Abstract
Despite considerable advances in pharmacological, surgical and technology-based cardiovascular therapy, left ventricular dysfunction and heart failure are increasingly prevalent health problems. Recent studies suggest that angiogenic gene therapy can restore perfusion in ischaemic myocardial tissue, and that the transfer of nonangiogenic genes may correct defects in calcium handling that contribute to abnormal contractile function in patients with heart failure; however, large clinical trials of gene therapy for treatment of left ventricular dysfunction and heart failure have yet to be completed, and only a small number of genes have been evaluated in patients. Researchers continue to investigate new genes, combinations of genes and approaches that combine gene and cell therapy, and to develop novel expression vectors and delivery systems; collectively, these refinements promise to improve both patient response and safety.
Keywords: Gene therapy, Heart failure, Left ventricular, dysfunction
Background
LVD and heart failure are increasingly important health problems despite considerable advances in pharmacological, surgical and technological approaches to treatment. Heart failure is the leading medical cause of hospitalization and is expected to cost the USA health care system $37.2 billion in direct and indirect expenses in 2009 [1]. The most common cause of LVD is coronary artery disease, followed by hypertension and valvular disease. Heart failure, which often develops in patients who survive myocardial infarction, is a debilitating disease with high morbidity and can lead to frequent hospital re-admissions. The prognosis for patients with chronic heart failure remains poor. Nonpharmacological therapies, such as heart transplantation and the use of implantable left ventricular assist devices, are considered appropriate only in later stages of disease progression and, consequently, are considered for a very small fraction of younger patients.
The impaired cardiac contractile function associated with heart failure can be attributed to declines in perfusion and to unfavourable cardiac remodelling. Because gene therapy is designed to restore perfusion, and newer treatments may also target abnormalities in the contractile function of viable cardiomyocytes, it may be considered a promising alternative for the treatment of LVD and heart failure. Here, we describe several genes that are currently under investigation, the advantages and disadvantages of the vectors and delivery routes that are used most frequently, and the implications of findings from preclinical and early clinical trials of cardiovascular gene therapy.
Gene candidates
Numerous proteins, growth factors, cytokines and calcium-transport regulators have been the focus of angiogenic and myogenic research. Several genes have been investigated in vitro and in animal models, and the promising results of these studies have justified the first clinical evaluations. A number of these genes are described below, but only a few have been used clinically.
Vascular endothelial growth factor
VEGF, which could be considered the prototype for angiogenic gene therapy [2], has been shown to improve LVD in animal models of both ischaemic [2] and pacing-induced heart failure [3]. VEGF-A (also called VEGF-1) was first discovered in 1989 [4] and is the founding member of the VEGF protein family. VEGF-A is upregulated during pathological vessel growth [5], but it possesses a diverse array of pro-angiogenic properties and is the isoform used most frequently in gene therapy. Several VEGF-A derivatives, each containing a different number of amino acids (e.g., VEGF-121, VEGF-165, VEGF-189, and VEGF-206), can induce angiogenesis in animal models [6]; their solubilities and binding characteristics (e.g., to heparin and the extracellular matrix) differ, which influences their ability to interact with target cells and, presumably, alters their angiogenic potency. VEGF-165 is the most promising for use in angiogenic therapy, because it is the most potent (100-fold more potent than VEGF-121) and can induce the developmental gradients required to pattern vessel growth [7].
Fibroblast growth factor
The FGF family includes 23 members, of which acidic FGF (FGF-1) and bFGF or FGF-2 are the best characterized. The biological activity of FGF is not well understood. FGF proteins are potent mitogens for a variety of cell types, including endothelial cells, vascular smooth muscle cells and fibroblasts, and FGF secretion stimulates the synthesis of proteases that contribute to angiogenesis by digesting the extracellular matrix. Unlike VEGF, FGF-1 and FGF-2 are not crucial for embryogenesis, and because they lack a secretory signal sequence, they enter the extracellular space only passively after cell damage. Nevertheless, both have been used successfully to induce angiogenesis [8], and the secreted FGF isoforms, such as FGF-4 and FGF-5, may have even more therapeutic potential.
Hypoxia-inducible factor
The metabolic stimuli associated with hypoxia and ischaemia induce expression of a variety of transcription factors that stimulate angiogenesis, and researchers have begun to investigate the genetic transfer of these factors as an alternative to growth-factor-based gene therapy. Because interactions between HIFs and hypoxia response elements trigger an “angiogenesis programme” by upregulating the expression of a number of growth factors and cytokines simultaneously, the therapeutic administration of HIF could enhance vascular growth by mimicking the natural angiogenic response. HIF1-alpha regulates the expression of SDF-1, which is critically involved both in the mobilization of angiogenic progenitor cells from the bone marrow to the peripheral circulation and in the recruitment of mobilized cells to ischaemic tissue [9]. However, HIF1-alpha can also induce cell death and may be significantly less potent than VEGF [10].
Sonic hedgehog
Recent experiments performed in our laboratory indicate that the embryonic hedgehog signalling pathway can be reactivated to combat ischaemia in adult mammals. Recombinant Shh protein induces a robust angiogenic effect by upregulating multiple angiogenic factors, including VEGF, in interstitial mesenchymal cells [11], and genetic transfer of Shh enhances the regeneration of ischaemic myocardium by inducing the expression of trophic factors, such as SDF-1, which increases the recruitment and incorporation of bone marrow-derived progenitor cells into the growing vasculature [12]. We have also shown that Gli3, a transcription factor targeted by Shh during hedgehog signalling, is strongly upregulated in the ischaemic tissue of adult mammals and may have a favourable effect on myogenesis and angiogenesis after an ischaemic insult [13]. Because Shh appears to trigger a cascade of pro-angiogenic factors, it may be particularly effective for angiogenic gene therapy.
Stromal cell-derived factor-1
SDF-1 (also called CXC CXCL12) is a 68-amino-acid protein of the CXC chemokine family. Two isoforms, SDF-1-alpha and SDF-1-beta, are encoded as splice variants of a single gene and are expressed by both endothelial cells and stem cells. The growth factor activity of SDF-1 has most often been linked to lymphopoiesis and myelopoiesis [14], but interactions between SDF-1 and its receptor CXCR4 also regulate progenitor cell trafficking, and SDF-1-alpha is essential for the recruitment of stem and progenitor cells to ischaemic tissue [15].
Factors that regulate calcium transport
Abnormalities in the function of molecules responsible for the rhythmic release and uptake of Ca2+ ions in myocytes contribute to impaired cardiac contractility in patients with heart failure [16], and because myocardial contractility is dependent on ventricular Ca2+ handling, genetic modification of these molecules could be a viable approach for treatment of heart failure. One of the key Ca2+ handling abnormalities in both humans and experimental models of heart failure is caused by a defect in sarcoplasmic reticulum function, and a large body of experimental evidence indicates that SERCA2a plays an important role in the progression of dilated cardiomyopathy. SERCA2a activity is known to decline in late-stage heart failure, and SERCA2a protein and messenger RNA levels are reduced in cardiac tissue isolated from the failing hearts of patients and animals with heart failure [17,18]. Furthermore, gene therapy with a pseudophosphorylated mutant of phospholamban, the principal regulator of SERCA2a, treated cardiomyopathy in hamsters and infarction-induced heart failure in rats successfully for 6 months or more [19,20]. Preclinical studies also indicate that increases in cardiac adenylyl cyclase content improve left ventricular function, attenuate deleterious remodelling and reduce mortality in both heart failure and acute-infarction models. Investigations of adenylyl cyclase type 6 gene transfer have progressed from studies in cultured cardiac myocytes to animal models of heart failure [21].
Gene delivery
Vectors
Unlike protein administration, gene therapy can lead to high, sustained protein levels; however, the effectiveness of gene transfer depends on the transfection efficiency — the amount of the gene internalized by cells in the target tissue — and the magnitude and endurance of subsequent expression. The cellular insertion and intracellular trafficking of the transgene is facilitated by vectors, which can be categorized as viral or nonviral. Nonviral plasmid vectors were used in early investigations because they are inexpensive, easily constructed, and generally considered safe; plasmid vectors do not initiate inflammation or an immune response and incur no risk of insertional mutagenesis. Plasmid DNA is taken up effectively and expressed by all mammalian cell types, including cardiomyocytes, in vivo; however, the transfection efficiency of plasmid vectors was low in randomized, controlled trials [22,23]. Nevertheless, plasmid vectors could be useful when short-term modification of gene expression may be beneficial, such as immediately after an acute cardiac event, or for initiating mechanisms that lead to progenitor cell recruitment and to the activation of resident stem cells. More recently, small interfering RNAs have become popular for inhibition studies and could provide a new option for nonviral gene manipulation; very high transfection efficiency can be achieved by administering decoy receptors or antibodies that circumvent blocking factors in cardiovascular tissue.
The viral vectors used most frequently for cardiovascular gene therapy are adenoviruses and AAVs; retroviral vectors were used in early studies, but their popularity has declined because of safety concerns. Compared with plasmid transfection, viral transfection into vessel walls and heart muscle is much more efficient [24], and virally transfected genes are typically expressed for a longer period of time — AAV-transfected genes can be expressed for months — although the duration varies depending on the virus used. In addition, viral vectors typically infect only a limited number of cell types, and this specificity (i.e., tropism) can be advantageous for cardiovascular therapy. Adenoviruses seem particularly effective for transfecting cardiomyocytes [25], and naturally occurring tropisms for vascular smooth muscle cells and cardiomyocytes are among the more useful characteristics of AAVs. However, viral vectors can generate an inflammatory or immunogenic response and may integrate into the cellular genome, which could increase cancer risk. Nevertheless, adenoviruses have a good safety record in cardiovascular clinical trials [22,26], and the likelihood of inflammation, immunogenicity and host-genome integration varies depending on the vector used.
Routes of administration
Clinical acceptance of gene therapy as a routine treatment option will require the development of standardized, practical delivery systems and techniques for delivering the gene to the tissues targeted most frequently, such as the myocardium and blood vessels. Genes can be injected directly into the targeted tissue for treatment of peripheral disease, but local delivery to the heart used to require open-chest surgery or thoracoscopy. Intracoronary administration or catheter-based delivery systems (e.g., navigation and catheter mapping technology) for transendocardial gene delivery to the myocardium are much less invasive and more feasible for patients with LVD and heart failure [27].
Gene therapy for ischaemic or nonischaemic left ventricular dysfunction and heart failure
To date, most cardiovascular gene therapies are designed to increase vascular growth and perfusion in ischaemic tissue (i.e., therapeutic angiogenesis) [28]; however, declines in heart function can also be attributable to adverse cardiac remodelling, which evolves from a wide variety of biological changes, including the loss of functional cardiomyocytes and disorganization of the contractile response. These abnormalities may be suitable targets for nonangiogenic gene therapies designed to prevent or suppress the development of heart failure (Table 1).
Table 1.
Therapeutic goals and underlying mechanisms for the treatment of left ventricular dysfunction and heart failure.
| Therapeutic goal | Mechanisms |
|---|---|
| Vascularization | Angiogenesis |
| Vasculogenesis | |
| Arteriogenesis | |
| Lymphangiogenesis | |
| Endothelial function | |
| Endothelial repair | |
| Re-endothelialization | |
| SMC proliferation matrix | |
| Production/degradation | |
| Apoptosis | |
| Cardiomyogenesis | Cardiomyocyte homeostasis |
| Cellular contraction | |
| Calcium | |
| Hypertrophy | |
| Fibrosis | |
| Apoptosis |
SMC: smooth muscle cell.
Angiogenic gene therapy
Long-term survival after myocardial infarction is now the most common cause of chronic heart failure, and despite significant medical advances, postischaemic heart failure remains a primary contributor to morbidity and mortality in the western world [29]. One of the primary goals for treating heart failure is improving perfusion in the ischaemic region, thereby preserving functional tissue and (perhaps) restoring function in viable, but dormant (i.e., “hibernating”) myocardium. Several laboratories have demonstrated that therapeutic vascular growth can be achieved in vivo by the genetic transfer of cytokines [2,30]. VEGF and, to a lesser extent, FGF are the most frequently studied and best-developed cytokines used in the clinical setting.
In the REVASC trial [31], 67 patients with coronary artery disease, severe angina and no conventional options for revascularization were randomized to receive direct intramyocardial gene transfer of adenoviral VEGF-121 (AdVEGF-121) via minithoracotomy or to continue receiving maximal medical treatment. Exercise time, the primary efficacy endpoint, was significantly greater in patients treated with AdVEGF-121 than in the control group (P = 0.026), and there was no significant difference between the two treatment groups in overall adverse event occurrence. In a phase 1 study, five ‘no-option’ patients with occlusive coronary artery disease and mild LVD received VEGF-165 plasmid DNA (pVEGF-165) via intramyocardial injection after thoracotomy. Patients experienced improvements in collateral vessel growth, myocardial perfusion, myocardial contractile function and clinical status [32]; the same treatment was associated with significant declines in mean ischaemic area in patients with chronic myocardial ischaemia and moderate LVD (mean ejection fraction, 44 ± 4%) [33,34]. In the Euroinject One trial, 80 ‘no-option’ patients with severe, stable, ischaemic heart disease received pVEGF-165 (0.5 mg) or a placebo plasmid; the therapy appeared to be safe and was associated with improved regional wall motion and a favourable anti-ischaemic effect [23], but did not improve significantly stress-induced abnormalities in myocardial perfusion.
The AGENT trials evaluated the intracoronary injection of AdFGF-4 in patients with stable coronary artery disease. Positive trends were observed in the two small phase 1/2 trials (AGENT 1 and 2) [35,36], but the larger, double-blind, placebo-controlled trials (AGENT 3 and 4) were halted when an interim analysis of data from the AGENT 3 trial indicated that the primary endpoint would not reach statistical significance. The interim analysis found no significant safety concerns, and pooled analyses of AGENT 3 and AGENT 4 data identified a sex-specific benefit: compared with women in the placebo treatment group, women who were treated with AdFGF-4 displayed significantly greater improvement in clinical status [37].
Collectively, the results of the REVASC (AdVEGF-121), Euroinject One (phVEGF-165) and AGENT trials (AdFGF-4) indicate that AdVEGF and AdFGF therapy are safe and may induce neovascularization in patients with myocardial ischaemia who lack options for revascularization surgery but have relatively preserved left ventricular ejection fractions; however, only limited improvements were observed in many cardiovascular measures. Longer follow-up periods may be necessary, and because heart failure is a progressive disease, patients may need to receive multiple treatments before significant benefits are observed in global cardiovascular variables.
Nonangiogenic gene therapy
Although most studies of cardiovascular gene therapy have investigated the use of angiogenic factors, many other genes are potential candidates for the treatment of heart failure. Gene therapy with recombinant AAV vectors coding for the expression of SERCA2a, which declines in patients with heart failure, was well tolerated in both small- and large-animal heart failure models, and restoration of SERCA2a levels to normal levels led to a significant improvement in cardiac function. These findings prompted initiation of the first-in-human phase 1/2 CUPID trial [38]. In the open-label portion of this ongoing study, nine patients with advanced heart failure received a single intracoronary infusion of recombinant AAV SERCA2a; the treatment was associated with an acceptable safety profile and with improvements in a number of symptomatic and functional variables, which supports initiation of the ensuing phase 2, double-blind, placebo-controlled study. A second, randomized, double-blind study (ClinicalTrials.gov identifier: NCT00534703) is investigating the safety and feasibility of SERCA2a gene therapy when delivered with the AAV6 vector and driven by the cytomegalovirus promoter (AAV6-CMV-SERCA2a). Sixteen patients with advanced heart failure who have received a left ventricular assist device will be randomized to receive AAV6-CMV-SERCA2a or placebo infusion into the coronary arteries, and the recovery of contractile function will be assessed during attempts to wean patients from the left ventricular assist device. The results will be assessed in conjunction with two studies in the USA: one of which delivers the same vector via direct injection into the myocardium during left ventricular assist device insertion, and another in which an AAV1-CMV-SERCA2a vector is administered percutaneously.
A substantial amount of data accumulated during the past several years suggests that adenylyl cyclase 6 expression may have unexpected but pronounced favourable effects for the treatment of cardiovascular disease [39]. A clinical study (ClinicalTrials.gov identifier: NCT00787059) is currently underway to determine whether a type 5 adenovirus encoding this gene can be administered safely and is potentially beneficial in patients with congestive heart failure.
Combination therapy
As the characterization of individual gene therapies becomes more complete, preclinical investigations designed to identify the potential complementary or synergistic effects achieved with combinations of therapies have been initiated. The outcomes of these studies will be determined, in part, by the same variables that influence the effectiveness of single-gene therapy, including the model species, the delivery vector, the organ and disease treated, and the genes delivered.
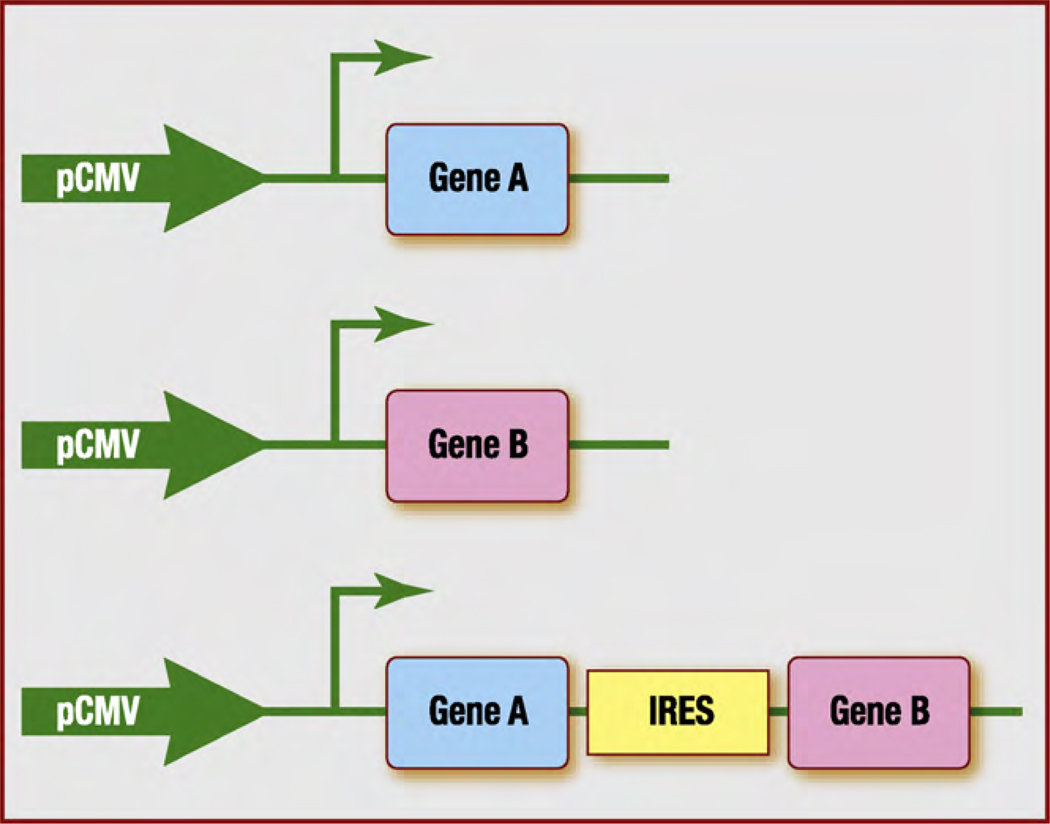
Two (or more) co-injected genes may not be expressed in the intended ratio, because one of the vectors could be preferentially silenced or removed [40]. Thus, therapeutic approaches that rely on combinations of genes will require the development of a gene transfer system that ensures the stable co-expression of both molecules. One system for inducing stable gene expression involves the use of IRESs. IRESs are structural elements located in the 5′ untranslated region of several mRNAs, where they permit the recruitment of translational machinery. These elements can be used to create expression cassettes that code for combinations of genes within a single mRNA sequence (Fig. 1). In a murine hindlimb ischaemia model, Rayssac et al. [41] showed that the expression of FGF-2 and Cyr61 was more stable when both genes were encoded by a single IRES-based vector rather than by two independent vectors, and vascular growth was more abundant, even though FGF-2 and Cyr61 protein levels were 5- to 10-fold lower in animals treated with the IRES vector. Furthermore, the vector encoding Cyr61 alone accelerates B16 melanoma growth in mice, whereas the IRES-based vector was not associated with carcinogenic effects. Thus, the risk-to-benefit ratio of this system appears to be very low and could likely be reduced even further, because many IRESs are tissue and/or context specific, and a third or fourth factor could be added to promote functional vessel growth with still lower doses of angiogenic factors.
Figure 1.
Combining gene therapies with an IRES-based system. With standard nonviral vectors, therapy that combines two genes (top and middle) requires the administration of two independent plasmids; however, the genes may not be expressed in the intended ratio, because one of the vectors could be preferentially silenced or removed. In an IRES-based system (bottom), a single vector can encode two (or more) genes separated by an IRES, thereby maintaining the intended expression ratio and decreasing the risk of adverse effects associated with the administration of several vectors. Collectively, these effects could substantially improve the risk-to-benefit ratio, which is of primary importance for potential clinical applications. IRES: internal ribosome entry site; pCMV: cytomegalovirus promoter.
Formation of a functional vascular network is a complex process involving multiple angiogenic factors that induce both capillary sprouting and the growth and remodelling of collateral arteries [42]. Experiments in animal models have shown that the recruitment and incorporation of endothelial progenitor cells can be increased with combinations of growth factors and/or cytokines [43], but combining granulocyte colony-stimulating factor-induced cell mobilization with intramyocardial VEGF injection did not improve heart function in humans, despite a significant increase in the number of circulating endothelial progenitor cells [23]. The authors concluded that higher VEGF gene doses and/or the administration of combinations of genes might be necessary to increase endothelial progenitor cell incorporation [44]. Alternatively, a single gene that regulates the expression of multiple angiogenic factors could be administered. For example, Shh, which is active during embryonic vasculogenesis, upregulates the expression of several angiogenic cytokines and enhances ventricular function in an animal model of myocardial infarction [12].
Like gene therapy, cell-based therapeutic approaches have not been completely characterized. Gene and cell therapy appear to have commonalities that could be exploited to enhance the benefit of each individual approach; furthermore, combined gene-cell therapy may be equally or even more beneficial at smaller doses than those required for either individual treatment, which may improve patient safety. Stem cells could be genetically modified before administration to improve the survival, differentiation and functional integration of both transplanted and endogenous cells, and advances in nanotechnology and tissue engineering have led to the development of pro-angiogenic matrices that deliver locally high concentrations of angiogenic growth factors or DNA that encodes growth factors [45]. These novel strategies may enable gene and cell therapy to be combined in a variety of tissue types for in situ biomedical applications.
From the bench to the bedside
A variety of biological, technical, methodological and disease-related factors have hindered the translation of gene therapy from the research laboratory to the clinic. In retrospect, the success of gene therapy in animal models may have generated unreasonably high expectations for its use in a clinical environment. Species-specific variations are unavoidable and chronic cardiovascular diseases are often polygenetic, so successful treatment with the onetime administration of a single gene appears unrealistic, especially as the disease has progressed for decades in most patients. The clinical success or failure of gene therapy is determined by the disease treated and the effectiveness of the gene itself, as well as the method of delivery and the delivery vector. Nonviral vectors are generally considered safe, but their transfection efficiency is low and, consequently, these vectors are of limited utility for treatments that require long-term expression. Viral vectors are efficiently transfected, but immunogenic and pathogenic concerns have prompted research into alternative, novel virus serotypes. Transfection efficiency is likely to increase as new vectors are identified, and methods that target vectors to specific tissues could also increase therapeutic potency and reduce adverse effects; however, targeted administration must overcome yet another set of technical limitations. A precise understanding of the mechanisms underlying neovascularization, including the time course and sequential roles of angiogenic and trophic factors, will enable researchers to better mimic the endogenous regenerative response.
Conclusions
Results from clinical trials suggest that cardiovascular gene therapy is safe but provides only limited improvements in global cardiovascular variables. However, only a small fraction of potential genetic targets have been investigated in patients, and large clinical trials of gene therapy for treatment of LVD and heart failure have yet to be completed. Furthermore, angiogenic gene therapy appears to restore perfusion in ischaemic myocardial tissue, and the transfer of nonangiogenic genes may correct the defects in calcium handling that contribute to abnormal contractile function in patients with heart failure. New genes, combinations of genes, expression vectors, delivery systems and approaches that combine gene and cell therapy continue to be developed and tested rigorously; collectively, these refinements promise to improve both patient response and safety.
Acknowledgements
We thank W. Kevin Meisner, Ph.D., ELS for editorial support. This work was supported in part by NIH grants R01 HL53354, R01 HL77428, R01 HL80137 and R01 HL95874 awarded to DouglasW. Losordo. Jerome Roncalli was supported by the French Federation of Cardiology. Jörn Tongers was supported by a Midwest Affiliate Postdoctoral Fellowship from the American Heart Association, the German Heart Foundation and Solvay Pharmaceuticals.
Abbreviations
- AAV
adeno-associated virus
- CMV
cytomegalovirus
- DNA
deoxyribonucleic acid
- FGF
fibroblast growth factor
- HIF
hypoxia-inducible factor
- IRES
internal ribosome entry sites
- LVD
left ventricular dysfunction
- RNA
ribonucleic acid
- SDF-1
stromal cell-derived factor-1
- SERCA2a
sarcoplasmic reticulum calcium adenosine triphosphatase
- Shh
sonic hedgehog
- VEGF
vascular endothelial growth factor
- CXCL12
chemokine ligand 12
- bFGF
basic FGF
- AdVEGF-121
adenovial VEGF-121
- AGENT
Angiogenic Gene Therapy
- AdFGF-4
adenoviral FGF-4
- CUPID
calcium upregulation percutaneous administration of gene therapy in cardiac disease
Footnotes
Conflict of interest statement
There are no conflicts of interest.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics – 2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Rissanen TT, Yla-Herttuala S. Current status of cardiovascular gene therapy. Mol Ther. 2007;15:1233–1247. doi: 10.1038/sj.mt.6300175. [DOI] [PubMed] [Google Scholar]
- 3.Leotta E, Patejunas G, Murphy G, et al. Gene therapy with adenovirus-mediated myocardial transfer of vascular endothelial growth factor 121 improves cardiac performance in a pacing model of congestive heart failure. J Thorac Cardiovasc Surg. 2002;123:1101–1113. doi: 10.1067/mtc.2002.121044. [DOI] [PubMed] [Google Scholar]
- 4.Leung DW, Cachianes G, Kuang WJ, et al. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 5.Rissanen TT, Vajanto I, Hiltunen MOetal, et al. Expression of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 (KDR/Flk-1) in ischemic skeletal muscle and its regeneration. Am J Pathol. 2002;160:1393–1403. doi: 10.1016/S0002-9440(10)62566-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeshita S, Weir L, Chen D, et al. Therapeutic angiogenesis following arterial gene transfer of vascular endothelial growth factor in a rabbit model of hindlimb ischemia. Biochem Biophys Res Commun. 1996;227:628–635. doi: 10.1006/bbrc.1996.1556. [DOI] [PubMed] [Google Scholar]
- 7.Yla-Herttuala S, Rissanen TT, Vajanto I, et al. Vascular endothelial growth factors: biology and current status of clinical applications in cardiovascular medicine. J Am Coll Cardiol. 2007;49:1015–1026. doi: 10.1016/j.jacc.2006.09.053. [DOI] [PubMed] [Google Scholar]
- 8.Ueno H, Li JJ, Masuda S, et al. Adenovirus-mediated expression of the secreted form of basic fibroblast growth factor (FGF-2) induces cellular proliferation and angiogenesis in vivo. Arterioscler Thromb Vasc Biol. 1997;17:2453–2460. doi: 10.1161/01.atv.17.11.2453. [DOI] [PubMed] [Google Scholar]
- 9.Ceradini DJ, Kulkarni AR, Callaghan MJetal, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 10.Carmeliet P, Dor Y, Herbert JM, et al. Role of HIF-1 alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 11.Pola R, Ling LE, Silver M, et al. The morphogen sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med. 2001;7:706–711. doi: 10.1038/89083. [DOI] [PubMed] [Google Scholar]
- 12.Kusano KF, Pola R, Murayama T, et al. Sonic hedgehog myocardial gene therapy: tissue repair through transient reconstitution of embryonic signalling. Nat Med. 2005;11:1197–1204. doi: 10.1038/nm1313. [DOI] [PubMed] [Google Scholar]
- 13.Renault MA, Roncalli J, Tongers J, et al. The hedgehog transcription factor Gli3 modulates angiogenesis. Circ Res. 2009;105:818–826. doi: 10.1161/CIRCRESAHA.109.206706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tashiro K, Tada H, Heilker R, et al. Signal sequence trap: a cloning strategy for secreted proteins and type I membrane proteins. Science. 1993;261:600–603. doi: 10.1126/science.8342023. [DOI] [PubMed] [Google Scholar]
- 15.Abbott JD, Huang Y, Liu D, et al. Stromal cell-derived factor-1 alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 16.Pieske B, Maier LS, Bers DM, et al. Ca2+ handling and sarcoplasmic reticulum Ca2+ content in isolated failing and nonfailing human myocardium. Circ Res. 1999;85:38–46. doi: 10.1161/01.res.85.1.38. [DOI] [PubMed] [Google Scholar]
- 17.Hasenfuss G, Reinecke H, Studer R, et al. Relation between myocardial function and expression of sarcoplasmic reticulum Ca(2+)-ATPase in failing and nonfailing human myocardium. Circ Res. 1994;75:434–442. doi: 10.1161/01.res.75.3.434. [DOI] [PubMed] [Google Scholar]
- 18.Schwinger RH, Bohm M, Schmidt U, et al. Unchanged protein levels of SERCA II and phospholamban but reduced Ca2+ uptake and Ca(2+)-ATPase activity of cardiac sarcoplasmic reticulum from dilated cardiomyopathy patients compared with patients with nonfailing hearts. Circulation. 1995;92:3220–3228. doi: 10.1161/01.cir.92.11.3220. [DOI] [PubMed] [Google Scholar]
- 19.Hoshijima M, Ikeda Y, Iwanaga Y, et al. Chronic suppression of heart-failure progression by a pseudophosphorylated mutant of phospholamban via in vivo cardiac rAAV gene delivery. Nat Med. 2002;8:864–871. doi: 10.1038/nm739. [DOI] [PubMed] [Google Scholar]
- 20.Iwanaga Y, Hoshijima M, Gu Y, et al. Chronic phospholamban inhibition prevents progressive cardiac dysfunction and pathological remodeling after infarction in rats. J Clin Invest. 2004;113:727–736. doi: 10.1172/JCI18716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai NC, Roth DM, Gao MH, et al. Intracoronary adenovirus encoding adenylyl cyclase VI increases left ventricular function in heart failure. Circulation. 2004;110:330–336. doi: 10.1161/01.CIR.0000136033.21777.4D. [DOI] [PubMed] [Google Scholar]
- 22.Hedman M, Hartikainen J, Syvanne M, et al. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio Angiogenesis Trial (KAT) Circulation. 2003;107:2677–2683. doi: 10.1161/01.CIR.0000070540.80780.92. [DOI] [PubMed] [Google Scholar]
- 23.Kastrup J, Jorgensen E, Ruck A, et al. Direct intramyocardial plasmid vascular endothelial growth factor-A165 gene therapy in patients with stable severe angina pectoris. A randomized double-blind placebo-controlled study: the Euroinject One trial. J Am Coll Cardiol. 2005;45:982–988. doi: 10.1016/j.jacc.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 24.Wright MJ, Wightman LM, Lilley C, et al. In vivo myocardial gene transfer: optimization, evaluation and direct comparison of gene transfer vectors. Basic Res Cardiol. 2001;96:227–236. doi: 10.1007/s003950170053. [DOI] [PubMed] [Google Scholar]
- 25.Nalbantoglu J, Pari G, Karpati G, et al. Expression of the primary coxsackie and adenovirus receptor is downregulated during skeletal muscle maturation and limits the efficacy of adenovirus-mediated gene delivery to muscle cells. Hum Gene Ther. 1999;10:1009–1019. doi: 10.1089/10430349950018409. [DOI] [PubMed] [Google Scholar]
- 26.Makinen K, Manninen H, Hedman M, et al. Increased vascularity detected by digital subtraction angiography after VEGF gene transfer to human lower limb artery: a randomized, placebo-controlled, double-blinded phase II study. Mol Ther. 2002;6:127–133. doi: 10.1006/mthe.2002.0638. [DOI] [PubMed] [Google Scholar]
- 27.Rutanen J, Rissanen TT, Markkanen JE, et al. Adenoviral catheter-mediated intramyocardial gene transfer using the mature form of vascular endothelial growth factor-D induces transmural angiogenesis in porcine heart. Circulation. 2004;109:1029–1035. doi: 10.1161/01.CIR.0000115519.03688.A2. [DOI] [PubMed] [Google Scholar]
- 28.Yla-Herttuala S, Alitalo K. Gene transfer as a tool to induce therapeutic vascular growth. Nat Med. 2003;9:694–701. doi: 10.1038/nm0603-694. [DOI] [PubMed] [Google Scholar]
- 29.Gheorghiade M, Bonow RO. Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation. 1998;97:282–289. doi: 10.1161/01.cir.97.3.282. [DOI] [PubMed] [Google Scholar]
- 30.Vincent KA, Jiang C, Boltje I, et al. Gene therapy progress and prospects: therapeutic angiogenesis for ischemic cardiovascular disease. Gene Ther. 2007;14:781–789. doi: 10.1038/sj.gt.3302953. [DOI] [PubMed] [Google Scholar]
- 31.Stewart DJ, Hilton JD, Arnold JM, et al. Angiogenic gene therapy in patients with nonrevascularizable ischemic heart disease: a phase 2 randomized, controlled trial of AdVEGF(121) (AdVEGF-121) versus maximum medical treatment. Gene Ther. 2006;13:1503–1511. doi: 10.1038/sj.gt.3302802. [DOI] [PubMed] [Google Scholar]
- 32.Losordo DW, Vale PR, Symes JF, et al. Gene therapy for myocardial angiogenesis: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation. 1998;98:2800–2804. doi: 10.1161/01.cir.98.25.2800. [DOI] [PubMed] [Google Scholar]
- 33.Vale PR, Losordo DW, Milliken CE, et al. Left ventricular electromechanical mapping to assess efficacy of phVEGF(165) gene transfer for therapeutic angiogenesis in chronic myocardial ischemia. Circulation. 2000;102:965–974. doi: 10.1161/01.cir.102.9.965. [DOI] [PubMed] [Google Scholar]
- 34.Vale PR, Losordo DW, Milliken CE, et al. Randomized, single-blind, placebo-controlled pilot study of catheter-based myocardial gene transfer for therapeutic angiogenesis using left ventricular electromechanical mapping in patients with chronic myocardial ischemia. Circulation. 2001;103:2138–2143. doi: 10.1161/01.cir.103.17.2138. [DOI] [PubMed] [Google Scholar]
- 35.Grines CL, Watkins MW, Helmer G, et al. Angiogenic Gene Therapy (AGENT) trial in patients with stable angina pectoris. Circulation. 2002;105:1291–1297. doi: 10.1161/hc1102.105595. [DOI] [PubMed] [Google Scholar]
- 36.Grines CL, Watkins MW, Mahmarian JJ, et al. A randomized, double-blind, placebo-controlled trial of Ad5FGF-4 gene therapy and its effect on myocardial perfusion in patients with stable angina. J Am Coll Cardiol. 2003;42:1339–1347. doi: 10.1016/s0735-1097(03)00988-4. [DOI] [PubMed] [Google Scholar]
- 37.Henry TD, Grines CL, Watkins MW, et al. Effects of Ad5FGF-4 in patients with angina: an analysis of pooled data from the AGENT 3 and AGENT 4 trials. J Am Coll Cardiol. 2007;50:1038–1046. doi: 10.1016/j.jacc.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 38.Jaski BE, Jessup ML, Mancini DM, et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J Card Fail. 2009;15:171–181. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hammond HK. Adenylyl cyclase gene transfer in heart failure. Ann N Y Acad Sci. 2006;1080:426–436. doi: 10.1196/annals.1380.032. [DOI] [PubMed] [Google Scholar]
- 40.Allera-Moreau C, Delluc-Clavieres A, Castano C, et al. Long-term expression of bicistronic vector driven by the FGF-1 IRES in mouse muscle. BMC Biotechnol. 2007;7:74. doi: 10.1186/1472-6750-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rayssac A, Neveu C, Pucelle M, et al. IRES-based vector co-expressing FGF-2 and Cyr61 provides synergistic and safe therapeutics of lower limb ischemia. Mol Ther. 2009;17:2010–2019. doi: 10.1038/mt.2009.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grundmann S, Piek JJ, Pasterkamp G, et al. Arteriogenesis: basic mechanisms and therapeutic stimulation. Eur J Clin Invest. 2007;37:755–766. doi: 10.1111/j.1365-2362.2007.01861.x. [DOI] [PubMed] [Google Scholar]
- 43.Kawamoto A, Murayama T, Kusano K, et al. Synergistic effect of bone marrow mobilization and vascular endothelial growth factor-2 gene therapy in myocardial ischemia. Circulation. 2004;110:1398–1405. doi: 10.1161/01.CIR.0000141563.71410.64. [DOI] [PubMed] [Google Scholar]
- 44.Ripa RS, Wang Y, Jorgensen E, et al. Intramyocardial injection of vascular endothelial growth factor-A165 plasmid followed by granulocyte-colony stimulating factor to induce angiogenesis in patients with severe chronic ischaemic heart disease. Eur Heart J. 2006;27:1785–1792. doi: 10.1093/eurheartj/ehl117. [DOI] [PubMed] [Google Scholar]
- 45.Shea LD, Smiley E, Bonadio J, et al. DNA delivery from polymer matrices for tissue engineering. Nat Biotechnol. 1999;17:551–554. doi: 10.1038/9853. [DOI] [PubMed] [Google Scholar]