Abstract
Recent genome-wide association studies have identified two host single-nucleotide polymorphisms (SNPs) near the IL28B gene (rs12979860 C/T and rs8099917 T/G) that are associated with sustained virological response in patients infected with the hepatitis C virus. Herein, we describe a rapid multiplexed dual-color fluorescence resonance energy transfer (FRET) probe assay that accurately genotypes for both SNPs simultaneously. A single-nucleotide extension assay was also developed for verification of genotypes. Agreement (100%) was observed in genotype calls between the FRET and single-nucleotide extension methods for both SNPs, yielding 100% analytical sensitivity and specificity. By using the FRET assay, 443 samples of varying ethnic backgrounds were genotyped and six different compound genotypes (rs12979860/rs8099917) were detected in whites, Asians, Middle Easterners, Hispanics, and African Americans, at the following frequencies: CC/TT (39.2%, 78.9%, 40.0%, 33.9%, and 16.8%), CT/TT (20.8%, 0%, 40%, 9.3%, and 37.0%), TT/TT (2.4%, 0%, 0%, 3.4%, and 35.3%), CT/TG (24.0%, 19.7%, 20%, 39.8%, and 3.4%), TT/TG (8.0%, 1.4%, 0%, 3.4%, and 5.9%), and TT/GG (5.6%, 0%, 0%, 10.2%, and 1.7%), respectively. The multiplexed FRET assay can be used to effectively genotype for both SNPs in a single tube, with high analytical sensitivity and specificity.
The global prevalence of hepatitis C virus (HCV) infection is approximately 3%.1 Only approximately 15% of those who contract HCV spontaneously clear the virus. The 85% of patients who become chronically infected are at risk for developing cirrhosis and hepatocellular carcinoma several decades after infection. HCV infection is the most common reason for liver transplantation in the United States.2 Standard-of-care treatment for fine hepatitis C consists of pegylated interferon and ribavirin; however, this treatment is expensive and successful in only 40% to 50% of individuals infected with HCV genotype 1.3 In addition, the treatment itself is difficult to tolerate. Individuals receiving therapy can experience serious adverse effects, ranging from psychological depression to bone marrow suppression, often resulting in premature withdrawal from treatment.4 Given the high cost of treatment, the poor response rate for HCV 1, and severe adverse effects, identifying factors that predict response to therapy would be valuable.
Recently, multiple independent research groups5–9 have performed genome-wide association studies and reported several single-nucleotide polymorphisms (SNPs) associated with spontaneous HCV clearance and response to treatment. These SNPs are located in the region of IL28B, IL28A, and IL29 interleukin genes on chromosome 19q13 and represent a recently discovered family of interferons known as type III or λ interferons. λ Interferons have inhibited HCV in vitro and are believed to trigger an antiviral cascade through the JAK-STAT pathway.10,11
Two biallelic SNPs rs12979860 (C/T) and rs8099917 (T/G), located upstream of IL28B, have a strong association with both spontaneous and treatment-induced HCV clearance. Recent studies5–8 found that individuals with two copies of the C allele (CC genotype) for the rs12979860 SNP were twofold more likely to respond to treatment, as defined by a sustained virological response (SVR), and that the C allele was also present 2.5 times more frequently in patients in whom the HCV infection spontaneously resolved. Conversely, individuals carrying the CT or TT genotype were less likely to respond to treatment. Other studies7–9 demonstrated that two copies of the T allele (TT genotype) for the rs8099917 SNP were strongly associated with natural HCV clearance and SVR. Similar to the rs12979860 pattern, the rs8099917 TG or GG genotype was less responsive to treatment.
Herein, we describe a specific, reproducible, single-tube genotyping method for simultaneous detection of the rs12979860 and rs8099917 SNPs using real-time PCR and sequence-specific dual-color fluorescence resonance energy transfer (FRET) probes, performed with the LightCycler (Roche Diagnostics, Indianapolis, IN). The method is based on the amplification of regions spanning the variants and simultaneous detection of the amplicons by hybridization with dual-color FRET probes, followed by melting curve analysis. The probes melt from the amplicons at a specific temperature that is characteristic for each allele, allowing for clear discrimination between the four alleles. Genotyping results were compared with results obtained using bidirectional sequencing or allele-specific single-nucleotide extension (SNE), essentially a minisequencing method. We also describe compound genotype and haplotype frequencies for the rs12979860 and rs8099917 SNPs determined in a set of samples of white, Asian, Middle Eastern, Hispanic, and African American descent.
Materials and Methods
DNA Samples
The FRET assay was validated using 152 genomic DNA samples, including four DNA samples with known rs8099917 genotypes (HapMap-CEU population submitted by Affymetrix: genomewidesnp 6.0), with these four samples being further genotyped for rs12979860 by traditional Sanger sequencing; 84 DNA samples from the white panel (Coriell Repositories, Coriell Institute for Medical Research, Camden, NJ); and 64 residual human DNA samples of unknown ethnicity, previously submitted to ARUP Laboratories (Salt Lake City, UT) for unrelated testing. FRET assay accuracy was further determined by sequencing 25 random samples for both SNPs.
To obtain allele and genotype frequencies across ethnic populations, an additional 359 DNA samples were genotyped with the FRET assay. These included samples from the Coriell Repositories Human Population Collections (10 Chinese, 10 Japanese, 10 South East Asian, 10 Middle Eastern, and 10 African American samples) and 309 residual clinical samples with self-reported ethnicity from ARUP Laboratories, for a total of 125 white, 71 Asian, 10 Middle Eastern, 118 Hispanic, and 119 African American samples. Clinical DNA samples were deidentified according to protocols approved by the University of Utah, Salt Lake City, Institutional Review Board.
SNP Genotyping by the FRET Method
PCR amplification and the thermal melting of DNA (Tm) were performed with a LightCycler. In a single-capillary tube, a multiplexed asymmetric PCR reaction amplifies the two fragments of interest: 175 bp for rs12979860 and 273 bp for rs8099917. Asymmetric PCR prevents competition between probe/amplicon and amplicon/amplicon hybridization by producing more copies of the strand that is complementary to, and will hybridize with, the probe. The FRET probes were designed using LightCycler Probe Design Software version 2.0 (Idaho Technology, Salt Lake City, UT). To attain optimal resolution of the heterozygous samples, the probes were designed to the strand that produces the greatest destabilization of the mismatch. Therefore, the sensor probe was designed to the C allele in rs12979860 because its melting temperature is higher than that of the mismatched hybrid of the T allele. The sensor probe was designed on the reverse strand to the G allele for rs8099917. Sequences of the respective primers and probes are described in Table 1. Primers were obtained from Integrated DNA Technology (Coralville, IA), and probes were obtained from Idaho Technology. An asymmetric PCR was performed in a 10-μL volume reaction. The master mix contained the following: 50 to 70 ng of genomic DNA, 1.2× LightCycler-DNA Master Hybridization Probes master mix (Roche Molecular Biochemicals, Indianapolis, IN), 3.0 mmol/L MgCl2 (including 1 mmol/L MgCl2 contributed by the LightCycler Master mix), 0.09 μmol/L rs12979860 forward primer, 0.3 μmol/L rs12979860 reverse primer, 0.35 μmol/L rs12979860 sensor and anchor probes, 0.5 μmol/L rs8099917 forward primer, 0.09 μmol/L rs8099917 reverse primer, and 0.45 μmol/L rs8099917 sensor and anchor probes. The LightCycler program consisted of 45 cycles of denaturation at 95°C (0 seconds), annealing at 60°C (10 seconds), and extension at 72°C (15 seconds), with a single acquisition mode. All heating and cooling steps during the PCR were performed with a temperature transition rate of 20°C/s. Melting curves were generated after denaturation at 95°C (120 seconds) and annealing at 45°C (120 seconds), with a rate of 20°C/s; an increasing temperature to 80°C at a rate of 0.1°C/s; and continuous fluorescence acquisition. Because of an emission wavelength overlap between the fluorescent dye LC-Red 640 and LC-Red 705, color compensation was performed using the Color Compensation Set (Roche Molecular Biochemicals), according to the manufacturer's instructions. The fluorimeter channel F2 was used to analyze the LC-Red 640 fluorescent signal for the rs12979860 alleles, and channel F3 was used to analyze the LC-Red 705 fluorescence for the rs8099917 alleles.
Table 1.
FRET and SNE Primer and Probe Sequences
| Primers and probes | Sequence |
|---|---|
| FRET Assay | |
| rs12979860 | |
| Forward | 5′-TGGGTACTGGCAGCGCA-3′ |
| Reverse | 5′-GCAGGCTCAGGGTCAATC-3′ |
| Sensor | 5′-CCGAAGGCG(C)GAACCAGG-3′ fluorescein |
| Anchor | LC-Red 640 5′-TGAATTGCACTCCGCGCTCCC-3′-C3 blocker |
| rs8099917 | |
| Forward | 5′-ATGGAGAGTTAAAGTAAGTCTTGTATTTCA-3′ |
| Reverse | 5′-TCTGGTATCAACCCCACCTC-3′ |
| Sensor | LC-Red 705 5′-TTGGGTGA(C)ATTGCTCACAGAAAGG-3′-C3 blocker |
| Anchor | 5′-CCAGCTACCAAACTGTATACAGCATGGTTCCA-3′ fluorescein |
| SNE Assay | |
| rs12979860 | |
| Forward | 5′-CCTAACCTCTGCACAGTCT-3′ |
| Reverse | 5′-GCGCGGAGTGCAATTCAA-3′ |
| Extension | 5′-AGCTCCCCGAAGGCG-3′ |
| rs8099917 | |
| Forward | 5′-TAACAATTTGTCACTGTTCCTCC-3′ |
| Reverse | 5′-GCAAATGAGAGATAATGGTAAGACATA-3′ |
| Extension | 5′-GTTTTCCTTTCTGTGAGCAAT-3′ |
The SNPs are underlined, boldfaced, and in parentheses.
DNA Sequencing
For sequencing, the following additional primers were designed: rs12979860, 5′-GATTCCTGGACGTGGATG-3′ (forward) and 5′-GCTCAGGGTCAATCACAGAAG-3′ (reverse); and rs8099917, 5′-TCACCATCCTCCTCTCATCC-3′ (forward) and 5′-TGCTGGGCCCTAACTGATAC-3′ (reverse). PCR amplification of rs12979860 and rs8099917 SNPs was performed separately in a 20-μL reaction consisting of 50 ng DNA; 1× Invitrogen buffer; 1.5 mmol/L MgCl2; 250 μmol/L each of dATP, dCTP, dGTP, and dTTP (where “d” indicates deoxy); 0.25 μmol/L of each primer; and 0.5 U of Platinum TaqDNA polymerase (Invitrogen, Carlsbad, CA). Cycling was performed in an ABI 9700 thermal cycler (Applied Biosystems, Weiterstadt, Germany) using the following conditions for rs12979860: one cycle at 95°C (2 minutes); five cycles at 95°C (30 seconds); annealing at 60°C (30 seconds), with a temperature decrease after one cycle of −0.5°C/cycle; and extension at 72°C (30 seconds). This was followed by 30 cycles at 94°C (30 seconds), annealing at 58°C (30 seconds), extension at 72°C (30 seconds), and a final elongation step at 72°C (2 minutes). Slightly different conditions were used to amplify the rs8099917, consisting of one cycle at 95°C (2 minutes); 13 cycles at 95°C (30 seconds); annealing at 64°C (30 seconds), with a temperature decrease after one cycle of −0.5°C/cycle; and extension at 72°C (30 seconds). This was followed by 22 cycles at 95°C (30 seconds), annealing at 58°C (30 seconds), extension at 72°C (30 seconds), and a final elongation step at 72°C (2 minutes). Excess primers and unincorporated dNTPs from the PCR product were removed with ExoSAP-IT treatment (USB Corp, Cleveland, OH). A sequencing reaction containing 6 μL of ExoSAP-IT–treated amplicon and 8 μL (1 μmol/L) of either the forward or the reverse primer (same as the PCR primers) was directly sequenced using the ABI BigDye Terminator Sequencing kit v2.1 (Applied Biosystems) and an automated capillary sequencer (ABI 3730; Applied Biosystems). A total of 25 samples were sequenced.
SNP Genotyping by the Minisequencing SNE Method
To further evaluate the accuracy of the FRET method, we developed a minisequencing (SNE) assay that interrogates both SNPs simultaneously. Four primers (Table 1) were designed to amplify the regions encompassing rs12979860 and rs8099917 SNPs. A multiplex PCR was performed in a 20-μL reaction volume. The master mix contained the following: 2 μL of DNA (20 to 50 ng/μL), 200 μmol/L of each dNTP, 0.20 μmol/L of each forward and reverse primer, 1× PCR buffer (Invitrogen), and 0.6 U of Platinum Taq polymerase (Invitrogen). Amplification was performed with an initial denaturation at 94°C (3 minutes); 13 cycles at 94°C (30 seconds); annealing at 62°C (30 seconds), with a temperature decrease after one cycle of −0.5°C/cycle; and extension at 72°C (30 seconds). This was followed by 22 cycles at 94°C (30 seconds), annealing at 56°C (30 seconds), and extension at 72°C (30 seconds) using the GeneAmp 9700 thermal reaction cycler (Applied Biosystems, Foster City, CA). Unincorporated primers and dNTPs were removed by incubating with 0.75 μL of ExoI (USB Corp) and 3 μL of shrimp alkaline phosphatase (USB Corp) at 37°C (60 minutes), followed by heat inactivation of the enzyme at 80°C for 15 minutes. A total of 152 samples were assessed with this method.
SNE Reaction
Two unlabeled extension primers (Integrated DNA Technologies) specific for rs12979860 C/T (15 bp) and rs8099917 T/G (25 bp) were designed (Table 1) to hybridize to the complementary sequence one base before the SNP of interest. The difference in primer sizes facilitates adequate separation of the multiplexed extended products by capillary electrophoresis. An SNE reaction was performed in a 10-μL volume, including 2 μL of the purified PCR product, 2.5 μL of the ABI Prism SNaP-shot multiplex kit (Applied Biosystems) that includes fluorescently labeled dideoxy-NTPs and AmpliTaq DNA Polymerase, and 2 μL of the pooled SNE extension primers at final concentrations of 0.1 μmol/L (rs12979860) and 0.05 μmol/L (rs8099917). The reactions were cycled using the following conditions: 25 cycles at 95°C (10 seconds), 55°C (5 seconds), and 60°C (30 seconds). The polymerase extends the primers by one nucleotide, adding a single dideoxy-NTP to the 3′ end. Samples were treated with 1 U of shrimp alkaline phosphatase at 37°C (45 minutes), followed by heat inactivation of enzyme at 80°C (15 minutes). The SNE products were resolved by electrophoresis on an automated ABI Prism 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA) using the GeneScan 120 LIZ internal size standard (Applied Biosystems), as previously described.12 Raw data were analyzed with GeneMapper software 4.0 (Applied Biosystems). A total of 152 samples were genotyped by minisequencing (SNE).
Reproducibility and Precision
To determine the precision of the Tm shifts for the rs12979860 and rs8099917 alleles, representative samples of each genotype were run in triplicate (within run) and in five individual runs on three different instruments (between runs). The Tm may vary slightly between samples because of variation in DNA or salt concentration. However, the difference in Tm between the wild-type and variant peaks (ΔTm) in heterozygous samples is more pronounced and reproducible.13 Both the average and SD of the Tm and ΔTm were calculated for within- and between-run reproducibility.
Results
In this study, we developed a LightCycler FRET genotyping assay for the simultaneous detection of IL28B rs12979860 and rs8099917 polymorphisms, which are associated with HCV infection treatment outcome. To validate the FRET method, 152 samples were also genotyped by a minisequencing (SNE) assay. A 25-sample subset of these samples was further confirmed for both SNPs by direct DNA sequencing. Figure 1 shows the probe-target melting curves for the C/T polymorphism in rs12979860 and the T/G polymorphism in rs8099917. The ΔTm values were highly reproducible: for within-run reproducibility, the rs12979860 ΔTm average was 10.5°C, with an SD of ±0.14°C, and the rs8099917 ΔTm average was 7.9°C, with an SD of ±0.05°C; for between-run reproducibility, the rs12979860 ΔTm average was 9.7°C, with an SD of ±0.78°C, and the rs8099917 ΔTm average was 7.8°C, with an SD of ±0.07°C. For the SNE assay, example results are shown in Figure 2.
Figure 1.
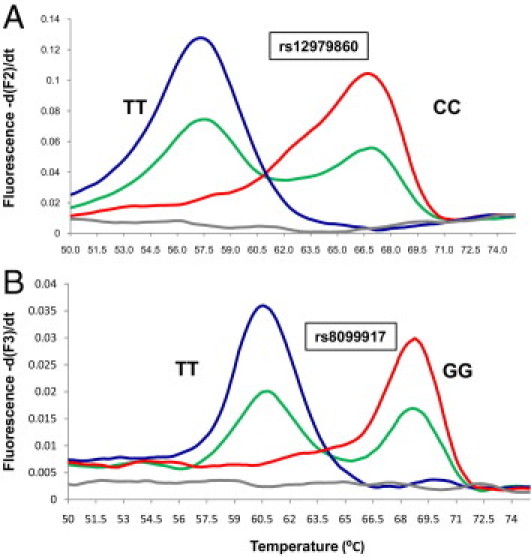
Multiplexed FRET rs12979860 and rs8099917 genotyping results. Melting analysis curves of multiplexed, asymmetric, real-time PCR and dual-color FRET probes of three individuals. A:rs12979860 homozygous for TT (blue) with Tm at 57.6°C, homozygous for CC (red) with Tm at 66.8°C, and heterozygous for TC (green) analyzed with the F2 channel. B:rs8099917 homozygous for TT (blue) with Tm at 61.9°C, homozygous for GG (red) with Tm at 69.3°C, and heterozygous for TG (green) analyzed with the F3 channel. The gray line indicates negative (no template) control.
Figure 2.
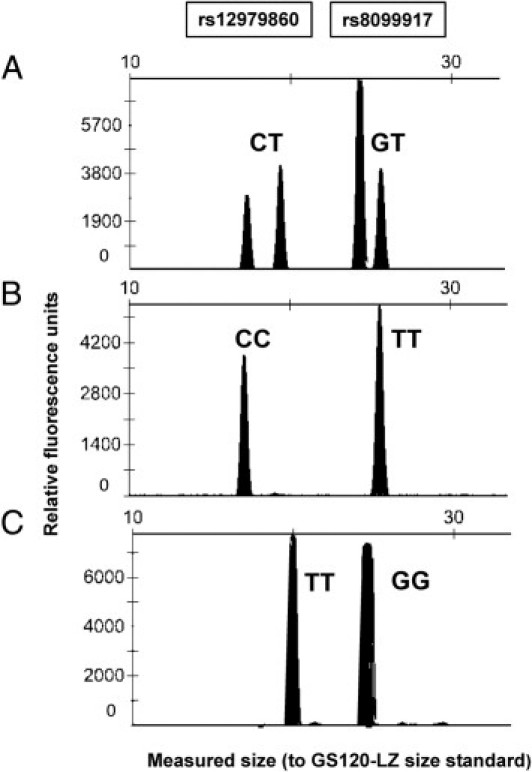
Multiplexed SNE genotyping results of rs12979860 and rs8099917. Electropherograms of the SNE reaction products are shown for three different individuals: rs12979860 heterozygous for CT; rs8099917 heterozygous for GT (A); rs12979860 homozygous for CC; rs8099917 homozygous for TT (B); and rs12979860 homozygous TT; rs8099917 homozygous for GG (C).
Concordance of 100% in genotype calls was obtained between the FRET and sequencing or SNE method. The analytical sensitivity and specificity for rs12979860 are 100% (95% confidence interval, 96.5% to 100%) and 100% (95% confidence interval, 96.1% to 100%), respectively; the analytical sensitivity and specificity for rs8099917 are 100% (95% confidence interval, 94.4% to 100%) and 100% (95% confidence interval, 97.3% to 100%), respectively. Both the allele and genotype frequencies of rs12979860 and rs8099917 were determined in five different ethnic groups: 125 whites, 71 Asians, 10 Middle Easterners, 118 Hispanics, and 119 African Americans. The observed genotype frequencies for each SNP were consistent with the Hardy-Weinberg equilibrium (P ≥ 0.6) for all populations, although a lower Hardy-Weinberg equilibrium (P = 0.06) was observed in African Americans. Figure 3 shows the frequencies of the six different compound genotypes (rs12979860/rs8099917) detected in all ethnic groups. Relative frequencies of the rs12979860 CC favorable genotype versus the combined-risk genotypes with one or no copies of the C allele (CT + TT) and the rs8099917 TT favorable genotype versus the combined-risk genotypes with one or no copies of the T allele (TG + GG) among the different populations are presented in Figure 4. Three haplotypes were observed among whites, Asians, Middle Easterners, Hispanics, and African Americans at the following frequencies: C-T (62%, 89%, 70%, 58%, and 37%), T-T (17%, 1%, 20%, 10%, and 57%), and T-G (22%, 11%, 10%, 32%, and 6%), respectively (Figure 5). The C-G haplotype was not found in any population.
Figure 3.
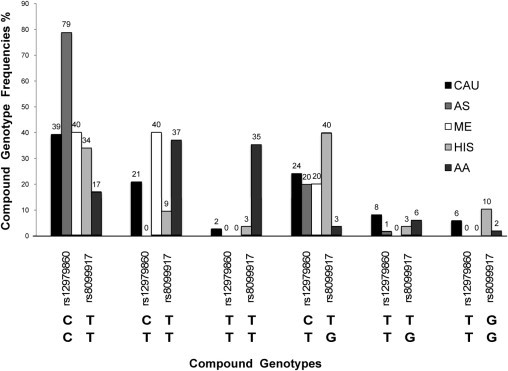
Histogram of frequencies of rs12979860/rs8099917 compound genotypes observed across ethnic populations. Genotype frequencies were obtained for whites (CAU; n = 125), Asians (AS; n = 71), Middle Easterners (ME; n = 10), Hispanics (HIS; n = 118), and African Americans (AA; n = 119). Six different rs12979860/rs8099917 compound genotypes are shown on the x axis. The rs12979860 C and the rs8099917 T favorable alleles associated with efficacy in virus clearance and treatment outcome.
Figure 4.
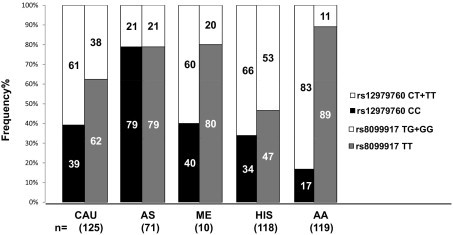
Frequency comparisons of rs12979760 CC and rs8099917 TT favorable genotypes, associated with efficacy in HCV virus clearance and treatment outcome; and rs12979760 CT + TT and rs8099917 TG + GG risk genotypes across different ethnic groups. CAU indicates white; AS, Asian; ME, Middle Eastern; HIS, Hispanic; and AA, African American.
Figure 5.
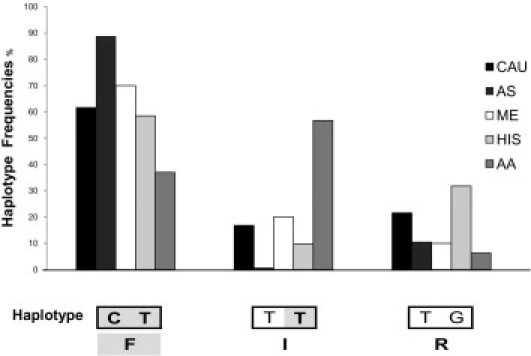
The distribution of rs12979860/rs8099917 haplotypes in whites (CAU), Asians (AS), Middle Easterners (ME), Hispanics (HIS), and African Americans (AA). Haplotype frequencies were obtained from the analysis of 886 chromosomes. Frequencies of C-T, T-T, and T-G haplotypes are shown on the x axis. The C-G haplotype was not observed. F indicates that a favorable haplotype is associated with efficacy in virus clearance and treatment outcome. The rs12979860 C and rs8099917 T favorable alleles are represented in bold and gray shaded. I indicates indeterminate haplotype with the presence of one risk allele (rs12979860 T) and the presence of the rs8099917 T favorable allele. R indicates that the risk haplotype is associated with the presence of two risk alleles (rs12979860 T and rs8099917 G).
Discussion
In the United States, HCV infection is the most prevalent chronic blood-borne disease (available at http://www.cdc.gov/hepatitis/HCV/index.htm, last accessed January 11, 2011). Identifying factors predicting which individuals are likely to respond to the expensive, difficult-to-tolerate, and marginally effective treatment for HCV 1 infection would be clinically useful. The recently published genome-wide association studies have provided insight into host genetic factors that are predictive of HCV disease progression and treatment outcome. Although several SNPs have an association with SVR and spontaneous HCV clearance, rs12979860 and rs8099917 have been identified by multiple groups with different patient cohorts. The FRET assay we developed for the simultaneous genotyping of these two SNPs is a single-tube rapid method with high analytical sensitivity and specificity, as shown by 100% concordance in genotype calls when compared with the SNE and traditional sequencing methods.
In this study, the genotype frequencies observed of individuals of different ethnicities uninfected with HCV are consistent with those of previous studies14,15 specifically investigating the IL28B SNPs and SVR in HCV-infected patients.
By using the FRET method for simultaneous genotyping of the two SNPs, three of the four possible haplotypes (ie, C-T, T-G, and T-T) were detected in all ethnic populations, a total of 443 samples. The presence of favorable alleles for both SNPs, C-T haplotype, predicts virus clearance and successful treatment outcome, whereas the presence of both risk alleles, T-G haplotype, predicts risk of chronicity and treatment failure (Figure 5). However, there are limited data16 for HCV infection outcome for the indeterminate T-T haplotype (T risk allele for rs12979860 and T favorable allele for rs8099917) that we detected in all ethnic populations. Additional studies are necessary to clarify the clinical significance of this haplotype and to determine the risk in individuals carrying a favorable allele for one, but not both, SNPs. Because the causative SNP has not been identified and the correlation between the two SNPs varies between ethnic populations, it may be useful to genotype for both SNPs. Although the precise mechanism of the SNP associations and HCV clearance remains unclear, the fact that the described SNPs are all located near interferon genes is consistent with the evidence supporting the critical role of the host's immune response in HCV infection outcome. Several factors are used to predict SVR, including viral genotype and baseline viral load, yet multivariate analysis shows that the IL28B genotype was the strongest pretreatment predictor of SVR in HCV 1–infected patients.17 Therefore, IL28B genotyping will likely be valuable for HCV management and would benefit from the availability of a simple accurate assay.
In conclusion, the multiplexed FRET assay described herein is rapid and accurately genotypes for both rs12979860 and rs8099917 in different ethnic populations. This multiplex genotyping method provides information not available with single SNP assays that can assist with patient management decisions by predicting the likelihood of HCV clearance and response to therapy.
Acknowledgment
We thank Mohamed Jama for excellent technical assistance with the SNE assay.
Footnotes
Supported by the Institute of Clinical and Experimental Pathology Associated Regional and University Pathologists (ARUP) Laboratories.
References
- 1.Ray Kim W. Global epidemiology and burden of hepatitis C. Microbes Infect. 2002;4:1219–1225. doi: 10.1016/s1286-4579(02)01649-0. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong G.L., Wasley A., Simard E.P., McQuillan G.M., Kuhnert W.L., Alter M.J. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 3.McHutchison J.G., Lawitz E.J., Shiffman M.L., Muir A.J., Galler G.W., McCone J., Nyberg L.M., Lee W.M., Ghalib R.H., Schiff E.R., Galati J.S., Bacon B.R., Davis M.N., Mukhopadhyay P., Koury K., Noviello S., Pedicone L.D., Brass C.A., Albrecht J.K., Sulkowski M.S. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009;361:580–593. doi: 10.1056/NEJMoa0808010. [DOI] [PubMed] [Google Scholar]
- 4.Fried M.W. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36:S237–S244. doi: 10.1053/jhep.2002.36810. [DOI] [PubMed] [Google Scholar]
- 5.Ge D., Fellay J., Thompson A.J., Simon J.S., Shianna K.V., Urban T.J., Heinzen E.L., Qiu P., Bertelsen A.H., Muir A.J., Sulkowski M., McHutchison J.G., Goldstein D.B. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 6.Suppiah V., Moldovan M., Ahlenstiel G., Berg T., Weltman M., Abate M.L., Bassendine M., Spengler U., Dore G.J., Powell E., Riordan S., Sheridan D., Smedile A., Fragomeli V., Muller T., Bahlo M., Stewart G.J., Booth D.R., George J. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;41:1100–1104. doi: 10.1038/ng.447. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka Y., Nishida N., Sugiyama M., Kurosaki M., Matsuura K., Sakamoto N., Nakagawa M., Korenaga M., Hino K., Hige S., Ito Y., Mita E., Tanaka E., Mochida S., Murawaki Y., Honda M., Sakai A., Hiasa Y., Nishiguchi S., Koike A., Sakaida I., Imamura M., Ito K., Yano K., Masaki N., Sugauchi F., Izumi N., Tokunaga K., Mizokami M. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105–1109. doi: 10.1038/ng.449. [DOI] [PubMed] [Google Scholar]
- 8.Thomas D.L., Thio C.L., Martin M.P., Qi Y., Ge D., O'Huigin C., Kidd J., Kidd K., Khakoo S.I., Alexander G., Goedert J.J., Kirk G.D., Donfield S.M., Rosen H.R., Tobler L.H., Busch M.P., McHutchison J.G., Goldstein D.B., Carrington M. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rauch A., Kutalik Z., Descombes P., Cai T., Di Iulio J., Mueller T., Bochud M., Battegay M., Bernasconi E., Borovicka J., Colombo S., Cerny A., Dufour J.F., Furrer H., Günthard H.F., Heim M., Hirschel B., Malinverni R., Moradpour D., Mullhaupt B., Witteck A., Beckmann J.S., Berg T., Bergmann S., Negro F., Telenti A., Bochud P.Y., Swiss Hepatitis C Cohort Study; Swiss HIV Cohort Study Genetic variation in IL28B is associated with chronic hepatitis C and treatment failure: a genome-wide association study. Gastroenterology. 2010;138:1338–1345. doi: 10.1053/j.gastro.2009.12.056. 1345.e1-1345.e7. [DOI] [PubMed] [Google Scholar]
- 10.Thio C.L., Thomas D.L. Interleukin-28b: a key piece of the hepatitis C virus recovery puzzle. Gastroenterology. 2010;138:1240–1243. doi: 10.1053/j.gastro.2010.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robek M.D., Boyd B.S., Chisari F.V. Lambda interferon inhibits hepatitis B and C virus replication. J Virol. 2005;79:3851–3854. doi: 10.1128/JVI.79.6.3851-3854.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jama M., Nelson L., Pont-Kingdon G., Mao R., Lyon E. Simultaneous amplification, detection, and analysis of common mutations in the galactose-1-phosphate uridyl transferase gene. J Mol Diagn. 2007;9:618–623. doi: 10.2353/jmoldx.2007.070027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyon E. Discovering rare variants by use of melting temperature shifts seen in melting curve analysis. Clin Chem. 2005;51:1331–1332. doi: 10.1373/clinchem.2005.051177. [DOI] [PubMed] [Google Scholar]
- 14.McCarthy J.J., Li J.H., Thompson A., Suchindran S., Lao X.Q., Patel K., Tillmann H.L., Muir A.J., McHutchison J.G. Replicated association between an IL28B gene variant and a sustained response to pegylated interferon and ribavirin. Gastroenterology. 2010;138:2307–2314. doi: 10.1053/j.gastro.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akuta N., Suzuki F., Hirakawa M., Kawamura Y., Yatsuji H., Sezaki H., Suzuki Y., Hosaka T., Kobayashi M., Saitoh S., Arase Y., Ikeda K., Chayama K., Nakamura Y., Kumada H. Amino acid substitution in hepatitis C virus core region and genetic variation near the interleukin 28B gene predict viral response to telaprevir with peginterferon and ribavirin. Hepatology. 2010;52:421–429. doi: 10.1002/hep.23690. [DOI] [PubMed] [Google Scholar]
- 16.Hayes C.N., Kobayashi M., Akuta N., Suzuki F., Kumada H., Abe H., Miki D., Imamura M., Ochi H., Kamatani N., Nakamura Y., Chayama K. HCV substitutions and IL28B polymorphisms on outcome of peg-interferon plus ribavirin combination therapy. Gut. 2011;60:261–267. doi: 10.1136/gut.2010.223495. [DOI] [PubMed] [Google Scholar]
- 17.Thompson A.J., Muir A.J., Sulkowski M.S., Ge D., Fellay J., Shianna K.V., Urban T., Afdhal N.H., Jacobson I.M., Esteban R., Poordad F., Lawitz E.J., McCone J., Shiffman M.L., Galler G.W., Lee W.M., Reindollar R., King J.W., Kwo P.Y., Ghalib R.H., Freilich B., Nyberg L.M., Zeuzem S., Poynard T., Vock D.M., Pieper K.S., Patel K., Tillmann H.L., Noviello S., Koury K., Pedicone L.D., Brass C.A., Albrecht J.K., Goldstein D.B., McHutchison J.G. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology. 2010;139:120–129.e18. doi: 10.1053/j.gastro.2010.04.013. [DOI] [PubMed] [Google Scholar]


