Abstract
The mechanisms by which cells spontaneously immortalized in tissue culture develop the capacity to form tumors in vivo likely embody fundamental processes in neoplastic development. The evolution of Madin–Darby canine kidney (MDCK) cells from presumptively normal kidney cells to immortalized cells that become tumorigenic represents an example of neoplastic development in vitro. Studies of the mechanisms by which spontaneously immortalized cells develop the capacity to form tumors would benefit from quantitative in vivo assays. Most mechanistic correlations are evaluated by using single-dose tumor-induction experiments, which indicate only whether cells are or are not tumorigenic. Here we used quantitative tumorigenicity assays to measure dose- and time-dependent tumor development in nude mice of 3 lots of unmodified MDCK cells. The results revealed lot-to-lot variations in the tumorigenicity of MDCK cells, which were reflected by their tumor-inducing efficiency (threshold cell dose represented by mean tumor-producing dose; log10 50% endpoints of 5.2 for vial 1 and 4.4 for vial 2, and a tumor-producing dose of 5.8 for vial 3) and mean tumor latency (vial 1, 6.6 wk; vial 2, 2.9 wk; and vial 3, 3.8 wk). These studies provide a reference for further characterization of the MDCK cell neoplastic phenotype and may be useful in delineating aspects of neoplastic development in vitro that determine tumor-forming capacity. Such data also are useful when considering MDCK cells as a reagent for vaccine manufacture.
Abbreviations: ATCC, American Type Culture Collection; MDCK, Madin–Darby canine kidney; TPD50, tumor-producing dose log10 50% endpoint
Evolution of immortalized cell populations growing in tissue culture to cells that become tumorigenic represents an accumulation of cellular phenotypic changes and selection for increased neoplastic traits. Characterizing the phenotypes expressed by immortalized (neoplastically transformed) cells growing in tissue culture, understanding the processes of neoplastic development as they occur during in vitro passage,6,30 and characterizing the nature of a tumorigenic phenotype can provide fundamental insights into the biology of neoplasia. The evolution of MDCK cells from presumptively normal kidney cells to immortalized cells that become tumorigenic is an example of neoplastic development in vitro. Toward the goal of understanding neoplastic development in vitro, we are characterizing the tumorigenic phenotype expressed by Madin–Darby canine kidney (MDCK) cells. The data presented here outline a quantitative analysis of the tumor-forming capacity exhibited by unmodified commercially obtained MDCK cells.
The establishment of the MDCK cell line in 1958 at the Naval Research Laboratory (University of California, Berkeley) was not described in a published report, although the methods used by that laboratory to establish mammalian cells in tissue culture and the application of these methods to establish 2 other cell lines, Madin–Darby bovine kidney cells and Madin–Darby ovine kidney cells, were the subjects of independent publications.17,18 At tissue-culture passage 49, the MDCK cell line was submitted to the Registry of Animal Cell Lines Certified by the Cell Culture Collection Committee (First Edition, 1964), the forerunner of the American Type Culture Collection (ATCC), to become Certified Cell Line 34. The description and the conditions used in the passage of the MDCK cells that were submitted to the ATCC as well as a report by Gaush and colleagues4 represent the information available on the establishment, early passage history, and karyotype of MDCK cells.
Over the years, there have been conflicting data regarding the tumorigenic capacity of MDCK cells. MDCK cells intravenously inoculated into chicken embryos formed tumors in the embryonic brain and nodules on the chorioallantoic membrane.12 However, these cells did not form progressively growing tumors in adult BALB/c nude mice,33 although viable MDCK cells persisted for as long as 2 mo at the injection site. In contrast, newborn BALB/c nude mice inoculated with MDCK cells developed nodules that expanded until the pups attained an adult body mass, at which point nodule expansion ceased; whether the nodules in these maturing mice resolved was not reported.33 Histology revealed that nodules that had developed from MDCK cells in newborn nude mice were adenocarcinomas.28 Their apparent immortality and reportedly nontumorigenic phenotype (at least in nude mice) led to the use of MDCK cells in studies addressing alteration of the neoplastic cell phenotype.1,8,10,11,23,35,38
In other studies, MDCK cells that were adapted to grow in suspension or on microcarrier beads formed tumors in nude mice. The MDCK cells adapted to grow in suspension formed tumors with doses of as few as 10 cells per mouse, whereas MDCK cells adapted to grow on microcarrier beads required doses of 105 (100,000) cells to form tumors in athymic nude mice.20,39,40,41 Notably, the dose of cells inoculated did not correlate with the incidence of tumors resulting from either of these MDCK cell derivatives. Furthermore, some mice failed to develop progressively growing tumors, and several tumor nodules that initially developed at the inoculation site regressed.
These variations in the reported tumor-forming capacities of MDCK cells prompted us to undertake a detailed evaluation of the capacity of MDCK cells to form tumors in vivo by using quantitative tumor-induction comparisons. The results showed that cells from 3 different lots of unmodified MDCK cells (obtained from the ATCC in 2004, 2006, and 2007) were all tumorigenic in athymic nude mice but that the tumor-forming capacity and tumor latency of the cells differed between lots. These data indicate that the well-known diversity of MDCK cell populations extends to differences in their tumorigencity and provides reference information that may be useful for future characterization of different MDCK cell lots and subclones. Such data provide the basis for a variety of purposes including studies of the biologic and molecular characteristics of the MDCK cell neoplastic phenotype as well as the evaluation of MDCK cells as reagents for vaccine manufacture.
Materials and Methods
Cell lines.
We obtained 3 vials of MDCK cells (NBL2 catalog no., CCL34) from ATCC (Manassas, VA). Vial 1 (lot no. 3563161; frozen 30 January 2004) was received at passage 55 and maintained in DMEM (Mediatech, Manassas, VA) supplemented with 10% FBS (lot no. AQG24425, ARB25716, ARB25880, ASE29663, or ASL31024; Hyclone, Logan, UT) and 2 mM L-glutamine (Mediatech; DMEM10). Vials 2 (lot no. 4398972; frozen Jan 20, 2006) and 3 (lot no. 7643577; frozen July 20, 2007) were received at passages 56 and 55, respectively, and maintained in ATCC-formulated EMEM supplemented with 10% FBS (EMEM10). Each of these cell lots was established at the ATCC by independent passage of cells from cell banks (‘token’ lots) generated from the original MDCK cell line for the purpose of preparing cell lots for commercial distribution. The lots represented by vials 1 and 2 were developed from the same token lot that was established at passage 52; vial 3 cells were established from a different token lot that was also at passage 52. Cells obtained from the ATCC were recovered by thawing in a 37 °C waterbath, expanded through 2 or 3 passages in culture, and frozen in liquid nitrogen (in culture medium with 7.5% DMSO; approximately 2 × 106 cells per vial) as small working cell banks. The cells in these 3 lots were shown to be free of 31 rodent agents and Mycoplasma species (Impact VIII PCR profile, Research Animal Diagnostic Laboratory, Columbia, MO). The cells were confirmed to be of canine origin by PCR using primers that recognize canine short interspersed elements.3 In addition, modal chromosome numbers obtained through cytogenetic analysis (Cell Culture Characterization Service, Orion Township, MI) were consistent with the cells being MDCK cells.
For in vivo assays, MDCK cells were propagated in 150 cm2 canted neck, vented flasks (catalog no. 430825, Corning Incorporated, Corning, NY) to confluence (2 × 107 cells per flask). To harvest cells, monolayers were washed twice with PBS (Mediatech) and trypsinized by incubation in 6 mL 0.25% trypsin with 0.53 mM EDTA at 37 °C. After the reaction was stopped by adding 6 mL DMEM10 or EMEM10, cell suspensions from individual flasks were combined, washed twice with PBS, and resuspended in 10 mL PBS. A 100-μL sample was taken from this cell suspension, diluted 1:20, and 20 to 25 μL was used to determine the viable cell concentration (Cellometer Auto T4, Nexcelom Bioscience, Lawrence, MA). Cells were brought to a concentration of 1 × 108 cells/mL in PBS. Nude mice were each inoculated with 0.1 mL (1 × 107 cells) of this cell suspension. For dose–response assays to determine the tumor-forming capacity of MDCK cells, the 1 × 108 cells/mL cell suspension was diluted 10-fold serially in PBS.
Animal studies.
Homozygous (nu/nu) female athymic nude mice (age, 4 to 6 wk) were obtained from the National Cancer Institute Animal Production Program (Frederick, MD). BALB/c nude mice (age, 4 to 6 wk) were obtained from Charles River Laboratories (Wilmington, MA). For experiments with newborn nude mice, heterozygous (nu/+) pregnant mice were obtained from the National Cancer Institute at approximately 19 d gestation. All mice were maintained on autoclaved water and γ-irradiated bedding and food (Prolab Isopro RMH 3000, PMI International, Brentwood, MO) in Micro-VENT caging systems (Allentown Inc., Allentown, NJ) on 12:12-h light:dark cycles at a room temperature of 18 to 22 °C. Husbandry procedures met all of the recommendations of the Guide for the Care and Use of Laboratory Animals.7 Adult mice were inoculated subcutaneously above the scapulae or by intraperitoneal injection with 0.1 mL cells in PBS. Newborn mice were inoculated subcutaneously over the scapulae with 0.1 mL cells in PBS between 24 and 48 h after birth. Control animals received 0.1 mL PBS. Mice were examined daily, and tumor development was monitored weekly for 6 mo. Tumor size was estimated visually (for tumors 10 mm and smaller) or determined in its maximal dimension by using calipers (for large tumors). Mice were euthanized and necropsied when tumors reached approximately 20 mm in any dimension (for subcutaneously inoculated mice) or when abdominal distention was noted (for intraperitoneally inoculated mice). The use of animals for these studies was approved by the Center for Biologics Evaluation and Research Animal Care and Use Committee.
Necropsy.
Tumor-bearing mice were euthanized by CO2 inhalation and topically disinfected by using at least 3 alternating washes with Wescodyne (Steris, St Louis, MO) and 70% ethanol. Tumors and major organs (spleen, kidney, liver, lungs, heart and mediastinum) were removed and fixed in 10% formalin. All formalin-fixed tissue samples were sent to American HistoLabs (Gaithersburg, MD) for paraffin embedding, sectioning, and staining with hematoxylin and eosin.
Data processing.
The tumorigenicity of MDCK cells was evaluated by measuring 2 independently expressed parameters that represent the tumorigenic phenotype: tumor-forming capacity and tumor latency.14 Tumor-forming capacity is defined by quantitative, dose–response, tumorigenicity assays that yield the tumor-producing dose as log10 50% endpoint (TPD50; estimated by the Spearman–Karber method22) values. TPD50 values represent the limiting or threshold dose of cells that form tumors in 50% of the animals. Tumor-incidence data used to estimate the TPD50 values for the cells from vials 1, 2, and 3 were obtained weekly and recorded in spread sheets (Excel, Microsoft, Redmond, WA). To compare tumor-forming capacity of the cell lines, mean TPD50 values obtained after 26 wk of observation were compared by a 2-tailed Student t test by using GraphPad Prism 4 (GraphPad Software, La Jolla, CA).
Tumor latency is the time to tumor appearance.14 Because the contribution of the tumor-latency trait to the neoplastic cell tumorigenic phenotype is not well established, we used 2 different methods to compare tumor latency. In one method, tumor-incidence data obtained weekly for cohorts of mice challenged with 10-fold serial dilutions of MDCK cells and used to estimate the TPD50 values were converted to survival curves14 by using GraphPad Prism 4. Because the occurrence of a tumor is a terminal event, these survival curves, which represent average percentage survival, also represent the average time to tumor appearance (tumor latency) at weekly intervals. Differences between the survival curves can be estimated by Kaplan–Meier analysis. Because the survival curves are derived from the tumor-incidence data (used to determine the TPD50) for cells from each vial, these curves characterize their tumorigenic phenotype14 as determined in the adult, athymic, nude mouse model.
The other method to evaluate tumor latency was to determine the time from inoculation to tumor appearance (in weeks) for each mouse in the dose–response assays. Average times to tumor appearance were estimated for each cell dose by dividing the total number of weeks (sum of number of weeks for each mouse) by the number of mice that developed tumors at that cell dose. Mean times for tumor appearance for each assay were developed by determining the means of the cell dose-determined averages. To compare tumor latency, the average means (of the replicate assays on the cells from each vial) were compared by 2-tailed Student t test by using GraphPad Prism 4.
Results
This study was done to expand the database on the tumorigenicity of unmodified MDCK cells and to characterize their tumorigenic phenotype. For this study, we compared the tumor-forming capacity and tumor latency of 3 lots of MDCK cells in athymic nude mice. Cells were assayed at 3 to 8 passages (58 through 63) beyond the passage levels of commercially obtained MDCK cells by using 2 strains of nude mice (athymic and BALB/c) and by 2 routes of inoculation (subcutaneous and intraperitoneal).
Tumor incidence as an indicator of phenotypic heterogeneity.
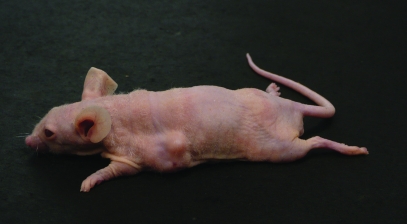
Adult, female, athymic nude mice were inoculated with a 10-fold series of doses (1 × 104 to 1 × 107 cells/mouse) of MDCK cells to determine dose response. Results from initial experiments relating tumor-cell dose to tumor incidence suggested lot-associated differences in tumor formation. To determine whether these differences were explained by assay-to-assay variations or differences among nude-mouse cohorts, replicate assays were done by using the different lots of MDCK cells in mice from different cohorts (Table 1; vial 1, experiments 1 through 4; vial 2, experiments 1 and 2; and vial 3, experiment 1) or the same cohort (Table 1; vials 1 and 2 in experiments 5 and 3, respectively). For purposes of assay and cell-lot comparison, TPD50 values were determined for cells from the lots represented by vials 1 through 3 (Table 1). The majority of tumors that developed in these experiments grew progressively to 20 mm, occasionally appearing as multinodular masses (Figure 1). However, in 3 of the experiments using vial 1 cells and 1 experiment using vial 2 cells, 4 of 140 and 1 of 79 tumors, respectively, regressed completely.
Table 1.
Dose–response data on the tumor-forming capacity of 3 different lots of CCL34 MDCK cells in adult athymic nude mice
| Experiment no. (passage [p] level) | Tumor incidence at indicated cell dose (log10) after subcutaneous inoculation (no. mice in which tumors formed/total no. of mice inoculated) |
TPD50a (log10) | Mean TPD50 (log10) | |||||
| 7 | 6 | 5 | 4 | 3 | ||||
| Vial 1 | 1 (p58) | 17/18b | 6/9 | 8/10 | 0/10 | not tested | 5.1 | 5.2 |
| 2 (p60) | 20/20 | 19/20 | 12/20 | not tested | not tested | 5.0 | ||
| 3 (p61) | 10/10 | 9/10b | 4/10b | 0/10 | not tested | 5.2 | ||
| 4 (p62) | 9/10b | 8/10 | 4/10 | 0/10 | not tested | 5.4 | ||
| 5 (p61) | 5/5 | 4/5 | 1/5 | 0/5 | not tested | 5.5 | ||
| Total incidence | 61/63 | 46/54 | 29/55 | 0/35 | ||||
| Vial 2 | 1 (p62) | 10/10 | 10/10 | 10/10 | 4/10 | 0/5 | 4.1 | 4.4 |
| 2 (p64) | 9/10b | 10/10 | 9/10 | 0/10 | 0/5 | 4.7 | ||
| 3 (p62) | 5/5 | 5/5 | 5/5 | 1/4 | not tested | 4.3 | ||
| Total incidence | 24/25 | 25/25 | 24/25 | 5/24 | 0/10 | |||
| Vial 3 | 1 (p61) | 10/10 | 7/10 | 0/10 | 0/10 | 0/10 | 5.8 | not applicable |
The difference between mean TPD50 values for vials 1 and 2 is significant (P = 0.0027).
The TPD50 represents the threshold (endpoint) cell dose required for tumor-forming activity by populations of neoplastic cells that express tumorigenic phenotypes.
Complete regression occurred with one tumor in these experiments.
Figure 1.
Adult athymic nude mouse with a multinodular tumor that developed from subcutaneous injection of 107 MDCK cells.
MDCK cells from vial 2 were more efficient in forming tumors in adult nude mice than were cells from vial 1 (mean TPD50: vial 2, 4.4 [25,000 cells]; vial 1, 5.2 [160,000 cells; P = 0.0027). In contrast, in a single assay, MDCK cells from vial 3 appeared to be less tumorigenic (TPD50, 5.8 [630,000 cells]) than were cells from either of the other 2 vials.
The 3 lots of MDCK cells differed in the threshold dose needed to form tumors in adult, athymic nude mice (Table 1). These differences were not due to animal variability, because vial 1 experiment 5 and vial 2 experiment 3 were tested in the same cohort of athymic nude mice and generated TPD50 values that were similar to those developed in different mouse cohorts over a 2-y period.
Tumor latency as an indicator of phenotypic heterogeneity.
Tumor latency is the time to tumor formation and is a characteristic of the neoplastic cell tumorigenic phenotype that can be expressed independently of the limiting or threshold cell dose.14 As an independent trait, tumor latency can be used to examine the heterogeneity of the phenotype. For this purpose, we examined the time to tumor formation required by MDCK cells from each of the vials (lots) used in this study (Table 2).
Table 2.
Time to tumor formation (tumor latency) of 3 different lots of MDCK cells in adult athymic nude mice
| Average time (wk) to appearance of tumors at indicated cell doses (log10) |
|||||||
| Experiment no. | 7 | 6 | 5 | 4 | Meana | Average mean | |
| Vial 1 | 1 | 1.4 | 10.8 | 14.1 | ND | 8.8 | 6.6 |
| 2 | 2.0 | 7.9 | 11.5 | ND | 7.1 | ||
| 3 | 1.0 | 4.5 | 7.6 | ND | 4.4 | ||
| 4 | 1.5 | 7.8 | 14.0 | ND | 7.8 | ||
| 5 | 1.6 | 7.5 | 6.0 | ND | 5.0 | ||
| Mean (range) | 1.5 (1–2) | 7. 7 (3–25) | 10.6 (7–26) | not done | |||
| Vial 2 | 1 | 1.0 | 1.6 | 2.8 | 7.5 | 3.2 | 2.9 |
| 2 | 1.0 | 1.7 | 3.8 | ND | 2.2 | ||
| 3 | 1.0 | 2.0 | 4.4 | 6.0 | 3.4 | ||
| Mean (range) | 1.0 (1) | 1.8 (1–3) | 3.7 (2–4) | 6.8 (5–9) | |||
| Vial 3 | 1 | 1.2 | 6.3 | ND | ND | 3.8 | not applicable |
ND, not determined due to the absence of tumors at the indicated cell dose
The difference in average mean tumor latency between vials 1 and 2 is statistically significant (P = 0.0043).
To evaluate average tumor latency, we determined the mean times to tumor appearance for each dose of MDCK cells and examined the differences in the averages of these means (Table 2). At cell doses of 106 per mouse, cells from vial 2 formed tumors more rapidly than did those from vial 1 (mean time to tumor: vial 2, 1.8 wk; vial 1, 7.7 wk; P = 0.0043). In the single assay with cells from vial 3, the average time to tumor appearance after receiving 106 cells per mouse was 6.3 wk compared with 7.7 wk for cells from vial 1.
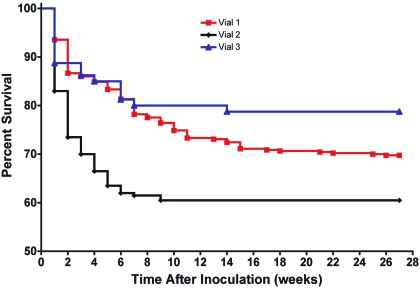
To further evaluate tumor latency, the tumor incidence data from Table 1 were converted to percentage survival and plotted over the 26-wk observation period (Figure 2). Because tumor development was the terminal event that was used to generate these survival curves, the curves could be used to compare average times to tumor appearance (tumor latency) and to represent the tumorigenic phenotype of the cells from a given vial. Inspection of the curves suggested differences between the cells in vials 1 and 2 (Kaplan–Meier, P ≤ 0.0001) and between those in vials 2 and 3 (P ≤ 0.0040). In contrast, differences between vials 1 and 3 appeared to be negligible (P = 0.1495), suggesting that the tumor latency traits expressed by the cells from these vials were similar.
Figure 2.
Average percentage survival (tumor latency) curves depicting tumor formation in adult athymic nude mice over time in response to the dose of MDCK cells injected from vial 1 (mean of 5 assays), vial 2 (mean of 3 assays), and vial 3 (1 assay). Average survival values were estimated from the tumor incidence recorded weekly. Kaplan–Meier analysis indicated that the differences between the average tumor-latency data for vial 1 compared with vial 2 were significant (P ≤ 0.0001) as were those between vials 2 and 3 (P ≤ 0.0040), whereas the differences between vials 1 and 3 were nonsignificant (P = 0.1495).
Because traits that determine the TPD50 and tumor latency are independent variables of the tumorigenic phenotype,14 our data indicate that MDCK cells from vial 3 were less efficient at tumor formation (higher TPD50) than were cells from vials 1 and 2 but expressed a tumor-latency trait that was similar to that of the cells in vial 1. In summary, the tumorigenic phenotypes expressed by MDCK cells from vials 1 and 3 were less aggressive than that of cells from vial 2, as evidenced by parameters of both tumor-forming capacity and tumor latency.
Tumor development in a different strain of nude mice and by a different inoculation route.
Because an earlier study showed that MDCK cells were nontumorigenic in adult BALB/c nude mice,33 but we found that MDCK cells were tumorigenic in the athymic nude mouse, which is on a Swiss mouse background, we repeated the tumorigenicity assay in adult BALB/c nude mice by using vial 1 cells. Tumors developed in 100% (10 of 10 mice at 107 cells per mouse), 80% (4 of 5 mice at 106 cells per mouse), 80% (4 of 5 mice at 105 cells per mouse), and 40% (2 of 5 mice at 104 cells/mouse) of the mice challenged with the indicated cell doses. Therefore, vial 1 cells produced tumors with approximately the same efficiency in BALB/c nude mice (TPD50, 4.9 [80,000 cells]) as was observed in athymic nude mice (TPD50, 5.2 [160,000 cells]).
A comparative experiment was done to determine whether MDCK cell tumor-forming capacity varied with the route of inoculation. Adult, athymic nude mice were inoculated intraperitoneally with 10-fold serial dilutions of cells from vial 1. Mice that developed abdominal distention were euthanized and necropsied to confirm tumor formation. Vial 1 cells injected intraperitoneally formed tumors with essentially the same efficiency (TPD50, 5.3 [200,000 cells]) as they did by subcutaneous inoculation (mean TPD50, 5.2 [160,000 cells]) in athymic nude mice.
Tumor formation in newborn nude mice.
Newborn nude mice are more sensitive than adult nude mice for tumorigenicity studies.19,33 Therefore, we evaluated newborn nude mice for their ability to support tumor formation by MDCK cells (vial 1). Tumors developed in 96% (47 of 49 mice at 107 cells per mouse), 97% (34 of 35 at 106 cells per mouse), 91% (20 of 22 mice at 105 cells per mouse), 86% (6 of 7 at 104 cells per mouse), and 14% (1 of 7 at 103 cells per mouse) of newborn mice challenged with the indicated doses of MDCK cells, yielding a TPD50 of 3.7 (5000 cells; Table 3). An indication of the increased sensitivity of the newborn nude mouse was that tumors developed in mouse pups injected with as few as 1000 cells, whereas in most experiments in adult mice, mice had to be inoculated with 105 cells or more (vial 1). The difference between the mean TPD50 values of tumors in adults and newborns was statistically significant (P = 0.0012).
Table 3.
Dose–response data on the tumor-forming capacity of vial 1 MDCK cells in newborn athymic nude mice
| Tumor incidence at indicated cell dose (log10; no. mice in which tumors formed/total no. of mice inoculated) |
||||||
| Experiment no. | 7 | 6 | 5 | 4 | 3 | TPD50 (log10) |
| 1 | NT | 1/1 | 5/5 | 5/5 | 1/4 | 3.3 |
| 2 | NT | 7/7 | Losta | 1/2 | 0/5 | 4.0 |
| 3 | NT | 14/15 | 9/11 | NT | NT | ND |
| Overall incidence | 47/49b | 34/35b | 20/22b | 6/7 | 1/9 | 3.7 |
ND, not determined; NT, not tested
Lost due to maternal neglect
bTotal includes data from independent single-dose assays
Necropsy and tumor histopathology of adult nude mice.
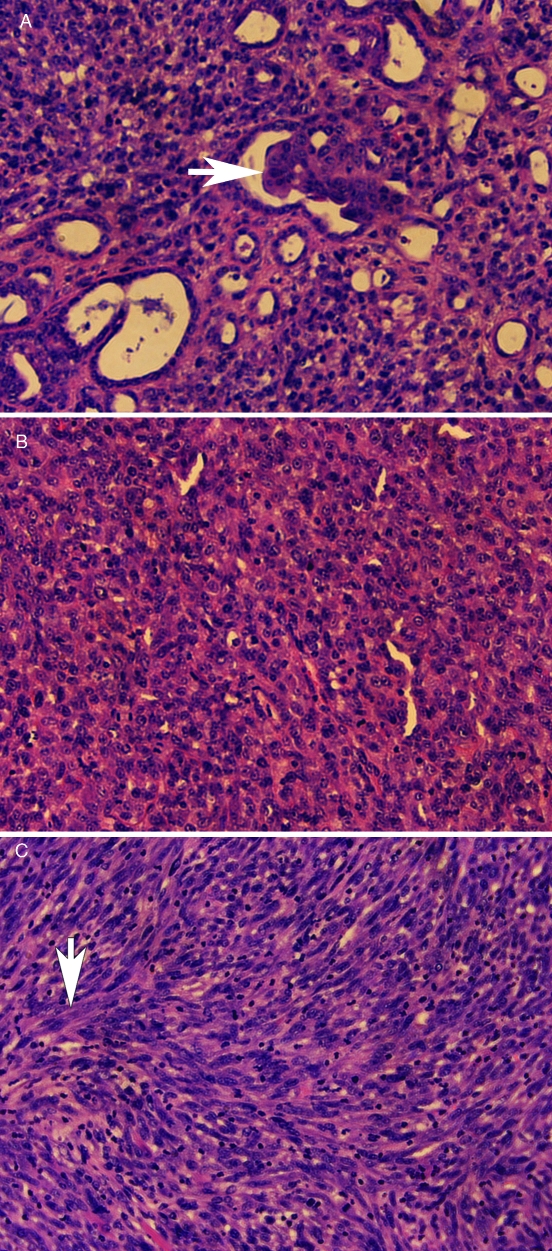
Nude mice were euthanized once the subcutaneous tumor growth progressed to 2 cm. None of the mice had tumor metastasis to the lungs or other organs by either gross or histopathologic examination. Many of the tumors that formed during this study had a tubular adenocarcinoma appearance. Tubular formation was irregular, and some tubules had small papillary ingrowths (Figure 3 A; arrow), resembling renal carcinomas. Other tumors had no evidence of tubule formation but instead had areas of densely packed polygonal cells (Figure 3 B) frequently accompanied by prominent areas of fusiform cells arranged in interwoven bundles (Figure 3 C; arrow). These histopathologic patterns were present in tumors that formed in both adult (Figure 3 A to C) and newborn athymic nude mice (data not shown).
Figure 3.
Histopathology of an MDCK cell tumor in an adult nude mouse. (A) An epithelial portion of tumor with irregular tubule formation, some with papillary ingrowths (arrow). (B) Tumor with polygonal cells. (C) Tumor with fusiform cells arranged in interwoven bundles (arrow). The tumors were fixed in formalin, paraffin-embedded, sectioned, stained with hematoxylin and eosin, and photographed under a 10× objective.
Discussion
Delineating the characteristics of the tumorigenic phenotype expressed by neoplastic cells growing in tissue culture requires tumor-incidence data derived from dose–response tumorigenicity assays.14,34 Such assays can be performed by using different animal models (for example, syngeneic animals, immunosuppressed allogeneic or immunosuppressed xenogeneic animals) or rodents exhibiting variations in immunocompetence (for example, athymic nude mice, SCID mice, NOD–SCID mice, CD3ϵ mice). Once these tumor-challenge models are set, observations of tumor development over time in dose–response assays can be used to define the efficiency (TPD50 at the limiting cell dose) and rate (latency) as 2 independent parameters of tumor formation that, when combined, provide a model-specific signature of the tumorigenic phenotype.14 Tumorigenic phenotypes characterized by this method provide baseline information for evaluating the significance of phenotypic changes that occur spontaneously or that are induced experimentally.
Our results characterize the tumorigenic phenotypes in adult athymic nude mice of 3 different lots of MDCK cells obtained from the ATCC. Despite the fact that the lots differed by only 1 passage level, differences were detected in their efficiencies in forming tumors in athymic nude mice. An earlier study33 found that MDCK cells failed to form progressively growing tumors in adult BALB/c nude mice (a mouse with a different genetic background to the athymic nude mouse) but did form nodules in newborn BALB/c nude mice that grew progressively until the pups reached maturity, at which time tumor growth ceased. The differences between our results and the earlier data33 prompted us to evaluate the tumor-forming capacity of our ATCC-derived MDCK cells in BALB/c nude mice. The ability of MDCK cells from vial 1 to form tumors did not appear to be dependent on the strain of the nude mouse, given that the adult BALB/c nude mice in our study supported progressive tumor formation (TPD50, 4.9 [80,000 cells]). In addition, similar to previous reports28,33, we found that MDCK cells form tumors when injected into newborn athymic nude mice. However, in contrast to the earlier studies, we did not note a cessation in tumor growth as the mice matured; tumors that developed from injection of 106 or fewer cells expanded progressively to 20 mm in diameter, at which point the mice were euthanized. Interestingly, MDCK cells injected at a dose of 107 cells in newborn athymic nude mice frequently did not develop into multilobular tumor masses within the subcutaneous tissue of the back but instead were highly invasive into the underlying musculature. The nude mouse pups carrying these invasive tumors developed a ‘failure-to-thrive’ syndrome, which will be described in another report.
Although some studies on the tumorigenicity of MDCK cells have failed to confirm their ability to form tumors in nude mice,31 other studies have found the cells capable of forming tumors in chicken embryos and nude mice.13,20 Collectively, the data published on MDCK cell tumorigenicity suggest that the phenotype can be quite variable. Our data support and extend these findings by showing that variations can occur in the expression of tumorigenic phenotypes as defined by dose–response tumorigenicity assays among sublines derived from the original MDCK cell line. Other studies have indicated that MDCK cells are heterogeneous with respect to other properties5,27,42 with subtypes that differ in their ciliation,24,42 expression of α1- and β2-adrenergic receptors,21 prostaglandin production,16 membrane architecture,27,32 electrophysical properties,5,27,42 susceptibility to transformation by alkaline stress, and sensitivity to renal hormones.5,27 In addition, MDCK cell populations have been noted to be composed of cells with epithelial and mesenchymal morphologies,27,42 findings that are consistent with our observations that cells from vials 1, 2, and 3 exhibit mixtures of epithelial and mesenchymal cell types with differences in the relative proportions of these types of cells. However, it is difficult to correlate this morphologic heterogeneity with the differences in their tumor-forming capacities, given that both epithelial and mesenchymal cells were present in the tumors formed in vivo by cells from these vials.
Although the molecular mechanisms that underlie the differences in the tumorigenic phenotypes of MDCK cells are unknown and are the subject of current studies, several explanations are possible. One possibility is that mutations conferring growth advantages and tumorigenic phenotypes may have developed in the MDCK cell population during its propagation, and the subcultures we received from the ATCC may represent populations of cells that had been selected for these evolving populations. Under these circumstances, the proportion of tumor-forming cells in the population would be expected to continue to increase during serial passage in our laboratory. However, the TPD50 values did not decrease (that is, tumorigenicity did not increase) during the serial passages in this study (Table 1), making this possibility unlikely.
Another possible explanation is that different cell lots contain varying subpopulations of stem cells that express tumorigenic phenotypes, each with a defined capacity (as exhibited by the TPD50 value) to form tumors in vivo. The proportion of tumor-forming cells in such populations might be expected to remain constant over the number of passages that occurred in the current study, as was apparent from the data on the cells from vials 1 and 2.
Other explanations for the heterogeneity of the tumorigenic phenotypes of these MDCK cells could involve epigenetic events. Because MDCK cells are composed of cells that have different sensitivities to hormones and other stimuli, as mentioned earlier, these cells may interact with their microenvironment differently, resulting in populations of tumorigenic and nontumorigenic cells or cell populations with different tumorigenic phenotypes. The proportions of the different subpopulations present in the 3 lots of MDCK cells may differ, yielding differences in their TPD50 values. In addition, epigenetic events might induce epithelial–mesenchymal transition and mesenchymal–epithelial transition. Both transitions have been reported to play a role in the induction of stem-cell properties,37 tumor development, and invasion and metastasis.9,25,26,36,37 Studies have been initiated to examine the possible role of subpopulation selection, the presence of tumor stem cells, and genetic and epigenetic processes in establishing the tumorigenic phenotypes expressed by the MDCK cells in vials 1, 2, and 3.
Our studies establish the heterogeneity of the tumorigenic phenotypes of 3 lots of MDCK cells. This degree of variability in the tumorigenicity of different lots of the same cell type at similar passage levels and maintained under similar conditions during limited tissue-culture passage did not occur with virus-transformed rodent cells or other neoplastic cell lines that have been studied.2,15,29 The application of the quantitative assays of tumorigenicity we present herein provides both the methodology and the reference data on MDCK cell-lot comparisons that can be used for an analysis of the in vivo relevance of candidate mechanisms emerging from future studies.
Acknowledgments
We thank Jerry Weir, Phil Krause, Arifa Khan, Robin Levis, and Haruhiko Murata for discussion or critical review of the manuscript.
This research was supported in part by a contract from the Division of Microbiology and Infectious Diseases of the National Institute of Allergy and Infectious Diseases through an interagency agreement with CBER/FDA and by the support of the James A and Marion C Grant Fund at the University of Illinois at Chicago.
The findings and conclusions in this article have not been formally disseminated by the Food and Drug Administration and should not be construed to represent any Agency determination or policy.
References
- 1.Bagnoli F, Buti L, Tompkins L, Covacci A, Amieva MR. 2005. Helicobacter pylori CagA induces a transition from polarized to invasive phenotypes in MDCK cells. Proc Natl Acad Sci USA 102:16339–16344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cook JL, Miura TA, Ikle DN, Lewis AM, Jr, Routes JM. 2003. E1A oncogene-induced sensitization of human tumor cells to innate immune defenses and chemotherapy-induced apoptosis in vitro and in vivo. Cancer Res 63:3435–3443 [PubMed] [Google Scholar]
- 3.Das M, Sakul H, Kong J, Acland GM, Pelletier J. 2000. A set of canine interrepeat sequence PCR markers for high-throughput genotyping. Physiol Genomics 4:13–24 [DOI] [PubMed] [Google Scholar]
- 4.Gaush CR, Hard WL, Smith TF. 1966. Characterization of an established line of canine kidney cells (MDCK). Proc Soc Exp Biol Med 122:931–935 [DOI] [PubMed] [Google Scholar]
- 5.Gekle M, Wunsch S, Oberleithner H, Silbernagl S. 1994. Characterization of 2 MDCK cell subtypes as a model system to study principal cell and intercalated cell properties. Pflugers Arch 428:157–162 [DOI] [PubMed] [Google Scholar]
- 6.Hennings H, Robinson VA, Michael DM, Pettit GR, Jung R, Yuspa SH. 1990. Development of an in vitro analogue of initiated mouse epidermis to study tumor promoters and antipromoters. Cancer Res 50:4794–4800 [PubMed] [Google Scholar]
- 7.Institute for Laboratory Animal Research 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 8.Jorda M, Vinyals A, Marazuela A, Cubillo E, Olmeda D, Valero E, Cano A, Fabra A. 2007. ID1 is induced in MDCK epithelial cells by activated Erk–MAPK pathway in response to expression of the Snail and E47 transcription factors. Exp Cell Res 313:2389–2403 [DOI] [PubMed] [Google Scholar]
- 9.Joyce JA, Pollard JW. 2009. Microenvironmental regulation of metastasis. Nat Rev Cancer 9:239–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadono Y, Okada Y, Namiki M, Seiki M, Sato H. 1998. Transformation of epithelial Madin–Darby canine kidney cells with p60(v-src) induces expression of membrane-type 1 matrix metalloproteinase and invasiveness. Cancer Res 58:2240–2244 [PubMed] [Google Scholar]
- 11.Kim KR, Yoshizaki T, Miyamori H, Hasegawa K, Horikawa T, Furukawa M, Harada S, Seiki M, Sato H. 2000. Transformation of Madin–Darby canine kidney (MDCK) epithelial cells by Epstein–Barr virus latent membrane protein 1 (LMP1) induces expression of Ets1 and invasive growth. Oncogene 19:1764–1771 [DOI] [PubMed] [Google Scholar]
- 12.Leighton J, Brada Z, Estes LW, Justh G. 1969. Secretory activity and oncogenicity of a cell line (MDCK) derived from canine kidney. Science 163:472–473 [DOI] [PubMed] [Google Scholar]
- 13.Leighton J, Estes LW, Mansukhani S, Brada Z. 1970. A cell line derived from normal dog kidney (MDCK) exhibiting qualities of papillary adenocarcinoma and of renal tubular epithelium. Cancer 26:1022–1028 [DOI] [PubMed] [Google Scholar]
- 14.Lewis AM, Jr, Alling DW, Banks SM, Soddu S, Cook JL. 1999. Evaluating virus-transformed cell tumorigenicity. J Virol Methods 79:41–50 [DOI] [PubMed] [Google Scholar]
- 15.Lewis AM, Jr, Cook JL. 1985. A new role for DNA virus early proteins in viral carcinogenesis. Science 227:15–20 [DOI] [PubMed] [Google Scholar]
- 16.Lewis MG, Spector AA. 1981. Differences in types of prostaglandins produced by 2 MDCK canine kidney cell sublines. Prostaglandins 21:1025–1032 [DOI] [PubMed] [Google Scholar]
- 17.Madin SH, Andriese PC, Darby NB. 1957. The in vitro cultivation of tissues of domestic and laboratory animals. Am J Vet Res 18:932–941 [PubMed] [Google Scholar]
- 18.Madin SH, Darby NB., Jr 1958. Established kidney cell lines of normal adult bovine and ovine origin. Proc Soc Exp Biol Med 98:574–576 [DOI] [PubMed] [Google Scholar]
- 19.Manohar M, Orrison B, Peden K, Lewis AM., Jr 2008. Assessing the tumorigenic phenotype of VERO cells in adult and newborn nude mice. Biologicals 36:65–72 [DOI] [PubMed] [Google Scholar]
- 20.Medema JK, Meijer J, Kersten AJ, Horton R. 2006. Safety assessment of Madin–Darby canine kidney cells as vaccine substrate. Dev Biol (Basel) 123:243–250, discussion 265–266 [PubMed] [Google Scholar]
- 21.Meier KE, Snavely MD, Brown SL, Brown JH, Insel PA. 1983. α1- and β2-adrenergic receptor expression in the Madin–Darby canine kidney epithelial cell line. J Cell Biol 97:405–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller RG. 1973. Nonparametric estimators of the mean tolerance in bioassay. Biometrika 60:535–542 [Google Scholar]
- 23.Oberleithner H, Westphale HJ, Gassner B. 1991. Alkaline stress transforms Madin–Darby canine kidney cells. Pflugers Arch 419:418–420 [DOI] [PubMed] [Google Scholar]
- 24.Pfaller W, Gstraunthaler G, Kersting U, Oberleithner H. 1989. Carbonic anhydrase activity in Madin–Darby canine kidney cells. Evidence for intercalated cell properties. Ren Physiol Biochem 12:328–337 [DOI] [PubMed] [Google Scholar]
- 25.Polyak K, Haviv I, Campbell IG. 2009. Coevolution of tumor cells and their microenvironment. Trends Genet 25:30–38 [DOI] [PubMed] [Google Scholar]
- 26.Polyak K, Weinberg RA. 2009. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 9:265–273 [DOI] [PubMed] [Google Scholar]
- 27.Richardson JC, Scalera V, Simmons NL. 1981. Identification of 2 strains of MDCK cells which resemble separate nephron tubule segments. Biochim Biophys Acta 673:26–36 [PubMed] [Google Scholar]
- 28.Rindler MJ, Chuman LM, Shaffer L, Saier MH., Jr 1979. Retention of differentiated properties in an established dog kidney epithelial cell line (MDCK). J Cell Biol 81:635–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Routes JM, Ryan JC, Ryan S, Nakamura M. 2001. MHC class I molecules on adenovirus E1A-expressing tumor cells inhibit NK cell killing but not NK cell-mediated tumor rejection. Int Immunol 13:1301–1307 [DOI] [PubMed] [Google Scholar]
- 30.Rubin H. 2008. Cell–cell contact interactions conditionally determine suppression and selection of the neoplastic phenotype. Proc Natl Acad Sci USA 105:6215–6221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schoenenberger CA, Zuk A, Kendall D, Matlin KS. 1991. Multilayering and loss of apical polarity in MDCK cells transformed with viral K-ras. J Cell Biol 112:873–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simmons NL. 1982. Cultured monolayers of MDCK cells: a novel model system for the study of epithelial development and function. Gen Pharmacol 13:287–291 [DOI] [PubMed] [Google Scholar]
- 33.Stiles CD, Desmond W, Chuman LM, Sato G, Saier MH. 1976. Growth control of heterologous tissue culture cells in the congenitally athymic nude mouse. Cancer Res 36:1353–1360 [PubMed] [Google Scholar]
- 34.Taghian A, Budach W, Zietman A, Freeman J, Gioioso D, Ruka W, Suit HD. 1993. Quantitative comparison between the transplantability of human and murine tumors into the subcutaneous tissue of NCr/Sed-nu/nu nude and severe combined immunodeficient mice. Cancer Res 53:5012–5017 [PubMed] [Google Scholar]
- 35.Taub M, Chuman UB, Rindler L, Saier MJ, Jr MH, Sato G. 1981. Alterations in growth requirements of kidney epithelial cells in defined medium associated with malignant transformation. J Supramol Struct Cell Biochem 15:63–72 [DOI] [PubMed] [Google Scholar]
- 36.Thiery JP. 2002. Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer 2:442–454 [DOI] [PubMed] [Google Scholar]
- 37.Thiery JP, Acloque H, Huang RY, Nieto MA. 2009. Epithelial–mesenchymal transitions in development and disease. Cell 139:871–890 [DOI] [PubMed] [Google Scholar]
- 38.U HS, Boerner P, Rindler MJ, Chuman L, Saier MH., Jr 1985. Characterization of chemically and virally transformed variants of Madin–Darby canine kidney (MDCK) epithelial cells. J Cell Physiol 122:299–307 [DOI] [PubMed] [Google Scholar]
- 39.United States of America Food and Drug Administration. [Internet] 2005. Proceedings of the FDA Vaccines and Related Biological Products Advisory Committee meeting. [Cited 16 November 2005]. Available at: www.fda.gov/ohrms/dockets/ac/05/transcripts/2005-4188t1.pdf
- 40.United States of America Food and Drug Administration. [Internet] 2005. Proceedings of the FDA Vaccines and Related Biological Products Advisory Committee meeting. [Cited 16 November 2005]. Available at: www.fda.gov/ohrms/dockets/ac/05/slides/5-4188S1_5.pdf
- 41.United States of America Food and Drug Administration. [Internet] 2005. Proceedings of the FDA Vaccines and Related Biological Products Advisory Committee meeting. [Cited 16 November 2005]. Available at: www.fda.gov/ohrms/dockets/ac/05/slides/5-4188S1_6.pdf
- 42.Valentich JD. 1981. Morphological similarities between the dog kidney cell line MDCK and the mammalian cortical collecting tubule. Ann N Y Acad Sci 372:384–405 [DOI] [PubMed] [Google Scholar]