Abstract
Background
In negative-pressure wound therapy (NPWT), a wound is covered with an airtight dressing, and negative pressure is applied. This is thought to promote healing. We evaluated NPWT with an updated, systematic review of the literature.
Methods
We systematically searched the PubMed and Cochrane Library databases for randomized, controlled trials (RCTs) of NPWT for the treatment of acute or chronic wounds. The primary outcome was complete wound closure.
Results
We found reports of 9 RCTs in addition to the 12 covered by earlier IQWiG reviews of this topic. Five of the 9 new trials involved NPWT systems that are not on the market. The frequency of complete wound closure is stated in only 5 of the 9 new reports; a statistically significant effect in favor of NPWT was found in only two trials.The results of 8 of the 9 new trials are hard to interpret, both because of apparent bias and because diverse types of wounds were treated.
Conclusion
Although there may be a positive effect of NPWT, we did not find clear evidence that wounds heal any better or worse with NPWT than with conventional treatment. Good RCTs are still needed to evaluate NPWT.
Negative-pressure wound therapy (NPWT) is a sealed wound-care system and is particularly indicated for large chronic persistent wounds and acute complicated wounds (1, 2). The system consists of an electronically controlled pump and a foam dressing that drains the wound. An adjustable negative pressure is applied via an airtight adhesive film that covers the wound. NPWT drains wound exudate and is thought to promote blood circulation and healing.
This systematic review aims to update the systematic reviews on NPWT previously published by the Institute for Quality and Efficiency in Health Care (Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen, IQWiG) (3– 5). The aim of these reports was to evaluate wound healing and adverse events following NPWT in comparison to conventional treatment in patients with acute or chronic wounds.
Methods
The reports within this systematic review were compiled in accordance with the principles of the PRISMA statement (e1).
Inclusion criteria
The research included randomized controlled trials (RCTs) involving patients with acute and chronic wounds. Because of the increasing number of RCTs conducted in recent years, non-randomized trials were not included in the evaluation. The intervention under examination was NPWT. As in the previous reports, studies of systems not commercially available were included in addition to commercially available systems. In the systems that were not commercially available, negative pressure was generated by a suction pump for chest drainage, a central vacuum system or Redon bottles, for example. The comparator treatment was conventional dressings, generally saline-soaked gauze dressings. There was no minimum number of patients per trial. There was no restriction on language or year of publication. However, articles in languages other than English or German were only included in the review if there were translations available that made it possible to assess the trials concerned.
Search strategy
Unlike the earlier IQWiG reports, this review included only RCTs (3, 4). The simplified search strategy used (eTable 1) identified all 12 RCTs already included in PubMed and the Cochrane Library’s Clinical Trials on November 7, 2010. EMBASE and CINAHL were not searched, as they had not yielded any additional relevant results in previous searches (6). The search results from the two included databases were imported into EndNote X3 (Thomson Reuters) and duplicates were deleted manually.
eTable 1. Search strategy*.
| ID | Search |
| #1 | “Negative-Pressure Wound Therapy” (Mesh) |
| #2 | “Vacuum” (Mesh) AND "Wound Healing” (Mesh) |
| #3 | negative pressure wound therapy |
| #4 | vacuum assisted closure |
| #5 | vacuum assisted wound |
| #6 | vacuum dressing |
| #7 | subatmospheric pressure |
| #8 | topical negative pressure |
| #9 | (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8) |
| #10 | “Random Allocation” (Mesh) |
| #11 | “Randomized Controlled Trials as Topic” (Mesh) |
| #12 | “Randomized Controlled Trial” (Publication Type) |
| #13 | randomiz* OR randomis* |
| #14 | (#10 OR #11 OR #12 OR #13) |
| #15 | (#9 AND #14) |
*Search strategy for research on November 7, 2010 in the electronic literature databases PubMed and the Cochrane Library´s Clinical Trials
The electronic trial registers ClinicalTrials.gov (URL: http://clinicaltrials.gov/; registration numbers: NCT followed by eight digits) and the International Standard Randomised Controlled Trial Number Register (URL: www.controlled-trials.com/; registration numbers: ISRCTN followed by eight digits) were searched for completed and ongoing trials on January 15, 2011, using the following search terms: “vacuum assisted closure;” “vac;” “negative pressure wound therapy;” “npwt.”
Study selection
First of all, articles were excluded on the basis of their title and abstract if these did not mention NPWT or it was clear that the trials were not randomized. The full text of the remaining articles was then examined. The reasons for excluding each individual study were recorded internally. All stages of study selection were performed independently by two separate individuals. Differences of opinion were discussed until a common decision could be made.
Potential for bias
The risk of bias within trials was examined using the criteria stated in Table 1. A positive answer in all five categories was established at the outset as indicating a low potential for bias. The potential effect of publication bias was assessed by updating a previous study (6) identifying all the trials that were terminated early.
Table 1. Potential for bias.
| RCT | Suitable allocation to groups*1 | Allocation to groups suitably concealed*2 | Assessment of endpoints blinded*3 | Reasons given for any data loss*4 | Adequate ITT analysis*5 | Potential for bias*6 |
| Commercially available systems | ||||||
| Blume 2008 (8) | + | + | – | + | – | High |
| Chio 2010 (9) | + | + | – | – | – | High |
| Keskin 2008 (10) | ? | ? | – | + | – | High |
| Stannard 2009 (11) | + | ? | – | + | – | High |
| Systems not commercially available | ||||||
| Bee 2008 (12) | + | + | – | + | – | High |
| Mody 2008 (13) | + | ? | – | – | – | High |
| Perez 2010 (14) | ? | ? | – | – | + | High |
| Saaiq 2010 (15) | + | ? | – | + | + | High |
| Sepúlveda 2009 (16)*7 | + | + | + | + | + | Low |
RCT: randomized controlled trial; +: Yes; –: No; ?: Unclear; ITT: intention-to-treat
*1Suitable allocation to groups: A precise description of the randomization sequence generating procedure was required (e.g. computer-generated lists).
*2Allocation to groups suitably concealed: Information on how allocation to groups was then blinded was required (e.g. centrally by telephone or using sealed, opaque envelopes).
*3Assessment of endpoints blinded: Information on who (patient and/or researcher) assessed which endpoint under blinding conditions (without knowing the group to which the patient had been allocated) was required.
*4Reasons given for any data loss: The requirement was either no data loss or, if data loss was reported, identification of all patients whose data could not be fully evaluated after randomization and the reasons for this (e.g. patients who dropped out before the beginning of treatment or during follow-up).
*5Adequate ITT analysis: Evaluation using the number of randomized patients as the size of the population was required.
*6Potential for bias: High or low; all five criteria had to be met for the potential for bias to be described as low.
*7Sepúlveda 2009: The blinded parameter was assessment of the percentage of wound granulation.
Data collection and analysis
All stages of data extraction were performed by one person (Frank Peinemann) and checked by another (Stefan Sauerland). Where there were differences of opinion, consensus was reached following discussion. The results were subjected to descriptive analysis. Study characteristics were extracted as shown in Table 2.
Table 2. Trial and patient characteristics in the newly-identified randomized controlled trials (RCTs).
| RCT | Sites | Recruitment period | FU (days) | Random‧ized patients | Dropout or LTFU | Dropout or LTFU by ITT | Mean age | Sex: % male | Mean wound surface area (cm2) | Comorbidities (%) |
| I vs. C | I vs. C | I vs. C | I vs. C | I vs. C | I vs. C | I vs. C | ||||
| Commercially available systems | ||||||||||
| Blume 2008 (8)*1 | 5 (USA) | 2002 to 2005 | 112 | 172 vs. 169 | 59 vs. 52 | 3 vs. 3 | 58 vs. 59 | 83 vs. 73 | 14 vs. 11 | n/a |
| Chio 2010 (9)*2 | 1 (USA) | 2007 to 2009 | 30 | 27 vs. 27 | 4 vs. 0 | 4 vs. 0 | 62 vs. 58 | 61 vs. 60 | 73 vs. 69 | 39 vs. 26 |
| Keskin 2008 (10)*3 | 1 (Turkey) | n/a | 10 | 20 vs. 20 | n/a | n/a | n/a | n/a | n/a | 0 vs. 0 |
| Stannard 2009 (11)*4 | 1 (USA) | 2001 to 2006 | 840 | 35 vs. 23 | 0 | 0 | n/a | 74 vs. 57 | 65 vs. 58 | n/a |
| Systems not commercially available | ||||||||||
| Bee 2008 (12) | 1 (USA) | 2003 to 2007 | n/a | 31 vs. 20 | 2 vs. 1 | 2 vs. 1 | 44 vs. 37 | 81 vs. 85 | n/a | n/a |
| Mody 2008 (13)*5 | 1 (India) | n/a | 214 | 19 vs. 36 | 5 vs. 15 | 4 vs. 3 | 54 | 72 | 67 vs. 121 | n/a |
| Perez 2010 (14)*6 | 1 (Haiti) | 2007 | n/a | 25 vs. 24 | 5 vs. 4 | 5 vs. 4 | 49 vs. 44 | 60 vs. 45 | 45 vs. 40 | 35 vs. 30 |
| Saaiq 2010 (15) | 1 (Pakistan) | 2007 to 2009 | n/a | 50 vs. 50 | n/a | n/a | 33 | 86 | 65 | n/a |
| Sepúlveda 2009 (16)*7 | 1 (Chile) | 2006 to 2007 | n/a | 12 vs. 12 | 0 | 0 | 62 vs. 62 | 83 vs. 75 | n/a | 42 vs. 33 |
LTFU: Lost to follow-up; ITT: intention to treat; I: Intervention group = negative-pressure wound therapy (NPWT); C: Control group; n/a: Not available;
*1Blume 2008: Allocation to groups: 172 NPWT vs. 169 control; NPWT/control group: 3/3 no NPWT + 1/5 LTFU + 54/43 trial terminated + 1/1 incomplete data = 59/52 dropout or LTFU
*2Chio 2010: Comorbidities were diabetes mellitus, peripheral vascular disease, hypothyroidism, and long-term steroid treatment.
*3Keskin 2008: Comorbidities were diabetes mellitus; 40 patients: mean age 38 years, 60% male.
*4Stannard 2009: Mean length of observation period (days); wound surface area was calculated from length and breadth.
*5Mody 2008: Mean length of observation period (days); dropouts after randomization, before treatment 4:3; LTFU before wound closure 1:12; mean wound surface area calculated on the basis of data on 4 wound categories.
*6Perez 2010: Comorbidities were diabetes mellitus.
*7Sepúlveda 2009: Comorbidities were dyslipidemia receiving drug treatment; proportion of patients with hypertension not stated.
Complete wound closure, a variable used both as raw data and as a Kaplan–Meier estimator, was the primary endpoint. The U.S. Food and Drug Administration’s (FDA) 2006 Guidance for Industry (7) defines complete wound closure as “skin closure without drainage or dressing requirements.” No meta-analysis was performed, as the primary trials were highly heterogenous.
The following dependent variables were used as secondary endpoints:
-
Adverse events, such as:
Death
Secondary amputations
Fistula formation
Wound infection
Time to complete wound closure
Reduction in wound size
Health-related quality of life.
Results
Search of the literature
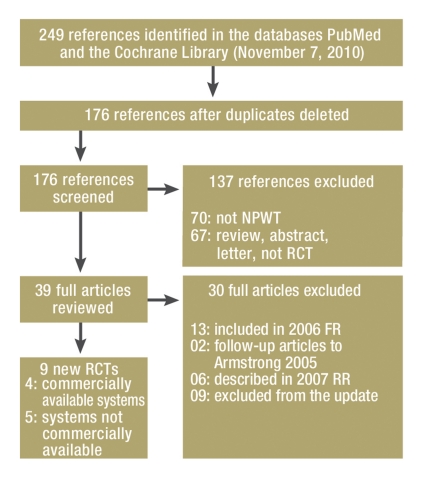
Of the 249 articles initially imported, 176 remained after duplicates had been deleted. In 137 cases it was clear from the title and/or abstract that the article did not meet the inclusion criteria (eFigure). A further 30 potentially relevant articles were excluded after they had been read in full. A total of nine new RCTs were identified in the updated search (8– 16). Five of the nine new RCTs examined systems that were not commercially available (12– 16). This left a total of 21 RCTs available for our research: seven (e2– e8) from IQWiG’s Final Report N04–03 (3), five RCTs in four articles (e9– e12) from IQWiG’s Rapid Report N06–02 (4), and nine RCTs from the updated search.
eFigure.
Search of the literature and trial selection:
NPWT: Negative-pressure wound therapy
RCT: Randomized controlled trial
FR: IQWiG Final Report 2006 (3)
RR: IQWiG Rapid Report 2007 (4)
Underlying data
An overview of trial characteristics is provided in Table 2. The mean age of the participants, most of whom were male, was generally over 50. The mean wound surface area was numerically slightly greater in intervention groups than in control groups in all trials in which this information was given separately. Detailed descriptions of inclusion and exclusion criteria, treatments under research, comparator treatments, analyzed endpoints, and their definitions can be found in eTables 2 and 3. In most trials comorbidities were not reported. The trials examined many different types of acute and chronic wound (eTable 4).
eTable 2. Trial characteristics: inclusion and exclusion criteria.
| RCT | Inclusion criteria | Exclusion criteria |
| Commercially available systems | ||
| Blume 2008 (8) | At least 18 years old, diabetes, foot ulcer at least 2 cm2, Wagner grade 2 or 3 | Active Charcot foot; ulcers not caused by electrical, chemical or radiation burns; collagen vascular diseases; neoplastic ulceration; untreated osteomyelitis; cellulitis; uncontrolled hyperglycemia with HbA1c above 12%; inadequate blood circulation in the legs; hyperbaric oxygen therapy; corticosteroid treatment, immunosuppressant treatment, or chemotherapy; growth factors; skin replacement less than 30 days after the beginning of the trial; enzymatic debridement; pregnant women; breastfeeding mothers |
| Chio 2010 (9) | Adults, status following removal of a free radial forearm flap | Not available |
| Keskin 2008 (10) | Age ≥ 18 years, traumatic leg wounds | Hemodynamic instability; lack of orientation or inability to cooperate |
| Stannard 2009 (11) | Age ≥ 18 years, severe open fractures requiring repeat debridement | Open fractures successfully closed after first operation; infected open fractures; incisions not treatable with NPWT; prisoners; pregnant women |
| Systems not commercially available | ||
| Bee 2008 (12) | Age ≥ 18 years, exploratory laparotomy following trauma or emergency surgery, indication for abdominal closure | Prisoners; pregnant women; life expectancy 7 days or less |
| Mody 2008 (13) | Acute or chronic wounds in the extremities or sacral region or abdominal wounds that could not be closed by initial surgery | Wounds in a part of the body where it would be hard to apply negative pressure; ischemic wounds; wounds with exposed intestine or blood vessels; wounds with necrotic tissue that could not be debrided; wounds with fistulas, osteomyelitis, neoplasia; contraindications according to manufacturer; anticoagulant treatment |
| Perez 2010 (14) | Individual acute or chronic wounds | Bone injuries; vascular ulcers |
| Saaiq 2010 (15) | Age ≥ 13 years, acute traumatic wounds up to 6 weeks old, wound surface area at least 9 cm2 | Diabetes mellitus, neoplasia, or increased tendency to bleed; need for flap surgery |
| Sepúlveda 2009 (16) | Age ≥ 18 years, type 2 diabetes mellitus, wounds following transmetatarsal amputation of 2 or more adjacent toes or the big toe; caused by infection or reduced blood circulation; adequate circulation in the affected leg; metatarsal pulse volume at least 5 mm, systolic blood pressure at least 15 mmHg, ankle–brachial index at least 0.5, foot pulse palpable or status following successful revascularization | Active Charcot foot; uncontrolled hyperglycemia with HbA1c above 12%; corticosteroid treatment, immunosuppressant treatment, or chemotherapy; severe nutritional disturbances with albumin levels below 2.1 mg/dL; growth factor treatment or hyperbaric oxygen therapy |
eTable 3. Trial characteristics: treatment and endpoints.
| RCT | Intervention | Comparator treatment | Patient-related endpoints (as defined by authors) |
| Commercially available systems | |||
| Blume 2008 (8) | Vacuum-assisted closure system, level of vacuum not stated | Saline-soaked gauze dressing | Primary: frequency of complete wound closure (100% reepithelialization) |
| Secondary: reduction in wound surface area, time to wound closure, decrease in adverse events, e.g. secondary amputations | |||
| Chio 2010 (9) | Vacuum-assisted closure system, continuous 125 mmHg vacuum; arm not immobilized | Saline-soaked gauze dressing; lower arm immobilized using splint | Surface area of unhealed part as proportion of total surface area of wound (not described as primary endpoint in article) |
| Keskin 2008 (10) | Vacuum-assisted closure system, intermittent 125 mmHg vacuum | Saline-soaked gauze dressing | Fear during treatment (not described as primary endpoint in article) |
| Stannard 2009 (11) | Vacuum-assisted closure system in addition to saline-soaked gauze dressing, size of vacuum not stated | Saline-soaked gauze dressing | Primary: frequency of deep wound infection, osteomyelitis, or wound dehiscence; no. of patients requiring 3 or more wound debridements. Thus there were several primary endpoints. |
| Secondary: time elapsing until wound suitably prepared for surgical closure | |||
| Systems not commercially available | |||
| Bee 2008 (12)* | Polyethylene film to cover the intestine, sponges on top of polyethylene film, suction tube connected to vacuum pump, wound site covered with an airtight adhesive film, continuous vacuum of 150 mmHg | Polyglactin mesh to cover opening in abdomen | Primary: frequency of delayed fascial closure: fistula formation, mortality, and cost |
| Mody 2008 (13) | Synthetic sponge dressing, suction tube connected to vacuum pump, wound site covered with an airtight adhesive film, intermittent vacuum of 125 mmHg | Saline-soaked gauze dressing | Primary: no. of days to complete secondary wound closure or delayed primary closure |
| Perez 2010 (14) | Hand-washing sponge covering wound, suction tube connected to vacuum pump, wound site covered with an airtight adhesive film, continuous vacuum of 100 mmHg | Saline-soaked gauze dressing | Primary: time to complete wound closure |
| Saaiq 2010 (15) | Synthetic sponge dressing, suction tube connected to vacuum pump, wound site covered with an airtight adhesive film, intermittent vacuum of 50 to 120 mmHg | Saline-soaked gauze dressing | Primary: acceptance of skin transplant |
| Secondary: time to wound healing, need for repeat skin transplant, duration of hospitalization | |||
| Sepúlveda 2009 (16) | Polyurethane foam covering wound, suction tube connected to vacuum pump, wound site covered with an airtight adhesive film, continuous vacuum of 100 mmHg | Saline-soaked gauze dressing, sometimes with the addition of hydrocolloid or alginate | Primary: no. of days to 90% wound granulation |
*Bee 2008: a few patients were treated using a vacuum-assisted closure system
eTable 4. Categories of wounds in included RCTs.
| RCT | Chronic open | Acute open | Covered | ||||||||
| Arteriosclerotic, diabetic ulcers | Chronic venous ulcers | Pressure sores | Other | Foot amp. | Post-trauma | Open abdominal | Fasciitis | Skin graft | Other | Split-skin graft | |
| Commercially available systems | |||||||||||
| Blume 2008 (8) | + | – | – | – | – | – | – | – | – | – | – |
| Chio 2010 (9) | – | – | – | – | – | – | – | – | + | – | – |
| Keskin 2008 (10) | – | – | – | – | – | + | – | – | – | – | – |
| Stannard 2009 (11) | – | – | – | – | – | + | – | – | – | – | – |
| Systems not commercially available | |||||||||||
| Bee 2008 (12) | – | – | – | – | – | – | + | – | – | – | – |
| Mody 2008 (13) | + | – | + | – | – | – | – | + | – | + | – |
| Perez 2010 (14) | + | + | – | + | – | + | – | + | – | + | – |
| Saaiq 2010 (15) | – | – | – | – | – | – | – | – | – | – | + |
| Sepúlveda 2009 (16) | – | – | – | – | + | – | – | – | – | – | – |
RCT: randomized controlled trial; + Yes; – No; amp.: Amputation; NPWT: Negative-pressure wound therapy; post-trauma: post-traumatic wounds
Potential for bias
Eight of the nine trials had a high potential for bias (Table 1). The conditions for generating randomization sequences and treatment blinding were unclear in some trials, with the result that random allocation of patients to treatment groups was not traceable in these trials. In eight of the nine trials it was not reported or not clear that endpoints had been measured in blinded conditions, although this was feasible. It should be stressed that quality-of-life results from non-blinded trials are prone to a particularly high potential for bias. Only one of the trials met all five criteria for low potential for bias. In six of the nine new trials, up to 20% of the data from randomized patients were not included in the evaluation, and there was thus no appropriate intention-to-treat analysis.
Investigation of publication bias revealed a further four RCTs that had been terminated early, in addition to the five that had already been reported on: NCT00121537, NCT00691821, NCT00837096, and NCT01108276.
The following reasons were given for terminating trials early:
Inclusion criteria not met
Patient withdrawal
Low recruitment levels
Changes in clinical practice
Errors in study planning.
Eight ongoing RCTs were also identified among the registered trials (NCT00582179, NCT00582998, NCT00635479, NCT01200563, NCT01191567, NCT00548314, and NCT00789659).
Primary endpoint
The proportion of patients with complete wound closure was reported in only five of the nine new trials (8, 12– 15) (Table 3). In four trials the difference between groups was statistically insignificant. Only two trials showed a statistically significant effect in favor of NPWT (15).
Table 3. Systematic review results on primary endpoint.
| RCT | Endpoint | NPWT | Control | p value | Evidence |
| Commercially available systems | |||||
| Blume 2008 (8) | No. of cases of complete wound closure; n (%) | 73 (43) | 48 (29) | p = 0.007 | Observation period 112 days; no data on 6 or 9 month follow-up |
| Chio 2010 (9) | n/a | n/a | n/a | n/a | – |
| Keskin 2008 (10) | n/a | n/a | n/a | n/a | – |
| Stannard 2009 (11) | n/a | n/a | n/a | n/a | – |
| Systems not commercially available | |||||
| Bee 2008 (12) | Abdominal wall closed by sewing together the fascia; n(%) | 15 (31) | 5 (26) | Insig. | Unclear figures in Table 2 of article: closure (total) 14 (48) but NPWT + control = 20 (70). No confidence interval or p value stated in article, only qualitative interpretation: no difference |
| Mody 2008 (13) | No. of cases of complete wound closure; n (%) | 7 (48) | 16 (48) | n/a | Percentages relate to no. of patients treated, not no. of patients randomized; few patient characteristics reported |
| Perez 2010 (14) | No. of cases of complete wound closure; n (%) | 18 (90) | 19 (95) | p = 0.302 | 30 days after wound closure or skin transplantation |
| Saaiq 2010 (15) | No. of cases of complete wound closure; n (%) | 45 (90) | 9 (18) | p <0.001 | 2 weeks after skin transplantation; 3 categories (2 weeks, 3 to 4 weeks and 5 or more weeks) instead of mean no. of days to wound healing |
| Sepúlveda 2009 (169) | n/a | n/a | n/a | n/a | – |
RCT: randomized controlled trial; –: None; n/a: not available; Insig.: statistically insignificant; NPWT: negative-pressure wound therapy
Secondary endpoints
Time to wound closure was reported in four of the nine new trials (8, 13, 14, 16) (Table 4). Three trials showed a statistically significant difference between groups in favor of NPWT (8, 14, 16), and in one trial the difference was statistically insignificant (13). There was a statistically significant difference in reduction in wound size in favor of NPWT in one of the nine new trials (8).
Table 4. Systematic review results on secondary endpoints.
| RCT | Endpoint | NPWT | Control | p value | Remarks |
| Commercially available systems | |||||
| Blume 2008 (8) | No. of days to complete wound closure; median (95% CI) | 96 (75 to 114) | >112 | p = 0.001 | Results based on Kaplan–Meier analysis |
| Blume 2008 (8) | Reduction in wound surface area (cm2) | – 4.3 | – 2.5 | p = 0.021 | Measured 28 days after beginning of treatment |
| Blume 2008 (8) | Secondary amputations; n (%) | 7 (4) | 17 (10) | p = 0.035 | – |
| Blume 2008 (8) | Mortality; n (%) | 3 (2) | 3 (2) | n/a | – |
| Blume 2008 (8) | No. of wound complications (edema, infection of wound, cellulitis, osteomyelitis); n (%) | 16 (10) | 11 (7) | – | No statistically significant difference in any individual adverse event |
| Chio 2010 (9) | No. of wound complications; n (%) | 7 (30) | 12 (44) | p = 0.816 | However, according to Table 2 of article NPWT 35% (8 of 23), not 30% (7 of 23) as stated in text |
| Keskin 2008 (10) | Increase in STAI; mean (SD) | 14.0 (2.3) | 2.6 (1.2) | p < 0.001 | Increase in fear during the first 10 days of treatment |
| Keskin 2008 (10) | Increase in HAM; mean (SD) | 4.4 (0.6) | 1.3 (0.6) | p < 0.001 | Increase in fear during the first 10 days of treatment |
| Stannard 2009 (11) | Wound complications: rate of deep wound infections; n (%) | 2 (5) | 7 (28) | p = 0.024 | – |
| Stannard 2009 (11) | Physical quality of life according to SF-36 after 6 months; mean (95% CI) | 43 (35 to 50) | 34 (29 to 39) | p = 0.049 | Results after 3 and 9 months also showed a statistically significant benefit for NPWT. |
| Stannard 2009 (11) | Mental quality of life according to SF-36 after 6 months; mean (95% CI) | n/a | n/a | n/a | Results after 3, 6, and 9 months all failed to show any statistically significant difference. |
| Systems not commercially available | |||||
| Bee 2008 (12) | Mortality; n (%) | 7 (26) | 5 (25) | n/a | 3 patients died within 7 days and were excluded from analysis. NPWT failed in a further 2 patients, who were successfully treated using the control therapy. |
| Bee 2008 (12) | Intestinal fistula formation; n (%) | (21) | (5) | p = 0.14 | – |
| Bee 2008 (12) | Abdominal abscess; n (%) | 12 (44) | 9 (47) | n/a | – |
| Mody 2008 (13) | Days to complete wound closure; mean (SD) | 36 (45) | 28 (19) | p = 0.66 | – |
| Mody 2008 (13) | No. of wound complications; n (%) | 6 (32) | 2 (6) | n/a | – |
| Perez 2010 (14) | Days to complete wound closure; mean (R) | 16 (14 to 23) | 25 (23 to 32) | p = 0.013 | – |
| Perez 2010 (14) | Secondary surgery rate | 7 | 4 | p = 0.038 | – |
| Saaiq 2010 (15) | 95% acceptance of skin transplant; n (%) | 45 (90) | 9 (18) | p < 0.001 | No patient characteristics reported for either treatment group. Categories from 1 to 3 given for endpoints. |
| Saaiq 2010 (15) | Mortality; n (%) | 0 | 0 | n/a | – |
| Saaiq 2010 (15) | No. of patients with complete wound closure within 2 weeks; n (%) | 45 (90) | 9 (18) | p < 0.001 | – |
| Saaiq 2010 (15) | No. of cases needing repeat skin transplantation: n (%) | 0 | 4 (8) | n/a | – |
| Sepúlveda 2009 (16) | Hospital mortality; n (%) | 0 | 0 | n/a | – |
| Sepúlveda 2009 (16) | Days to 90% wound granulation; mean (SD) | 19 (6) | 32 (14) | p = 0.007 | – |
RCT: Randomized controlled trial; –: not applicable; HAM: Hamilton Rating Scale; CI: Confidence interval; SF 36: short form (36) health survey; n/a: not available; NPWT: negative-pressure wound therapy; STAI: State-Trait Anxiety Inventory; R: range; SD: standard deviation
Adverse events were investigated in eight of the nine new trials (8, 9, 11, 12). Statistically significant differences in favor of NPWT were reported in three trials. The adverse events concerned were secondary amputations (8), the proportion of patients with deep wound infections (11), and the secondary surgery rate (14).
Differences in mortality rates between treatment groups were statistically insignificant (8, 12, 15, 16). This was also the case for most of the wound complication rates in four trials (8, 9, 12, 13).
One trial investigated health-related quality of life, using questionnaires (11). The results for the physical component (following treatment) were better in the NPWT group, and the difference was statistically significant. For the mental component, meanwhile, the results were comparable.
Another trial revealed more fear of treatment, e.g. due to possible pain, in the NPWT group than in the control group, and the difference was statistically significant (10).
Summary of results
Table 5shows the qualitative results of all 21 RCTs included in the present systematic review, in terms of the endpoints studied. The quantitative results of the 12 older RCTs covered in IQWiG reports can be found in the corresponding publications (3, 4), and the results of the nine new RCTs are shown in Table 3 and 4.
Table 5. Summary of results of all 21 randomized controlled trials (RCTs) included to date.
| RCT | Primary endpoint | Secondary endpoints | |||
| Complete wound closure | Time to complete wound closure | Reduction in wound size*1 | Mortality | Other adverse events*2, *3 | |
| Armstrong 2005 (e2) | +++ | +++ | n/a | (+) | (+) |
| Bee 2008 (12) | (+) | n/a | n/a | (–) | (–) |
| Blume 2008 (8) | +++ | +++ | +++ | (+) | (+) |
| Braakenburg 2006 (e9) | n/a | (+) | (+) | (+) | (–) |
| Chio 2010 (9) | n/a | n/a | n/a | n/a | (+) |
| Eginton 2003 (e3) | n/a | n/a | +++ | n/a | n/a |
| Ford 2002 (e4) | (–) | n/a | (+) | n/a | (–) |
| Joseph 2000 (e5) | n/a | +++ | +++ | n/a | +++ |
| Keskin 2008 (10)*4 | n/a | n/a | n/a | n/a | n/a |
| Llanos 2006 (e10) | n/a | n/a | +++ | n/a | n/a |
| Mody 2008 (13) | (–) | (–) | n/a | n/a | (–) |
| Moisidis 2004 (e6) | n/a | n/a | n/a | n/a | n/a |
| Mouës 2004 (e7) | n/a | (+) | +++ | n/a | n/a |
| Perez 2010 (14) | (–) | +++ | n/a | n/a | –- |
| Saaiq 2010 (15) | +++ | +++ | n/a | 0 | n/a |
| Sepúlveda 2009 (16) | n/a | +++ | n/a | 0 | n/a |
| Stannard 2006a (e11)*5 | n/a | n/a | n/a | n/a | (–) |
| Stannard 2006b (e11) | n/a | n/a | n/a | n/a | 0 |
| Stannard 2009 (11)*6 | n/a | n/a | n/a | n/a | –- |
| Vuerstaek 2006 (e12) | n/a | +++ | +++ | (–) | (–) |
| Wanner 2003 (e8) | n/a | (+) | (+) | n/a | n/a |
+++: statistically significant difference in favor of negative-pressure wound therapy (NPWT); –-: statistically significant difference in favor of comparator treatment; (+): insignificant difference in favor of NPWT; (–): insignificant difference in favor of comparator treatment; 0: no difference; n/a: not available
*1Wound size: Surface area or volume, statistically significant results reported primarily
*2Pain: Braakenburg 2006 referred to a study on pain but did not report its results; Vuerstaek 2006 reported a statistically significant benefit of NPWT, but there were already statistically significant differences in pain between the two groups initially (pain was lower at baseline in the NPWT group). This was not suitably taken into account in the evaluation.
*3Further results that were only reported singly and were therefore not included in the table: amputations: Armstrong 2005: (+); Blume 2008: +++; Braakenburg 2006 (+). Quality of life: Keskin 2008: –-; Stannard 2009: +++; Vuerstaek 2006: –- (in the first week of treatment)
*4Keskin 2008: fear during the first 10 days
*5Stannard 2006a: trial on hematomas; Stannard 2006b: trial on incisions in fractures
*6Stannard 2009: statistically significant difference after 3, 6 and 9 months
Discussion
Primary endpoint: complete wound closure
The results on complete wound closure are not homogenous, and it is impossible to be sure that NPWT performs better than the control treatments. Effects in favor of NPWT were reported in some trials, and no opposing effects could be detected in other trials. As a result, other systematic reviews also currently conclude that an additional benefit of NPWT in comparison to other types of wound treatment has not been proved (17, 18).
Secondary endpoints
Time to wound closure: In terms of the endpoint “time to wound closure,” effects in favor of NPWT groups were reported in most cases. However, there were considerable differences between trials in terms of the methods used to measure and evaluate wound closure; particularly problematic is the fact that no blinding was used when this endpoint was measured. In addition, most trials did not investigate whether wounds that had healed successfully actually remained closed in the longer term. The results thus cannot be interpreted as showing definitively that any one treatment is superior.
Adverse events: The results on adverse events were not homogenous. For some specific complications, such as secondary amputations, statistically significant effects in favor of NPWT groups were reported, but for a number of other adverse events no statistically significant difference was detected. No opposing results, i.e. statistically significant effects in favor of comparator groups, were recorded.
The difference between the number of patients included in trials and the number of patients treated worldwide is particularly striking when describing adverse effects. Data from the RCTs are of only limited use in evaluating the frequency of adverse events. It would be more appropriate if sufficiently large-scale RCTs were conducted.
The FDA recently issued a report on six deaths and 77 other complications that were reported within a two-year period in connection with NPWT (19). All the deaths were caused by acute hemorrhages, and known contraindications for NPWT (e.g. a large blood vessel exposed) had clearly been overlooked. Many of the deaths occurred in outpatient care or care homes, which highlights the need to monitor therapy. In this regard, it should be noted that trials of NPWT were generally conducted in hospitals.
Potential for bias
Of the nine included RCTs, eight have a high potential for bias. This limits the value of the results on the endpoints reported on. The difficulties of conducting RCTs and the arguments for and against including non-randomized trials when assessing medical devices and surgeries have been extensively described (e13, e14).
Heterogeneity
Strikingly, almost all the trials of commercially available NPWT systems were conducted in the USA. It seems that in developing countries the commercially available systems are very difficult to afford, and as a result such countries have developed their own NPWT systems, sometimes from very simple materials. Trials of these are now being conducted. This wide variety of NPWT systems makes the data considerably more difficult to interpret, although it is still largely unclear whether or not there are genuine differences between commercially available NPWT systems and those that are not commercially available. Also, the treatments administered to comparator groups (conventional dressings) were defined in different ways in different trials, probably as a result of differences between patient populations. This too can cause heterogeneity between trials and so limit the comparability of trial results.
Publication bias
According to the website of manufacturer KCI (August 2010), NPWT has been prescribed to more than 3 million patients, and some 600 peer-reviewed articles have been published on the subject. This and the low total number of RCTs make it astonishing that despite the frequency of acute and chronic wounds and the widespread use of NPWT a considerable number of trials have apparently had to be terminated due to recruitment problems. Although it seems that some planned RCTs had not even been started or were terminated soon after they began, the fact that there are RCTs on which nothing has been published casts doubt on the completeness of the data available for assessment of the benefits of NPWT.
Summary
Pool of trials
As the wounds for which NPWT is used vary greatly in their etiology, chronicity, size, and location, there is considerable variation between trials in the selection and definition of endpoints. This alone makes a quantitative summary of all trials of questionable value. In trials that provide results on wound healing, these results are mostly favorable for NPWT. These results are partly supported by statistically significant effects regarding the primary endpoint “complete wound closure” and the secondary endpoints “time to complete wound closure,” “reduction in wound size,” and “amputations.”
The results on overall mortality and total adverse events are inconsistent. A considerable proportion of the total deaths were probably not caused by treatment. Individual adverse events are reported too infrequently and inconsistently for conclusions to be drawn. Also, the group of adverse events as a whole is highly heterogenous. Some of the few results on quality of life are limited to the endpoint “fear” alone. Only a few trials investigated pain (in particular when dressings were changed).
The available pool of only 21 RCTs remains too small to provide a clear answer to the question of whether or not NPWT is superior to conventional wound treatment. The difficulty of interpreting the RCTs is caused essentially by the heterogeneity of the various indications for NPWT on the one hand, and the considerable qualitative and quantitative shortcomings of the trials on the other.
Outlook
Germany’s statutory health insurers jointly invited tenders for two RCTs on NPWT in July 2010 (20, e15). Patients with diabetic foot ulcers or iatrogenic wounds must be randomized to receive NPWT or conventional wound treatment, and patient numbers must be sufficient in each treatment group and for each indication. It is hoped that these trials will provide the further evidence needed for a decision on NPWT to be made. Eight other ongoing registered RCTs were also identified.
Conclusion
Although NPWT may have a positive effect on wound healing, there is no proof that it is either superior or inferior to conventional wound treatment. Further RCTs of good methodological quality are required.
Key Messages.
An update of a systematic review of the literature yielded a total pool of 21 randomized controlled trials (RCTs) on the subject of wound closure.
The incidence of complete wound closure was used as the primary endpoint.
A statistically significant difference in the primary endpoint was reported in only one trial.
A high potential for bias and diagnostic heterogeneity make the results difficult to interpret. Further RCTs of good methodological quality are therefore required.
Negative-pressure wound therapy (NPWT) may have a positive effect on wound healing.
Acknowledgments
Translated from the original German by Caroline Devitt, MA.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
This article was sponsored by the Institute for Quality and Efficiency in Health Care (Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen, IQWiG).
References
- 1.Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Annals of plastic surgery. 1997;38:563–577. [PubMed] [Google Scholar]
- 2.Fleischmann W, Strecker W, Bombelli M, Kinzl L. Vakuumversiegelung zur Behandlung des Weichteilschadens bei offenen Frakturen. Unfallchirurg. 1993;96:488–492. [PubMed] [Google Scholar]
- 3.IQWiG. Abschlussbericht N04-03. Köln: IQWiG Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen; 2006. Vakuumversiegelungstherapie von Wunden. www.iqwig.de. [Google Scholar]
- 4.IQWiG. Rapid Report N06-02. 2007. Vakuumversiegelungstherapie von Wunden. www.iqwig.de. [Google Scholar]
- 5.Gregor S, Maegele M, Sauerland S, Krahn JF, Peinemann F, Lange S. Negative pressure wound therapy: a vacuum of evidence? Arch Surg. 2008;143:189–196. doi: 10.1001/archsurg.2007.54. [DOI] [PubMed] [Google Scholar]
- 6.Peinemann F, McGauran N, Sauerland S, Lange S. Negative pressure wound therapy: potential publication bias caused by lack of access to unpublished study results data. BMC Med Res Methodol. 2008;8 doi: 10.1186/1471-2288-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.FDA. Chronic cutaneous ulcer and burn wounds—developing products for treatment. 2006. Draft guidance for industry. www.fda.gov/ [DOI] [PubMed] [Google Scholar]
- 8.Blume PA, Walters J, Payne W, Ayala J, Lantis J. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trial. Diabetes Care. 2008;31:631–636. doi: 10.2337/dc07-2196. [DOI] [PubMed] [Google Scholar]
- 9.Chio EG, Agrawal A. A randomized, prospective, controlled study of forearm donor site healing when using a vacuum dressing. Otolaryngol Head Neck Surg. 2010;142:174–178. doi: 10.1016/j.otohns.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Keskin M, Karabekmez FE, Yilmaz E, Tosun Z, Savaci N. Vacuum-assisted closure of wounds and anxiety. Scand J Plast Reconstr Surg Hand Surg. 2008;42:202–205. doi: 10.1080/02844310802091586. [DOI] [PubMed] [Google Scholar]
- 11.Stannard JP, Volgas DA, Stewart R, McGwin G, Jr, Alonso JE. Negative pressure wound therapy after severe open fractures: a prospective randomized study. J Orthop Trauma. 2009;23:552–557. doi: 10.1097/BOT.0b013e3181a2e2b6. [DOI] [PubMed] [Google Scholar]
- 12.Bee TK, Croce MA, Magnotti LJ, Zarzaur BL, Maish GO, 3rd, Minard G, et al. Temporary abdominal closure techniques: a prospective randomized trial comparing polyglactin 910 mesh and vacuum-assisted closure. J Trauma. 2008;65:337–342. doi: 10.1097/TA.0b013e31817fa451. [DOI] [PubMed] [Google Scholar]
- 13.Mody GN, Nirmal IA, Duraisamy S, Perakath B. A blinded, prospective, randomized controlled trial of topical negative pressure wound closure in India. Ostomy Wound Manage. 2008;54:36–46. [PubMed] [Google Scholar]
- 14.Perez D, Bramkamp M, Exe C, von Ruden C, Ziegler A. Modern wound care for the poor: a randomized clinical trial comparing the vacuum system with conventional saline-soaked gauze dressings. Am J Surg. 2010;199:14–20. doi: 10.1016/j.amjsurg.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 15.Saaiq M, Hameed Ud D, Khan MI, Chaudhery SM. Vacuum-assisted closure therapy as a pretreatment for split thickness skin grafts. J Coll Physicians Surg Pak. 2010;20:675–679. [PubMed] [Google Scholar]
- 16.Sepúlveda G, Espindola M, Maureira M, Sepúlveda E, Ignacio Fernández J, Oliva C, et al. Negative-pressure wound therapy versus standard wound dressing in the treatment of diabetic foot amputation. A randomised controlled trial. Cir Esp. 2009;86:171–177. doi: 10.1016/j.ciresp.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 17.AHRQ. Negative pressure wound therapy devices. Technology assessment report, project ID. WNDT1108. 2009 www.ahrq.gov/ [Google Scholar]
- 18.Ubbink DT, Westerbos SJ, Evans D, Land L, Vermeulen H. Topical negative pressure for treating chronic wounds. Cochrane Database Syst Rev. 2008;(3) doi: 10.1002/14651858.CD001898.pub2. CD001898. [DOI] [PubMed] [Google Scholar]
- 19.FDA. www.fda.gov/ 2009. Serious complications associated with negative pressure wound therapy systems. [Google Scholar]
- 20.EUTED. European Union Tenders Electronic Daily. 2010. Vergabeverfahren Studie zur Vakuumversiegelungstherapie. www.ted.europa. [Google Scholar]
- e1.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009 6 e1000097. [PMC free article] [PubMed] [Google Scholar]
- e2.Armstrong DG, Lavery LA. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet. 2005;366:1704–1710. doi: 10.1016/S0140-6736(05)67695-7. [DOI] [PubMed] [Google Scholar]
- e3.Eginton MT, Brown KR, Seabrook GR, Towne JB, Cambria RA. A prospective randomized evaluation of negative-pressure wound dressings for diabetic foot wounds. Ann Vasc Surg. 2003;17:645–649. doi: 10.1007/s10016-003-0065-3. [DOI] [PubMed] [Google Scholar]
- e4.Ford CN, Reinhard ER, Yeh D, Syrek D, De Las Morenas A, Bergman SB, et al. Interim analysis of a prospective, randomized trial of vacuum-assisted closure versus the healthpoint system in the management of pressure ulcers. Ann Plast Surg. 2002;49:55–61. doi: 10.1097/00000637-200207000-00009. [DOI] [PubMed] [Google Scholar]
- e5.Joseph E, Hamori CA, Bergman S, Roaf E, Swann NF, Anastasi GW. A prospective randomized trial of vacuum-assisted closure versus standard therapy of chronic nonhealing wounds Wounds. A Compendium of Clinical Research and Practice. 2000;12:60–67. [Google Scholar]
- e6.Moisidis E, Heath T, Boorer C, Ho K, Deva AK. A prospective, blinded, randomized, controlled clinical trial of topical negative pressure use in skin grafting. Plast Reconstr Surg. 2004;114:917–922. doi: 10.1097/01.prs.0000133168.57199.e1. [DOI] [PubMed] [Google Scholar]
- e7.Mouës CM, Vos MC, van den Bemd GJ, Stijnen T, Hovius SE. Bacterial load in relation to vacuum-assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen. 2004;12:11–17. doi: 10.1111/j.1067-1927.2004.12105.x. [DOI] [PubMed] [Google Scholar]
- e8.Wanner MB, Schwarzl F, Strub B, Zaech GA, Pierer G. Vacuum-assisted wound closure for cheaper and more comfortable healing of pressure sores: a prospective study. Scand J Plast Reconstr Surg Hand Surg. 2003;37:28–33. doi: 10.1080/713796078. [DOI] [PubMed] [Google Scholar]
- e9.Braakenburg A, Obdeijn MC, Feitz R, van Rooij IA, van Griethuysen AJ, Klinkenbijl JH. The clinical efficacy and cost effectiveness of the vacuum-assisted closure technique in the management of acute and chronic wounds: a randomized controlled trial. Plast Reconstr Surg. 2006;118:390–397. doi: 10.1097/01.prs.0000227675.63744.af. discussion 8-400. [DOI] [PubMed] [Google Scholar]
- e10.Llanos S, Danilla S, Barraza C, Armijo E, Pineros JL, Quintas M, et al. Effectiveness of negative pressure closure in the integration of split thickness skin grafts: a randomized, double-masked, controlled trial. Ann Surg. 2006;244:700–705. doi: 10.1097/01.sla.0000217745.56657.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e11.Stannard JP, Robinson JT, Anderson ER, McGwin G, Jr, Volgas DA, Alonso JE. Negative pressure wound therapy to treat hematomas and surgical incisions following high-energy trauma. J Trauma. 2006;60:1301–1306. doi: 10.1097/01.ta.0000195996.73186.2e. [DOI] [PubMed] [Google Scholar]
- e12.Vuerstaek JD, Vainas T, Wuite J, Nelemans P, Neumann MH, Veraart JC. State-of-the-art treatment of chronic leg ulcers: A randomized controlled trial comparing vacuum-assisted closure (VA.C.) with modern wound dressings. J Vasc Surg. 2006;44:1029–1037. doi: 10.1016/j.jvs.2006.07.030. discussion 38. [DOI] [PubMed] [Google Scholar]
- e13.Gottrup F, Apelqvist J. The challenge of using randomized trials in wound healing. Br J Surg. 2010;97:303–304. doi: 10.1002/bjs.7030. [DOI] [PubMed] [Google Scholar]
- e14.Hartling L, McAlister FA, Rowe BH, Ezekowitz J, Friesen C, Klassen TP. Challenges in systematic reviews of therapeutic devices and procedures. Ann Intern Med. 2005;142(12 Pt 2):1100–1111. doi: 10.7326/0003-4819-142-12_part_2-200506211-00010. [DOI] [PubMed] [Google Scholar]
- e15.AOK. Pressemitteilung Allgemeine Ortskrankenkassen. 2010. Studie zur Vakuumversiegelungstherapie bei chronischen Wunden ausgeschrieben. www.aok-bv.de/ [Google Scholar]