Abstract
Targeting of radionuclides with antibodies, or radioimmunotherapy, has been an active field of research spanning nearly 50 years, evolving with advancing technologies in molecular biology and chemistry, and with many important preclinical and clinical studies illustrating the benefits, but also the challenges, which all forms of targeted therapies face. There are currently two radiolabeled antibodies approved for the treatment of non-Hodgkin lymphoma, but radioimmunotherapy of solid tumors remains a challenge. Novel antibody constructs, focusing on treatment of localized and minimal disease, and pretargeting are all promising new approaches that are currently under investigation.
Keywords: bispecific antibody, cancer, monoclonal antibody, pretargeting, radioimmunotherapy, radionuclide
Antibodies are integral agents of our immune system, primarily used to identify and aid in the clearing of foreign pathogens. While antibodies have been examined for many years as possible cancer therapeutics, early trials with murine monoclonal antibodies were disappointing [1–3]. However, as many as 22 antibodies are now approved for clinical use in a variety of indications, with many more under investigation [4–6]. While antibody-dependent cellular cytotoxicity (ADCC) and complement activation play a role in the success of some unconjugated antibodies, identification of pathways required for cell growth has opened new possibilities for using antibodies as relatively nontoxic agents that can alter these processes and control tumor progression. Still, on their own, relatively few antibodies alter patient survival significantly, but are becoming increasingly important adjuncts, being administered along with standard chemotherapy to enhance the overall response/survival. Thus, interest in developing antibody conjugates to enhance efficacy continues. In this article, we review the use of antibodies conjugated with radionuclides, known as radioimmunotherapy (RAIT), for the treatment of cancer.
Investigations on the use of antibody- conjugated radionuclides began in the early 1950s [7], but it took nearly 25 more years before these feasibility studies came to clinical fruition, demonstrating first that antibodies could selectively localize cancer [8,9], and then illustrating their therapeutic potential [10]. Clinical studies began with the evaluation of 131I-labeled antibodies, but in combination with chemotherapy [11,12]. This effort drew criticism because the efficacy of the 131I-labeled antibody alone had not been established and, therefore, future clinical trials focused on monotherapy with radiolabeled antibodies [13]. The initial clinical trials focused on using radioiodinated antibodies but, over time, advances in chelation chemistry have allowed many new therapeutic radionuclides to be explored (Figure 1 & Table 1) [14,15].
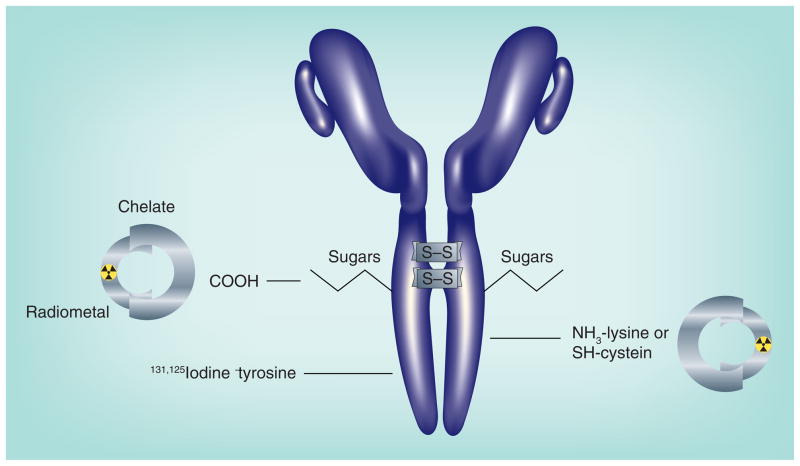
Figure 1. Radionuclides are attached to antibodies principally by two methods.
Radioiodine is bound to aromatic rings, primarily to tyrosine, in the presence of a mild oxidative agent, such as iodogen or chloramine-T. The ε amino group of lysine can be modified to accept a metal-binding chelate, which is then loaded with a radiometal. Exposing IgG to a mild reducing agent can split disulfide bonds, allowing the coupling of chelate or other compounds to the reactive sulfhydryl. To ensure amino acids within the antigen-binding sites of the antibody are not altered, carbohydrates, commonly found on the IgG’s CH2 domain, can be modified to accept a chelate.
Table 1.
Therapeutic radionuclides for radioimmunotherapy.
| Radionuclide | Energy (MeVmax)† | Range† | Half-life |
|---|---|---|---|
| β | |||
| 90Yttrium | 2.28 | 11.3 mm | 2.7 days |
| 131Iodine | 0.61 | 2.3 mm | 8.0 days |
| 177Lutetium | 0.50 | 1.8 mm | 6.7 days |
| 188Rhenium | 2.12 | 10.4 mm | 0.7 days |
| 67Copper | 0.58 | 2.1 mm | 2.6 days |
| α | |||
| 213Bismuth | 8.3 | 60–85 μm | 0.8 h |
| 211Astatine | 6.8 | 7.2 h | |
| 225Actinium | 6.8 | 10 days | |
| Auger-electron | |||
| 125Iodine | 2–500 nm | 60.5 days | |
As reported by Kassis [270].
MeVmax: Maximum range of particulate energy in tissue.
Hematological malignancies
The first major advance in RAIT occurred with hematological malignancies, starting with a report that a fractionated dosing regimen using 131I-labeled anti-HLA-DR antibody (Lym-1) achieved remarkable regressions of bulky masses, primarily in patients with non-Hodgkin lymphoma (NHL) [16,17]. Subsequent trials reported success with 131I-labeled anti-CD37 IgG, anti-CD20 IgG, and anti-CD22 IgG in lymphoma [18–22]. In all instances, RAIT was limited by hematologic toxicity, because the radiolabeled antibody cleared slowly from the blood, exposing the radiosensitive bone marrow to a continuous source of low-dose radiation. When the dose was escalated to myeloablative levels with help of bone marrow grafting, a significant number of patients achieved complete objective responses for extended durations [22]. Excellent responses were also reported with nonmyeloablative doses of an 131I-labeled anti-CD20 antibody [21].
These studies claimed that adding unlabeled antibody to the radioimmunoconjugate resulted in a more ‘favorable biodistribution’ [18,21–23]. At low protein doses, the radiolabeled antibody cleared into the spleen quickly, where a sizeable number of normal B cells reside, which also express these antigens, but patients with bulky disease also cleared the antibody quickly. Predosing with unconjugated antibody blocked the rapid uptake in this antigen sink and slowed the radiolabeled antibody’s blood clearance, which, in turn, gave the radioimmunoconjugate more time to localize to more tumor sites. A similar finding was confirmed in clinical investigations with a 90Y-labeled anti-CD20 antibody [24], selecting 250 mg/m2 as the preferred predose of unlabeled antibody [25]. While examining the optimal protein dose for an 131I-anti-CD20 IgG, Kaminski et al. noted that some patients given a predose of 685 mg of anti-B1 (tositumomab) actually experienced tumor shrinkage after receiving only an imaging dose of the 131I-labeled anti-CD20 antibody [21,26]. Preclinical studies also noted antitumor activity with the unlabeled antibody in xenograft models [27]. Shortly thereafter, clinical studies confirmed the antitumor effects by another unlabeled, chimeric anti-CD20 antibody, later known as rituximab [28].
The 90Y- and 131I-labeled anti-CD20 antibodies, now known as 90Y-ibritumomab tiuxetan (Zevalin™, Spectrum Pharmaceuticals, CA, USA) and 131I-tositumomab (Bexxar®, GlaxoSmithKline, NC, USA), respectively, are the only radiolabeled antibodies approved for cancer therapy in the USA, being registered to treat chemotherapy-refractive, follicular NHL, with or without transformation. 131I-tositumomab requires a pretherapy imaging study in order to establish the prescribed therapy dose. 90Y-ibritumomab is administered at a fixed dose of 0.4 mCi/kg, but requires a dose reduction to 0.3 mCi/kg if platelet counts are below 150,000. Neither treatment is recommended for patients with more than 25% bone marrow involvement, since these patients have an increased risk of more severe hematologic toxicity.
Antitumor responses in NHL occur at relatively low radiation-absorbed doses (e.g., much less than 1000 cGy) [29–32]. Although clear evidence for a dose-response relationship is lacking, recent data using 3D dosimetry and radiobiological modeling have provided reasonable positive predictive values for response versus mean absorbed dose and equivalent uniform dose [33]. Radiation dose–response relationships are probably confounded by the fact that each of these treatments uses substantial amounts of their respective unconjugated anti-CD20 IgG, which are therapeutically active, yet the radioimmunoconjugate is able to enhance the response. For example, a randomized trial comparing the efficacy of rituximab (Genentech, CA, USA) (375 mg/m2 four-times weekly) to 90Y-ibritumomab tiuxetan (250 mg/m2 chimeric rituximab two-times weekly with the radiolabeled murine anti-CD20) found superior objective response with RAIT [34]. 131I-tositumomab was also more active than unlabeled murine anti-B1 antibody [35]. Research also has shown that these antibodies can enhance a tumor cell’s sensitivity to radiation and chemotherapy [36–40], and thus in these treatments, responses probably represent a combination of the unconjugated antibody and targeted radiation. Unfortunately, the data from the randomized trial did not find significant difference in the duration of response or time to progression for RAIT over the unconjugated antibody therapy, so clinical acceptance for this treatment has been tempered, even though durable responses have been reported for patients who achieved a complete response following RAIT [41,42].
A number of other issues have hindered the acceptance of RAIT. For example, the incidence of secondary cancers and of myelodysplastic syndrome (MDS) is a concern, but there is considerable evidence suggesting that the overall risk for RAIT is no higher than chemotherapy [43–45]. There were concerns that the hematologic toxicity associated with RAIT would reduce a patient’s tolerance to chemotherapy, yet clinical studies contradicted this [46]. Kaminski et al. reported encouraging response rates and durations with 131I-tositumomab given as a front line treatment [47]. In addition, 28 patients were able to receive a second 131I-tositumomab treatment with no additional toxicity, with all of the 18 patients who responded to the first treatment responding to the second treatment [48,49]. While RAIT as a stand-alone treatment for follicular lymphoma has not garnered much attention, an increasing number of clinical trials are incorporating RAIT successfully with chemotherapy or, occasionally, external beam therapy [50–63]. There is also interest in using RAIT in high-dose therapy regimens with chemotherapy and external beam radiation (or possibly as a replacement for whole-body radiation) in cytoreductive marrow conditioning regimens [43,64–76].
Several other radioantibody conjugates have been tested clinically in hematologic malignancies. A few centers reported success with 131I-rituximab [77,78]. One recent study administered two doses of 131I-rituximab, given approximately 6 weeks apart, commencing 6 weeks after completion of a standard unconjugated rituximab therapy [78]. They reported a 90% overall response with a 50% complete response and a median time to progression of 20 months, which was significantly longer than the last chemotherapy regimen the patients had received prior to qualifying for this study. Lym-1, a murine anti-HLA-DR10 antibody, was examined as an 131I-labeled (Oncolym, Peregrine Pharmaceuticals, CA, USA) and 90Y-labeled conjugate with promising activity in initial clinical testing [79,80], but is no longer being pursued for commercial development. A 131I-labeled anti-CD45 antibody is being tested in patients who will undergo a myeloablative procedure because CD45 is expressed on many different types of white blood cells [81]. A Phase I/II trial for 90Y-labeled epratuzumab (humanized anti-CD22 IgG; Immunomedics, Inc., NJ, USA), administered as fractionated two- or three-weekly injections, found that 11 out of 12 (92%) of the follicular lymphoma patients receiving a total dose of more than 30 mCi/m2 had a complete response, with a median duration of 24.6 months [82]. In addition, ten patients with follicular lymphomas who were previously refractory to an anti-CD20-containing therapeutic regimen achieved a median progression-free survival of 21.5 months, with nine of these patients (90%) obtaining a complete response. Encouraging responses were also noted in patients with aggressive forms of NHL.
Preclinical studies suggest that further improvements could be achieved by combining the 90Y-labeled anti-CD22 IgG with unconjugated anti-CD20 antibody therapy [83]. As mentioned previously, both of the currently approved anti-CD20 RAIT agents administer a substantial amount of unlabeled anti-CD20 IgG prior to the radiolabeled anti-CD20 (as much as 900 mg is administered prior to the therapeutic radioimmunoconjugate) [84]. This procedure was reported to enhance tumor visualization and tumor dosimetry, derived in small, separate groups of patients, suggesting that the radiation-absorbed dose was not affected [23,24]. However, preclinical studies have indicated that unlabeled anti-CD20 can reduce tumor uptake of radiolabeled IgG [83,85]. By using a radioimmunoconjugate that binds to a different, yet B-cell-specific, antigen, both agents would have an equal ability to localize in the tumor without competing for binding sites. If the unconjugated anti-CD20 IgG is administered sometime before the radiolabeled anti-CD22 (e.g., 1–3 weeks), it also would reduce the normal B-cell sink that could affect the radiolabeled anti-CD22 targeting. Animal studies have also suggested that a consolidation course of unlabeled anti-CD20 antibody therapy after RAIT could amplify the response and its duration [86].
Radioimmunotherapy with β-emitting radionuclides increases the risk for severe myelosuppression, because the β-emissions, which can travel several millimeters, cause collateral damage to nontargeted normal tissues (Table 1). However, shorter-range radionuclides, such as α-emitters, travel less than 0.1 mm (i.e., a few cells deep) and, thus, would be a better choice in treating cancer in the blood or bone marrow. Early clinical investigations focused on 213Bi-labeled anti-CD33 IgG in myeloid leukemia [87–91]; however, even though leukemic cells were rapidly localized, 213Bi’s short physical half-life (46 min) presents logistical issues for conjugate preparation and is not ideally suited for an IgG when tumors are less accessible. Investigators have speculated that a more rapid targeting system might be necessary [92,93]. Longer-lived α-emitters, such as 211At (7.2 h), and 225Ac, which has a 10-day half-life and emits four daughter α-particles during its decay, are also being explored as a better match for an IgG. However, issues with renal toxicity need to be addressed [94–101]. Targeted α-particle therapy is being explored in more easily accessible settings, such as the peritoneal cavity or the cavity left following primary brain cancer surgery [102–106], or to the tumor vasculature [107–111], where the radionuclide would be more quickly localized to the disease.
Auger-emitters, such as 125I, 67Ga and 111In, have such a limited range that they are only effective when deposited very near or within the nucleus [112]. Therefore, they are active only against single cells, but could be suitable for micrometastatic disease, even in the bone marrow, because of the limited potential for collateral damage [113,114]. One of the few systems where Auger-emitters have been used successfully has been with an anti-CD74 antibody [115]. In this system, even though there are a small number of antigen sites on the cell surface, the antigen is constantly recycling, thereby transporting and emptying the antibody with its radioactive payload inside the cell and then returning to cell surface, where it is available to bind additional antibody. Activity was also reported for 111In-labeled antibodies to the EGF receptor (EGFR) and HER-2 [116].
Nonhematologic malignancies
Clinical trials in advanced solid tumors rarely progress beyond Phase I/II because, even though there is occasional evidence for tumor shrinkage, most fail to meet accepted criteria of an objective response [117–121]. However, two agents did proceed to more advanced clinical testing in solid tumors. The first was pemtumomab (R1549; Antisoma PLC, UK), a 90Y-labeled murine anti-HMFG1 (MUC-1) murine antibody that was administered intraperitoneally to ovarian cancer patients with measurable and occult disease. Promising data were first reported from a Phase II trial, where this agent (18 mCi/m2) was administered to 21 women with stage Ic–IV ovarian cancer, who had no detectable disease after completing surgery and a platinum-based chemotherapy regimen [122]. Unfortunately, this experience was not confirmed in a large, Phase III, randomized study [123]. A pivotal trial performed in China showed more promising results in 107 lung cancer patients that were given an 131I-labeled chimeric antitumor necrosis therapy IgG (Shanghai MediPharm Biotech Co. Ltd, China) [124]. A total of 62 patients received two intravenous injections spaced 2–4 weeks apart (0.8 mCi/kg), while another 45 patients received an intratumoral injection of 0.8 mCi/kg. Not surprisingly, patients receiving the intratumoral administration had less hematologic toxicity than the systemically administered group. However, the overall response rates were similar (~35%), with most having partial responses. Because the trial was designed to evaluate response only at 10 weeks post-treatment, the full duration of these responses, and the impact on survival, are not known. This agent is not approved for this indication in the USA, but early clinical trials in the USA using this antibody labeled with 131I (Cotara, Peregrine Pharmaceuticals Inc., CA, USA) for the treatment of brain and colorectal cancers have been reported [125–127].
Perhaps one of the more obvious issues with RAIT in solid tumors is that, unlike lymphoma, most of the antibodies used, are themselves, unable to affect tumor growth. Preclinical and clinical studies have been examining the possibility of using trastuzumab, cetuximab or panitumumab [95,128–138], but their binding specificity might limit their utility. For example, imaging studies have indicated that trastuzumab has enhanced uptake in the heart wall, but dosimetry estimates suggested this might not be dose limiting [137]. Encouraging clinical responses, using a trastuzumab–drug conjugate, may provide additional incentive to evaluate the radioimmunoconjugate [139]. Improved responses with 90Y-labeled panitumumab in a head and neck cancer model was also reported recently [140]. In addition to ensuring tumors express antigen, cetuximab or panitumumab radioimmunoconjugates should also require patient selection based on wild-type KRAS status in order to enhance the prospects that the unconjugated antibody will provide additional therapeutic response [141].
Owing to a lack of evidence for significant benefit in patients with advanced and disseminated solid tumors, efforts are being undertaken to find ways to: improve antibody uptake (both quantitatively and in a more uniform distribution); enhance the tumor’s sensitization to radiation; or administer RAIT when less disease is present.
Meredith et al. combined an 131I-labeled anti-carcinoembryonic antigen (CEA) antibody (COL-1) with 131I-CC49 anti-tumor-associated glycoprotein (TAG)-72 IgG, because immunohistology demonstrated that the combination gave a more homogeneous distribution within the tumor than either antibody alone [142]. In addition, patients received IFN-α, which had been reported to enhance CEA and TAG-72 expression in gastrointestinal tumors. Tumor imaging was judged to be excellent in most cases, and in comparison with other trials that used only 131I-CC49, the combination of anti-CEA and anti-TAG-72 antibodies, together with interferon, appeared to result in a modest increase in the absorbed dose to the tumor; however, no objective responses were reported. When using antibody combinations, one needs to be cognizant that while this might enhance the uniformity of distribution, the absolute uptake of the antibody combination may be less than that of the single antibody with the highest uptake, which was illustrated well by Pagel et al. using three non-cross-reactive antilymphoma antibodies [143].
Enhancing uptake by modifying the tumor’s vascular properties through hyperthermia, radiation, or biologically active compounds has been widely investigated [144–150], but these manipulations usually have only a modest effect on antibody uptake. Locoregional administration, such as intraperitoneal and intracranial, is another example of modifying the route of administration in order to give an antibody a ‘first-pass’ opportunity to bind to the tumor, enhancing uptake [151]. Early preclinical data demonstrated an advantage for intraperitoneal injection, particularly, but not conditionally, when there is malignant ascites [152,153]. One of the earliest clinical studies to examine route of administration coinjected 131I/125I-B72.3 anti-TAG-72 IgG intraperitoneally and intravenously. It was concluded that peritoneal implants were more likely to benefit from the intraperitoneal injection, whereas nonimplants (i.e., those metastases in the peritoneal cavity derived from hematogeneous spread) were more likely to have higher uptake by intravenous injection [154]. A clinical trial using only intraperitoneally administered 177Lu-CC49 anti-TAG-72 IgG in ovarian cancer noted a trend for improved responses only in patients with minimal disease, which is expected based on the short-range β-emission of 177Lu [155]. This group also examined the combination of intraperitoneally administered 90Y-CC49 with IFN-α and paxlitaxel (for upregulation of TAG-72 and radiosensitization, respectively) [156]. Two partial responses were noted in patients who had measurable disease at the time of treatment, while four out of of 11 patients who had no measurable disease were disease-free from 9 to 24 months following therapy.
As mentioned previously, a Phase III trial of intraperitoneal RAIT for ovarian cancer with a 90Y-antibody failed to improve survival in patients who had been surgically debulked and had received primary chemotherapy [123]. Whether this failure was related to using the long-range-emitting 90Y in a potentially suboptimal setting of minimal or microscopic disease could be debated, particularly because a retrospective analysis showed relapse was less likely to occur in the peritoneal cavity in the RAIT-treated patients, and primarily in a subgroup of patients who had residual disease following surgery [157]. These results are very important since they reflect a very common finding for regional therapies of all kinds; namely, cancer is a systemic disease that, when treated locally, can lead to impressive local responses, but often needs to be coupled with a potent systemic treatment to affect survival. Thus, while a number of investigators are considering shorter-ranged β-emitters or even α-emitters for less toxic and more directed localized treatment [158–162], improved survival may be achieved only when the treatment includes an effective systemic counterpart.
In contrast to peritoneal involvement, brain cancer does not usually spread to other organs, making localized treatment ideal, particularly when there is a solitary lesion at diagnosis. Clinical studies have examined treatments where RAIT is injected directly into the tumor, but more often treatment is administered to eradicate locally advanced disease after a solitary mass is surgically removed [104]. A real advantage for the latter approach is that, unlike the peritoneal cavity, radiolabeled antibodies placed directly in the surgical cavity reside almost entirely in this cavity, sparing exposure of other organs. Clinical studies with an 131I-labeled antitenascin antibody for the treatment of glioblastoma multiforme (GBM; Neuradiab, Bradmer Pharmaceuticals, Inc., ON, Canada) have been encouraging, with the median survival rivaling that of brachytherapy or steriotactic radiosurgery, but with a lower rate of radionecrosis [163–166]. A correlation between the radiation-absorbed dose delivered to the rim of the cavity, where the radioantibody was deposited, and tumor recurrence or radionecrosis was also reported, including various parameters that reflected a positive outcome for the patient [167]. Encouraging survival results were also reported for the same agent when administered locally in newly diagnosed patients, so as to boost radiation exposure to the surgical margins in combination with conventional external beam radiation and chemotherapy [168]. Early clinical testing of 188Re-labeled humanized anti-EGFR antibody (nimotuzumab, Biocon, Bangalore, India) and an 211At-antitenascin antibody has also been reported [106,169,170]. In addition to intracranial RAIT, radiolabeled antibodies are being administered intrathecally to treat disease in the CNS [138,171–175]. Although not given compartmentally, Quang et al. reported positive outcomes using intravenous or intra-arterial injections of 125I-labeled 425, an anti-EGFR antibody, to patients following surgical resection of their primary brain cancer and in concert with external beam therapy [176]. In a recent overview of a Phase II experience of patients who received three weekly intravenous treatments of 125I-anti-EGFR (~50 mCi/dose) following surgery and radiation therapy or with temozolomide therapy, the median survival was 15.7 months, which was improved over a contemporaneous control group that did not receive RAIT, but since the study was not randomized, the control group was not well balanced [177]. Indeed, the median survival for GBM patients following surgical resection with subsequent radiotherapy and temozolomide is 14.6 months [178]. With additional improvements being reported for the standard treatment of recurrent GBM by the addition of bevacizumab [179], and with new clinical trials now examining this combination in first-line therapy, RAIT will need to do better if it is to be integrated in these currently approved regimens. However, because of its good safety profile, it may still become an important part of GBM treatment, particularly when administered compartmentally.
Early preclinical studies had demonstrated that RAIT rarely eradicated large tumors, but cures could be achieved when tumors of minimal or microscopic size were treated, and, just as importantly, effective treatment against microscopic metastatic disease could be erased if the animals also had bulky tumors at the time of treatment [180,181]. Thus, it is reasonable for clinical trials to turn to indications where the extent of disease is minimal or microscopic (i.e., adjuvant). Liersch et al. reported trial results using an 131I-humanized anti-CEACAM5 IgG in 23 patients who had undergone liver resection for metastatic colorectal cancer [182]. At the median follow-up time of 91 months, 19 assessable patients had a median survival of 58 months, while in a contemporaneous group of similar patients who had not received RAIT after liver resection, the median survival was only 31 months [183].
Radioimmunotherapy is also being combined with other agents to enhance therapeutic prospects in solid tumors. Most efforts in solid tumors have focused on adding a sub-therapeutic, rather than a therapeutic dose of drugs, such as paclitaxel, docetaxel, topotecan and gemcitabine, as demonstrated in models for breast, colorectal, pancreatic and prostate cancers, enhancing responses to RAIT [184–189]. As with any combination modality, there are numerous dosing and scheduling issues required to optimize the therapy [190,191]. Panijdeh et al. showed the feasibility of cotargeting RAIT with chemotherapy through antibody-directed enzyme-prodrug therapy (ADEPT), using an engineered single chain (scFv) of the A33 anti-gpA33 antigen fused with cytosine deaminase, a prodrug-converting enzyme [192]. We recently reported enhanced antitumor activity by combining an effective antibody–drug conjugate with RAIT [193]. In this approach, the targeting was performed with antibodies identifying different antigens coexpressed in pancreatic cancer xenografts, but effective therapy could also be achieve using antibodies against the same target. Interestingly, the antibody–drug conjugate (IgG-SN-38) could be administered at a dose that was effective alone, but also with the maximum tolerated dose of RAIT without appreciably altering toxicity. Clinically, a number of feasibility trials combining RAIT with chemotherapy have been reported [120,194–196]. Most recently, a Phase Ib clinical trial was reported to have promising results with a 90Y-labeled antibody (clivatuzumab tetraxetan; Immunomedics, NJ, USA) directed against a pancreatic cancer mucin used in combination with low-dose gem-citabine as a front line treatment for patients with metastatic pancreatic cancer [197,198]. In this protocol, patients are administered three weekly doses of the 90Y-antibody, each dose followed by 200 mg/m2 of gemcitabine. Interestingly, the fractionated dosing regimen has been tolerated at a cumulative dose of 45 mCi/m2 (3 × 15 mCi/m2), whereas as single-dose treatment, the maximum tolerated dose was just 20 mCi/m2 [199]. Investigators reported that 68% of the patients had disease control (i.e., stable and partial responses) across all dose levels, starting with 3 × 6.5 mCi/m2 of the 90Y-antibody [198]. While this and other reports have demonstrated the safety of these combinations, it is too early to determine if any of these approaches will provide the benefit necessary for RAIT to become an acceptable treatment for solid tumors.
Most therapy trials have used whole IgG, not only because it can be easily manufactured and handled, but preclinical models found that IgG has the highest tumor uptake when compared to other forms, such as enzymatically cleaved F(ab′)2 or Fab′ fragments or various engineered forms (Table 2) [200–202]. IgG’s slow blood clearance encourages high tumor uptake, but this also exposes the highly radiation-sensitive red marrow to a continuous source of low-dose radiation, causing myelosuppression well before a tumoricidal dose is achieved (except in radiosensitive hematopoietic diseases). Tumor uptake is also affected by others factors. For example, tumors have an abnormal vascular system that is ‘leaky’, allowing macromolecules to pass more easily, which is why even a nonspecific antibody will have a higher concentration in the tumor than in most normal tissues [203–205]. However, tumors can have reduced lymphatics, and so internal pressure builds within the tumor, which impedes the free flow of molecules throughout the tumor mass, with larger molecules being affected more than smaller ones. Movement of targeted molecules (i.e., not just antibodies) is also affected most significantly by the ‘binding-site barrier’ (i.e., when a molecule binds to its target, it will be retained at this location, usually being concentrated in the perivascular space) [206–208]. Movement of molecules with higher affinity within the tumor is more affected than ones with lower affinity, albeit higher-affinity antibody may have enhanced uptake [206,208,209]. This restricted localization can be overcome to some degree by increasing the protein dose [210,211], but excessive unlabeled product could compete and reduce the amount of radiolabeled product in the tumor. Nevertheless, concentrating radiation in the perivascular space may cause the local destruction of the blood vessels, which, in turn, could affect tumor progression.
Table 2.
Targeting properties of various antibody forms.
| IgG | F(ab′)2 | Fab′ | scFv | |
|---|---|---|---|---|
| Physical properties | ||||
| Estimated molecular weight (kDa) | 150 | 100 | 50 | 25 |
| Biological properties | ||||
| Relative biological half-life in blood† | 1 day | 2 days | 3 h | 4 min |
| Target organ | Liver | Liver | Kidneys | Kidneys |
| Tumor-binding properties | ||||
| Relative uptake‡ | 1 | 2 | 3 | 4 |
| Relative duration‡ | 1 | 2 | 3 | 4 |
| Time to optimum accretion | Day(s) | Day(s) | Hour(s) | Hour(s) |
Antibody half-life in blood.
Highest = 1; lowest = 4.
scFv: Single-chain Fv fragment.
Reducing bone marrow exposure by hastening the clearance of the antibody from the blood, but with the same tumor retention as the IgG, has been attempted using anti-antibodies or extracorporeal adsorption procedures [212,213]. The extracorporeal method avoids the formation of immune complexes in the body that will inevitably redirect the radioimmunoconjugate to the liver and spleen, greatly increasing their radiation exposure. This method has been promising in preclinical testing, but whether sufficient escalation in the administered dose will bring about a significant improvement in responses, clinically, is still to be determined.
Single chains (~25,000 Da) and diabodies (~50,000 Da), which are smaller monovalent and divalent binding proteins, respectively, and a variety of other types of constructs, are gaining interest as possible alternatives to intact IgG. Constructs with divalent binding are generally preferred over monovalent ones, because they usually have a longer retention time in the tumor [208,214,215]. These constructs invariably have a faster clearance from the blood than intact IgG, because of their physical size, but also because most lack the Fc portion of the IgG that has regions capable of binding the neonatal Fc receptor, which is used by an intact IgG to traverse vascular endothelium, allowing it to stay in the blood for long periods [216]. Smaller antibody forms are better able to traverse the vascular channels, yielding a more rapid tumor uptake, superior tumor/nontumor ratios and potentially more uniform distribution as compared with IgG, but they too are susceptible to the binding-site barrier effect. In addition, the faster a protein clears from the blood, the less time is allotted for target interaction; therefore, tumor uptake for these constructs is lower than an IgG. Faster clearance reduces red marrow exposure, allowing the total administered activity to be increased. While this increase does not often permit higher doses to the tumor, rapid tumor uptake delivers the radiation at a higher dose rate, which is more cytocidal than a low dose rate [217]. With the advent of molecular engineering, a variety of constructs with more favorable pharmacokinetics and tumor uptake, such as CH2-deleted antibodies and point-mutated scFv–Fc fusion proteins that avoid neonatal Fc receptor binding, have been described [216,218].
The primary deterrent for using molecules less than 50,000 Da is that they are cleared through the kidneys, which raises concern for renal toxicity, particularly when a radionuclide that is reabsorbed and retained by the kidney, such as radiometals, is used. Behr et al. reported that a high predose of cationic amino acids could significantly reduce renal tubular reabsorption of radiometal-labeled Fab′, and demonstrated that higher doses could be administered to mice with less renal toxicity [219]. This, and other approaches, have been used to reduce the risk for renal toxicity [220], but since renal uptake often far exceeds tumor uptake, the compensation afforded by these methods, which can be as much as a twofold reduction, may not be sufficient to yield positive tumor:kidney ratios. Because indications of renal toxicity are not manifested for many months, or even years, after treatment, careful monitoring is required for extended periods.
With all the different antibody forms made possible through molecular engineering, it is important to keep in mind that radionuclide selection may vary based on the antibody’s biodistribution, tumor retention and blood clearance properties [221,222]. For example, 131I remains an isotope of interest for compounds that clear quickly with elimination primarily through the kidneys, since it does not have as long a residence time as a radiometal conjugated to an antibody. However, smaller antibody constructs that have a shorter retention time in the tumor than an IgG (e.g., ≤1 day vs >3 days, respectively) would not benefit from the longer physical half-life of 131I, namely approximately 8 days. The long physical half-life of 131I is also of little use for antibodies that rapidly internalize, with the cells not retaining the radionuclide sufficiently to take advantage of the longer half-life. In this situation, 177Lu seems to be a reasonable replacement for 131I, since it has similar half-life and β-energy, albeit 131I can be coupled to antibodies that would allow it to be retained in cells [223]. Some conjugation processes can affect antibody binding, which can affect radionuclide selection, and even after radiolabeling, stability and retention of binding must be diligently tested. Thus, there are a number of factors that contribute to the selection of the most appropriate radionuclide for a radioimmunoconjugate, and while there are many radionuclides in nature to choose from, availability and cost have limited the choices for clinical use.
Pretargeted radioimmunotherapy
In the mid-1980s, pretargeting was introduced as a way to improve tumor:blood ratios (Figure 2). The initial concept envisioned a bispecific (bs) monoclonal antibody (mAb) capable of binding a tumor antigen and a chelate [224]. The bs mAb would not be radiolabeled, and thus no exposure would occur while it localized to the tumor. Since there was ample clinical experience with radiolabeled chelates, such as diethylene triamine pentaacetic acid (DTPA) or ethylene-diaminetetraacetic acid (EDTA), demonstrating these agents cleared very quickly from the blood and tissues with minimal residual activity remaining in the body, the radioactivity would be carried by the chelate loaded with a radionuclide. Thus, the antichelate binding arm of the bs mAb that had been pretargeted to the tumor would serve as a receptor for the radiolabeled chelate. The small size and inert properties of the radiolabeled chelate allowed it to traverse blood vessels and distribute in the fluidic volume within minutes, and then just as quickly be eliminated, thereby minimizing nontarget tissue exposure. A significant improvement to bs mAb pretargeting was made by Le Doussal et al., who determined that radiolabeled compounds bearing two haptens were more stably bound in the tumor than a monovalent compound, since they can cross-link two adjacent bs mAbs on the tumor cell surface (Figure 2). This is a concept known as the affinity enhancement system (AES) [225].
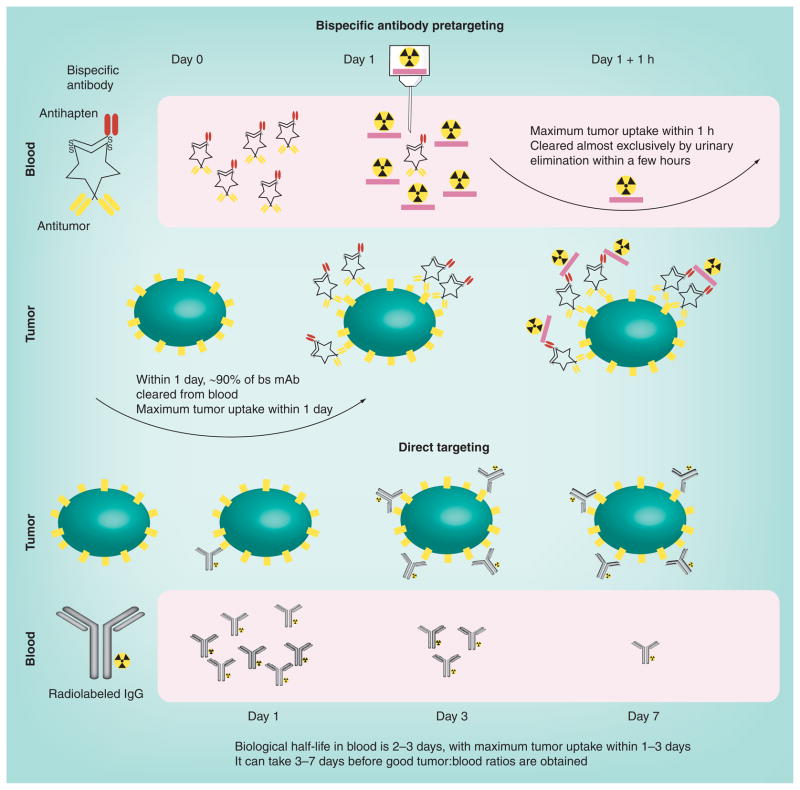
Figure 2. Comparison of bispecific pretargeting with direct targeting.
For pretargeting, an unlabeled bs mAb is used (shown is a tri-Fab recombinant structure described in Figure 3). In patients, 90% of this antibody is cleared from the blood, and animal studies have demonstrated that maximum tumor uptake occurs within approximately 6 h. The radioactivity is introduced on a small peptide (~four amino acids) that contains two haptens to help stabilize binding within the tumor (affinity enhancement). The peptide also contains structures that will bind the radionuclide. The radiolabeled hapten–peptide is administered when the bs mAb is sufficiently cleared from the blood, and clears exceptionally fast from the blood and body by urinary excretion, reaching peak tumor uptake within 1 h. Tumor:blood ratios are highly favorable within a few hours. In direct targeting, the isotope is on the antibody. Shown here is an IgG that will clear very slowly from the blood, gradually building its concentration in the tumor, so tumor:blood ratios remain relatively low over the first few days.
bs mAb: Bispecific monoclonal antibody.
Several other pretargeting methods have been investigated, but they all strive to overcome the limitation of the slow blood clearance of a directly radiolabeled IgG by separating antibody targeting from the delivery of the radionuclide [226]. The first was based on avidin (mammalian) or streptavidin (bacterial) in conjunction with biotin in a variety of configurations [227]. This system appeared to be ideally suited for pretargeting, since the avidins could bind as many as four biotin molecules with an exceptionally high binding constant (10−15 M), the reagents could be produced in plentiful amounts, were nontoxic and amenable to chemical modification for coupling to antibodies or radiolabeling. Two avidin/biotin-based pretargeting configurations were examined clinically. One system consisted of three agents, starting with a streptavidin-conjugated antibody (later replaced with a recombinant streptavidin–scFv) that was administered for 1–3 days to localize the tumor [228–231]. A clearing agent then was given to remove residual streptavidin antibody from the blood. The clearing step was essential because the ultrahigh affinity of biotin for avidin would have caused the subsequently administered radiolabeled biotin to bind primarily to the conjugate in the blood, reducing its ability to reach the tumor. The other approach uses four agents, beginning with a biotinylated antibody [232]. Avidin, a glycosylated protein, is used as a clearing agent, but it is administered along with streptavidin, a nonglycosylated bacterial protein. The avidins will bind to the tumor-localized biotinylated antibody, serving as a bridge to link it to radiolabeled biotin that is administered 1 day later. The primary issue with pretargeting methods that rely on avidin/streptavidin is the immunogenicity of these foreign proteins [233,234]. A third type of pretargeting system is also under development, which uses complementary synthetic DNA analogs, known as morpholinos, as bridging agents [235,236]. These morpholino compounds should have a low immunogenicity and therefore, when this system is fully optimized, it could also lead to further improvements in radionuclide targeting.
Since the pretargeting agent acts as a surrogate receptor that will bind the radiolabeled compound, it must remain accessible, either on the cell surface or within the interstitial space of the tumor. Therefore, antigens known to internalize might not be best for pretargeting [237]. However, we have evaluated an anti-Trop-2 bs mAb that can be used effectively in pretargeting even though the bs mAb internalizes [238]. Thus, antigens that internalize should not be dismissed as potential agents for pretargeting.
The initial antihapten antibodies used in a bs mAb pretargeting approach bound with high specificity to metal-binding chelates, but chelates are often so specific for certain metals that an antibody raised against one chelate–metal complex might not bind as well when loaded with a different radiometal. To avoid this complication, the chelate would first need to be loaded with its appropriate metal so it would bind well to the antichelate–metal complex, and then the peptide portion of the divalent hapten complex could be radiolabeled. For example, tyrosine in the peptide core can be radioiodinated. A different ligand capable of binding 99mTc/188Re has been coupled to a di(In)DTPA-peptide [225,239,240]. However, a more practical approach is to use an antibody against a synthetic compound that is not involved in the binding of the radionuclide, allowing radionuclide binding to be controlled by another constituent within the hapten–peptide complex. The approach has been successfully used with an antibody to histamine-succinyl-glycine (HSG) [241]. Di-HSG-peptide structures have been developed for binding radioiodine, 90Y, 177Lu, 111In, 67/68Ga, 18F, 99mTc and 188Re [242–245].
Regardless of the pretargeting approach taken, data have consistently shown that pre-targeting delivers as much, or more, radioactivity to a tumor as a directly radiolabeled antibody, but with much less exposure to the red marrow [228,246]. In addition, radiolabeled biotin or hapten/peptide localizes in the tumor very quickly, maximizing the number of decays occurring in the tumor rather than elsewhere in the body. Thus, pretargeting increases the dose rate to the tumor as compared with a directly radiolabeled IgG that can take 1–2 days to reach maximum accretion. Radiolabeled biotin and hapten/peptides also have far less renal accretion than a radiometal-labeled antibody fragment, providing favorable tumor:kidney ratios. Pretargeting systems might also avoid problems when circulating antigen is present. With a directly radiolabeled antibody, the antibody–antigen complexes formed in the blood are processed primarily by the liver, increasing hepatic exposure. In pretargeting, if the complexes are cleared and processed before the radiolabeled compound is administered, hepatic uptake would be minimized. Pretargeting approaches might be more amenable to combination therapies, particularly with agents that cause severe hematologic toxicities. Several studies have shown combinations with chemotherapeutic radiosensitizing agents and antiangiogenic agents improve responses [149,247–249].
Most clinical experience has been limited to Phase I trials designed to determine optimal targeting conditions, with subsequent accrual to determine the maximum tolerated dose. One Phase II trial examined a dose of 110 mCi/m2 of 90Y-DOTA-biotin pretargeted with a NR-LU-10, an anti-EpCam (or EGP-2) IgG–streptavidin conjugate in advanced colorectal cancer [230]. Disappointingly, no significant responses were observed and radiation-absorbed tumor doses reported in just two patients were only approximately 500 and 2900 cGy, respectively. Hematologic toxicity was mostly mild to moderate, but because the anti-EpCam antibody bound to the normal colon, a substantial fraction of the radiation was delivered to the intestine, causing severe diarrhea that was dose-limiting. With most of the radioactivity being removed from the body by renal filtration, it was not surprising that some evidence of renal toxicity was observed in this trial. More recently, a recombinant protein of streptavidin with four CC49 (anti-TAG-72) single chains has been tested in patients [250]. Dosimetry from this study suggests that if 110 mCi/m2 of 90Y-biotin was tolerated, colorectal tumors could receive 5000 cGy or more in some patients.
Hematologic toxicity was dose limiting in clinical trials using a bs mAb pretargeting approach [251], as well as avidin/biotin approach [252,253]. Investigators found medullary thyroid cancer patients developed hematologic toxicity at lower doses than other cancer patients. Other studies revealed that these patients often have tumor involvement in the bone and bone marrow that is not appreciated at the time of enrollment, and this might explain the lower tolerance [254]. Despite this limitation, a retrospective analysis of the efficacy of the pretargeting procedure in medullary thyroid cancer patients found a statistically significant increase in the survival of a subset of patients who had a calcitonin doubling time of 2 years or less [255]. Investigators speculated that enhanced survival might have been attributed by the control of micrometastatic bone marrow disease by the pretargeted therapy that used an 131I-labeled hapten-peptide.
Pretargeting will also most likely have its greatest impact in patients with more radiosensitive tumors, with minimal disease, or in locoregional applications. In one trial, previously treated glioblastoma (n = 16) or astrocytoma (n = 8) patients, who, after having a second debulking, were given two intracranial treatments of a biotinylated antitenascin antibody followed by 90Y-DOTA-biotin. Two patients had a partial response and four had a mixed response (25% overall response rate) [256]. In another trial, 37 patients with grade III gliomas or glioblastoma (grade IV) were divided into two groups. All had no radiological evidence of disease following surgical debulking and radiotherapy with or without chemotherapy, but one group of 19 patients also received an intravenous adjuvant pretargeted therapy. The median survival of the glioblastoma patients who did not receive the additional pretargeting procedure was 8 months (n = 12), while the median survival in the group given pretargeted therapy was 33.5 months (n = 8). The median survival for six patients with grade III glioma was 33 months, but the median survival for the 11 grade III glioma patients who received adjuvant-pretargeted therapy could not be estimated, since only two had died at the time the report was filed [253].
Encouraging preclinical studies using a streptavidin- or a bs mAb-pretargeting procedure, targeting CD20 in NHL xenografts, have been reported [257]. It appears that an anti-CD45–streptavidin fusion protein is also being developed, primarily in a transplant setting, since this antigen is more broadly expressed in blood cells than CD20s restricted presence on B cells [258]. Our group has also demonstrated that administering an anti-CD20 antibody therapy after RAIT, or pretargeted RAIT, improves responses [86]. Thus, with its ability to exert better responses with much less hematologic toxicity, pretargeting could represent a significant improvement over the existing approved, directly radiolabeled, anti-CD20 IgG agents.
We are now embarking on clinical studies with newly engineered, humanized, recombinant tri-Fab bs mAbs that bind divalently to a tumor antigen, and with the added specificity for binding the HSG hapten (Figure 3) [259]. Preclinical studies have found that these recombinant bs mAbs, prepared using a unique dock and lock technique, to be excellent pretargeting agents. For example, for imaging, small animal imaging studies showed pretargeting with an 124I-labeled di-HSG-peptide revealed very small (0.3 mm) nodules of a human colonic tumor disseminated in the lungs of nude mice, while 18F-fluor-deoxyglucose (18F-FDG) failed to disclose these lesions [245]. Specificity is also improved, with animal studies showing a bs mAb pretargeting procedure does not localize an inflammatory lesion, but will target a tumor in the same animal, while 18F-FDG shows uptake in both lesions [243]. Pretargeting’s low background activity is also better suited for specific detection of tumors in the peritoneal cavity [260]. As previously mentioned, therapeutically we found pretargeting has an advantage over direct targeting in models of colorectal and pancreatic cancers, as well as NHL [246,249,261]. Initial clinical studies found that these novel tri-Fab bs mAbs are cleared very quickly from the blood, despite their similar molecular size to an IgG [262]. Data suggested the bs mAb was stable in vivo, so their rapid clearance is probably related to lacking the Fc portion of the antibody. The bs mAb localized in the tumor, and initial clinical results with the radiolabeled hapten-peptide have shown targeting of known tumor masses even though the procedure has not yet been optimized.
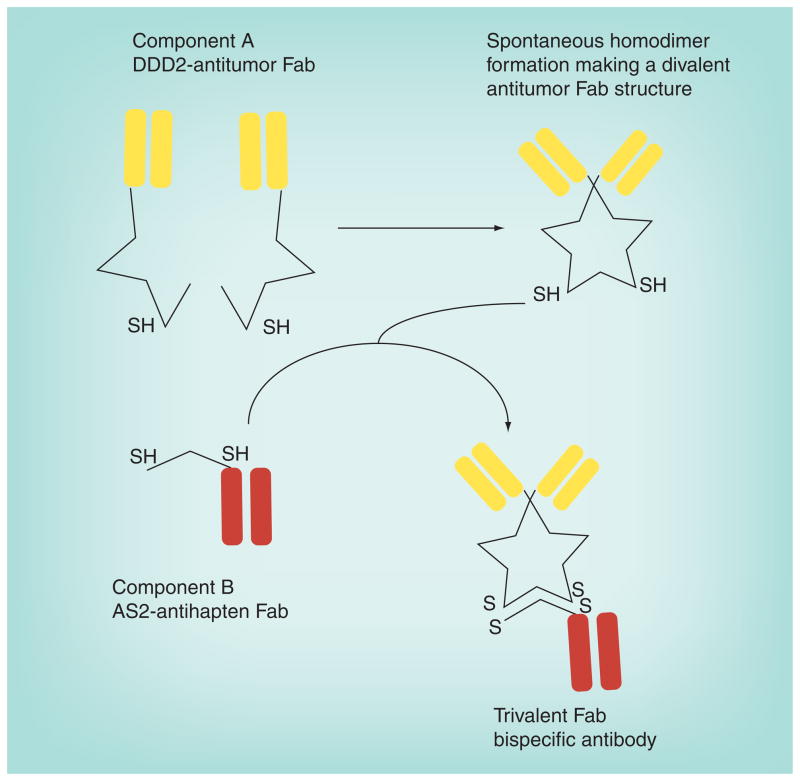
Figure 3. A humanized recombinant bispecific antibody formed by the ‘dock and lock’ method.
The bs mAb consists of two proteins. The first is a Fab, specific for a tumor antigen, modified with a peptide structure known as the DDD, which was modified to introduce a cysteine residue in a strategic location (DDD2). The DDD2 residues have a natural affinity for binding to each other to form homodimers, resulting in a divalent Fab structure. The second protein is the Fab modified with an anchoring domain (AD) that will specifically dock within the dimerized DDD2 structure. The AD segment also had cysteine residues strategically placed so that when the docking occurs, the cysteine residues on the DDD2 and AD2 will lock into place.
bs mAb: Bispecific monoclonal antibody; DDD: Dimerization docking domain. Adapted from [259].
Conclusion & future perspective
Radioimmunotherapy is a promising modality, with well-documented preclinical studies attesting to its clinical prospects in numerous indications. Clinical studies have been encouraging in NHL, but the slow adoption of the first RAIT products is disconcerting, although there are encouraging studies from Europe indicating that RAIT can be effectively integrated in a multimodality approach. There are also encouraging developments with new therapeutics for lymphoma that combine RAIT and effective antibody therapy in a manner that could enhance the overall response and duration without affecting toxicity. New therapies with α-emitters may still provide greatly needed improvement in the treatment of leukemias.
Integrating RAIT into the treatment of solid tumors will be more challenging, but there are a number of promising developments. Using antibodies that have therapeutic activity could improve efficacy, but preclinical data have emphasized that RAIT would be best utilized in situations where the extent of disease is minimal, even micrometastatic, or localized. RAIT is well suited for these situations since, except for manageable hematologic toxicity, it is well tolerated, and the treatment is not given over a protracted period of time, as are most chemotherapy regimens. Programs evaluating localized treatment of brain cancers are well advanced and have shown encouraging results. However, there have been equally encouraging results in using RAIT as an adjuvant treatment following surgical resection of hepatic metastases in colorectal cancer, but early results from clinical studies with fractionated RAIT combined with gemcitabine have shown surprising responses even in patients with advanced pancreatic cancer.
While there continues to be some questions concerning long-term safety issues for RAIT, data in NHL patients have upheld the view that RAIT has no higher risks than currently used chemotherapy regimens. Thus, in our view, RAIT remains a viable treatment option for cancer, and there are even interesting new developments for treating various forms of infectious disease that should be considered [263–269].
Radioimmunotherapy is one of the few treatment modalities where the deposition of the therapeutic can be readily detected and measured in tumors and tissues. Thus, molecular imaging to select and monitor patients can be adapted for most agents. While tumors are often readily detected, there are still difficulties in predicting responses based on dosimetry and radiobiological models. Improving these basic understandings would provide RAIT with an essential advantage if these models could better predict toxicity and efficacy. We need to develop a better understanding of how to best administer these treatments (single or fractionated), how often they can be administered (multiple cycles) and how can they be best integrated with other agents to enhance the overall response. This undertaking is formidable, requiring considerable preclinical and clinical testing to refine these models, which would probably vary for different cancers and treatments.
New molecularly engineered antibodies may provide a better balance in achieving high tumor accretion with reduced tissue exposure. Currently, preclinical studies have indicated that pretargeting strategies can boost efficacy by optimizing tumor uptake, while greatly minimizing normal tissue exposure, which could enhance responses in hematologic and nonhematologic malignancies. By reducing radiation exposure to the normal tissues, pretargeting may be better poised to be combined with other treatment modalities. Whether the added complexity will be worth the effort is an issue resolved only by additional clinical testing.
Radioimmunotherapy is truly a multidisciplinary technology, requiring the skills of medical, surgical and radiation oncologists, nuclear medicine physicians and physicists to coordinate and manage patients undergoing these treatments. While the experience in building a user base for the approved radiolabeled therapeutics in lymphoma has highlighted some difficulties, particularly in the USA, these hurdles can be overcome as treatment outcomes improve. Thus, even though RAIT might be considered the grandfather of many of the advances in molecularly targeted therapeutics over the past 10 years, it remains a promising technology with new opportunities to advance cancer treatment.
Executive summary.
Background
The targeting of therapeutic radionuclides conjugated to antibody (radioimmunotherapy) has a 50-year history for enhancing response over unconjugated antibodies.
Therapeutic radionuclides have different properties that can be used to optimize the response in various clinical situations.
Hematologic malignancies
High sensitivity to radiation has allowed for maximum therapeutic benefit in patients with follicular non-Hodgkin lymphoma.
Therapeutically active unconjugated antibodies probably enhance overall response.
Two radiolabeled antibody products are approved for human use; several other agents are under investigation.
New trials are finding additional advantages when radioimmunotherapy is given for consolidation therapy.
Nonhematologic malignancies
Nonhematologic malignancies are more challenging to treat owing to higher resistance to radiation.
Localized delivery (e.g., intracranial for localized brain cancer or intraperitoneal for ovarian cancer), treatment of residual disease and combination with radiosensitizing chemotherapeutics are promising areas of investigation.
Engineered antibodies are providing new opportunities.
Pretargeted radioimmunotherapy
Pretargeting is a multistep process that separates antibody targeting from the radionuclide targeting.
Tumor uptake that can sometimes rival that of an IgG but is capable of reaching maximum accretion within 1 h, while substantially minimizing tissue and red marrow exposure, is a hallmark of all pretargeting procedures.
Bispecific antibodies with radiolabeled peptides and streptavidin conjugates/fusion proteins with radiolabeled biotin have been examined clinically with promising initial results.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
David Goldenberg is employed as an officer and is a stockholder of Immunomedics, Inc., which develops RAIT products. Robert Sharkey and David Goldenberg are supported from NIH grants P01 CA103985 and R01 CA107088. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
- 1.Grossbard ML, Press OW, Appelbaum FR, Bernstein ID, Nadler LM. Monoclonal antibody-based therapies of leukemia and lymphoma. Blood. 1992;80(4):863–878. [PubMed] [Google Scholar]
- 2.Sharkey RM, Burton J, Goldenberg DM. Radioimmunotherapy of non-Hodgkin’s lymphoma: a critical appraisal. Expert Rev Clin Immunol. 2005;1(1):47–62. doi: 10.1586/1744666X.1.1.47. [DOI] [PubMed] [Google Scholar]
- 3.Sharkey RM, Goldenberg DM. Perspectives on cancer therapy with radiolabeled monoclonal antibodies. J Nucl Med. 2005;46(Suppl 1):S115–S127. [PubMed] [Google Scholar]
- 4.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10(5):317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson AL, Dhimolea E, Reichert JM. Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov. 2010;9(10):767–774. doi: 10.1038/nrd3229. [DOI] [PubMed] [Google Scholar]
- 6.Reichert JM. Antibodies to watch in 2010. MAbs. 2010;2(1):84–100. doi: 10.4161/mabs.2.1.10677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pressman D, Korngold L. The in vivo localization of anti-Wagner-osteogenic-sarcoma antibodies. Cancer. 1953;6(3):619–623. doi: 10.1002/1097-0142(195305)6:3<619::aid-cncr2820060319>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 8.Goldenberg DM, DeLand F, Kim E, et al. Use of radiolabeled antibodies to carcinoembryonic antigen for the detection and localization of diverse cancers by external photoscanning. N Engl J Med. 1978;298(25):1384–1386. doi: 10.1056/NEJM197806222982503. [DOI] [PubMed] [Google Scholar]
- 9▪.Goldenberg DM, Sharkey RM. Radioactive antibodies: a historical review of selective targeting and treatment of cancer. Hosp Pract. 2010;38(4):82–93. doi: 10.3810/hp.2010.06.300. Overview of the progress in radioimmunotherapy (RAIT) [DOI] [PubMed] [Google Scholar]
- 10.Goldenberg DM, Gaffar SA, Bennett SJ, Beach JL. Experimental radioimmunotherapy of a xenografted human colonic tumor (GW-39) producing carcinoembryonic antigen. Cancer Res. 1981;41(11 Pt 1):4354–4360. [PubMed] [Google Scholar]
- 11.Order SE, Klein JL, Ettinger D, et al. Phase I–II study of radiolabeled antibody integrated in the treatment of primary hepatic malignancies. Int J Radiat Oncol Biol Phys. 1980;6(6):703–710. doi: 10.1016/0360-3016(80)90226-6. [DOI] [PubMed] [Google Scholar]
- 12.Order SE, Klein JL, Ettinger D, et al. Use of isotopic immunoglobulin in therapy. Cancer Res. 1980;40(8 Pt 2):3001–3007. [PubMed] [Google Scholar]
- 13.Larson SM, Carrasquillo JA, Krohn KA, et al. Localization of 131I-labeled p97-specific Fab fragments in human melanoma as a basis for radiotherapy. J Clin Invest. 1983;72:2101–2114. doi: 10.1172/JCI111175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gansow OA, Brechbiel MW, Mirzadeh S, Colcher D, Roselli M. Chelates and antibodies: current methods and new directions. Cancer Treat Res. 1990;51:153–171. doi: 10.1007/978-1-4613-1497-4_7. [DOI] [PubMed] [Google Scholar]
- 15.Moi MK, DeNardo SJ, Meares CF. Stable bifunctional chelates of metals used in radiotherapy. Cancer Res. 1990;50(Suppl 3):S789–S793. [PubMed] [Google Scholar]
- 16.DeNardo SJ, DeNardo GL, O’Grady LF, et al. Treatment of B cell malignancies with 131I Lym-1 monoclonal antibodies. Int J Cancer Suppl. 1988;3:96–101. [PubMed] [Google Scholar]
- 17.DeNardo SJ, DeNardo GL, O’Grady LF, et al. Treatment of a patient with B cell lymphoma by I-131 LYM-1 monoclonal antibodies. Int J Biol Markers. 1987;2(1):49–53. doi: 10.1177/172460088700200107. [DOI] [PubMed] [Google Scholar]
- 18.Press OW, Eary JF, Badger CC, et al. Treatment of refractory non-Hodgkin’s lymphoma with radiolabeled MB-1 (anti-CD37) antibody. J Clin Oncol. 1989;7(8):1027–1038. doi: 10.1200/JCO.1989.7.8.1027. [DOI] [PubMed] [Google Scholar]
- 19.Bernstein ID, Eary JF, Badger CC, et al. High dose radiolabeled antibody therapy of lymphoma. Cancer Res. 1990;50(Suppl 3):S1017–S1021. [PubMed] [Google Scholar]
- 20.Goldenberg DM, Horowitz JA, Sharkey RM, et al. Targeting, dosimetry, and radioimmunotherapy of B-cell lymphomas with iodine-131-labeled LL2 monoclonal antibody. J Clin Oncol. 1991;9(4):548–564. doi: 10.1200/JCO.1991.9.4.548. [DOI] [PubMed] [Google Scholar]
- 21.Kaminski MS, Zasadny KR, Francis IR, et al. Radioimmunotherapy of B-cell lymphoma with 131I-anti-B1 (anti-CD20) antibody. N Engl J Med. 1993;329(7):459–465. doi: 10.1056/NEJM199308123290703. [DOI] [PubMed] [Google Scholar]
- 22.Press OW, Eary JF, Appelbaum FR, et al. Radiolabeled-antibody therapy of B-cell lymphoma with autologous bone marrow support. N Engl J Med. 1993;329(17):1219–1224. doi: 10.1056/NEJM199310213291702. [DOI] [PubMed] [Google Scholar]
- 23.Wahl RL. Tositumomab and 131I therapy in non-Hodgkin’s lymphoma. J Nucl Med. 2005;46(Suppl 1):S128–S140S. [PubMed] [Google Scholar]
- 24.Knox SJ, Goris ML, Trisler K, et al. Yttrium-90-labeled anti-CD20 monoclonal antibody therapy of recurrent B-cell lymphoma. Clin Cancer Res. 1996;2(3):457–470. [PubMed] [Google Scholar]
- 25.Witzig TE, White CA, Wiseman GA, et al. Phase I/II trial of IDEC-Y2B8 radioimmunotherapy for treatment of relapsed or refractory CD20+ B-cell non-Hodgkin’s lymphoma. J Clin Oncol. 1999;17(12):3793–3803. doi: 10.1200/JCO.1999.17.12.3793. [DOI] [PubMed] [Google Scholar]
- 26.Kaminski MS, Zasadny KR, Francis IR, et al. Iodine-131-anti-B1 radioimmunotherapy for B-cell lymphoma. J Clin Oncol. 1996;14(7):1974–1981. doi: 10.1200/JCO.1996.14.7.1974. [DOI] [PubMed] [Google Scholar]
- 27.Buchsbaum DJ, Wahl RL, Glenn SD, Normolle DP, Kaminski MS. Improved delivery of radiolabeled anti-B1 monoclonal antibody to Raji lymphoma xenografts by predosing with unlabeled anti-B1 monoclonal antibody. Cancer Res. 1992;52(3):637–642. [PubMed] [Google Scholar]
- 28.McLaughlin P, Grillo-Lopez AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16(8):2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 29.Koral KF, Dewaraja Y, Clarke LA, et al. Tumor-absorbed-dose estimates versus response in tositumomab therapy of previously untreated patients with follicular non-Hodgkin’s lymphoma: preliminary report. Cancer Biother Radiopharm. 2000;15(4):347–355. doi: 10.1089/cbr.2000.15.347. [DOI] [PubMed] [Google Scholar]
- 30.Sgouros G, Squeri S, Ballangrud AM, et al. Patient-specific, 3-dimensional dosimetry in non-Hodgkin’s lymphoma patients treated with 131I-anti-B1 antibody: assessment of tumor dose-response. J Nucl Med. 2003;44(2):260–268. [PubMed] [Google Scholar]
- 31.Sharkey RM, Brenner A, Burton J, et al. Radioimmunotherapy of non-Hodgkin’s lymphoma with 90Y-DOTA humanized anti-CD22 IgG (90Y-epratuzumab): do tumor targeting and dosimetry predict therapeutic response? J Nucl Med. 2003;44(12):2000–2018. [PubMed] [Google Scholar]
- 32.Koral KF, Dewaraja Y, Li J, et al. Update on hybrid conjugate-view SPECT tumor dosimetry and response in 131I-tositumomab therapy of previously untreated lymphoma patients. J Nucl Med. 2003;44(3):457–464. [PubMed] [Google Scholar]
- 33.Dewaraja YK, Schipper MJ, Roberson PL, et al. 131I-tositumomab radioimmunotherapy: initial tumor dose-response results using 3-dimensional dosimetry including radiobiologic modeling. J Nucl Med. 2010;51(7):1155–1162. doi: 10.2967/jnumed.110.075176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34▪.Witzig TE, Gordon LI, Cabanillas F, et al. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphoma. J Clin Oncol. 2002;20(10):2453–2463. doi: 10.1200/JCO.2002.11.076. First randomized clinical trial to demonstrate the superior antitumor effects of radiolabeled anti-CD20 antibody to rituximab, leading to the approval of 90Y-ibritumomab tiuxetan. [DOI] [PubMed] [Google Scholar]
- 35.Davis TA, Kaminski MS, Leonard JP, et al. The radioisotope contributes significantly to the activity of radioimmunotherapy. Clin Cancer Res. 2004;10(23):7792–7798. doi: 10.1158/1078-0432.CCR-04-0756. [DOI] [PubMed] [Google Scholar]
- 36.Cardarelli PM, Quinn M, Buckman D, et al. Binding to CD20 by anti-B1 antibody or F(ab′)2 is sufficient for induction of apoptosis in B-cell lines. Cancer Immunol Immunother. 2002;51(1):15–24. doi: 10.1007/s00262-001-0247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du Y, Honeychurch J, Cragg MS, et al. Antibody-induced intracellular signaling works in combination with radiation to eradicate lymphoma in radioimmunotherapy. Blood. 2004;103(4):1485–1494. doi: 10.1182/blood-2003-06-2037. [DOI] [PubMed] [Google Scholar]
- 38.Hernandez MC, Knox SJ. Radiobiology of radioimmunotherapy: targeting CD20 B-cell antigen in non-Hodgkin’s lymphoma. Int J Radiat Oncol Biol Phys. 2004;59(5):1274–1287. doi: 10.1016/j.ijrobp.2004.02.065. [DOI] [PubMed] [Google Scholar]
- 39.Kapadia NS, Engles JM, Wahl RL. In vitro evaluation of radioprotective and radiosensitizing effects of rituximab. J Nucl Med. 2008;49(4):674–678. doi: 10.2967/jnumed.107.043752. [DOI] [PubMed] [Google Scholar]
- 40.Ivanov A, Krysov S, Cragg MS, Illidge T. Radiation therapy with tositumomab (B1) anti-CD20 monoclonal antibody initiates extracellular signal-regulated kinase/mitogen-activated protein kinase-dependent cell death that overcomes resistance to apoptosis. Clin Cancer Res. 2008;14(15):4925–4934. doi: 10.1158/1078-0432.CCR-07-5072. [DOI] [PubMed] [Google Scholar]
- 41.Witzig TE, Molina A, Gordon LI, et al. Long-term responses in patients with recurring or refractory B-cell non-Hodgkin lymphoma treated with yttrium90 ibritumomab tiuxetan. Cancer. 2007;109(9):1804–1810. doi: 10.1002/cncr.22617. [DOI] [PubMed] [Google Scholar]
- 42.Buchegger F, Antonescu C, Delaloye AB, et al. Long-term complete responses after 131I-tositumomab therapy for relapsed or refractory indolent non-Hodgkin’s lymphoma. Br J Cancer. 2006;94(12):1770–1776. doi: 10.1038/sj.bjc.6603166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gopal AK, Gooley TA, Maloney DG, et al. High-dose radioimmunotherapy versus conventional high-dose therapy and autologous hematopoietic stem cell transplantation for relapsed follicular non-Hodgkin lymphoma: a multivariable cohort analysis. Blood. 2003;102(7):2351–2357. doi: 10.1182/blood-2003-02-0622. [DOI] [PubMed] [Google Scholar]
- 44.Bennett JM, Kaminski MS, Leonard JP, et al. Assessment of treatment-related myelodysplastic syndromes and acute myeloid leukemia in patients with non-Hodgkin lymphoma treated with tositumomab and iodine I131 tositumomab. Blood. 2005;105(12):4576–4582. doi: 10.1182/blood-2004-12-4690. [DOI] [PubMed] [Google Scholar]
- 45.Czuczman MS, Emmanouilides C, Darif M, et al. Treatment-related myelodysplastic syndrome and acute myelogenous leukemia in patients treated with ibritumomab tiuxetan radioimmunotherapy. J Clin Oncol. 2007;25(27):4285–4292. doi: 10.1200/JCO.2006.09.2882. [DOI] [PubMed] [Google Scholar]
- 46.Ansell SM, Schilder RJ, Pieslor PC, et al. Antilymphoma treatments given subsequent to yttrium90 ibritumomab tiuxetan are feasible in patients with progressive non-Hodgkin’s lymphoma: a review of the literature. Clin Lymphoma. 2004;5(3):202–204. doi: 10.3816/clm.2004.n.028. [DOI] [PubMed] [Google Scholar]
- 47▪.Kaminski MS, Tuck M, Estes J, et al. 131I-tositumomab therapy as initial treatment for follicular lymphoma. N Engl J Med. 2005;352(5):441–449. doi: 10.1056/NEJMoa041511. The only clinical trial using RAIT as a frontline treatment for non-Hodgkin lymphoma. [DOI] [PubMed] [Google Scholar]
- 48.Kaminski MS, Estes J, Zasadny KR, et al. Radioimmunotherapy with iodine 131I tositumomab for relapsed or refractory B-cell non-Hodgkin lymphoma: updated results and long-term follow-up of the University of Michigan experience. Blood. 2000;96(4):1259–1266. [PubMed] [Google Scholar]
- 49.Kaminski MS, Radford JA, Gregory SA, et al. Re-treatment with I-131 tositumomab in patients with non-Hodgkin’s lymphoma who had previously responded to I-131 tositumomab. J Clin Oncol. 2005;23(31):7985–7993. doi: 10.1200/JCO.2005.01.0892. [DOI] [PubMed] [Google Scholar]
- 50.Press OW, Unger JM, Braziel RM, et al. A Phase 2 trial of CHOP chemotherapy followed by tositumomab/iodine I131 tositumomab for previously untreated follicular non-Hodgkin lymphoma: Southwest Oncology Group Protocol S9911. Blood. 2003;102(5):1606–1612. doi: 10.1182/blood-2003-01-0287. [DOI] [PubMed] [Google Scholar]
- 51.Leonard JP, Coleman M, Kostakoglu L, et al. Abbreviated chemotherapy with fludarabine followed by tositumomab and iodine I131 tositumomab for untreated follicular lymphoma. J Clin Oncol. 2005;23(24):5696–5704. doi: 10.1200/JCO.2005.14.803. [DOI] [PubMed] [Google Scholar]
- 52.Press OW, Unger JM, Braziel RM, et al. Phase II trial of CHOP chemotherapy followed by tositumomab/iodine I-131 tositumomab for previously untreated follicular non-Hodgkin’s lymphoma: five-year follow-up of Southwest Oncology Group Protocol S9911. J Clin Oncol. 2006;24(25):4143–4149. doi: 10.1200/JCO.2006.05.8198. [DOI] [PubMed] [Google Scholar]
- 53▪.Morschhauser F, Radford J, Van Hoof A, et al. Phase III trial of consolidation therapy with yttrium-90-ibritumomab tiuxetan compared with no additional therapy after first remission in advanced follicular lymphoma. J Clin Oncol. 2008;26(32):5156–5164. doi: 10.1200/JCO.2008.17.2015. Clinical trial showing the potential utility of a RAIT treatment in consolidation therapy for non-Hodgkin lymphoma. [DOI] [PubMed] [Google Scholar]
- 54.Zinzani PL, Tani M, Fanti S, et al. A Phase II trial of CHOP chemotherapy followed by yttrium90 ibritumomab tiuxetan (Zevalin) for previously untreated elderly diffuse large B-cell lymphoma patients. Ann Oncol. 2008;19(4):769–773. doi: 10.1093/annonc/mdm560. [DOI] [PubMed] [Google Scholar]
- 55.Jacobs SA, Swerdlow SH, Kant J, et al. Phase II trial of short-course CHOP-R followed by 90Y-ibritumomab tiuxetan and extended rituximab in previously untreated follicular lymphoma. Clin Cancer Res. 2008;14(21):7088–7094. doi: 10.1158/1078-0432.CCR-08-0529. [DOI] [PubMed] [Google Scholar]
- 56.Zinzani PL, Tani M, Fanti S, et al. A Phase 2 trial of fludarabine and mitoxantrone chemotherapy followed by yttrium-90 ibritumomab tiuxetan for patients with previously untreated, indolent, nonfollicular, non-Hodgkin lymphoma. Cancer. 2008;112(4):856–862. doi: 10.1002/cncr.23236. [DOI] [PubMed] [Google Scholar]
- 57.Zinzani PL, Tani M, Pulsoni A, et al. Fludarabine and mitoxantrone followed by yttrium-90 ibritumomab tiuxetan in previously untreated patients with follicular non-Hodgkin lymphoma trial: a Phase II non-randomised trial (FLUMIZ) Lancet Oncol. 2008;9(4):352–358. doi: 10.1016/S1470-2045(08)70039-1. [DOI] [PubMed] [Google Scholar]
- 58.Morschhauser F, Dreyling M, Rohatiner A, Hagemeister F, Bischof Delaloye A. Rationale for consolidation to improve progression-free survival in patients with non-Hodgkin’s lymphoma: a review of the evidence. Oncologist. 2009;14(Suppl 2):17–29. doi: 10.1634/theoncologist.2009-S2-17. [DOI] [PubMed] [Google Scholar]
- 59.Hainsworth JD, Spigel DR, Markus TM, et al. Rituximab plus short-duration chemotherapy followed by Yttrium-90 Ibritumomab tiuxetan as first-line treatment for patients with follicular non-Hodgkin lymphoma: a Phase II trial of the Sarah Cannon Oncology Research Consortium. Clin Lymphoma Myeloma. 2009;9(3):223–228. doi: 10.3816/CLM.2009.n.044. [DOI] [PubMed] [Google Scholar]
- 60.Alduaij W, Illidge TM. Radioimmunotherapy: strategies for the future in indolent and aggressive lymphoma. Curr Oncol Rep. 2009;11(5):363–370. doi: 10.1007/s11912-009-0049-8. [DOI] [PubMed] [Google Scholar]
- 61.Burdick MJ, Neumann D, Pohlman B, et al. External beam radiotherapy followed by 90Y-ibritumomab tiuxetan in relapsed or refractory bulky follicular lymphoma. Int J Radiat Oncol Biol Phys. 2010 doi: 10.1016/j.ijrobp.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 62.Link BK, Martin P, Kaminski MS, et al. Cyclophosphamide, vincristine, and prednisone followed by tositumomab and iodine-131-tositumomab in patients with untreated low-grade follicular lymphoma: eight-year follow-up of a multicenter Phase II study. J Clin Oncol. 2010;28(18):3035–3041. doi: 10.1200/JCO.2009.27.8325. [DOI] [PubMed] [Google Scholar]
- 63.Zinzani PL, Rossi G, Franceschetti S, et al. Phase II trial of short-course R-CHOP followed by 90Y-ibritumomab tiuxetan in previously untreated high-risk elderly diffuse large B-cell lymphoma patients. Clin Cancer Res. 2010;16(15):3998–4004. doi: 10.1158/1078-0432.CCR-10-0162. [DOI] [PubMed] [Google Scholar]
- 64.Gopal AK, Rajendran JG, Petersdorf SH, et al. High-dose chemo-radioimmunotherapy with autologous stem cell support for relapsed mantle cell lymphoma. Blood. 2002;99(9):3158–3162. doi: 10.1182/blood.v99.9.3158. [DOI] [PubMed] [Google Scholar]
- 65.Winter JN. Combining yttrium 90-labeled ibritumomab tiuxetan with high-dose chemotherapy and stem cell support in patients with relapsed non-Hodgkin’s lymphoma. Clin Lymphoma. 2004;5(Suppl 1):S22–S26. doi: 10.3816/clm.2004.s.005. [DOI] [PubMed] [Google Scholar]
- 66.Vose JM, Bierman PJ, Enke C, et al. Phase I trial of iodine-131 tositumomab with high-dose chemotherapy and autologous stem-cell transplantation for relapsed non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23(3):461–467. doi: 10.1200/JCO.2005.05.117. [DOI] [PubMed] [Google Scholar]
- 67.Ferrucci PF, Vanazzi A, Grana CM, et al. High activity 90Y-ibritumomab tiuxetan (Zevalin) with peripheral blood progenitor cells support in patients with refractory/resistant B-cell non-Hodgkin lymphomas. Br J Haematol. 2007;139(4):590–599. doi: 10.1111/j.1365-2141.2007.06869.x. [DOI] [PubMed] [Google Scholar]
- 68.Molina A, Krishnan A, Fung H, et al. Use of radioimmunotherapy in stem cell transplantation and posttransplantation: focus on yttrium90 ibritumomab tiuxetan. Curr Stem Cell Res Ther. 2007;2(3):239–248. doi: 10.2174/157488807781696230. [DOI] [PubMed] [Google Scholar]
- 69.Gopal AK, Rajendran JG, Gooley TA, et al. High-dose 131itositumomab (anti-CD20) radioimmunotherapy and autologous hematopoietic stem-cell transplantation for adults ≥ 60 years old with relapsed or refractory B-cell lymphoma. J Clin Oncol. 2007;25(11):1396–1402. doi: 10.1200/JCO.2006.09.1215. [DOI] [PubMed] [Google Scholar]
- 70.Krishnan A, Nademanee A, Fung HC, et al. Phase II trial of a transplantation regimen of yttrium-90 ibritumomab tiuxetan and high-dose chemotherapy in patients with non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26(1):90–95. doi: 10.1200/JCO.2007.11.9248. [DOI] [PubMed] [Google Scholar]
- 71.Shimoni A, Zwas ST, Oksman Y, et al. Ibritumomab tiuxetan (Zevalin) combined with reduced-intensity conditioning and allogeneic stem-cell transplantation (SCT) in patients with chemorefractory non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2008;41(4):355–361. doi: 10.1038/sj.bmt.1705919. [DOI] [PubMed] [Google Scholar]
- 72.Zhang MM, Gopal AK. Radioimmunotherapy-based conditioning regimens for stem cell transplantation. Semin Hematol. 2008;45(2):118–125. doi: 10.1053/j.seminhematol.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Devizzi L, Guidetti A, Tarella C, et al. High-dose yttrium-90-ibritumomab tiuxetan with tandem stem-cell reinfusion: an outpatient preparative regimen for autologous hematopoietic cell transplantation. J Clin Oncol. 2008;26(32):5175–5182. doi: 10.1200/JCO.2008.16.8294. [DOI] [PubMed] [Google Scholar]
- 74.Gisselbrecht C, Vose J, Nademanee A, Gianni AM, Nagler A. Radioimmunotherapy for stem cell transplantation in non-Hodgkin’s lymphoma: in pursuit of a complete response. Oncologist. 2009;14(Suppl 2):41–51. doi: 10.1634/theoncologist.2009-S2-41. [DOI] [PubMed] [Google Scholar]
- 75.Bethge WA, Lange T, Meisner C, et al. Radioimmunotherapy with yttrium-90-ibritumomab tiuxetan as part of a reduced intensity conditioning regimen for allogeneic hematopoietic cell transplantation in patients with advanced non-Hodgkin lymphoma: results of a Phase II study. Blood. 2010;116(10):1795–1802. doi: 10.1182/blood-2010-02-270538. [DOI] [PubMed] [Google Scholar]
- 76.Kang BW, Kim WS, Kim C, et al. Yttrium-90-ibritumomab tiuxetan in combination with intravenous busulfan, cyclophosphamide, and etoposide followed by autologous stem cell transplantation in patients with relapsed or refractory B-cell non-Hodgkin’s lymphoma. Invest New Drugs. 2010;28(4):516–522. doi: 10.1007/s10637-009-9283-z. [DOI] [PubMed] [Google Scholar]
- 77.Turner JH, Martindale AA, Boucek J, Claringbold PG, Leahy MF. 131I-anti CD20 radioimmunotherapy of relapsed or refractory non-Hodgkins lymphoma: a Phase II clinical trial of a nonmyeloablative dose regimen of chimeric rituximab radiolabeled in a hospital. Cancer Biother Radiopharm. 2003;18(4):513–524. doi: 10.1089/108497803322287583. [DOI] [PubMed] [Google Scholar]
- 78.Illidge TM, Bayne M, Brown NS, et al. Phase 1/2 study of fractionated 131I-rituximab in low-grade B-cell lymphoma: the effect of prior rituximab dosing and tumor burden on subsequent radioimmunotherapy. Blood. 2009;113(7):1412–1421. doi: 10.1182/blood-2008-08-175653. [DOI] [PubMed] [Google Scholar]
- 79.DeNardo GL, DeNardo SJ, Lamborn KR, et al. Low-dose, fractionated radioimmunotherapy for B-cell malignancies using 131I-Lym-1 antibody. Cancer Biother Radiopharm. 1998;13(4):239–254. doi: 10.1089/cbr.1998.13.239. [DOI] [PubMed] [Google Scholar]
- 80.O’Donnell RT, Shen S, Denardo SJ, et al. A Phase I study of 90Y-2IT-BAD-Lym-1 in patients with non-Hodgkin’s lymphoma. Anticancer Res. 2000;20(5C):3647–3655. [PubMed] [Google Scholar]
- 81.Matthews DC, Appelbaum FR, Eary JF, et al. Phase I study of 131I-anti-CD45 antibody plus cyclophosphamide and total body irradiation for advanced acute leukemia and myelodysplastic syndrome. Blood. 1999;94(4):1237–1247. [PubMed] [Google Scholar]
- 82.Morschhauser F, Kraeber-Bodere F, Wegener WA, et al. High rates of durable responses with anti-CD22 fractionated radioimmunotherapy: results of a multicenter, Phase I/II study in non-Hodgkin’s lymphoma. J Clin Oncol. 2010;28(23):3709–3716. doi: 10.1200/JCO.2009.27.7863. [DOI] [PubMed] [Google Scholar]
- 83▪.Mattes MJ, Sharkey RM, Karacay H, Czuczman MS, Goldenberg DM. Therapy of advanced B-lymphoma xenografts with a combination of 90Y-anti-CD22 IgG (epratuzumab) and unlabeled anti-CD20 IgG (veltuzumab) Clin Cancer Res. 2008;14(19):6154–6160. doi: 10.1158/1078-0432.CCR-08-0404. Preclinical evidence for improved response by combining radiolabeled anti-CD22 IgG with unconjugated anti-CD20 antibody therapy. [DOI] [PubMed] [Google Scholar]
- 84.Sharkey RM, Press OW, Goldenberg DM. A re-examination of radioimmunotherapy in the treatment of non-Hodgkin lymphoma: prospects for dual-targeted antibody/radioantibody therapy. Blood. 2009;113(17):3891–3895. doi: 10.1182/blood-2008-11-188896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gopal AK, Press OW, Wilbur SM, Maloney DG, Pagel JM. Rituximab blocks binding of radiolabeled anti-CD20 antibodies (Ab) but not radiolabeled anti-CD45 Ab. Blood. 2008;112(3):830–835. doi: 10.1182/blood-2008-01-132142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sharkey RM, Karacay H, Johnson CR, et al. Pretargeted versus directly targeted radioimmunotherapy combined with anti-CD20 antibody consolidation therapy of non-Hodgkin lymphoma. J Nucl Med. 2009;50(3):444–453. doi: 10.2967/jnumed.108.058602. [DOI] [PubMed] [Google Scholar]
- 87.Jurcic JG, Scheinberg DA. Radioimmunotherapy of hematological cancer: problems and progress. Clin Cancer Res. 1995;1(12):1439–1446. [PubMed] [Google Scholar]
- 88.McDevitt MR, Sgouros G, Finn RD, et al. Radioimmunotherapy with α-emitting nuclides. Eur J Nucl Med. 1998;25(9):1341–1351. doi: 10.1007/s002590050306. [DOI] [PubMed] [Google Scholar]
- 89.Nikula TK, McDevitt MR, Finn RD, et al. α-emitting bismuth cyclohexylbenzyl DTPA constructs of recombinant humanized anti-CD33 antibodies: pharmacokinetics, bioactivity, toxicity and chemistry. J Nucl Med. 1999;40(1):166–176. [PubMed] [Google Scholar]
- 90.Sgouros G, Ballangrud AM, Jurcic JG, et al. Pharmacokinetics and dosimetry of an α-particle emitter labeled antibody: 213Bi-HuM195 (anti-CD33) in patients with leukemia. J Nucl Med. 1999;40(11):1935–1946. [PubMed] [Google Scholar]
- 91.Jurcic JG, Larson SM, Sgouros G, et al. Targeted α particle immunotherapy for myeloid leukemia. Blood. 2002;100(4):1233–1239. [PubMed] [Google Scholar]
- 92.Zalutsky MR, Pozzi OR. Radioimmunotherapy with α-particle emitting radionuclides. Q J Nucl Med Mol Imaging. 2004;48(4):289–296. [PubMed] [Google Scholar]
- 93.Mulford DA, Scheinberg DA, Jurcic JG. The promise of targeted α-particle therapy. J Nucl Med. 2005;46(Suppl 1):S199–S204. [PubMed] [Google Scholar]
- 94▪.McDevitt MR, Ma D, Lai LT, et al. Tumor therapy with targeted atomic nanogenerators. Science. 2001;294(5546):1537–1540. doi: 10.1126/science.1064126. Novel α-emitting therapy. [DOI] [PubMed] [Google Scholar]
- 95.Borchardt PE, Yuan RR, Miederer M, McDevitt MR, Scheinberg DA. Targeted actinium-225 in vivo generators for therapy of ovarian cancer. Cancer Res. 2003;63(16):5084–5090. [PubMed] [Google Scholar]
- 96.Yao Z, Zhang M, Garmestani K, et al. Pretargeted α emitting radioimmunotherapy using 213Bi 1,4,7,10-tetraazacyclododecane-N,N′,N″,N‴-tetraacetic acid-biotin. Clin Cancer Res. 2004;10(9):3137–3146. doi: 10.1158/1078-0432.ccr-03-0171. [DOI] [PubMed] [Google Scholar]
- 97.Jaggi JS, Seshan SV, McDevitt MR, et al. Renal tubulointerstitial changes after internal irradiation with α-particle-emitting actinium daughters. J Am Soc Nephrol. 2005;16(9):2677–2689. doi: 10.1681/ASN.2004110945. [DOI] [PubMed] [Google Scholar]
- 98.Jaggi JS, Seshan SV, McDevitt MR, et al. Mitigation of radiation nephropathy after internal α-particle irradiation of kidneys. Int J Radiat Oncol Biol Phys. 2006;64(5):1503–1512. doi: 10.1016/j.ijrobp.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 99.Sofou S, Kappel BJ, Jaggi JS, et al. Enhanced retention of the α-particle-emitting daughters of actinium-225 by liposome carriers. Bioconjug Chem. 2007;18(6):2061–2067. doi: 10.1021/bc070075t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Miederer M, Scheinberg DA, McDevitt MR. Realizing the potential of the actinium-225 radionuclide generator in targeted α particle therapy applications. Adv Drug Deliv Rev. 2008;60(12):1371–1382. doi: 10.1016/j.addr.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Song H, Hobbs RF, Vajravelu R, et al. Radioimmunotherapy of breast cancer metastases with α-particle emitter 225Ac: comparing efficacy with 213Bi and 90Y. Cancer Res. 2009;69(23):8941–8948. doi: 10.1158/0008-5472.CAN-09-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Senekowitsch-Schmidtke R, Schuhmacher C, Becker KF, et al. Highly specific tumor binding of a 213Bi-labeled monoclonal antibody against mutant E-cadherin suggests its usefulness for locoregional α-radioimmunotherapy of diffuse-type gastric cancer. Cancer Res. 2001;61(7):2804–2808. [PubMed] [Google Scholar]
- 103.Elgqvist J, Andersson H, Back T, et al. Therapeutic efficacy and tumor dose estimations in radioimmunotherapy of intraperitoneally growing OVCAR-3 cells in nude mice with (211)At-labeled monoclonal antibody MX35. J Nucl Med. 2005;46(11):1907–1915. [PubMed] [Google Scholar]
- 104▪.Zalutsky MR. Current status of therapy of solid tumors: brain tumor therapy. J Nucl Med. 2005;46(Suppl 1):S151–S156. Overview of localized RAIT to brain cancer. [PubMed] [Google Scholar]
- 105.Andersson H, Cederkrantz E, Back T, et al. Intraperitoneal α-particle radioimmunotherapy of ovarian cancer patients: pharmacokinetics and dosimetry of 211At-MX35 F(ab′)2 – a Phase I study. J Nucl Med. 2009;50(7):1153–1160. doi: 10.2967/jnumed.109.062604. [DOI] [PubMed] [Google Scholar]
- 106.Zalutsky MR, Reardon DA, Akabani G, et al. Clinical experience with α-particle emitting 211At: treatment of recurrent brain tumor patients with 211At-labeled chimeric antitenascin monoclonal antibody 81C6. J Nucl Med. 2008;49(1):30–38. doi: 10.2967/jnumed.107.046938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Singh Jaggi J, Henke E, Seshan SV, et al. Selective α-particle mediated depletion of tumor vasculature with vascular normalization. PLoS One. 2007;2(3):E267. doi: 10.1371/journal.pone.0000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kennel SJ, Chappell LL, Dadachova K, et al. Evaluation of 225Ac for vascular targeted radioimmunotherapy of lung tumors. Cancer Biother Radiopharm. 2000;15(3):235–244. doi: 10.1089/108497800414329. [DOI] [PubMed] [Google Scholar]
- 109.Kennel SJ, Mirzadeh S, Eckelman WC, et al. Vascular-targeted radioimmunotherapy with the α-particle emitter 211At. Radiat Res. 2002;157(6):633–641. doi: 10.1667/0033-7587(2002)157[0633:vtrwta]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 110.Kennel SJ, Huang Y, Zeng WB, Stuckey A, Wall J. Kinetics of vascular targeted monoclonal antibody. Curr Drug Deliv. 2010;7(5):428–435. doi: 10.2174/156720110793566227. [DOI] [PubMed] [Google Scholar]
- 111.Zhu X, Palmer MR, Makrigiorgos GM, Kassis AI. Solid-tumor radionuclide therapy dosimetry: new paradigms in view of tumor microenvironment and angiogenesis. Med Phys. 2010;37(6):2974–2984. doi: 10.1118/1.3431999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mattes MJ. The mechanism of killing of B-lymphoma cells by 111In-conjugated antibodies. Int J Radiat Biol. 2008;84(5):389–399. doi: 10.1080/09553000801998867. [DOI] [PubMed] [Google Scholar]
- 113.Mattes MJ. Radionuclide-antibody conjugates for single-cell cytotoxicity. Cancer. 2002;94(Suppl 4):1215–1223. doi: 10.1002/cncr.10288. [DOI] [PubMed] [Google Scholar]
- 114.Ong GL, Elsamra SE, Goldenberg DM, Mattes MJ. Single-cell cytotoxicity with radiolabeled antibodies. Clin Cancer Res. 2001;7(1):192–201. [PubMed] [Google Scholar]
- 115.Michel RB, Brechbiel MW, Mattes MJ. A comparison of 4 radionuclides conjugated to antibodies for single-cell kill. J Nucl Med. 2003;44(4):632–640. [PubMed] [Google Scholar]
- 116.Mattes MJ, Goldenberg DM. Therapy of human carcinoma xenografts with antibodies to EGFr and HER-2 conjugated to radionuclides emitting low-energy electrons. Eur J Nucl Med Mol Imaging. 2008;35(7):1249–1258. doi: 10.1007/s00259-008-0731-3. [DOI] [PubMed] [Google Scholar]
- 117.Tempero M, Leichner P, Dalrymple G, et al. High-dose therapy with iodine-131-labeled monoclonal antibody CC49 in patients with gastrointestinal cancers: a Phase I trial. J Clin Oncol. 1997;15(4):1518–1528. doi: 10.1200/JCO.1997.15.4.1518. [DOI] [PubMed] [Google Scholar]
- 118.Wong JYC, Chu DZ, Yamauchi DM, et al. A Phase I radioimmunotherapy trial evaluating 90yttrium-labeled anti-carcinoembryonic antigen (CEA) chimeric T84.66 in patients with metastatic CEA-producing malignancies. Clin Cancer Res. 2000;6(10):3855–3863. [PubMed] [Google Scholar]
- 119.Tempero M, Leichner P, Baranowska-Kortylewicz J, et al. High-dose therapy with 90yttrium-labeled monoclonal antibody CC49: a Phase I trial. Clin Cancer Res. 2000;6(8):3095–3102. [PubMed] [Google Scholar]
- 120.Wong JY, Shibata S, Williams LE, et al. A Phase I trial of 90Y-anti-carcinoembryonic antigen chimeric T84.66 radioimmunotherapy with 5-fluorouracil in patients with metastatic colorectal cancer. Clin Cancer Res. 2003;9(16 Pt 1):5842–5852. [PubMed] [Google Scholar]
- 121.Tagawa ST, Beltran H, Vallabhajosula S, et al. Anti-prostate-specific membrane antigen-based radioimmunotherapy for prostate cancer. Cancer. 2010;116(Suppl 4):1075–1083. doi: 10.1002/cncr.24795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Epenetos AA, Hird V, Lambert H, Mason P, Coulter C. Long term survival of patients with advanced ovarian cancer treated with intraperitoneal radioimmunotherapy. Int J Gynecol Cancer. 2000;10(Suppl 1):44–46. doi: 10.1046/j.1525-1438.2000.99510.x. [DOI] [PubMed] [Google Scholar]
- 123.Verheijen RH, Massuger LF, Benigno BB, et al. Phase III trial of intraperitoneal therapy with yttrium-90-labeled HMFG1 murine monoclonal antibody in patients with epithelial ovarian cancer after a surgically defined complete remission. J Clin Oncol. 2006;24(4):571–578. doi: 10.1200/JCO.2005.02.5973. [DOI] [PubMed] [Google Scholar]
- 124.Chen S, Yu L, Jiang C, et al. Pivotal study of iodine-131-labeled chimeric tumor necrosis treatment radioimmunotherapy in patients with advanced lung cancer. J Clin Oncol. 2005;23(7):1538–1547. doi: 10.1200/JCO.2005.06.108. [DOI] [PubMed] [Google Scholar]
- 125.Shapiro WR, Carpenter SP, Roberts K, Shan JS. 131I-chTNT-1/B mAb: tumour necrosis therapy for malignant astrocytic glioma. Expert Opin Biol Ther. 2006;6(5):539–545. doi: 10.1517/14712598.6.5.539. [DOI] [PubMed] [Google Scholar]
- 126.Street HH, Goris ML, Fisher GA, et al. Phase I study of 131I-chimeric(ch) TNT-1/B monoclonal antibody for the treatment of advanced colon cancer. Cancer Biother Radiopharm. 2006;21(3):243–256. doi: 10.1089/cbr.2006.21.243. [DOI] [PubMed] [Google Scholar]
- 127.Shapiro WR, Judy K, Patel SJ, et al. Open-label, dose confirmation, and dosimetry study of interstitial 131I-chTNT-1/B MAb for the treatment of recurrent glioblastoma multiforme (GBM): final results. J Clin Oncol. 2010;28(Suppl 15) Abstract 2039. [Google Scholar]
- 128.Milenic DE, Wong KJ, Baidoo KE, et al. Cetuximab: preclinical evaluation of a monoclonal antibody targeting EGFR for radioimmunodiagnostic and radioimmunotherapeutic applications. Cancer Biother Radiopharm. 2008;23(5):619–631. doi: 10.1089/cbr.2008.0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Niu G, Sun X, Cao Q, et al. Cetuximab-based immunotherapy and radioimmunotherapy of head and neck squamous cell carcinoma. Clin Cancer Res. 2010;16(7):2095–2105. doi: 10.1158/1078-0432.CCR-09-2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rades D, Wolff C, Nadrowitz R, et al. Radioactive EGFR antibody cetuximab in multimodal cancer treatment: stability and synergistic effects with radiotherapy. Int J Radiat Oncol Biol Phys. 2009;75(4):1226–1231. doi: 10.1016/j.ijrobp.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 131.Tijink BM, Laeremans T, Budde M, et al. Improved tumor targeting of anti-epidermal growth factor receptor nanobodies through albumin binding: taking advantage of modular nanobody technology. Mol Cancer Ther. 2008;7(8):2288–2297. doi: 10.1158/1535-7163.MCT-07-2384. [DOI] [PubMed] [Google Scholar]
- 132.Tijink BM, Neri D, Leemans CR, et al. Radioimmunotherapy of head and neck cancer xenografts using 131I-labeled antibody L19-SIP for selective targeting of tumor vasculature. J Nucl Med. 2006;47(7):1127–1135. [PubMed] [Google Scholar]
- 133.Costantini DL, Chan C, Cai Z, Vallis KA, Reilly RM. 111In-labeled trastuzumab (Herceptin) modified with nuclear localization sequences (NLS): an Auger electron-emitting radiotherapeutic agent for HER2/neu-amplified breast cancer. J Nucl Med. 2007;48(8):1357–1368. doi: 10.2967/jnumed.106.037937. [DOI] [PubMed] [Google Scholar]
- 134.Palm S, Enmon RM, Jr, Matei C, et al. Pharmacokinetics and biodistribution of 86Y-trastuzumab for 90Y dosimetry in an ovarian carcinoma model: correlative MicroPET, MRI. J Nucl Med. 2003;44(7):1148–1155. [PubMed] [Google Scholar]
- 135.Tolmachev V, Orlova A, Pehrson R, et al. Radionuclide therapy of HER2-positive microxenografts using a 177Lu-labeled HER2-specific Affibody molecule. Cancer Res. 2007;67(6):2773–2782. doi: 10.1158/0008-5472.CAN-06-1630. [DOI] [PubMed] [Google Scholar]
- 136.Milenic DE, Wong KJ, Baidoo KE, et al. Targeting HER2: a report on the in vitro and in vivo pre-clinical data supporting trastuzumab as a radioimmunoconjugate for clinical trials. MAbs. 2010;2(5):550–564. doi: 10.4161/mabs.2.5.13054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wong JY, Raubitschek A, Yamauchi D, et al. A pretherapy biodistribution and dosimetry study of indium-111-radiolabeled trastuzumab in patients with human epidermal growth factor receptor 2-overexpressing breast cancer. Cancer Biother Radiopharm. 2010;25(4):387–394. doi: 10.1089/cbr.2010.0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Boskovitz A, McLendon RE, Okamura T, et al. Treatment of HER2-positive breast carcinomatous meningitis with intrathecal administration of α-particle-emitting 211At-labeled trastuzumab. Nucl Med Biol. 2009;36(6):659–669. doi: 10.1016/j.nucmedbio.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Krop IE, Beeram M, Modi S, et al. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol. 2010;28(16):2698–2704. doi: 10.1200/JCO.2009.26.2071. [DOI] [PubMed] [Google Scholar]
- 140.Liu Z, Liu Y, Jia B, et al. Epidermal growth factor receptor-targeted radioimmunotherapy of human head and neck cancer xenografts using 90Y-labeled fully human antibody panitumumab. Mol Cancer Ther. 2010;9(8):2297–2308. doi: 10.1158/1535-7163.MCT-10-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hecht JR, Mitchell E, Neubauer MA, et al. Lack of correlation between epidermal growth factor receptor status and response to Panitumumab monotherapy in metastatic colorectal cancer. Clin Cancer Res. 2010;16(7):2205–2213. doi: 10.1158/1078-0432.CCR-09-2017. [DOI] [PubMed] [Google Scholar]
- 142.Meredith RF, Khazaeli MB, Plott WE, et al. Phase II study of dual 131I-labeled monoclonal antibody therapy with interferon in patients with metastatic colorectal cancer. Clin Cancer Res. 1996;2(11):1811–1818. [PubMed] [Google Scholar]
- 143.Pagel JM, Pantelias A, Hedin N, et al. Evaluation of CD20, CD22, and HLA-DR targeting for radioimmunotherapy of B-cell lymphomas. Cancer Res. 2007;67(12):5921–5928. doi: 10.1158/0008-5472.CAN-07-0080. [DOI] [PubMed] [Google Scholar]
- 144.Buchegger F, Roth A, Allal A, et al. Radioimmunotherapy of colorectal cancer liver metastases: combination with radiotherapy. Ann NY Acad Sci. 2000;910:263–269. doi: 10.1111/j.1749-6632.2000.tb06714.x. discussion 269–270. [DOI] [PubMed] [Google Scholar]
- 145.Kinuya S, Yokoyama K, Hiramatsu T, et al. Optimal timing of administration of hyperthermia in combined radioimmunotherapy. Cancer Biother Radiopharm. 2000;15(4):373–379. doi: 10.1089/cbr.2000.15.373. [DOI] [PubMed] [Google Scholar]
- 146.Li XF, Kinuya S, Yokoyama K, et al. Benefits of combined radioimmunotherapy and anti-angiogenic therapy in a liver metastasis model of human colon cancer cells. Eur J Nucl Med Mol Imaging. 2002;29(12):1669–1674. doi: 10.1007/s00259-002-0997-9. [DOI] [PubMed] [Google Scholar]
- 147.Pallela VR, Rao SP, Thakur ML. Interferon-α-2b immunoconjugate for improving immunoscintigraphy and immunotherapy. J Nucl Med. 2000;41(6):1108–1113. [PubMed] [Google Scholar]
- 148.Pedley RB, El-Emir E, Flynn AA, et al. Synergy between vascular targeting agents and antibody-directed therapy. Int J Radiat Oncol Biol Phys. 2002;54(5):1524–1531. doi: 10.1016/s0360-3016(02)03923-8. [DOI] [PubMed] [Google Scholar]
- 149.Kraeber-Bodere F, Bodet-Milin C, Niaudet C, et al. Comparative toxicity and efficacy of combined radioimmunotherapy and antiangiogenic therapy in carcinoembryonic antigen-expressing medullary thyroid cancer xenograft. J Nucl Med. 2010;51(4):624–631. doi: 10.2967/jnumed.109.070714. [DOI] [PubMed] [Google Scholar]
- 150.Meyer T, Gaya AM, Dancey G, et al. A Phase I trial of radioimmunotherapy with 131I-A5B7 anti-CEA antibody in combination with combretastatin-A4-phosphate in advanced gastrointestinal carcinomas. Clin Cancer Res. 2009;15(13):4484–4492. doi: 10.1158/1078-0432.CCR-09-0035. [DOI] [PubMed] [Google Scholar]
- 151.Sharkey RM, Goldenberg DM. Use of antibodies and immunoconjugates for the therapy of more accessible cancers. Adv Drug Deliv Rev. 2008;60(12):1407–1420. doi: 10.1016/j.addr.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rowlinson G, Snook D, Busza A, Epenetos AA. Antibody-guided localization of intraperitoneal tumors following intraperitoneal or intravenous antibody administration. Cancer Res. 1987;47(24 Pt 1):6528–6531. [PubMed] [Google Scholar]
- 153.Wahl RL, Barrett J, Geatti O, et al. The intraperitoneal delivery of radiolabeled monoclonal antibodies: studies on the regional delivery advantage. Cancer Immunol Immunother. 1988;26(3):187–201. doi: 10.1007/BF00199929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Colcher D, Esteban J, Carrasquillo JA, et al. Complementation of intracavitary and intravenous administration of a monoclonal antibody (B72.3) in patients with carcinoma. Cancer Res. 1987;47(15):4218–4224. [PubMed] [Google Scholar]
- 155.Alvarez RD, Partridge EE, Khazaeli MB, et al. Intraperitoneal radioimmunotherapy of ovarian cancer with 177Lu-CC49: a Phase I/II study. Gynecol Oncol. 1997;65(1):94–101. doi: 10.1006/gyno.1996.4577. [DOI] [PubMed] [Google Scholar]
- 156.Meredith RF, Khazaeli MB, Macey DJ, et al. Phase II study of interferon-enhanced 131I-labeled high affinity CC49 monoclonal antibody therapy in patients with metastatic prostate cancer. Clin Cancer Res. 1999;5(Suppl 10):S3254–S3258. [PubMed] [Google Scholar]
- 157.Oei AL, Verheijen RH, Seiden MV, et al. Decreased intraperitoneal disease recurrence in epithelial ovarian cancer patients receiving intraperitoneal consolidation treatment with yttrium-90-labeled murine HMFG1 without improvement in overall survival. Int J Cancer. 2007;120(12):2710–2714. doi: 10.1002/ijc.22663. [DOI] [PubMed] [Google Scholar]
- 158.Andersson H, Elgqvist J, Horvath G, et al. Astatine-211-labeled antibodies for treatment of disseminated ovarian cancer: an overview of results in an ovarian tumor model. Clin Cancer Res. 2003;9(10 Pt 2):S3914–S3921S. [PubMed] [Google Scholar]
- 159.Elgqvist J, Andersson H, Back T, et al. Fractionated radioimmunotherapy of intraperitoneally growing ovarian cancer in nude mice with 211At-MX35 F(ab′)2: therapeutic efficacy and myelotoxicity. Nucl Med Biol. 2006;33(8):1065–1072. doi: 10.1016/j.nucmedbio.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 160.Elgqvist J, Johansson BR, Partheen K, Danielsson A. Tumor cure probability during α-RIT of ovarian cancer with different radiation sensitivity. Anticancer Res. 2010;30(7):2545–2551. [PubMed] [Google Scholar]
- 161.Milenic DE, Garmestani K, Brady ED, et al. α-particle radioimmunotherapy of disseminated peritoneal disease using a 212Pb-labeled radioimmunoconjugate targeting HER2. Cancer Biother Radiopharm. 2005;20(5):557–568. doi: 10.1089/cbr.2005.20.557. [DOI] [PubMed] [Google Scholar]
- 162.Aarts F, Bleichrodt RP, Oyen WJ, Boerman OC. Intracavitary radioimmunotherapy to treat solid tumors. Cancer Biother Radiopharm. 2008;23(1):92–107. doi: 10.1089/cbr.2007.0412. [DOI] [PubMed] [Google Scholar]
- 163.Reardon DA, Akabani G, Coleman RE, et al. Phase II trial of murine 131I-labeled antitenascin monoclonal antibody 81C6 administered into surgically created resection cavities of patients with newly diagnosed malignant gliomas. J Clin Oncol. 2002;20(5):1389–1397. doi: 10.1200/JCO.2002.20.5.1389. [DOI] [PubMed] [Google Scholar]
- 164.Reardon DA, Akabani G, Coleman RE, et al. Salvage radioimmunotherapy with murine iodine-131-labeled antitenascin monoclonal antibody 81C6 for patients with recurrent primary and metastatic malignant brain tumors: Phase II study results. J Clin Oncol. 2006;24(1):115–122. doi: 10.1200/JCO.2005.03.4082. [DOI] [PubMed] [Google Scholar]
- 165.Reardon DA, Quinn JA, Akabani G, et al. Novel human IgG2b/murine chimeric antitenascin monoclonal antibody construct radiolabeled with 131I and administered into the surgically created resection cavity of patients with malignant glioma: Phase I trial results. J Nucl Med. 2006;47(6):912–918. [PubMed] [Google Scholar]
- 166.McLendon RE, Akabani G, Friedman HS, et al. Tumor resection cavity administered iodine-131-labeled antitenascin 81C6 radioimmunotherapy in patients with malignant glioma: neuropathology aspects. Nucl Med Biol. 2007;34(4):405–413. doi: 10.1016/j.nucmedbio.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Akabani G, Cokgor I, Coleman RE, et al. Dosimetry and dose-response relationships in newly diagnosed patients with malignant gliomas treated with iodine-131-labeled anti-tenascin monoclonal antibody 81C6 therapy. Int J Radiat Oncol Biol Phys. 2000;46(4):947–958. doi: 10.1016/s0360-3016(99)00500-3. [DOI] [PubMed] [Google Scholar]
- 168.Reardon DA, Zalutsky MR, Akabani G, et al. A pilot study: 131I-antitenascin monoclonal antibody 81C6 to deliver a 44-Gy resection cavity boost. Neuro Oncol. 2008;10(2):182–189. doi: 10.1215/15228517-2007-053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zalutsky MR, Reardon DA, Pozzi OR, Vaidyanathan G, Bigner DD. Targeted α-particle radiotherapy with 211At-labeled monoclonal antibodies. Nucl Med Biol. 2007;34(7):779–785. doi: 10.1016/j.nucmedbio.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Casaco A, Lopez G, Garcia I, et al. Phase I single-dose study of intracavitary-administered nimotuzumab labeled with 188Re in adult recurrent high-grade glioma. Cancer Biol Ther. 2008;7(3):333–339. doi: 10.4161/cbt.7.3.5414. [DOI] [PubMed] [Google Scholar]
- 171.Kramer K, Kushner BH, Modak S, et al. Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. J Neurooncol. 2010;97(3):409–418. doi: 10.1007/s11060-009-0038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Iwamoto FM, Schwartz J, Pandit-Taskar N, et al. Study of radiolabeled indium-111 and yttrium-90 ibritumomab tiuxetan in primary central nervous system lymphoma. Cancer. 2007;110(11):2528–2534. doi: 10.1002/cncr.23077. [DOI] [PubMed] [Google Scholar]
- 173.Kramer K, Humm JL, Souweidane MM, et al. Phase I study of targeted radioimmunotherapy for leptomeningeal cancers using intra-Ommaya 131-I-3F8. J Clin Oncol. 2007;25(34):5465–5470. doi: 10.1200/JCO.2007.11.1807. [DOI] [PubMed] [Google Scholar]
- 174.Maza S, Kiewe P, Munz DL, et al. First report on a prospective trial with yttrium-90-labeled ibritumomab tiuxetan (Zevalin) in primary CNS lymphoma. Neuro Oncol. 2009;11(4):423–429. doi: 10.1215/15228517-2008-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Doolittle ND, Jahnke K, Belanger R, et al. Potential of chemoimmunotherapy and radioimmunotherapy in relapsed primary central nervous system (CNS) lymphoma. Leuk Lymphoma. 2007;48(9):1712–1720. doi: 10.1080/10428190701493902. [DOI] [PubMed] [Google Scholar]
- 176.Quang TS, Brady LW. Radioimmunotherapy as a novel treatment regimen: 125I-labeled monoclonal antibody 425 in the treatment of high-grade brain gliomas. Int J Radiat Oncol Biol Phys. 2004;58(3):972–975. doi: 10.1016/j.ijrobp.2003.09.096. [DOI] [PubMed] [Google Scholar]
- 177.Li L, Quang TS, Gracely EJ, et al. A Phase II study of anti-epidermal growth factor receptor radioimmunotherapy in the treatment of glioblastoma multiforme. J Neurosurg. 2010;113(2):192–198. doi: 10.3171/2010.2.JNS091211. [DOI] [PubMed] [Google Scholar]
- 178.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 179.Moustakas A, Kreisl TN. New treatment options in the management of glioblastoma multiforme: a focus on bevacizumab. Onco Targets Ther. 2010;3:27–38. doi: 10.2147/ott.s5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Blumenthal RD, Sharkey RM, Haywood L, et al. Targeted therapy of athymic mice bearing GW-39 human colonic cancer micrometastases with 131I-labeled monoclonal antibodies. Cancer Res. 1992;52(21):6036–6044. [PubMed] [Google Scholar]
- 181.Boerman OC, Sharkey RM, Blumenthal RD, Aninipot RL, Goldenberg DM. The presence of a concomitant bulky tumor can decrease the uptake and therapeutic efficacy of radiolabeled antibodies in small tumors. Int J Cancer. 1992;51(3):470–475. doi: 10.1002/ijc.2910510322. [DOI] [PubMed] [Google Scholar]
- 182▪.Liersch T, Meller J, Kulle B, et al. Phase II trial of carcinoembryonic antigen radioimmunotherapy with 131I-labetuzumab after salvage resection of colorectal metastases in the liver: five-year safety and efficacy results. J Clin Oncol. 2005;23(27):6763–6770. doi: 10.1200/JCO.2005.18.622. Clinical trial illustrating the prospects for using RAIT in an adjuvant setting for colorectal cancer. [DOI] [PubMed] [Google Scholar]
- 183.Liersch T, Meller J, Bittrich M, et al. Update of carcinoembryonic antigen radioimmunotherapy with 131I-labetuzumab after salvage resection of colorectal liver metastases: comparison of outcome to a contemporaneous control group. Ann Surg Oncol. 2007;14(9):2577–2590. doi: 10.1245/s10434-006-9328-x. [DOI] [PubMed] [Google Scholar]
- 184.DeNardo SJ, Kukis DL, Kroger LA, et al. Synergy of Taxol and radioimmunotherapy with yttrium-90-labeled chimeric L6 antibody: efficacy and toxicity in breast cancer xenografts. Proc Natl Acad Sci USA. 1997;94(8):4000–4004. doi: 10.1073/pnas.94.8.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kelly MP, Lee FT, Tahtis K, et al. Radioimmunotherapy with α-particle emitting 213Bi-C-functionalized trans-cyclohexyl-diethylenetriaminepentaacetic acid-humanized 3S193 is enhanced by combination with paclitaxel chemotherapy. Clin Cancer Res. 2007;13(18 Pt 2):S5604–S5612. doi: 10.1158/1078-0432.CCR-07-1071. [DOI] [PubMed] [Google Scholar]
- 186.Milenic DE, Garmestani K, Brady ED, et al. Potentiation of high-LET radiation by gemcitabine: targeting HER2 with trastuzumab to treat disseminated peritoneal disease. Clin Cancer Res. 2007;13(6):1926–1935. doi: 10.1158/1078-0432.CCR-06-2300. [DOI] [PubMed] [Google Scholar]
- 187.Costantini DL, Bateman K, McLarty K, Vallis KA, Reilly RM. Trastuzumab-resistant breast cancer cells remain sensitive to the auger electron-emitting radiotherapeutic agent 111In-NLS-trastuzumab and are radiosensitized by methotrexate. J Nucl Med. 2008;49(9):1498–1505. doi: 10.2967/jnumed.108.051771. [DOI] [PubMed] [Google Scholar]
- 188.Al-Ejeh F, Darby JM, Brown MP. Chemotherapy synergizes with radioimmunotherapy targeting La autoantigen in tumors. PLoS One. 2009;4(2):E4630. doi: 10.1371/journal.pone.0004630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kelly MP, Lee ST, Lee FT, et al. Therapeutic efficacy of 177Lu-CHX-A″-DTPA-hu3S193 radioimmunotherapy in prostate cancer is enhanced by EGFR inhibition or docetaxel chemotherapy. Prostate. 2009;69(1):92–104. doi: 10.1002/pros.20856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Blumenthal RD, Leone E, Goldenberg DM. Tumor-specific dose scheduling of bimodal radioimmunotherapy and chemotherapy. Anticancer Res. 2003;23(6C):4613–4619. [PubMed] [Google Scholar]
- 191.DeNardo SJ, Kroger LA, Lamborn KR, et al. Importance of temporal relationships in combined modality radioimmunotherapy of breast carcinoma. Cancer. 1997;80(Suppl 12):2583–2590. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2583::aid-cncr34>3.3.co;2-8. [DOI] [PubMed] [Google Scholar]
- 192.Panjideh H, Da Silva Coelho VC, Dernedde J, et al. Biodistribution and efficacy of 131I-A33scFv::CDy, a recombinant antibody-enzyme protein for colon cancer. Int J Oncol. 2008;32(4):925–930. [PubMed] [Google Scholar]
- 193.Karacay H, Govindan SV, Sharkey RM, Goldenberg DM. Combining antibody-targeted radiation (radioimmunotherapy) and antibody-SN-38 conjugates (ADC) improves pancreatic cancer therapy. Proc Am Assoc Cancer Res. 2010 Abstract 5543. [Google Scholar]
- 194.Sharkey RM, Hajjar G, Yeldell D, et al. A Phase I trial combining high-dose 90Y-labeled humanized anti-CEA monoclonal antibody with doxorubicin and peripheral blood stem cell rescue in advanced medullary thyroid cancer. J Nucl Med. 2005;46(4):620–633. [PubMed] [Google Scholar]
- 195.Forero A, Meredith RF, Khazaeli MB, et al. Phase I study of 90Y-CC49 monoclonal antibody therapy in patients with advanced non-small cell lung cancer: effect of chelating agents and paclitaxel co-administration. Cancer Biother Radiopharm. 2005;20(5):467–478. doi: 10.1089/cbr.2005.20.467. [DOI] [PubMed] [Google Scholar]
- 196.Richman CM, DeNardo SJ, O’Donnell RT, et al. High-dose radioimmunotherapy combined with fixed, low-dose paclitaxel in metastatic prostate and breast cancer by using a MUC-1 monoclonal antibody, m170, linked to indium-111/yttrium-90 via a cathepsin cleavable linker with cyclosporine to prevent human anti-mouse antibody. Clin Cancer Res. 2005;11(16):5920–5927. doi: 10.1158/1078-0432.CCR-05-0211. [DOI] [PubMed] [Google Scholar]
- 197.Pennington K, Guarion MJ, Serafini AN, et al. Multicenter study of radiosensitizing gemcitabine combined with fractionated radioimmunotherapy for repeated treatment cycles in advanced pancreatic cancer. J Clin Oncol. 2009;27(Suppl 15):231. Abstract 4620. [Google Scholar]
- 198.Pennington KL, Guarion MJ, Sheikh A, et al. Repeated treatment cycles of fractionated radioimmunotherapy (RAIT) combined with low-dose radiosensitizing gemcitabine (Gem) in advanced pancreatic cancer (APC). Presented at: 2010 Gastrointestinal Cancer Symposium; Orlando, FL, USA. 22–24 January 2010. [Google Scholar]
- 199.Gulec AA, Cohen SJ, Pennington KL, et al. Treatment of advanced pancreatic carcinoma with 90Y-clivatuzumab tetraxetan (humanized anti-pancreatic mucin antibody, hPAM4): a Phase I dose escalation trial. Clin Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-10-2579. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Colcher D, Pavlinkova G, Beresford G, et al. Pharmacokinetics and biodistribution of genetically-engineered antibodies. Q J Nucl Med. 1998;42(4):225–241. [PubMed] [Google Scholar]
- 201.Milenic DE, Yokota T, Filpula DR, et al. Construction, binding properties, metabolism, and tumor targeting of a single-chain Fv derived from the pancarcinoma monoclonal antibody CC49. Cancer Res. 1991;51(23 Pt 1):6363–6371. [PubMed] [Google Scholar]
- 202.Sharkey RM, Motta-Hennessy C, Pawlyk D, Siegel JA, Goldenberg DM. Biodistribution and radiation dose estimates for yttrium- and iodine-labeled monoclonal antibody IgG and fragments in nude mice bearing human colonic tumor xenografts. Cancer Res. 1990;50(8):2330–2336. [PubMed] [Google Scholar]
- 203.Baxter LT, Jain RK. Transport of fluid and macromolecules in tumors. II. Role of heterogeneous perfusion and lymphatics. Microvasc Res. 1990;40(2):246–263. doi: 10.1016/0026-2862(90)90023-k. [DOI] [PubMed] [Google Scholar]
- 204.Baxter LT, Jain RK. Transport of fluid and macromolecules in tumors. III. Role of binding and metabolism. Microvasc Res. 1991;41(1):5–23. doi: 10.1016/0026-2862(91)90003-t. [DOI] [PubMed] [Google Scholar]
- 205.Primus FJ, Wang RH, Goldenberg DM, Hansen HJ. Localization of human GW-39 tumors in hamsters by radiolabeled heterospecific antibody to carcinoembryonic antigen. Cancer Res. 1973;33(11):2977–2982. [PubMed] [Google Scholar]
- 206▪.Fujimori K, Covell DG, Fletcher JE, Weinstein JN. Modeling analysis of the global and microscopic distribution of immunoglobulin G, F(ab′)2, and Fab in tumors. Cancer Res. 1989;49(20):5656–5663. Binding-site barrier and its impact on antibody penetration in tumors; a concept relevant to all targeted therapies. [PubMed] [Google Scholar]
- 207.Weinstein JN, van Osdol W. Early intervention in cancer using monoclonal antibodies and other biological ligands: micropharmacology and the ‘binding site barrier’. Cancer Res. 1992;52(Suppl 9):S2747–S2751. [PubMed] [Google Scholar]
- 208.Rudnick SI, Adams GP. Affinity and avidity in antibody-based tumor targeting. Cancer Biother Radiopharm. 2009;24(2):155–161. doi: 10.1089/cbr.2009.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Schlom J, Eggensperger D, Colcher D, et al. Therapeutic advantage of high-affinity anticarcinoma radioimmunoconjugates. Cancer Res. 1992;52(5):1067–1072. [PubMed] [Google Scholar]
- 210.Blumenthal RD, Fand I, Sharkey RM, et al. The effect of antibody protein dose on the uniformity of tumor distribution of radioantibodies: an autoradiographic study. Cancer Immunol Immunother. 1991;33(6):351–358. doi: 10.1007/BF01741594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Boerman OC, Sharkey RM, Wong GY, et al. Influence of antibody protein dose on therapeutic efficacy of radioiodinated antibodies in nude mice bearing GW-39 human tumor. Cancer Immunol Immunother. 1992;35(2):127–134. doi: 10.1007/BF01741860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Goldenberg DM, Sharkey RM, Ford E. Anti-antibody enhancement of iodine-131 anti-CEA radioimmunodetection in experimental and clinical studies. J Nucl Med. 1987;28(10):1604–1610. [PubMed] [Google Scholar]
- 213.Martensson L, Nilsson R, Ohlsson T, et al. Reduced myelotoxicity with sustained tumor concentration of radioimmunoconjugates in rats after extracorporeal depletion. J Nucl Med. 2007;48(2):269–276. [PubMed] [Google Scholar]
- 214.Colcher D, Goel A, Pavlinkova G, et al. Effects of genetic engineering on the pharmacokinetics of antibodies. Q J Nucl Med. 1999;43(2):132–139. [PubMed] [Google Scholar]
- 215.Olafsen T, Wu AM. Antibody vectors for imaging. Semin Nucl Med. 2010;40(3):167–181. doi: 10.1053/j.semnuclmed.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216▪.Olafsen T, Kenanova VE, Wu AM. Tunable pharmacokinetics: modifying the in vivo half-life of antibodies by directed mutagenesis of the Fc fragment. Nat Protoc. 2006;1(4):2048–2060. doi: 10.1038/nprot.2006.322. Novel alterations in antibody structure to affect biodistribution and clearance. [DOI] [PubMed] [Google Scholar]
- 217.Behr TM, Memtsoudis S, Sharkey RM, et al. Experimental studies on the role of antibody fragments in cancer radioimmunotherapy: influence of radiation dose and dose rate on toxicity and anti-tumor efficacy. Int J Cancer. 1998;77(5):787–795. doi: 10.1002/(sici)1097-0215(19980831)77:5<787::aid-ijc19>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 218.Slavin-Chiorini DC, Kashmiri SV, Schlom J, et al. Biological properties of chimeric domain-deleted anticarcinoma immunoglobulins. Cancer Res. 1995;55(Suppl 23):S5957–S5967. [PubMed] [Google Scholar]
- 219.Behr TM, Sharkey RM, Sgouros G, et al. Overcoming the nephrotoxicity of radiometal-labeled immunoconjugates: improved cancer therapy administered to a nude mouse model in relation to the internal radiation dosimetry. Cancer. 1997;80(Suppl 12):2591–2610. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2591::aid-cncr35>3.3.co;2-a. [DOI] [PubMed] [Google Scholar]
- 220.Vegt E, de Jong M, Wetzels JF, et al. Renal toxicity of radiolabeled peptides and antibody fragments: mechanisms, impact on radionuclide therapy, and strategies for prevention. J Nucl Med. 2010;51(7):1049–1058. doi: 10.2967/jnumed.110.075101. [DOI] [PubMed] [Google Scholar]
- 221.Geerlings MW. Radionuclides for radioimmunotherapy: criteria for selection. Int J Biol Markers. 1993;8(3):180–186. doi: 10.1177/172460089300800308. [DOI] [PubMed] [Google Scholar]
- 222.Mausner LF, Srivastava SC. Selection of radionuclides for radioimmunotherapy. Med Phys. 1993;20(2 Pt 2):503–509. doi: 10.1118/1.597045. [DOI] [PubMed] [Google Scholar]
- 223.Stein R, Govindan SV, Mattes MJ, et al. Improved iodine radiolabels for monoclonal antibody therapy. Cancer Res. 2003;63(1):111–118. [PubMed] [Google Scholar]
- 224.Reardan DT, Meares CF, Goodwin DA, et al. Antibodies against metal chelates. Nature. 1985;316(6025):265–268. doi: 10.1038/316265a0. [DOI] [PubMed] [Google Scholar]
- 225▪.Le Doussal JM, Martin M, Gautherot E, Delaage M, Barbet J. In vitro and in vivo targeting of radiolabeled monovalent and divalent haptens with dual specificity monoclonal antibody conjugates: enhanced divalent hapten affinity for cell-bound antibody conjugate. J Nucl Med. 1989;30(8):1358–1366. Clinical evaluation of pretargeting for the treatment of medullary thyroid cancer demonstrating survival advantage. [PubMed] [Google Scholar]
- 226▪.Goldenberg DM, Sharkey RM, Paganelli G, Barbet J, Chatal JF. Antibody pretargeting advances cancer radioimmunodetection and radioimmunotherapy. J Clin Oncol. 2006;24(5):823–834. doi: 10.1200/JCO.2005.03.8471. Comprehensive review of pretargeting methods. [DOI] [PubMed] [Google Scholar]
- 227.Hnatowich DJ, Virzi F, Rusckowski M. Investigations of avidin and biotin for imaging applications. J Nucl Med. 1987;28(8):1294–1302. [PubMed] [Google Scholar]
- 228.Axworthy DB, Reno JM, Hylarides MD, et al. Cure of human carcinoma xenografts by a single dose of pretargeted yttrium-90 with negligible toxicity. Proc Natl Acad Sci USA. 2000;97(4):1802–1807. doi: 10.1073/pnas.97.4.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Breitz HB, Weiden PL, Beaumier PL, et al. Clinical optimization of pretargeted radioimmunotherapy with antibody-streptavidin conjugate and 90Y-DOTA-biotin. J Nucl Med. 2000;41(1):131–140. [PubMed] [Google Scholar]
- 230.Knox SJ, Goris ML, Tempero M, et al. Phase II trial of yttrium-90-DOTA-biotin pretargeted by NR-LU-10 antibody/streptavidin in patients with metastatic colon cancer. Clin Cancer Res. 2000;6(2):406–414. [PubMed] [Google Scholar]
- 231.Schultz J, Lin Y, Sanderson J, et al. A tetravalent single-chain antibody-streptavidin fusion protein for pretargeted lymphoma therapy. Cancer Res. 2000;60(23):6663–6669. [PubMed] [Google Scholar]
- 232.Paganelli G, Malcovati M, Fazio F. Monoclonal antibody pretargetting techniques for tumour localization: the avidin-biotin system. International Workshop on Techniques for Amplification of Tumour Targetting. Nucl Med Commun. 1991;12(3):211–234. doi: 10.1097/00006231-199103000-00006. [DOI] [PubMed] [Google Scholar]
- 233.Goldenberg DM, Chang CH, Sharkey RM, et al. Radioimmunotherapy: is avidin-biotin pretargeting the preferred choice among pretargeting methods? Eur J Nucl Med Mol Imaging. 2003;30(5):777–780. doi: 10.1007/s00259-002-1089-6. [DOI] [PubMed] [Google Scholar]
- 234.Paganelli G, Chinol M. Radioimmunotherapy: is avidin-biotin pretargeting the preferred choice among pretargeting methods? Eur J Nucl Med Mol Imaging. 2003;30(5):773–776. doi: 10.1007/s00259-002-1090-0. [DOI] [PubMed] [Google Scholar]
- 235.Liu G, Dou S, Mardirossian G, et al. Successful radiotherapy of tumor in pretargeted mice by 188Re-radiolabeled phosphorodiamidate morpholino oligomer, a synthetic DNA analogue. Clin Cancer Res. 2006;12(16):4958–4964. doi: 10.1158/1078-0432.CCR-06-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Liu G, Dou S, Pretorius PH, et al. Pretargeting CWR22 prostate tumor in mice with MORF-B72.3 antibody and radiolabeled cMORF. Eur J Nucl Med Mol Imaging. 2008;35(2):272–280. doi: 10.1007/s00259-007-0606-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Pagel JM, Orgun N, Hamlin DK, et al. A comparative analysis of conventional and pretargeted radioimmunotherapy of B-cell lymphomas by targeting CD20, CD22, and HLA-DR singly and in combinations. Blood. 2009;113(20):4903–4913. doi: 10.1182/blood-2008-11-187401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Karacay H, Sharkey RM, Rossi EA, et al. A new tri-Fab recombinant bispecific antibody (bsMAb) for pretargeting epithelial cancers: studies with TF12 and 111In-labeled hapten-peptide (IMP 288) in ovarian cancer. J Nucl Med. 2010;51(Suppl 2) Abstract 1148. [Google Scholar]
- 239.Karacay H, McBride WJ, Griffiths GL, et al. Experimental pretargeting studies of cancer with a humanized anti-CEA x murine anti-[In-DTPA] bispecific antibody construct and a 99mTc-/188Re-labeled peptide. Bioconjug Chem. 2000;11(6):842–854. doi: 10.1021/bc0000379. [DOI] [PubMed] [Google Scholar]
- 240.McBride WJ, Zanzonico P, Sharkey RM, et al. Bispecific antibody pretargeting PET (immunoPET) with an 124I-labeled hapten-peptide. J Nucl Med. 2006;47(10):1678–1688. [PubMed] [Google Scholar]
- 241.Janevik-Ivanovska E, Gautherot E, Hillairet de Boisferon M, et al. Bivalent hapten-bearing peptides designed for iodine-131 pretargeted radioimmunotherapy. Bioconjug Chem. 1997;8(4):526–533. doi: 10.1021/bc970083h. [DOI] [PubMed] [Google Scholar]
- 242.McBride WJ, D’Souza CA, Sharkey RM, et al. Improved 18F labeling of peptides with a fluoride–aluminum–chelate complex. Bioconjug Chem. 2010;21(7):1331–1340. doi: 10.1021/bc100137x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Schoffelen R, Sharkey RM, Goldenberg DM, et al. Pretargeted immunopositron emission tomography imaging of carcinoembryonic antigen-expressing tumors with a bispecific antibody and a 68Ga- and 18F-labeled hapten peptide in mice with human tumor xenografts. Mol Cancer Ther. 2010;9(4):1019–1027. doi: 10.1158/1535-7163.MCT-09-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Sharkey RM, Cardillo TM, Rossi EA, et al. Signal amplification in molecular imaging by pretargeting a multivalent, bispecific antibody. Nat Med. 2005;11(11):1250–1255. doi: 10.1038/nm1322. [DOI] [PubMed] [Google Scholar]
- 245.Sharkey RM, Karacay H, Vallabhajosula S, et al. Metastatic human colonic carcinoma: molecular imaging with pretargeted SPECT, PET in a mouse model. Radiology. 2008;246(2):497–507. doi: 10.1148/radiol.2462070229. [DOI] [PubMed] [Google Scholar]
- 246.Karacay H, Brard PY, Sharkey RM, et al. Therapeutic advantage of pretargeted radioimmunotherapy using a recombinant bispecific antibody in a human colon cancer xenograft. Clin Cancer Res. 2005;11(21):7879–7885. doi: 10.1158/1078-0432.CCR-05-1246. [DOI] [PubMed] [Google Scholar]
- 247.Kraeber-Bodere F, Sai-Maurel C, Campion L, et al. Enhanced antitumor activity of combined pretargeted radioimmunotherapy and paclitaxel in medullary thyroid cancer xenograft. Mol Cancer Ther. 2002;1(4):267–274. [PubMed] [Google Scholar]
- 248.Graves SS, Dearstyne E, Lin Y, et al. Combination therapy with pretarget CC49 radioimmunotherapy and gemcitabine prolongs tumor doubling time in a murine xenograft model of colon cancer more effectively than either monotherapy. Clin Cancer Res. 2003;9 (10 Pt 1):3712–3721. [PubMed] [Google Scholar]
- 249.Karacay H, Sharkey RM, Gold DV, et al. Pretargeted radioimmunotherapy of pancreatic cancer xenografts: TF10–90Y-IMP-288 alone and combined with gemcitabine. J Nucl Med. 2009;50(12):2008–2016. doi: 10.2967/jnumed.109.067686. [DOI] [PubMed] [Google Scholar]
- 250.Shen S, Forero A, LoBuglio AF, et al. Patient-specific dosimetry of pretargeted radioimmunotherapy using CC49 fusion protein in patients with gastrointestinal malignancies. J Nucl Med. 2005;46(4):642–651. [PubMed] [Google Scholar]
- 251.Kraeber-Bodere F, Rousseau C, Bodet-Milin C, et al. Targeting, toxicity, and efficacy of 2-step, pretargeted radioimmunotherapy using a chimeric bispecific antibody and 131I-labeled bivalent hapten in a Phase I optimization clinical trial. J Nucl Med. 2006;47(2):247–255. [PubMed] [Google Scholar]
- 252.Paganelli G, Grana C, Chinol M, et al. Antibody-guided three-step therapy for high grade glioma with yttrium-90 biotin. Eur J Nucl Med. 1999;26(4):348–357. doi: 10.1007/s002590050397. [DOI] [PubMed] [Google Scholar]
- 253.Grana C, Chinol M, Robertson C, et al. Pretargeted adjuvant radioimmunotherapy with yttrium-90-biotin in malignant glioma patients: a pilot study. Br J Cancer. 2002;86(2):207–212. doi: 10.1038/sj.bjc.6600047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Mirallie E, Vuillez JP, Bardet S, et al. High frequency of bone/bone marrow involvement in advanced medullary thyroid cancer. J Clin Endocrinol Metab. 2005;90(2):779–788. doi: 10.1210/jc.2004-1500. [DOI] [PubMed] [Google Scholar]
- 255.Chatal JF, Campion L, Kraeber-Bodere F, et al. Survival improvement in patients with medullary thyroid carcinoma who undergo pretargeted anti-carcinoembryonic-antigen radioimmunotherapy: a collaborative study with the French Endocrine Tumor Group. J Clin Oncol. 2006;24(11):1705–1711. doi: 10.1200/JCO.2005.04.4917. [DOI] [PubMed] [Google Scholar]
- 256.Paganelli G, Bartolomei M, Ferrari M, et al. Pre-targeted locoregional radioimmunotherapy with 90Y-biotin in glioma patients: Phase I study and preliminary therapeutic results. Cancer Biother Radiopharm. 2001;16(3):227–235. doi: 10.1089/10849780152389410. [DOI] [PubMed] [Google Scholar]
- 257.Forero A, Weiden PL, Vose JM, et al. Phase 1 trial of a novel anti-CD20 fusion protein in pretargeted radioimmunotherapy for B-cell non-Hodgkin lymphoma. Blood. 2004;104(1):227–236. doi: 10.1182/blood-2003-09-3284. [DOI] [PubMed] [Google Scholar]
- 258.Green DJ, Pagel JM, Nemecek ER, et al. Pretargeting CD45 enhances the selective delivery of radiation to hematolymphoid tissues in nonhuman primates. Blood. 2009;114(6):1226–1235. doi: 10.1182/blood-2009-03-210344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Rossi EA, Goldenberg DM, Cardillo TM, et al. Stably tethered multifunctional structures of defined composition made by the dock and lock method for use in cancer targeting. Proc Natl Acad Sci USA. 2006;103(18):6841–6846. doi: 10.1073/pnas.0600982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Schoffelen R, Franssen G, McBride WJ, et al. Pretargeted immunoPET for imaging human colonic cancer in a mouse model. J Nucl Med. 2010;51(Suppl 2) Abstract 61. [Google Scholar]
- 261.Sharkey RM, Karacay H, Litwin S, et al. Improved therapeutic results by pretargeted radioimmunotherapy of non-Hodgkin’s lymphoma with a new recombinant, trivalent, anti-CD20, bispecific antibody. Cancer Res. 2008;68(13):5282–5290. doi: 10.1158/0008-5472.CAN-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262▪.Sharkey RM, Rossi EA, McBride WJ, Chang CH, Goldenberg DM. Recombinant bispecific monoclonal antibodies prepared by the dock-and-lock strategy for pretargeted radioimmunotherapy. Semin Nucl Med. 2010;40(3):190–203. doi: 10.1053/j.semnuclmed.2009.12.002. Overview of preclinical and clinical studies using novel recombinant bispecific antibody for pretargeting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Dadachova E, Wang XG, Casadevall A. Targeting the virus with radioimmunotherapy in virus-associated cancers. Cancer Biother Radiopharm. 2007;22(3):303–308. doi: 10.1089/cbr.2007.344. [DOI] [PubMed] [Google Scholar]
- 264.Dadachova E. Radioimmunotherapy of infection with Bi-labeled antibodies. Curr Radiopharm. 2008;1(3):234–239. doi: 10.2174/1874471010801030234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Dadachova E, Casadevall A. Host and microbial cells as targets for armed antibodies in the treatment of infectious diseases. Curr Opin Investig Drugs. 2008;9(2):184–188. [PubMed] [Google Scholar]
- 266.Bryan RA, Jiang Z, Huang X, et al. Radioimmunotherapy is effective against high-inoculum Cryptococcus neoformans infection in mice and does not select for radiation-resistant cryptococcal cells. Antimicrob Agents Chemother. 2009;53(4):1679–1682. doi: 10.1128/AAC.01334-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Rivera J, Nakouzi AS, Morgenstern A, et al. Radiolabeled antibodies to Bacillus anthracis toxins are bactericidal and partially therapeutic in experimental murine anthrax. Antimicrob Agents Chemother. 2009;53(11):4860–4868. doi: 10.1128/AAC.01269-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Dadachova E, Casadevall A. Radioimmunotherapy of infectious diseases. Semin Nucl Med. 2009;39(2):146–153. doi: 10.1053/j.semnuclmed.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Bryan RA, Jiang Z, Howell RC, et al. Radioimmunotherapy is more effective than antifungal treatment in experimental cryptococcal infection. J Infect Dis. 2010;202(4):633–637. doi: 10.1086/654813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Kassis AI. Therapeutic radionuclides: biophysical and radiobiologic principles. Semin Nucl Med. 2008;38(5):358–366. doi: 10.1053/j.semnuclmed.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]