Abstract
Background
When inhaling medication, it is essential that drug particles are delivered to all sites of lung inflammation, including the peripheral airways. The aim of this study was to assess the lung deposition and lung distribution of beclomethasone dipropionate (BDP)/formoterol (100/6 μg), both dissolved in hydrofluoroalkane (HFA) and delivered by pressurized metered dose inhaler (pMDI) in healthy subjects, asthmatic, and chronic obstructive pulmonary disease (COPD) patients, to investigate how the in vitro characteristics of the formulation translate into the in vivo performance in diseases with different airway obstruction.
Methods
Healthy volunteers (n = 8), persistent asthmatics (n = 8), and patients with stable COPD (n = 8) completed this open-label, single-dose parallel-group study. Each patient received one single treatment of four puffs of 99 mTc-labeled BDP/formoterol formulation. The correlation between particle size distribution of radioactivity and of the drugs in the radiolabeled formulation was validated. Intra- and extrapulmonary deposition, amount of exhaled drug, and the central to peripheral ratio (C/P) were calculated immediately after inhalation. Patients' lung function and pharmacokinetic parameters were also assessed up to 24 h post-dose.
Results
The average lung deposition of BDP/formoterol was 34.08 ± 9.30% (relative to nominal dose) in healthy subjects, 30.86 ± 8.89% in asthmatics, and 33.10 ± 8.90% in COPD patients. Extrathoracic deposition was 53.48% ± 8.95, 57.64% ± 9.92 and 54.98% ± 7.01, respectively. C/P ratios of 1.42 ± 0.32 in healthy subjects, 1.96 ± 0.43 in asthmatics, and 1.94 ± 0.69 for COPD patients confirmed drug distribution to all regions of the lungs. Forced expiratory volume in 1 sec (FEV1) increased in all groups after BDP/formoterol inhalation, but was more evident in the patient groups. No significant correlation between baseline lung function and drug deposition was observed. Formoterol, BDP, and beclomethasone 17 monopropionate (B17MP) plasma profiles were comparable between groups.
Conclusion
Inhalation of BDP/formoterol HFA (100/6 μg) produces high and homogeneous deposition of BDP and formoterol in the airways, regardless of pathophysiological condition.
Key words: asthma, beclomethasone dipropionate, chronic obstructive pulmonary disease, extra fine, formoterol, hydrofluoroalkane, lung deposition, small airways
Introduction
Inhaled corticosteroids (ICSs) and long-acting β2-agonists (LABAs) are the pharmacological mainstays for treating obstructive lung disease. Their combination is currently recommended for those asthmatics not adequately controlled on ICS alone,(1) and for chronic obstructive pulmonary disease (COPD) patients with severe disease who suffer from repeated exacerbations.(2) The complementary clinical effects of ICSs and LABAs are well documented, and include improved lung function, symptom control, patient compliance, reduction in exacerbation rate, and risk of therapy discontinuation, compared to either agent administered alone.(3) The rationale for their combined use derives from the hypothesis that ICSs and LABAs can mutually potentiate their effects at the molecular and receptor levels when given together, thus optimizing each other's beneficial actions in the airways.(4)
Inhalation is the preferred route of administration of asthma and COPD medications because it delivers drugs directly into the airways, while minimizing systemic side effects. The pressurized metered dose inhaler (pMDI) is the most frequently prescribed inhaler device. Historically, its major limitations are the relatively large drug particles generated, low lung deposition (10–20%) and the fast-moving aerosol, which necessitates coordination of inspiration and actuation, making it difficult to correctly use the inhaler, and increasing the risk of high deposition of drug in the pharynx.(5–7) However, with technologic improvements in both pMDI formulation and design, the desired aerosol size distribution, spray impact force, and mass of drug available per shot can be achieved.(8) Inhalation of smaller drug particles leads to increased total lung deposition, farther distal airway penetration, and more peripheral lung deposition,(9) which would be beneficial for asthmatic and COPD patients since the peripheral airways are an important site of inflammation in both diseases.(10,11)
An extrafine fixed combination formulation of the ICS beclomethasone dipropionate (BDP) and the LABA formoterol (100/6 μg) both dissolved in a hydrofluoroalkane (HFA) propellant and delivered by pMDI (Foster®, Chiesi Farmaceutici, Italy) has recently been developed using Modulite® technology. This technology enables the manipulation of inhaled HFA-based solution formulations, and has the potential to eliminate many of the limitations associated with pMDI use.(12) In addition, by tailoring the particle size, the Modulite® allows the development of extrafine formulations, replacing existing drugs at a reduced nominal dose.(13) For the same clinical effect, with BDP/formoterol HFA extrafine, the BDP dose is 2.5-fold lower than the conventional BDP chlorofluorocarbon (CFC) product: 400 μg BDP extrafine was clinically equivalent to 1000 μg of the reference BDP CFC formulation.(14,15) Each actuation of BDP/formoterol (100/6 μg) delivers 86.4 μg of BDP and 5 μg of formoterol.(16) In addition, the ratio of the two drugs (mean ratio 17.6) was maintained at each stage of the Anderson Cascade Impactor, suggesting the likelihood of their co-deposition in the lungs.(17)
Although the clinical efficacy of the fixed extrafine combination of BDP/formoterol has been established in a number of studies,(18–20) its lung deposition pattern has never been investigated. The aim of this study was to assess the lung deposition and lung distribution of BDP/formoterol HFA pMDI in healthy subjects, asthmatic, and COPD patients, to investigate how the in vitro characteristics of the formulation translate into the in vivo performance in diseases with different airway obstruction.
Materials and Methods
Study design
This was an open, uncontrolled, non-randomized single-dose study (study number: 2006-005557-30), consisting of one single treatment of four puffs of BDP/formoterol (100/6 μg) HFA combination delivered by pMDI, yielding a total dose of 400 μg BDP and 24 μg formoterol. The primary endpoint was intrapulmonary deposition of BDP/formoterol (% of nominal dose). Secondary endpoints included extrathoracic deposition, amount of BDP/formoterol exhaled, residual drug remaining in the device (all as % of nominal dose), central/peripheral ratio (C/P, an index of regional lung deposition), and variance of pixel counts (VAR, an index of homogeneity of deposition within the lung). Lung function and pharmacokinetic parameters were also assessed. Safety was assessed by documenting all adverse events that occurred during the study. The study was carried out in accordance with the Declaration of Helsinki (1996), the ICH Harmonized Tripartite Guideline for Good Clinical Practice (GCP) and with applicable regulatory requirements. The study protocol was approved by an Independent Ethics Committee (Ethikkommission der Bayerischen Landesärzte-kammer).
Subject selection
Both male and female subjects, without childbearing potential, and the ability to properly use a pMDI were recruited. Healthy and asthmatic subjects were required to be aged 21–70 years and be non-smokers or ex-smokers for at least 1 year (previous smoking history of <5 pack years). Asthmatic patients were required to have moderate persistent or severe persistent asthma;(1) a forced expiratory volume in 1 sec (FEV1) ≥30% and <80% predicted, and an FEV1 reversibility ≥12% and at least 200 mL of the initial value 30 min after inhalation of salbutamol (200 μg) at screening. Patients with stable COPD, aged 40–70 years with a minimum smoking history of 10 pack years were recruited. COPD patients were required to have an FEV11 ≥30% and <50% of predicted values, an FEV1/forced vital capacity (FVC) ≤70% documented at screening visit, and an FEV1 reversibility of <12% of the initial value 30-min postinhalation of salbutamol (200 μg) at screening. All subjects gave their written informed consent.
Subjects were excluded from the study if they had clinically relevant abnormal laboratory values, and clinically significant and uncontrolled cardiac, hepatic, renal, gastrointestinal, endocrine, metabolic, neurologic, or psychiatric disorders. Asthmatic and COPD patients were excluded if they changed the dose or type of asthma/COPD medication within 4 weeks prior to the screening visit, and if they had experienced an exacerbation in the last 4 weeks.
No LABA, long-acting anticholinergic drugs, theophylline, or BDP were allowed 72 h prior to inhalation of study medication. In addition, no β-blockers in the previous 24 h and no inhaled steroids (with the exception of BDP) in the previous 12 h were permitted. No short-acting anticholinergics or β2-agonist drugs were permitted within 8 h prior to inhalation of test medication.
Radiolabeling
The BDP/formoterol combination HFA formulation was labeled with 99 mTechnetium (99 mTc), prior to inhalation. As both drugs are dissolved in solution it was assumed that the radioactivity was evenly distributed between the two drugs. 99 mTechnetium was eluted as sodium pertechnetate (NaTcO4) in saline solution from a commercially available technetium generator (Tyco Healthcare, Germany). Depending on the specific activity of the NaTcO4 solution, a volume of 0.2 to 2 mL was filled into a 20-mL glass vial. Any amount less than 2 mL was filled up to 2 mL with water for injection. A fourfold amount of 3-pentanone was added to extract 99 mTc from the saline solution. After separation of the liquid phases, the 3-pentanone layer was removed and heated in a beaker to approximately 100°C until all the liquid had evaporated. After cooling to room temperature, 400 μL of ethanol was added to the beaker, so that the ethanol could take up the 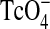 .
.
A canister, cooled down to −80°C (8–24 h), was opened using a spike, and 100 μL of the radioactive ethanol solution placed inside it. The canister was closed with a rivet and a special Viton sealing, and was equilibrated for 30 min to room temperature.
Validation of the labeling procedure
The labeled canisters were analyzed in terms of radioactive output, radioactive particle size distribution, and particle size distribution of drug content. An eight-stage Andersen Cascade Impactor was used to confirm that radiolabeling did not change the particle size distribution of the product. Ten puffs of radiolabeled BDP/formoterol HFA formulation were fired into the impactor. The 99 mTC radioactivity on each impactor stage, on the impactor throat, and in the actuator was measured using a scintillation counter (AM2005; MED, Germany). After radioactive measurement, all plates were washed and the BDP/formoterol amount on each stage was analyzed using high-performance liquid chromatography (HPLC). Additionally, measurement of the unlabeled formulation was performed using a HPLC System with UV detector (Dionex, Germany). The mass median aerodynamic diameter (MMAD), geometric standard deviation (GSD), fine particle fraction (FPF; particles <4.7 μm), and delivered dose (DD), expressed as percentage of the nominal dose, were determined by using the CITDAS evaluation software version 2.0 (Copley Scientific Ltd, UK). On each study day, the HFA spray was analyzed in terms of radioactive output, radioactive particle size distribution, and particle size distribution of the active ingredients. The radioactive data of output and particle size distribution were used for releasing the batch.
Test drug inhalation
In order to control and to standardize the inhalation flow, subjects were trained to use the pMDI properly prior to test drug inhalation, using a placebo inhaler connected to a flow meter, to avoid an inhalation that was either too forceful or too slow. Using this flow meter, subjects were instructed to start a long and deep inhalation with an inspiratory flow of approximately 30 L/min, which corresponds to the inhalation flow during normal breathing. Inhalation of the radiolabeled combination test drug formulation was subsequently performed without measurement of the actual inhalation flow rate. Patients inhaled four shots of BDP/formoterol (100/6 μg) using the breathing pattern they had learned with the placebo inhaler and flow meter. No more than 8 MBq 99 mTC was administered to each subject during the study. At the end of the inspiration, subjects were asked to breath-hold for 10 sec, and then to exhale into an exhalation filter.
Assessment of drug deposition
Immediately after inhalation of the radiolabeled test drug formulation, a planar gamma camera image (posterior) was taken for each subject using a Siemens Diacam gamma camera with a field of view of 53 × 40 cm and a low-energy parallel hole collimator. An 81 mKrypton-ventilation scan was also obtained to define the lung borders and lung fields. Using the regions of interest (ROI) defined from this ventilation scan, the following parameters were measured:
The radioactivity emitted by the inhaler (AI). Prior to the inhalation by each patient, four shots were fired into a filter and radioactivity was determined using a gamma scintillation counter. This radioactivity was defined as the amount of radioactivity the patient had inhaled (AI).
The count rates measured for the lung region (CL).
The count rates measured for the extrathoracic region including oropharynx, trachea, esophagus, and stomach (CET).
The radioactivity on the exhalation filter (AEX).
The radioactivity on the actuator after inhalation (AAC).
From these activity data, the following parameters were calculated:
Absolute activity deposited in the lungs
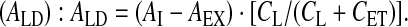
Lung deposition (DL) relative to nominal dose (ex-valve):
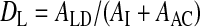 .
.Absolute activity deposited in the extrathoracic region (AETD):
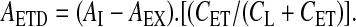 .
.Extrathoracic deposition (DE) relative to nominal dose (ex-valve):
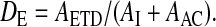 .
.Fraction of activity remaining on the actuator (DAC) relative to nominal dose (ex valve):
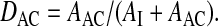 .
.Fraction of exhaled activity (MEX) relative to nominal dose:
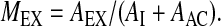 .
.
C/P ratio
For determination of C/P ratio of deposited activity after inhalation, whole lung rectangular ROIs for each lung were drawn at the boundaries of the Krypton ventilation scan (defined at 15% of the peak Krypton counts for the entire lung). Central ROIs, with dimensions equal to half of the whole lung ROIs width and one half of its height, were positioned on the interior boundary of the lung, centered by height so that the central ROI was 25% of the area of the whole lung ROI. The peripheral region (P) was that area lying between the central and whole lung outline. These regions were displayed over the aerosol deposition (99 mTc) and Krypton (Kr) scan to determine the counts in each region. The ratio of C/P counts was then determined and normalized by the C/P ratio for the Krypton scan, (C/P)Kr;
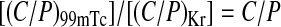 |
for the right and left lung.
This normalization was done to account for the difference in relative lung area and thickness between the central and peripheral regions. While both the central and peripheral regions overlay alveoli and intermediate/small airways, the central region also incorporates large bronchial airways not present in the peripheral region. Therefore, decreases in C/P reflect a decrease in large, bronchial airway deposition relative to intermediate/small bronchi/bronchioles and alveolated airspaces.
As an additional analysis for homogeneity of deposition, the variance of pixel counts (VAR) in the lung (number of pixels vs. counts/pixel) was assessed. Again, using the boundaries of the Krypton scan for each subject (at 15% of the peak Krypton counts for the entire lung), outline ROIs were prepared for the right and left lungs. Within each lung's outline ROI the mean and variance of counts/pixel were determined as the standard deviation (or square root of variance) divided by the mean. As the VAR decreases, homogeneity of deposition within the lung improves. Tissue attenuation correction was performed according to Pitcairn et al.(21) Count rate measured for the lung region (CL) and for the extrathoracic region (CET) were corrected for attenuation.
Attenuation factors for lung (ACFL) and stomach (ACFS) were calculated from the thorax thickness (T) using the following equation:
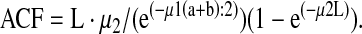 |
For the lung attenuation factor (ACFL) the following parameters were used:
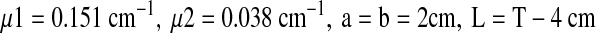 |
For the stomach attenuation factor (ACFS) the parameters were:
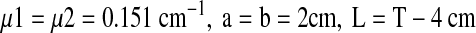 |
Spirometry
All lung function parameters were measured using a Jaeger-Masterlab (Cardinal Health, Würzburg, Germany). The parameters assessed were FEV1, FVC, and mid-expiratory flow (MEF) at 25, 50, and 75% vital capacity: MEF25, MEF50, and MEF75. These parameters were measured at screening and on administration day pre-dose (after at least 10-min rest, with patients sitting and with the nose clipped), and at 15 and 30 min, 1, 2, 4, 6, 8, and 24 h post-dose. For FEV1 and FVC, three technically satisfactory measurements were done for each patient, and the highest value recorded. If consecutive values differed by ≥200 mL, up to eight measurements were made and the largest value reported. For MEF, the values were derived from the best of the three curves (i.e. the greatest sum FEV1 + FVC). Predicted values were calculated according to the formula of the European Coal and Steel Community.
Pharmacokinetic measurements
Blood samples were taken pre-dose and at 15 and 30 min, 1, 1.5, 2, 3, 4, 6, 8, 10, and 24 h after dosing. Plasma was separated by centrifugation at approximately 4°C and 2500 rpm for 15 min and stored at −80°C for formoterol assay and at −20°C for BDP/beclomethasone 17 monopropionate (B17MP) assay. Plasma samples were analyzed for the determination of formoterol, BDP, and B17MP using validated liquid chromatography–tandem mass spectrometry (LC-MS-MS) methods with limit of quantitation (LOQ) of 2 pg/mL for formoterol and 20 pg/mL for BDP and B17MP. The following pharmacokinetic parameters were calculated from the individual plasma drug concentration versus time profiles: maximum plasma concentration (Cmax), time to maximum plasma concentration (tmax), area under the plasma concentration versus time curve observed from 0 to 30 min (AUC0–30 min) and from 0 to 24 h (AUC0–24 h), calculated using the linear trapezoidal rule. AUC0–24 h was used to evaluate the overall exposure to the active ingredients. The AUC0–30 min was considered an index of lung absorption because it is unlikely that significant amounts of the swallowed fraction of drug can reach the systemic circulation in the first 30 min after inhalation. Other authors have previously demonstrated that plasma levels of inhaled drugs measured during the lag phase of oral absorption are indicative of the lung deposition.(22,23) This was also confirmed in a previous study investigating the systemic exposure of BDP/formoterol HFA pMDI before and after charcoal block administration, which is a well-recognized technique able to prevent oral absorption without influencing lung absorption.(24) In that study, similar AUC30 min values were observed before and after charcoal block administration, and plasma concentrations decreased rapidly after the first 30 min post-inhalation with charcoal block as oral absorption of the drug was prevented.(24) Cmax and tmax are indicators of the rate of absorption.
Data analysis
Differences between subject groups for each endpoint parameter were tested by an analysis of variance using a linear model with “group” and “patient” as independent variables, and assuming random effects using the “mixed” SAS procedure. Correlations between baseline lung function and deposition parameters were tested using Spearman rank correlation analysis.
A sample size of 8 subjects to detect differences in lung deposition between groups of approximately 30% (paired t-test), was roughly estimated on the basis of a previous study showing a deposition of formoterol HFA of 35 ± 7% in patients with severe COPD.(25)
Results
Patients
A total of 25 subjects (21 male and 4 female) were recruited into the study. Of these subjects, 8 were healthy (mean age: 46 ± 13 years), 8 were asthmatic (mean age: 51 ± 16 years), and 9 had COPD (mean age 61 ± 7 years). One patient in the COPD group who experienced a moderate ischalgia was discontinued from the study before treatment. Baseline data are presented in Table 1.
Table 1.
Patient Baseline Characteristics
| |
Group |
||
|---|---|---|---|
| Variable | Healthy subjects (n = 8) | Asthma patients (n = 8) | COPD patients (n = 9) |
| Age (years) | 46.13 ± 12.51 | 51.25 ± 16.23 | 61.33 ± 6.78 |
| Height (cm) | 179.38 ± 7.25 | 171.13 ± 9.46 | 172.66 ± 3.61 |
| Weight (kg) | 79.75 ± 8.60 | 73.88 ± 6.33 | 72.88 ± 16.18 |
| PY (years) | 1.50 ± 1.31 | 0.38 ± 1.06 | 52.88 ± 22.49 |
| FEV1 (L) | 4.29 ± 0.83 | 2.35 ± 0.90 | 1.37 ± 0.19 |
| FEV1 (% predicted) | 112.13 ± 11.89 | 70.75 ± 8.33 | 43.67 ± 7.26 |
| MEF25 (L/sec) | 8.25 ± 0.94 | 2.73 ± 1.21 | 1.22 ± 0.46 |
| MEF50 (L/sec) | 4.43 ± 1.10 | 1.51 ± 0.72 | 0.56 ± 0.16 |
| MEF75 (L/sec) | 1.42 ± 0.39 | 0.57 ± 0.36 | 0.23 ± 0.04 |
| FEV1/FVC | 76.50 ± 9.17 | 58.94 ± 5.60 | 42.24 ± 9.17 |
Results are expressed as mean ± standard deviation.
COPD, chronic obstructive pulmonary disease; PY, pack years; FEV1, forced expiratory volume in 1 sec; MEF25, maximal expiratory flow at 25% vital capacity; MEF50, maximal expiratory flow at 50% vital capacity; MEF75, maximal expiratory flow at 75% vital capacity; FVC, forced vital capacity.
Radiolabeling validation
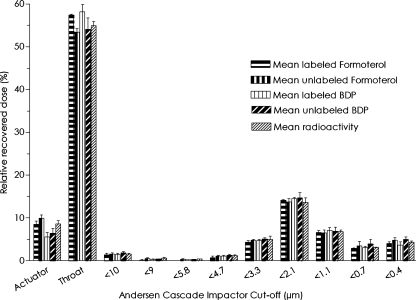
Particle size distributions of both unlabeled and labeled BDP/formoterol formulations were in close agreement. The MMAD, FPF, GSD, and DD for the MDI were similar for the unlabeled and labeled formulations (Table 2 and Fig. 1). The DDs (±standard deviation) of formoterol were 4.86 ± 0.28 μg and 5.01 ± 0.16 μg for the unlabeled and labeled formulation, respectively. Similarly, the DDs of BDP were 82.6 ± 6.79 μg and 84.8 ± 3.48 μg for the unlabeled and labeled formulation, respectively. The MMAD values were in even closer agreement being 1.30 ± 0.10 μm for formoterol for both labeled and unlabeled formulations, as well as for BDP for the unlabeled formulation, and 1.37 ± 0.06 μm for labeled BDP formulation. These results confirm that the labeling procedure did not change the properties of the product.
Table 2.
Mass Median Aerodynamic Diameter (MMAD), Geometric Standard Deviation (GSD), Fine Particle Dose (FPD, Stage Three-Filter), Fine Particle Fraction (FPF ), and Delivered Dose (DD) of Both Labeled and Unlabeled BDP/Formoterol Hfa Formulation
| MMAD (μm) | GSD | FPD (active substance μg) | FPF (% metered dose) | DD | |
|---|---|---|---|---|---|
| Formoterol: unlabeled formulation (HPLC detection) | 1.30 ± 0.10 | 1.97 ± 0.12 | 1.85 ± 0.10 | 34.4 ± 1.86 | 4.86 ± 0.28a |
| BDP: unlabeled formulation (HPLC detection) | 1.30 ± 0.10 | 2.00 ± 0.00 | 32.6 ± 2.34 | 37.0 ± 2.95 | 82.6 ± 6.79a |
| Formoterol: labeled formulation (HPLC detection) | 1.30 ± 0.10 | 1.80 ± 0.10 | 1.79 ± 0.04 | 32.6 ± 0.26 | 5.01 ± 0.16a |
| BDP: labeled formulation (HPLC detection) | 1.37 ± 0.06 | 1.90 ± 0.00 | 30.6 ± 0.05 | 34.1 ± 1.32 | 84.8 ± 3.48a |
| 99 mTC radioactivity: labeled formulation (radioactivity detection) | 1.33 ± 0.06 | 1.97 ± 0.06 | — | 33.9 ± 1.26 | 1171 ± 102b |
μg.
kBq.
Results are expressed as mean ± standard deviation.
BDP, beclomethasone dipropionate; HFA, hydrofluoroalkane; HPLC. high-performance liquid chromatography.
FIG. 1.
Amount of unlabeled and labeled beclomethasone dipropionate (BDP)/formoterol formulation, and radioactivity found on the different stages of the cascade impactor.
Deposition data
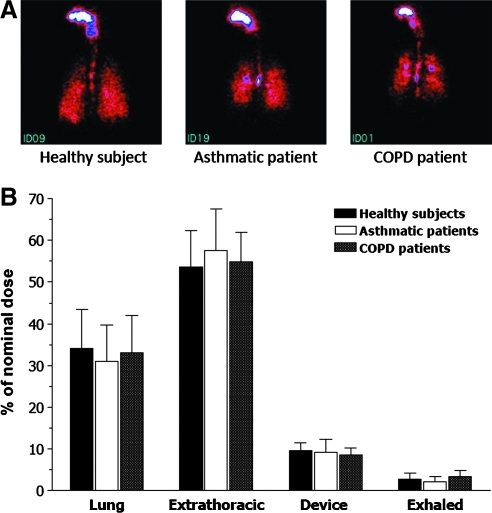
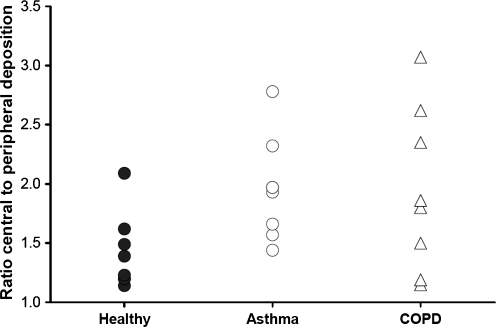
Neither lung deposition nor extrathoracic deposition of BDP/formoterol HFA significantly differed between the study groups. Mean lung deposition (± standard deviation) was 34.08 ± 9.30% of the nominal dose in healthy subjects, 30.86 ± 8.89% in asthmatics and 33.10 ± 8.90% in COPD patients (Table 3 and Fig. 2). Mean extrathoracic deposition was also similar for the three groups, ranging from 53.48 ± 8.95% of the nominal dose in healthy subjects, 54.98 ± 7.01% in COPD patients and 57.64 ± 9.92% in asthmatics (Table 3 and Fig. 2). The mean ratio of central to peripheral deposition (C/P) was significantly (p = 0.046) higher in asthmatics (1.96 ± 0.43) compared to healthy subjects (1.42 ± 0.32) (Table 3 and Fig. 3). Conversely, the difference between the mean C/P ratio in COPD patients (1.94 ± 0.69) and in healthy subjects just missed statistical significance (p = 0.051) (Table 3 and Figure 3). The variance of pixel counts indicated a more heterogeneous deposition in the lungs of patients compared with healthy subjects. In particular, the difference between COPD patients (0.0029 ± 0.0019) and healthy subjects (0.0016 ± 0.0007) was statistically significant (p = 0.043) (Table 3). The amount of exhaled BDP/formoterol or drug fraction remaining on the device did not differ significantly between groups (Table 3). There was no statistically significant correlation between FEV1 at baseline and lung deposition (r = 0.19; p = 0.38), extrathoracic deposition (r = −0.20; p = 0.34) or C/P ratio (r = −0.32; p = 0.13).
Table 3.
Deposition in Healthy Subjects, Asthmatic, and COPD Patients following Administration of One Single Dose of Four Puffs of the BDP/Formoterol HFA (100/6 μg) Radiolabeled Formulation
| |
Group |
||
|---|---|---|---|
| Variable | Healthy subjects (n = 8) | Asthma patients (n = 8) | COPD patients (n = 8) |
| Lung deposition (% nominal dose) | 34.08 ± 9.30 | 30.86 ± 8.89 | 33.10 ± 8.90 |
| (20.00–43.80) | (21.50–47.40) | (14.00–43.60) | |
| Extrathoracic deposition (% nominal dose) | 53.48 ± 8.95 | 57.64 ± 9.92 | 54.98 ± 7.01 |
| (42.00–66.70) | (43.50–69.30) | (45.00–69.80) | |
| C/P | 1.42 ± 0.32 | 1.96 ± 0.43* | 1.94 ± 0.69 |
| (1.14–2.09) | (1.44–2.78) | (1.15–3.07) | |
| VAR (pixel counts) | 0.0016 ± 0.0007 | 0.0023 ± 0.0006 | 0.0029 ± 0.0019** |
| (0.0008–0.0030) | (0.0017–0.0032) | (0.0011–0.0060) | |
| Amount exhaled (% nominal dose) | 2.79 ± 1.46 | 2.18 ± 1.26 | 3.41 ± 1.49 |
| (1.30–5.50) | (0.90–4.10) | (2.00–6.20) | |
| Residuals in the device (% nominal dose) | 9.68 ± 1.90 | 9.30 ± 2.96 | 8.53 ± 1.79 |
| (7.40–12.30) | (7.10–13.90) | (6.40–11.20) | |
p = 0.046 versus healthy subjects.
p = 0.043 versus healthy subjects.
Results are presented as mean ± standard deviation (range).
BDP, beclomethasone dipropionate; HFA, hydrofluoroalkane; COPD, chronic obstructive pulmonary disease; C/P, central to peripheral ratio; VAR, variance of deposition in the lungs.
FIG. 2.
(A) Scintigraphy in individual subject and (B) histogram showing mean (+standard deviation) drug deposition in healthy subjects (n = 8), asthma patients (n = 8), and COPD patients (n = 8) after a single inhalation of four puffs of beclomethasone dipropionate/formoterol (100/6 μg) HFA pMDI.
FIG. 3.
Central to peripheral deposition (C/P) in healthy subjects (n = 8), asthma patients (n = 8), and COPD patients (n = 8) after a single inhalation of four puffs of beclomethasone dipropionate/formoterol (100/6 μg) HFA pMDI.
Lung function
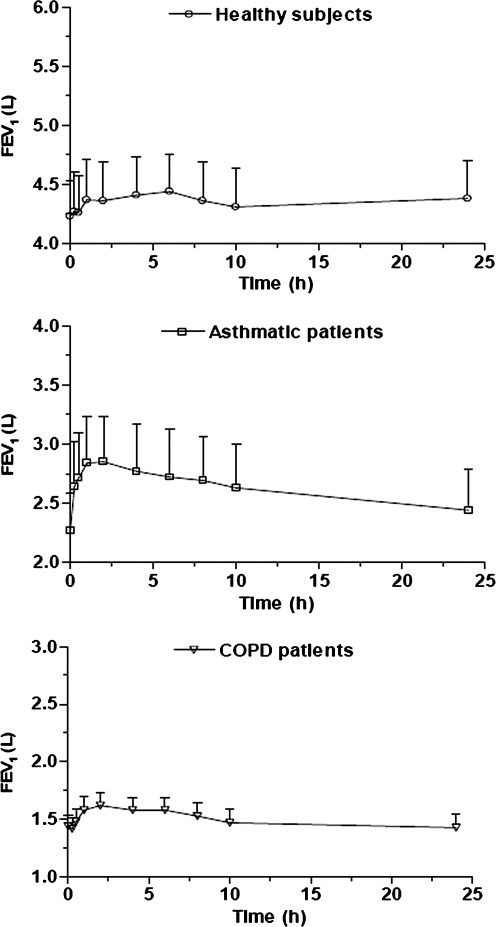
Administration of BDP/formoterol produced an FEV1 increase in each group, but a more evident bronchodilator effect was achieved in asthmatic and COPD patients (Fig. 4). In healthy subjects, the maximum increase in FEV1 over baseline values was 5%, occurring at 6 h and corresponding to approximately 200 mL. In asthmatic and COPD patients the maximum FEV1 increase was 25.6% (approx. 300 mL) and 12.5% (approx. 180 mL) respectively, both of which occurred 2 h post-dose (Fig. 4).
FIG. 4.
Mean forced expiratory volume in 1 sec (FEV1) over time (±standard error) in healthy subjects (n = 8), asthmatic (n = 8), and COPD patients (n = 8) after a single inhalation of four puffs of beclomethasone dipropionate/formoterol (100/6 μg) HFA pMDI.
Pharmacokinetics
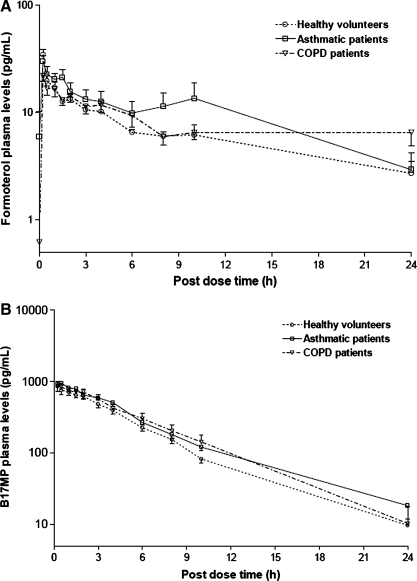
Comparable formoterol, BDP, and B17MP plasma profiles were observed during the 24 h after inhalation of the study drug (Table 4 and Fig. 5). BDP is rapidly metabolized to B17MP, and so was not detectable in plasma 1.5 h after dosing. The mean (±standard deviation) maximum plasma concentration of B17MP was 929.0 ± 309.7 pg/mL in healthy subjects, 1047.3 ± 221.9 pg/mL in asthmatics and 1016.4 ±265.6 pg/mL in COPD patients and was reached at median tmax of 0.5 h in healthy subjects and asthmatics and 0.37 h in COPD patients. The formoterol maximum plasma concentration was also similar for healthy (34.6 ± 11.5 pg/mL) and asthmatics (31.2 ± 11.1 pg/mL) and slightly lower for COPD patients (23.9 ± 5.2 pg/mL). The median tmax of formoterol was 0.25 h in healthy subjects and asthmatics and 0.75 h in patients with COPD. The mean (± standard deviation) systemic exposure of formoterol and B17MP over 30 min post-dose, taken as an index of lung absorption,(22,23) were similar in the healthy, asthmatic, and COPD groups, respectively (formoterol AUC0–30 min 11.1 ±3.2, 10.1 ± 5.0, and 7.2 ±2.8 h*pg/mL; B17MP AUC0–30 min 306.2 ± 110.8, 349.3 ± 79.8, 321.2 ± 113.0 h*pg/mL), reflecting the comparable lung deposition of the drugs (Table 4). The area under the time curve of B17MP and formoterol plasma levels (mean ± standard deviation) were roughly comparable in healthy subjects, patients with asthma, and patients with COPD, respectively, with a trend for a slight increase in systemic exposure in patients (formoterol AUC0–24 h: 142.8 ± 37.6, 229.9 ± 169.0, and 183.1 ± 70.6 h*pg/mL; B17MP AUC0–24 h 4185.2 ± 1127.2, 5199.0 ± 867.2, and 5221.1 ± 2091.7 h*pg/mL).
Table 4.
BDP, B17MP, and Formoterol Pharmacokinetic Parameters in Healthy Subjects, Asthmatic, and COPD Patients following Administration of One Single Dose of Four Puffs of the BDP/Formoterol HFA (100/6 μg) Combination
| Healthy subjects (n = 8) | Asthma patients (n = 8) | COPD patients (n = 8) | |
|---|---|---|---|
| BDP | |||
| tmax (h) | 0.25 (0.25–0.25) | 0.25 (0.25–0.25) | 0.25 (0.25–0.25) |
| Cmax (pg/mL) | 278.6 ± 107.0 | 214.0 ± 181.4 | 475.3 ± 299.8 |
| AUC0–24 h (h*pg/mL) | 95.3 ± 34.3 | 73.5 ± 60.6 | 159.6 ± 100.8 |
| B17MP | |||
| tmax (h) | 0.5 (0.25–2.00) | 0.5 (0.25–1.50) | 0.37 (0.25–2.00) |
| Cmax (pg/mL) | 929.0 ± 309.7 | 1047.3 ± 221.9 | 1016.4 ± 265.6 |
| AUC0–30 min (h*pg/mL) | 306.2 ± 110.8 | 349.3 ± 79.8 | 321.2 ± 113.0 |
| AUC0–24 h (h*pg/mL) | 4185.2 ± 1127.2 | 5199.0 ± 867.2 | 5221.1 ± 2091.7 |
| Formoterol | |||
| tmax (h) | 0.25 (0.25–1) | 0.25 (0.25–10) | 0.75 (0.25–24) |
| Cmax (pg/mL) | 34.6 ± 11.5 | 31.2 ± 11.1 | 23.9 ± 5.2 |
| AUC0–30 min (h*pg/ml) | 11.1 ± 3.2 | 10.1 ± 5.0 | 7.2 ± 2.8 |
| AUC0–24 h (h*pg/mL) | 142.8 ± 37.6 | 229.9 ± 169.0 | 183.1 ± 70.6 |
Results are expressed as mean ± standard deviation [except tmax – median (range)]
BDP, beclomethasone dipropionate; B17MP, BDP metabolite; HFA, hydrofluoroalkane; COPD, chronic obstructive pulmonary disease; tmax, time to maximum plasma concentration; Cmax, maximum plasma concentration; AUC0–30 min, area under the plasma concentration versus time curve observed from 0 to 30 min; AUC0–24 h, area under the plasma concentration time curve observed from 0 to 24 h.
FIG. 5.
Log-transformed (A) Formoterol and (B) B17MP plasma profiles (mean values ± standard error) of healthy subjects (n = 8), asthmatic (n = 8), and COPD patients (n = 8) after a single inhalation of four puffs of beclomethasone dipropionate/formoterol (100/6 μg) HFA pMDI.
Safety and tolerability
In total, 12 adverse events were observed. They were all of mild or moderate intensity. Two patients experienced mild headache. Other adverse events included an abnormal laboratory value, cough and dyspnoea, common cold, urinary tract infection, phlebitis, and hand trembling/vertigo. One COPD patient experienced a moderate ischalgia and a moderate pleurisy, and was discontinued from the study before treatment. Three adverse events were considered related to the study medication, but none were serious in nature.
Discussion
This study showed that a large amount of the inhaled BDP/formoterol extrafine HFA fixed combination was deposited into the lungs (31–34%), with a low variability between healthy subjects, asthmatic, and COPD patients, confirming efficient lung delivery regardless of pathophysiological condition. Drug distribution was observed throughout the lung, including the peripheral airways, where at least one-third of the drug was deposited (41% in healthy subjects and 34% in asthmatic and COPD patients), indicating that the increased airway obstruction in patients had a moderate impact on the pattern of deposition (C/P ratio, VAR). The increase in FEV1 confirmed a prolonged pharmacodynamic effect of the combination. In this study, bidimensional gamma scintigraphy was used. This method provides limited spatial resolution of the lung. The spatial distribution of the formulation is under investigation using the segmentation/CFD combination technique.
The pulmonary deposition of extrafine formulations of ICS and LABAs administered as single agents have already been investigated.(25–28) In healthy volunteers, extrafine BDP–HFA lung deposition ranged from 55–60% of the emitted dose (44–48% of the nominal dose) compared with just 4–7% of the emitted dose following CFC–BDP inhalation.(26,27) In mild asthmatics, lung deposition of extrafine BDP–HFA from a breath-activated device (Autohaler®) was 60% of the emitted dose (48% of the nominal dose) compared to 56–59% of the emitted dose (45–47% of the nominal dose) for patients using a pMDI. This percentage fell to 37% of the emitted dose (30% of the nominal dose) in subjects with poor inhalation technique.(26,28) The lung deposition of extrafine formoterol HFA in healthy volunteers, asthmatic, and COPD patients has previously been reported as 31, 34, and 35% of the nominal dose, respectively.(25) These data agree with our findings, and suggest comparable lung deposition in the different populations. The addition of a spacer device to a pMDI is one way to improve lung deposition and reduce oropharyngeal deposition. The lung deposition values for the BDP/formoterol extrafine HFA pMDI observed in the present study are comparable to, or higher than, those reported for pMDIs plus spacers.(29)
The deposition pattern of inhaled drugs depends on the complex interaction between device, formulation, and inhalation technique.(7) It is important that the therapeutic agent reaches the lung periphery for several reasons. First, accumulating evidence shows that in asthma, airway inflammation, and remodeling occur both in large and small airways,(30) with more severe inflammatory processes present in the peripheral compared with the central airways.(10) Additionally, in COPD the peripheral airways are the main site of obstruction.(11) Second, corticosteroid receptors and β2-adrenergic receptors are present throughout the airways;(31, 32) thus, the ICS/LABA synergistic interaction at the molecular level might occur at various cellular types in the lungs. Last, extrafine BDP alone has already been shown to reduce candidate markers of small airway inflammation.(33) Devices that generate smaller particles will give a more peripheral deposition of drug.(9)
Pressurized MDIs are the most frequently prescribed inhaler device, and HFA formulations capable of delivering extrafine drug particles are currently available. A pMDI delivering extrafine drug particles has the potential to eliminate problems of decreased pulmonary deposition previously described for pMDIs,(34) as smaller drug particles should stay suspended longer in the inspiratory air of patients, and reduce the effects of incorrect pMDI technique.(35) Leach and colleagues(28) compared the lung delivery of HFA–BDP from a breath-activated inhaler (QVAR Autohaler) with that from a press and breath pMDI used both correctly and incorrectly. They showed that although the degree of lung deposition was decreased as patients demonstrated poor inhaler technique, patients with poor technique still received a large dose of BDP (≥37%) compared with lung deposition values of 4–7% for CFC BDP MDIs. With smaller drug particle sizes, the speed of the inhalation maneuver is also not critical to lung deposition. Usmani and colleagues(9) showed a greater total lung deposition and farther distal airway penetration with small (1.5 μm) albuterol particles during slow inhalation.
In the present study, the deposition pattern in the lung confirmed a drug distribution throughout the airways, including both large and small airways, in all three groups. This is due to the fact that small particles generated by BDP/formoterol HFA are deposited predominantly by sedimentation. Deposition by impaction in extrathoracic airways and at sites of obstruction in the asthmatic or COPD lung is therefore much smaller compared to larger drug particles.(25) Consequently, BDP/formoterol extrafine fixed combination, provides a homogeneous distribution of both active drugs throughout the entire bronchial tree, irrespective of pathophysiological condition. This finding is consistent with the data obtained with another extrafine formulation in patients with very mild asthma (mean percent predicted FEV1 of 91%).(28) Despite the impaired level of airway obstruction of the patients enrolled in the present study (mean FEV1 of 70% in the asthmatics group and 43% in the COPD group) the extrafine formulation has proven to deliver a considerable amount of drug to the lung periphery.
For extrafine formulations of corticosteroids, the risk that higher lung deposition and peripheral distribution might lead to higher systemic exposure is reasonable. In this regard, a recently published pharmacokinetics study compared the systemic exposure of BDP/formoterol extrafine, used at the same dose and in the same formulation as in the present investigation, with an equipotent regimen of BDP nonextrafine plus formoterol extrafine given via separate inhalers.(36) The study showed that, although comparable formoterol systemic exposure was observed after the two treatments, the 24-h systemic exposure of B17MP was 35% lower with the BDP/formoterol extrafine fixed combination than with the extemporary combination, where BDP was non-extrafine. In addition, the exposure of B17MP in the first 30 min, reported to be an index of pulmonary absorption,(23) was 86% greater with the extrafine fixed combination than with the separate components.(36) Therefore, these data indicate that, despite the fixed combination of BDP/formoterol delivering more drug to the lungs, it results in a lower systemic exposure when compared with an equipotent regimen of non-extrafine BDP plus formoterol.(36)
This is the first study investigating the lung deposition profile of a fixed combination ICS/LABA, and correlating this pattern to the lung function at baseline of patients with different obstructive diseases. No significant correlation was detected between baseline lung function and drug deposition, suggesting that the improved lung deposition afforded by the HFA formulation is independent of patients' lung function and is, instead, a consequence of small particle size. The large VAR in COPD patients, however, indicates a larger heterogeneity in lung deposition profile in this population. Similarly, no correlation was found between baseline lung function and lung deposition of formoterol HFA in healthy volunteers, asthmatic, or COPD patients in a previously performed study.(25) This result is not surprising, as lung function parameters such as FEV1 and peak expiratory flow (PEF) reflect central airways patency. Conversely, symptom improvement would be a good indication of improved lung deposition, and this has already been shown with BDP HFA extrafine aerosol in asthmatic patients.(37)
Interestingly, the efficacy of extrafine BDP/formoterol HFA fixed combination has been shown to be superior to equipotent doses of non extrafine BDP plus formoterol administered via separate inhalers in improving clinical measures of asthma control.(18) Superiority of a fixed combination over the same drugs given separately has not been reported with budesonide/formoterol or fluticasone propionate/salmeterol.(38,39) This observation with BDP/formoterol HFA over other fixed ICS/LABA combinations is likely due to its unique extrafine solution formulation that delivers the drug throughout the bronchial tree, as shown in the present study. In addition, as both BDP and formoterol in the extrafine formulation under investigation have a similar particle size distribution in vitro,(36) it is likely that, in vivo, they are codeposited throughout all districts of the bronchial tree.(16) This, in turn, may permit the positive interaction between BDP and formoterol similar to what has already been described both at the receptor and molecular levels in various experimental models.(40–42)
Formoterol, BDP, and B17MP plasma profiles were comparable in all three groups, in agreement with the deposition results. BDP was rapidly metabolized to B17MP, and was below the LOQ at 1.5 h post-dose. A previous study in healthy volunteers comparing extrafine BDP/formoterol to separately administered non-extrafine BDP CFC (250 μg) and formoterol 24 μg, showed that B17MP systemic exposure during the first 30 min post-administration were 86% higher with BDP/formoterol than with the separate components.(36) As this measure is considered an indicator of pulmonary absorption,(22,23) the good lung deposition of the extrafine combination reported in the present study is in line with this interpretation. Formoterol systemic exposure was comparable when administered as a fixed combination or separately.(36) Formoterol and B17MP rapid absorption has previously been observed in asthmatic patients.(43)
BDP/formoterol HFA fixed combination was well tolerated. No serious adverse events were reported in any of the study groups. High dose BDP/formoterol HFA fixed combination (10 puffs of 100/6 μg for 7 days) was also well tolerated and exhibited a safety profile generally similar to formoterol alone when administered in high doses to stable asthmatic patients.(43) The safety profile of fixed combination BDP/formoterol does not differ from that of other available ICS/LABA fixed combinations.(19,20)
In conclusion, our results indicate that BDP/formoterol (100/6 μg) extrafine formulation is efficiently delivered to the lung, produces high lung deposition, low variability, and homogeneous distribution of BDP and formoterol throughout the airways, regardless of pathophysiological condition and independent of lung function. Assuming that the radioactive label is uniformly distributed within the BDP/formoterol HFA formulation (as indicated by the good agreement between the distribution of radioactive label and of the drugs in this study), these results indicate that both components are distributed throughout the lung, including the peripheral airways, which in turn increases the potential for synergistic interaction.
Acknowledgments
This study was sponsored by Chiesi Farmaceutici. Thanks to Dr. Ruth Murray for assistance in writing this document.
Author Disclosure Statement
W. De Backer: Prof De Backer has previously been employed by Chiesi as a principal investigator of another clinical study. A. Devolder: no conflict of interest exists. G. Poli, D. Acerbi, R. Monno, and F. Mariotti are all employees of Chiesi Farmaceutici, which sponsored the study. C. Herpich, K. Sommerer, and T. Meyer are all employees of Inamed Research, the Contract Research Organization, employed by Chiesi, responsible for coordinating the clinical trial.
References
- 1.Global Initiative for Asthma (GINA) http://www.GINA.com http://www.GINA.com
- 2.Global Strategy for the Diagnosis, Management and Prevention of COPD Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2007. http://www.goldcopd.org http://www.goldcopd.org
- 3.Van Noord JA. Schreurs AJM. Mol SJM. Mulder PGH. Addition of salmeterol versus doubling the dose of fluticasone propionate in patients with mild to moderate asthma. Thorax. 1999;54:207–212. doi: 10.1136/thx.54.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting beta2-agonists and corticosteroids. Eur Respir J. 2002;19:182–191. doi: 10.1183/09031936.02.00283202. [DOI] [PubMed] [Google Scholar]
- 5.Barnes PJ. Inhaled glucocorticoids for asthma. N Engl J Med. 1995;332:868–875. doi: 10.1056/NEJM199503303321307. [DOI] [PubMed] [Google Scholar]
- 6.Lenney J. Innes JA. Crompton GK. Inappropriate inhaler use: assessment of use and patient preference of seven inhalation devices. EIDICI. Respir Med. 2000;94:496–500. doi: 10.1053/rmed.1999.0767. [DOI] [PubMed] [Google Scholar]
- 7.Nicolini G. Scichilone N. Bizzi A. Papi A. Fabbri LM. Beclomethasone/formoterol fixed combination for the management of asthma: patient considerations. Ther Clin Risk Manage. 2008;4:855–864. doi: 10.2147/tcrm.s3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newman SP. Principles of metered-dose inhaler design. Respir Care. 2005;50:1177–1190. [PubMed] [Google Scholar]
- 9.Usmani OS. Biddiscombe MF. Barnes PJ. Regional lung deposition and bronchodilator response as a function of β2-agonist particle size. Am J Respir Crit Care Med. 2005;172:1497–1504. doi: 10.1164/rccm.200410-1414OC. [DOI] [PubMed] [Google Scholar]
- 10.Hamid Q. Song Y. Kotsimbos TC. Minshall E. Bai TR. Hegele RG. Hogg JC. Inflammation of small airways in asthma. J Allergy Clin Immunol. 1997;100:44–51. doi: 10.1016/s0091-6749(97)70193-3. [DOI] [PubMed] [Google Scholar]
- 11.Hogg JC. Chu F. Utokaparch S. Woods R. Elliott WM. Buzatu L. Cherniack RM. Rogers RM. Sciurba FC. Coxson HO. Paré PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 12.Fabbri LM. Nicolini G. Olivieri D. Papi A. Inhaled Beclomethasone dipropionate/formoterol extra-fine fixed combination in the treatment of asthma: evidence and future perspectives. Expert Opin Pharmacother. 2008;9:479–490. doi: 10.1517/14656566.9.3.479. [DOI] [PubMed] [Google Scholar]
- 13.Acerbi D. Brambilla G. Kottakis I. Advances in asthma and COPD management: delivering CFC-free inhaled therapy using Modulite technology. Pulm Pharmacol Ther. 2007;20:290–303. doi: 10.1016/j.pupt.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Busse WW. Brazinsky S. Jacobson K. Stricker W. Schmitt K. Vanden Burgt J. Donnell D. Hannon S. Colice GL. Efficacy response of inhaled beclomethasone dipropionate in asthma is proportional to dose and is improved by formulation with a new propellant. J Allergy Clin Immunol. 1999;104:1215–1222. doi: 10.1016/s0091-6749(99)70016-3. [DOI] [PubMed] [Google Scholar]
- 15.Rigamonti E. Kottakis I. Pelc M. Grzelewska Rzymowska I. Feschenko Y. Comparison of a new extra-fine beclomethasone dipropionate HFA 134a-formulated pMDI with a standard BDP CFC pMDI in adults with moderate persistent asthma. Eur Respir J. 2006;28(Suppl. 50):P1236. [Google Scholar]
- 16.Dhillon S. Keating GM. Beclomethasone dipropionate/formoterol: in an HFA-propelled metered-dose inhaler. Drugs. 2006;66:1475–1483. doi: 10.2165/00003495-200666110-00005. [DOI] [PubMed] [Google Scholar]
- 17.Lewis D. Brambilla G. Church T. Meakin B. Beclomethasone dipropionate and formoterol association within a combination HFA solution MDI. Respir Drug Deliv. 2006;3:939–942. [Google Scholar]
- 18.Huchon G. Magnussen H. Chuchalin A. Dymek L. Bonnet Gonod F. Bousquet J. Lung function and asthma control with beclomethasone and formoterol in a single inhaler. Respir Med. 2009;103:41–49. doi: 10.1016/j.rmed.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Papi A. Paggiaro PL. Nicolini G. Vignola AM. Fabbri LM. Inhaled Combination Asthma Treatment versus SYmbicort (ICAT SY) Study Group: beclomethasone/formoterol versus budesonide/formoterol combination therapy in asthma. Eur Respir J. 2007;29:682–689. doi: 10.1183/09031936.00095906. [DOI] [PubMed] [Google Scholar]
- 20.Papi A. Paggiaro P. Nicolini G. Vignola AM. Fabbri LM ICAT SE study group. Beclomethasone/formoterol vs fluticasone/salmeterol inhaled combination in moderate to severe asthma. Allergy. 2007;62:1182–1188. doi: 10.1111/j.1398-9995.2007.01493.x. [DOI] [PubMed] [Google Scholar]
- 21.Pitcairn GR. Newman SP. Tissue attenuation corrections in γ scintigraphy. J Aerosol Med. 1997;10:187–198. [Google Scholar]
- 22.Silkstone VL. Corlett SA. Chrystyn H. Determination of the relative bioavailability of salbutamol to the lungs and systemic circulation following nebulization. Br J Clin Pharmacol. 2002;54:115–119. doi: 10.1046/j.1365-2125.2002.01632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chrystyn H. Methods to identify drug deposition in the lungs following inhalation. Br J Clin Pharmacol. 2001;51:289–299. doi: 10.1046/j.1365-2125.2001.01304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poli G. Acerbi D. Rusca A. Pharmacokinetics and lung bioavailability of Foster using the standard actuator or a spacer. Eur Respir J. 2007;30(Suppl 51):350. [Google Scholar]
- 25.Haussermann S. Acerbi D. Brand P. Herpich C. Poli G. Sommerer K. Meyer T. Lung deposition of formoterol HFA (Atimos/Forair) in healthy volunteers, asthmatic and COPD patients. J Aerosol Med. 2007;20:331–341. doi: 10.1089/jam.2007.0613. [DOI] [PubMed] [Google Scholar]
- 26.Leach CL. Davidson PJ. Boudreau RJ. Improved airway targeting with the CFC-free HFA-beclomethasone metered dose inhaler compared with CFC beclomethasone. Eur Respir J. 1998;12:1346–1353. doi: 10.1183/09031936.98.12061346. [DOI] [PubMed] [Google Scholar]
- 27.Leach CL. Davidson PJ. Hassselquist BE. Boudreau RJ. Lung deposition of hydrofluoroalkane 132a beclomethasone is greater than that of chlorofluorocarbon fluticasone and chlorofluorocarbon beclomethasone: a cross over study in healthy volunteers. Chest. 2002;122:510–516. doi: 10.1378/chest.122.2.510. [DOI] [PubMed] [Google Scholar]
- 28.Leach CL. Davidson PJ. Hasselquist BE. Boudreau RJ. Influence of particle size and patient dosing technique on lung deposition of HFA-beclomethasone from a metered dose inhaler. J Aerosol Med. 2005;18:379–385. doi: 10.1089/jam.2005.18.379. [DOI] [PubMed] [Google Scholar]
- 29.Selroos O. Pietinalho A. Riska H. Delivery devices for inhaled asthma medication. Clin Immunother. 1996;6:273–299. [Google Scholar]
- 30.Tulic MK. Christodoulopoulos P. Hamid Q. Small airway inflammation in asthma. Respir Res. 2001;2:333–339. doi: 10.1186/rr83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adcock IM. Gilbey T. Gelder CM. Chung KF. Barnes PJ. Glucocorticoid receptor localization in normal and asthmatic lung. Am J Respir Crit Care Med. 1996;154:771–782. doi: 10.1164/ajrccm.154.3.8810618. [DOI] [PubMed] [Google Scholar]
- 32.Spina D. Rigby PJ. Paterson JW. Goldie RG. Autoradiographic localization of beta-adrenoceptors in asthmatic human lung. Am Rev Respir Dis. 1989;140:1410–1415. doi: 10.1164/ajrccm/140.5.1410. [DOI] [PubMed] [Google Scholar]
- 33.Hauber H. Taha R. Bergeron C. Migouno V. Hamid Q. Olivenstein R. Effects of hydrofluoroalkane and dry powder-formulated corticosteroids on sputum inflammatory markers in asthmatic patients. Can Respir J. 2006;13:73–78. doi: 10.1155/2006/648392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newman SP. Weisz AW. Talee N. Clarke SW. Improvement of drug delivery with a breath-activated aerosol for patients with poor inhaler technique. Thorax. 1991;46:712–716. doi: 10.1136/thx.46.10.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindgren S. Bake B. Larsson S. Clinical consequences of inadequate inhalation technique in asthma therapy. Eur J Respir Dis. 1987;70:93–98. [PubMed] [Google Scholar]
- 36.Bousquet J. Poli G. Acerbi D. Monno R. Ramael S. Nollevaux F. Systemic exposure and implications for lung deposition with an extra-fine HFA beclometasone dipropionate/formoterol fixed combination. Clinical Pharmacokinet. 2009;48:347–358. doi: 10.2165/00003088-200948060-00001. [DOI] [PubMed] [Google Scholar]
- 37.Van Schayck CP. Donnell D. The efficacy and safety of QVAR (hydrofluoroalkane-beclomethasone dipropionate extra-fine aerosol in asthma (part 1): an update of clinical experience in adults. Int J Clin Pract. 2004;58:678–688. doi: 10.1111/j.1368-5031.2004.00273.x. [DOI] [PubMed] [Google Scholar]
- 38.Chapman KR. Ringdal N. Backer V. Palmqvist M. Saarelainen S. Briggs M. Salmeterol, fluticasone propionate (50/250 microg) administered via combination Diskus inhaler: as effective as when given via separate Diskus inhalers. Can Respir J. 1999;6:45–51. doi: 10.1155/1999/894803. [DOI] [PubMed] [Google Scholar]
- 39.Noonan M. Rosenwasser LJ. martin P. O'Brien CD. O'Dowd L. Efficacy and safety of budesonide and formoterol in one pressurised metered-dose inhaler in adults and adolescents with moderate to severe asthma: a randomised clinical trial. Drugs. 2006;66:2235–2254. doi: 10.2165/00003495-200666170-00006. [DOI] [PubMed] [Google Scholar]
- 40.Profita M. Gagliardo R. Di Giorgi R. Pompeo F. Gjomarkaj M. Nicolini G. Bousquet J. Vignola AM. Biochemical interaction between the effects of beclometasone dipropionate/formoterol, and salbutamol or formoterol in sputum cells from mild to moderate asthmatics. Allergy. 2005;60:323–329. doi: 10.1111/j.1398-9995.2005.00702.x. [DOI] [PubMed] [Google Scholar]
- 41.Razzetti R. Bergamaschi M. Villetti G. Bolzoni P. Civelli M. Berti F. Rossoni G. Formoterol and beclomethasone dipropionate interact positively in antagonising bronchoconstriction and inflammation in the lung. Pharmacol Res. 2007;55:426–432. doi: 10.1016/j.phrs.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 42.Descalzi D. Folli C. Nicolini G. Riccio AM. Gamalero C. Scordamaglia F. Canonica GW. Anti-proliferative and anti-remodelling effect of beclomethasone dipropionate, formoterol and salbutamol alone or in combination in primary human bronchial fibroblasts. Allergy. 2008;63:432–437. doi: 10.1111/j.1398-9995.2007.01582.x. [DOI] [PubMed] [Google Scholar]
- 43.Singh D. Piccinno A. Borrill Z. Poli G. Acerbi D. Meuleners L. Woodcock A. Tolerability of high cumulative doses of the HFA Modulite beclomethasone dipropionate/formoterol combination inhaler in asthmatic patients. Pulm Pharmacol Ther. 2008;21:551–557. doi: 10.1016/j.pupt.2008.01.001. [DOI] [PubMed] [Google Scholar]