Abstract
Purpose
Xanthogranulomatous pyelonephritis is an uncommon disorder of unknown etiology that is characterized by extensive destruction of the involved kidney. It is being increasingly recognized as an important cause of renal morbidity around the world.
Materials and Methods
This retrospective study was undertaken to review the xanthogranulomatous pyelonephritis cases presented at our tertiary care referral center in Bangalore, India.
Results
A total of 16 biopsy-proven cases of xanthogranulomatous pyelonephritis from October 2007 to March 2010 treated at our institute were included in the study. There were 10 females and 6 males with a mean age of 51.5 years. Flank pain was the most common presenting symptom followed by fever. All patients had unilateral disease and underwent total nephrectomy of the affected nonfunctional kidney.
Conclusions
Xanthogranulomatous pyelonephritis is a chronic and unusual infectious inflammatory condition involving the renal parenchyma. The definite treatment is nephrectomy. Early identification and prompt treatment of this relatively benign and uncommon condition is important to minimize morbidity and mortality.
Keywords: Nephrectomy, Pyelonephritis, Xanthogranulomatous
INTRODUCTION
Xanthogranulomatous pyelonephritis (XGP) is an uncommon and distinct type of chronic infective pyelonephritis in which yellow, lobulated masses diffusely replace the renal architecture. The disease is characterized by accumulation of foamy histiocytes, macrophages with mature adipocytes, and occasional giant cells [1]. The condition is commonly seen in middle-aged female patients, especially diabetics. A higher incidence of about 18% has also been reported in the pediatric age range [2,3].
First described by Schlagehaufer in 1916, XGP occurs in 1% of all renal infection [4]. The disease often mimics other inflammatory or neoplastic renal disorders; hence, it has been described as a great imitator owing to its variant clinical presentation.
Unlike chronic pyelonephritis, it may spread to the perinephric tissue with formation of abscesses and even fistulas [5]. The involved areas of the kidney are eventually destroyed, leading to nonfunctioning of the kidney. Surgical intervention in the form of nephrectomy is the only definitive treatment.
We retrospectively reviewed the cases presented at our tertiary care referral center for a better understanding of the clinicopathological profile of XGP with an aim to improve the outcome in future cases.
MATERIALS AND METHODS
All pathologically proven cases of XGP during the period of October 2007 to March 2010 in our institute were included in this retrospective review. A total of 16 cases were diagnosed as XGP during this period. All patients had undergone total nephrectomy. The preoperative workup included routine biochemical and hematological tests along with urine culture. All patients underwent ultrasound, intravenous pyelography (IVP), and renal isotope (DTPA) scanning.
Routine gross examination of the specimen was done. The specimens were fixed on formalin and processed. The sections were stained with H&E. The cases were reviewed with respect to symptoms, laboratory, and histopathologic data.
RESULTS
The study comprised 16 cases, 10 females and 6 males with q mean age of 51.5±17.04 years. The youngest patient in the series was 15 years and the oldest was 73 years. The highest incidence was found in the fifth decade of life in both males and females. The condition was more common in females with a ratio of 1.6:1. All patients had unilateral disease predominantly affecting the left kidney (9 cases out of the 16).
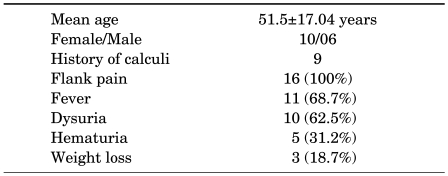
All patients had loin pain as the main presenting complaint followed by fever (68.7%) and dysuria (62.5%). The other complaints were hematuria (31.2%), anorexia (31.2%), and weight loss (18.7%). The duration of symptoms ranged from 6 weeks to 7 years (mean, 18 months). On examination, renal angle tenderness could be elicited in 10 cases and a palpable lump associated with pyonephrosis in 5 cases. The clinical presentations are given in Table 1.
TABLE 1.
Demographics and presenting symptoms
The laboratory results showed the presence of anemia in all cases with a mean hemoglobin of 8.7 g/dl and a raised erythrocyte sedimentation rate above 90 mm/hour. Eleven cases (70%) showed leukocytosis and none had azotemia. Pyuria and bacteremia were present in about 70% of cases. Associated microscopic hematuria was seen in five cases. Urine culture reports were available for 10 patients, of whom 6 patients had growth of gram-negative organisms [E. coli (4 cases) and proteus (2 cases)] and 4 cases had no growth. One case was associated with a tumor (renal cell carcinoma), and renal stones (staghorn or otherwise) were present in 10 cases. Radiologically, all patients underwent ultrasound and IVP. On ultrasonography, an enlarged kidney with thinned out parenchyma with a dilated pelvicaliceal system was found. All patients had a nonfunctioning or poorly functioning involved kidney on IVP confirmed by DTPA renal scan. All patients underwent total nephrectomy and the postoperative period was uneventful.
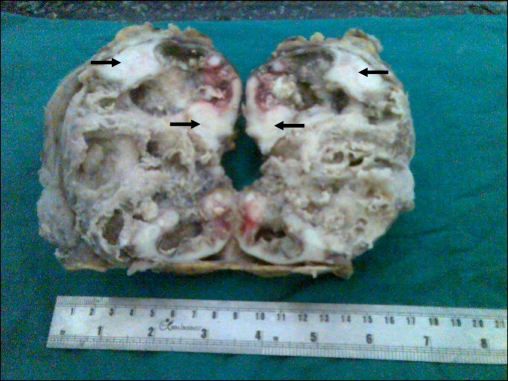
Gross pathology showed increased perinephric fat and adherent capsules in 6 cases, whereas in the remaining 10 cases the capsule was stripped off easily (Fig. 1). Nine cases were associated with calculi: staghorn in eight cases and ureteric calculi in two cases. In all specimens, the corticomedullary tissue was replaced by yellow nodular areas of fatty tissue with diffuse dilatation of the pelvicaliceal system.
FIG. 1.
Cut surface of the kidney with variegated appearance. Arrows correspond to clear vacuolated white areas of xanthogranulomatous pyelonephritis.
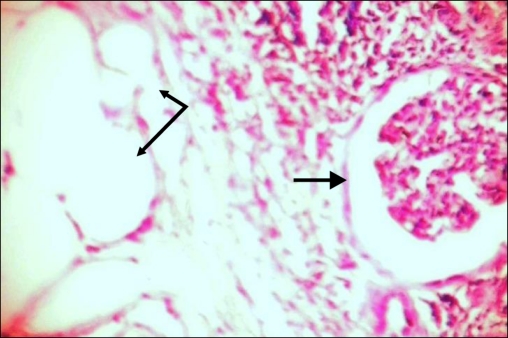
Microscopically, there was diffuse, granulomatous, inflammatory infiltrate consisting of dense lymphoid aggregates with large sheets of foamy histiocytes and neutrophils with plasma cells (Fig. 2). There was a background of chronic pyelonephritis characterized by focal, variable renal tubular atrophy, fibrosis, and chronic inflammation. There were varied areas containing mature adipocytes and congested blood vessels.
FIG. 2.
Bold arrows show glomeruli and the other two arrows show mature adipocytes.
DISCUSSION
XGP is becoming a well-recognized cause of renal morbidity, with more cases being reported in the literature. The worldwide reported incidence varies from 0.6% to 1% of all cases of pyelonephritis. The disease is prevalent in all age groups and is more common in females. In our series, a slight female preponderance was noted in the age distribution from 15 to 73 years [6,7].
The disease process affects the whole of the kidney; focal forms are rare. The lesion is generally unilateral with both the right and the left kidney affected with equal frequency. Bilateral lesions, although rare, have a fatal outcome [8,9]. All 16 cases reported here were unilateral and the affected kidney was nonfunctional.
The exact pathogenesis is still not known, but a number of predisposing factors have been implicated. These include recurrent urinary tract infection, genitourinary obstruction, altered immunological anomalies, and abnormal lipid metabolism. In our series, we found recurrent urinary tract infections, obstruction, and nephrolithiasis as the main factors responsible for the development of XGP [10-13].
All our patients had pain (abdominal/flank) as the primary presenting complaint followed by fever, dysuria, and weight loss, as reported in previous studies [14]. None of the patients had azotemia because of compensatory functioning of the opposite kidney.
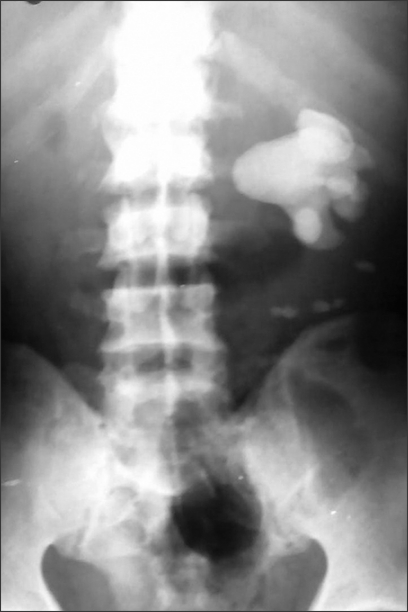
Radiologically, plain X-ray of the abdomen revealed the presence of calculi, especially staghorn calculi (Fig. 3). On ultrasonography, loss of parenchyma with hydronephrosis is usually seen. A nonfunctioning or poorly functioning kidney is the most common finding on IVP and DTPA renal scan.
FIG. 3.
X-ray of abdomen showing staghorn calculi.
XGP is generally a disease limited to the affected kidney, but spread to adjacent tissues has also been seen. According to the extent of involvement of the adjacent tissue, Malek and Elder have classified this disease into three stages [15]:
Stage I: Nephric. Disease confined to renal parenchyma only.
Stage II: Nephric and perinephric. Disease process involves renal parenchyma along with perinephric fat.
Stage III: Nephric and perinephric. Disease extending into adjacent structure or diffuse retroperitoneum.
XGP is often misdiagnosed as a renal tumour (renal cell carcinoma). One of our patients had both concomitant renal cell carcinoma and XGP in the same kidney. This is a very rare presentation and only a few such cases have been reported [16].
Reports of complications such as renocolic fistula and psoas/paranephric abscess have been described. In our series, none of the patients had any sort of complications, and all patients underwent total nephrectomy of the affected kidney [17-20].
The varied clinical presentation and paucity of definite diagnostic tests make the diagnosis difficult to confirm. However, studies have shown that clinical laboratory findings when combined with imaging modalities such as computed tomography scanning and magnetic resonance imaging can help to identify this fulminate kidney infection preoperatively [21]. As in other studies, our results show that XGP is common in middle-aged female patients. Most of these patients had a history of recurrent urinary tract infection, urinary tract obstruction, and urolithiasis, all of which are probable predisposing factors for the development of XGP.
Identifying this subpopulation of patients, aggressively treating the urinary tract infection with appropriate antibiotics, and evaluating and relieving the urinary obstruction is important. Early diagnosis may limit the disease process and associated morbidity, thus leading to a good outcome.
CONCLUSIONS
Early diagnosis and prompt treatment play a crucial role in minimizing the morbidity and mortality rates of XGP. The treatment of choice for diffuse XGP is surgery and consists of nephrectomy with resection of all other involved tissue. Appropriate antibiotic treatment is essential to prevent complications. The prognosis is generally good; most patients recover without any morbidity.
Footnotes
The authors have nothing to disclose.
References
- 1.Tilkoff-Rubin NE, Cotran RS, Rubin RH. Urinary tract infection, pyelonephritis, and reflux nephropathy. In: Brenner BM, editor. Brenner & Rector's The Kidney. 7th ed. Philadelphia: Saunders; 2004. pp. 1554–1555. [Google Scholar]
- 2.Matthews GJ, McLorie GA, Churchill BA, Steckler RE, Khoury AE. Xanthogranulomatous pyelonephritis in pedriatric patients. J Urol. 1995;153:1958–1959. [PubMed] [Google Scholar]
- 3.Lee HN, Kim KH, Ryu IW, Han MC, Chung WS. Xanthogranulomatous pyelonephritis in an infant. Korean J Urol. 2006;47:1367–1370. [Google Scholar]
- 4.Parsons MA, Harris SC, Longstaff AJ, Grainger RG. Xanthogranulomatous pyelonephritis: a pathological, clinical and aetiological analysis of 87 cases. Diagn Histopathol. 1983;6:203–219. [PubMed] [Google Scholar]
- 5.Jung TS, Cho KH, Yang WJ, Song YS, Park YH, Park SM, et al. Xanthogranulomatous pyelonephritis with spontaneous nephrocutaneous fistula. Korean J Urol. 2008;49:1158–1160. [Google Scholar]
- 6.Grainger RG, Longstaff AJ, Parsons MA. Xanthogranulomatous pyelonephritis: a reappraisal. Lancet. 1982;1:1398–1401. doi: 10.1016/s0140-6736(82)92511-9. [DOI] [PubMed] [Google Scholar]
- 7.Rosi P, Selli C, Carini M, Rosi MF, Mottola A. Xanthogranulomatous pyelonephritis: clinical experience with 62 cases. Eur Urol. 1986;12:96–100. doi: 10.1159/000472589. [DOI] [PubMed] [Google Scholar]
- 8.Smith FR. Bilateral xanthogranulomatous pyelonephritis. Br J Urol. 1981;53:81. doi: 10.1111/j.1464-410x.1981.tb03134.x. [DOI] [PubMed] [Google Scholar]
- 9.McDonald GS. Xanthogranulomatous pyelonephritis. J Pathol. 1981;133:203–213. doi: 10.1002/path.1711330304. [DOI] [PubMed] [Google Scholar]
- 10.Eastham J, Ahlering T, Skinner E. Xanthogranulomatous pyelonephritis: clinical findings and surgical considerations. Urology. 1994;43:295–299. doi: 10.1016/0090-4295(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 11.Tolia BM, Iloreta A, Freed SZ, Fruchtman B, Bennett B, Newman HR. Xanthogranulomatous pyelonephritis: detailed analysis of 29 cases and a brief discussion of atypical presentations. J Urol. 1981;126:437–442. doi: 10.1016/s0022-5347(17)54566-8. [DOI] [PubMed] [Google Scholar]
- 12.Van Vlem B, Billiouw JM. Images in clinical medicine. Xanthogranulomatous pyelonephritis. N Engl J Med. 2000;342:1572. doi: 10.1056/NEJM200005253422105. [DOI] [PubMed] [Google Scholar]
- 13.Kim YJ, Huh JS. Clinical characteristics of xanthogranulomatous pyelonephritis. Korean J Urol. 2004;45:935–940. [Google Scholar]
- 14.Levy M, Baumal R, Eddy AA. Xanthogranulomatous pyelonephritis in children. Etiology, pathogenesis, clinical and radiologic features, and management. Clin Pediatr (Phila) 1994;33:360–366. doi: 10.1177/000992289403300609. [DOI] [PubMed] [Google Scholar]
- 15.Malek RS, Elder JS. Xanthogranulomatous pyelonephritis: a critical analysis of 26 cases and of the literature. J Urol. 1978;119:589–593. doi: 10.1016/s0022-5347(17)57559-x. [DOI] [PubMed] [Google Scholar]
- 16.Papadopoulos I, Wirth B, Wand H. Xanthogranulomatous pyelonephritis associated with renal cell carcinoma. Report on two cases and review of the literature. Eur Urol. 1990;18:74–76. doi: 10.1159/000463873. [DOI] [PubMed] [Google Scholar]
- 17.Matsuoka Y, Arai G, Ishimaru H, Takagi K, Aida J, Okada Y. Xanthogranulomatous pyelonephritis with a renocolic fistula caused by a parapelvic cyst. Int J Urol. 2006;13:433–435. doi: 10.1111/j.1442-2042.2006.01326.x. [DOI] [PubMed] [Google Scholar]
- 18.Alan C, Ataus S, Tunç B. Xanthogranulamatous pyelonephritis with psoas abscess: 2 cases and review of the literature. Int Urol Nephrol. 2004;36:489–493. doi: 10.1007/s11255-004-0858-5. [DOI] [PubMed] [Google Scholar]
- 19.Sherman SC, Limkakeng A. Xanthogranulomatous pyelonephritis with a nephrocutaneous fistula. J Emerg Med. 2005;29:337–338. doi: 10.1016/j.jemermed.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 20.Park S, Ji YH, Cheon SH, Kim YM, Moon KH. Urachal xanthogranuloma: laparoscopic exicision with minimal incision. Korean J Urol. 2009;50:714–717. [Google Scholar]
- 21.Tiu CM, Chou YH, Chiou HJ, Lo CB, Yang JY, Chen KK, et al. Sonographic features of xanthogranulomatous pyelonephritis. J Clin Ultrasound. 2001;29:279–285. doi: 10.1002/jcu.1034. [DOI] [PubMed] [Google Scholar]