Abstract
Botulism is a rare, life-threatening paralytic disease of both humans and animals that is caused by botulinum neurotoxins (BoNT). Botulism is confirmed in the laboratory by the detection of BoNT in clinical specimens, contaminated foods, and cultures. Despite efforts to develop an in vitro method for botulinum toxin detection, the mouse bioassay remains the standard test for laboratory confirmation of this disease. In this study, we evaluated the use of a nonlethal mouse toe-spread reflex model to detect BoNT spiked into buffer, serum, and milk samples. Samples spiked with toxin serotype A and nontoxin control samples were injected into the left and right extensor digitorum longus muscles, respectively. Digital photographs at 0, 8, and 24 h were used to obtain objective measurements through effective paralysis scores, which were determined by comparing the width-to-length ratio between right and left feet. Both objective measurements and clinical observation could accurately identify over 80% of animals injected with 1 LD50 (4.3 pg) BoNT type A within 24 h. Half of animals injected with 0.5 LD50 BoNT type A and none injected with 0.25 LD50 demonstrated localized paralysis. Preincubating the toxin with antitoxin prevented the development of positive effective paralysis scores, demonstrating that (1) the effect was specific for BoNT and (2) identification of toxin serotype could be achieved by using this method. These results suggest that the mouse toe-spread reflex model may be a more humane alternative to the current mouse bioassay for laboratory investigations of botulism.
Abbreviations: BoNT, botulinum neurotoxin; EDL, extensor digitorum longus muscle; EPS, effective paralysis score; GBS, gelatin phosphate-buffered collecting fluid; MBA, mouse bioassay
Botulism is a rare but life-threatening disease marked by descending bilateral flaccid paralysis. This distinctive paralysis is the result of the action of botulinum neurotoxins (BoNT), which inhibit acetylcholine release at the neuromuscular junction.18 In humans, the clinical presentation typically includes dry mouth, blurry or double vision, difficulty in swallowing, and flaccid facial expression. Progression of the disease is marked by paralysis of the limbs (first arms then legs) and, if untreated, death can result from respiratory paralysis.11 Botulism can occur by ingestion of preformed toxin in contaminated food (foodborne), growth of toxigenic Clostridia spp., and production of toxin in infected tissue (wound) or from colonization of the gut (infant, adult toxemia).18 In addition to natural occurrences of botulism, BoNT are a potential weapon of bioterrorism.4
An equine-derived heptavalent antitoxin is distributed by the Centers for Disease Control through an Investigational New Drug protocol for treatment of all naturally occurring noninfant cases of botulism in the United States.7 A human-derived pentavalent (A, B, C, D, and E) immunoglobulin intravenous product is available through the California Infant Botulism Prevention and Treatment Program for treatment of infant botulism.5 Botulinum antitoxin neutralizes only the toxin molecules that are not yet bound to nerve endings and therefore is more effective if administered early in the course of illness.18 Rapid diagnosis and treatment (hospital supportive care and administration of antitoxin) are crucial for patient recovery.
BoNT are produced by Clostridium botulinum and rare strains of C. baratii, C. butyricum, and C. argentinense. There are 7 serologically distinct BoNT, designated A through G, but only serotypes A, B, E, and F are associated with botulism disease in humans.18 The toxin is secreted as a multimeric complex as large as 900 kDa that contains the neurotoxin as well as nontoxic auxiliary proteins. The neurotoxin is a 150-kDa dichain metalloproteinase.15 The 100-kDa heavy chain is responsible for binding and translocating the neuroprotein across the synaptic membrane. The 50-kDa light chain inhibits neurotransmitter release by cleaving the proteins that aid in the release of acetylcholine required for muscle contraction.15
Laboratory confirmation of botulism is based on the identification of BoNT in clinical specimens and cultures or in remnants of food consumed by the patient.6 The mouse bioassay (MBA), a lethal assay, is the standard procedure for detection of BoNT. Mice are injected intraperitoneally with a sample suspected of containing toxin and monitored for as long as 4 d. BoNT potency is defined by the MBA and, by definition, the limit of detection is 1 LD50. This value represents the results of serial dilutions of BoNT in which each dilution is injected intraperitoneally into 8 to 10 mice; 1 LD50 is the amount of toxin that produces botulism in 50% of the mice at a single dilution (for example, if 10 mice were injected with 1 LD50, only 5 would be expected to show signs of botulism).8 Although the MBA typically is positive within 24 h, many clinical specimens have low levels of toxin (less than 4 LD50/mL) which may not be evident within 4 d.10 If a sample is positive for botulinum neurotoxin, mice develop ruffled fur, a narrowed waist (due to respiratory failure), and muscle weakness.11 The toxin type can be determined by adding type-specific antitoxins to aliquots of the diluted sample; mice injected with a toxin-containing sample that also contains antitoxin do not exhibit symptoms of botulism.
The MBA has been used to detect BoNT in a wide variety of matrices, including serum, stool, vomitus, gastric content, enema wash with sterile water, food, tissue samples, and culture supernatants.6,10 Although few materials cause interference in the ability to detect toxin, various components (drugs, antibiotics) or competing organisms present in the sample can interfere with identification by causing nonspecific systemic effects after intraperitoneal injection into mice. In addition, fecal proteinases may degrade BoNT and lead to false-negative results.11 Regardless, the MBA is a highly robust test and is the only approved BoNT detection procedure in the United States for clinical samples.18
Although an effective laboratory procedure, the MBA is time-consuming because test animals must be monitored for up to 96 h.6 A single specimen requires as many as 10 mice because specimen aliquots must be incubated with different neutralizing antisera, and test samples (specimen plus antiserum) must be tested in duplicate. Frequently more than 45 samples are received in the botulism laboratory during an investigation of foodborne botulism involving only 3 or 4 patients, thereby requiring more than 450 animals at a cost of approximately $1600 for animals and per diem fees for 7 d (3 d of quarantine and 4 test days). In contrast, testing these same samples by ELISA would cost approximately $1000 only (an estimated 38% cost reduction), and test results would be available within 5 h (an estimated 95% reduction in time to test result). Most clinical laboratories would not have sufficient animal facility space to test this number of specimens concurrently, thus increasing outbreak laboratory response time, but such a laboratory could test at least 100 samples daily by ELISA (according to personnel at the Centers for Disease Control Botulism Diagnostic Laboratory). In addition, there is concern regarding ethical issues of the MBA and growing interest in alternative methods of BoNT detection.10
In vitro methods of BoNT detection include ELISA and mass spectrometry. With results available within hours, ELISA is more rapid than the MBA, and the technology is widely available in public health laboratories. However, the botulinum toxin ELISA detects only 4 toxin types, is less sensitive than the MBA, and demonstrates crossreactivity.18 Mass spectrometry is a rapid and sensitive in vitro assay that can detect the enzymatic activity of the toxin. However, the required equipment is expensive, matrix effects are unknown, and detection currently is limited to known toxin types.10 The mechanism of action of BoNT on neuronal cells involves several steps, including binding to the cell, translocation across the cell membrane, and enzymatic cleavage of one of the SNARE proteins involved in the release of acetylcholine, which involved in muscle contraction.15 No in vitro method developed so far has been proven to be a complete replacement for the MBA because none incorporates all mechanisms of BoNT action. Laboratories still must confirm results of in vitro methods by using the MBA.10 So although in vitro methods can be used to reduce the number of animals required for testing, these methods do not eliminate the requirement for the MBA in clinical laboratories investigating botulism cases. Other investigators have developed ex vivo methods and nonlethal in vivo animal models.10 The ex vivo methods still require animals, but the laboratory procedures are considered more humane because tests are not performed on live animals. However, because they require nonstandard equipment and skills not readily available at state health departments, these procedures are impractical for clinical laboratories investigating botulism outbreaks.
Measurement of BoNT by local paralysis effect was first demonstrated by intramuscular injection in the gastrocnemius muscle of mice.17 These studies, as well as those in chicks, inspired research on the therapeutic treatment of strabismus in nonhuman primates, in which picogram quantities of BoNT led to long-lasting paralysis of a single targeted muscle with no systemic side effects.13 This pivotal study led to approval of BoNT for human therapeutic use.12 Recent studies have targeted the mouse inguinocrual muscle14 and rat extensor digitorum longus (EDL) muscle2,3 as alternatives to the traditional MBA for toxin potency testing and for the investigation of potential therapeutic treatment of botulism. In the mouse inguinocrual model, the level of paralysis is measured by assigning a visually determined score; this model and assessment method was found to equal the accuracy and precision of traditional mouse model potency measurement. However, score assessment varied between observers.14 Previous studies involving injection of 1 mLD50 into the EDL muscle in rats demonstrated that toxin levels sufficient to cause death in intraperitoneally injected mice remained localized and did not cause systemic effects.2,3 The absence of systemic effects after injection of BoNT into the rat EDL muscle suggests that adaptation of this method to a variety of sample matrices may provide a refinement to the MBA by eliminating systemic effects that cause animal stress. This approach to refinement of the MBA could be transferred to many laboratories, because unique equipment would not be needed and many state health departments currently have staff with laboratory animal handling experience. However, the use of rats would be cost-prohibitive for routine use by state health department laboratories due to increased costs of animals and larger housing requirements.
We present here a nonlethal BoNT detection assay that is based on previous work in rats2,3 and targets the mouse EDL muscle. The use of a mouse model that reduces or eliminates systemic effects, which cause stress to animals, would provide a much needed refinement to the traditional MBA yet maintain positive aspects of BoNT detection, including (1) incorporating all known mechanisms of BoNT action and (2) retaining the ability to detect not-yet discovered toxin serotypes. Paralysis of the EDL muscle results in the absence of a reflex toe spread, which can be assessed visually. Because visual assessment can vary between observers, we measured and scored toe spread from photographs taken before and after injection of BoNT. For this study, we used BoNT type A because it is the most common serotype in human botulism cases.16 Our hypothesis for this study is that the toe-spread reflex model in rats can be adapted to mice and that by using an objective, quantifiable measure of toe spread after targeted injection of the mouse EDL muscle, this method can be used to identify BoNT type A in samples submitted to clinical laboratories investigating botulism outbreaks.
Materials and Methods
Animals.
This study was conducted in an AAALAC-accredited facility at the Centers for Disease Control and Prevention (Atlanta, GA). All procedures conformed to policies and procedures approved by the facility's IACUC and to the Guide for the Care and Use of Laboratory Animals.9 Female Crl:CD1(ICR) outbred mice (Mus musculus) (age, 4 to 6 wk) were purchased from Charles River Laboratories (Kingston, NY) and Taconic Farms (Germantown, NY). Health screening was performed on indirect sentinels on a quarterly rotation, according to vendor reports. At the time of this study, the colony was known to be free of Sendai virus, pneumonia virus of mice, mouse minute virus, mouse parvovirus, lymphocytic choriomeningitis virus, ectromelia virus, Salmonella spp., Streptococcus moniliformis, Staphylococcus aureus, Streptococcus pneumoniae, cilia-associated respiratory bacillus, Clostridium rodentium, Corynebacterium kutscheri, Helicobacter bilis, Helicobacter spp., and ectoparasites. In addition, the colony was free of helminths, Giardia spp., Spironucleus spp., and other protozoa. Mice were group-housed at 5 per cage, under 12:12-h light:dark cycles in static polycarbonate microisolation rodent cages on paper chip bedding (Shepherd Specialty Papers, Watertown, TN). Water and rodent chow (Lab Diet 5001, PMI, St Louis, MO) were available ad libitum with supplemental food enrichment (Bonanza Bounti-Buffet, Hartz Mountain Corporation, Secaucus, NJ). Cotton nesting squares (Nestlets, Ancare, Bellmore, NY), Shepherd Shacks (Pharmaserve, Framingham, MA), and rodent houses (Igloos, Bioserve, Frenchtown, NJ) were provided as environmental enrichment. Room temperature and humidity were maintained at 64 to 79 °F (17.8 to 26.1 °C) and 30% to 70%, respectively. Cage changes were performed twice weekly, and mice were observed at photography time points for any clinical signs in addition to left foot paralysis.
Toxin.
Purified dichain BoNT type A was purchased from Metabiologics (Madison, WI), the concentration of which was 1 mg/mL (2.2 × 108 LD50/mL), as determined by the manufacturer. Stock BoNT toxin was diluted in gelatin phosphate-buffered collecting fluid (GBS) to 0.33 LD50/µL (1 LD50 per mouse; 4.3 pg per mouse), as previously used.2,3 This solution was 2fold serially diluted with GBS to obtain the following toxin levels: 0.25 LD50/µL (0.75 LD50 per mouse, 3.2 pg per mouse), 0.17 LD50/µL (0.50 LD50 per mouse, 2.2 pg per mouse), 0.08 LD50/µL (0.25 LD50 per mouse, 1.1 pg per mouse), and 0.04 LD50/µL (0.125 LD50 per mouse, 0.5 pg per mouse).
Intramuscular injections.
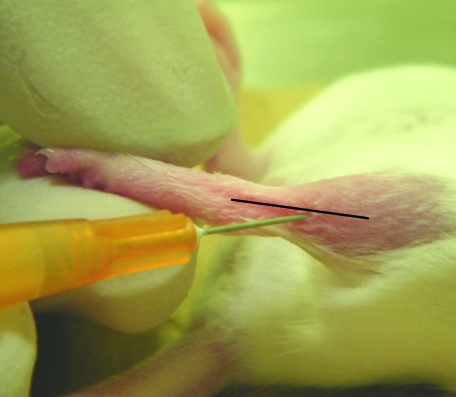
All mice were anesthetized with 5% isofluorane (Isothesia, Butler Animal Health Supply, Dublin, OH) and maintained with 3% isofluorane by using a rodent anesthesia unit. Mice were placed in dorsal recumbency while anesthetized. The left and right hindlimbs were shaved and sprayed with 70% ethanol. The foot to be injected was held so that the leg was straight. Each sample was loaded into a 10-µL Hamilton syringe with a 26-gauge disposable needle and injected on the lateral aspect of the leg, proximal to the hock joint, at an approximate 45° angle (Figure 1).
Figure 1.
Injection of the EDL muscle. The injection is made near the lateral aspect of the leg, distal to the patella, at an approximate 45° angle.
Determination of toxin detection range.
Mice were injected with 3 µL GBS in the right EDL muscle and 3 µL BoNT type A in the left EDL muscle; 5 mice each were injected with 0.125 LD50, 5 mice each received 0.25 LD50, 10 mice each received 0.5 LD50, 10 mice each were injected with 0.75 LD50, and 15 mice each received 1 LD50.
Neutralization assays.
Antitoxin–toxin mixture was prepared by adding 1 µL C. botulinum Type A Horse Serum A (Lot 00-0056L, Centers for Disease Control) to 30 µL BoNT type A (0.33LD50/µL); 5 mice each were injected in the left EDL muscle with 3 µL of the antitoxin–toxin mixture (1 LD50 per mouse; 4.3 pg per mouse) and with 3 µL GBS in the right EDL muscle. In addition, 5 control mice were injected in the left EDL muscle with 3 µL BoNT type A (1 LD50 per mouse) and with 3 µL GBS in the right EDL muscle.
Determination of matrix effects.
Normal human pooled serum was purchased from Fisher BioReagents (Pittsburgh, PA), and milk was purchased from a local grocery store. Milk was prepared according to standardized procedure.6 Spiked serum and milk were prepared by adding 1 µL BoNT type A (220 LD50/µL) to 666 µL serum or milk. Each of 5 mice was injected in the left EDL muscle with 3 µL of the spiked sample (1 LD50 per mouse; 4.3 pg per mouse) and with 3 µL GBS in the right EDL muscle; 5 control mice were injected in the left EDL muscle with 3 µL unspiked sample and with 3 µL GBS in the right EDL muscle.
Measurement of toe spread.
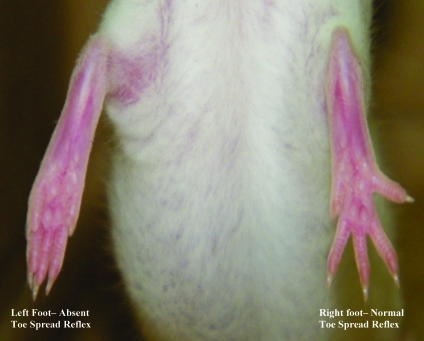
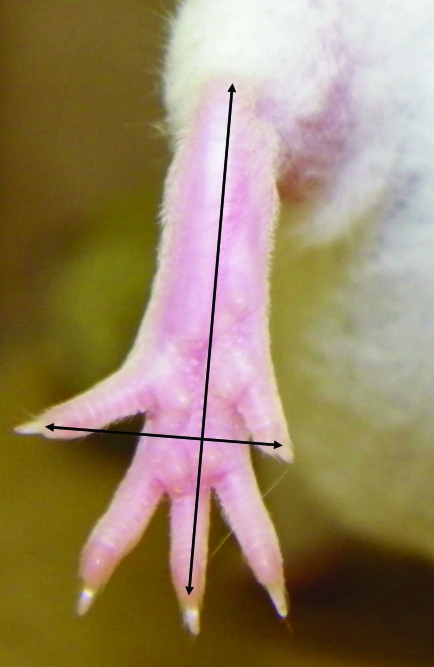
The toe-spread reflex was observed by lifting the mouse by the base of the tail, with the hindlimbs hanging free, and allowing the forelimbs to rest on the workbench. This positioning caused the mice to extend their hindlimbs and spread the toes. To obtain the photographs, mice were restrained in a holding device, and the camera was positioned directly outside of the device. The photographs were taken by the same person throughout the duration of the study. Toe spread was assessed at 0, 8, and 24 h by direct observation and documented by obtaining at least 10 photographs per mouse. Figure 2 illustrates normal (right foot) and absent (left foot) toe-spread reflex. Photographs were analyzed by using ImageJ software (http//imagej.nih.gov).1 The width of the foot was defined as the distance between the tips of the first and fifth toes; the length of the foot was defined as the distance from the heel to the tip of the middle toe (Figure 3). Toe-spread ratios were calculated by dividing foot width by foot length. Left and right width–length ratios were obtained by averaging toe-spread ratios from at least 10 photographs, to further analyze data and compare results among all injected mice. Right width–length ratios at 0, 8, and 24 h were divided by the corresponding left width–length ratios to yield right–left ratios at each time point. The effective paralysis score (EPS) at 8 or 24 h was calculated by subtracting the right–left ratio at 8 or 24 h, respectively, from that at 0 h. Clinical observation was used to establish the EPS that differentiated toxin-injected from nontoxin-injected mice; an EPS greater than 0.30 was considered to be a positive result (that is, toe-spread reflex absent).
Figure 2.
Loss of toe-spread reflex in the left foot after injection with botulinum toxin type A. The toe-spread reflex is present in the right foot after injection with GBS.
Figure 3.
Measurement of the toe-spread reflex. The width of the foot was defined as the distance between the tips of the first and fifth toes; the length of the foot was defined as the distance from the heel to the tip of the middle toe.
Euthanasia.
Immediately after the 24-h photographs were obtained, mice were euthanized by CO2 inhalation followed by cervical dislocation.
Statistical analysis.
Toe-spread ratios at 0, 8, and 24 h were obtained from at least 10 photographs per animal and SD calculated using Excel 2007 (Microsoft, Redmond, WA). Differences among ratios were examined by using the Mann–Whitney test at a significance level of 0.05.
Results
Determination of toxin detection range.
Groups of mice were injected in the left EDL muscle with varying levels of BoNT type A (1, 0.75, 0.5, 0.25, and 0.125 LD50) to determine the concentration range at which the toxin could be detected; no systemic effects were observed at these concentrations of BoNT. GBS was injected in the right EDL muscle as a control. Right and left foot width–length and right–left ratios, used to calculate EPS, were obtained by averaging toe-spread ratios from at least 10 photographs of each mouse; the SD for these ratios were all lower than 0.09.
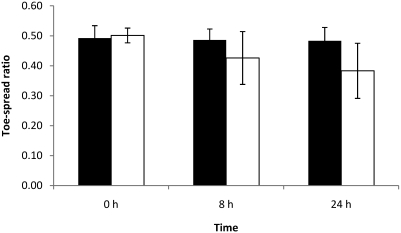
Absence of toe-spread reflex at 24 h was observed in 12 of the 15 mice injected with 1 LD50. In addition, 8 mice of those 12 mice also showed loss of toe-spread reflex at 8 h. Animals with observable loss of toe-spread reflex had EPS at 8 and 24 h that were greater than 0.30 (data not shown). At this toxin dose, the left width–length ratio was significantly (P < 0.05) lower than the right width–length ratio at both 8 and 24 h (Figure 4).
Figure 4.
Right (black column) and left (white column) width–length ratios at 0, 8, and 24 h of mice each injected with 0.125 LD50 BoNT type A. Left EDL muscles were injected with 3 µL BoNT type A, and right EDL muscles were injected with 3 µL GBS. Differences between right and left width–length ratios were not significant.
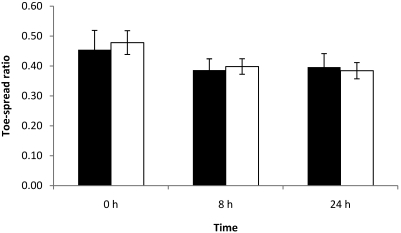
Two of the 10 mice injected with 0.75 LD50 showed loss of toe-spread reflex at both 8 and 24 h, and the corresponding EPS of these mice were greater than 0.30 (data not shown). In mice that retained a toe-spread response, left and right width–length ratios remained constant at all time points, and EPS were lower than 0.30. Differences between left and right width–length ratios were not statistically significant at any time point for this dose level (Figure 5).
Figure 5.
Right (black column) and left (white column) width–length ratios at 0, 8, and 24 h of mice each injected with 0.25 LD50 BoNT type A. Left EDL muscles were injected with 3 µL BoNT type A, and right EDL muscles were injected with 3 µL GBS. Differences between right and left width–length ratios were not significant.
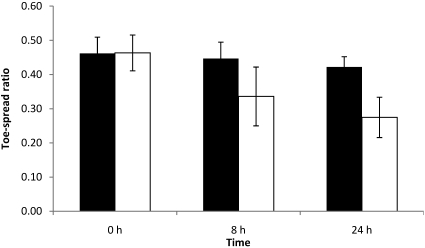
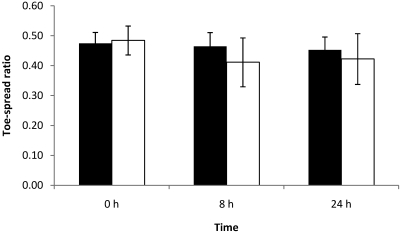
The toe-spread reflex at 24 h was reduced or absent in 5 of the 10 mice injected with 0.5 LD50; 3 of these 5 mice had abnormal spread reflexes at 8 h as well. Left width–length ratios were significantly (P < 0.05) lower than right width–length ratios at 24 h (Figure 6); the EPS at 8 and 24 h of the mice with an abnormal toe-spread reflex were greater than 0.30 (data not shown).
Figure 6.
Right (black column) and left (white column) width–length ratios at 0, 8, and 24 h of mice each injected with 0.5 LD50 BoNT type A. Left EDL muscles were injected with 3 µL BoNT type A, and right EDL muscles were injected with 3 µL GBS. At 24 h, left width–length ratios were significantly (*, P < 0.05) lower than were right width–length ratios.
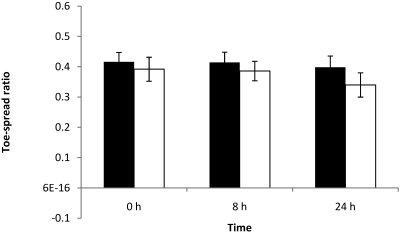
None of the 5 mice injected with 0.25 LD50 showed any effect in the toe-spread reflex (Figure 7). Furthermore, all width–length ratios remained constant among time points, and EPS at 8 and 24 h did not exceed 0.30 (data not shown).
Figure 7.
Right (black column) and left (white column) width–length ratios at 0, 8, and 24 h of mice each injected with 0.75 LD50 BoNT type A. Left EDL muscles were injected with 3µL BoNT type A, and right EDL muscles were injected with 3 µL GBS. Differences between right and left width–length ratios were not significant.
Only 1 of the 5 mice injected with 0.125 LD50 showed an abnormal toe-spread reflex at 24 h, with an EPS greater than 0.30. All 5 mice had normal responses at 8 h, and EPS at 8 and 24 h for all mice with normal responses were lower than 0.30. All width–length ratios for these mice remained equivalent, and differences were not statistically significant (Figure 8).
Figure 8.
Right (black column) and left (white column) width–length ratios at 0, 8, and 24 h of mice each injected with 1 LD50 BoNT type A. Left EDL muscles were injected with 3 µL BoNT type A, and right EDL muscles were injected with 3 µL GBS. Left width–length ratios were significantly (*, P < 0.05) lower at 8 and 24 h than were right width–length ratios.
Neutralization assay.
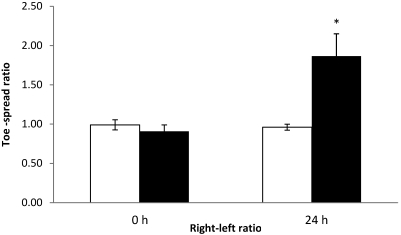
To investigate whether the neutralization effects of antitoxin would prevent paralysis of the EDL muscle, 5 mice were injected with toxin neutralized with antitoxin; 5 additional mice were injected with toxin only, as controls. As expected, all mice that received the antitoxin–toxin solution showed normal toe-spread reflex at 24 h, whereas all 5 control (toxin only) mice exhibited loss of toe-spread reflex. At 24 h, the right–left ratios of mice that received the antitoxin–toxin mixture were significantly (P < 0.05) different from those of toxin-only control mice (Figure 9); the SD for the width–length ratios (used to calculate the right–left ratios) were all lower than 0.07 (data not shown). In addition, the EPS at 24 h of toxin-only mice were all greater than 0.30, whereas those of the mice that received the neutralized toxin were all lower than 0.30 (data not shown).
Figure 9.
Right–left ratios at 0 and 24 h of mice injected with BoNT neutralized with antitoxin (white column) or with toxin only (control; black column). In the neutralized toxin group, the left EDL muscles were injected with 3 µL of the toxin–antitoxin mixture. In the control group, the left EDL muscles were injected with 3 µL BoNT type A at 1 LD50 per mouse. In both groups, the right EDL muscles were injected with 3 µL GBS. *, value differs significantly (P < 0.05) from that of the GBS control group.
Determination of matrix effects.
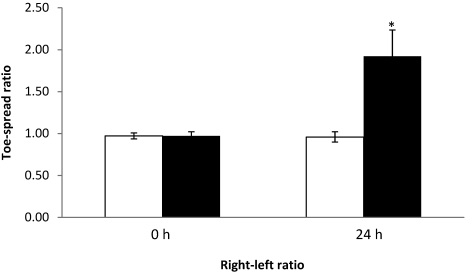
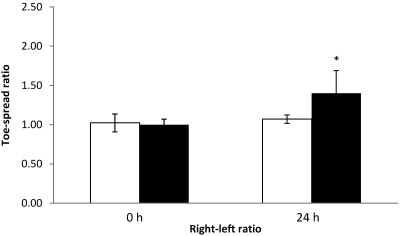
To investigate the effects of serum and milk on detection of BoNT, 5 mice each were injected with either serum or milk that had been spiked with 1 LD50 BoNT type A; an additional 5 mice were injected with serum or milk only, as controls. All 5 mice injected with spiked serum and 3 of the 5 mice injected with spiked milk exhibited loss of the toe-spread reflex at 24 h. All of the control mice had normal toe-spread responses. At 24 h, the right–left ratios of mice injected with spiked serum (Figure 10) or milk (Figure 11) were significantly (P < 0.05) different from those of respective control mice, and EPS at 24 h were greater than 0.30 in mice that received toxin (data not shown). The SD for all width–length ratios were lower than 0.06 (data not shown).
Figure 10.
Right–left ratios at 0 and 24 h of control mice injected with nonspiked serum (control, white column) and mice injected with serum spiked with BoNT (black column). In the nonspiked control group, the left EDL muscles were injected with 3 µL serum. In the spiked serum group, the left EDL muscles were injected with 3 µL of the spiked serum (1 LD50 per mouse). Both groups received GBS in the right EDL muscle. *, value differs significantly (P < 0.05) from that of the GBS control group.
Figure 11.
Right–left ratios at 0 and 24 h of control mice injected with nonspiked milk (white column) and mice injected with milk spiked with BoNT (black column). In the nonspiked control group, the left EDL muscles were injected with 3 µL nonspiked milk. In the spiked milk group, the left EDL muscles were injected with 3 µL of spiked milk (1 LD50 per mouse). Both groups received GBS in the right EDL muscle. *, value differs significantly (P < 0.05) from that of the GBS control group.
Discussion
Injection of BoNT type A dichain toxin into the EDL of mice caused localized paralysis of the hindfoot at 24 h (and at 8 h in some cases). Observations were not documented beyond the 24-h time point because the purpose of this study was to determine whether this refinement of the MBA could identify the presence of toxin within 24 h of injection. Localized paralysis was identified through clinical observation of mice's ability to use the foot and by objective measurement of the changes in the toe-spread reflex. A toxin level of 1 LD50 was identified more consistently than were lower toxin doses. Localized paralysis was prevented by pretreating toxin samples with type-specific antitoxin, thereby demonstrating that paralysis was specific to the presence of BoNT. Toxin was detected when in the presence of buffer, serum, or milk. Injection of these matrices alone did not cause any changes in the mice's ability to use their feet or in EPS, further demonstrating the specificity of this method. No systemic paralysis was observed, suggesting that the injected toxin remained localized to the injection site.
The MBA has been used to confirm botulism cases since the early 1900s and remains the standard method for these investigations.18 The MBA is used by both public health clinical laboratories and food regulatory agencies for both investigating outbreaks and establishing hazard control requirements for commercial food products.6,8 Alternative methods, including animal model refinements (ex vivo and more humane in vivo models), which reduce animal requirements, and in vitro methods, which eliminate dependence on animals, have been developed, but none has proven to be a complete replacement for the MBA or its associated toxin-potency test, the mouse neutralization assay.10 None of the studied alternatives incorporates all aspects of the mechanisms of action of BoNT, a metalloprotease, which include: (1) specific binding to the neuronal cell, (2) translocation across the membrane, and (3) enzymatic cleavage of the specific proteins responsible for the acetylcholine release required for muscle contraction.15 In addition, no alternative has been identified that can be used for the variety of samples matrices encountered in the botulism laboratory, including serum, stool cultures, and an endless list of food matrices including home-canned and commercial food products, all of which have been tested successfully by using the MBA.6 However, the MBA has several limitations. Although a positive test result is usually available within 24 h, low levels of toxin may require as long as 4 d. In addition, the MBA requires the use of large numbers of mice. Finally, the test can cause animal stress unless mice are euthanized before late-stage botulism, which involves respiratory insufficiency and failure.11
The toe-spread reflex model is comparable to the MBA in that a positive result is dependent on all components of BoNT action, including binding, translocation, and enzymatic activity to prevent release of acetylcholine. This dependence makes the toe-spread reflex model superior to in vitro alternatives to the MBA that address only a single functional component (for example, endopeptidase-based methods that only identify enzymatic activity) or that lack assessment of toxin activity (for example, ELISA). In addition, the toe-spread reflex model may be a superior alternative to methods that depend on antibodies to capture toxin prior to detection. These antibody-dependent methods can only be used to detect previously identified BoNT for which a sufficient supply of antibody is available; these alternative methods likely would fail to identify new BoNT serotypes. Potential, yet-to-be identified BoNT serotypes could be detected by the toe-spread reflex model, as with the MBA, because the paralytic effects of any BoNT would be recognized. Our studies demonstrated that the toe-spread reflex model could detect BoNT type A toxin in buffer, serum, or milk. Although additional studies are required, these results suggest that the toe-spread reflex model may be as robust as the MBA in limiting nonspecific matrix effects. The toe-spread reflex model may even be superior to the MBA in limiting nonspecific reactions, given that effects are restricted to a localized area close to the injection site, whereas some clinical specimens and food substances cause systemic nonspecific effects that can interfere with the detection of botulinum toxin by MBA. Additional studies with a larger variety of samples, including stool specimens and a variety of food samples, are needed to more fully understand potential matrix effects on the performance of this MBA refinement. We anticipate that the effect on the toe-spread reflex will be the same regardless of toxin serotype (that is, 1 LD50 of toxin serotype A equals 1 LD50 of toxin serotype B), because their mechanisms of action are equivalent.15,18 However, studies are needed to ensure that the time at which a change in the toe-spread reflex is observed doesn't vary among serotypes.
The MBA is dependent on observation by a skilled analyst of botulism signs that result from BoNT exposure in mice. Investigators previously used the toe-spread reflex model in rats to investigate small molecules with potential as therapeutic agents against botulism.2,3 In these studies, purified BoNT with a known, consistent concentration in a defined buffer matrix were injected into rats such that consistent observable effects of toxin could be identified. Previous studies also showed that measured differences between the width-to-length toe-spread ratio of rats and mice injected with toxin alone or with a potential toxin blocker could be used to identify possible therapeutic products.2,3 However, the variable level of BoNT in outbreak investigation samples and the variety of complex matrices makes clinical observation of minimal paralysis effects more difficult. Therefore dependence on clinical observation increases the requirement for highly skilled analysts who reliably can identify the subtle signs of paralysis, which may be concentration-dependent and can differentiate signs of botulism from those of illness not due to BoNT. Our study was designed to determine whether an objective measurement could be used to identify BoNT-injected mice, thereby potentially reducing dependency on clinical observations. We anticipated that foot width–length and right–left ratios would vary between mice, and these objective measurements were reproducible (coefficients of variation of 11% and 8%, respectively). In addition, variability within animals was low (SD < 0.08), suggesting that objective measurements may be useful in identifying the presence of toxin in samples. Clinical observation was used to establish the EPS (greater than 0.30) that differentiated between toxin-injected and nontoxin-injected mice. Overall, clinical observations correlated with the established minimal EPS. However, clinical observation records of 4 out the 60 mice tested indicated only partial paralysis with an EPS in excess of 0.30; EPS was less than 0.40 in 3 of these 4 mice. Furthermore, 2 of these results occurred at the 8-h time point and the other 2 at 24 h. Regardless, the combined results suggest that objective measurement of mouse toe spread is a reliable indicator of BoNT at the 1 LD50 concentration in the injected sample and may indicate early effects of toxin not easily identified by clinical observation alone.
Most (80%) of mice injected with 1 LD50 (4.3 pg) had a paralytic effect in the injected hindfoot at 24 h, as demonstrated by both clinical observation and objective measurement (EPS) of the ability of the animal to spread the toes. Most (67%) of the mice with positive EPS at 24 h were also positive at 8 h. Having additional affected mice at the 24-h time point was not unexpected, because previous studies with this method demonstrated that increasing the study time length can improve identification of toxin.2,3 A high number (50%) of mice were paralysis-positive when they received 0.50 LD50, but minimal to no effect was observed in mice injected with 0.75 LD50, 0.25 LD50, or 0.125 LD50. Why fewer animals injected with 0.75 LD50 had a positive EPS than did those injected with 0.50 LD50, even after repeated testing, is unclear. The 2 mice with a positive EPS at 24 h after injection of 0.75 LD50 were also positive at 8 h. This result suggests that the level of toxin in the samples was sufficient for a paralysis effect in the toe-spread reflex model and that perhaps the delivery of toxin into the muscle is variable. Our study used blind injection into the mouse EDL muscle. Although the hair was shaved from the injection site, this muscle is very difficult to visualize, and injection errors can occur. In previous studies,2,3 the rat EDL was revealed through an incision before injection. This incision was necessary to obtain absolute accuracy of the injection site to evaluate potential therapeutic compounds. Our study was designed to assess the usefulness of the toe-spread reflex model in a clinical laboratory investigating botulism outbreaks. The amount of time required to reveal the muscle in animals would be prohibitive in an outbreak investigations setting. In addition, the use of rats, with their larger EDL target, instead of mice would make tests costs too high. Further studies are needed to determine whether changes can be made to the blind injection technique to improve accuracy so that a lower amount of toxin can be detected reliably.
BoNT potency is defined by the MBA, and by definition, the limit of detection is 1 LD50. This value represents the results of serial dilutions of BoNT in which each dilution is injected (intraperitoneally) into 8 to 10 mice. 1 LD50 is defined as the amount of toxin that produces botulism in 50% of the mice at a single dilution (for example, if 10 mice were injected with 1 LD50, only 5 would be expected to show signs of botulism).8 The weight of toxin that is in 1 LD50 varies with the purity of the toxin (150 kDa for the dichain to 900 kDa for the complex) and its specific production lot. 1 LD50 of dichain toxin is equivalent to approximately 5 pg;10 1 LD50 for the toxin used in the current study was equivalent to 4.3 pg. In our study, a toxin level of 1 LD50 consistently caused hindfoot paralysis in mice; therefore the toe-spread reflex model is equivalent to the MBA when actual toxin levels are compared. However, in the MBA, a much larger sample volume (usually 0.5 mL) that that we used is injected into each of 2 mice, resulting in a minimal detectable sample toxin concentration of 1 LD50/mL. The injection volume used in our study was 3 µL, so that the minimal sample toxin concentration reproducibly detected was 333 LD50/mL, which was the lowest concentration of toxin previously injected into the rat EDL.2 The lowest concentration of toxin that we detected was 83 LD50/mL (0.25 LD50), but the paralysis effect was not consistent in all animals injected with this level of toxin. Consistent detection of toxin at 333 LD50/mL is adequate for most foods and cultures commonly encountered in an outbreak investigations laboratory; however, toxin levels in serum are frequently less than 125 LD50/mL and often approach 1 LD50/mL. One explanation of the less-than-desired limit of detection we obtained may be related to how a positive EPS measurement was determined. The assigned minimum EPS of 0.30 was based on clinical observations of the spread of the toes and on any observable loss of test mice's ability to use the foot. Further studies using samples blinded to the investigator are needed to better define the minimum EPS that can predict the presence of toxin perhaps even before clinical observations identify changes in the mouse's ability to use the foot.
In conclusion, the results of the current study suggest that the toe-spread reflex model is a viable alternative to the MBA for botulism investigations, including determining toxin serotype. This method incorporates all components of the mechanisms of action of BoNT and provides results within 24 h. The toe-spread reflex method provides a refinement to the standard MBA by minimizing stress in mice, because systemic effects of BoNT injection did not occur. Additional studies are needed to improve the limit of detection before the toe-spread reflex method can be recommended for routine use.
Acknowledgments
We thank Dr S Deshpande (USAMRICD) and Dr James Apland (USAMRICD) for demonstrating the technique for local injection of the EDL muscle and eliciting of the toe-spread reflex and Ms Fiona Brabazon (USAMRICD) for her technical assistance with photographing hindlimbs and use of ImageJ software. We also thank Dr Nathaniel Powell (CDC), Ms Yvonne Reed (CDC), and Ms Abiola Aminu for their assistance in this protocol.
This publication was supported by funds made available from the Office for Public Health Preparedness and Response (Centers for Disease Control and Prevention, Atlanta, GA). Additional funds were made available from the CDC Laboratory Animal Medicine Residency Program, Division of Scientific Resources, National Center for Emerging Zoonotic and Infectious Disease. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- 1.Abramoff MD, Magelhaes PJ, Ram SJ. 2004. Image processing with ImageJ. Biophotonics International 11:36–42 [Google Scholar]
- 2.Adler M, Keller JE, Sheridan RE, Deshpande SS. 2001. Persistence of botulinum neurotoxin A demonstrated by sequential administration of serotypes A and E in rat EDL muscle. Toxicon 39:233–243 [DOI] [PubMed] [Google Scholar]
- 3.Adler M, MacDonald DA, Sellin LC, Parker GW. 1996. Effect of 3, 4-diaminopyridine on rat extensor digitorum longus muscle paralyzed by local injection of botulinum neurotoxin. Toxicon 34:237–249 [DOI] [PubMed] [Google Scholar]
- 4.Arnon SS, Schecter R, Inglesby MD, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Hauer J, Layton M, Lillibridge S, Osterholm MT, Otoole T, Parker G, Perl T, Russell PK, Swerdlow DL, Tonat K. 2001. Botulinum toxin as a biological weapon: medical and public health management. J Am Med Assoc 285:1059–1070 [DOI] [PubMed] [Google Scholar]
- 5.Arnon SS, Schecter R, Maslanka SE, Jewell NP, Hatheway CL. 2006. Human botulism immune globulin for the treatment of infant botulism. N Engl J Med 354:462–471 [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention 1998. Botulism in the United States, 1899–1996: handbook for epidemiologists, clinicians, and laboratory workers. Atlanta (GA): Centers for Disease Control and Prevention. [Google Scholar]
- 7.Centers for Disease Control and Prevention [Internet] 2010. Investigational heptavalent botulinum antitoxin (HBAT) to replace licensed botulinum antitoxin AB and investigational botulinum antitoxin E. Morbidity and Mortality Weekly Report (MMWR). [Cited 25 September 2010]. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/mm5910a4.htm [PubMed]
- 8.Food and Drug Administration. [Internet] 2001. Bacteriological analytical manual. Chapter 17. Clostridium botulinum. [Cited 13 September 2010]. Available at: http://www.fda.gov/Food/ScienceResearch/LaboratoryMethods/BacteriologicalAnalyticalManualBAM/UCM070879
- 9.Institute for Laboratory Animal Research 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 10.Interagency Coordinating Committee on the Validation of Alternative Methods. [Internet] 2008. Report on the ICCVAM–NICEATM/ECVAM scientific workshop on alternative methods to refine, reduce, or replace the mouse LD50 assay for botulinum toxin testing. [Cited 25 September 2010]. Available at: http://iccvam.niehs.nih.gov/methods/biologics/bot_workshop.htm
- 11.Lindström M, Korkeala H. 2006. Laboratory diagnostics of botulism. Clin Microbiol Rev 19:298–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott AB. 1981. Botulinum toxin injection of eye muscles to correct strabismus. Trans Am Ophthalmol Soc 79:734–770 [PMC free article] [PubMed] [Google Scholar]
- 13.Scott AB. 2004. Development of botulinum toxin therapy. Dermatol Clin 22:131–133 [DOI] [PubMed] [Google Scholar]
- 14.Sesardic D, Mclellan K, Ekong TA, Das RG. 1996. Refinement and validation of an alternative bioassay for potency for potency testing of therapeutic botulinum type A toxin. Pharmacol Toxicol 78:283–288 [DOI] [PubMed] [Google Scholar]
- 15.Simpson LL. 2004. Identification of the major steps in botulinum toxin action. Annu Rev Pharmacol Toxicol 44:167–193 [DOI] [PubMed] [Google Scholar]
- 16.Sobel J, Tucker N, Sulka A, McLaughlin J, Maslanka S. 2004. Foodborne botulism in the United States, 1990–2000. Emerg Infect Dis 10:1606–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugiyama H, Brenner SL, Dasgupta BR. 1975. Detection of Clostridium botulinum toxin by local paralysis elicited with intramuscular challenge. Appl Microbiol 30:420–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu PA, Maslanka SE, St Louis ME, Swerdlow DL. 2009. Botulism, p 159–176 : Abrutyn E, Brachman PS. Bacterial infections of humans, 4th ed New York (NY): Springer [Google Scholar]