Abstract
Leprosy, also known as Hansen’s disease, is a chronic infectious disease caused by Mycobacterium leprae in which susceptibility to the mycobacteria and its clinical manifestations are attributed to the host immune response. Even though leprosy prevalence has decreased dramatically, the high number of new cases indicates active transmission. Owing to its singular features, M. leprae infection is an attractive model for investigating the regulation of human immune responses to pathogen-induced disease. Leprosy is one of the most common causes of nontraumatic peripheral neuropathy worldwide. The proportion of patients with disabilities is affected by the type of leprosy and delay in diagnosis. This article briefly reviews the clinical features as well as the immunopathological mechanisms related to the establishment of the different polar forms of leprosy, the mechanisms related to M. leprae–host cell interactions and prophylaxis and diagnosis of this complex disease. Host genetic factors are summarized and the impact of the development of interventions that prevent, reverse or limit leprosy-related nerve impairments are discussed.
Keywords: leprosy, Mycobacterium leprae, Schwann cells, thalidomide, TNF-α
Leprosy: clinical features & classification
The principal mode of transmission of Mycobacterium leprae is probably by aerosol spread of nasal secretions and uptake through nasal or respiratory mucosa [1]. The incubation period of the disease ranges from 3 to 10 years. M. leprae primarily invades Schwann cells (SCs) in the peripheral nerves leading to nerve damage and the development of disabilities [2].
As described by Ridley and Jopling, disease classification is defined within two poles with transition between the clinical forms [3]. Clinical, histopathological and immunological criteria identify five forms of leprosy: tuberculoid polar form (TT), borderline tuberculoid (BT), mid-borderline (BB), borderline lepromatous (BL) and lepromatous polar leprosy (LL).
Regarding the histopathological aspects of leprosy, skin lesions from tuberculoid patients are characterized by inflammatory infiltrate containing well-formed granulomas with differentiated macrophages, epithelioid and giant cells and a predominance of CD4+ T cells at the lesion site, with low or absent bacteria. Patients show a vigorous specific immune response to M. leprae with a Th1 profile, IFN-γ production and a positive skin test (Lepromin or Mitsuda reaction). On the other pole, lepromatous patients present with several skin lesions with a preponderance of CD8+ T cells in situ, absence of granuloma formation, high bacterial load and a flattened epidermis [4]. The number of bacilli from a newly diagnosed lepromatous patient can reach 1012 bacteria per gram of tissue. Patients with LL leprosy have a CD4:CD8 ratio of approximately 1:2 with a predominant Th2 type response and high titers of anti-M. leprae antibodies. Cell-mediated immunity against M. leprae is either modest or absent, characterized by negative skin test and diminished lymphocyte proliferation [5,6].
For therapeutic purposes, patients were divided into two groups: paucibacillary (TT, BT) and multibacillary (mid-borderline (BB), BL, LL) [7]. Later, it was recommended that the classification was based on the number of lesions, less than or equal to five for paucibacillary (PB) and greater than five for the multibacillary (MB) form.
The diagnosis of leprosy is primarily clinical and based on one or more of three signs: hypopigmented or reddish patches with definite loss of sensation, thickened peripheral nerves and acid-fast bacilli detected on skin smears or biopsy material [8]. Identification of bacteria is supported by detection of the bacilli through staining of slit-skin or lymph smears and skin biopsies. The bacillary index (BI) is a logarithmic scale (ranging from 1–6+) quantifying the density of M. leprae on lymph smears and it is used to assess bacterial load for classification and response to treatment.
The disease is curable and treated with multi-drug therapy (MDT). According to the WHO guidelines, rifampicin (600 mg, once monthly), clofazimine (300 mg, once monthly and 50 mg daily) and dapsone (100 mg daily) are used for treatment of MB patients, and lasts 1 year. For PB patients, only rifampicin (600 mg, monthly) and dapsone (100 mg daily) are employed for a period of 6 months.
In individuals unable to take clofazimine or dapsone, other agents, such as fluoroquinolones, ofloxacin, moxifloxacin or pefloxacin, minocycline, and the macrolide clarithromycin, are active against M. leprae and can all potentially be used as second-line agents [2].
Reactional episodes in leprosy
More striking is the occurrence of leprosy reactions, which are acute episodes of clinical inflammation occurring during the chronic course of disease. Leprosy reactions are a challenging problem because they increase morbidity due to nerve damage even after the conclusion of MDT. They are classified as type I (reversal reaction; RR) or type II (erythema nodosum leprosum; ENL) reactions. Type I reaction occurs in borderline patients (BT, mid-borderline and BL) whereas ENL only occurs in BL and LL forms.
Reactions are interpreted as a shift in patients’ immunologic status. Chemotherapy, pregnancy, concurrent infections, and emotional and physical stress have been identified as predisposing conditions to reactions [9]. Rarely severe enough to require hospitalization, both types have been found to cause nerve inflammation (neuritis), representing the primary cause of irreversible deformities.
Type 1 reaction is characterized by edema and erythema of existing skin lesions, the formation of new skin lesions, neuritis and additional sensory and motor loss. Edema of the hands, feet and face can also be a feature of reaction, but systemic symptoms are unusual. The presence of an inflammatory infiltrate with a predominance of CD4+ T cells, differentiated macrophages and thickened epidermis has been observed in RR and in the tuberculoid form.
Type II reaction is characterized by the appearance of tender, erythematous, subcutaneous nodules located on apparently normal skin, and is frequently accompanied by systemic symptoms, such as fever, malaise, enlarged lymph nodes, anorexia, weight loss, arthralgia and edema. Additional organs including the testes, joints, eyes and nerves may also be affected. Furthermore, a patient may present significant leukocytosis that typically recedes after the reactional state has subsided. Some reports have confirmed the presence of high levels of proinflammatory cytokines such as TNF-α, IL-6 and IL-1β in the sera of ENL patients, suggesting that these pleiotropic inflammatory cytokines may be at least partially responsible for the clinical manifestations of a type II reaction [10,11].
Treatment of reactions is aimed at controlling the acute inflammation, easing pain and reversing eye and nerve damage. MDT should be continued during the reactional episodes. For type 1 reactions, treatment relies on oral steroids. Even though different regimens have been employed, prednisone can be used at 0.5–1 mg/kg weight daily decreased by 5–10 mg every 2–4 weeks after demonstration of improvement [8]. However, there is no consensus about the dose or duration of treatment [12]. One randomized controlled trial demonstrated that patients treated with a 5 month course of prednisone were less likely to need additional steroid treatment than those treated with a 3 month course of the drug [12]. Patients with a type 1 reaction have relatively slow response to therapy and variation of individual responses [13].
The majority of ENL reactions require immunosuppression. The more severe ones require high doses of steroids, usually starting with prednisone 60 mg daily [14,15]. The recurrent nature of the condition means that steroid-induced side effects may become a significant problem. Thalidomide 300–400 mg daily has a dramatic effect in controlling ENL and preventing recurrences [16]. Clofazimine and pentoxifylline have both been used in ENL [16,17], but they are less effective than prednisone or thalidomide [18–20]. Because of the teratogenic effects of thalidomide, the use of prednisone and clofazimine has been reinforced by the WHO for treatment of reactions.
Prednisone, one drug with potent anti-inflammatory properties, remains the drug of choice for treatment of neuritis owing to its ability to reduce nerve edema, exert an immunosuppressive effect and decrease postinflammatory scar formation, all important for improving nerve function. Moreover, when detected and treated in time, peripheral neuropathy may not progress into deformity and may even reverse initial impairments.
Thalidomide, a racemic glutamic acid analog, was first synthesized in Germany in 1954, and subsequently marketed in Europe, Australia and Canada as a sedative (with less potential for overdose compared with barbiturates) and antiemetic. Owing to its properties, it was rapidly used in pregnant women but because of teratogenicity, it was taken off the market in 1961 [21]. After a search for the cause of birth defects, thalidomide was found to inhibit angiogenesis [22,23] and its mechanism of teratogenicity was recently unraveled [24]. In this work, cereblon was identified as a thalidomide-binding protein. This protein forms an E3 ubiquitin ligase complex with damaged DNA-binding protein 1. Thalidomide binding to cereblon inhibits the associated ubiquitin ligase activity [24].
The use of thalidomide was approved by the FDA in the USA in 1998 for the treatment of the cutaneous manifestations of moderate to severe ENL and suppression of the cutaneous manifestations of ENL recurrences. Several reports have demonstrated thalidomide effectiveness in treating various inflammatory and dermatological conditions, such as ENL and graft versus host disease [16,21], and it plays a role in vascular remodeling [21,23]. Adverse events associated with the use of thalidomide, some of which can be dose-related, include somnolence, peripheral neuropathy, constipation and rash. Some of the described mechanisms of action of thalidomide involve a decrease of TNF-α, IL-1β and IL-6 production [25,26], and diminished density of TNF-induced cell-surface adhesion molecules ICAM-1, VCAM-1 and E-selectin on human endothelial cells, it also has antiangiogenic properties that seem to be independent of their immunomodulatory effects, among others [21,27]. Treatment of ENL with thalidomide or pentoxifylline also decreased gene expression at the lesion site [28,29].
Since thalidomide safety has been questioned, several thalidomide analogs, with the same pharmacological effects and reduced side effects have been developed [27,30,31]. Potential efficacy of the drug and its analogs has been observed in human trials for relapse/refractory multiple myeloma, leukemia, glioma, metastatic melanoma, pancreatic and prostate cancer [21,27,32]. However, whether they can be used to treat ENL with the same efficacy when compared with thalidomide still needs to be determined.
Epidemiology
Over the last 20 years, the WHO has implemented an intense and coordinated worldwide MDT program to treat leprosy. MDT, which cures the infection, has led to the understanding that leprosy can be effectively treated before disability develops [2]. Since 1985, more than 14 million individuals have received MDT [201].
The MDT program has reduced the prevalence of M. leprae infection to less than one case per 10,000 in 90% of the endemic countries where leprosy was considered to be a public health problem [201]. Despite achievements reached with MDT, new case detection rates are still high [33, 201]. Altogether, 244,796 new cases of leprosy were detected during 2009 and the registered prevalence at the beginning of 2010 was 211,903 [202]. Among new cases, 55% are multibacillary patients and 9% occur in children. India and Brazil account for 54% and 15%, respectively. Among highly populated countries, Brazil, Nepal and Timor-Leste have not eliminated leprosy [202]. In adults, the lepromatous type of leprosy is more common in men than in women after puberty, with a male to female ratio of 2:1. In children, the tuberculoid form predominates, and no sex preference exists. The occurrence of disease in children reflects disease transmission in the community and efficiency of the control programs [34].
In 2009, the global leprosy program established a sentinel surveillance network to monitor drug resistance although the proportion of resistant bacteria is small. Data have been systematically collected in six endemic countries: Brazil, China, Colombia, India, Myanmar and Vietnam. Accordingly, relapse rates following MDT are acceptably low (≤10%); they vary from 0 to 2.5% in PB leprosy and 0–7.7% in MB patients. The higher rates are observed in patients with the higher bacterial load at pretreatment (BI > 4) [35,203].
There has been concern that co-infection with HIV might exacerbate the pathogenesis of leprosy lesions and/or lead to increased susceptibility to leprosy as it is seen with tuberculosis. However, to date, HIV infection has not been reported to increase susceptibility to leprosy, impact on immune response to M. leprae or to have a significant effect on the pathogenesis of neural or skin lesions [36,37]. By contrast, initiation of antiretroviral treatment has been reported to be associated with activation of subclinical M. leprae infection and exacerbation of existing leprosy lesions (type 1 reaction) likely as part of immune reconstitution inflammatory syndrome [38–40].
Genomics of mycobacteria
Many mycobacteria are ubiquitously found in soil and water, can be isolated from the environment and are termed nontuberculous mycobacteria, whereas a few members (M. leprae, Mycobacterium tuberculosis (MTB), M. ulcerans) are major human and animal pathogens. Since the discovery of M. leprae by Armauer Hansen (1873) and MTB by Robert Koch (1883), approximately 130 mycobacterial species have been described [41]. Mycobacteria are known for their notoriously slow growth but among them, they are subsequently divided into rapid (M. smegmatis, M. abscessus, M. chelonae) and slow growers (M. avium, M. kansasii), with doubling times between 2 and 6 h, and approximately 24 h for those belonging to the MTB complex. The record of bacterial slow growth is attained by M. leprae, which doubles approximately every 14 days. Surprisingly, M. leprae has not yet been successfully cultured in vitro [41,42].
The availability of mycobacterial genomes in the past few years has provided a huge pool of information and careful analysis continues to provide insights into the biology and virulence determinants of the major mycobacterial species. The first mycobacterial genome sequenced was MTB H37Rv (originally isolated in 1905) [43]. The genome of M. leprae has also been sequenced in totality [44]. Comparison of the M. leprae genome with that of MTB revealed that the leprosy bacillus underwent extensive reductive evolution resulting in functional loss of approximately 2000 genes, nearly half of its metabolic functions and absence of entire metabolic pathways that are functional in MTB. It presents with less than 50% coding capacity, it consists of 3,268,203 bp genome size (MTB has 4,411,532 bp), 2770 total genes and 1605 protein-coding sequences (MTB has 3991). A notable feature of the M. leprae genome is the exceptionally large number of pseudogenes (1115). The remaining M. leprae genes help to define the minimal gene set necessary for in vivo survival of this mycobacterial pathogen as well as genes potentially required for infection and pathogenesis seen in leprosy. Interestingly, M. leprae pseudogenes are highly expressed as RNA and their expression levels seem to change following macrophage infection [45].
Recently, a newly identified mycobacterium, M. lepromatosis sp, which causes disseminated leprosy has been described [46,47]; its significance is still not clearly understood.
Work by Monot et al. described 16 single nucleotide polymorphisms, allowing the classification of subtypes of M. leprae with limited geographic distribution [48]. When comparing strains from Brazil, Thailand and the USA, little genomic diversity was found, which suggests that leprosy has arisen from infection with a single clone that has passed through a recent evolutionary bottleneck. A traceable route to the origin of leprosy is also proposed [49].
Variable number tandem repeats allow population-based genotyping analysis on clinical samples of M. leprae, and investigation of transmission and strain variation in different regions [50,51]. Nevertheless, it has been demonstrated that variable number tandem repeat profiles may vary among isolates obtained from different lesions in one particular patient [52].
Genetic determinants of host response to M. leprae
Many studies have pointed out the genetic predisposition of the host to the development of diseases such as leprosy and tuberculosis. It is suggested that human genetic factors may influence the acquisition of leprosy and the clinical course of disease [53]. Single nucleotide polymorphism-association studies showed a low lymphotoxin-α (LTA)-producing allele as a major genetic risk factor for early onset leprosy [54]. Other hits found SNPs to be associated with disease and/or the development of reactions in several genes, such as vitamin D receptor (VDR), TNF-α, IL-10, IFN-γ, HLA genes and TLR1 [55–58]. On the other hand, linkage studies have identified polymorphic risk factors in the promoter region shared by two genes: PARK2, coding for an E3-ubiquitin ligase designated Parkin, and PACRG [59], This study revealed a new ubiquitin-dependent pathway of immunity to infection with M. leprae.
A frequently occurring SNP in TLR1 (the 602S allele), which impairs receptor trafficking and function, has been described and seems to play a protective role in the context of clinical leprosy [60]. Previous studies has demonstrated that TLR2 mediates the innate immune recognition of M. leprae [61]. TLR2 polymorphisms are associated with susceptibility to leprosy and/or leprosy reactions. Bochud et al. demonstrated that the TLR2 microsatellite polymorphism as well as a TLR2 SNP (597C→T) both influenced susceptibility to RR [62]. No associations were demonstrated with occurrence of neuritis or ENL and the observations are consistent among three different ethnic groups. These data suggest that TLR2 is differentially involved in the clinical manifestations of leprosy. Since TLR2 requires TLR1 or TLR6 as co-receptors, mutations in the TLR1 or TLR6 genes may have a stronger effect on susceptibility to leprosy or leprosy type.
Several polymorphisms located near the 3′UTR of the VDR gene (BsmI, ApaI and TaqI) are related to the stability or transcriptional activity of VDR mRNA, whereas a polymorphism located in the translation initiation codon (FokI) gives rise to a three-amino acid difference in the VDR length that affects protein function [63–64]. The TaqI polymorphism was associated with clinical subtypes of leprosy in one study conducted in Calcutta, India [65]. In this work the TT genotype was associated with the tuberculoid leprosy form characterized by a strong cellular response but not with resistance to leprosy per se, which was associated with heterozygosity for VDR genotype. However, Sapkota et al. have described inconclusive results about the relevance of VDR-FokI gene polymorphism in the development of a RR [66]. The authors did not find association between VDR-TaqI and specific subtypes of leprosy. Controversies over the VDR acting as a susceptibility factor in Mycobacterium sp. infections may be the result of incorrect population stratification [67]. Therefore, differences in ethnic backgrounds of the study population, sample size, other aspects of study design, or altered virulence of M. leprae in different geographic locations should be considered. Sapkota et al. discussed the possibility that the effect of TaqI polymorphism might be related to its close linked loci (including ApaI or BsmI) [66] which contribute variably to disease phenotype across populations.
Recently, the first genome-wide association study for leprosy demonstrated a significant association between polymorphisms found in seven genes and susceptibility to disease [68]. The association between SNPs in CCDC122, C13orf31, NOD2, TNFSF15, HLA-DR, LRRK2 and RIPK2 were stronger for MB leprosy. One attractive mechanism pointed out in the study links the NOD2-mediated signaling pathway to susceptibility to infection with M. leprae. One additional study from Berrington et al. also suggests that NOD2 genetic variants are associated with susceptibility to leprosy and the development of reactions (RR and ENL) [69].
Early diagnostics & prophylaxis
Proximity to leprosy patients is an important determinant of transmission [1,70]. The relative risk for leprosy disease in household contacts is 8–10 for lepromatous disease and 2–4 for the tuberculoid forms. As leprosy prevalence falls in a community, the relative importance of household transmission increases; this association might justify prophylactic therapy in family or other close contacts of leprosy patients.
The true incidence of leprosy is difficult to assess and the rate of infection with M. leprae in a community cannot be measured, by contrast with MTB, for which the annual rate of infectivity is estimated from surveys following the use of tuberculin skin test (TST or purified protein derivative [PPD]) reactivity.
Immunological tests have been used as complementary tools in disease diagnosis. The lepromin skin test is useful to assess the ability to mount a granulomatous response following injection of killed M. leprae and reading is performed after 28 days. BT and TT patients show a positive response (> 5 mm) whereas LL typically has no response.
Another test evaluates antibody response against the phenolic glycolipid-1 (PGL-1) of M. leprae. Even though serologic tests alone cannot provide a definitive diagnosis, it is helpful to aid in patient classification when the results are considered together with clinical information. Anti-PGL-1 antibodies (if detected by ELISA or through the dipstick assay) have been used in several studies showing that leprosy patients at the lepromatous end of the spectrum form large amounts of antibodies (seropositivity 80–100%), while patients at the tuberculoid end show specific immunoglobulin at much lower levels (30–60%) [71–73]. The use of a new M. leprae serum lateral flow test was easier to manage and results correlated the concentration of anti-PGL-1 in patients’ peripheral blood with their bacillary load [74]. It may be used to classify patients as MB or PB and in the monitoring of treatment efficacy [73,74]. This test can be also used for diagnostic procedures to identify bacterial burden in healthy individuals from endemic areas and in household contacts of leprosy patients [75].
Another diagnostic tool relies on sensitive PCR assays that detect highly conserved M. leprae-specific genes in biological samples targeting the 16S rRNA or the complex 85 genes [76,77]. The assay has been applied to support monitoring disease status in leprosy patients or in those who present with nerve damage in the absence of skin lesions [78].
Evaluation of the cell-mediated immune response based on IFN-γ release assays have been used to identify M. leprae-specific antigens able to discriminate asymptomatic disease and/or early stage of infection in endemic countries. It has been demonstrated that M. leprae peptides discriminate contacts and patients from healthy controls better [79,80]. However, they induce low levels of IFN-γ compared with proteins, especially if evaluated by whole blood assays [81]. Peptides that provide specific responses in leprosy patients from an endemic setting could potentially be developed into a rapid diagnostic test for the early detection of M. leprae infection and epidemiological surveys of the incidence of leprosy, of which little is known. One of the hurdles hampering T-cell-based diagnostic tests is that M. leprae antigens cross-react at the T-cell level with antigens present in other mycobacteria, such as MTB or M. bovis BCG.
One additional challenging topic refers to strategies to be applied and how to proceed when potentially infected contacts are identified. Immunoprophylaxis with BCG is routinely given to infants and there is good evidence that this affords some protection against leprosy [82]. In Brazil, BCG vaccination of leprosy contacts is a routine procedure mandated by the Leprosy Control Program. One recent study showed a good protective effect (50–80%) of the vaccine against development of the lepromatous forms of leprosy [83]. Revaccination with BCG in the general population or immunization in contacts of known leprosy [84] has been tried in a research setting to look for any further protective effect. Importantly, meta-analyses of BCG protection against leprosy indicate that the current vaccine could impact on leprosy control, mainly as a two-dose vaccination [82,85].
On the other hand, chemoprophylaxis has been employed as a mechanism to interrupt the chain of transmission and early stages of infection. Single-dose rifampicin was shown to have some protective effect [86]. It was also observed that protection from BCG given in infancy was additional to protection gained from rifampicin chemoprophylaxis [87]. Since both interventions are known to have a preventive effect, it may be expected that such approaches, the combined effect of chemoprophylaxis with single dose rifampicin and immunoprophylaxis with BCG, in contacts of new cases of leprosy may be complimentary, and even long-lasting. This type of research needs to be further encouraged.
Immunology of reactional episodes
Reversal reaction appears to be a naturally occurring delayed-type hypersensitivity response to M. leprae. Clinically, it is characterized by ‘upgrading’ of the clinical picture towards the tuberculoid pole, including a reduction in bacillary load. Immunologically, it is characterized by the development of strong skin test reactivity as well as lymphocyte responsiveness and a predominant Th1 response [88,89]. RR episodes have been associated with the infiltration of IFN-γ and TNF-secreting CD4+ lymphocytes in skin lesions and nerves, resulting in edema and painful inflammation [90,91]. As a result of this T-cell response, diagnostic assays have been proposed to predict higher risk patients for type 1 or type 2 reaction. Some immunologic markers were described suggesting CXCL10 as a potential tool for discriminating RR [92].
More recently, Massone et al. have demonstrated no difference in FoxP3 expression between unreactional patients whereas a significant increase in FoxP3 staining was observed in RR patients compared with ENL and patients with nonreactional leprosy, implying a role for regulatory T cells in RR [93].
As for ENL, even though pathogenesis is thought to be related to the deposition of immune complexes [94], evidence indicating that cell-mediated immunity plays a role in ENL is unclear. An enhanced pattern of immune response has been detected in leprosy patients during the reactional episodes concomitant with increased levels of TNF-α, IL-1β and IFN-γ and other cytokines in type I and II reactions [95–97]. In addition, C-reactive protein, amyloid A protein and α-1 antitrypsin have all been reported to be elevated in ENL patients’ sera [98]. Nevertheless, despite the data accumulated so far, the critical mechanisms that initiate either type of reaction have not been clearly determined.
Neutrophils constitute a common finding in lesions from patients undergoing type II reaction or ENL. A massive infiltrate of polymorphonuclear cells (PMN) in the lesions is only observed during ENL and some patients present with high numbers of neutrophils in the blood as well. One pioneering study from our group [99] demonstrated that neutrophils obtained from patients with type II reaction presented accelerated ex vivo apoptosis compared with cells from nonreactional lepromatous patients or normal donors. Moreover, following stimulation with lipopolysaccharide, lipoarabinomannan or M. leprae, PMNs were able to secrete IL-8 and TNF-α; cytokine production by PMNs was inhibited by thalidomide, an effective drug for treatment of ENL [99]. Thus, neutrophils may contribute to the bulk of TNF production that is associated with tissue damage in leprosy. More recently, microarray analysis demonstrated that the mechanism of neutrophil recruitment in ENL involves the enhanced expression of E-selectin and IL-1β, likely leading to neutrophil adhesion to endothelial cells [100]; again, an effect of thalidomide on PMN function was observed since this drug inhibited the neutrophil recruitment pathway. Altogether, the data highlight some of the possible mechanisms for thalidomide’s efficacy in treating type 2 reaction.
TNF-α may augment the immune response towards the elimination of the pathogen and/or mediate the pathologic manifestations of the disease. TNF-α can be induced following stimulation of cells with total, or components of, M. leprae, namely lipoarabinomannan (the mycobacteria ‘lipopolysaccharide’-like component) a potent TNF inducer [101]. In addition, mycolyl arabinogalactan–peptidoglycan complex of Mycobacterium species, the protein–peptidoglycan complex and muramyl dipeptide all elicit significant TNF-α release [101].
Mycobacterium leprae induces apoptosis in monocytes and SCs [102,103]. In addition, it was also demonstrated that proteasome function is important for M. leprae-induced apoptosis [104], and that thalidomide and pentoxifylline can prevent cell death and induction of proapoptotic genes by the mycobacteria [99,102]. Induction of apoptosis in SCs was validated following the observation of apoptotic SCs in the lesions of leprosy patients [105]. On the other hand, murine macrophages infected with live M. leprae demonstrated little apoptosis, while higher rates of cell death was observed when macrophages were infected with dead mycobacteria [106]. Differences in host sensitivity regarding the outcome of infection may reflect the divergence of results. In human cells and tissue, the production of inflammatory cytokines and induction of apoptosis induced by M. leprae can contribute to lower mycobacteria viability at the lesion site, and/or it can contribute to increase tissue and nerve damage.
M. leprae & SC interaction
Schwann cells are a major target for infection by M. leprae leading to injury of the nerve, demyelination and consequent disability. The molecular basis of these interactions has just started to be unraveled. In vitro – cultures of nervous tissue – and in vivo experiments – animal models – have confirmed the M. leprae tropism for peripheral nerves. Binding of M. leprae to SCs induces demyelination and loss of axonal conductance [107]. Using Rag-1−/− mice, which lack B and T lymphocytes, it was demonstrated that M. leprae attachment to the SC surface is sufficient to cause demyelination in peripheral nerves. Conversion of SC from the myelinated to nonmyelinated phenotype is directly related to an increased proliferative capacity [107].
ST88–14 is a scwhannoma SC line immortalized by Yan and colleagues [108]. Over the last decade, the cell line has been used by researchers as a model for M. leprae/SC interaction.
Accumulated studies have suggested cell surface molecules are involved in M. leprae adhesion to the SC surface. It has been shown that M. leprae can invade SCs by a specific laminin-binding protein of 21 kDa [109] in addition to PGL-1 [110]. PGL-1, a major unique glycoconjugate on the M. leprae surface, binds laminin-2, which explains the predilection of the bacterium for peripheral nerves [110]. In conjunction, data have been validated with the use of co-culture axon-SCs in experimental models, the use of the human SC line and of human primary SCs.
The identification of the M. leprae-targeted SC receptor, dystroglycan (DG), suggests a role for this molecule in early nerve degeneration [111]. α-DG is a component of the DG complex involved in the pathogenesis of muscular dystrophies. M. leprae specifically binds to α-DG only in the presence of the G domain of the α2 chain of laminin-2 [111,112]. Thus, M. leprae may use linkage between the extracellular matrix and cytoskeleton through laminin-2 and α-DG for its interaction with SCs [112,113].
Mycobacterium leprae-induced demyelination is a result of direct bacterial ligation to neuregulin receptor, ErbB2 and Erk1/2 activation, and subsequent MAP kinase signaling and proliferation [114]. M. leprae-induces activation of ErbB2 receptor tyrosine kinase (RTK) signaling without ErbB2–ErbB3 heterodimerization, a previously unknown mechanism that bypasses the neuregulin–ErbB3-mediated ErbB2 phosphorylation [114]. MEK-dependent Erk1 and Erk2 signaling is identified as a downstream target of M. leprae-induced ErbB2 activation that mediates demyelination.
One more recent study suggests a biological role for 9-O-acetyl GD3 in the infection of SCs with M. leprae [115]. 9-O-acetyl GD3 ganglioside is an acetylated glycolipid present in the cell membrane of many types of vertebrate cells and seems to play an important role in the development, differentiation and regeneration of the nervous system [115].
Additional mechanisms that contribute to damage to the peripheral nerve have been investigated. SC ST88–14 and staining of SCs at the lesion skin site demonstrated the expression of Toll-like receptors (TLRs) on SCs [105]. TLRs can therefore be activated by M. leprae lipoproteins and, consequently, lead to apoptosis and cytokine release [105].
Matrix metalloproteinases (MMPs) mediate demyelination and breakdown of the blood–nerve barrier in peripheral neuropathies. MMPs and tissue inhibitor of metalloproteinase 1 gene expression and secretion were studied in SCs stimulated with M. leprae and TNF-α, and in nerve biopsies [116,117]. M. leprae induced upregulation of MMP-2 and MMP-9 and increased secretion of these enzymes in cultured ST88–14 cells. M. leprae, through the induction of TNF-α, induces NF-κB-dependent transcription in human SCs, a phenomena that is inhibited by thalidomide [118].
To investigate potential mechanisms of nerve injury in leprosy, studies on ninjurin 1, an adhesion molecule involved in nerve regeneration in leprosy were performed [119]. It was demonstrated that M. leprae stimulates in vitro upregulation of ninjurin mRNA in cultured SCs and blood cells from leprosy patients. mRNA and protein expression were also detected in leprosy nerve biopsies. A polymorphism (asp110ala) was investigated in a case–control study and no association was found with leprosy. SNP ala110 was also associated with nerve functional impairment (NFI) and with lower mRNA levels.
Mechanisms of M. leprae uptake & nonresponsiveness in leprosy
Macrophages are one of the most abundant host cells to come in contact with mycobacteria. Pathogenic mycobacteria have adopted various strategies to invade their hosts, but the exact mechanism of internalization of the bacteria by phagocytic cells is unknown. Phagocytosis of M. leprae by monocyte-derived macrophages can be mediated by complement receptors CR1 (CD35), CR3 (CD11b/CD18) and CR4 (CD11c/CD18) [120] and is regulated by protein kinase [121].
One additional component that is involved in early interactions between human innate immune cells and a variety of pathogens is dendritic cell-specific ICAM-3 grabbing non-integrin (DC-SIGN). DC-SIGN (CD209) is a C-type lectin expressed by immature myeloid dendritic cells found in blood and tissue, by some populations of macrophages, and has been associated with Th2 responses [122]. It can mediate entry of HIV into the cells and acts as a receptor for a range of pathogens, including MTB, Ebola virus and others [123,124]. DC-SIGN binds MTB and M. bovis BCG via the mycobacterial cell wall component mannosecapped lipoarabinomannan (ManLAM).
Nonresponsiveness towards M. leprae seems to correlate with a Th2 cytokine profile. Previously, a more predominant expression of DC-SIGN-positive cells was noted in LL lesions and of CD1b+ cells in tuberculoid leprosy [125]. Work from our group and others showed that DC-SIGN can act as an entry receptor for M. leprae, as it does for MTB, through the cell wall component lipoarabinomannan [126,127]. Moreover, abundant DC-SIGN expression was found in LL but not BT leprosy. DC-SIGN was expressed on virtually all M. leprae-containing cells, providing further evidence for its role as a receptor.
More recently, expression of DC-SIGN on SCs as described co-localizing with the SC marker, 2′, 3′-cyclic nucleotide 3′-phospho diesterase (CNPase), detected in the peripheral nerve in skin biopsies [128]. CD209 was upregulated on SC by IL-4 but not IFN-γ, and facilitated M. leprae binding. The above mentioned data indicate that CD209 provides a common mechanism by which macrophages and SCs bind to M. leprae.
The antigen-specific T-cell anergy observed in lepromatous patients is not fully understood but it is known that lepromatous patients are capable of responding to antigens other than M. leprae [129,130]. Many hypotheses have been raised and have been the focus of intense research.
CD1-restricted T-cell subsets that appear to recognize only mycobacterial lipids and glycolipids were implied to play a role in nonresponsiveness in leprosy [131]. Another study performed by Agrewala et al. suggested an involvement of defective co-receptor signaling [132]. The work demonstrated that downregulation of B7–1 and CD28 in BL/LL are probably responsible for the defective T-cell response induced by M. leprae antigens. In addition, the immune events that characterize the innate response during M. leprae infection are not completely understood.
TLR2–TLR1 heterodimers seem to mediate cell activation by killing M. leprae. Synthetic lipopeptides representing the 19 kDa and 33 kDa lipoproteins of M. leprae activated both monocytes and dendritic cells. TLR activation by M. leprae peptides triggered the production of proinflammatory cytokines including TNF-α and IL-12, which instruct the adaptive Th1 cytokine response [133]. Additional studies demonstrated that the M. leprae 33 kDa lipoprotein and M. leprae major membrane protein II, also a lipoprotein, when triggering TLR2 responses, required the acyl functions and the polypeptide region for optimal activity [134]. Accordingly, it has been demonstrated that TLR2 and TLR1 were expressed more in lesions from tuberculoid patients compared with lepromatous patients [133]. It was also observed that TLR2/1 ligation, triggered by mycobacterial 19 kDa lipopeptide may drive the high level of DC-SIGN expression as seen in LL, possibly via induction of IL-15 [125].
It was reported that in vitro, M. leprae plays an active role in controlling the release of cytokines from monocytes by producing both positive and negative regulatory signals via multiple signaling pathways involving PI3K, NF-κB and caspase 1 [135]. Downregulation of macrophage function seems to be a feature of the pathogenic mechanisms induced by the mycobacteria. Similarly in SCs, M. leprae infection is related to modulation of host kinases [136].
Previous data reported the defective activation of M. leprae-burdened macrophages to be reversible by indomethacin, and a similar block in IFN-γ activation was observed in normal macrophages treated with PGE2 [137]. Macrophages from lepromatous leprosy, but not from healthy controls or tuberculoid patients, when in the presence of M. leprae, released high level of PGE2. Corroborating these results, Misra et al. demonstrated that monocytes from lepromatous but not tuberculoid leprosy patients released soluble factors containing IL-10 and PGE2, which inhibited M. leprae-induced in vitro lymphocyte proliferation [138].
Several studies have demonstrated the involvement of the enzyme indoleamine 2, 3-dioxygenase (IDO) as a mediator of suppression of T-cell response, indicating further that biochemical changes due to the catabolism of tryptophan may affect the proliferation of these cells. IDO is an IFN-γ-induced enzyme [139–141]. Other cytokines, such as TNF-α and IL-10 are able to induce IDO, although with less efficiency than IFN-γ [142]. The role of IDO as an immunoregulator in leprosy is under investigation
In the absence of an appropriate experimental model to study leprosy, the human leprosy lesion has been used for decades in an attempt to unravel the complex clinical presentation of the disease. Of the innate immune cytokines known to regulate macrophage function, tuberculoid lesions express IL15, whereas lepromatous lesions are characterized by the expression of IL-10 [143], prompting the comparison of the IL-15- and IL-10-induced macrophage differentiation. IL-10 induced the phagocytic pathway, whereas IL-15 induced the vitamin D-dependent antimicrobial pathway, yet cells were less phagocytic.
One recent work demonstrated that macrophage programs for phagocytosis and antimicrobial responses are distinct and differentially regulated in leprosy [144]. Following phagocytosis, it is clear that a variety of antimicrobial mechanisms are at play to kill pathogens, including the generation of nitric oxide and superoxide radicals, and in humans, the vitamin D-dependent induction of antimicrobial peptides, including cathelicidin [143]. There is evidence that the vitamin D antimicrobial pathway may contribute to the outcome in leprosy. Analysis of gene expression profiles in leprosy lesions indicates that genes encoding for key components of the vitamin D antimicrobial pathway were differentially expressed in tuberculoid lesions compared with lepromatous ones [144].
Mycobacterial infection of macrophages also triggers the induction and accumulation of host-derived oxidized phospholipids, as observed in both the disseminated leprosy lesions and in vitro [145]. Oxidized phospholipids impaired host innate immune responses to mycobacterial infection, including TLR-induced cytokine and antimicrobial responses. The ability of mycobacteria to promote lipid body formation appears to be dependent on TLR2 signaling and peroxisome proliferator-activated receptor γ activation [146].
Future perspective
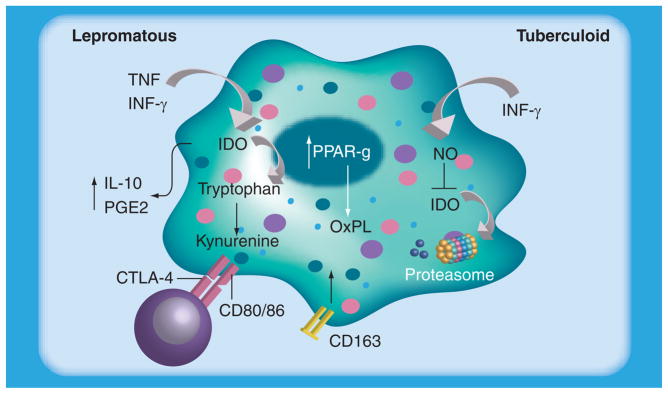
Several studies have pointed out the molecular and biochemical mechanisms involved in immunosuppression observed during leprosy. In addition, the molecular pathways involved during the reactional episodes have been described by several authors. Since macrophages are the principal mycobacteria host cell, knowledge of the mediators of suppression and/or activation (Figure 1) are important for the development of more effective strategies against the disease.
Figure 1. Lepromatous macrophages have increased expression and activity of IDO that is probably induced by mycobacterial components of cytokines such as TNF or IFN-γ.
Increased IL-10 and PGE2 levels were observed in LL macrophages. LL macrophages also present increased expression of the scavenger receptor CD163 and oxidized phospholipids, which is related to PPAR-γ induction by the mycobacteria. By contrast, in tuberculoid macrophages the decrease in IDO expression was probably due to increased nitric oxide and other reactive radicals, which may contribute to the control of bacillary load. IDO: Indoleamine-pyrrole 2,3-dioxygenase; LL: Lepromatous leprosy; NO: Nitric oxide; OxPL: Oxidized phospholipid; PGE2: Prostaglandin E2.
The knowledge accumulated so far suggests that innate immune cells are responsible for determining the polar forms in leprosy. Host factors, which regulate immune responses and signaling pathways, may determine the type of disease. In addition, keratinocytes, cytokines and the epidermis have been shown to play a role in the tissue pattern observed in leprosy and in leprosy reactional lesions [147,148].
In that sense, the ability to isolate ex vivo macrophages from skin biopsies is of note [149]. The feasibility of obtaining cells from leprosy lesions and keeping them in long-term culture has been demonstrated [149,150]. These procedures may open new pathways to study the interaction between M. leprae and human macrophages.
Executive summary.
Epidemiology & clinical issues
Leprosy or Hansen’s disease is primarily a neurological and dermatological disease caused by Mycobacterium leprae with an incubation period that ranges from 3 to 10 years. 244,796 new cases were detected during 2009 and prevalence was 211,903.
Leprosy is the leading cause of nontraumatic peripheral neuropathy worldwide. Development of neuropathy is the result of M. leprae infection of Schwann cells (SCs), which leads to demyelination and axonal degeneration, frequently leading to deformities.
The disease is for the most part curable; multidrug therapy lasts for 1 year for multibacillary lepromatous patients and 6 months for the paucibacillary forms.
Host genetic factors
The first genome-wide association study for leprosy demonstrated significant association of polymorphisms in seven genes with susceptibility to disease. One attractive mechanism pointed to the NOD2-mediated signaling pathway as a relevant pathway for pathogenesis.
Reactional episodes & treatment
During the chronic course of leprosy, reactions (erythema nodosum leprosum and reversal reaction) are acute inflammatory conditions that lead to exacerbation of disease and neuritis.
The multibacillary forms of leprosy correlate to T-cell anergy toward the M. leprae. Reactions may be explained by enhanced immune response and production of cytokines.
Treatment for reactions uses mainly steroids and/or thalidomide; other drugs that show some clinical effect are pentoxifylline and clofazimine.
Early treatment is crucial for preventing nerve damage and the progression of peripheral neuropathy into deformity.
Mechanism of nerve injury
Leprosy is one of the most common causes of nontraumatic neuropathy; one ultimate consequence if not properly prevented is nerve injury, which represents a major source of patient morbidity.
Schwann cells are a major target for infection by M. leprae leading to injury of the nerve, demyelination and consequent disability; conversion from the myelinated to nonmyelinated phenotype in SC is related with increased proliferation.
M. leprae can invade SCs by a specific laminin-binding protein of 21 kDa in addition to phenolic glycolipid-1.
M. leprae and SCs interact through linkage between the extracellular matrix and cytoskeleton, through laminin-2 and α-dystroglycan, respectively.
Prophylaxis
Household contacts are at higher risk for developing the disease, mainly among contacts of newly identified multibacillary patients.
One major challenge is to identify tools able to discriminate asymptomatic disease and/or early stage of infection.
Immunological tests, serological test (detection of anti-phenolic glycolipid-1 antibodies) and IFN-γ release assays, have been applied to define among household contacts those who are at risk of developing leprosy.
BCG vaccination of leprosy contacts may afford some protection against leprosy; single-dose rifampicin has also been shown to have some protective effect.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
This work was supported by IOC/FIOCRUZ, CNPq, Brazil, and in part by the Intramural Research Program of NIH/NIAID. The authors’ group at the Rockefeller University, NY, USA, had a patent received for the work on thalidomide action developed in 1990/1991. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Noordeen SK. Elimination of leprosy as a public health problem. Indian J Lepr. 1994;66:1–10. [PubMed] [Google Scholar]
- 2.Britton WJ, Lockwood DN. Leprosy. Lancet. 2004;363:1209–1219. doi: 10.1016/S0140-6736(04)15952-7. [DOI] [PubMed] [Google Scholar]
- 3.Ridley DS, Jopling WH. Classification of leprosy according to immunity A five- group system. Int J Lepr. 1966;34:255–273. [PubMed] [Google Scholar]
- 4.Van Voorhis WC, Kaplan G, Sarno EN, et al. The cutaneous infiltrates of leprosy: cellular characteristics and the predominant T-cell phenotypes. N Engl J Med. 1982;307:1593–1597. doi: 10.1056/NEJM198212233072601. [DOI] [PubMed] [Google Scholar]
- 5.Modlin RL, Hofman FM, Taylor CR, Rea TH. T lymphocyte subsets in the skin lesions of patients with leprosy. J Am Acad Dermatol. 1983;8:182–189. doi: 10.1016/s0190-9622(83)70021-6. [DOI] [PubMed] [Google Scholar]
- 6.Wallach D, Flageul B, Bach MA, Cottenot F. The cellular content of dermal leprous granulomas: an immuno-histological approach. Int J Lepr. 1984;52:318–326. [PubMed] [Google Scholar]
- 7.WHO. Chemotherapy of leprosy for control programmes. World Health Organ Tech Rep Ser. 1982;675:1–33. [PubMed] [Google Scholar]
- 8.Walker SL, Lockwood DN. The clinical and immunological features of leprosy. Br Med Bull. 2006:77–78. 103–121. doi: 10.1093/bmb/ldl010. [DOI] [PubMed] [Google Scholar]
- 9.Lienhardt C, Fine PE. Type 1 reaction, neuritis and disability in leprosy What is the current epidemiological situation? Lepr Rev. 1994;65:9–33. doi: 10.5935/0305-7518.19940002. [DOI] [PubMed] [Google Scholar]
- 10.Sarno EN, Grau GE, Vieira LM, Nery JA. Serum levels of tumour necrosis factor-α and interleukin-1 β during leprosy reactional states. Clin Exp Immunol. 1991;84:103–108. [PMC free article] [PubMed] [Google Scholar]
- 11.Khanolkar-Young S, Rayment N, Brickell PM, et al. Tumour necrosis factor-α (TNF-α) synthesis is associated with the skin and peripheral nerve pathology of leprosy reversal reactions. Clin Exp Immunol. 1995;99:196–202. doi: 10.1111/j.1365-2249.1995.tb05532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker SL, Lockwood DN. Leprosy type 1 (reversal) reactions and their management. Lepr Review. 2008;79:372–386. [PubMed] [Google Scholar]
- 13.Lockwood DN. Steroids in leprosy type 1 reactions: mechanisms of action and effectiveness. Lepr Rev. 2000;71(Suppl):S111–S114. doi: 10.5935/0305-7518.20000080. [DOI] [PubMed] [Google Scholar]
- 14.Croft RP, Nicholls PG, Steyerberg EW, Richardus JH, Withington SG, Smith WC. A clinical prediction rule for nerve function impairment in leprosy patients revisited after 5 years of follow-up. Lepr Rev. 2003;74:35–41. [PubMed] [Google Scholar]
- 15.Girdhar BK, Girdhar A, Chakma JK. Advances in the treatment of reactions in leprosy. Indian J Lepr. 2007;79:121–134. [PubMed] [Google Scholar]
- 16.Walker SL, Waters MF, Lockwood DN. The role of thalidomide in the management of erythema nodosum leprosum. Lepr Rev. 2007;78:197–215. [PubMed] [Google Scholar]
- 17.Sarno EN, Nery JAC, Sampaio EP. Is pentoxifylline a viable alternative in the treatment of ENL? Int J Lepr. 1995;63:570–571. [PubMed] [Google Scholar]
- 18.Sales AM, de Matos HJ, Nery JA, Duppre NC, Sampaio EP, Sarno EN. Double-blind trial of the efficacy of pentoxifylline vs thalidomide for the treatment of type II reaction in leprosy. Braz J Med Biol Res. 2007;40:243–248. doi: 10.1590/s0100-879x2007000200011. [DOI] [PubMed] [Google Scholar]
- 19.Kaur I, Dogra S, Narang T, De D. Comparative efficacy of thalidomide and prednisolone in the treatment of moderate to severe erythema nodosum leprosum: a ramdomized study. Australas J Dermatol. 2009;50:181–185. doi: 10.1111/j.1440-0960.2009.00534.x. [DOI] [PubMed] [Google Scholar]
- 20.Saber M, Bourassa-Fulop C, Bouffard D, Provost N. Canadian case report of erythema nodosum leprosum successfully treated with prednisone and thalidomide. J Cutan Med Surg. 2010;14:95–99. doi: 10.2310/7750.2009.08094. [DOI] [PubMed] [Google Scholar]
- 21.Teo SK. Properties of thalidomide and its analogues: implications for anticancer therapy. AAPS J. 2005;7:E14–E19. doi: 10.1208/aapsj070103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lebrin F, Srun S, Raymond K, et al. Thalidomide stimulates vessel maturation and reduces epistaxis in individuals with hereditary hemorrhagic telangiectasia. Nat Med. 2010;16:420–428. doi: 10.1038/nm.2131. [DOI] [PubMed] [Google Scholar]
- 23.Akhurst RJ. Taking thalidomide out of rehab. Nat Med. 2010;16:370–372. doi: 10.1038/nm0410-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24▪.Ito T, Ando H, Suzuki T, et al. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327:1345–1350. doi: 10.1126/science.1177319. Describes the mechanism of thalidomide teratogenicity. [DOI] [PubMed] [Google Scholar]
- 25.Sampaio EP, Sarno EN, Galilly R, Cohn ZA, Kaplan G. Thalidomide selectively inhibits tumor necrosis factor α production by stimulated human monocytes. J Exp Med. 1991;173:699–703. doi: 10.1084/jem.173.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moreira AL, Sampaio EP, Zmuidzinas A, Frindt P, Smith KA, Kaplan G. Thalidomide exerts its inhibitory action on tumor necrosis factor α by enhancing mRNA degradation. J Exp Med. 1993;177:1675–1680. doi: 10.1084/jem.177.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson KC. Lenalidomide and thalidomide: mechanisms of action – similarities and differences. Semin Hematol. 2005;42:S3–S8. doi: 10.1053/j.seminhematol.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Sampaio EP, Kaplan G, Miranda A, et al. The influence of thalidomide on the clinical and immunological manifestation of erythema nodosum leprosum. J Infect Dis. 1993;168:408–414. doi: 10.1093/infdis/168.2.408. [DOI] [PubMed] [Google Scholar]
- 29.Moraes MO, Sarno EN, Teles RM, et al. Anti-inflammatory drugs block cytokine mRNA accumulation in the skin and improve the clinical condition of reactional leprosy patients. J Invest Dermatol. 2000;115:935–941. doi: 10.1046/j.1523-1747.2000.00158.x. [DOI] [PubMed] [Google Scholar]
- 30.Corral LG, Muller GW, Moreira AL, et al. Selection of novel analogs of thalidomide with enhanced tumor necrosis factor α inhibitory activity. Mol Med. 1996;2:506–515. [PMC free article] [PubMed] [Google Scholar]
- 31.Fujimoto H, Noguchi T, Kobayashi H, Miyachi H, Hashimoto Y. Effects of immunomodulatory derivatives of thalidomide (IMiDs) and their analogs on cell-differentiation, cyclooxygenase activity and angiogenesis. Chem Pharm Bull (Tokyo) 2006;54:855–860. doi: 10.1248/cpb.54.855. [DOI] [PubMed] [Google Scholar]
- 32.Gandhi AK, Kang J, Capone L, et al. Dexamethasone synergizes with lenalidomide to inhibit multiple myeloma tumor growth, but reduces lenalidomide-induced immunomodulation of T and NK cell function. Curr Cancer Drug Targets. 2010;10:155–167. doi: 10.2174/156800910791054239. [DOI] [PubMed] [Google Scholar]
- 33.Lockwood DN. Leprosy elimination: a virtual phenomenon or a reality? BMJ. 2002;324:1516–1518. doi: 10.1136/bmj.324.7352.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao AG. Study of leprosy in children. Indian J Lepr. 2009;81:195–197. [PubMed] [Google Scholar]
- 35.Balagon MF, Cellona RV, dela Cruz EC, et al. Long-term risk of relapse of multibacillary leprosy after completion of 2-year multiple drug therapy (WHO-MDT) in Cebu, Philippines. Am J Trop Med Hyg. 2009;81:895–899. doi: 10.4269/ajtmh.2009.09-0189. [DOI] [PubMed] [Google Scholar]
- 36.Sampaio EP, Caneshi JRT, Nery JAC, et al. Cellular immune response to Mycobacterium leprae infection in human immunodeficiency virus-infected individuals. Infect Immun. 1995;63:1848–1854. doi: 10.1128/iai.63.5.1848-1854.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nery JAC, Sampaio EP, Galhardo MCG, et al. M. leprae–HIV co-infection: pattern of immune response in vivo and in vitro. Indian J Lepr. 2000;72:155–167. [PubMed] [Google Scholar]
- 38.Sarno EN, Illarramendi X, Nery JA, et al. HIV–M. leprae interaction: can HAART modify the course of leprosy? Public Health Rep. 2008;123:206–212. doi: 10.1177/003335490812300213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deps PD, Lockwood DN. Leprosy occurring as immune reconstitution syndrome. Trans R Soc Trop Med Hyg. 2008;102:966–968. doi: 10.1016/j.trstmh.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 40.Couppie P, Domergue V, Clyti E, et al. Increased evidence of leprosy following HAART initiation: a manifestation of the immune reconstitution disease. AIDS. 2009;23:1599–1600. doi: 10.1097/QAD.0b013e32832bb5b7. [DOI] [PubMed] [Google Scholar]
- 41.Rastogi N, Legrand E, Sola C. The mycobacteria: an introduction to nomenclature and pathogenesis. Rev Sci Tech. 2001;20:21–54. doi: 10.20506/rst.20.1.1265. [DOI] [PubMed] [Google Scholar]
- 42.Gutierrez MC, Supply P, Brosch R. Pathogenomics of mycobacteria. Genome Dyn. 2009;6:198–210. doi: 10.1159/000235772. [DOI] [PubMed] [Google Scholar]
- 43.Cole ST, Brosch R, Parkhill J, et al. Deciphering the biology of M. tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 44▪.Cole ST, Eiglmeier K, Parkhill J, et al. Massive gene decay in the leprosy bacillus. Nature. 2001;409:1007–1011. doi: 10.1038/35059006. The genome sequence of Mycobacterium tuberculosis and Mycobacterium leprae highlighted new lines of research for better understanding the biology of the bacteria. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki K, Nakata N, Bang PD, Ishii N, Makino M. High level expression of pseudogenes in Mycobacterium leprae. FEMS Microbiol Lett. 2006;259:208–214. doi: 10.1111/j.1574-6968.2006.00276.x. [DOI] [PubMed] [Google Scholar]
- 46.Han XY, Seo YH, Sizer KC, et al. A new Mycobacterium species causing diffuse lepromatous leprosy. Am J Clin Pathol. 2008;130:856–864. doi: 10.1309/AJCPP72FJZZRRVMM. [DOI] [PubMed] [Google Scholar]
- 47.Han XY, Sizer KC, Thompson EJ, et al. Comparative sequence analysis of Mycobacterium leprae and the new leprosy-causing Mycobacterium lepromatosis. J Bacteriol. 2009;191:6067–6074. doi: 10.1128/JB.00762-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Monot M, Honoré N, Garnier T, et al. Comparative genomic and phylogeographic analysis of Mycobacterium leprae. Nat Genet. 2009;41:1282–1289. doi: 10.1038/ng.477. [DOI] [PubMed] [Google Scholar]
- 49.Maiden MC. Putting leprosy on the map. Nat Genet. 2009;41:1264–1266. doi: 10.1038/ng1209-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gillis T, Vissa V, Matsuoka M, et al. Characterization of short tandem repeats for genotyping Mycobacterium leprae. Lepr Rev. 2009;80:250–260. [PubMed] [Google Scholar]
- 51.Kimura M, Sakamuri RM, Groathouse NA, et al. Rapid variable-number tandem-repeat genotyping for Mycobacterium leprae clinical specimens. J Clin Microbiol. 2009;47:1757–1766. doi: 10.1128/JCM.02019-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monot M, Honore N, Baliere C, et al. Are variable-number tandem repeats appropriate for genotyping Mycobacterium leprae? J Clin Microbiol. 2008;46:2291–2297. doi: 10.1128/JCM.00239-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53▪.Alter A, Alcaïs A, Abel L, Schurr E. Leprosy as a genetic model for susceptibility to common infectious diseases. Hum Genet. 2008;123:227–235. doi: 10.1007/s00439-008-0474-z. A comprehensive overview of how complex genetics of diseases like leprosy and tuberculosis can be and the multi-factorial genetic profile that needs to be identified. [DOI] [PubMed] [Google Scholar]
- 54.Alcaïs A, Alter A, Antoni G, et al. Stepwise replication identifies a low-producing lymphotoxin-α allele as a major risk factor for early-onset leprosy. Nat Genet. 2007;39:517–522. doi: 10.1038/ng2000. [DOI] [PubMed] [Google Scholar]
- 55.Santos AR, Suffys PN, Vanderborght PR, et al. TNFα and IL-10 promoter polymorphisms in leprosy: association with disease susceptibility. J Infect Dis. 2002;186:1687–1691. doi: 10.1086/345366. [DOI] [PubMed] [Google Scholar]
- 56.Mira MT, Alcais A, di Pietrantonio T, et al. Segregation of HLA/TNF region is linked to leprosy clinical spectrum in families displaying mixed leprosy subtypes. Genes Immun. 2003;4:67–73. doi: 10.1038/sj.gene.6363911. [DOI] [PubMed] [Google Scholar]
- 57.Misch EA, Macdonald M, Ranjit C, et al. Human TLR1 deficiency is associated with impaired mycobacterial signaling and protection from leprosy reversal reaction. PLoS Negl Trop Dis. 2008;2:E231–E239. doi: 10.1371/journal.pntd.0000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cardoso CC, Pereira AC, Brito-de-Souza VN, et al. IFNG +874 T>A single nucleotide polymorphism is associated with leprosy among Brazilians. Hum Genet. 2010;128:481–490. doi: 10.1007/s00439-010-0872-x. [DOI] [PubMed] [Google Scholar]
- 59.Mira MT, Alcaïs A, Nguyen VT, et al. Susceptibility to leprosy is associated with PARK2 and PACRG. Nature. 2004;427:636–640. doi: 10.1038/nature02326. [DOI] [PubMed] [Google Scholar]
- 60.Johnson C, Lyle EA, Omueti KO, et al. Cutting edge: a common polymorphism impairs cell surface trafficking and functional responses of TLR1 but protects against leprosy. J Immunol. 2007;178:7520–7524. doi: 10.4049/jimmunol.178.12.7520. [DOI] [PubMed] [Google Scholar]
- 61.Bochud P-Y, Hawn TR, Aderem A. Cutting edge: a Toll-like receptor 2 polymorphism that is associated with lepromatous leprosy is unable to mediate mycobacterial signaling. J Immunol. 2003;170:3451–3454. doi: 10.4049/jimmunol.170.7.3451. [DOI] [PubMed] [Google Scholar]
- 62.Bochud P-Y, Hawn TR, Siddiqui MR, et al. Toll-like receptor 2 (TLR2) polymorphisms are associated with reversal reaction in leprosy. J Infect Dis. 2008;197:253–261. doi: 10.1086/524688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morrison NA, Qi JC, Tokita A, et al. Prediction of bone density from vitamin D receptor alleles. Nature. 1994;367:284–287. doi: 10.1038/367284a0. [DOI] [PubMed] [Google Scholar]
- 64.van Etten E, Verlinden L, Giulietti A, et al. The vitamin D receptor gene FokI polymorphism: functional impact on the immune system. Eur J Immunol. 2007;37:395–405. doi: 10.1002/eji.200636043. [DOI] [PubMed] [Google Scholar]
- 65.Roy S, Frodsham A, Saha B, et al. Association of vitamin D receptor genotype with leprosy type. J Infect Dis. 1999;179:187–191. doi: 10.1086/314536. [DOI] [PubMed] [Google Scholar]
- 66.Sapkota BR, Macdonald M, Berrington WR, et al. Association of TNF, MBL, and VDR polymorphisms with leprosy phenotypes. Hum Immunol. 2010;71:992–998. doi: 10.1016/j.humimm.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goulart LR, Ferreira FR, Goulart IM. Interaction of TaqI polymorphism at exon 9 of the vitamin D receptor gene with the negative lepromin response may favor the occurrence of leprosy. FEMS Immunol Med Microbiol. 2006;48:91–98. doi: 10.1111/j.1574-695X.2006.00128.x. [DOI] [PubMed] [Google Scholar]
- 68▪.Zhang FR, Huang W, Chen SM, et al. Genomewide association study of leprosy. N Engl J Med. 2009;361:2609–2618. doi: 10.1056/NEJMoa0903753. This is the first genome-wide study in leprosy identifying a role for the NOD2 signaling pathway in the pathogenesis of the disease. [DOI] [PubMed] [Google Scholar]
- 69.Berrington WR, Macdonald M, Khadge S, et al. Common polymorphisms in the NOD2 gene region are associated with leprosy and its reactive states. J Infect Dis. 2010;201:1422–1435. doi: 10.1086/651559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Beers SM, Hatta M, Klatser PR. Patient contact is the major determinant in incident leprosy: implications for future control. Int J Lepr. 1999;67:119–128. [PubMed] [Google Scholar]
- 71.Moura RS, Calado KL, Oliveira ML, Bührer-Sékula S. Leprosy serology using PGL-I: a systematic review. Rev Soc Bras Med Trop. 2008;41(Suppl 2):11–18. doi: 10.1590/s0037-86822008000700004. [DOI] [PubMed] [Google Scholar]
- 72.Bührer-Sékula S, Smits HL, Gussenhoven GC, van Ingen CW, Klatser PR. A simple dipstick assay for the detection of antibodies to phenolic glycolipid-I of Mycobacterium leprae. Am J Trop Med Hyg. 1998;58:133–136. doi: 10.4269/ajtmh.1998.58.133. [DOI] [PubMed] [Google Scholar]
- 73.Zenha EM, Ferreira MA, Foss NT. Use of anti-PGL-1 antibodies to monitor therapy regimes in leprosy patients. Braz J Med Biol Res. 2009;42:968–972. doi: 10.1590/s0100-879x2009001000016. [DOI] [PubMed] [Google Scholar]
- 74.Bührer-Sékula S, Illarramendi X, Teles RB, et al. The additional benefit of the ML flow test to classify leprosy patients. Acta Trop. 2009;111:172–176. doi: 10.1016/j.actatropica.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 75.Bührer-Sékula S, van Beers S, Oskam L, et al. The relation between seroprevalence of antibodies against phenolic glycolipid-I among school children and leprosy endemicity in Brazil. Rev Soc Bras Med Trop. 2008;41(Suppl 2):81–88. doi: 10.1590/s0037-86822008000700017. [DOI] [PubMed] [Google Scholar]
- 76.Martinez AN, Britto CFPC, Jardim MR, et al. Detection of Mycobacterium leprae DNA in skin biopsy samples of leprosy patients: evaluation of real time and conventional PCR targeting complex 85 genes. J Clin Microbiol. 2006;44:3154–3159. doi: 10.1128/JCM.02250-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bang PD, Suzuki K, Phuong le T, et al. Evaluation of polymerase chain reaction-based detection of Mycobacterium leprae for the diagnosis of leprosy. J Dermatol. 2009;36:269–276. doi: 10.1111/j.1346-8138.2009.00637.x. [DOI] [PubMed] [Google Scholar]
- 78.Jardim MM, Antunes SG, Wildenbeest JG, et al. PGL-I as an accessory test for the diagnosis of pure neural leprosy. Lepr Rev. 2005;76:232–240. [PubMed] [Google Scholar]
- 79.Geluk A, van der Ploeg J, Teles RO, et al. Rational combination of peptides derived from different Mycobacterium leprae proteins improves sensitivity for immunodiagnosis of M. leprae infection. Clin Vaccine Immunol. 2008;15:522–533. doi: 10.1128/CVI.00432-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Spencer JS, Dockrell HM, Kim HJ, et al. Identification of specific proteins and peptides in Mycobacterium leprae sui for the selective diagnosis of leprosy. J Immunol. 2005;175:7930–7938. doi: 10.4049/jimmunol.175.12.7930. [DOI] [PubMed] [Google Scholar]
- 81.Geluk A, van der Ploeg-van Schip JJ, van Meijgaarden KE, et al. Enhancing sensitivity of detection of immune responses to Mycobacterium leprae peptides in whole-blood assays. Clin Vaccine Immunol. 2010;17:993–1004. doi: 10.1128/CVI.00046-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Setia MS, Steinmaus C, Ho CS, Rutherford GW. The role of BCG in prevention of leprosy: a meta-analysis. Lancet Infect Dis. 2006;6:162–170. doi: 10.1016/S1473-3099(06)70412-1. [DOI] [PubMed] [Google Scholar]
- 83.Düppre NC, Camacho LAB, Cunha SS, et al. Effectiveness of BCG vaccination among leprosy contacts: a cohort study. Trans R Soc Trop Med Hyg. 2008;102:631–638. doi: 10.1016/j.trstmh.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 84.Sharma P, Mukherjee R, Talwar GP, et al. Immunoprophylactic effects of the anti-leprosy Mw vaccine in household contacts of leprosy patients: clinical field trials with a follow up of 8–10 years. Lepr Rev. 2005;76:127–143. [PubMed] [Google Scholar]
- 85.Merle CS, Cunha SS, Rodrigues LC. BCG vaccination and leprosy protection: review of current evidence and status of BCG in leprosy control. Expert Rev Vaccines. 2010;9:209–222. doi: 10.1586/erv.09.161. [DOI] [PubMed] [Google Scholar]
- 86.Moet FJ, Pahan D, Oskam L, Richardus JH. Effectiveness of single dose rifampicin in preventing leprosy in close contacts of patients with newly diagnosed leprosy: cluster randomised controlled trial. BMJ. 2008;336:761–764. doi: 10.1136/bmj.39500.885752.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schuring RP, Richardus JH, Pahan D, Oskam L. Protective effect of the combination BCG vaccination and rifampicin prophylaxis in leprosy prevention. Vaccine. 2009;27:7125–7128. doi: 10.1016/j.vaccine.2009.09.054. [DOI] [PubMed] [Google Scholar]
- 88.Bjune G, Barnetson RS, Ridley DS, Kronvall G. Lymphocyte transformation test in leprosy; correlation of the response with inflammation of lesions. Clin Exp Immunol. 1976;25:85–94. [PMC free article] [PubMed] [Google Scholar]
- 89.Rea TH, Levan NE. Current concepts in the immunology of leprosy. Arch Dermatol. 1977;113:345–352. [PubMed] [Google Scholar]
- 90.Khanolkar-Young S, Rayment N, Brickell PM, et al. Tumour necrosis factor-α (TNF-α) synthesis is associated with the skin and peripheral nerve pathology of leprosy reversal reactions. Clin Exp Immunol. 1995;99:196–202. doi: 10.1111/j.1365-2249.1995.tb05532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Little D, Khanolkar-Young S, Coulthart A, Suneetha S, Lockwood DN. Immunohistochemical analysis of cellular infiltrate and gamma interferon, interleukin-12, and inducible nitric oxide synthase expression in leprosy type 1 (reversal) reactions before and during prednisolone treatment. Infect Immun. 2001;69:3413–3417. doi: 10.1128/IAI.69.5.3413-3417.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stefani MM, Guerra JG, Sousa AL, et al. Potential plasma markers of Type 1 and Type 2 leprosy reactions: a preliminary report. BMC Infect Dis. 2009;9:75–79. doi: 10.1186/1471-2334-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Massone C, Nunzi E, Ribeiro-Rodrigues R, et al. T regulatory cells and plasmocytoid dendritic cells in Hansen disease: a new insight into pathogenesis? Am J Dermatopathol. 2010;32:251–256. doi: 10.1097/DAD.0b013e3181b7fc56. [DOI] [PubMed] [Google Scholar]
- 94.Bjorvatn B, Barnetson RS, Kronvall G, Zubler RH, Lambert PH. Immune complexes and complement hypercatabolism in patients with leprosy. Clin Exp Immunol. 1976;26:388–396. [PMC free article] [PubMed] [Google Scholar]
- 95.Sreenivasan P, Misra RS, Wilfred D, Nath I. Lepromatous leprosy patients show T helper 1-like cytokine profile with differential expression of interleukin-10 during type 1 and 2 reactions. Immunology. 1998;95:529–536. doi: 10.1046/j.1365-2567.1998.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nath I, Vemuri N, Reddi AL, et al. The effect of antigen presenting cells on the cytokine profiles of s and reactional lepromatous leprosy patients. Immunol Lett. 2000;75:69–76. doi: 10.1016/s0165-2478(00)00271-6. [DOI] [PubMed] [Google Scholar]
- 97.Moraes MO, Sampaio EP, Nery JAC, Saraiva BCG, Alvarenga FBF, Sarno EN. Sequencial erythema nodosum leprosum and reversal reaction with similar lesional cytokine mRNA patterns in a borderline leprosy patient. Brit J Dermatol. 2001;144:175–181. doi: 10.1046/j.1365-2133.2001.03970.x. [DOI] [PubMed] [Google Scholar]
- 98.Kahawita IP, Lockwood DN. Towards understanding the pathology of erythema nodosum leprosum. Trans R Soc Trop Med Hyg. 2008;102:329–337. doi: 10.1016/j.trstmh.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 99.Oliveira RB, Moraes MO, Oliveira EB, Sarno EN, Nery JA, Sampaio EP. Neutrophils isolated from leprosy patients release TNF-α and exhibit accelerated apoptosis in vitro. J Leukoc Biol. 1999;65:364–371. doi: 10.1002/jlb.65.3.364. [DOI] [PubMed] [Google Scholar]
- 100.Lee DJ, Li H, Ochoa MT, et al. Integrated pathways for neutrophil recruitment and inflammation in leprosy. J Infect Dis. 2010;201:558–569. doi: 10.1086/650318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barnes PF, Chatterjee D, Brennan PJ, Rea TH, Modlin RL. Tumor necrosis factor production in patients with leprosy. Infect Immun. 1992;60:1441–1446. doi: 10.1128/iai.60.4.1441-1446.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hernandez MO, Neves I, Sales JS, Carvalho DS, Sarno EN, Sampaio EP. Induction of apoptosis in monocytes by Mycobacterium leprae in vitro: a possible role for tumour necrosis factor-α. Immunology. 2003;109:156–164. doi: 10.1046/j.1365-2567.2003.01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Oliveira RB, Sampaio EP, Antas PRZ, Teles RMB, Aarestrup F, Sarno EN. Cytokines and Mycobacterium leprae induce apoptosis in human Schwann cells. J Neuropathol Exp Neurol. 2005;64:882–890. doi: 10.1097/01.jnen.0000182982.09978.66. [DOI] [PubMed] [Google Scholar]
- 104.Fulco TO, Lopes UG, Sarno EN, Sampaio EP, Saliba AM. The proteasome function is required for Mycobacterium leprae-induced apoptosis and cytokine secretion. Immunol Lett. 2007;110:82–85. doi: 10.1016/j.imlet.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 105.Oliveira RB, Ochoa MT, Sieling PA, et al. Expression of Toll-like receptor 2 on human Schwann cells: a mechanism of nerve damage in leprosy. Infect Immun. 2003;71:1427–1433. doi: 10.1128/IAI.71.3.1427-1433.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lahiri R, Randhawa B, Krahenbuhl JL. Infection of mouse macrophages with viable Mycobacterium leprae does not induce apoptosis. J Infect Dis. 2010;201:1736–1742. doi: 10.1086/652499. [DOI] [PubMed] [Google Scholar]
- 107.Rambukkana A, Zanazzi G, Tapinos N, Salzer JL. Contact-dependent demyelination by Mycobacterium leprae in the absence of immune cells. Science. 2002;296:927–931. doi: 10.1126/science.1067631. [DOI] [PubMed] [Google Scholar]
- 108.Yan N, Ricca C, Fletcher J, Glover T, Seizinger BR, Manne V. Farnesyltransferase inhibitors block the neurofibromatosis type I (NF1) malignant phenotype. Cancer Res. 1995;55:3569–3575. [PubMed] [Google Scholar]
- 109.Marques MA, Antônio VL, Sarno EN, Brennan PJ, Pessolani MC. Binding of α2-laminins by pathogenic and non-pathogenic mycobacteria and adherence to Schwann cells. J Med Microbiol. 2001;50:23–28. doi: 10.1099/0022-1317-50-1-23. [DOI] [PubMed] [Google Scholar]
- 110.Ng V, Zanazzi G, Timpl R, et al. Role of the cell wall phenolic glycolipid-1 in the peripheral nerve predilection of Mycobacterium leprae. Cell. 2000;103:511–524. doi: 10.1016/s0092-8674(00)00142-2. [DOI] [PubMed] [Google Scholar]
- 111.Rambukkana A, Yamada H, Zanazzi G, et al. Role of α-dystroglycan as a Schwann cell receptor for Mycobacterium leprae. Science. 1998;282:2076–2079. doi: 10.1126/science.282.5396.2076. [DOI] [PubMed] [Google Scholar]
- 112▪.Rambukkana A, Salzer JL, Yurchenco PD, Tuomanen EI. Neural targeting of Mycobacterium leprae mediated by the G domain of the laminin-α2 chain. Cell. 1997;88:811–821. doi: 10.1016/s0092-8674(00)81927-3. Pioneering study defining receptor and binding molecules that mediate M. leprae binding to Schwann cells. [DOI] [PubMed] [Google Scholar]
- 113.Shimoji Y, Ng V, Matsumura K, Fischetti VA, Rambukkana A. A 21-kDa surface protein of Mycobacterium leprae binds peripheral nerve laminin-2 and mediates Schwann cell invasion. Proc Natl Acad Sci USA. 1999;96:9857–9862. doi: 10.1073/pnas.96.17.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tapinos N, Ohnishi M, Rambukkana A. ErbB2 receptor tyrosine kinase signaling mediates early demyelination induced by leprosy bacilli. Nat Med. 2006;12:961–966. doi: 10.1038/nm1433. [DOI] [PubMed] [Google Scholar]
- 115.Ribeiro-Resende VT, Ribeiro-Guimaraes ML, Lemes RM, et al. Involvement of 9-O-acetyl GD3 ganglioside in Mycobacterium leprae infection of Schwann cells. J Biol Chem. 2010;285(44):34086–34096. doi: 10.1074/jbc.M110.147272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Teles RM, Antunes SL, Jardim MR, et al. Expression of metalloproteinases (MMP-2, MMP-9, and TACE) and TNF-α in the nerves of leprosy patients. J Peripher Nerv Syst. 2007;12:195–204. doi: 10.1111/j.1529-8027.2007.00139.x. [DOI] [PubMed] [Google Scholar]
- 117.Oliveira AL, Antunes SL, Teles RM, et al. Schwann cells producing matrix metalloproteinases under Mycobacterium leprae stimulation may play a role in the outcome of leprous neuropathy. J Neuropathol Exp Neurol. 2010;69:27–39. doi: 10.1097/NEN.0b013e3181c6515c. [DOI] [PubMed] [Google Scholar]
- 118.Pereira RM, Calegari-Silva TC, Hernandez MO, et al. Mycobacterium leprae induces NF-κB-dependent transcription repression in human Schwann cells. Biochem Biophys Res Commun. 2005;335:20–26. doi: 10.1016/j.bbrc.2005.07.061. [DOI] [PubMed] [Google Scholar]
- 119.Cardoso CC, Martinez AN, Guimarães PE, et al. Ninjurin 1 asp110ala single nucleotide polymorphism is associated with protection in leprosy nerve damage. J Neuroimmunol. 2007;190:131–138. doi: 10.1016/j.jneuroim.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 120.Schlesinger LS, Horwitz MA. Phagocytosis of Mycobacterium leprae by human monocyte-derived macrophages is mediated by complement receptors CR1 (CD35), CR3 (CD11b/CD18), and CR4 (CD11c/CD18) and IFN-γ activation inhibits complement receptor function and phagocytosis of this bacterium. J Immunol. 1991;147:1983–1994. [PubMed] [Google Scholar]
- 121.Prabhakaran K, Harris EB, Randhawa B. Regulation by protein kinase of phagocytosis of Mycobacterium leprae by macrophages. J Med Microbiol. 2000;49:339–342. doi: 10.1099/0022-1317-49-4-339. [DOI] [PubMed] [Google Scholar]
- 122.Soilleux EJ, Morris LS, Leslie G, et al. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J Leukoc Biol. 2002;71:445–457. [PubMed] [Google Scholar]
- 123.Geijtenbeek TB, van Kooyk Y. Pathogens target DC-SIGN to influence their fate: DC-SIGN functions as a pathogen receptor with broad specificity. APMIS. 2003;111:698–714. doi: 10.1034/j.1600-0463.2003.11107803.x. [DOI] [PubMed] [Google Scholar]
- 124.Maeda N, Nigou J, Herrmann JL, et al. The cell surface receptor DC-SIGN discriminates between Mycobacterium species through selective recognition of the mannose caps on lipoarabinomannan. J Biol Chem. 2003;278:5513–5516. doi: 10.1074/jbc.C200586200. [DOI] [PubMed] [Google Scholar]
- 125.Krutzik SR, Tan B, Li H, et al. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat Med. 2005;11:653–660. doi: 10.1038/nm1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Soilleux EJ, Sarno EN, Hernandez MO, et al. DC-SIGN association with the Th2 environment of lepromatous lesions: cause or effect? J Pathol. 2006;209:182–189. doi: 10.1002/path.1972. [DOI] [PubMed] [Google Scholar]
- 127.Barreiro LB, Quach H, Krahenbuhl J, et al. DC-SIGN interacts with Mycobacterium leprae but sequence variation in this lectin is not associated with leprosy in the Pakistani population. Hum Immunol. 2006;67:102–107. doi: 10.1016/j.humimm.2006.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Teles RM, Krutzik SR, Ochoa MT, Oliveira RB, Sarno EN, Modlin RL. IL-4 regulates the expression of CD209 and subsequent uptake of Mycobacterium leprae by Schwann cells in human leprosy. Infect Immun. 2010;78:4634–4643. doi: 10.1128/IAI.00454-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kaplan G, Weinstein DE, Steinman RM, et al. An analysis of in vitro T cell responsiveness in lepromatous leprosy. J Exp Med. 1985;162:917–929. doi: 10.1084/jem.162.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sarno EN, Espinosa M, Sampaio EP, et al. Immunological responsiveness to M. leprae and BCG antigens in 98 leprosy patients and their household contacts. Braz J Med Biol Res. 1988;21:461–470. [PubMed] [Google Scholar]
- 131.Sieling PA, Jullien D, Dahlem M, et al. CD1 expression by dendritic cells in human leprosy lesions: correlation with effective host immunity. J Immunol. 1999;162:1851–1858. [PubMed] [Google Scholar]
- 132.Agrewala JN, Kumar B, Vohra H. Potential role of B7–1 and CD28 molecules in immunosuppression in leprosy. Clin Exp Immunol. 1998;111:56–63. doi: 10.1046/j.1365-2249.1998.00463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Krutzik SR, Ochoa MT, Sieling PA, et al. Activation and regulation of Toll-like receptors 2 and 1 in human leprosy. Nat Med. 2003;9:525–532. doi: 10.1038/nm864. [DOI] [PubMed] [Google Scholar]
- 134.Jain M, Petzold CJ, Schelle MW, et al. Lipidomics reveals control of Mycobacterium tuberculosis virulence lipids via metabolic coupling. Proc Natl Acad Sci USA. 2007;104:5133–5138. doi: 10.1073/pnas.0610634104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sinsimer D, Fallows D, Peixoto B, Krahenbuhl J, Kaplan G, Manca C. Mycobacterium leprae actively modulates the cytokine response in naive human monocytes. Infect Immun. 2010;78:293–300. doi: 10.1128/IAI.00816-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Alves L, de Mendonça Lima L, da Silva Maeda E, et al. Mycobacterium leprae infection of human Schwann cells depends on selective host kinases and pathogen-modulated endocytic pathways. FEMS Microbiol Lett. 2004;238:429–437. doi: 10.1016/j.femsle.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 137.Sibley LD, Krahenbuhl JL. Mycobacterium leprae-burdened macrophages are refractory to activation by γ interferon. Infect Immun. 1987;55:446–450. doi: 10.1128/iai.55.2.446-450.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Misra N, Selvakumar M, Singh S, et al. Monocyte derived IL-10 and PGE2 are associated with the absence of Th 1 cells and in vitro T cell suppression in lepromatous leprosy. Immunol Lett. 1995;48:123–128. doi: 10.1016/0165-2478(95)02455-7. [DOI] [PubMed] [Google Scholar]
- 139.Mellor AL, Munn DH. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol Today. 1999;20:469–473. doi: 10.1016/s0167-5699(99)01520-0. [DOI] [PubMed] [Google Scholar]
- 140.Uyttenhove C, Pilotte L, Théate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 141.Popov A, Schultze JL. IDO-expressing regulatory dendritic cells in cancer and chronic infection. J Mol Med. 2008;86:145–160. doi: 10.1007/s00109-007-0262-6. [DOI] [PubMed] [Google Scholar]
- 142.Schröcksnadel K, Wirleitner B, Winkler C, Fuchs D. Monitoring tryptophan metabolism in chronic immune activation. Clin Chim Acta. 2006;364:82–90. doi: 10.1016/j.cca.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 143.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 144.Montoya D, Cruz D, Teles RMB, et al. Divergence of macrophage phagocytic and antimicrobial programs in leprosy. Cell Host Microbe. 2009;6:343–353. doi: 10.1016/j.chom.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cruz D, Watson AD, Miller CS, et al. Host-derived oxidized phospholipids and HDL regulate innate immunity in human leprosy. J Clin Invest. 2008;118:2917–2928. doi: 10.1172/JCI34189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Almeida PE, Silva AR, Maya-Monteiro CM, et al. Mycobacterium bovis Bacillus Calmette-Guérin infection induces TLR2-dependent peroxisome proliferator-activated receptor γ expression and activation: functions in inflammation, lipid metabolism, and pathogenesis. J Immunol. 2009;183:1337–1345. doi: 10.4049/jimmunol.0900365. [DOI] [PubMed] [Google Scholar]
- 147.Sampaio EP, Moraes MO, Nery JAC, Santos AR, Mattos HJ, Sarno EN. Pentoxifylline decreases in vivo and in vitro TNFα production in lepromatous leprosy patients with ENL. Clin Exp Immunol. 1998;111:300–308. doi: 10.1046/j.1365-2249.1998.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Teles RM, Moraes MO, Geraldo NT, Salles AM, Sarno EN, Sampaio EP. Differential TNFα mRNA regulation detected in the epidermis of leprosy patients. Arch Dermatol Res. 2002;294:355–362. doi: 10.1007/s00403-002-0340-0. [DOI] [PubMed] [Google Scholar]
- 149.Sarno EN, Sampaio EP, Moreira AL, Alvarenga FBF. Isolation and functional characterization of mononuclear phagocytes from human lepromatous lesion. Rev Soc Bras Med Trop. 1987;20:205–207. doi: 10.1590/s0037-86821987000400004. [DOI] [PubMed] [Google Scholar]
- 150.Moura DF, Teles RM, Ribeiro-Carvalho MM, et al. Long-term culture of multibacillary leprosy macrophages isolated from skin lesions: a new model to study Mycobacterium leprae–human cell interaction. Br J Dermatol. 2007;157:273–283. doi: 10.1111/j.1365-2133.2007.07992.x. [DOI] [PubMed] [Google Scholar]
Websites
- 201.WHO and Novartis deliver free leprosy treatment for all patients worldwide. www.who.int/mediacentre/news/releases/2005/pr57/en/index.html.
- 202.WHO. Global leprosy situation. Weekly epidemiological record. 2010;35:337–348. www.who.int/wer/2010/wer8535.pdf.
- 203.WHO. Surveillance of drug resistance in leprosy: 2009. Weekly epidemiological record. 2010;29:281–284. www.ilep.org.uk/fileadmin/uploads/documents/wer/wer8529.pdf. [PubMed]