Abstract
CD45+ and collagen I–positive (Col+) fibrocytes are implicated in fibrogenesis in skin, lungs, and kidneys. Fibrocyte migration in response to liver injury was investigated using bone marrow (BM) from chimeric mice expressing luciferase (Col-Luc→wt) or green fluorescent protein (Col-GFP→wt) under control of the α1(I) collagen promoter and enhancer, respectively. Monitored by luciferase expression, recruitment of fibrocytes was detected in CCl4-damaged liver and in spleen. Migration of CD45+Col+ fibrocytes was regulated by chemokine receptors CCR2 and CCR1, as demonstrated, respectively, by 50% and 25% inhibition of fibrocyte migration in Col-LucCCR2−/−→wt and Col-LucCCR1−/−→wt mice. In addition to CCR2 and CCR1, egress of BM CD45+Col+ cells was regulated by transforming growth factor-β1 (TGF-β1) and liposaccharide in vitro and in vivo, which suggests that release of TGF-β1 and increased intestinal permeability have important roles in fibrocyte trafficking. In the injured liver, fibrocytes gave rise to (myo)fibroblasts. In addition, a BM population of CD45+Col+ cells capable of differentiation into fibrocytes in culture was identified. Egress of CD45+Col+ cells from BM was detected in the absence of injury or stress in aged mice but not in young mice. Development of liver fibrosis was also increased in aged mice and correlated with high numbers of liver fibrocytes. In conclusion, in response to liver injury, fibrocytes migrate from BM to the liver. Their migration is regulated by CCR2 and CCR1 but is compromised with age.
Hepatic fibrosis is characterized by activation of extracellular matrix producing myofibroblasts and accumulation of collagen type I.1 It is closely associated with release of transforming growth factor-β1 (TGF-β1), the major fibrogenic cytokine, and with translocation of bacterial products into the blood.2 Although hepatic stellate cells (HSCs) are believed to be a major source of collagen type I in the fibrotic liver,1 bone marrow (BM)–derived fibrocytes, defined by simultaneous expression of CD45 and collagen type I,3 are also implicated in the pathogenesis of liver fibrosis.4 Fibrocytes contribute to fibrogenesis of skin, lungs, kidneys, and liver.5 It has been demonstrated that CD45+ and collagen α1(I)+ fibrocytes migrate to the injured liver in response to cholestasis4 and comprise approximately 3% to 5% of the cells producing collagen type I. Despite low numbers, inhibition of fibrocyte recruitment via administration of human serum amyloid protein,6 a natural inhibitor of fibrocyte migration and differentiation, effectively attenuated fibrosis in lungs,7 kidneys,8 and liver (our unpublished observation), emphasizing an important role for fibrocytes in fibrogenesis of parenchymal organs. A Phase II clinical trial is under way to assess the effectiveness of human serum amyloid protein in postsurgical fibrosis in patients with glaucoma. Fibrocytes have been isolated from fibrotic tissues, spleen, and peripheral blood.3,9 Cultured blood-derived fibrocytes are spindle-shaped and obtain a myofibroblast phenotype on differentiation on plastic or in response to TGF-β1.9 In addition to collagen, fibronectin, and vimentin, fibrocytes express CD45, CD34, MHCII, CD11b, Gr1, CD54, CD80, and CD86 and secrete growth factors (TGF-β1 and monocyte chemotactic protein 1) that promote deposition of extracellular matrix.9,10
Migration of fibrocytes to fibrotic lungs and kidneys is regulated on several levels including profibrogenic growth factors (eg, TGF-β1) and chemokines (CCL2, CCL3, and CCL12). Reduced fibrocyte recruitment has been observed in CCR5−/− and CCR2−/− mice11,12 and correlates with attenuation of lung fibrosis. Migration of fibrocytes to fibrotic kidneys is regulated by chemokine receptors CCR2, CCR7, and CXCR413,14 and is highly restricted to damaged organs.4,13,14
Recruitment of fibrocytes to the site of injury is not only an important biomarker of tissue fibrosis but contributes to the host response.15 However, little is known about the migration of fibrocyte precursors from BM to injured liver. The present study charts fibrocyte migration to the target organ or organs in response to fibrogenic liver injury.
Materials and Methods
Mice
C57BL/6 and CCR2−/− mice (004888 on Bl/6 background) were purchased from Jackson Laboratory (Bar Harbor, ME), and CCR1−/− mice (004087-M on BL/6 background) were purchased from Taconic Farms, Inc. (Hudson, NY).
Generation of Collagen-α1(I)–Luciferase Transgenic Mice
Collagen-α1(I)–luciferase (Col-Luc) transgenic mice express luciferase under the control of the collagen-α1(I) promoter/enhancer. The transgenic construct was generated using pGL3(R2.1)-Basic vector containing the hlucP+ reporter gene (synthetic firefly luciferase including hPEST; Promega Corp., Madison, WI). Collagen α1(I) promoter/enhancer was inserted into the plasmid using KpnI and BglII restriction enzymes. The transgenic construct was excised using KpnI and SalI unique restriction enzymes and microinjected into fertilized C57BL/6J × CBA F1 hybrid embryos, which were implanted in pseudo-pregnant Swiss Webster foster mothers. The offspring (founders) were genotyped using Southern blot analysis HindIII digested tail DNA with a 32P-deoxyadenosine triphosphate–labeled luciferase probe. Luciferase expression was determined in the total protein extracts from tail snips using a microplate reader (FLUOstar Optima; BMG Labtech GmbH, Offenburg, Germany). All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of California, San Diego.
Generation of Fibrocyte-Specific Chimeric Mice Transplanted with Col-Luc BM
To generate fibrocyte-specific mice, BM from chimeric mice transplanted with Col-Luc (Col-Luc→wt mice) or chimeric mice transplanted with Col–green fluorescent protein BM (Col-GFP→wt mice) was transplanted into lethally irradiated (1200 rad) wild-type mice (C57/Bl6).
Induction of Liver Injury and Lipopolysaccharide Therapy in Mice
Liver injury was induced via intragastric gavage using CCl4 (diluted 1:4 in corn oil, 150 μL, 12 times over 6 weeks), intravenous infection with TGF-β1–expressing or control adenovirus (1 × 108 PFU) for 72 hours, or surgical ligation of the common bile duct (BDL).4 Lipopolysaccharide (LPS), 6 μg/g weight, was administered i.v. in mice.
Transwell Migration Assay
BM CD45+Col+ cells were placed in the upper chamber of the polyethylene terephthalate track-etched membrane (HTS FluoroBlock Insert, 8.0 μm pore size; BD Biosciences, Franklin Lakes, NJ), and 5 ng/mL TGF-β1 or 1 ng/mL LPS was placed in the bottom chamber. The number of green fluorescent protein (GFP)–positive cells that transmigrated to the bottom chamber was analyzed using fluorescent microscopy after 18 hours, and was calculated relative to the passive transmigration.
Imaging of Fibrocyte-Specific Col-Luc→wt Mice
Bioluminescence was produced in Col-Luc→wt mice via the reaction in situ between firefly luciferase and D-luciferin (3 mg/200 μL i.v.; Xenogen Corp., Alameda, CA), and detected using the IVIS-200 Optical Imaging System (Xenogen Corp.). Images were acquired for 4 minutes using “photon” unit selection, analyzed using Living Image 3.0 software (Xenogen Corp.), and presented as total photon flux [photons per second per square centimeter per steradian (p/s/cm2/sr)].
Immunofluorescence and IHC
Formalin-fixed frozen liver tissues, hepatic fibrocytes,4 and BM cells were isolated as described4 and stained with anti-luciferase antibody (Abcam, Inc., Cambridge, MA), anti–α-smooth muscle actin (SMA) antibody (Abcam, Inc.), anti-CD45 (eBioscience, Inc., San Diego, CA), and anti–collagen type I antibody (sc-25974; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). The images were obtained using Olympus IX71 and Olympus FV1000 confocal microscopes (Olympus Corp., Tokyo, Japan).
Live Liver Microscopy
CCl4-treated Col-GFP→wt mice were euthanized via anesthesia overdose. The portal vein was cannulated with a PE 10 tube, and 5 mg/mL red fluorescent tetramethylrhodamine-dextran (molecular weight, 150 kDa) was injected. The large liver lobe was exteriorized and viewed using an upright microscope (E600FN; Nikon Instruments, Inc., Melville, NY) equipped with a multiphoton system (Radiance 2100; Bio-Rad Laboratories, Inc., Hercules, CA). Excitation was set at 840 nm, and images of 1-μm optical sections were acquired using a 40× water immersion objective, and spectrally separated. Second harmonic signal of collagen was detected in the blue channel, and two-photon fluorescence of GFP and tetramethylrhodamine-dextran in green and red, respectively.
Flow Cytometry
Cell sorting for CD45-PE+ and Col-GFP+ BM cells was performed using a MoFlo (Beckman Coulter, Inc., Fullerton, CA). Phenotyping of CD45+Col+ BM cells was performed using a FACSCanto Flow Cytometry System (BD Biosciences, San Jose, CA) using the antibodies from eBioscience and Cytofix/Cytoperm Fixation and Permeabilization Solution (BD Biosciences).
Whole-Mouse Genome Gene Expression Microarray
Splenic fibrocytes from young (6 weeks; n = 7) and aged (13 to 15 months; n = 5) mice treated using 6 μg/g LPS were compared using the Whole Mouse Genome Microarray (Agilent Technologies, Inc., Santa Clara, CA). Total RNA was isolated using RNeasy columns (Qiagen, Inc., Valencia, CA), labeled, and hybridized to a microarray (4×44K). Each sample was analyzed in duplicate. Slides were scanned using the GZ505B scanner and analyzed using Gene Spring Software (both from Agilent Technologies, Inc.). The data were normalized using a modified LOESS (locally weighted scatterplot smoothing) program, and functional enrichment analysis was performed using DAVID (Database for Annotation, Visualization, and Integrated Discovery) (http://david.abcc.ncifcrf.gov). Expression of selected genes was confirmed using RT-PCR and flow cytometry.
Quantitative RT-PCR
Total RNA was isolated from cells using RNeasy columns (Qiagen, Inc.). First-strand cDNA was synthesized using SuperScript III and random hexamers (Invitrogen Corp., Carlsbad, CA). Samples were run using a real-time PCR cycler (AB1 PRISM 7300; Applied Biosystems, Inc., Foster City, CA). Gene expression levels were calculated after normalization to the standard housekeeping gene GAPDH using the ΔΔCt method as described by the manufacturer (Invitrogen Corp.) and were expressed as relative mRNA levels compared with control. The results are given as mean ± SEM. P < 0.0001 was considered statistically significant.
Statistical Analysis
Statistical analysis for significant differences was performed using one-way analysis of variance with a Bonferroni post test when comparing different time points. When the design of experiments involved analysis of different genotypes over time, two-way analysis of variance with a Bonferroni post test was used. Unless otherwise stated, the number of mice included in the experiments was 10. Statistical analysis was performed using PRISM version 5.0b for Mac (GraphPad Software, Inc., San Diego, CA) and IBM SPSS Statistics version 19 for Windows (SPSS, Inc., Chicago, IL).
Results
Sustained Liver Injury Triggers Fibrocyte Recruitment and Differentiation Into (Myo)fibroblasts
To investigate recruitment of CD45+Col+ cells in response to hepatotoxic liver injury, BMT chimeric Col-GFP→wt mice4 were generated via transplantation of collagen α1(I) promoter/enhancer-GFP+ BM16 into lethally-irradiated wild-type mice and then subjected to liver injury induced by CCl4 (16 injections over 8 weeks; Figure 1A). Monitored by GFP expression, BM-derived Col+ cells were detected in fibrotic liver of CCl4-treated but not control (corn oil–treated) Col-GFP→wt mice. Live liver microscopy of fibrotic liver revealed simultaneous presence of GFP+ myofibroblast-like and round fibrocytes (Figure 1B; see also Supplemental Videos S1 and S2 at http://ajp.amjpathol.org), which suggests that sustained liver injury triggers fibrocyte differentiation into (myo)fibroblasts in situ. In concordance, isolated liver fibrocytes rapidly obtained myofibroblastic phenotype in culture (Figure 1C). Similar to HSCs, fibrocyte-derived myofibroblasts up-regulated α-SMA in culture but are differentiated from HSCs by lack of vitamin A.1
Figure 1.
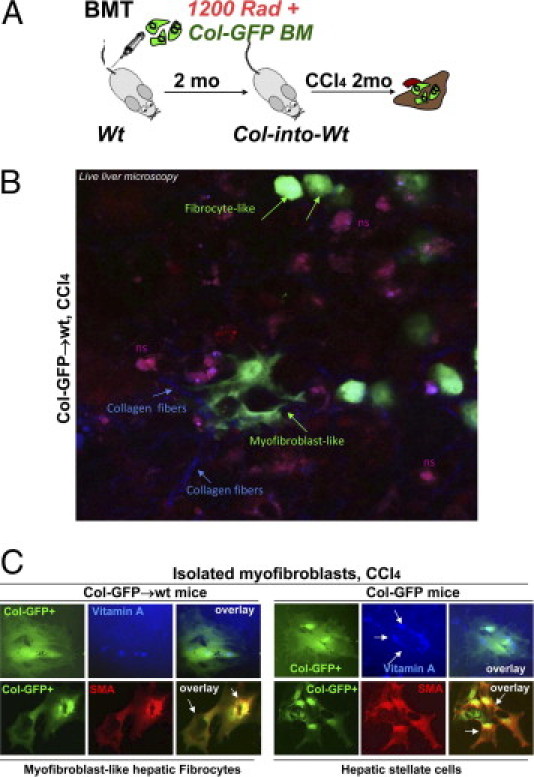
Liver fibrocytes have potential to differentiate into (myo)fibroblasts. A: Study design. BMT chimeric Col-GFP→wt mice were subjected to CCl4 for 2 months. B: Round and (myo)fibroblast-like collagen α1(I)–GFP+ cells (green arrows) were detected using fluorescent microscopy in fibrotic livers of CCl4-treated Col-GFP→wt mice (n = 4). Collagen fibers (blue arrows) are shown. ns, Nonspecific autofluorescence of fibrotic livers. C: GFP+ (myo)fibroblasts from CCl4-treated Col-into-wt mice were compared with GFP+ HSCs from CCl4-treated Col-GFP mice using fluorescent microscopy for expression of vitamin A (blue) and α-SMA (red).
Generation of Col-Luc→wt Mice
To study fibrocyte migration in real time in live mice, the collagen α1(I) promoter/enhancer16 was used to drive expression of luciferase in mice (Col-Luc) (Figure 2, A and B). To achieve luciferase expression only in fibrocytes and their precursors, BM from Col-Luc mice was transplanted into lethally irradiated wild-type recipients (Figure 2C). Two months later, Col-Luc→wt mice were subjected to CCl4 injury (16 injections over 8 weeks) and compared with untreated (Figure 2C) or wild-type littermates (data not shown). Significant luciferase activity was detected only in CCl4-treated Luc+ mice, and corresponded to the liver, spleen, and patchy structures in small and large intestine.
Figure 2.
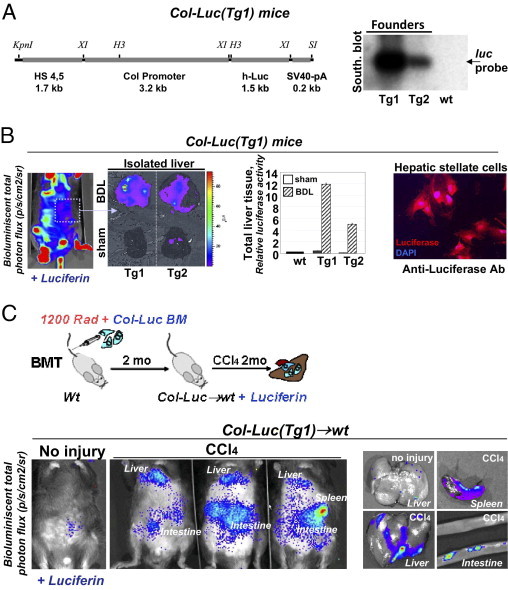
Generation of BM chimeric Col-Luc→wt mice. A: Transgenic construct consists of collagen α1(I) enhancer (1.7 kb), promoter (3.2 kb), and hlucP+ (1.5 kb) (left panel). Southern blot analysis with Luc-specific probe (luc, 1 kb) identified Tg1 and Tg2 transgenic founders (right panel). B: Luc expression is detected in Col-Luc mice at i.v. administration of D-Luc using Ivis-200 by up-regulation of bioluminescent signal (p/s/cm2/sr) and conformed by luciferase assay and immunostaining of activated HSCs with anti-Luc antibody. C: Generation of chimeric Col-Luc→wt mice. Migration of Luc+ cells in abdominal area is detected in liver, spleen, and intestine of CCl4-treated Col-Luc→wt mice. Data are given as p/s/cm2/sr. Representative images are shown.
Luc+ Fibrocytes and their Precursors are Recruited in Response to CCl4 in Col-Luc→wt Mice
Fibrocyte recruitment into the abdominal area was studied during the onset (2 weeks of injections), development (2 months of injections), and resolution (2 weeks after last injection) of CCl4-induced liver fibrosis in Col-Luc→wt mice (Figure 3A). The first bioluminescent signal was detected in these mice (n = 10) at 7 days after onset of CCl4 injury, and peaked at day 14, reflecting a fibrocyte flux into the abdomen in response to acute injury (Figure 3B; see also Supplemental Figure S1 at http://ajp.amjpathol.org). Luc+ fibrocytes were present in the portal area of fibrotic liver, as confirmed at immunostaining of fibrocytes in the isolated hepatic lymphocyte fraction4 with anti-luciferase and anti-CD45+ antibodies (Figure 3C). An unexpected second peak was detected at 2 weeks after the last administration of CCl4 (Figure 3B), corresponding to the recovery phase. These findings are in concordance with the dual role of CD11b+ monocytes and macrophages in liver fibrosis,17 indicating that CD11b+ fibrocytes may be controlled through similar pathways as myeloid inflammatory cells.
Figure 3.
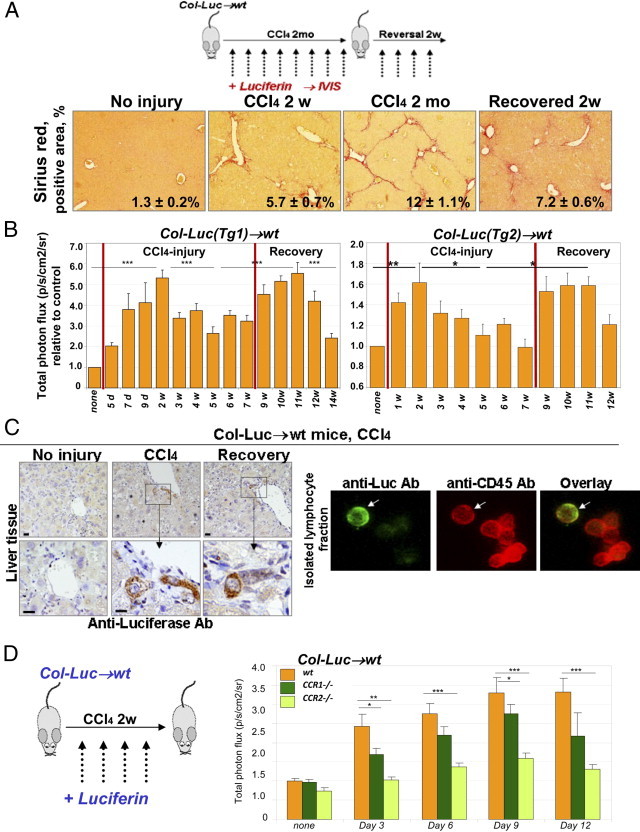
CCR2 and CCR1 facilitate fibrocyte recruitment in response to CCl4. A: Study design (top). Col-Luc→wt mice were treated with CCl4 and recovered from fibrosis. Sirius red staining (bottom) determined the total collagen deposition at chosen times (mean ± SEM; P < 0.05). B: CCl4-treated Col-Luc→wt mice were monitored using bioluminescence. Luc+ cells egress BM in two phases corresponding to acute injury and recovery (n = 10). *P < 0.05, **P < 0.01, and ***P < 0.001. Groups compared are none and 2w, 2w and 5w, 5w and 11w, and 11w and 14w for Col-Luc(Tg1)-Luc mice. Graphic representation of the biphastic egress of fibrocytes and precursors in response to CCl4. C: Livers and fibrocyte-containing fraction4 from Col-Luc→wt mice are stained with anti-Luc and anti-CD45 antibodies. Scale bars = 50 μm. D: Recruitment of Luc+ cells was studied in LucCCR2−/−→wt mice (n = 10) and LucCCR1−/−→wt mice (n = 10) mice and compared with Lucwt-into-wt mice in response to CCl4 (2 weeks). Bars represent mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001. Groups compared are wt and LucCCR2−/−→wt mice, and wt and LucCCR1−/−→wt mice.
CCR2 and CCR1 Regulate Fibrocyte Migration in Response to CCl4-Induced Liver Injury
Fibrocytes express CCR1, CCR2, CCR4, CCR5, and CCR7,11–14 and their migration is regulated in part by chemokines.5 To dissect the mechanism of fibrocyte trafficking in response to liver injury, a CCR1 (or CCR2) receptor knockout mouse was crossed with a Col-Luc mouse and used for BM transplantation into lethally irradiated wild-type mice (Figure 3D). Chimeric Col-LucCCR1−/−→wt and Col-LucCCR2−/−→wt mice were used to monitor migration of Luc+ receptor-deficient BM cells and compared with wild-type Col-Lucwt→wt mice. Compared with Lucwt→wt mice, LucCCR1−/−→wt mice demonstrated an approximately 25% reduction in bioluminescent signal in response to CCl4, and bioluminescent signal was decreased by approximately 50% (P < 0.05) in LucCCR2−/−→wt mice.
BM Population of CD45+Col+ Cells Differentiates Into Fibrocytes in Vitro
BM transplantation experiments in mice indicate that fibrocytes have a hematopoietic origin. Consistently, fibrocytes are isolated by outgrowth of cultures of peripheral mononuclear or splenic cells3 and are described as spindle-shaped CD45 and Col+ cells,3 which share expression of some markers with cells of myelomonocytic lineage18 and undergo several stages of maturation.19 Meanwhile, the BM counterpart of tissue fibrocytes has not been identified. To look for fibrocyte precursors, we examined the BM of BMT chimeric Col-GFP→wt mice,4 in which all BM-derived Col+ cells are labeled by GFP expression.16
Monitored by GFP expression, CD45+Col+ cells were detected in the BM (0.2% ± 0.1%) of untreated Col-GFP→wt mice (Figure 4A). In response to hepatotoxic liver injury (CCl4, 16 injections over 8 weeks), their number increased to 1.8% (0.6%) of total BM cells (Figure 4, A and C). They expressed CD11b (82% ± 7%), Ly-6C (75% ± 6%), F4/80 (25% ± 5%), and CD115 (53% ± 6%; Figure 4, A and B), low levels of CD14, and CD68 (Figure 4D), resembling CD11b+Ly6c+CCR2+CD115+ monocytes.20 Similar to mature fibrocytes,18 CD45+Col+ cells expressed chemokine receptors CCR1 (15% ± 5%) and CCR2 (18% ± 4%; Figure 4A), major histocompatibility complex class II (18% ± 10%), and hematopoietic markers CD34, CD133, CD105, and Thy1.1 (Figure 4, B and 4D).20
Figure 4.
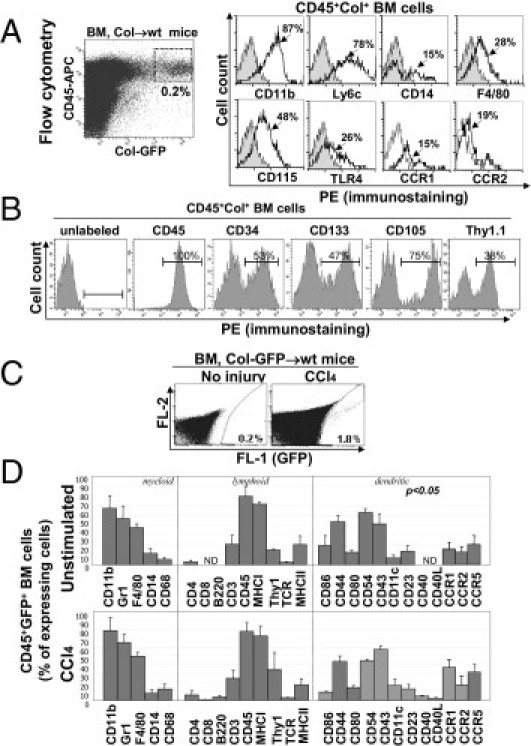
BM CD45+Col+ cells represent precursors of liver fibrocytes. A: Flow cytometry. BM CD45+Col+ cells constitute 0.2% of total BM cells in untreated Col-GFP→wt mice (dot plot) and express monocyte CD11b+Ly6c+CCR2+CD115+ markers (histograms). B: BM CD45+Col+ cells from untreated Col-GFP→wt mice express markers of hematopoietic progenitors. C: The number of CD45+Col+ cells is increased in Col-GFP→wt mice in response to CCl4. D: Comparative analysis of antigen profile of BM CD45+Col+ cells with and without CCl4 liver injury. Bars represent mean ± SEM fluorescent intensity.
To test whether BM CD45+Col+ cells possess fibrocyte features, CD45+Col+ cells were purified from BM by cell sorting4 for double-positive CD45+ and Col-GFP+ cells (Figure 5A) and characterized by structure and gene expression. Expression of collagen type I in purified BM CD45+Col+ cells was confirmed by positive staining with anti–collagen type I antibody (Figure 5A) but not with isotype-matched control antibody (data not shown).
Figure 5.
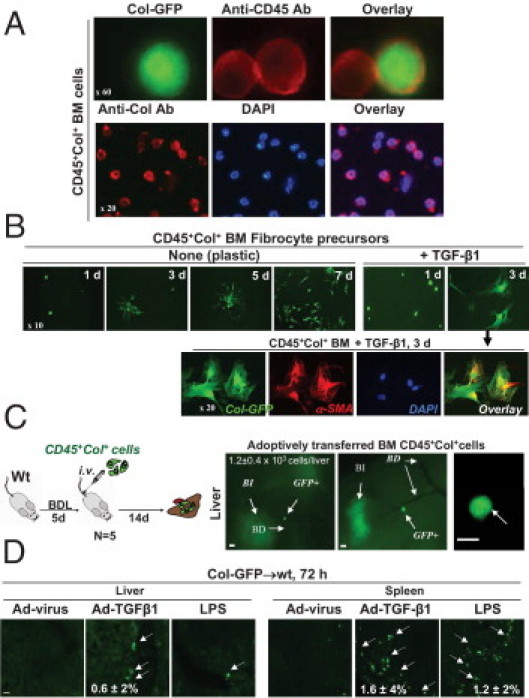
BM CD45+Col+ FPs is regulated by TGF-β1 and LPS. A: Isolated BM GFP+ cells expressed CD45 and collagen type I, detected at IHC. B: BM CD45+Col+ cells, cultured on plastic in RPMI plus 10% fetal calf serum with or without 5 ng/mL TGF-β1 were stained using anti–α-SMA antibody. C: Adoptively transferred BM CD45+Col+ cells migrated specifically to fibrotic livers (n = 6) in BDL mice. Whole liver sections, bile ducts (BD), bile infarcts (BI), and adoptively transferred GFP+ are shown (arrows). Scale bars = 50 μm. D: Col-GFP→wt mice (n = 5 mice per group) are infected i.v. with TGF-β1–expressing adenovirus (Ad–TGF-β1, 1 × 108 PFU) or control adenovirus (Ad-virus), or injected with 6 μg/g LPS. In vivo migration of fibrocytes (arrows) is examined at 72 hours after injury.
Differentiation of BM CD45+Col+ cells was further characterized in vitro. Immediately after isolation, BM CD45+Col+ cells exhibited a round phenotype and expressed CD45 (Figure 5A), but similar to fibrocytes, rapidly attained a spindle shape in culture (Figure 5B). After 7 days in culture, BM CD45+Col+ cells gave rise to classic collagen-expressing spindle-shaped fibroblast-like cells, as demonstrated at fluorescent microscopy (Figure 5B). This process was accelerated by TGF-β1,21 and differentiation of BM CD45+Col+ cells into GFP+α-SMA+ myofibroblasts was detected after 3 days in culture (Figure 5B and reference 4). Our data are in concordance with current methods of fibrocyte culturing,18,21 and BM CD45+Col+ cells may represent precursors of fibrocytes, which mature into fibrocytes on appropriate stimulation in vitro.
BM-Derived Fibrocytes Migrate Specifically to Injured Liver
The ability of CD45+GFP+ BM cells to migrate to injured liver was tested in a series of adoptive transfer experiments (Figure 5C; see also Supplemental Figure S2 at http://ajp.amjpathol.org). Cells (1 × 105 cells per mouse) were purified, by cell sorting for double-positive CD45+ and GFP+ cells, from BM of CCl4-treated Col-GFP mice, and injected i.v. into BDL wild-type mice (n = 5). BDL was chosen because it produces “localized” liver injury and, unlike CCl4, does not induce fibrocyte migration to the lungs or kidneys.4 At 14 days after the adoptive transfer, GFP+ cells (1.2 ± 0.4 × 103 cells per liver) migrated to the portal tracts of livers of the BDL mice (Figure 5C), as calculated by the number of GFP+ cells in serial liver sections of BDL wt mice. Meanwhile, no GFP+ cells were detected in the lungs or kidneys of these BDL mice (data not shown) because wells as injection of GFP+ cells into wild-type mice without injury did not result in fibrocyte engraftment (Figure S2). In comparison, adoptively transferred activated Col+ HSCs did not migrate to the injured livers but localized in the lungs (Figure S2).
TGF-β1 and LPS Induce Migration of Fibrocytes to Spleen and Liver
Treatment of BM chimeric Col-GFP→wt mice with LPS (6 μg/g) or TGF-β1 (via an expressing adenovirus at 1 × 108 PFU) induced rapid (within 72 hours) migration of fibrocytes to spleen and liver, as demonstrated using fluorescent microscopy for GFP+ cells (Figure 5D). Corroborating these in vivo results, both TGF-β1 and LPS elicited chemotaxis of BM CD45+Col+ cells in vitro, increasing transwell migration by 4.5- and 6-fold, respectively (see Supplemental Figure S3 at http://ajp.amjpathol.org). As expected, TGF-β1–induced migration of these cells was associated with Smad2/3 phosphorylation and nuclear translocation (Figure S3).
Recruitment of Fibrocytes Is Impaired in Aged Mice
In aged mice, differentiation of myeloid (versus lymphoid) lineage prevailed.22 Hepatic fibrosis was also accelerated in aged mice23 (see Supplemental Figure S4 at http://ajp.amjpathol.org). We compared migration of fibrocytes in young (6 to 8 weeks) and aged (older than 20 months) mice. Compared with young untreated mice, aged untreated mice demonstrated a 4.5-fold increase in the bioluminescent signal (Figure 6A), which suggests that the biggest difference between young and aged mice is the high baseline of fibrocyte accumulation in aged mice. Meanwhile, in response to CCl4 injury (12 times over 6 weeks), recruitment of fibrocytes was observed in both young and aged mice, but with a slightly higher increase in fibrocyte flux in aged mice versus young mice (Δ17% ± 5%; P < 0.05; Figure 6A). These findings were confirmed in untreated Col-GFP→wt mice, and detected recruitment of CD45+Col+ fibrocyte-like cells to spleen (0.8% ± 0.3%) and Peyer's patches (Figure 6B; see also Supplemental Figures S5 and S6 at http://ajp.amjpathol.org) in aged mice under normal conditions. Moreover, more GFP+ cells were detected in the spleens (4.5% ± 0.7%) and livers (2.5% ± 0.8%) of aged mice (versus 2.4% ± 0.6% and 1.2% ± 7%, respectively, in young mice) in response to CCl4-induced injury (12 times over 6 weeks), which suggests that increased recruitment of fibrocytes to fibrotic livers in aged mice may be a factor that exacerbates liver fibrosis in aged rodents23 (Figure S4). In addition, there are age-related differences in regulation of fibrocyte or precursor migration (Table 1).
Figure 6.
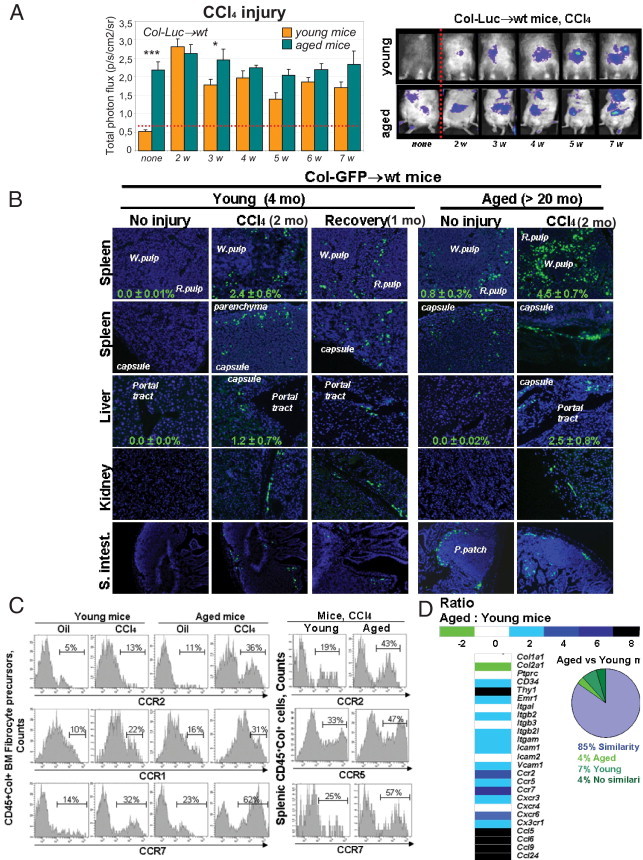
Egress of fibrocyte-like cells to spleen occurs without injury in aged mice. A: Migration of Luc+ cells was compared in young (6 to 8 weeks, n = 10) and aged (older than 20 months, n = 10) Col-Luc→wt mice before and during CCl4-induced liver injury. Data are given as mean ± SEM p/s/cm2/sr. *P < 0.05, ***P < 0.01. Young and aged mice were compared at each time point. B: Migration of fibrocytes into peripheral organs in Col-GFP→wt mice was demonstrated using fluorescent microscopy for GFP+ and CD45+ cells. The mean ± SEM percentage of GFP+ cells was calculated for total splenocytes (n = 4 per group). P < 0.05. C: Flow cytometry analysis of BM and splenic CD45+Col+ cells from young and aged Col-GFP mice revealed increased expression of CCR2 and CCR7. D: CD45+Col+ cells isolated from spleens of LPS-treated young and aged Col-GFP mice were analyzed using the mRNA microarray.
Table 1.
Detection of CD45+Col+ Fibrocyte-like Cells Using Flow Cytometry in Young Versus Aged Mice
| Injury | Cell Type | Young Mice |
Aged Mice |
||
|---|---|---|---|---|---|
| BM, % | Spleen, % | BM, % | Spleen, % | ||
| None | CD45+Col+ | 0.2 ± 0.1 | 0.001 ± 0 | 0.6 ± 0.1 | 1.3 ± 0.4 |
| CD19+ | 17 ± 1.2 | 12 ± 3 | |||
| CD4+ | 3.5 ± 0.2 | 1.9 ± 0.4 | |||
| CCl4 (2 mo) | CD45+Col+ | 1.8 ± 0.6 | 3.1 ± 0.7 | 0.9 ± 0.2 | 4.6 ± 0.6 |
| CD19+ | 7.6 ± 0.4 | 3.5 ± 0.5 | |||
| CD4+ | 1.8 ± 0.2 | 1.2 ± 0.3 | |||
BM and spleens from young and aged untreated or CCl4-treated Col-GFP→wt mice were analyzed using flow cytometry for the presence of CD45+Col+ cells. CD19+ cells represent B cells, CD4+ cells represent T cells, CD45+Col+ cells (bold) represent fibrocyte-like cells. Data are given as the mean ± SEM of fluorescence intensity.
BM and Splenic CD45+Col+ Cells Up-Regulate Chemokine Receptors With Age
Higher expression of CCR2 and CCR7 was detected in BM and spleen CD45+Col+ cells of untreated aged mice versus young mice (Figure 6C), and was further increased in response to CCl4 injury. CD45+Col+ cells were isolated from spleens of LPS-treated young and aged mice, and their gene expression profile was analyzed. CD45+Col+ cells from young and aged mice share 85% similarity (Figure 6D). It was observed that mRNA levels of chemokine receptors CCR5 (increased twofold), CXCR3 (increased twofold), CX3CR1 (increased twofold), CCR2 (increased fourfold), CCR6 (increased fourfold), and CCR7 (increased sixfold) were increased in cells from aged mice, as confirmed by RT-PCR analysis (see Supplemental Figure S7 at http://ajp.amjpathol.org). Moreover, mRNA levels of integrins (Itgb2, Itgb21, and Itgam) and adhesion molecules (Icam1 and Vcam1) were increased twofold in aged mice. In addition, up-regulation of mRNA levels of interferon-γ and its cognate receptor 1 (Ifng and Ifngr1, each increased sixfold), and chemokines CCL5, CCL6, CCL9, and CCL24 (increased eightfold) was detected in cells from aged mice (Figure 6E; see also Supplemental Tables S1 and S2 at http://ajp.amjpathol.org). Considered together, the data suggest that increased expression of chemokine receptors in aged mice is associated with abnormal regulation of BM CD45+Col+ cells in response to injury or stress.
Discussion
Fibrocytes are defined by the co-expression of CD45 and collagen type I.3 They are recruited to the site of injury in all types of tissue fibrosis and actively participate in the fibrogenic process. However, little is known about the migration of fibrocytes from BM to injured liver. Using BM chimeric Col-GFP→wt and Col-Luc→wt mice, migration of fibrocytes was monitored in models of liver injury to reveal the following. Both cholestatic4 and hepatotoxic liver injury recruits fibrocytes to the liver. Optimal recruitment of fibrocytes to the liver and spleen is regulated by CCR2 and CCR1. BM contains a population of CD45+Col+ fibrocyte-like cells. Adaptively transferred BM-derived CD45+Col+ cells, but not HSCs, migrate to the injured liver. TGF-β1 induces differentiation of BM Col+CD45+ cells in myofibroblasts, similar to Col+CD45+ cells in blood and spleen. TGF-β and LPS induce migration of fibrocytes in vivo and in culture. Age-related up-regulation of chemokine receptor expression leads to egress of CD45+Col+ fibrocyte-like cells from the BM to lymphoid organs in the absence of injury. Accelerated fibrosis in aged mice correlates with increased recruitment of fibrocytes to fibrotic liver.
Migration of Fibrocytes Is Mediated by Chemokine Receptors
Fibrocytes have been implicated in the pathogenesis of fibrogenic diseases in lungs, kidneys, and liver.9,13,24 They have potential to differentiate into (myo)fibroblasts in vivo in response to CCl4-induced liver injury4 and into hepatic fibrosis in Abcb4-deficient mice.25 Inhibition of fibrocyte recruitment by human serum amyloid protein significantly attenuates fibrogenesis in liver (our unpublished observation), lungs,7 and kidneys.8 Similar to BDL, hepatotoxic injury triggers migration of fibrocytes to the damaged liver. Unlike BDL, CD45+Col+ cells were detected in kidneys and lungs, which suggests that CCl4 induces a systemic effect. In addition, CD45+Col+ cells were detected in lymphoid organs such as spleen, a phenomenon previously described in kidney and liver fibrosis.4,13,14,25 Recruitment of CD45+Col+ fibrocytes peaked in the extramedullary tissues in two phases corresponding to the acute phase and regression of fibrosis. Egress of fibrocytes from BM in the acute phase is mediated by signaling via CCR2. These data are in concordance with previous studies in lung and kidney fibrosis.24,26 Dissimilar to lungs and kidneys, recruitment of fibrocytes to fibrotic liver is also regulated by CCR1, which is up-regulated in CCl4-treated mice.
Recent studies have demonstrated that BDL- and CCl4-induced fibrosis is markedly reduced in CCR2−/−, CCR1−/−, and TLR4−/− mice.27–29 However, although BM chimeric mice exhibiting CCR2−/− or CCR1−/− BM demonstrated less macrophage and fibrocyte recruitment in response to liver injury, individual deficiency of CCR2 or CCR1 (or TLR4) in hematopoietic cells has little or no effect on extent of hepatic fibrosis.
Identification of CD45+Col+ Fibrocyte-Like Cells in the BM Compartment
Based on the ability of the collagen α1(I)-GFP transgene to drive GFP expression specifically in CD45+Col+ cells but not other cells of the hematopoietic system4 such as activated macrophages,18 we identified a population of fibrocyte-like cells that share expression of some markers with CD11b+Ly6c+CCR2+CD115+ monocytes but attain a spindle shape in culture and differentiate into myofibroblasts on TGF-β1 stimulation. BM CD45+Col+ cells resemble fibrocytes phenotypically (expression of specific markers and antigens) and functionally (ability to migrate toward TGF-β1 and LPS gradient and to differentiate into myofibroblasts), and, therefore, may contribute to liver fibrosis. For example, increased numbers of BM CD45+Col+ fibrocyte-like cells in aged mice correlate with the severity of fibrosis.
Migration of Fibrocytes and BM Fibrocyte-Like Cells Changes With Age
Recruitment of fibrocytes to the spleen and to a lesser extent to liver was increased with age. The hematopoietic system undergoes specific age-related changes including prevalence of myeloid over lymphoid lineage, reduced ability to adhere to the BM stroma, and mismatch in the DNA repair function,22 whereas progenitor activities of hematopoietic stem cells is likely preserved.30 Age-associated changes in monocyte or macrophage function (“inflamm-aging”) is a major contributor to low-grade chronic inflammation.22 Consistent with the myelomonocytic origin of fibrocytes, increased numbers of Col+CD45+ cells were observed in the BM in aged C57BL/6 mice. In contrast to young mice, fibrocytes populated the spleen in the absence of injury or stress in aged mice, and their egress from BM was associated with increased expression of chemokine receptors CCR1, CCR2, and CCR7 and adhesion molecules (ICAM1). The highest number of fibrocytes in the liver was detected in response to injury in aged mice. Liver fibrosis is accelerated with age,23 and fibrocytes may contribute to increased fibrosis as both inflammatory and collagen-producing cells. In support of these findings, the blood in healthy aged persons contains increased concentrations of CD45+ collagen type 1–positive fibrocytes, which suggests that fibrocytes are associated with aging.31
Acknowledgments
We thank Stella Stefanova and Karin Diggle for technical support.
Footnotes
Supported by the National Institutes of Health (grants GM41804, AA15055, DK72237, AI077780, and 2 P50 AA011999-11 NIH/NIAAA/USC), the American Liver Foundation (2006 Liver Scholar Research Award), and Rotation Program of the Medical Faculty, RWTH University, Aachen, Germany.
D.S. and Y.H.P. acquired the data; D.R. and C.K.G. analyzed the microarrays; J.L. and J.B. performed microscopy; D.A.B. provided critical revision of the manuscript; and T.K. designed the study and wrote the manuscript.
Supplemental material for this article can be found on http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2011.03.049.
Supplementary data
Biphastic migration of fibrocytes to abdominal area in response to CCl4. Luciferase expression is visualized in Col-Luc→wt mice upon i.v. administration of D-luciferin. Upregulation of bioluminescent signal (p/s/cm2/sr) was detected by Ivis-200 and analyzed in each individual mouse throughout the duration of experiment. Mice were analyzed over a time period of 11 weeks, the bioluminescent signal peaked after 2d and 8th weeks. Representative images of three individual mice (N1, N2, N3) are shown. Scale represents color coded photon counts over the region of interest over time (p/s/ cm2/sr).
doptively transferred activated GFP+ HSCs do not migrate to the liver of BDL-injured wild type mice. In vivo activated HSCs were isolated from Col-GFP mice and i.v. transplanted (2 x 105 cells) into BDL-injured wild type mice. Mice were sacrificed 14 days later, liver, lungs and spleen were examined for the presence of myofibroblasts-like cells. GFP+ cells were detected in the lungs, but not livers of the wild type mice. Scale bars represent 50 μm.
TGF-β1 and LPS have chemoattractive effect on CD45+Col+ cells in vitro. Transwell migration of isolated BM CD45+Col+ cells is performed with TGF-β1 (5 ng/ml) or LPS (1 μg/ml) added to the bottom chamber, left panel. The number of BM GFP+ cells from CCl4-injured mice (BM-CCl4) in the bottom chamber was assessed 18 h after culturing. Bars represent mean ± SEM of four independent experiments, *p<0.001. TGF-β1 induces nuclear translocation of phospho-Smad2/3 in sort purified CD45+Col+ cells, stimulated with TGF-β1 (2 ng/ml) for 1h at 37°C, right panel. Scale bars represent 50 μm.
Liver fibrosis is exacerbated in aged mice. Liver tissues from CCl4-treated (2 mo) young and aged Col-GFP mice were compared by fluorescent microscopy for collagen α1(I) (GFP) expression or total collagen deposition (Sirius red staining).
CD45+Col+ cells surround lymphoid patches in intestine. Representative sections from small and large intestine of CCl4-treated (2 mo) Col-GFP→wt mice, CD45+Col+ cells (GFP) and nuclei (DAPI) are shown. Scale bars represent 50 μm.
Col-GFP+ cells in spleen, liver, kidneys and intestine express CD45. Tissues from CCl4-treated aged mice were stained with anti-CD45 antibody (red). Co-localization of CD45 in GFP+ cells is shown with arrows, bars represent 50 μm.
Gene expression analysis of genes expressed in Col+GFP+ cells by RT-PCR. Relative mRNA levels are calculated for fold induction of gene expression in aged mice in comparison with young mice, after normalization to GAPDH gene using the ΔΔ CT method. Genes are listed as Fibronectin 1(Fn1), S100a4 (fibroblast specific protein, FSP-1), p<0.0001. RT-PCR was performed using individual mRNA preparations (n=3), each represented a pool of two-three mice.
References
- 1.Kisseleva T., Brenner D.A. Fibrogenesis of parenchymal organs. Proc Am Thorac Soc. 2008;5:338–342. doi: 10.1513/pats.200711-168DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seki E., De Minicis S., Osterreicher C.H., Kluwe J., Osawa Y., Brenner D.A., Schwabe R.F. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 3.Bucala R., Spiegel L.A., Chesney J., Hogan M., Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 4.Kisseleva T., Uchinami H., Feirt N., Quintana-Bustamante O., Segovia J.C., Schwabe R.F., Brenner D.A. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol. 2006;45:429–438. doi: 10.1016/j.jhep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Strieter R.M., Gomperts B.N., Keane M.P. The role of CXC chemokines in pulmonary fibrosis. J Clin Invest. 2007;117:549–556. doi: 10.1172/JCI30562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pilling D., Buckley C.D., Salmon M., Gomer R.H. Inhibition of fibrocyte differentiation by serum amyloid P. J Immunol. 2003;171:5537–5546. doi: 10.4049/jimmunol.171.10.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray L.A., Rosada R., Moreira A.P., Joshi A., Kramer M.S., Hesson D.P., Argentieri R.L., Mathai S., Gulati M., Herzog E.L., Hogaboam C.M. Serum amyloid P therapeutically attenuates murine bleomycin-induced pulmonary fibrosis via its effects on macrophages. PLoS One. 2010;5:e9683. doi: 10.1371/journal.pone.0009683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castaño A.P., Lin S.L., Surowy T., Nowlin B.T., Turlapati S.A., Patel T., Singh A., Li S., Lupher M.L., Jr, Duffield J.S. Serum amyloid P inhibits fibrosis through Fc gamma R-dependent monocyte-macrophage regulation in vivo. Sci Transl Med. 2009 doi: 10.1126/scitranslmed.3000111. [DOI] [PMC free article] [PubMed] [Google Scholar]; 5ra131
- 9.Quan T.E., Cowper S., Wu S.P., Bockenstedt L.K., Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36:598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Bellini A., Mattoli S. The role of the fibrocyte, a bone marrow–derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 2007;87:858–870. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- 11.Moore B.B., Kolodsick J.E., Thannickal V.J., Cooke K., Moore T.A., Hogaboam C., Wilke C.A., Toews G.B. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol. 2005;166:675–684. doi: 10.1016/S0002-9440(10)62289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore B.B., Murray L., Das A., Wilke C.A., Herrygers A.B., Toews G.B. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol. 2006;35:175–181. doi: 10.1165/rcmb.2005-0239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakai N., Wada T., Yokoyama H., Lipp M., Ueha S., Matsushima K., Kaneko S. Secondary lymphoid tissue chemokine (SLC/CCL21)/CCR7 signaling regulates fibrocytes in renal fibrosis. Proc Natl Acad Sci USA. 2006;103:14098–14103. doi: 10.1073/pnas.0511200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakai N., Wada T., Matsushima K., Bucala R., Iwai M., Horiuchi M., Kaneko S. The renin-angiotensin system contributes to renal fibrosis through regulation of fibrocytes. J Hypertens. 2008;26:780–790. doi: 10.1097/HJH.0b013e3282f3e9e6. [DOI] [PubMed] [Google Scholar]
- 15.Uehara H, Nakagawa T, Katsuno T, Sato T, Isono A, Noguchi Y, Saito Y: Emergence of fibrocytes showing morphological changes in the inflamed colonic mucosa. Dig Dis Sci 55:253–260 [DOI] [PubMed]
- 16.Yata Y., Scanga A., Gillan A., Yang L., Reif S., Breindl M., Brenner D.A., Rippe R.A. DNase I-hypersensitive sites enhance alpha1(I) collagen gene expression in hepatic stellate cells. Hepatology. 2003;37:267–276. doi: 10.1053/jhep.2003.50067. [DOI] [PubMed] [Google Scholar]
- 17.Duffield J.S., Forbes S.J., Constandinou C.M., Clay S., Partolina M., Vuthoori S., Wu S., Lang R., Iredale J.P. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilling D., Fan T., Huang D., Kaul B., Gomer R.H. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One. 2009;4:e7475. doi: 10.1371/journal.pone.0007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pilling D., Gomer R.H. Regulatory pathways for fibrocyte differentiation: Fibrocytes: New Insights Into Tissue Repair and Systemic Fibroses, ch 3. In: Bucala R., editor. World Scientific Publishing Co Pte Ltd; Singapore: 2007. pp. 37–60. [Google Scholar]
- 20.Serbina N.V., Jia T., Hohl T.M., Pamer E.G. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abe R., Donnelly S.C., Peng T., Bucala R., Metz C.N. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 22.Geiger H., Rudolph K.L. Aging in the lympho-hematopoietic stem cell compartment. Trends Immunol. 2009;30:360–365. doi: 10.1016/j.it.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Medeiros M.V., Freitas L.A., Andrade Z.A. Differences in hepatic pathology resulting from bile duct obstruction in young and old rats. Braz J Med Biol Res. 1988;21:75–83. [PubMed] [Google Scholar]
- 24.Phillips R.J., Burdick M.D., Hong K., Lutz M.A., Murray L.A., Xue Y.Y., Belperio J.A., Keane M.P., Strieter R.M. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roderfeld M., Rath T., Voswinckel R., Dierkes C., Dietrich H., Zahner D., Graf J., Roeb E. Bone marrow transplantation demonstrates medullar origin of CD34+ fibrocytes and ameliorates hepatic fibrosis in Abcb4-/- mice. Hepatology. 2010;51:267–276. doi: 10.1002/hep.23274. [DOI] [PubMed] [Google Scholar]
- 26.Balmelli C., Alves M.P., Steiner E., Zingg D., Peduto N., Ruggli N., Gerber H., McCullough K., Summerfield A. Responsiveness of fibrocytes to toll-like receptor danger signals. Immunobiology. 2007;212:693–699. doi: 10.1016/j.imbio.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Seki E., de Minicis S., Inokuchi S., Taura K., Miyai K., van Rooijen N., Schwabe R.F., Brenner D.A. CCR2 promotes hepatic fibrosis in mice. Hepatology. 2009;50:185–197. doi: 10.1002/hep.22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seki E., De Minicis S., Gwak G.Y., Kluwe J., Inokuchi S., Bursill C.A., Llovet J.M., Brenner D.A., Schwabe R.F. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest. 2009;119:1858–1870. doi: 10.1172/JCI37444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seki E., Uchinami H., Osawa Y., Brenner D.A., Schwabe R.F. TLR4 mediates inflammation and fibrogenesis after bile duct ligation. Hepatology. 2005;42:265A–266A. [Google Scholar]
- 30.Donnini A., Re F., Orlando F., Provinciali M. Intrinsic and microenvironmental defects are involved in the age-related changes of Lin - c-kit+ hematopoietic progenitor cells. Rejuvenation Res. 2007;10:459–472. doi: 10.1089/rej.2006.0524. [DOI] [PubMed] [Google Scholar]
- 31.Mathai S.K., Gulati M., Peng X., Russell T.R., Shaw A.C., Rubinowitz A.N., Murray L.A., Siner J.M., Antin-Ozerkis D.E., Montgomery R.R., Reilkoff R.A., Bucala R.J., Herzog E.L. Circulating monocytes from systemic sclerosis patients with interstitial lung disease show an enhanced profibrotic phenotype. Lab Invest. 2010;90:812–823. doi: 10.1038/labinvest.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Biphastic migration of fibrocytes to abdominal area in response to CCl4. Luciferase expression is visualized in Col-Luc→wt mice upon i.v. administration of D-luciferin. Upregulation of bioluminescent signal (p/s/cm2/sr) was detected by Ivis-200 and analyzed in each individual mouse throughout the duration of experiment. Mice were analyzed over a time period of 11 weeks, the bioluminescent signal peaked after 2d and 8th weeks. Representative images of three individual mice (N1, N2, N3) are shown. Scale represents color coded photon counts over the region of interest over time (p/s/ cm2/sr).
doptively transferred activated GFP+ HSCs do not migrate to the liver of BDL-injured wild type mice. In vivo activated HSCs were isolated from Col-GFP mice and i.v. transplanted (2 x 105 cells) into BDL-injured wild type mice. Mice were sacrificed 14 days later, liver, lungs and spleen were examined for the presence of myofibroblasts-like cells. GFP+ cells were detected in the lungs, but not livers of the wild type mice. Scale bars represent 50 μm.
TGF-β1 and LPS have chemoattractive effect on CD45+Col+ cells in vitro. Transwell migration of isolated BM CD45+Col+ cells is performed with TGF-β1 (5 ng/ml) or LPS (1 μg/ml) added to the bottom chamber, left panel. The number of BM GFP+ cells from CCl4-injured mice (BM-CCl4) in the bottom chamber was assessed 18 h after culturing. Bars represent mean ± SEM of four independent experiments, *p<0.001. TGF-β1 induces nuclear translocation of phospho-Smad2/3 in sort purified CD45+Col+ cells, stimulated with TGF-β1 (2 ng/ml) for 1h at 37°C, right panel. Scale bars represent 50 μm.
Liver fibrosis is exacerbated in aged mice. Liver tissues from CCl4-treated (2 mo) young and aged Col-GFP mice were compared by fluorescent microscopy for collagen α1(I) (GFP) expression or total collagen deposition (Sirius red staining).
CD45+Col+ cells surround lymphoid patches in intestine. Representative sections from small and large intestine of CCl4-treated (2 mo) Col-GFP→wt mice, CD45+Col+ cells (GFP) and nuclei (DAPI) are shown. Scale bars represent 50 μm.
Col-GFP+ cells in spleen, liver, kidneys and intestine express CD45. Tissues from CCl4-treated aged mice were stained with anti-CD45 antibody (red). Co-localization of CD45 in GFP+ cells is shown with arrows, bars represent 50 μm.
Gene expression analysis of genes expressed in Col+GFP+ cells by RT-PCR. Relative mRNA levels are calculated for fold induction of gene expression in aged mice in comparison with young mice, after normalization to GAPDH gene using the ΔΔ CT method. Genes are listed as Fibronectin 1(Fn1), S100a4 (fibroblast specific protein, FSP-1), p<0.0001. RT-PCR was performed using individual mRNA preparations (n=3), each represented a pool of two-three mice.


