 ± 896.4 cm vs. E. coli 3,731 ± 763.9 cm; p = 0.585). The data from the distance traveled in the locomotor test indicate that E. coli injection during GD 17 does not alter gross motor activity in the resulting offspring.
Motor coordination and balance was assessed by the rotating rotarod over a period of 3 days tested at prepuberty (PND 30–32) and again at adulthood (PND 60–62). Prenatal E. coli injection significantly reduced the time spent on the rotating rotarod at speeds of 15 rpm in prepubertal rats (p = 0.001). Post hoc analysis indicated that animals in the E. coli group spent significantly less time on the rotarod on the first day of testing at 15 rpm (p = 0.009) (fig. 7a). There were no statistically significant differences between the groups when the speed was increased to 30 rpm at any day (p = 0.987) (fig. 7c). When adult animals were assessed at 15 rpm, there were no statistically significant differences between the groups in time spent on the rotarod at any day (p = 0.257) (fig. 7b). When the speed was increased to 30 rpm, animals in the E. coli group had significantly decreased time spent on the rotarod compared to animals in the saline group on all 3 days (p = 0.001) (fig. 7d).
Fig. 7.
Motor coordination and balance at PND 30–32 and 60–62. Male rats were tested for motor coordination and balance. At PND 30–32, E. coli-induced IUI significantly decreased the time spent on the rotarod at 15 rpm (a) during the first day of testing. There were no statistically significant differences on subsequent days of testing, or when the speed was increased to 30 rpm (c). At PND 60–62, E. coli-induced IUI significantly decreased the time spent on the rotarod at 15 rpm (b) during the first day of testing, and on all 3 days of testing at 30 rpm (d). Data are represented as average time spent on the rotarod at each indicated speed. At each time point, saline n = 15 and E. coli n = 15. * Significantly different from corresponding saline group as indicated by two-way repeated measures ANOVA and post hoc Bonferroni-corrected t tests (p < 0.01).
Discussion
In the present study, to investigate the effects of prenatal infection on cerebellar motor functions, we examined motor function as well as Purkinje cell density and size in rats presented with an immune challenge prenatally. In addition, we also examined levels of calbindin in the cerebellum as calbindin has been found to play a crucial physiological role in motor coordination [19]. The cerebellum, which is involved in motor and balance coordination as well as learning and memory processes, is particularly vulnerable to developmental insults as it is one of the last brain regions to fully mature. Our results demonstrate that prenatal infection decreases Purkinje cell density and volume as well as probably decreasing calbindin expression in Purkinje cells. The decrease in calbindin in Purkinje cells is also accompanied by impairments in motor coordination and balance in rats from the early postnatal period through adulthood.
Purkinje cells are generated primarily between embryonic days 14 and 16 in rats, but continue maturation into the early postnatal period [20,21]. E. coli injection administered at GD 17 decreased Purkinje cell density and volume in the current study. It is important to note that since the Purkinje cell serves as the only output from the cerebellar cortex, a reduction in density and volume can result in permanent effects on motor coordination and balance as well as cognitive functions associated with the cerebellum. Interestingly, these results are different from those in animal models of gestational stress. When pregnant Sprague-Dawley rats were restrained for 6 h on GD 7 and 14, the resultant offspring actually had an increase in Purkinje cell density and volume [22]. Animal models of fetal alcohol syndrome have also shown that ethanol consumption during pregnancy decreases Purkinje cell density and responsiveness, as do animal models being administered β2-adrenoceptor agonists in the earlier postnatal period [23,24]. This would suggest that not only does the timing of an adverse event during pregnancy affect Purkinje cell development and formation, but so does the challenge. These studies indicate that the cerebellum and, in particular, Purkinje cells are sensitive to developmental alterations during the late gestational period through the early postnatal period. Since this period of development coincides with Purkinje cell migration, any toxicant, regardless of its mechanism of action, poses a threat to development. Studies using the Borna disease virus have found that up to 75% of Purkinje cells are lost 7 months after infection [25], indicating that viral administration in the early postnatal period has long-lasting effects on Purkinje cell development. We also found similar results in that, despite the expected age-related decrease in Purkinje cells [26,27], prenatal E. coli infection still caused a significant decrease in the packing density of Purkinje cells. These studies lend further confirmation to the view that insults during pregnancy have long-term effects on the developing cerebellum.
Calbindin expression is decreased in Purkinje cells from animal models using viral strains of influenza or Borna disease [19,28]. As Purkinje cells are the only cerebellar neurons to express calbindin, the decrease in the levels of calbindin found in the present study indicates that IUI decreases calbindin in Purkinje cells. The decrease in calbindin protein expression may be due to the decreased number of Purkinje cells in the IUI group. However, our immunohistochemical studies also suggest that the surviving individual Purkinje cells express lower levels of calbindin (fig. 4). This is, to our knowledge, the first study to indicate that prenatal E. coli injection decreases calbindin expression in Purkinje cells. It is possible that the decrease in Purkinje cells and calbindin seen in the current study are responsible for the decreased time spent on the rotarod as well as for the poor performance in the sensorimotor tests.
Previous studies have shown that decreases in calbindin and/or Purkinje cells result in impaired motor coordination on the rotarod but do not affect general locomotion [16,22,29]. The data from the current study are in agreement since prenatal E. coli injection has no significant effects on general locomotor behavior, but causes deficits in sensorimotor behavior and rotarod performance. The accelerating rotarod test is commonly used to assess balance and coordination, but it is not the most sensitive behavioral test to identify primary cerebellar defects. In order to delineate between defects in muscle, upper motor neuron and cerebellar motor control, other tests would need to be performed, tests such as gait and fluid-licking behavior [30]. To determine if more specific motor circuits were affected, sensorimotor tests such as negative geotaxis as well as forelimb placing and grasping along with surface righting are direct behavioral outputs of the exteroceptive, vestibular and proprioceptive systems modulated by the cerebellum [31,32]. Animals injected with E. coli showed delays in these same tests, indicating that IUI selectively affects cerebellar systems that regulate those behavioral outputs.
Animals from the E. coli group spent significantly less time on the rotarod compared to animals from the control group, implying that the decrease in Purkinje cells and the decrease in calbindin are also associated with motor impairments. At PND 30, E. coli animals were not able to retain their balance at 15 rpm on the first day of testing. However, after their first trial, they were able to maintain their balance at a faster speed (30 rpm), and showed no impairments on the subsequent days of testing. When assessed at PND 60, these same animals were able to maintain their balance at 15 rpm, but not at 30 rpm, regardless of the testing day. This is one of the first studies to show that E. coli given in utero decreases balance and coordination on the rotarod.
Models using calbindin–/– mice or Purkinje cell-specific conditional calbindin–/– mice have shown motor coordination problems, suggesting a physiologic link between calbindin and motor coordination [19,29,33]. Despite both calbindin and parvalbumin serving as calcium-binding proteins in Purkinje cells, only calbindin deficiencies are associated with impairments in motor coordination. When mice are knocked out for calbindin, parvalbumin or both, only knockout mice for calbindin display deficits in the rotarod and runway assays [34]. A decrease in Purkinje cells can contribute to decreased calcium regulation in the cerebellum. Studies examining calcium homeostasis in calbindin–/– mice indicate that calbindin is used to shape fast calcium transients in Purkinje cell dendrites. These dendrites are used for motor behavior and potentially sensorimotor integration and motor learning [35].
One major limitation to this study is the lack of female pups. We have shown that intrauterine E. coli injection does not lead to any significant differences between male and female pups in sensorimotor behavior (unpublished data); as such, this is a study of male offspring only. Several studies have shown that there are gender-related differences due to inflammation during pregnancy and environmental rearing which can lead to differences in behavior and locomotion as well as altering brain structures [36,37,38]. As such, it is important that future studies include both males and females, so that the effects of IUI and brain development can be studied in both males and females.
Infection during pregnancy has been shown to lead to a series of diverse neuropathological changes in the resulting offspring [39,40]. Meyer et al. [41] showed that depending on the time of maternal immune challenge, different behavioral abnormalities occur. Another recent study using viral and bacterial reagents also concluded that the timing of administration and nature of the infectious agent produced different behavioral responses in neonates and adult rats [42]. The presence of inflammatory cytokines such as interleukin-1β, tumor necrosis factor-α and interleukin-6 have all been implicated in causing neuronal damage to the developing brain as a result of neonatal or early postnatal infection [6,10,43]. The fact that the rat cerebellum has a slower development than the cerebral cortex and undergoes extensive development during the postnatal period makes it a more vulnerable target for prenatal infection occurring during the last period of pregnancy [44,45]. As the release of inflammatory cytokines in the presence of infection has been shown to cross the blood-placenta barrier, thus impairing the prenatal blood-brain barrier, it is possible that inflammatory cytokines released due to IUI at critical stages of cerebellum development contribute to Purkinje cell death, density and volume [46,47,48,49]. We have previously shown lesions, myelination deficits, astrogliosis as well as disturbances in oligodendrocyte development in the cerebral cortex as a result of IUI [11,12,50]. As such, future studies need to be done to determine if IUI also creates these same white matter deficits in the cerebellum. Studies in fetal sheep have found that there is selective white matter injury in the cerebellum which does not always involve damage to Purkinje cells [13,51]. The timing and source of infection seem to determine the type of pathological and behavioral changes that will be seen [41,51].
The present study suggests several important clinical implications. For instance, postmortem brain studies on autism have shown that there is a reduction in the number of Purkinje cells as compared to nonautistic cases [52,53]. Purkinje cell deficits and reduced cerebellar volume have also been implicated in schizophrenia and Huntington's disease and models of fetal alcohol syndrome [50,54,55]. This is one of the first studies to show that prenatal E. coli injection results in long-term motor impairment, and that this impairment may be mediated by alterations in Purkinje cell development. The results from this study, along with studies focusing on viral mechanisms of cerebellar damage, suggest that infection during the late prenatal period and the associated immune system responses may target the still developing cerebellum. The possibility that immune response is associated with infection of the cerebellum remains an important area of investigation.
Supplementary Material
Supplementary Materials
Acknowledgements
This study was supported in part by grant No. RR17701 to I.P. and departmental support from the Department of Obstetrics and Gynecology at the University of Mississippi Medical Center.
References
- 1.Nelson K, Willoughby R. Overview: infection during pregnancy and neurologic outcome in the child. Ment Retard Dev Disabil Res Rev. 2002;8:1–2. doi: 10.1002/mrdd.10010. [DOI] [PubMed] [Google Scholar]
- 2.Girard S, Kadhim H, Beaudet N, Sarret P, Sebire G. Developmental motor deficits induced by combined fetal exposure to lipopolysaccharide and early neonatal hypoxia/ischemia: a novel animal model for cerebral palsy in very premature infants. Neuroscience. 2009;158:673–682. doi: 10.1016/j.neuroscience.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 3.Dammann O, Kuban K, Leviton A. Perinatal infection, fetal inflammatory response, white matter damage and cognitive limitations in children born preterm. Ment Retard Dev Disabil Res Rev. 2002;8:46–50. doi: 10.1002/mrdd.10005. [DOI] [PubMed] [Google Scholar]
- 4.Debillon T, Gras-Leguen C, Vérielle V, Winer N, Caillon J, Rozé J, Gressens P. Intrauterine infection induces programmed cell death in rabbit periventricular white matter. Pediatr Res. 2000;47:736–742. doi: 10.1203/00006450-200006000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Bell M, Hallenbeck J. Effects of intrauterine inflammation on developing rat brain. J Neurosci Res. 2002;70:570–579. doi: 10.1002/jnr.10423. [DOI] [PubMed] [Google Scholar]
- 6.Cai Z, Pan Z, Pang Y, Evans O, Rhodes P. Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopolysaccharide administration. Pediatr Res. 2000;47:64–72. doi: 10.1203/00006450-200001000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Dobbing J. The later growth of the brain and its vulnerability. Pediatrics. 1974;53:2–6. [PubMed] [Google Scholar]
- 8.Volpe J. Cerebellum of the premature infant: rapidly developing, vulnerable, clinically important. J Child Neurol. 2009;24:1085–1104. doi: 10.1177/0883073809338067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang C, Chang F, Yu C, Ko H, Chen H. Assessment of fetal cerebellar volume using three-dimensional ultrasound. Ultrasound Med Biol. 2000;26:981–988. doi: 10.1016/s0301-5629(00)00225-8. [DOI] [PubMed] [Google Scholar]
- 10.Wallace K, Lopez J, Wells A, Shaffery J, Paul I, Bennett W. Interleukin-10/Ceftriaxone prevents E. coli-induced delays in sensorimotor task learning and spatial memory in neonatal and adult Sprague-Dawley rats. Brain Res Bull. 2010;81:141–148. doi: 10.1016/j.brainresbull.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodts-Palenik S, Wyatt-Ashmead J, Pang Y, Thigpen B, Cai Z, Rhodes P, Martin J, Granger J, Bennett W. Maternal infection-induced white matter injury is reduced by treatment with interleukin-10. Am J Obstet Gynecol. 2004;191:1387–1392. doi: 10.1016/j.ajog.2004.06.093. [DOI] [PubMed] [Google Scholar]
- 12.Pang Y, Rodts-Palenik S, Cai Z, Bennett W, Rhodes P. Suppression of glial activation is involved in the protection of IL-10 on maternal E. coli-induced neonatal white matter injury. Dev Brain Res. 2005;157:141–149. doi: 10.1016/j.devbrainres.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Hutton L, Yan E, Yawno T, Castillow-Melendez M, Hirst J, Walker D. Injury of the developing cerebellum: a brief review of the effects of endotoxin and asphyxia challenges in the late-gestational sheep fetus. Cerebellum. 2007:1–10. doi: 10.1007/s12311-014-0602-3. [DOI] [PubMed] [Google Scholar]
- 14.Pierce K, Courchesne E. Evidence for a cerebellar role in reduced exploration and stereotyped behavior. Biol Psychiatry. 2001;49:655–664. doi: 10.1016/s0006-3223(00)01008-8. [DOI] [PubMed] [Google Scholar]
- 15.Beny M, McConnell P, Sievers J, Price S, Anwar A. Factors influencing the growth of cerebellar neural networks. Bibl Anat. 1981;19:1–51. [PubMed] [Google Scholar]
- 16.Sajdel-Sulkowska E, Nguon K, Sulkowski Z, Rosen G, Baxter M. Purkinje cell loss accompanies motor impairment in rats developing at altered gravity. Neuroreport. 2005;16:2037–2040. doi: 10.1097/00001756-200512190-00014. [DOI] [PubMed] [Google Scholar]
- 17.Gunderson H. Stereology of arbitrary particles: a review of unbiased number and size estimators and the presentation of some new ones, in memory of William R Thompson. J Microsc. 1986;143:3–45. [PubMed] [Google Scholar]
- 18.Dunham N, Miya T. A note on a simple apparatus for detecting neurological deficits in rats and mice. J Am Pharm Assoc Am Pharm Assoc (Baltim) 1957;46:208–209. doi: 10.1002/jps.3030460322. [DOI] [PubMed] [Google Scholar]
- 19.Barski J, Hartmann J, Rose C, Hoebeek F, Morl K, Noll-Hussong D, Zeeuw C, Konnerth A, Meyer M. Calbindin in cerebellar Purkinje cells is a critical determinant of the precision of motor coordination. J Neurosci. 2003;23:3469–3477. doi: 10.1523/JNEUROSCI.23-08-03469.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berry M, McConnell P, Sievers J, Price S, Anwar A. Factors influencing the growth of cerebellar neural networks. Bibl Anat. 1981;19:1–51. [PubMed] [Google Scholar]
- 21.Altman J, Bayer S. Development of the Cerebellar System. New York: CRC; 1997. [Google Scholar]
- 22.Ulupinar E, Yucel F, Ortug G. The effects of prenatal stress on the Purkinje cell neurogenesis. Neurotoxicol Teratol. 2006;28:86–94. doi: 10.1016/j.ntt.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Rhodes M, Seidler F, Abdel-Rahman A, Tate C, Nyska A, Rincavage H, Slotkink T. Terbutaline is a developmental neurotoxicant: effects on neuroproteins and morphology in cerebellum, hippocampus, and somatosensory cortex. J Pharm Exp Ther. 2004;308:529–537. doi: 10.1124/jpet.103.060095. [DOI] [PubMed] [Google Scholar]
- 24.West J, Parnell S, Chen W, Cudd T. Alcohol-mediated Purkinje cell loss in the absence of hypoxemia during the third trimester in an ovine model system. Alcohol Clin Exp Res. 2001;25:1051–1057. [PubMed] [Google Scholar]
- 25.Eisenman L, Brothers R, Tan M, Kean R, Dickson G, Dietzschold B, Hooper D. Neonatal Borna disease virus infection in the rat causes a loss of Purkinje cells in the cerebellum. J Neurovirol. 1999;5:181–189. doi: 10.3109/13550289909022000. [DOI] [PubMed] [Google Scholar]
- 26.Amenta F, Valle M, Vega J, Zaccheo D. Age-related structural changes in rat cerebellar cortex: effects of choline alfoscerate treatment. Meth Ageing Dev. 1991;61:173–186. doi: 10.1016/0047-6374(91)90015-r. [DOI] [PubMed] [Google Scholar]
- 27.Larsen J, Skalicky M, Viidik A. Does long-term physical exercise counteract age-related Purkinje cell loss? A stereological study of rat cerebellum. J Comp Neurol. 2000;428:213–222. [PubMed] [Google Scholar]
- 28.Shi L, Smith S, Malkova N, Tse D, Su Y, Patterson P. Activation of the maternal immune system alters cerebellar development in the offspring. Brain Behav Immun. 2009;23:116–123. doi: 10.1016/j.bbi.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Airaksinen M, Eilers J, Garaschuk O, Thoenen H, Konnerth A, Meyer M. Ataxia and altered dendritic calcium signaling in mice carrying a targeted null mutation of the calbindin D28k gene. Proc Natl Acad Sci USA. 1997;94:1488–1493. doi: 10.1073/pnas.94.4.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heck D, Zhao Y, Roy S, LeDoux M, Reiter L. Analysis of cerebellar function in Ube3a-deficient mice reveals novel genotype-specific behaviors. Hum Mol Genet. 2008;17:2181–2189. doi: 10.1093/hmg/ddn117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Altman J, Sudarshan K. Postnatal development of locomotion in the laboratory rat. Anim Behav. 1975;23:869–920. doi: 10.1016/0003-3472(75)90114-1. [DOI] [PubMed] [Google Scholar]
- 32.Geisler H, Westerga J, Gramsbergen A. Development of posture in the rat. Acta Neurobiol. 1993;53:517–523. [PubMed] [Google Scholar]
- 33.Barski J, Dethleffsen K, Meyer M. Cre recombinase expression in cerebellar Purkinje cells. Genesis. 2000;28:93–98. [PubMed] [Google Scholar]
- 34.Farre-Castany M, Schwaller B, Gregory P, Barski J, Mariethoz C, Eriksson J, Tetko I, Wolfer D, Celio M, Schmutz I, Albrecht U, Villa A. Differences in locomotor behavior revealed in mice deficient for the calcium-binding proteins parvalbumin, calbindin D-28k or both. Behav Brain Res. 2007;172:250–261. doi: 10.1016/j.bbr.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Schwaller B, Meyer M, Schiffmann S. ‘New’ functions for ‘old’ proteins: the role of the calcium-binding proteins calbindin D-28k, calretinin and parvalbumin, in cerebellar physiology – studies with knockout mice. Cerebellum. 2002;1:241–258. doi: 10.1080/147342202320883551. [DOI] [PubMed] [Google Scholar]
- 36.Nguon K, Ladd B, Baxter M, Sajdel-Sulkowska E. Sexual dimorphism in cerebellar structure, function, and response to environmental perturbations. Prog Brain Res. 2005;148:343–351. doi: 10.1016/S0079-6123(04)48027-3. [DOI] [PubMed] [Google Scholar]
- 37.Dahlgren J, Nilsson C, Jennische E, Ho H, Eriksson E, Niklasson A, Björntorp P, Albertsson Wikland K, Holmäng A. Prenatal cytokine exposure results in obesity and gender-specific programming. Am J Physiol Endocrinol Metab. 2001;281:E326–E334. doi: 10.1152/ajpendo.2001.281.2.E326. [DOI] [PubMed] [Google Scholar]
- 38.Jonasson Z. Meta-analysis of sex differences in rodent models of learning and memory: a review of behavioral and biological data. Neurosci Biobehav Rev. 2005;28:811–825. doi: 10.1016/j.neubiorev.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Brown A, Begg M, Gravenstein S, Schaefer C, Wyatt R, Bresnahan M, Babulas V, Susser E. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61:774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- 40.Tohmi M, Tsuda N, Watanabe Y, Kakita A, Nawa H. Perinatal inflammatory cytokine challenge results in distinct neurobehavioral alterations in rats: implication in psychiatric disorders of developmental origin. Neurosci Res. 2004;50:67–75. doi: 10.1016/j.neures.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee B, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fortier M, Luheshi G, Boksa P. Effects of prenatal infection on prepulse inhibition in the rat depend on the nature of the infectious agent and the stage of pregnancy. Behav Brain Res. 2007;181:270–277. doi: 10.1016/j.bbr.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 43.Yoon B, Kim C, Romero R, Jun J, Park K, Choi S, Chi J. Experimentally induced intrauterine infection causes fetal brain white matter lesions in rabbits. Am J Obstet Gynecol. 1997;177:797–802. doi: 10.1016/s0002-9378(97)70271-0. [DOI] [PubMed] [Google Scholar]
- 44.Valentino K, Jones E. Morphological and immunocytochemical identification of macrophages in the developing corpus callosum. Anat Embryol (Berl) 1981;163:157–172. [Google Scholar]
- 45.Murabe Y, Sano Y. Morphological studies on neuroglia. VI. Postnatal development of microglia cells. Cell Tissue Res. 1982;225:469–485. doi: 10.1007/BF00214798. [DOI] [PubMed] [Google Scholar]
- 46.Quagliarello V, Wispelwey B, Long W, Scheld W. Recombinant human interleukin-1 induces meningitis and blood-brain barrier injury in the rat. J Clin Invest. 1991;87:1360–1366. doi: 10.1172/JCI115140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Urakubo A, Jarskog L, Lieberman J, Gilmore J. Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophr Res. 2001;47:27–36. doi: 10.1016/s0920-9964(00)00032-3. [DOI] [PubMed] [Google Scholar]
- 48.Hornig M, Weissenbock H, Horscroft N, Lipkin W. An infection-based model of neurodevelopmental damage. Proc Natl Acad Sci USA. 1999;96:12102–12107. doi: 10.1073/pnas.96.21.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sauder C, de la Torre J. Cytokine expression in the rat central nervous system following perinatal Borna disease virus infection. J Neuroimmunol. 1999;96:29–45. doi: 10.1016/s0165-5728(98)00272-0. [DOI] [PubMed] [Google Scholar]
- 50.Bottmer C, Bachmann S, Pantel J, Essig M, Amann M, Schad L, Magnotta V, Schröder J. Reduced cerebellar volume and neurological soft signs in first-episode schizophrenia. Psychiatry Res. 2005;140:239–250. doi: 10.1016/j.pscychresns.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 51.Dean J, Farrag D, Zahkouk S, El Zawahry E, Hagberg H, Kjellmer I, Mallard C. Cerebellar white matter injury following systemic endotoxemia in preterm fetal sheep. Neuroscience. 2009;160:606–615. doi: 10.1016/j.neuroscience.2009.02.071. [DOI] [PubMed] [Google Scholar]
- 52.Greig PC, Murtha AP, Jimmerson CJ, Herbert WN, Roitman-Johnson B, Allen J. Maternal serum interleukin-6 during pregnancy and during term and preterm labor. Obstet Gynecol. 1997;90:465–469. doi: 10.1016/s0029-7844(97)00294-9. [DOI] [PubMed] [Google Scholar]
- 53.Fatemi S, Halt A, Realmuto G, Earle J, Kist D, Thuras P, Merz A. Purkinje cell size is reduced in cerebellum of patients with autism. Cell Mol Neurobiol. 2002;22:171–175. doi: 10.1023/a:1019861721160. [DOI] [PubMed] [Google Scholar]
- 54.Jeste D, Barban L, Parisi J. Reduced Purkinje cell density in Huntington's disease. Exp Neurol. 1984;85:78–86. doi: 10.1016/0014-4886(84)90162-6. [DOI] [PubMed] [Google Scholar]
- 55.Cebolla A, Cheron G, Hourez R, Bearzatto B, Dan B, Servais L. Effects of maternal alcohol consumption during breastfeeding on motor and cerebellar Purkinje cell behavior in mice. Neurosci Lett. 2009;455:4–7. doi: 10.1016/j.neulet.2009.03.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials