Abstract
Liver X receptors (LXR)-α,β regulate intracellular cholesterol homeostasis and inhibit inflammatory gene expression. We studied the effects of the LXRα,β-agonist GW3965 on acute and chronic organ damage in the F344-LEW rat kidney transplantation model. In addition, to gain LXR isoform and cell-specific insights BALB/c kidneys were transplanted into mice with macrophage overexpression of LXRα (mLXRα-tg) and evaluated 7 and 42 days after transplantation. After 56 days GW3965 improved significantly function and morphology of rat kidney allografts by substantial reduction of mononuclear cell infiltrate and fibrosis; in vitro GW3965 reduced inflammatory activity of bone marrow–derived macrophages (BMDMs) and alloreactivity of T cells. Kidneys transplanted into mLXRα-tg mice were also protected from development of chronic allograft dysfunction. Similarly to GW3965-activated BMDMs, mLXRα-tg macrophages secreted significantly less monocyte chemoattractant protein 1 and macrophage inflammatory protein 1β. Interestingly, 7 days after transplantation, when the total number of intragraft macrophages did not differ, evidently more arginase 1– and mannose receptor C type 1–positive cells were found in LXR rat and mice kidney allografts; in vitro both LXR activation by GW3965 and mLXRα overexpression accentuated the induction of alternative activation of BMDMs by IL-4/IL-13, suggesting an additional mechanism by LXRs to prevent graft damage. The results highlight the relevance of macrophage LXRα in allograft rejection and prevention of fibrosis.
Chronic renal allograft dysfunction, characterized by atrophic and fibrotic changes, is thought to be the result of complex interactions between innate and adaptive immune responses and parenchymal cells.1 Acting synergistically with lymphocytes, macrophages contribute to both innate and acquired immunity.2,3 The contribution of macrophages to the pathogenesis of renal fibrosis is well documented, although, depending on the inflammatory microenvironment, macrophages may change their phenotype and take on an anti-inflammatory, reparative, and matrix remodeling role.3–5 Recently, macrophages expressing arginase 1 (Arg1) have been found to reduce T-helper cell 2 (Th2)–mediated liver inflammation and fibrosis.6 We have shown that Ccr5 deficiency could induce the alternative activation (M2) pathway in macrophages; the resulting M2 phenotype apparently had an important role in the attenuation of chronic renal allograft rejection.7
Liver X receptors (LXRs) are lipid-ligand–activated nuclear receptors. The two LXR isotypes, α and β, have high-sequence homology and are activated by oxidized cholesterols (oxysterols) or synthetic agonists such as T0901317 or GW3965.8,9 LXRβ is ubiquitously expressed, whereas LXRα is restricted to certain organs and cells such as liver, adrenal glands, intestine, adipose tissue, lung, and kidney.10 Macrophages express both LXRs; in lymphocytes the dominating isotype seems to be LXRβ.11
After ligand binding LXRs form permissive heterodimers with the retinoid X receptor. These dimers control transcriptional programs involved in lipid metabolism and inflammation.10,12 In murine macrophages ligand-activated LXRs have been shown to blunt the expression of inflammatory genes, such as iNOS, COX-2, and MMP-9, and various chemokines [eg, monocyte chemoattractant protein (MCP)-1] in response to lipopolysaccharide, tumor necrosis factor-α, and IL-1β.13–15 In RAW cells and primary mouse macrophages LXRα has been documented to positively regulate arginase II, a gene that may have anti-inflammatory effects through antagonism of nitric oxide signaling.16 It has been reported that the transcriptional regulation of intracellular cholesterol homeostasis by LXRβ influences lymphocyte proliferation and acquired immune responses.11 Collectively, these data have led us to expect a relevant immunomodulatory activity of LXRs in chronic fibrosing inflammation, which we exemplarily analyzed in renal allografts with chronic damage.
We have studied the effects of ligand activation of LXRs by GW3965 on acute and chronic rejection phenomena of F344-to Lew rat renal allografts. In addition, we have investigated whether in a fully major histocompatibility complex–mismatched model of mouse renal transplantation the cell-specific transgenic expression of LXRα in recipient macrophages might affect renal allograft rejection. Our results have shown that the LXR agonist GW3965 could prevent the development of chronic lesions in rat renal allografts by a reduction of the mononuclear cell infiltrate and by reduced intragraft pro-inflammatory/profibrotic gene [MCP-1/CCL2, macrophage inflammatory protein (MIP)-1β/CCL4] expression. Macrophage LXRα had an important effect on chronic organ damage because kidneys transplanted into mice with selective overexpression of LXRα in macrophages showed considerably better function and morphology 42 days after transplantation. In addition to the quantitative reduction of the pro-inflammatory macrophage phenotype, we could detect the induction of anti-inflammatory alternatively activated macrophages by LXRs during the acute phase of rejection which may have contributed to prevention of late graft damage.
Materials and Methods
Animals
Male inbred Lewis (LEW, RT11) and Fisher (F344, RT11v1) rats as well as BALB/c mice were purchased from Charles River GmbH, Sulzfeld, Germany. Mice with macrophage overexpression of LXRα (C57BL/6 background) were provided by Daniel Teupser (University of Leipzig, Germany)17 and bred in our animal facility. These mice were designated as mLXRα-tg. Animal experiments were performed according to German laws on animal protection.
Kidney Transplantation
Rats
Transplantation was performed under ether drop anesthesia.18 The left kidney of the donor rat (F344) was isolated, perfused with ice-cold isotonic sodium chloride solution, excised, and transplanted orthotopically into a weight-matched (200 to 220 g) Lewis recipient. In the recipient, the left renal vein and artery were mobilized and clamped, the ureter was cut, and the left kidney was excised. End-to-end anastomoses of renal vessels and of ureter, without ureteral stenting, were performed with 10-0 nonabsorbable nylon sutures. Total ischemic time of the donor kidney varied between 30 and 45 minutes. The right kidney was left in place to enhance rejection and damage to the transplant by avoidance of potential endogenous immunosuppressive effects of renal insufficiency and by a reduction of the work load of the transplanted kidney18,19; to obtain functional parameters of the graft at the end of the experiments, the right kidney was removed 48 hours before sacrifice. The animals were fed a standard rat chow without or with the synthetic LXR agonist GW3965 (20 mg/kg of body weight/day)12 and sacrificed 7 or 56 days after transplantation. The dose of 20 mg/kg of body weight/day GW3965 corresponds to a dose by which anti-inflammatory effects of the substance have been found.15,20
Mice
Mice (male, 8 to 10 weeks old, 20 to 25 g) were anesthetized with Tribromoethanol (Avertin) intraperitoneally. Kidneys from BALB/c(H-2d) donor mice were orthotopically transplanted into C57BL/6 (H-2b) wild-type (WT) or mLXRα-tg recipients as described.21 Briefly, the abdomen of the donor was opened through a midline incision, the left kidney with its vessels was attached to a segment of the aorta, and the vena cava along with ureter was removed en bloc. The donor aorta and inferior vena cava were then anastomosed end-to-side to the recipient abdominal aorta and inferior vena cava below the level of the native renal vessels, respectively. The native left kidney was removed before revascularization. Donor and recipient ureters were anastomosed at the ureteropelvic junction end to end. The native right kidney was removed after grafting. Mice were sacrificed on day 7 or day 42 after transplantation.
All transplanted kidneys with hydronephrosis, which was evaluated both macroscopically and by light microscopy, were excluded from the experimental groups.
Biochemical Analysis
For measurements of serum creatinine and albuminuria animals were kept in metabolic cages 24 hours before the end of the experiment. Serum creatinine (enzymatic determination), urea, cholesterol, triglycerides, glutamate-oxalate-transaminase, glutamate-pyruvate-transferase, alkaline phosphatase, and albumin in urine were analyzed by a Hitachi 9-17-E autoanalyzer (Hitachi, Frankfurt, Germany).22
Histology
Renal allografts were removed in deep anesthesia, quickly blotted free of blood, weighed, and processed as required for histology, immunohistology, and molecular analysis. For histology and immunohistology the kidneys were cut into 1-mm coronal slices and immersion fixed in 4% formaldehyde in PBS, Methacarn (rat kidney samples), or zinc solution (mice kidney samples) and then embedded in paraffin. In addition tissue slices were snap frozen in liquid nitrogen and stored at −80°C.
Light microscopy was performed on 3-μm sections stained by PAS. Kidneys were evaluated for evidence of acute and chronic vascular, glomerular, and tubulointerstitial damage as previously described.17,23 In short, acute vascular injury was assessed as 0, indicating no injury; 0.5, sticking of mononuclear cells to the endothelium; 1, subendothelial location of mononuclear cells; 2, inflammation of the media, including transmural infiltration; or 3, fibrinoid necrosis of the vessel wall or thrombosis of the vessel or both in addition to the inflammatory reaction. Chronic vascular injury was evaluated as negative (−) or positive (+), determined as narrowing of the luminal area by fibrous thickening of the subendothelial space with or without the presence of foam cells and given as percentage of positive vessels. Acute glomerular injury was defined as 0, indicating no injury; 0.5, sticking of mononuclear cells to the capillary endothelium in <50% of the convolutes; 1, sticking of mononuclear cells to the capillary endothelium in >50% of the convolute; 2, mesangiolysis with or without sticking of mononuclear cells; or 3, aneurysm, thrombosis, or necrosis of the capillary loops. Chronic glomerular injury was defined as 0, indicating no sclerosis; 0.5, sclerosis of <25% of capillary loops; 1, sclerosis of 26% to 50% of the capillary loops; 2, sclerosis of 51% to 75% of the capillary loops; or 3, sclerosis of >75% of the capillary loops. The acute and chronic glomerular injury was evaluated in ≥50 glomeruli per sections. Chronic tubulointerstitial damage was defined as broadening of the basement membrane of the tubuli with flattened epithelium, tubular atrophy, and interstitial matrix increase; it was judged as 0.5, indicating focal chronic damage, and 1, diffuse chronic damage. Tubulointerstitial inflammation was judged as 0, indicating no mononuclear cells in the interstitium; 0.5, focal mononuclear cell infiltration in the interstitium; 1, focal mononuclear infiltration in the interstitium with tubulitis; 2, diffuse mononuclear cell infiltration of the interstitium; or 3, diffuse mononuclear cell infiltration of the interstitium with tubulitis. Tubulitis was defined as ≥1 mononuclear cells/tubular cross section. A tubulointerstitial inflammation index was defined as the percentage of fields with respective degree of the injury encountered in 10 fields (objective 20×) of cortex and outer stripe of outer medulla. The final tubulointerstitial inflammation score was calculated as the sum of all specific indices, whereby the index of fields with degree 0.5 was multiplied by 0.5, that of degree 1 × 1, that of degree 2 × 2, and that of degree 3 × 3. Vascular and glomerular injury were scored in an analogous pattern.17,23
Morphometric analysis was performed with the use of a semiautomatic image analyzing system (Leica Q600 Qwin, Cambridge, UK) on sections stained with Goldner-Masson-Trichrom to quantify scared areas in tubulointerstitium: 10 randomly selected fields (objective 10×) of cortex and outer stripe of outer medulla were evaluated. Results were expressed as a percentage of the total tubulointerstitial area, obtained after exclusion of glomeruli.18
IHC
Immunohistochemical (IHC) staining was done on sections of frozen or paraffin-embedded kidney samples. The antibodies used for rat tissues included mouse anti-rat monoclonal antibodies against ED1, CD4, CD8 (Serotec, Oxford, UK), Ki-67 (clone MIB-5; Dianova, Hamburg, Germany), rat anti-rat FoxP3 (NatuTec, Frankfurt, Germany), rabbit anti-rat polyclonal antibodies against collagen I (Biogenesis, Poole, UK), collagen III (Chemicon, Temecula, CA), C4d (Hycult Biotech, Beutelsbach, Germany), and antibodies to α-smooth muscle actin (α-SMA) (mouse ascites fluid; Sigma, Schnelldorf, Germany). Mouse kidneys were stained with rat anti-mouse monoclonal antibodies against F4/80 (Serotec), CD3 (Santa Cruz Biotechnology, Santa Cruz, CA), CD4 (BD Biosciences Pharmingen, San Diego, CA), FoxP3 (NatuTec), α-SMA (Sigma), and rabbit anti-mouse against collagen I/III (Biogenesis). Anti-Arg1 (BD Biosciences Pharmingen), anti-mannose receptor-1 (CD206; Abcam) monoclonal antibodies were used to detect markers of alternatively activated macrophages.
Positive glomerular cells were counted in ≥50 glomerular cross sections and given as the mean per glomerular section; interstitial positive cells were counted in 20 high-power fields (×40) of cortex and outer medulla and recorded as mean per high-power field. The intensity of the staining for collagen was evaluated as not detectable (degree 0), faint (degree 1), moderate (degree 2), and intense staining (degree 3). Scores were calculated as described.18
Bone Marrow–Derived Macrophages
Generation of murine bone marrow–derived macrophages (BMDMs) of WT and mLXRα-tg mice was performed according to standard protocols.24,25 In brief, mouse femurs were dissected, and each bone was flushed with 10 mL of PBS. A bone marrow cell suspension was collected and centrifuged. Pellets were resuspended in RPMI 1640 medium supplemented by 20% macrophage colony-stimulating factor–containing L929 medium. The cells were plated on non-TC–treated 10-cm petri dishes and incubated at 37°C/5% CO2. Fresh medium was provided at days 3 and 5, and experiments were performed at day 7. After pre-incubation with dimethyl sulfoxide (DMSO; 0.05%) (WT and mLXRα-tg) or 3 μmol/L GW3965 dissolved in DMSO (WT) for 16 hours, macrophages were stimulated with murine IL-4 and IL-13 (10 ng/mL; PrepoTech) overnight. Cells and supernatant fluids were collected for further analyses.
Mixed Lymphocyte Reaction
Splenic T cells and T cell–depleted splenocytes were prepared as described.26 Briefly, spleens from BALB/c (n = 4) and C57BL/6 mice (n = 4) were mechanically disrupted in 6-well plates with 5 mL of digestion solution [RPMI 1640, 10 mmol/L HEPES, 0.1% bovine serum albumin (BSA), 0.5 mg/mL collagenase IA, and 4.5 kU/mL DNase I; all from Sigma-Aldrich] and incubated for 15 minutes at 37°C and 5% CO2. After repetitive pipetting, cell suspensions were sieved through filters with 100-μm and 30-μm pore sizes (BD, Heidelberg, Germany). Purified splenic T cells and T cell–depleted splenocytes were prepared with magnetic bead–coupled anti-CD90.2 antibodies (clone 30-H12; Miltenyi Biotec, Galdbach, Germany) and with magnetically activated cell sorter mass spectrometric columns and separators (both Miltenyi Biotec) according to the manufacturer's instructions. After pretreatment with DMSO 0.05% or 3 μmol/L GW3965 dissolved in DMSO WT for 16 hours 105 T cells purified from C57BL/6 mice were incubated with 105 T cell–depleted splenocytes from BALB/c mice. Culture supernatant fluids were harvested after 24 and 72 hours, and concentrations of IL-2 and interferon-γ (IFN-γ) were analyzed as described below.
Cytometric Bead Array for Measurement of Cytokines/Chemokines
Supernatant fluids of BMDM and splenocyte/T cell co-cultures were measured for MCP-1, MIP-1β and IL-2, and IFN-γ, respectively, by FACSCalibur with BD CBA Flex-Set bead assays (BD). Sample files were analyzed by FCAP Array 1.0.1 software.
RNA Extraction and Real-Time RT-PCR
Total RNA was extracted from the kidney allografts and macrophages (BMDMs) with the method of Chomczynski and Sacchi27 (n = four to six animals/group). RNA quality was checked by a RNA6000 Nanochip (Agilent Technologies, Waldbronn, Germany). Total RNA (10 μg) was digested with DNase I according to standard protocol. Total RNA (3 μg; DNA free) was used for the first-strand cDNA synthesis with the use of Superscript II Reverse Transcriptase and oligo d(T)12–18 as primer (LifeTechnologies, Karlsruhe, Germany). Real-time PCR was performed by LightCycler with the use of LightCyler-FastStart DNA MasterSYBR Green I kit (Roche Diagnostics, Mannheim, Germany) as described.18 The primer sequences for target genes are shown in Table 1.
Table 1.
Sequences of Primers Used for Real-Time RT-PCR Analysis
| Gene | Sense | Antisense |
|---|---|---|
| Rat | ||
| Cyclophilin | 5′-AGGTGAAAGAAGGCATAGC-3′ | 5′-TTACAGGGTATTGCGAGCAG-3′ |
| MCP-1/CCL2 | 5′-GCTGACCCCAATAAGGAATG-3′ | 5′-GTTGTGGAAAAGAGAGTGGATG-3′ |
| MIP-1β | 5′-GCTCTGACCCTCCCACTTC-3′ | 5′-ACTCATTGACCCAGGGCTC-3′ |
| IL-4 | 5′-ACGGCAACAAGGAACACCAC-3′ | 5′-TTCAGACCGCTGACACCTCTAC-3′ |
| IL-10 | 5′-CATGGGTCTTGGGAAGAGAA-3′ | 5′-GCTTTCGAGACTGGAAGTGG-3′ |
| IL-13 | 5′-CATGGTATGGAGCGTGGAC-3′ | 5′-GAGGCCTTTTGGTTACAGAGG-3′ |
| MRC1 | 5′-AGTGGTCATCGTGGTCCTTC-3′ | 5′-AATGACCGCGATGCTCATTCT-3′ |
| Mouse | ||
| GAPDH | 5′-ACTCCCACTCTTCCACCTTC-3′ | 5′-GGTCCAGGGTTTCTTTACTCC-3′ |
| Tubulin | 5′-TCTCTCACCCTCGCCTTCTA-3′ | 5′-GGGTCCCAGGTCTACGAACA-3′ |
| MCP-1/CCL2 | 5′-ACCAAGCTCAAGAGAGAGG-3′ | 5′-ACATTCAAAGGTGCTGAAGAC-3′ |
| MIP-1β | 5′-GCTGTTTCTCTTACACCTCC-3′ | 5′-ACTCATGTACTCAGTGACCC-3′ |
| IL-4 | 5′-TCCACGGATGCGACAAAAAT-3′ | 5′-TTCTTCTTCAAGCATGGAGT-3′ |
| IL-10 | 5′-ACCTGGCAAACAAAATGAGG-3′ | 5′-CTCTGACCTGCTGTCATGGA-3′ |
| IL-13 | 5′-CTCACTGGCTCTGGGCTTCA-3′ | 5′-CTCATTAGAAGGGGCCGTGG-3′ |
| Arg-1 | 5′-ACCACGGCAGTGGCTTTAACC-3′ | 5′-GGTTTTCATGTGGCGCATTC-3′ |
| MRC1 | 5′-GCGTTGCACATACCTCAAGA-3′ | 5′-GCTAAATGATCGCATGCTCA-3′ |
| Chi3l3/Ym1 | 5′-GAAGGAGCCACTGAGGTCTG-3′ | 5′-CACGGCACCTCCTAAATTGT-3′ |
| Fizz1 | 5′-TCCCAGTGAATACTGATGAGA-3′ | 5′-CCACTCTGGATCTCCCAAGA-3′ |
| Pdcd1lg2 | 5′-GCCACACGTGAGTTAT-3′ | 5′-TTGAACATGCCAAGCT-3′ |
Statistical Analysis
All data were presented as mean ± SEM. Data were analyzed by the non-parametric Mann-Whitney U-test or unpaired t-test as appropriate. A P value <0.05 was considered to show a significant difference between two groups.
Results
Plasma Lipid Levels and Hepatic Enzyme Activities
At day 7 after transplantation treatment with GW3965 led to a moderate increase of triglyceride levels (data not shown). As presented in Table 2, at day 56 after transplantation no significant differences in triglyceride and cholesterol levels between groups were found, but GW3965 led to an increase of alkaline phosphatase activity, a phenomenon previously reported.28 Consistent with earlier data in mice with overexpression of LXRα in macrophages, plasma lipids did not change in comparison to WT littermates.17
Table 2.
Biochemical Data in Experimental Groups
| Groups | Cholesterin (mg/dL) | Triglyceride (mg/dL) | GOT (U/L) | GPT (U/L) | ALP (U/L) |
|---|---|---|---|---|---|
| Control, 56 days | 118.8 ± 6.7 | 53.6 ± 2.9 | 83.0 ± 5.0 | 12.2 ± 2.2 | 66.2 ± 7.8 |
| GW3965, 56 days | 107.3 ± 4.7 | 46.6 ± 3.6 | 77.9 ± 4.7 | 8.2 ± 1.1 | 82.6 ± 6.3 |
| WT, 42 days | 128.6 ± 5.5 | 74.1 ± 8.6 | ND | ND | ND |
| mLXR-tg, 42 days | 117.8 ± 7.3 | 72.8 ± 10.3 | ND | ND | ND |
Values are mean ± SEM.
ALP, alkaline phosphatase; GOT, glutamate oxalate transaminase; GPT, glutamate pyruvate transferase; ND, no data.
Ligand Activation of LXR by GW3965 Prevents Chronic Allograft Dysfunction in F344-LEW Kidney Grafts
Administration of GW3965 for 7 days after transplantation led to a moderate reduction of glomerular and vascular rejection scores in kidney allografts without reaching statistical significance (Figure 1A). As presented in Figure 1 graft structure and function deteriorated dramatically between day 7 and day 56 in untreated animals. At day 56 after transplantation vascular and glomerular changes, as well as interstitial inflammation, fibrosis, and tubular atrophy, were strikingly lower in rats with the LXR agonist (Figure 1, A and C). Graft excretory functional parameters serum creatinine, urea, and albuminuria were approximately threefold lower, showing a significantly improved renal function in comparison to rats without LXR activation (Figure 1D).
Figure 1.
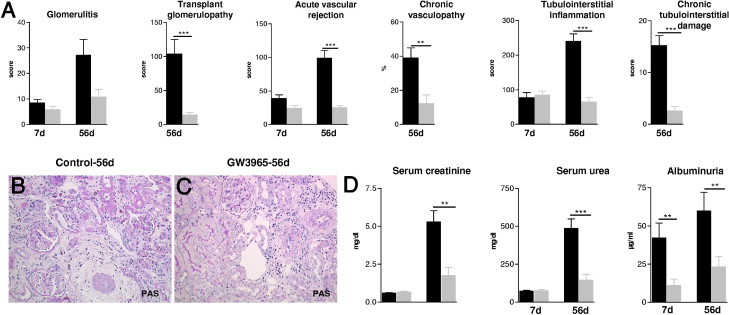
The effects of LXR activation by GW3965 on graft structure and function of Fisher-LEW kidney allografts 7 and 56 days after transplantation. A: Glomerular, vascular, and tubulointerstitial damage scores were significantly reduced 56 days after transplantation by GW3965. No significant differences were detected at day 7 after transplantation. B: Representative micrograph of an untreated renal allograft 56 days after transplantation showing a pre-glomerular artery with significant obliteration by subendothelial matrix increase and perivascular fibrosis, glomeruli with segmental extensive broadening of peripheral basement membrane, and tubulointerstitium with increased interstitial mononuclear cell infiltrate and matrix, surrounding collapsed atrophic tubules. C: Micrograph of an allograft with LXR activation presenting pre-glomerular arteries with only few mononuclear cells sticking to endothelium and moderate perivascular mononuclear cell infiltrate; glomeruli with few mononuclear cells in the capillary lumen, surrounding tubulointerstitium with focal sparse mononuclear cell infiltrate; majority of tubules are differentiated. Original magnification, ×200 (B and C; PAS staining). D: Graphs presenting functional parameters (serum creatinine, urea, albuminuria) of kidney allografts of bilaterally nephrectomized rats, significantly improved 56 days after transplantation by GW3965. Mean ± SEM; **P < 0.01, ***P < 0.001 versus controls.
At day 7 the number of graft-infiltrating mononuclear cells did not differ between controls and GW rats (Figure 2). In contrast, at day 56 after transplantation the mononuclear cell infiltrate consisting of ED1+ macrophages (Δ50%), CD4+ and, to a lesser extent, CD8+ T lymphocytes (Δ50% to 60% in tubulointerstitium) was significantly diminished by administration of the LXR agonist (Figure 2). The FoxP3+ subpopulation of CD4+ T cells was not significantly different neither at day 7 nor at day 56 after transplantation between treated and untreated grafts (Figure 2). Given the ability of LXRs to modulate cell proliferation,9,29,30 we performed immunostaining for Ki-67. Significantly less proliferating cells were found by LXR-agonist in the interstitial area of kidney grafts 56 days after transplantation (control versus GW: 31.5 ± 5.3 versus 6.3 ± 0.6 cells/high-power field of interstitium; P < 0.001).
Figure 2.
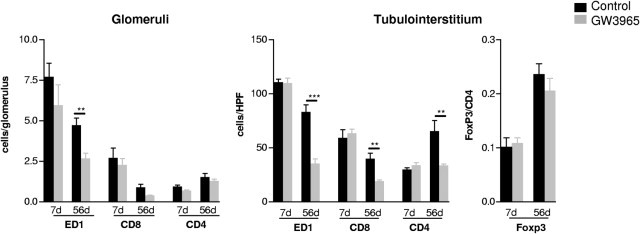
The effects of LXR activation by GW3965 on mononuclear cell infiltrate in rat kidney allografts 7 and 56 days after transplantation. Phenotyping of the mononuclear cell infiltrate of rat kidney allografts by IHC evidenced a significant reduction of ED1+ macrophages (glomeruli and tubulointerstitium) as well as CD8+ and CD4+ T cells in the tubulointerstitial area of kidney allografts 56 days after transplantation by GW3965; the number of FoxP3+ T cells was also lower in kidney allografts at this time point. The magnitude of glomerular and tubulointerstitial mononuclear cell infiltrate was not significantly different between controls and GW3965-treated grafts 7 days after transplantation. Mean ± SEM; **P < 0.01, ***P < 0.001 versus controls.
Myofibroblasts (α-SMA+) in the interstitium of kidney allografts decreased significantly with LXR activation for 56 days (Figure 3, A–C). Interstitial fibrosis evaluated by Goldner-Masson-Trichrom was up to sixfold lower (control versus GW: 49.5% ± 1.5% versus 8.2% ± 2.1% of interstitium; P < 0.01); in addition, the extracellular matrix components collagen I and III were reduced threefold to fourfold by the LXR agonist (Figure 3, D–I).
Figure 3.
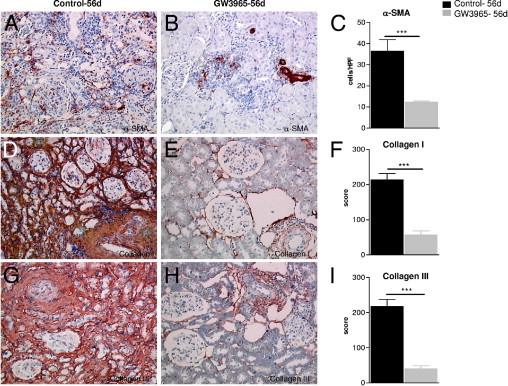
Effects of LXR activation by GW3965 on fibroblasts (A–C) and extracellular matrix proteins (D–I) in rat kidney allografts 56 days after transplantation. Micrograph showing the high number of α-SMA+ (A) myofibroblasts in an untreated kidney allograft; considerably less α-SMA+ fibroblasts can be seen in GW3965 transplants 56 days after transplantation (B). Diffuse deposition of collagen I (D) and III (G) in the tubulointerstitium of control renal allografts 56 days after transplantation. By GW3965 a significant reduction of tubulointerstitial collagen I (E) and III (H) was seen. The results of semiquantitative evaluation (see Materials and Methods) are presented as mean ± SEM; ***P < 0.001 versus controls (C, F, and I). Original magnification, ×200 (A, B, D, E, G, and H; avidin biotin complex staining).
The mRNA expression of IL-4, IL-10, IL-13, MCP-1, and MIP-1β were analyzed by real-time RT PCR. Severn days after transplantation there were no significant differences in the intragraft mRNA expression levels of these cytokines/chemokines with the exception of IL-13, which was significantly higher (see Supplemental Table S1 at http://ajp.amjpathol.org). At day 56 cytokine expression was significantly lower in the allografts treated with LXR agonist, further showing the relevant reduction of inflammation and fibrosis in these kidneys (Table 3).
Table 3.
Effects of GW3965 on mRNA Expression of Cytokines/Chemokines in Rat Kidney Allografts 56 Days after Transplantation
| Groups | IL-4 | IL-10 | IL-13 | MCP-1 | MIP-1β |
|---|---|---|---|---|---|
| Control | 1.07 ± 0.2 × 10−2 | 0.32 ± 0.1 | 0.62 ± 0.1 × 10−2 | 6.79 ± 2.8 | 0.20 ± 0.1 |
| GW3965 | 0.53 ± 0.1 × 10−2⁎ | 0.11 ± 0.1 | 0.27 ± 0.1 × 10−2 | 0.32 ± 0.3⁎ | 0.02 ± 0.0† |
Values were normalized to cyclophilin mRNA levels and are shown as mean ± SEM; n = 5 to 6 animals per group.
P < 0.05 versus controls.
P < 0.01 versus controls.
IHC for C4d was performed to test whether the LXR stimulation by GW3965 could modulate the antibody-mediated rejection in rat kidney allografts. An evident linear or granular positivity of both of peritubular capillaries could be observed in control allografts 56 days after transplantation; this was almost totally missing in GW3965 transplants (Figure 4).
Figure 4.
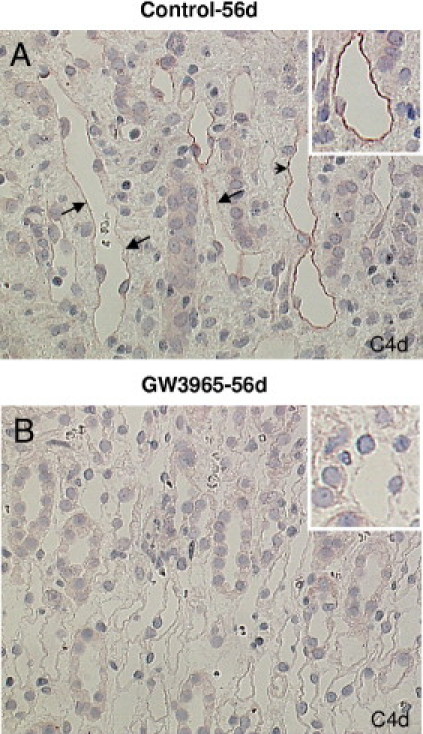
Effects of LXR activation by GW3965 on antibody-mediated rejection in rat kidney allografts 56 days after transplantation. Micrograph showing linear (arrowhead) and granular (arrows) C4d-stained peritubular capillaries in control renal allografts 56 days after transplantation (A). By GW3965 no stained capillaries could be observed (B). Original magnification, ×400 (avidin biotin complex staining).
Overexpression of LXRα in Recipient Macrophages (mLXRα-tg) Is Associated with Reduced Damage in Kidney Allografts
To study the role of macrophage LXRα in allograft rejection kidneys of BALB/c mice were transplanted into recipients with transgenic overexpression of LXRα in macrophages (mLXRα-tg). Transgenic animals have been shown to express selectively and increased levels of LXRα in macrophages and brain.17
In this fully major histocompatibility complex–mismatched model of renal transplantation characteristic features of acute (7 days after transplantation) and chronic (day 42) damage have developed.7,21 Histologic evaluation showed a moderate amelioration of acute glomerular and vascular rejection at day 7 but a significant reduction of all acute (P < 0.001) and chronic (P < 0.05) rejection scores in kidney grafts of mLXRα-tg recipients at day 42 after transplantation (Figure 5, A–C). Fibrosis was reduced (Goldner-Masson-Trichrom–stained kidney sections: WT versus mLXRα-tg, 19.9% ± 2.6% versus 7.3% ± 1.0% of interstitium; P < 0.05). Correspondingly, serum creatinine and urea were significantly lower (Figure 5D).
Figure 5.
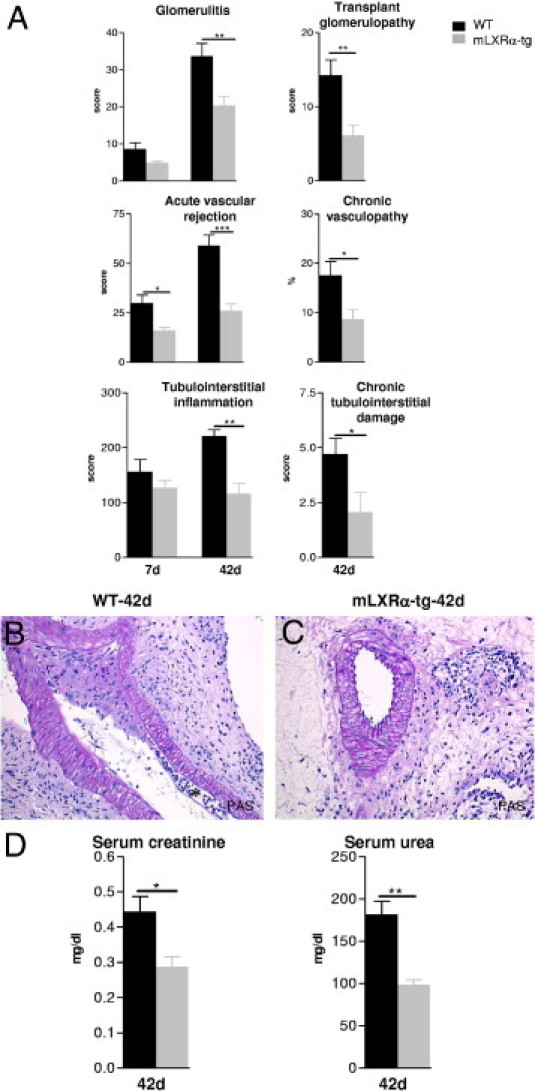
The effects of LXRα overexpression in recipient macrophages (mLXRα-tg) on structure (A–C) and excretory function (D) of mice renal allografts 7 and 42 days after transplantation. A: Significant amelioration of acute and chronic glomerular, vascular, and tubulointerstitial changes in kidney allografts of mLXRα-tg recipients in comparison to grafts of WT recipients 42 days after transplantation. Mean ± SEM; *P < 0.05, **P < 0.01, and ***P < 0.001 mLXRα-tg versus WT. B: A pre-glomerular artery of a graft transplanted into WT recipient showing an important constriction of the vessel lumen by a subendothelial mononuclear cell infiltrate and matrix increase. Subendothelial foam cells also can be observed (asterisk). Important perivascular fibrosis with mononuclear cell infiltrate. C: Regular aspect of a pre-glomerular artery of a graft transplanted into mLXRα-tg recipient. Perivascular fibrosis and mononuclear cell infiltrate are reduced. D: Graft excretory functional parameters (serum creatinine, urea) of mLXRα-tg recipients were significantly lower compared with grafts of WT recipients 42 days after transplantation. Mean ± SEM; *P < 0.05, **P < 0.01 mLXRα-tg versus WT. Original magnification: ×200 (B and C; PAS staining).
A relevant decrease of F4/80+ macrophages was detected in mLXRα-tg recipients at day 42 after transplantation (P < 0.01 for glomeruli; P < 0.001 for interstitium; Figure 6). The number of interstitial CD3+ lymphocytes was also significantly lower in comparison to grafts of WT recipients (Figure 6). The α-SMA+ myofibroblasts and collagen I/III were found to be significantly less expressed in mLXRα-tg recipients (Table 4).
Figure 6.
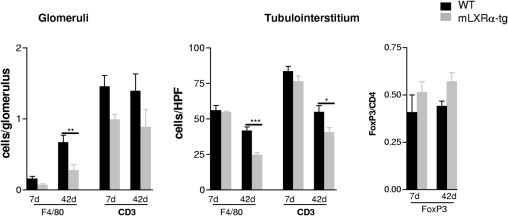
Effects of LXRα overexpression in recipient macrophages (mLXRα-tg) on mononuclear cell infiltrate of mice renal allografts 7 and 42 days after transplantation. Graphs presenting a significant reduction of F4/80+ macrophages (glomeruli and tubulointerstitium) and CD3+ T cells (tubulointerstitium) in mice kidney allografts of mLXRα-tg recipients 42 days after transplantation in comparison to graft of WT recipients. The number of interstitial FoxP3+ T cells did not change in grafts of mLXRα-recipients. Mean ± SEM; *P < 0.05, **P < 0.01, and ***P < 0.001 mLXRα-tg versus WT.
Table 4.
Myofibroblasts and Collagen Deposition in Kidney Allografts of mLXRα-tg and WT Recipients 42 Days after Transplantation
| Groups | α-SMA (cells/HPF) | Collagen I/III (score) |
|---|---|---|
| WT | 16.8 ± 2.4 | 171.6 ± 12.6 |
| mLXRα-tg | 10.2 ± 1.7⁎ | 94.3 ± 12.0† |
Values are mean ± SEM.
HPF, high-power field.
P < 0.05 versus controls.
P < 0.01 versus controls.
Seven days after transplantation IL-4, IL-10, and IL-13 mRNA expression levels were higher in grafts of mLXRα-tg recipients (see Supplemental Table S2 at http://ajp.amjpathol.org). At day 42 after transplantation, corresponding to the overall attenuated inflammation, both the mRNA expression of Th1 and Th2 response-associated pro-inflammatory genes (IL-4, MCP-1, MIP-1β) was significantly lower in kidneys of mLXRα-tg recipients in comparison to grafts of WT recipients. No statistically significant differences were noted in the expression of IL-10 and IL-13 at this time point (Table 5).
Table 5.
Effects of LXRα Overexpression in Macrophages on mRNA Expression of Cytokines/Chemokines in Mouse Kidney Allografts 42 Days after Transplantation
| Groups | IL-4 | IL-10 | IL-13 | MCP-1 | MIP-1β |
|---|---|---|---|---|---|
| WT | 0.11 ± 0.0 × 10−2 | 0.35 ± 0.1 × 10−2 | 0.77 ± 0.1 × 10−3 | 1.71 ± 0.6 × 10−2 | 0.03 ± 0.0 |
| mLXRα-tg | 0.02 ± 0.0 × 10−2⁎ | 0.22 ± 0.1 × 10−2 | 0.88 ± 0.2 × 10−3 | 0.16 ± 0.1 × 10−2† | 0.01 ± 0.0⁎ |
Values were normalized to mRNA expression of GAPDH and are shown as mean ± SEM; n = 4 to 6 animals per group.
P < 0.05 versus controls.
P < 0.01 versus controls.
Reduced Inflammatory Activity of BMDMs by LXRs
It has been reported that ligand activation of LXRs in macrophages dampens its inflammatory phenotype.14–16 To test whether similar changes can be observed also in macrophages overexpressing LXRα, we measured the secretion of classical mediators of inflammation in the supernatant fluid of BMDMs of mLXRα-tg mice. In parallel BMDMs pretreated with GW3965 were analyzed. Similarly to macrophages with GW3965, mLXRα-tg macrophages secreted significantly less MCP-1 and MIP-1β (Figure 7).
Figure 7.
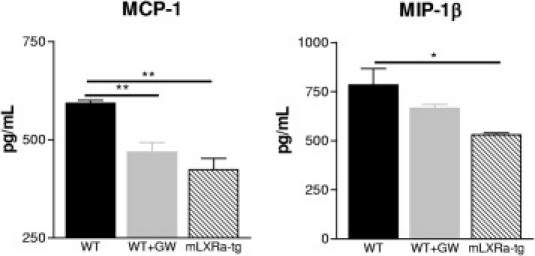
In vitro effects of GW3965 and LXRα overexpression on inflammatory activity of BMDMs. BMDMs were pre-incubated with DMSO (WT and mLXRα-tg) or 3 μmol/L GW3965 dissolved in DMSO (WT) for 16 hours. Both GW3965 treatment and LXRα overexpression resulted in secretion of significantly lower amounts of MCP-1 and MIP-1β after 12 hours. Bar diagrams show mean values ± SEMs for three different experiments; *P < 0.05, **P < 0.01 versus WT.
Markers of Alternative Macrophage Activation Induced by LXRs
Markers of alternatively activated macrophages were evaluated by IHC and real-time RT-PCR in vivo and in vitro. A significant increase in the number of interstitial Arg1+ cells and an evident increase in the mRNA expression of mannose receptor C type 1 (Mrc1) was found in GW3965-treated rat allografts 7 days after transplantation (Figure 8). This could be of functional relevance because at this time point no differences in the number of intragraft ED1+ macrophages between untreated and treated grafts were found. Consonant with the overall reduction of monocytes/macrophages at day 56 by GW3965, Arg1+ interstitial macrophages were also decreased in number at this chronic stage after transplantation (Figure 2, and Figure 8, A–D). A similar expression pattern was observed in kidney allografts of mice with the macrophage-specific LXRα transgene: 7 days after transplantation Arg1+ and Mrc1+ cells were significantly more numerous, whereas the number of overall F4/80+ macrophages remained unchanged. Forty-two days after transplantation parallel to the reduced number of F4/80+ macrophages the number of cells positive for markers of alternative activation (Arg1, Mrc1) was significantly lower in grafts of mLXRα-tg recipients (Figure 8, E and F). In vitro in BMDM GW3965 accentuated IL-4 and IL-13 induced M2 marker genes Arg1, Mrc1, chitinase 3-like 3 (Chi3l3/Ym1), found in inflammatory zone 1 (Fizz1) and programed cell death 1 ligand 2 (Pdcd1lg2). This effect depended on the LXRα isoform because LXRα-tg macrophages showed similar alternative activation of macrophages as those activated by the general LXR ligand, GW3965 (see Supplemental Figure S1 at http://ajp.amjpathol.org).
Figure 8.
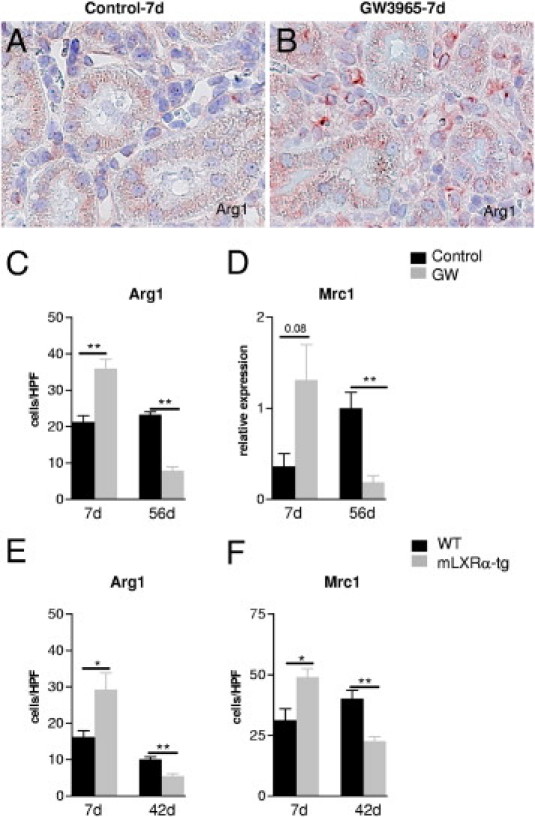
Effects of LXR activity on markers of alternatively activated macrophages in kidney allografts. A higher number of Arg1+ cells in a rat kidney allograft with GW3965 (B) in comparison to an untreated graft (A) 7 days after transplantation. Quantification of Arg1+ cells (C) and the mRNA expression of the M2 marker gene Mrc1 (D) in rat kidney allografts 7 and 56 days after transplantation without and with GW3965. Number of Arg1+ (E) and Mrc1+ cells (F) in mouse kidney allografts of WT and mLXRα-recipients 7 and 42 days after transplantation. Note that the total macrophage population at day 7 after transplantation is similar in the control and GW groups but strongly reduced only in LXR rat and mouse kidney allografts at days 56 and 42, respectively. Mean ± SEM cells/high-power field. *P < 0.05 GW versus controls; **P < 0.01 mLXRα-tg versus WT. mRNA expression of Mrc1 in rat kidney allografts was normalized to cyclophilin mRNA (real-time RT-PCR).
Reduced T-Cell Response to Alloantigens by LXRs in Vitro
In addition to macrophages lymphocytes are prominent players of allograft rejection and might be targets of the LXR agonist. To test the effects of LXR stimulation on the reactivity of T cells in an alloantigen mixed lymphocyte reaction, T cell–depleted splenocytes from BALB/c mice were co-cultured with T cells purified from spleens of C57BL/6 mice. GW3965-pretreated T cells secreted significantly lower amounts of IL-2 and IFN-γ as measured in the cell-culture supernatant fluids after 24 and 72 hours of co-incubation (Figure 9).
Figure 9.
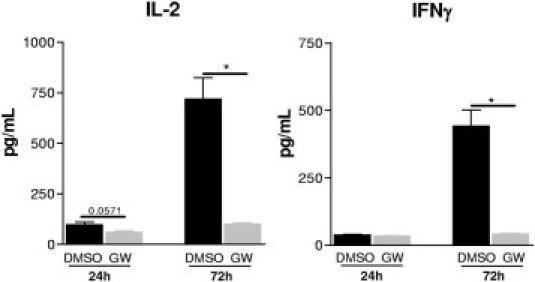
In vitro effects of LXR activation by GW3965 on T-cell response to alloantigens. Mixed lymphocyte reaction was performed with co-incubating T cell–depleted splenocytes from BALB/c mice with T cells purified from spleens of C57BL/6 mice. GW3965-pretreated T cells (3 μmol/L for 16 hours) secreted significantly lower amounts of IL-2 and IFN-γ, as measured in the cell culture supernatant fluids after 24 and 72 hours co-incubation. Mean ± SEM; n = 4; *P < 0.05 versus DMSO.
Discussion
The Fisher 344 to Lewis rat renal transplantation model is a model of allograft rejection between rat strains differing in minor histocompatibility antigens This transplantation model has been shown to exhibit several features of chronic allograft damage encountered in human allografts treated with immunosuppressants and thereby lacking the full alloreactivity of an untreated major histocompatibility mismatched graft.18,31,32 Macrophages constitute a major fraction of mononuclear cells in the chronic phase of this model comparable to the high number of macrophages in human renal transplants with transplant glomerulopathy and chronic tubulointerstitial damage.3,18 LXR activation by the synthetic agonist GW3965 administered for 56 days after transplantation inhibited the development of advanced chronic changes in rat kidney grafts; graft excretory function was correspondingly preserved. The significant improvement of chronic damage by LXR activation was associated with an important decrease of the mononuclear cell infiltrate.
Studies have characterized LXRs as key regulators of macrophage function.14–16 Macrophage accumulation in the transplanted kidney, through recruitment of blood monocytes and subsequent proliferation within the graft, typically precedes and accompanies fibrosis of the grafted organ.1,3
Administration of GW3965 for 56 days reduced infiltrating monocytes/macrophages (ED1+) in the tubulointerstitium of rat kidney allografts by 60% and diminished the intragraft expression of pro-inflammatory/profibrotic genes (eg, MCP-1, MIP-1β). Correspondingly to these in vivo data and in accordance with earlier reports, in BMDMs, treatment with GW3965 reduced the secretion of MCP-1 and MIP-1β. These data show that a suppressed inflammatory response of intragraft macrophages by LXR activation might be an important mechanism in prevention of graft fibrosis. The regulation of inflammatory signaling by LXRs in macrophages is thought to be mainly mediated by transrepression.15 Ligand activation of LXRs in murine macrophages has been shown to inhibit the induction of inflammatory genes (eg, iNOS, IL-1β, MCP-1) in response to lipopolysaccharide or cytokines (IL-1β and tumor necrosis factor-α) in part via blockade of NF-κB signaling.13,15 In addition to macrophages the number of intragraft CD4+ and CD8+ T cells was also significantly lower in the LXR group on day 56 after transplantation. These effects may involve a diminished T-lymphocyte activation and proliferation in kidney allografts by the LXR agonist. Performing a mixed lymphocyte reaction we found a reduced alloreactivity of GW3965-pretreated T cells. Bensinget et al11 could show that LXRβ is an intrinsic regulator of lymphocyte proliferation. By altering cellular sterol content through the cholesterol transporter, ATP-binding cassette transporter G1 (ABCG1), an LXR target gene, loss of LXRβ expression increased the proliferative capacity of lymphocytes, leading to enhanced homeostatic and antigen-driven responses; the opposite was observed by ligand activation of the receptor.11 In addition, vascular cells might have been affected. Blaschke et al33 have shown that LXR ligands suppress proliferation of vascular smooth muscle cells by the suppression of mitogen-induced degradation of the cyclin-dependent kinase inhibitor; this mechanism may have contributed to the reduced severity of chronic graft vasculopathy in GW3965-treated allografts. Thus, the prevention of fibrotic changes in rat kidney allografts by the nonselective LXR agonist is likely to be a suppression of activity of T cells, monocytes/macrophages, and resident renal cells. In addition, a damped antibody response by GW3965, evidenced by reduced C4d immunostaining, can also be considered to contribute to the beneficial effects of LXR activation in chronic kidney allograft rejection.
To investigate which LXR isoform is important for the modulation of macrophage activity in chronic allograft dysfunction, kidneys were transplanted into mice with selective overexpression of LXRα in macrophages (mLXRα-tg). In an earlier study we have demonstrated that macrophage overexpression of LXRα achieved by transgenic expression of the mouse LXRα cDNA under the control of a chicken lysozyme promoter resulted in the activation of LXRα target genes (ABCA1, ABCG1).17 Activated macrophages can produce higher amounts of endogenous ligands of LXRs [eg, 24(S)-hydroxycholesterol, 22(R)-hydroxycholesterol, 24(S),25-epoxycholesterol], which in the state of overexpressed receptor apparently lead to a biologically relevant activation without further administration of an exogenous ligand.34–36 Because the majority of kidney injury–associated macrophages are derived from the circulating monocyte pool,37 these experiments provided cell- and LXR isoform-specific information about LXR signaling in allograft rejection. These data about isoform-specific effects of LXRs are of practical interest for the generation of isoform-specific pharmacologic modulators without potential adverse metabolic effects (hypertriglyceridemia) ascribed to nonselective agonists.
In this fully major histocompatibility complex–mismatched mouse transplantation model kidney allografts of recipients with LXRα overexpression in macrophages had significantly reduced chronic graft damage shown by a strongly lowered F4/80+ macrophage, moderately lowered CD3+ lymphocytic intragraft infiltrate, and by less fibrosis. The lower expression of MCP-1, MIP-1β, and IL-4 reflected reduced Th1 and Th2 responses in the grafts of mLXRα-tg mice at day 42 after transplantation. In vitro, similarly to the GW3965-treated BMDMs, macrophages of the transgenic mice secreted less MCP-1 and MIP-1β. These results clearly pointed to a blunted pro-inflammatory macrophage phenotype by macrophage LXRα.
Chronic allograft dysfunction might involve not only a sustained pro-inflammatory reaction but also a failure of anti-inflammatory control mechanisms.1,2 The prevention of late chronic changes in rat renal allograft by GW3965 without reduction of the degree of mononuclear cell infiltrate in the early phase of rejection raised the question of whether LXR activation modulated the phenotype of the mononuclear cell infiltrate at this time point. Treg-mediated immunosuppression is an unlikely explanation for the improved late graft histology and function, because Foxp3+ T cells in renal allografts were not significantly different at day 7 after transplantation between the two groups and were even lower in grafts with GW3965 at day 56. Macrophages can switch from a classically activated state (M1) to an alternatively activated state (M2) on specific signals.4,38 M2 cells can generate Arg1, which suppresses inflammation by inhibiting the production of pro-inflammatory nitric oxide.38,39 Moreover, Arg1-expressing macrophages have been shown to suppress Th2 cytokine–driven liver inflammation and fibrosis; these effects were independent of IL-10 and transforming growth factor β1 and could be correlated to inhibited T-cell proliferation by Arg1-expressing macrophages in vitro.6 We have recently found a correlation of improved chronic renal transplantation outcome to alternative macrophage activation in kidney allografts of CCR5-deficient recipients.7 These data suggested an until now unappreciated role of alternatively activated macrophages in reduction of chronic renal injury. The higher number of Arg1- and Mrc1-expressing cells by LXR activation in kidney allografts 7 days after transplantation led us to assume that LXR might modulate the induction or activation of alternatively activated macrophages. With the use of IL-4– and IL-13– stimulated BMDMs, we have found the M2 marker genes (Arg1, Mrc1, Ym1, Fizz1, Pdcd1lg2) up-regulated in GW3965-treated as well as LXRα transgene macrophages. In addition, the higher expression of IL-4 (mouse) and IL-13 (rat and mouse) in kidney allografts 7 days after transplantation by LXR is analogous to published data that LXR activation can promote polarization of T cells to Th2 response.29 In the chronic phase of allograft rejection no evidence of increased alternative macrophage activation could be evidenced in kidney allografts. One explanation for that might be the overall reduced number of macrophages as well as cytokine levels by LXRs at this later time point.
In conclusion, our studies have shown that LXR activation by the synthetic ligand GW3965 could prevent chronic renal allograft damage. In addition, our data indicated a relevant role specifically of macrophage LXRα in the suppression of chronic renal allograft damage.
Footnotes
Supported by grants from the Deutsche ForschungsgemeinschaftSFB 938, G/P (H.-J.G and S.P.), GR 880/3 (H.-J.G.) and EU grant INNOCHEM (H.-J.G.).
Supplemental material for this manuscript can be found at http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2011.03.019.
Supplementary data
In vitro effects of LXR activation by GW3965 and overexpression of LXRα on the expression of markers of alternatively activated macrophages with the use of BMDMs. mRNA expression of marker genes of alternatively activated macrophages (Arg1, Mrc1, Ym1, Fizz1, Pdcd1lg2) in BMDMs of WT (without and with pre-incubation with GW3965 3 μmol/L for 16 hours) and mLXRα-tg mice after stimulation with IL4 and IL13 (each 10 ng/mL) for 12 hours. Values represent mean ± SEM (normalized to tubulin mRNA) for three mice measured in duplicates. The IL4/IL13 stimulation induced an increase in the expression of M2 marker genes and was accentuated by pre-incubation of the WT macrophages by GW3965. LXRα overexpression also resulted in an increase in the expression of M2 marker genes in comparison to IL4/IL13-stimulated WT macrophages. Mean ± SEM; *P < 0.05 versus WT + IL4/IL13, and #P < 0.05 mLXRα-tg versus WT + IL4/IL13 + GW.
References
- 1.Yates P.J., Nicholson M.L. The aetiology and pathogenesis of chronic allograft nephropathy. Transpl Immunol. 2006;16:148–157. doi: 10.1016/j.trim.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Joosten S.A., Sijpkens Y.W., van Kooten C., Paul L.C. Chronic renal allograft rejection: pathophysiologic considerations. Kidney Int. 2005;68:1–13. doi: 10.1111/j.1523-1755.2005.00376.x. [DOI] [PubMed] [Google Scholar]
- 3.Wyburn K.R., Jose M.D., Wu H., Atkins R.C., Chadban S.J. The role of macrophages in allograft rejection. Transplantation. 2005;80:1641–1647. doi: 10.1097/01.tp.0000173903.26886.20. [DOI] [PubMed] [Google Scholar]
- 4.Martinez F.O., Helming L., Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y., Wang Y.P., Zheng G., Lee V.W., Ouyang L., Chang D.H., Mahajan D., Coombs J., Wang Y.M., Alexander S.I., Harris D.C. Ex vivo programmed macrophages ameliorate experimental chronic inflammatory renal disease. Kidney Int. 2007;72:290–299. doi: 10.1038/sj.ki.5002275. [DOI] [PubMed] [Google Scholar]
- 6.Pesce J.T., Ramalingam T.R., Mentink-Kane M.M., Wilson M.S., El Kasmi K.C., Smith A.M., Thompson R.W., Cheever A.W., Murray P.J., Wynn T.A. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 2009;5:e1000371. doi: 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dehmel S., Wang S., Schmidt C., Kiss E., Loewe R.P., Chilla S., Schlöndorff D., Gröne H.J., Luckow B. Chemokine receptor Ccr5 deficiency induces alternative macrophage activation and improves long-term renal allograft outcome. Eur J Immunol. 2010;40:267–278. doi: 10.1002/eji.200939652. [DOI] [PubMed] [Google Scholar]
- 8.Schultz J.R., Tu H., Luk A., Repa J.J., Medina J.C., Li L., Schwendner S., Wang S., Thoolen M., Mangelsdorf D.J., Lustig K.D., Shan B. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14:2831–2838. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins J.L., Fivush A.M., Watson M.A., Galardi C.M., Lewis M.C., Moore L.B., Parks D.J., Wilson J.G., Tippin T.K., Binz J.G., Plunket K.D., Morgan D.G., Beaudet E.J., Whitney K.D., Kliewer S.A., Willson T.M. Identification of a nonsteroidal liver X receptor agonist through parallel array synthesis of tertiary amines. J Med Chem. 2002;45:1963–1966. doi: 10.1021/jm0255116. [DOI] [PubMed] [Google Scholar]
- 10.Repa J.J., Mangelsdorf D.J. The liver X receptor gene team: potential new players in atherosclerosis. Nat Med. 2002;8:1243–1248. doi: 10.1038/nm1102-1243. [DOI] [PubMed] [Google Scholar]
- 11.Bensinger S.J., Bradley M.N., Joseph S.B., Zelcer N., Janssen E.M., Hausner M.A., Shih R., Parks J.S., Edwards P.A., Jamieson B.D., Tontonoz P. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134:97–111. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong C., Tontonoz P. Coordination of inflammation and metabolism by PPAR and LXR nuclear receptors. Curr Opin Genet Dev. 2008;18:461–467. doi: 10.1016/j.gde.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogawa S., Lozach J., Benner C., Pascual G., Tangirala R.K., Westin S., Hoffmann A., Subramaniam S., David M., Rosenfeld M.G., Glass C.K. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell. 2005;122:707–721. doi: 10.1016/j.cell.2005.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castrillo A., Joseph S.B., Marathe C., Mangelsdorf D.J., Tontonoz P. Liver X receptor-dependent repression of matrix metalloproteinase-9 expression in macrophages. J Biol Chem. 2003;278:10443–10449. doi: 10.1074/jbc.M213071200. [DOI] [PubMed] [Google Scholar]
- 15.Joseph S.B., Castrillo A., Laffitte B.A., Mangelsdorf D.J., Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9:213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 16.Marathe C., Bradley M.N., Hong C., Lopez F., Ruiz de Galarreta C.M., Tontonoz P., Castrillo A. The arginase II gene is an anti-inflammatory target of liver X receptor in macrophages. J Biol Chem. 2006;281:32197–32206. doi: 10.1074/jbc.M605237200. [DOI] [PubMed] [Google Scholar]
- 17.Teupser D., Kretzschmar D., Tennert C., Burkhardt R., Wilfert W., Fengler D., Naumann R., Sippel A.E., Thiery J. Effect of macrophage overexpression of murine liver X receptor-alpha (LXR-alpha) on atherosclerosis in LDL-receptor deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:2009–2015. doi: 10.1161/ATVBAHA.108.175257. [DOI] [PubMed] [Google Scholar]
- 18.Adams J., Kiss E., Arroyo A.B., Bonrouhi M., Sun Q., Li Z., Gretz N., Schnitger A., Zouboulis C.C., Wiesel M., Wagner J., Nelson P.J., Gröne H.J. 13-cis retinoic acid inhibits development and progression of chronic allograft nephropathy. Am J Pathol. 2005;167:285–298. doi: 10.1016/S0002-9440(10)62973-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bedke J., Kiss E., Schaefer L., Behnes C.L., Bonrouhi M., Gretz N., Horuk R., Diedrichs-Moehring M., Wildner G., Nelson P.J., Gröne H.J. Beneficial effects of CCR1 blockade on the progression of chronic renal allograft damage. Am J Transplant. 2007;7:527–537. doi: 10.1111/j.1600-6143.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- 20.Li N., Rivéra-Bermúdez M.A., Zhang M., Tejada J., Glasson S.S., Collins-Racie L.A., Lavallie E.R., Wang Y., Chang K.C., Nagpal S., Morris E.A., Flannery C.R., Yang Z. LXR modulation blocks prostaglandin E2 production and matrix degradation in cartilage and alleviates pain in a rat osteoarthritis model. Proc Natl Acad Sci U S A. 2010;107:3734–3739. doi: 10.1073/pnas.0911377107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang S., Schmaderer C., Kiss E., Schmidt C., Bonrouhi M., Porubsky S., Gretz N., Schaefer L., Kirschning C.J., Popovic Z.V., Gröne H.J. Recipient Toll-like receptors contribute to chronic graft dysfunction by both MyD88- and TRIF-dependent signaling. Dis Model Mech. 2010;3:92–103. doi: 10.1242/dmm.003533. [DOI] [PubMed] [Google Scholar]
- 22.Keppler A., Gretz N., Schmidt R., Kloetzer H.M., Groene H.J., Lelongt B., Meyer M., Sadick M., Pill J. Plasma creatinine determination in mice and rats: an enzymatic method compares favorably with a high-performance liquid chromatography assay. Kidney Int. 2007;71:74–78. doi: 10.1038/sj.ki.5001988. [DOI] [PubMed] [Google Scholar]
- 23.Kiss E., Adams J., Grone H.J., Wagner J. Isotretinoin ameliorates renal damage in experimental acute renal allograft rejection. Transplantation. 2003;76:480–489. doi: 10.1097/01.TP.0000066354.31050.5A. [DOI] [PubMed] [Google Scholar]
- 24.Weischenfeldt J., Porse B. Bone marrow-derived macrophages (BMM): isolation and applications. CSH Protoc. 2008 doi: 10.1101/pdb.prot5080. [DOI] [PubMed] [Google Scholar]
- 25.Gersuk G.M., Razai L.W., Marr K.A. Methods of in vitro macrophage maturation confer variable inflammatory responses in association with altered expression of cell surface dectin-1. J Immunol Methods. 2008;329:157–166. doi: 10.1016/j.jim.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Porubsky S., Wang S., Kiss E., Dehmel S., Bonrouhi M., Dorn T., Luckow B., Brakebusch C., Gröne H.J. Rhoh deficiency reduces peripheral T-cell function and attenuates allogenic transplant rejection. Eur J Immunol. 2011;41:76–88. doi: 10.1002/eji.201040420. [DOI] [PubMed] [Google Scholar]
- 27.Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 28.Chisholm J.W., Hong J., Mills S.A., Lawn R.M., Jeffrey W. The LXR ligand T0901317 induces severe lipogenesis in the db/db diabetic mouse. J Lipid Res. 2003;44:2039–2048. doi: 10.1194/jlr.M300135-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Geyeregger R., Shehata M., Zeyda M., Kiefer F.W., Stuhlmeier K.M., Porpaczy E., Zlabinger G.J., Jäger U., Stulnig T.M. Liver X receptors interfere with cytokine-induced proliferation and cell survival in normal and leukemic lymphocytes. J Leukoc Biol. 2009;86:1039–1048. doi: 10.1189/jlb.1008663. [DOI] [PubMed] [Google Scholar]
- 30.Vedin L.L., Lewandowski S.A., Parini P., Gustafsson J.A., Steffensen K.R. The oxysterol receptor LXR inhibits proliferation of human breast cancer cells. Carcinogenesis. 2009;30:575–579. doi: 10.1093/carcin/bgp029. [DOI] [PubMed] [Google Scholar]
- 31.von Toerne C., Schmidt C., Adams J., Kiss E., Bedke J., Porubsky S., Gretz N., Lindenmeyer M.T., Cohen C.D., Gröne H.J., Nelson P.J. Wnt pathway regulation in chronic renal allograft damage. Am J Transplant. 2009;9:2223–2239. doi: 10.1111/j.1600-6143.2009.02762.x. [DOI] [PubMed] [Google Scholar]
- 32.White E., Hildemann W.H., Mullen Y. Chronic kidney allograft reactions in rats. Transplantation. 1969;8:602–617. doi: 10.1097/00007890-196911000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Blaschke F., Leppanen O., Takata Y., Caglayan E., Liu J., Fishbein M.C., Kappert K., Nakayama K.I., Collins A.R., Fleck E., Hsueh W.A., Law R.E., Bruemmer D. Liver X receptor agonists suppress vascular smooth muscle cell proliferation and inhibit neointima formation in balloon-injured rat carotid arteries. Circ Res. 2004;95:e110–e123. doi: 10.1161/01.RES.0000150368.56660.4f. [DOI] [PubMed] [Google Scholar]
- 34.Hultén L.M., Lindmark H., Diczfalusy U., Björkhem I., Ottosson M., Liu Y., Bondjers G., Wiklund O. Oxysterols present in atherosclerotic tissue decrease the expression of lipoprotein lipase messenger RNA in human monocyte-derived macrophages. J Clin Invest. 1996;97:461–468. doi: 10.1172/JCI118436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rowe A.H., Argmann C.A., Edwards J.Y., Sawyez C.G., Morand O.H., Hegele R.A., Huff M.Y. Enhanced synthesis of the oxysterol 24(S),25-epoxycholesterol in macrophages by inhibitors of 2,3-oxidosqualene: lanosterol cyclase. Circ Res. 2003;93:717–725. doi: 10.1161/01.RES.0000097606.43659.F4. [DOI] [PubMed] [Google Scholar]
- 36.Shibata N., Glass C.K. Regulation of macrophage function in inflammation and atherosclerosis. J Lipid Res. 2009;50(Suppl):S277–S281. doi: 10.1194/jlr.R800063-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ricardo S.D., van Goor H., Eddy A.A. Macrophage diversity in renal injury and repair. J Clin Invest. 2008;118:3522–3530. doi: 10.1172/JCI36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varin A., Gordon S. Alternative activation of macrophages: immune function and cellular biology. Immunobiology. 2009;214:630–641. doi: 10.1016/j.imbio.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Porcheray F., Viaud S., Rimaniol A.C., Léone C., Samah B., Dereuddre-Bosquet N., Dormont D., Gras G. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol. 2005;142:481–489. doi: 10.1111/j.1365-2249.2005.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In vitro effects of LXR activation by GW3965 and overexpression of LXRα on the expression of markers of alternatively activated macrophages with the use of BMDMs. mRNA expression of marker genes of alternatively activated macrophages (Arg1, Mrc1, Ym1, Fizz1, Pdcd1lg2) in BMDMs of WT (without and with pre-incubation with GW3965 3 μmol/L for 16 hours) and mLXRα-tg mice after stimulation with IL4 and IL13 (each 10 ng/mL) for 12 hours. Values represent mean ± SEM (normalized to tubulin mRNA) for three mice measured in duplicates. The IL4/IL13 stimulation induced an increase in the expression of M2 marker genes and was accentuated by pre-incubation of the WT macrophages by GW3965. LXRα overexpression also resulted in an increase in the expression of M2 marker genes in comparison to IL4/IL13-stimulated WT macrophages. Mean ± SEM; *P < 0.05 versus WT + IL4/IL13, and #P < 0.05 mLXRα-tg versus WT + IL4/IL13 + GW.


