Abstract
Progressive splenomegaly is a hallmark of visceral leishmaniasis in humans, canids, and rodents. In experimental murine visceral leishmaniasis, splenomegaly is accompanied by pronounced changes in microarchitecture, including expansion of the red pulp vascular system, neovascularization of the white pulp, and remodeling of the stromal cell populations that define the B-cell and T-cell compartments. Here, we show that Ly6C/G+ (Gr-1+) cells, including neutrophils and inflammatory monocytes, accumulate in the splenic red pulp during infection. Cell depletion using monoclonal antibody against either Ly6C/G+ (Gr-1; RB6) or Ly6G+ (1A8) cells increased parasite burden. In contrast, depletion of Ly6C/G+ cells, but not Ly6G+ cells, halted the progressive remodeling of Meca-32+ and CD31+ red pulp vasculature. Strikingly, neither treatment affected white pulp neovascularization or the remodeling of the fibroblastic reticular cell and follicular dendritic cell networks. These findings demonstrate a previously unrecognized compartment-dependent selectivity to the process of splenic vascular remodeling during experimental murine visceral leishmaniasis, attributable to Ly6C+ inflammatory monocytes.
Leishmaniasis, caused by protozoan parasites (genus Leishmania), is a major neglected disease in resource-poor countries. The species L. donovani and L. infantum are responsible for visceral leishmaniasis, the most severe form of the disease, which if untreated is almost universally fatal.1 In mouse models, as with human and canine disease, the spleen is the site of chronic infection.2 As infection progresses, marked splenomegaly develops, associated with extensive remodeling of the lymphoid tissue microarchitecture. Elements of the marginal zone are either lost or disrupted in their spatial organization,3 and follicular dendritic cells (FDCs) and fibroblastic reticular cells (FRCs), the key stromal elements that underpin B cell follicle integrity and form the T-cell zone conduit system, respectively,4, 5 are progressively denuded.
More recently, we and others have demonstrated that, in murine experimental visceral leishmaniasis (EVL)6 and in canine VL,7 there are pronounced changes to the splenic vasculature that accompany splenomegaly. Notably, in EVL, Meca-32+ red pulp vessels are greatly enlarged and show signs of angiogenesis. In the white pulp, neovascularization leads to the appearance of numerous CD31+αSMA-1+ vessels that develop coincident with a breakdown in the organization of the FRC and FDC networks.6 Studies in wild-type mice treated with neutralizing antibodies and in tumor necrosis factor (TNF)-deficient mice have implicated the proinflammatory cytokine tumor necrosis factor (which is overexpressed by macrophages and many other cells during EVL) as a mediator of white pulp stromal cell remodeling.3, 4 However, little is known about the regulation of vascular remodeling within the red pulp or neovascularization in the white pulp.
Both neutrophils and inflammatory monocytes have recently attracted much interest in the study of leishmaniasis, by acting as a host cells during infection8, 9, 10 and by playing a variety of regulatory roles that affect late stages of both visceral and cutaneous disease.10, 11, 12 More recently, neutrophils that express matrix metalloproteinase 9 (MMP-9),13, 14, 15 as well as inflammatory monocytes,16 have been implicated in the regulation of angiogenesis in a variety of experimental models of disease. In the context of EVL, however, it is currently unknown whether either cell population plays a role in the splenic tissue remodeling that occurs during progressive disease.
In the present study, therefore, we sought to determine the effects of depletion of these cells on the progressive changes to splenic architecture that are characteristic of this disease. With use of flow cytometry and confocal microscopy, we show that Ly6C/Ghigh neutrophils and Ly6C/Gint inflammatory monocytes are located within the splenic red pulp and that their number increases during infection. Depletion of either Ly6C/Gint/high cells [using Gr-1 monoclonal antibody (mAb) RB6] or Ly6G+ cells (using mAb 1A8) from day 21 to day 28 of infection increased splenic parasite load, but these treatments had distinctly different effects on splenic remodeling. Gr-1 administration led to a selective arrest in red pulp vascular remodeling, whereas neovascularization and the disruption of stromal cell networks in the white pulp continued unabated. In contrast, administration of mAb 1A8 had no effect on any aspect of splenic remodeling that we measured. These data demonstrate for the first time that the regulation of splenic vascular remodeling during EVL occurs in a strictly compartmentalized manner and is independent of mechanisms that control parasite burden.
Materials and Methods
Mice and Infection
Female C57BL/6 mice, 6 to 8 weeks old, were obtained from Charles River UK (Margate, UK) and were housed under specific-pathogen-free conditions. L. donovani (LV9) amastigotes were obtained from the spleens of infected Syrian hamsters, as described previously.17 Mice were injected with 3 × 107 amastigotes i.v. via the lateral tail vein. At day 21 and day 24 after infection, mice were administered 0.2 mg i.p. of Ly6C/G-specific Gr-1 (RB6-8C5) or 0.25 mg Ly6G-specific (1A8) mAbs or their isotype control antibodies (AFRC Mac-5 or Mac-4, respectively). Mice were sacrificed by cervical dislocation at day 28 and spleens were removed for analysis. All experiments were approved by the institutional ethics committee and were performed under U.K. Home Office license.
IHC
Spleen sections (10-μm thick) cut from spleens of both uninfected and infected mice were fixed in ice-cold acetone or 2% paraformaldehyde at room temperature for 15 minutes. Paraformaldehyde-fixed tissue sections were then blocked and permeabilized by 0.1% bovine serum albumin (w/w), 0.1% (v/v) saponin in PBS for 1 hour at room temperature. For MMP-9 localization, sections were double-stained with biotinylated rat anti-MMP-9 (R&D Systems, Minneapolis, MN) and with allophycocyanin-conjugated Gr-1, F4/80, or CD169 (eBioscience, San Diego, CA) primary antibodies, followed by streptavidin Alexa Fluor 488 or streptavidin Alexa Fluor 647. Slides were washed three times with 0.1% (v/v) saponin in PBS. The sections were dehydrated after staining, mounted with antifade solution (Molecular Probes, Eugene, OR), and visualized using a Zeiss Axioplan LSM 510 confocal microscope. For the assessment of vascular changes, sections were stained using biotinylated CD31 (eBioscience) or purified Meca-32 (BD Pharmingen, San Diego, CA) followed by Alexa Fluor 488 streptavidin and fluorescein isothiocyanate goat anti-rat antibody (both from Invitrogen, Carlsbad, CA). Localization of Gr-1+ and F4/80+ cells was performed using biotinylated mAb followed by streptavidin conjugated to Alexa Fluor 546 and Alexa Fluor 647 conjugated mAb, respectively. Data were analyzed by quantitative morphometry, as described previously.6
In Situ Zymography
In situ zymography with fluorescein isothiocyanate-labeled dye-quenched gelatin (Enz Chek; Molecular Probes, Eugene, OR) was used to determine the localization of MMP-9 activity in spleen sections. Unfixed sections (8-μm thick) were thawed, preincubated in PBS at room temperature, and incubated with PBS containing 40 μg/mL dye-quenched gelatin (1 mg/mL) in a humidified chamber overnight. At the end of the incubation period, sections were washed three times with PBS and then were dehydrated and mounted with antifade solution. As controls, a general MMP inhibitor, 1,10-phenanthroline (10 mmol/L; Sigma-Aldrich, St. Louis, MO), was added to the PBS/dye-quenched gelatin mixture and applied to frozen cryostat sections. Nonspecific serine protease activity was blocked using phenylmethylsulfonyl fluoride (Sigma-Aldrich). Images were acquired with a Zeiss Axioplan LSM 510 confocal microscope.
Flow Cytometry and Intracellular Enzyme Detection
Splenic cells from naïve or infected mice were homogenized in RPMI and cells were counted on hemocytometer. Then 1 to 2 × 106 cells per tube were resuspended in ice-cold PBS buffer consisting of 5 mmol/L EDTA, 1% fetal calf serum, and 1% NaNO3. All stainings were performed on ice. Splenic cells were surface stained with phycoerythrin or allophycocyanin conjugated Gr-1, Ly6G-phycoerythrin, Ly6C-fluorescein isothiocyanate, CD11b-Pacific blue, CD11c phycoerythrin-Cy7, and MHCII-allophycocyanin. GR-1 stained cells were washed twice with EDTA/fetal calf serum/NaNO3, resuspended in the same buffer, and incubated 15 minutes on ice with 2% paraformaldehyde. Fixed cells were then resuspended in PBS/EDTA/fetal calf serum/NaNO3 containing 0.5% saponin and intracellularly labeled with biotinylated anti-MMP-9. Biotinylated antibodies were visualized with fluorescein isothiocyanate-streptavidin. Background staining was judged from appropriate isotype control mAbs. Flow cytometric analysis was performed using a CyAn flow cytometry system (Beckman Coulter, High Wycombe, UK) and Summit 4.0 software.
Statistical Analysis
Statistical analysis was performed using a nonpaired Student's t-test and two-way analysis of variance. P < 0.05 was considered significant. All experiments were repeated independently at least twice.
Results
CD11b+Ly6C+ Cells Accumulate in the Spleen During L. donovani Infection
In the spleen of naïve mice, neutrophils can be distinguished from monocytes/DC using a combination of CD11b and Ly6G (Figure 1A). Within the CD11b+Ly6G− monocyte/DC population, considerable heterogeneity was found in expression of Ly6C, as has been observed by others.18 During infection with L. donovani, the relative balance of neutrophils to monocyte/DC shifts from a ratio of approximately 1:1 to approximately 1:6, reflecting recruitment of inflammatory CD11b+Ly6G− monocytes/DC cells that are more homogeneous for Ly6C expression (Figure 1B). By immunohistochemistry (IHC), two populations of Ly6C/G+ cells were also readily distinguished (Figure 1, C and D), both localized within the red pulp. Ly6C/Gbright cells, almost invariably with polymorphonuclear appearance, were found throughout the red pulp in naïve mice (Figure 1C), whereas most Ly6C/Gdull cells were concentrated in the subcapsular red pulp and therefore likely represent the resident splenic monocyte pool.18 In contrast, in the spleens of infected mice, large nests of Gr-1dull cells could also readily be observed throughout the red pulp (Figure 1D).
Figure 1.
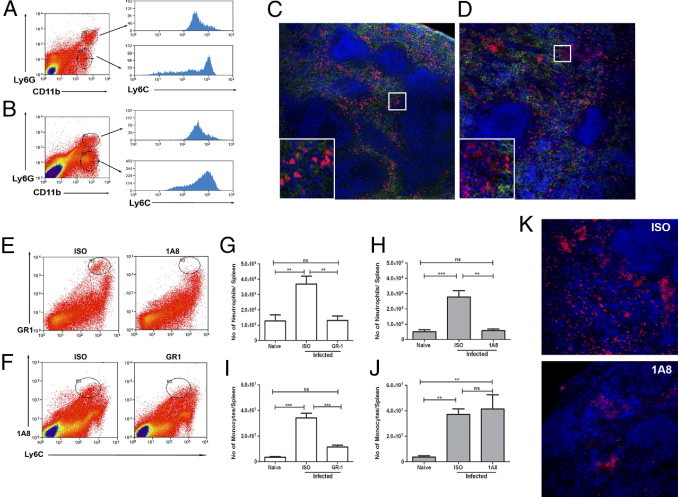
Identification of Ly6C/G+ cells in EVL. Spleens from L. donovani infected mice were removed at day 28 after infection and analyzed alongside spleens from naïve mice by flow cytometry and by IHC. A and B: Spleen cells from naïve (A) and infected (B) mice were stained with Ly6G and CD11b and the expression of Ly6C was determined on neutrophils (upper histograms) and monocytes/DC (lower histograms). C and D: Sections from naïve (C) and infected (D) mice were stained for Ly6C/G (Gr-1; red) and F4/80 (green) and counterstained with DAPI (blue). Original magnification, ×100. Insets show boxed areas at higher magnification: Gr-1bright neutrophils (C) and a nest of F4/80−Gr-1dull cells with two neutrophils in close proximity (D). Original magnification, ×630. E: Spleen cells from infected mice treated with 1A8 or isotype control antibody were stained with Gr-1 and Ly6C. Gr-1+Ly6C+ neutrophils are highlighted in oval gated regions. F: Spleen cells from infected mice treated with Gr-1 or isotype control antibody were stained with 1A8 and Ly6C. 1A8+Ly6C+ neutrophils are highlighted in oval gated regions. G–J: Absolute numbers of splenic neutrophils (G and H) and monocytes/DC (I and J) in spleens of naïve mice and infected mice were determined using Ly6G (1A8) and Ly6C for Gr-1-depleted mice (G and I) and using Ly6C and Gr-1 for 1A8-depleted mice (H and J). Data represent means ± SEM from 7 mice per group, pooled from two independent experiments for Gr-1 depletion and 4 mice per group from one experiment for 1A8 depletion. Similar results were seen in an additional experiment in which 1A8-depleted mice were analyzed using CD11b and Ly6C (data not shown). **P < 0.01, ***P < 0.001. ns, not significant. Peripheral blood neutrophils were depleted by 91 ± 4% after 1A8 and 98 ± 0.5% after Gr-1 depletion (data not shown). K: Spleen sections from infected mice administered either isotype control (ISO) or 1A8 were stained for Gr-1 (red) and DAPI (blue). Original magnification, ×200.
Previous reports have suggested monocytes, macrophages, and neutrophils as potent cellular sources of proangiogenic MMP-9 during inflammation.19 In the present study, however, IHC analysis, in situ zymography, and flow cytometry all revealed that in both naïve mice and infected mice MMP-9 was colocalized almost exclusively with Gr-1bright cells, with minimal MMP-9 expression in Gr-1int monocytes/DC even after neutrophil depletion (see Supplemental Figure S1 at http://ajp.amjpathol.org).
To determine the potential role of these two myeloid populations in regulating vascular remodeling during infection, we compared the effects of either depleting both populations (using Gr-1) or just Ly6G+ neutrophils (using 1A8). Neutrophil depletion was similar in mice treated with either mAb (Figure 1, E–H), whereas monocytes/DC were depleted only after administration of Gr-1 (Figure 1, I and J). Immunohistology of 1A8-treated mice showed an almost complete absence of Gr-1bright cells, a finding that, together with the flow analysis, helped to confirm that most Gr-1dull cells detected by this method were monocytes/DC (Figure 1K). Consistent with previous studies,11 administration of Gr-1 mAb during established infection led to an increase in splenic parasite burden: 195 ± 17 versus 304 ± 40 Leishman-Donovan units (LDU) at day 28 after infection in isotype-treated versus Gr-1-treated mice, respectively (n = 10 mice; P < 0.02). Similarly, administration of 1A8 also increased parasite burden at day 28, from 53 ± 12 LDU to 168 ± 33 LDU (n = 7 mice; P < 0.001).
Ly6C+ Cells are Selectively Involved in Red Pulp Vascular Remodeling
To assess the effects of monocyte and neutrophil depletion on splenic vascular remodeling, we performed quantitative IHC on spleen sections from Gr-1 and 1A8 treated mice (Figure 2). Meca-32 is a pan endothelial marker that recognizes red and white pulp vasculature and the endothelium of the marginal sinus in naïve mice.6 As previously reported,6 Meca-32+ vessels increased in abundance in the red pulp of mice infected with L. donovani (Figure 2A). Administration of Gr-1 mAb to mice from day 21 to day 28 after infection reduced the level of Meca-32 expression in red pulp to that seen in control uninfected mice (Figure 2, A and C). In the red pulp, some large-caliber vessels also stained for CD31 (data not shown), and the increase in CD31 in the red pulp observed in infected mice was also arrested after administration of Gr-1 mAb (Figure 2E). Strikingly, when Ly6G+ cells alone were depleted using 1A8, no effects were observed on either of these two measures of splenic red pulp vascular remodeling (Figure 2, A, D, and F). These findings indicate that vascular remodeling in the red pulp is likely to be dependent on the presence of inflammatory Ly6C+ monocytes/DC.
Figure 2.
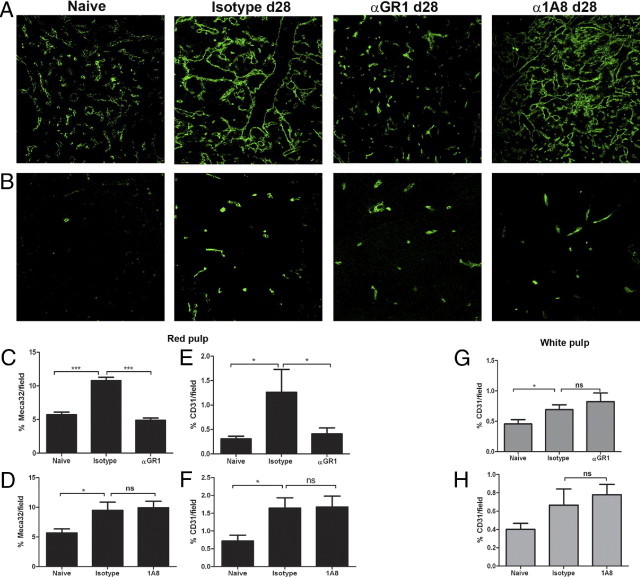
Ly6C+ inflammatory monocytes/DC promote vascular remodeling in the red pulp. Spleens from mice infected with L. donovani were removed at day 28 after infection and analyzed alongside spleens from naïve mice by IHC. A and B: Spleen sections from naïve mice and infected mice treated with isotype control, Gr-1, or 1A8 from day 21 to day 28 after infection were stained with Meca-32 (A) or with CD31 (B). Original magnification, ×200. C–H: Computer-assisted quantitative morphometry was performed to determine the expression of Meca-32 (C and D) and of CD31 (E and F) in the red pulp and also the expression of CD31 in the white pulp (G and H) of mice treated with Gr-1 (C, E, and G) or 1A8 (D, F, and H). Data were generated from multiple sections examined from at least 5 mice per group. *P < 0.05, **P < 0.01, and ***P < 0.001.
Neither Ly6C/G+ nor Ly6G+ Cells Affect Vascular and Stromal Cell Remodeling in the White Pulp
Remodeling of the white pulp compartment also occurs during EVL.6, 20 In the white pulp, CD31 is expressed only on the central arteriole in naïve mice, but with infection it is expressed also in the neovasculature that emerges during progressive infection.6 Strikingly, neither administration of Gr-1 or 1A8 mAb had any effect on the neovascularization of the white pulp as measured by CD31+ vessels (Figure 2, B, G, and H).
Loss of the FDC and FRC stromal cell networks is also characteristic of EVL-induced lymphoid tissue remodeling and underpins a loss of T- and B-cell compartmentalization.20 We also assessed, therefore, whether this process was regulated by either Ly6C/G+ or Ly6G+ cells. Neither administration of Gr-1 nor 1A8 was able to reverse the disruption seen in T- and B-cell compartments (Figure 3A). Similarly, loss of FRC networks, measured using gp38 (Figure 3, B, C, and D), and loss of FDC networks, measured using FDCM1 (Figure 3, B, E, and F), was equivalent in both Gr-1 and 1A8-treated mice. Thus, by all criteria measured, white pulp remodeling was independent of the presence or absence of neutrophils and monocytes, consistent with the exclusion of these cells from the white pulp.
Figure 3.
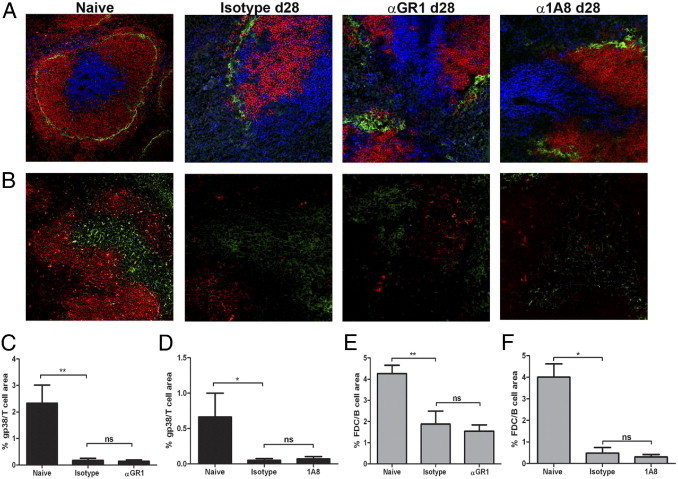
Neither Ly6C+ inflammatory monocytes/DC nor Ly6G+ neutrophils are responsible for white pulp remodeling. Spleens from L. donovani infected mice were removed at day 28 after infection and were analyzed, alongside spleens from naïve mice, by IHC. A and B: Spleen sections from naïve mice and infected mice treated with isotype control, Gr-1, or 1A8 from day 21 to day 28 of infection were stained to identify marginal metallophilic macrophages (CD169; green), B cells (B220; red), and T cells (CD3; blue) (A) and FRC (gp38, green) and FDC (FDCM1, red) (B). Original magnification, ×200. C–F: Computer-assisted quantitative morphometry was performed to determine the expression of gp38 (C and D) and FDCM1 (E and F) in mice treated with Gr-1 (C and E) and 1A8 (D and F). Data were generated from multiple sections examined from at least 5 mice per group. *P < 0.05, **P < 0.01.
Discussion
The present findings provide a number of new insights into the pathogenesis of EVL-associated splenomegaly. Most importantly, we show that Ly6C+ inflammatory monocytes/DC located in the red pulp play a major role in remodeling the red pulp vasculature during ongoing splenomegaly. The observation that neither neovascularistion of the white pulp nor white pulp stromal cell networks were affected by monocyte/DC (or neutrophil) depletion suggests strongly that these cells can modify only their immediate environment.
Our data derived from this model of infection-induced splenic vascular remodeling are in contrast to a number of recent reports that implicated neutrophils as major agents of vascular remodeling: i) neutrophil-derived MMP-9 was able to direct angiogenesis in collagen onplants grafted onto the chorioallantoic membrane of chick embryos,21 ii) neutrophil depletion caused a significant attenuation of microvessel growth in a model of brain focal angiogenesis induced by AAV-VEGF gene transfer,22 and iii) neutrophil depletion reduced the number of angiogenic islet dysplasias in RIP1-Tag2 mice.23 However, Gr-1 has often been used as a tool for neutrophil depletion in vivo and, as the present findings clearly indicate, the partial loss of monocytes that occurs as a result of Gr-1 administration can have profound functional consequences.
The present findings add to a growing body of evidence that implicates Ly6C+CD11b+ monocytes/DC/myeloid suppressor cells as regulators of vascular growth and remodeling. For example, such cells are closely associated with the vasculature found in spontaneous prostate cancers in TRAMP mice and produce a range of proangiogenic factors, including MMP-9.24 Gr-1dullCD11b+ cells have been implicated in ischemia-associated neovascularization, with adoptive transfer of these cells improving local blood flow in mice with femoral artery dissection.25 CD11b+ macrophages have also been implicated in inflammation-induced angiogenesis and lymphangiogenesis in a model of cutaneous leishmaniasis, based on in vivo depletion studies using clodronate liposomes.16 Even taking into account variations in phenotypic analysis, differences in methods of in vivo depletion, and different endpoints for scoring vascular remodeling, it is difficult to reconcile these data with a single angiogenic stage of monocyte/macrophage/DC differentiation. Rather, it seems likely that the potential for vascular remodeling exists within mononuclear phagocytes at multiple stages of their differentiation, influenced by local environmental factors.
In both macrophages and neutrophils, MMPs (and MMP-9 in particular) have been implicated as proangiogenic factors,13, 21, 24 and human monocytes can be stimulated to produce this metalloproteinase by either alternative IL-4-mediated activation or IL-10-directed deactivation.26 Both cytokines are readily detectable during EVL.27 Taken together, these considerations led us to study the expression of MMP-9 in the spleens of L. donovani infected mice. Surprisingly, we were unable to demonstrate MMP-9 expression in any splenic mononuclear phagocyte population, whether by flow cytometry, IHC, or gelatinolytic assays. In neutrophils, however, MMP-9 was abundant. The absence of detectable levels of MM9 in splenic macrophages of mice infected with L. donovani would be consistent with the low levels of alternative activation seen in L. donovani infection,28 but may also reflect tissue and/or species-specific differences in MMP-9 expression by macrophages. For example, F4/80+ peritoneal macrophages expressed significantly more MMP-9 than did neutrophils during the early stages of zymosan-induced peritonitis,29 consistent with a recent report that peritoneal macrophages infected in vitro with L. chagasi and cocultured with hepatocytes secrete elevated levels of active MMP-9.30 Similarly, whereas in that study resident peritoneal neutrophils did not express detectable MMP-9,29 in the present study neutrophils represent the only detectable MMP-9+ population in the naïve spleen. Although the apparently tight regulation of MMP-9 expression during EVL is of interest, in the present context it appears that MMP-9 is unlikely to play a major role in red pulp vascular remodeling.
In summary, we have shown that splenic vascular remodeling during EVL takes place in a compartment-specific manner, with red pulp remodeling being regulated directly or indirectly by Ly6C+ inflammatory monocytes recruited to this site during infection. Although our findings provide an important mechanistic insight into the vascularization process that accompanies splenomegaly, the compartment-specific activity of these monocytes also indicates that some disparate mechanism or mechanisms must be involved in neovascularization of the white pulp.6 Further studies are now required to define the cells and factors involved in the latter process.
Acknowledgment
We thank the staff of the Biological Services Facility at the Department of Biology, University of York, for animal husbandry.
Footnotes
Supported by grants from the Wellcome Trust and the Medical Research Council (P.M.K.). Supported also in part by the Overseas Research Fellowship Program (2214) of the Scientific and Technological Research Council of Turkey.
P.Y. and J.D. contributed equally to the present work.
Supplemental material for this article can be found at http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2011.03.009.
Current address of A.M., GlaxoSmithKline Biologicals, Hamilton, Montana.
Supplementary data
MMP-9 expression in the spleen of naïve and L. donovani infected mice. A and B: Spleen sections, 8 μm thick, from naïve or day 28 infected B6 mice were stained with Gr-1 and for intracellular MMP-9. Nuclei were counterstained with DAPI (blue). MMP-9 (green) was restricted in distribution to Gr-1bright cells (red) in the red pulp in both naïve (A) and infected (B) mice. C and D: MMP-9+ cells (green), identified as neutrophils by nuclear morphology, were interspersed with F4/80+ red pulp macrophages (red) in both naïve (C) and infected (D) mice. Nuclei are counterstained with DAPI (blue). Scale bar = 20 μm. E.In situ zymography showing strong MMP-9 activity in the red pulp of naïve (left) and infected (right) mice. F. Spleen cells from naïve mice (left graphs) and day 28 infected mice (right graphs) were stained for Gr-1 and intracellular MMP-9 (upper graphs) or an isotype control mAb (ISO; lower graphs) and examined by flow cytometry. Oval gates represent Gr-1bright MMP-9+ cells and show representative frequency. Rectangular gates represent Gr-1dull/neg cells, including monocytes. G. Spleen cells from infected mice treated with isotype control (left graphs) or 1A8 (right graphs) and stained and gated for Gr-1/ISO and MMP-9/ISO as in F. The percentage of Gr-1dull monocytes/DC expressing MMP-9 was 0.4 ± 0.1 in ISO-treated infected mice and was 0.3 ± 0.1 in 1A8-depleted mice.
References
- 1.Chappuis F., Sundar S., Hailu A., Ghalib H., Rijal S., Peeling R.W., Alvar J., Boelaert M. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol. 2007;5:873–882. doi: 10.1038/nrmicro1748. [DOI] [PubMed] [Google Scholar]
- 2.Engwerda C.R., Ato M., Kaye P.M. Macrophages, pathology and parasite persistence in experimental visceral leishmaniasis. Trends Parasitol. 2004;20:524–530. doi: 10.1016/j.pt.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Engwerda C.R., Ato M., Cotterell S.E., Mynott T.L., Tschannerl A., Gorak-Stolinska P.M., Kaye P.M. A role for tumor necrosis factor-alpha in remodeling the splenic marginal zone during Leishmania donovani infection. Am J Pathol. 2002;161:429–437. doi: 10.1016/s0002-9440(10)64199-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ato M., Stäger S., Engwerda C.R., Kaye P.M. Defective CCR7 expression on dendritic cells contributes to the development of visceral leishmaniasis. Nat Immunol. 2002;3:1185–1191. doi: 10.1038/ni861. [DOI] [PubMed] [Google Scholar]
- 5.Smelt S.C., Engwerda C.R., McCrossen M., Kaye P.M. Destruction of follicular dendritic cells during chronic visceral leishmaniasis. J Immunol. 1997;158:3813–3821. [PubMed] [Google Scholar]
- 6.Dalton J.E., Maroof A., Owens B.M., Narang P., Johnson K., Brown N., Rosenquist L., Beattie L., Coles M., Kaye P.M. Inhibition of receptor tyrosine kinases restores immunocompetence and improves immune-dependent chemotherapy against experimental leishmaniasis in mice. J Clin Invest. 2010;120:1204–1216. doi: 10.1172/JCI41281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexandre-Pires G., Pais D., Correia M., Pina J.A. Leishmaniosis–a report about the microvascular and cellular architecture of the infected spleen in Canis familiaris. Microsc Res Tech. 2006;69:227–235. doi: 10.1002/jemt.20267. [DOI] [PubMed] [Google Scholar]
- 8.Peters N.C., Egen J.G., Secundino N., Debrabant A., Kimblin N., Kamhawi S., Lawyer P., Fay M.P., Germain R.N., Sacks D. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies [Erratum appeared in Science 2008, 322:1634] Science. 2008;321:970–974. doi: 10.1126/science.1159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thalhofer C.J., Chen Y., Sudan B., Love-Homan L., Wilson M.E. Leukocytes infiltrate the skin and draining lymph nodes in response to the protozoan Leishmania infantum chagasi. Infect Immun. 2011;79:108–117. doi: 10.1128/IAI.00338-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Trez C., Magez S., Akira S., Ryffel B., Carlier Y., Muraille E. iNOS-producing inflammatory dendritic cells constitute the major infected cell type during the chronic Leishmania major infection phase of C57BL/6 resistant mice. PLoS Pathog. 2009;5(6):e1000494. doi: 10.1371/journal.ppat.1000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smelt S.C., Cotterell S.E., Engwerda C.R., Kaye P.M. B cell-deficient mice are highly resistant to Leishmania donovani infection, but develop neutrophil-mediated tissue pathology. J Immunol. 2000;164:3681–3688. doi: 10.4049/jimmunol.164.7.3681. [DOI] [PubMed] [Google Scholar]
- 12.Tacchini-Cottier F., Zweifel C., Belkaid Y., Mukankundiye C., Vasei M., Launois P., Milon G., Louis J.A. An immunomodulatory function for neutrophils during the induction of a CD4+ Th2 response in BALB/c mice infected with Leishmania major. J Immunol. 2000;165:2628–2636. doi: 10.4049/jimmunol.165.5.2628. [DOI] [PubMed] [Google Scholar]
- 13.Ardi V.C., Van den Steen P.E., Opdenakker G., Schweighofer B., Deryugina E.I., Quigley J.P. Neutrophil MMP-9 proenzyme, unencumbered by TIMP-1, undergoes efficient activation in vivo and catalytically induces angiogenesis via a basic fibroblast growth factor (FGF-2)/FGFR-2 pathway. J Biol Chem. 2009;284:25854–25866. doi: 10.1074/jbc.M109.033472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung Y.W., Zindl C.L., Lai J.F., Weaver C.T., Chaplin D.D. MMP induced by Gr-1+ cells are crucial for recruitment of Th cells into the airways. Eur J Immunol. 2009;39:2281–2292. doi: 10.1002/eji.200838985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jablonska J., Leschner S., Westphal K., Lienenklaus S., Weiss S. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model [Erratum appeared in J Clin Invest 2010, 120:4163] J Clin Invest. 2010;120:1151–1164. doi: 10.1172/JCI37223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horst A.K., Bickert T., Brewig N., Ludewig P., van Rooijen N., Schumacher U., Beauchemin N., Ito W.D., Fleischer B., Wagener C., Ritter U. CEACAM1+ myeloid cells control angiogenesis in inflammation. Blood. 2009;113:6726–6736. doi: 10.1182/blood-2008-10-184556. [DOI] [PubMed] [Google Scholar]
- 17.Cotterell S.E., Engwerda C.R., Kaye P.M. Enhanced hematopoietic activity accompanies parasite expansion in the spleen and bone marrow of mice infected with Leishmania donovani. Infect Immun. 2000;68:1840–1848. doi: 10.1128/iai.68.4.1840-1848.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swirski F.K., Nahrendorf M., Etzrodt M., Wildgruber M., Cortez-Retamozo V., Panizzi P., Figueiredo J.L., Kohler R.H., Chudnovskiy A., Waterman P., Aikawa E., Mempel T.R., Libby P., Weissleder R., Pittet M.J. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parks W.C., Wilson C.L., López-Boado Y.S. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 20.Ato M., Maroof A., Zubairi S., Nakano H., Kakiuchi T., Kaye P.M. Loss of dendritic cell migration and impaired resistance to Leishmania donovani infection in mice deficient in CCL19 and CCL21. J Immunol. 2006;176:5486–5493. doi: 10.4049/jimmunol.176.9.5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ardi V.C., Kupriyanova T.A., Deryugina E.I., Quigley J.P. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci USA. 2007;104:20262–20267. doi: 10.1073/pnas.0706438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hao Q., Chen Y., Zhu Y., Fan Y., Palmer D., Su H., Young W.L., Yang G.Y. Neutrophil depletion decreases VEGF-induced focal angiogenesis in the mature mouse brain. J Cereb Blood Flow Metab. 2007;27:1853–1860. doi: 10.1038/sj.jcbfm.9600485. [DOI] [PubMed] [Google Scholar]
- 23.Nozawa H., Chiu C., Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci USA. 2006;103:12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dudley A.C., Udagawa T., Melero-Martin J.M., Shih S.C., Curatolo A., Moses M.A., Klagsbrun M. Bone marrow is a reservoir for proangiogenic myelomonocytic cells but not endothelial cells in spontaneous tumors. Blood. 2010;116:3367–3371. doi: 10.1182/blood-2010-02-271122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim J.A., March K., Chae H.D., Johnstone B., Park S.J., Cook T., Merfeld-Clauss S., Broxmeyer H.E. Muscle-derived Gr1(dim)CD11b(+) cells enhance neovascularization in an ischemic hind limb mouse model. Blood. 2010;1169:1623–1626. doi: 10.1182/blood-2009-08-237040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lolmede K., Campana L., Vezzoli M., Bosurgi L., Tonlorenzi R., Clementi E., Bianchi M.E., Cossu G., Manfredi A.A., Brunelli S., Rovere-Querini P. Inflammatory and alternatively activated human macrophages attract vessel-associated stem cells, relying on separate HMGB1- and MMP-9-dependent pathways. J Leukoc Biol. 2009;85:779–787. doi: 10.1189/jlb.0908579. [DOI] [PubMed] [Google Scholar]
- 27.Engwerda C.R., Smelt S.C., Kaye P.M. An in vivo analysis of cytokine production during Leishmania donovani infection in scid mice. Exp Parasitol. 1996;84:195–202. doi: 10.1006/expr.1996.0105. [DOI] [PubMed] [Google Scholar]
- 28.Hassan M.F., Zhang Y., Engwerda C.R., Kaye P.M., Sharp H., Bickle Q.D. The Schistosoma mansoni hepatic egg granuloma provides a favorable microenvironment for sustained growth of Leishmania donovani. Am J Pathol. 2006;169:943–953. doi: 10.2353/ajpath.2006.051319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolaczkowska E., Barteczko M., Plytycz B., Arnold B. Role of lymphocytes in the course of murine zymosan-induced peritonitis. Inflamm Res. 2008;57:272–278. doi: 10.1007/s00011-007-7131-1. [DOI] [PubMed] [Google Scholar]
- 30.Costa J.D., Nogueira de Melo A.C., Vermelho A.B., Meirelles Mde N., Porrozzi R. In vitro evidence for metallopeptidase participation in hepatocyte damage induced by Leishmania chagasi-infected macrophages. Acta Trop. 2008;106:175–183. doi: 10.1016/j.actatropica.2008.03.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MMP-9 expression in the spleen of naïve and L. donovani infected mice. A and B: Spleen sections, 8 μm thick, from naïve or day 28 infected B6 mice were stained with Gr-1 and for intracellular MMP-9. Nuclei were counterstained with DAPI (blue). MMP-9 (green) was restricted in distribution to Gr-1bright cells (red) in the red pulp in both naïve (A) and infected (B) mice. C and D: MMP-9+ cells (green), identified as neutrophils by nuclear morphology, were interspersed with F4/80+ red pulp macrophages (red) in both naïve (C) and infected (D) mice. Nuclei are counterstained with DAPI (blue). Scale bar = 20 μm. E.In situ zymography showing strong MMP-9 activity in the red pulp of naïve (left) and infected (right) mice. F. Spleen cells from naïve mice (left graphs) and day 28 infected mice (right graphs) were stained for Gr-1 and intracellular MMP-9 (upper graphs) or an isotype control mAb (ISO; lower graphs) and examined by flow cytometry. Oval gates represent Gr-1bright MMP-9+ cells and show representative frequency. Rectangular gates represent Gr-1dull/neg cells, including monocytes. G. Spleen cells from infected mice treated with isotype control (left graphs) or 1A8 (right graphs) and stained and gated for Gr-1/ISO and MMP-9/ISO as in F. The percentage of Gr-1dull monocytes/DC expressing MMP-9 was 0.4 ± 0.1 in ISO-treated infected mice and was 0.3 ± 0.1 in 1A8-depleted mice.


