Abstract
Purpose of review
Premenopausal women have a lower risk and incidence of hypertension and cardiovascular disease (CVD) compared to age-matched men and this sex advantage for women gradually disappears after menopause, suggesting that sexual hormones play a cardioprotective role in women. However, randomized prospective primary or secondary prevention trials failed to confirm that hormone replacement therapy (HRT) affords cardioprotection. This review highlights the factors that may contribute to this divergent outcome and could reveal why young or premenopausal women are protected from CVD and yet postmenopausal women do not benefit from HRT.
Recent findings
In addition to the two classical estrogen receptors, ERα and ERβ, a third, G-protein-coupled estrogen receptor GPR30, has been identified. New intracellular signaling pathways and actions for the cardiovascular protective properties of estrogen have been proposed. In addition, recent Women’s Health Initiative (WHI) studies restricted to younger postmenopausal women showed that initiation of HRT closer to menopause reduced the risk of CVD. Moreover, dosage, duration, the type of estrogen and route of administration all merit consideration when determining the outcome of HRT.
Summary
HRT has become one of the most controversial topics related to women’s health. Future studies are necessary if we are to understand the divergent published findings regarding HRT and develop new therapeutic strategies to improve the quality of life for women.
Keywords: cardiovascular disease, estrogen, hormonal replacement therapy
Introduction
Cardiovascular disease (CVD) is the leading cause of death in the United States and differs significantly depending on age and sex. Observational studies have demonstrated that both the incidence of CVD and resultant morbidity and mortality are much less in premenopausal women compared to age-matched men, and this sex advantage for women becomes far less or disappears with increased age and reduced estrogen levels after menopause [1–4]. Observational studies also show that postmenopausal women who receive hormone replacement therapy (HRT) have a lower rate of CVD and cardiac death than those not receiving HRT [5,6]. However, two randomized prospective primary or secondary prevention trials, the Women’s Health Initiative (WHI) [7] and the Heart and Estrogen/Progestin Replacement Study (HERS I and II) [8,9], showed that HRT may actually increase the risk and events of CVD in postmenopausal women. The reasons for this paradoxical characterization of HRT as both beneficial and detrimental remain unclear. Many potential factors may contribute to the adverse outcome, among them the age of patients, preexisting CVD and/or risk, when HRT was initiated, the type of HRT given (conjugated equine estrogen with progestin), dosage, and the thromboembolic properties of estrogen and progestin [6,10–13]. Overall, the use of HRT has become one of the most controversial topics related to women’s health, making it all the more urgent to clarify whether estrogens (and/or HRT) prevent or promote CVD, as well as the mechanism(s) involved.
Sex differences in cardiovascular disease
Blood pressure is typically lower in premenopausal women than men; however, after menopause it increases to levels similar to or higher than age-matched men [2,4]. Approximately 75% of women over 60 years of age are hypertensive [14]. Comparison of cohorts from the National Health and Nutrition Examination Survey (NHANES) III (1988–1994) with NHANES IV (1999–2002) showed that over the time period from 1994 to 2002, the percentage of hypertensive patients decreased among men but increased among women [15]. Indeed, the percentage of individuals with uncontrolled hypertension was also higher in women than men (55.9 ± 1.4 vs. 50.8 ± 2.1%), despite the fact that a higher percentage of women than men reported having their blood pressure measured within the previous 6 months [15]. It is not clear why hypertension is less well controlled in women than men despite more frequent blood pressure monitoring, but this observation suggests the mechanism(s) responsible may differ in men and women.
Premenopausal women also have a much lower incidence and prevalence of heart and renal disease compared to men of the same age [4,16–18]. This sex difference in favor of women also gradually disappears after menopause, indeed cardiovascular risk becomes even higher in older women [3,19]. Although it has been debated whether the loss of cardiorenal protection in postmenopausal women is related to aging, loss of female hormones or both, substantial studies indicate that reduced levels of ovarian hormones constitute a major risk factor for development of CVD [1,4,16,17,20]. The recent Nurse’s Health Study [21•] and the WISE Study [22], as well as others [23], have demonstrated that early menopause in young women due to ovarian dysfunction or bilateral oophorectomy is associated with increased risk of CVD compared to women with normal endogenous estrogen levels. In animal models of CVD, females exhibited a lower mortality, less vascular injury, better preserved cardiovascular function and slower progression to decompensated heart failure, the differences being narrowed or abolished by ovariectomy or deficiency of endogenous estrogen [24••,25–27].
Endogenous estrogen may have a cardioprotective effect in men as well. In men, significant amounts of estrogen can be produced via conversion of C19 androgenic steroids to 17β-estradiol by the enzyme aromatase. In healthy young men inhibition of aromatase lowers plasma 17β-estradiol, and is associated with decreased flow-mediated dilatation of the brachial artery [28]. Similarly, aromatase knockout mice demonstrated impaired endothelial function [29]. Supplemental estrogen in men attenuated volume overload-induced structural and functional remodeling [30] and slowed the progression of left ventricular dysfunction to heart failure post-myocardial infarction (MI) [31]. Taken together, the evidence suggests that the differences in cardioprotection between men and women may be attributable largely to the protective effect of estrogen in women.
Cardioprotective mechanisms of estrogen
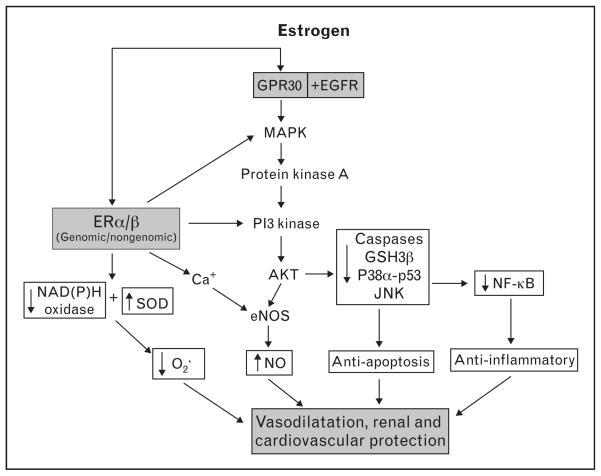
Two classical estrogen receptor subtypes, ERα and ERβ, have been identified in the heart and vasculature [32,33]. The long-term effects of estrogen may be mediated by both ERα and ERβ through alteration of gene expression and protein synthesis (genomic action) [34], whereas the rapid nongenomic effect of estrogen may involve calcium-mediated activation of endothelial nitric oxide synthase [35], cGMP and intracellular signal transduction pathways [32,33] (Fig. 1). Recently, a third membrane-bound and G-protein-coupled estrogen receptor (GPER), GPR30, has been identified. In addition to estrogen, other hormones/factors such as progestin, genistein and estrogen antagonist/selective estrogen receptor modulators (SERMs) tamoxifen and ICI 182780 have been shown to act as GPR30 ligand; however, their affinity to GPR30 is 10-fold to 100-fold less than estrogen [36]. Studies have demonstrated estrogen acting via GPR30 and transactivation of epidermal growth factor receptors (EGFR) inducing rapid signal transduction, including activation of MAP kinase (MAPK), protein kinase A (PKA) and PI3 kinase (PI3K) [36]. In the heart, activation of GPR30 with the specific agonist G1 reduced ischemia/reperfusion injury and preserved cardiac function acting through PI3K-dependent Akt pathways [37••], suggesting a cardioprotective role of this newly defined estrogen receptor (Fig. 1).
Figure 1. Possible mechanisms responsible for estrogen-mediated vasodilatation, renal and cardiovascular protection.
The multifaceted mechanisms of estrogen involve (a) acting on estrogen receptor-α (ERα) and ERβ to reduce synthesis of NADPH oxidase and increase synthesis of endothelial nitric oxide synthase (eNOS) and superoxide dismutase (SOD), thereby decreasing superoxide and increasing NO production and bioavailability (genomic effect); (b) rapidly activating eNOS via a calcium-mediated mechanism(s) without altering gene expression (nongenomic effect), leading to NO/cGMP release and vascular relaxation; (c) activating Akt via MAP kinase (MAPK)–PI3 kinase (PI3K) pathways, reducing apoptosis and enhancing cell survival and (d) reducing NF-κB activation/translocation via p38α-mediated p53 phosphorylation and JNK1/2-mediated signaling pathways, inhibiting chemokine/cytokine transcription and decreasing inflammation. In addition, estrogen acts on the membrane-bound and G-protein-coupled estrogen receptor (GPER) GPR30 associated with transactivation of epidermal growth factor receptors, which induces rapid signal transduction, including activation of MAPK, protein kinase A (PKA) and PI3K, leading to cardiovascular protection.
There are other recently discovered mechanisms by which estrogens could provide cardioprotection. Estrogen has been shown to increase expression of superoxide dismutase and inhibit NADPH oxidase activity, thereby reducing oxidative stress [26,32,38]. Estrogen acting via ERβ increases protein S-nitrosylation, a common post-translational protein modification, leading to cardioprotection [39]. Inflammation is considered a key element in the pathogenesis of hypertension, atherosclerosis and development of coronary heart disease (CHD), and estrogen has been reported to reduce inflammatory markers [32,40]. Estrogen also attenuates afterload- or agonist-induced cardiac hypertrophy via inhibition of calcineurin, hypertrophic transcription factor NF-AT, and MAPK signaling pathways [41]. In addition, estrogen has a profound antiapoptotic and pro-survival effect on cardiomyocytes via activation of Akt and inhibition of caspase-3, GSK-3β [42], p38α-mediated p53 phosphorylation, and JNK1/2-mediated NF-κB activation [43] (Fig. 1). Moreover, estrogen has been shown to promote endothelial progenitor cell mobilization [44] and enhance mesenchymal stem cell-mediated vascular endothelial growth factor (VEGF) release [45,46•], improving endothelial and myocardial functional after ischemia.
Hormonal replacement therapy
Observational studies have suggested that HRT decreases the risk of CVD and reduces mortality in postmenopausal women with heart disease [5,47]. However, large-scale clinical trials, the HERS I and II [8,9] and WHI [7], showed an unfavorable outcome of HRT on CVD risk and events. So far, we know of no good explanation as to why young or premenopausal women are protected from CVD and yet postmenopausal women do not benefit from HRT. The controversy over the risks and benefits of HRT in primary prevention of CVD continues, and much of the debate has focused on the age of postmenopausal women enrolled in these trials, when HRT is initiated, duration of the replacement, dosage and the form of estrogen used [6,10–13].
Age and timing of hormonal replacement therapy initiation
In most observational studies, women started HRT around the time of menopause (which occurs on average at 51 years), whereas the WHI trials examined postmenopausal women ranging from 50 to 79 who began HRT at an average age of 63.3 years; 67% of them were 60–79 years of age, and 73% of this aged population had never received HRT. Presumably many of them already had atherosclerotic plaques and thus were predisposed to thrombosis. These preexisting conditions, even subclinical, may have had a profound impact on the outcome of HRT use, when the goal was primary prevention. Indeed, the later published ‘Coronary Artery Calcium Study’ (WHI-CACS) [48], that was restricted to postmenopausal women aged 50–59 years, showed that HRT initiated in these younger women reduced coronary artery calcification and the prevalence of subclinical CHD. Furthermore, a secondary analysis of the WHI data set [49] showed that women who began HRT closer to menopause tended to have a reduced risk of CVD. More recently, a cohort study with long-term follow-up showed that women who underwent bilateral ovariectomy before age 45 had increased cardiovascular mortality, and this risk was significantly lowered by treatment with estrogen through age 45 or longer [23]. In monkeys, starting HRT in early menopause reduced coronary artery atherosclerosis by about 50–70%, whereas delaying initiation of HRT for 2 years (about 6 years in human terms) blunted this protection [50]. Taken together, these studies support the hypothesis that estrogen therapy may have a cardiovascular benefit when initiated early after the onset of menopause.
There is evidence that atherosclerosis involves an ongoing inflammatory response, which is more profound during the early years of menopause [40,51,52]. Cytokine production has been shown to increase in the early years following menopause but thereafter declines to within the premenopausal range [52,53•]. Estrogen reportedly reduces interleukin (IL)-1, IL-6, IL-18, C-reactive protein, tissue necrosis factor (TNF)-α and increases macrophage colony-stimulating factor, a cytokine that lowers plasma cholesterol levels by enhancing clearance of low-density lipoprotein [53•,54–56]. Thus since inflammatory responses are higher in early postmenopause while absence or reduction of endogenous estrogen may accelerate the progression of atherosclerosis, the timing of HRT with respect to onset of menopause may have important ramifications regarding its efficacy in preventing or delaying the progression of atherosclerosis and CVD. Alternatively, some of the WHI data may be explained by the fact the HRT has been shown to increase indicators of inflammation, such as TNF-α [53•]. Future studies will be necessary to ferret out the contribution of HRT to inflammation in postmenopausal women at various ages.
Duration of hormonal replacement therapy
Re-analysis of the WHI data set [49] shows that younger postmenopausal women given relatively short-term HRT (<10 years) tended to have reduced risk and incidence of CVD, but this protection gradually disappeared in succeeding years. It is reported that short-term HRT (2–3 years) reduced CVD mortality by 30%, associated with significantly reduced severity of atherosclerotic lesions [57]. Klaiber et al. [58] studied the effect of HRT on serum estradiol levels in women soon after menopause (average 12.9 months) vs. a long time after menopause (average 78 months) and found that estradiol was 46% higher after long duration HRT. It is known that some of the adverse effects of estrogen, such as increased breast and endometrial cancer and venous thrombosis, correlated positively with plasma estradiol levels; however, the cardiovascular consequences due to HRT-induced excessive increase in plasma estradiol are not well established and need further investigation.
Dosage
Most clinical trials use only one dose of estrogen (0.625 mg conjugated equine estrogen, CEE) plus one dose of progestin (2.5 mg medroxyprogesterone acetate, MPA). Choice of CEE dosage is largely based on data from prospective studies showing that it takes at least 0.625 mg/day to significantly increase bone mineral density. However, it is not clear whether this is an appropriate dosage for preventing or reducing CVD risk. In a prospective study [5], 0.3 mg/day decreased major coronary events in women compared to untreated controls, whereas 0.625 mg or more CEE combined with progestin increased the risk of stroke. Genant et al. [59] and Villa et al. [60•] showed that low-dose estrogen slightly increased plasma estradiol but improved endothelial function and lipid profile without endometrial hyperplasia, while higher doses increased plasma estradiol two-fold to three-fold and were associated with endometrial hyperplasia. Unfortunately, they did not examine the dose effect of estrogen on vascular calcification and risk of CVD. In monkeys fed an atherogenic diet, 0.3 mg CEE reduced coronary artery atherosclerosis comparable to the standard dose [61]. We recently reported that in mice with intact cardiac function or myocardial infarction, low-dose estrogen tended to be cardioprotective whereas at higher doses that increased plasma estradiol beyond the physiological levels, estrogen increased mortality, worsened cardiac function and caused severe damage to the kidney, including hydronephrosis, severe albuminuria, renal tubular dilatation and glomerulosclerosis. High dose of estrogen also caused ascites, hepatomegaly and fluid retention in the uterine horns [62•,63••]. High doses of estrogen also raised testosterone significantly, although the mechanisms by which estrogen replacement increases testosterone are not clear. Taken together, it is rational to speculate that estrogen dosage may have a significant impact on its outcome on the heart, vasculature and kidney.
Estrogen only vs. standard hormonal replacement therapy
It has been questioned whether the adverse effect of HRT on thromboembolism is attributable to the presence of progestin. It is reported that CEE doubled flow-mediated and endothelium-dependent vasodilatation in postmenopausal women and this effect was reversed by MPA [64]. In estrogen-deficient cynomolgus monkeys fed high-fat diet, CEE reduced coronary artery plaque formation and this effect was abolished by MPA [65]. The WHI-CACS survey showed that CEE alone significantly reduced coronary artery calcification [48]. In women with hysterectomy, CEE reduced CHD by 44% in younger postmenopausal women (50–59 years), although no overall cardiovascular protective effects were evidenced [66].
The ongoing Kronos Early Estrogen Prevention Study (KEEPS) [67••], a prospective, randomized and placebo-controlled multicenter trial, is designed to determine the effect of oral CEE or transdermal 17β-estradiol with or without progestin on atherosclerosis and coronary calcification in women within 3 years of menopause. KEEPS is expected to provide valuable information on timing, route and protocols of HRT for prevention of CVD in women.
Conclusion
Whether estradiol protects women from CVD is still unknown. Studies in women previous to the HERS I and II and the WHI and many animal studies suggested that indeed both estradiol and HRT protected women from CVD via various mechanisms. The fact that premenopausal women have a lower incidence of CVD than men also implicates the sex steroids in women as playing a role in this protection. If that is true, we then need to determine why aging in women prevents HRT from being as effective in protecting against CVD as endogenous estradiol and progesterone. Are there age-related changes in estrogen receptors, intracellular signaling, or genomics that alter the response to HRT in older postmenopausal women? What role do increases in androgens unopposed by estrogens in postmenopausal women play in mediating CVD? In addition, what role does obesity –which is reaching epidemic proportions in perimenopausal and postmenopausal women – play in mediating the response to HRT? Future studies will be necessary to answer these important questions and lead to more effective therapeutic strategies to improve the quality of life for women following menopause.
Key points
The fact that young or premenopausal women are protected from cardiovascular disease and yet postmenopausal women do not benefit from HRT has made HRT one of the most controversial topics related to women’s health.
Age, time of initiation, duration of replacement therapy, dosage, route of administration and presence or absence of progestin all need to be taken into consideration.
Future studies are needed to clarify whether agerelated changes in estrogen receptors, intracellular signaling, or genomics alter the response to HRT in older postmenopausal women.
New and more effective therapeutic strategies are urgently needed to improve the quality of life for women.
Acknowledgments
This work was supported by the following grants: NIH HL078951 (XPY) and NIH HL66194, HL66072 and HL51971 (JFR).
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 197).
- 1.Bittner V. Menopause, age, and cardiovascular risk: a complex relationship. J Am Coll Cardiol. 2009;54:2374–2375. doi: 10.1016/j.jacc.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Coylewright M, Reckelhoff JF, Ouyang P. Menopause and hypertension: an age-old debate. Hypertension. 2008;51:952–959. doi: 10.1161/HYPERTENSIONAHA.107.105742. [DOI] [PubMed] [Google Scholar]
- 3.Kim ES, Menon V. Status of women in cardiovascular clinical trials. Arterioscler Thromb Vasc Biol. 2009;29:279–283. doi: 10.1161/ATVBAHA.108.179796. [DOI] [PubMed] [Google Scholar]
- 4.Reckelhoff JF, Maric C. Sex and gender differences in cardiovascular-renal physiology and pathophysiology. Steroids. 2010;75:745–746. doi: 10.1016/j.steroids.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 5.Grodstein F, Manson JE, Colditz GA, et al. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933–941. doi: 10.7326/0003-4819-133-12-200012190-00008. [DOI] [PubMed] [Google Scholar]
- 6.Rosano GM, Vitale C, Fini M. Cardiovascular aspects of menopausal hormone replacement therapy. Climacteric. 2009;12 (Suppl 1):41–46. doi: 10.1080/13697130903012306. [DOI] [PubMed] [Google Scholar]
- 7.Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 8.Hulley S, Grady D, Bush T, et al. for the Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 9.Hulley S, Furberg C, Barrett-Connor E, et al. for the HERS Research Group. Noncardiovascular disease outcomes during 6.8 years of hormone therapy. Heart and Estrogen/progestin Replacement Study follow-up (HERS II) J Am Med Assoc. 2002;288:58–66. doi: 10.1001/jama.288.1.58. [DOI] [PubMed] [Google Scholar]
- 10.Hodis HN. Assessing benefits and risks of hormone therapy in 2008: new evidence, especially with regard to the heart. Cleve Clin J Med. 2008;75 (Suppl 4):S3–S12. doi: 10.3949/ccjm.75.suppl_4.s3. [DOI] [PubMed] [Google Scholar]
- 11.Schnatz PF. Hormonal therapy: does it increase or decrease cardiovascular risk? Obstet Gynecol Surv. 2006;61:673–681. doi: 10.1097/01.ogx.0000238674.98471.bb. [DOI] [PubMed] [Google Scholar]
- 12.Haines CJ, Farrell E. Menopause management: a cardiovascular risk-based approach. Climacteric. 2010;13:328–339. doi: 10.3109/13697130903450154. [DOI] [PubMed] [Google Scholar]
- 13.Harman SM. Estrogen replacement in menopausal women: recent and current prospective studies, the WHI and the KEEPS. Gend Med. 2006;3:254–269. doi: 10.1016/s1550-8579(06)80214-7. [DOI] [PubMed] [Google Scholar]
- 14.Barton M, Meyer MR. Postmenopausal hypertension: mechanisms and therapy. Hypertension. 2009;54:11–18. doi: 10.1161/HYPERTENSIONAHA.108.120022. [DOI] [PubMed] [Google Scholar]
- 15.Kim JK, Alley D, Seeman T, et al. Recent changes in cardiovascular risk factors among women and men. J Womens Health (Larchmt) 2006;15:734–746. doi: 10.1089/jwh.2006.15.734. [DOI] [PubMed] [Google Scholar]
- 16.Rosano GM, Vitale C, Marazzi G, Volterrani M. Menopause and cardiovascular disease: the evidence. Climacteric. 2007;10 (Suppl 1):19–24. doi: 10.1080/13697130601114917. [DOI] [PubMed] [Google Scholar]
- 17.Wake R, Yoshiyama M. Gender differences in ischemic heart disease. Recent Pat Cardiovasc Drug Discov. 2009;4:234–240. doi: 10.2174/157489009789152249. [DOI] [PubMed] [Google Scholar]
- 18.Silbiger S, Neugarten J. Gender and human chronic renal disease. Gend Med. 2008;5 (Suppl A):S3–S10. doi: 10.1016/j.genm.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Anderson RD, Pepine CJ. Gender differences in the treatment for acute myocardial infarction: bias or biology? Circulation. 2007;115:823–826. doi: 10.1161/CIRCULATIONAHA.106.685859. [DOI] [PubMed] [Google Scholar]
- 20.Matthews KA, Crawford SL, Chae CU, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009;54:2366–2373. doi: 10.1016/j.jacc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21•.Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113:1027–1037. doi: 10.1097/AOG.0b013e3181a11c64. A prospective study that demonstrates that bilateral oophorectomy before age 50 years was associated with an increased risk of all-cause mortality, CHD and stroke. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bairey Merz CN, Johnson BD, Sharaf BL, et al. Hypoestrogenemia of hypothalamic origin and coronary artery disease in premenopausal women: a report from the NHLBI-sponsored WISE study. J Am Coll Cardiol. 2003;41:413–419. doi: 10.1016/s0735-1097(02)02763-8. [DOI] [PubMed] [Google Scholar]
- 23.Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16:15–23. doi: 10.1097/gme.0b013e31818888f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24••.Wang F, Keimig T, He Q, et al. Augmented healing process in female mice with acute myocardial infarction. Gend Med. 2007;4:230–247. doi: 10.1016/s1550-8579(07)80043-x. A mouse study showing that females had low cardiac rupture and mortality, augmented healing and better reserved cardiac function after myocardial infarction. [DOI] [PubMed] [Google Scholar]
- 25.Dent MR, Tappia PS, Dhalla NS. Gender differences in apoptotic signaling in heart failure due to volume overload. Apoptosis. 2010;15:499–510. doi: 10.1007/s10495-009-0441-8. [DOI] [PubMed] [Google Scholar]
- 26.Lagranha CJ, Deschamps A, Aponte A, et al. Sex differences in the phosphorylation of mitochondrial proteins result in reduced production of reactive oxygen species and cardioprotection in females. Circ Res. 2010;106:1681–1691. doi: 10.1161/CIRCRESAHA.109.213645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Javeshghani D, Schiffrin EL, Sairam MR, Touyz RM. Potentiation of vascular oxidative stress and nitric oxide-mediated endothelial dysfunction by high-fat diet in a mouse model of estrogen deficiency and hyperandrogenemia. J Am Soc Hypertens. 2009;3:295–305. doi: 10.1016/j.jash.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Lew R, Komesaroff P, Williams M, et al. Endogenous estrogens influence endothelial function in young men. Circ Res. 2003;93:1127–1133. doi: 10.1161/01.RES.0000103633.57225.BC. [DOI] [PubMed] [Google Scholar]
- 29.Kimura M, Sudhir K, Jones M, et al. Impaired acetylcholine-induced release of nitric oxide in the aorta of male aromatase-knockout mice. Regulation of nitric oxide production by endogenous sex hormones in males. Circ Res. 2003;93:1267–1271. doi: 10.1161/01.RES.0000103172.98986.25. [DOI] [PubMed] [Google Scholar]
- 30.Gardner JD, Murray DB, Voloshenyuk TG, et al. Estrogen attenuates chronic volume overload induced structural and functional remodeling in male rat hearts. Am J Physiol Heart Circ Physiol. 2010;298:H497–H504. doi: 10.1152/ajpheart.00336.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cavasin MA, Tao ZY, Yu AL, Yang X-P. Testosterone enhances early cardiac remodeling after myocardial infarction, causing rupture and degrading cardiac function. Am J Physiol Heart Circ Physiol. 2006;290:H2043–H2050. doi: 10.1152/ajpheart.01121.2005. [DOI] [PubMed] [Google Scholar]
- 32.Xing D, Nozell S, Chen YF, et al. Estrogen and mechanisms of vascular protection. Arterioscler Thromb Vasc Biol. 2009;29:289–295. doi: 10.1161/ATVBAHA.108.182279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Babiker FA, De Windt LJ, van Eickels M, et al. Estrogenic hormone action in the heart: regulatory network and function. Cardiovasc Res. 2002;53:709–719. doi: 10.1016/s0008-6363(01)00526-0. [DOI] [PubMed] [Google Scholar]
- 34.Barkhem T, Nilsson S, Gustafsson JA. Molecular mechanisms, physiological consequences and pharmacological implications of estrogen receptor action. Am J Pharmacogenom. 2004;4:19–28. doi: 10.2165/00129785-200404010-00003. [DOI] [PubMed] [Google Scholar]
- 35.Ruehlmann DO, Mann GE. Rapid nongenomic vasodilator actions of oestrogens and sex steroids. Curr Med Chem. 2000;7:533–541. doi: 10.2174/0929867003375038. [DOI] [PubMed] [Google Scholar]
- 36.Prossnitz ER, Maggiolini M. Mechanisms of estrogen signaling and gene expression via GPR30. Mol Cell Endocrinol. 2009;308:32–38. doi: 10.1016/j.mce.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37••.Deschamps AM, Murphy E. Activation of a novel estrogen receptor, GPER, is cardioprotective in male and female rats. Am J Physiol Heart Circ Physiol. 2009;297:H1806–H1813. doi: 10.1152/ajpheart.00283.2009. A recent study showing that activation of the newly discovered estrogen receptor, GPER, improves cardiac function and reduces infarct size. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang QG, Raz L, Wang R, et al. Estrogen attenuates ischemic oxidative damage via an estrogen receptor alpha-mediated inhibition of NADPH oxidase activation. J Neurosci. 2009;29:13823–13836. doi: 10.1523/JNEUROSCI.3574-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin J, Steenbergen C, Murphy E, Sun J. Estrogen receptor-beta activation results in S-nitrosylation of proteins involved in cardioprotection. Circulation. 2009;120:245–254. doi: 10.1161/CIRCULATIONAHA.109.868729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reckelhoff JF. Cardiovascular disease, estrogen deficiency, and inflammatory cytokines. Hypertension. 2006;48:372–373. doi: 10.1161/01.HYP.0000235866.97871.9d. [DOI] [PubMed] [Google Scholar]
- 41.Donaldson C, Eder S, Baker C, et al. Estrogen attenuates left ventricular and cardiomyocyte hypertrophy by an estrogen receptor-dependent pathway that increases calcineurin degradation. Circ Res. 2009;104:265–275. doi: 10.1161/CIRCRESAHA.108.190397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang F, He Q, Sun Y, et al. Female adult mouse cardiomyocytes are protected against oxidative stress. Hypertension. 2010;55:1172–1178. doi: 10.1161/HYPERTENSIONAHA.110.150839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu CJ, Lo JF, Kuo CH, et al. Akt mediates 17beta-estradiol and/or estrogen receptor-alpha inhibition of LPS-induced tumor necresis factor-alpha expression and myocardial cell apoptosis by suppressing the JNK1/2-NFkappaB pathway. J Cell Mol Med. 2009;13:3655–3667. doi: 10.1111/j.1582-4934.2009.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erwin GS, Crisostomo PR, Wang Y, et al. Estradiol-treated mesenchymal stem cells improve myocardial recovery after ischemia. J Surg Res. 2009;152:319–324. doi: 10.1016/j.jss.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baruscotti I, Barchiesi F, Jackson EK, et al. Estradiol stimulates capillary formation by human endothelial progenitor cells: role of estrogen receptor-alpha/beta, heme oxygenase 1, and tyrosine kinase. Hypertension. 2010;56:397–404. doi: 10.1161/HYPERTENSIONAHA.110.153262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46•.Bolego C, Rossoni G, Fadini GP, et al. Selective estrogen receptor-alpha agonist provides widespread heart and vascular protection with enhanced endothelial progenitor cell mobilization in the absence of uterotrophic action. FASEB J. 2010;24:2262–2272. doi: 10.1096/fj.09-139220. New mechanisms that may be responsible for the cardioprotective action of estrogen. [DOI] [PubMed] [Google Scholar]
- 47.Grodstein F, Stampfer MJ, Colditz GA, et al. Postmenopausal hormone therapy and mortality. N Engl J Med. 1997;336:1769–1775. doi: 10.1056/NEJM199706193362501. [DOI] [PubMed] [Google Scholar]
- 48.Manson JE, Allison MA, Rossouw JE, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591–2602. doi: 10.1056/NEJMoa071513. [DOI] [PubMed] [Google Scholar]
- 49.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 50.Clarkson TB, Anthony MS, Morgan TM. Inhibition of postmenopausal atherosclerosis progression: a comparison of the effects of conjugated equine estrogens and soy phytoestrogens. J Clin Endocrinol Metab. 2001;86:41–47. doi: 10.1210/jcem.86.1.7151. [DOI] [PubMed] [Google Scholar]
- 51.Nuedling S, Kahlert S, Loebbert K, et al. Differential effects of 17beta-estradiol on mitogen-activated protein kinase pathways in rat cardiomyocytes. FEBS Lett. 1999;454:271–276. doi: 10.1016/s0014-5793(99)00816-9. [DOI] [PubMed] [Google Scholar]
- 52.Yasui T, Maegawa M, Tomita J, et al. Changes in serum cytokine concentrations during the menopausal transition. Maturitas. 2007;56:396–403. doi: 10.1016/j.maturitas.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 53•.Georgiadou P, Sbarouni E. Effect of hormone replacement therapy on inflammatory biomarkers. Adv Clin Chem. 2009;47:59–93. doi: 10.1016/s0065-2423(09)47003-3. A comprehensive review outlines the effects of HRT on inflammatory biomarkers. [DOI] [PubMed] [Google Scholar]
- 54.Oztas E, Kurtay G. Effects of raloxifene on serum macrophage colony-stimulating factor and interleukin-18 levels in postmenopausal women younger than 60 years. Menopause. 2010;17:1188–1193. doi: 10.1097/gme.0b013e3181e04a18. [DOI] [PubMed] [Google Scholar]
- 55.Karim R, Stanczyk FZ, Hodis HN, et al. Associations between markers of inflammation and physiological and pharmacological levels of circulating sex hormones in postmenopausal women. Menopause. 2010;17:785–790. [PMC free article] [PubMed] [Google Scholar]
- 56.Kamada M, Irahara M, Maegawa M, et al. Postmenopausal changes in serum cytokine levels and hormone replacement therapy. Am J Obstet Gynecol. 2001;184:309–314. doi: 10.1067/mob.2001.109940. [DOI] [PubMed] [Google Scholar]
- 57.Alexandersen P, Tankó LB, Bagger YZ, et al. The long-term impact of 2–3 years of hormone replacement therapy on cardiovascular mortality and atherosclerosis in healthy women. Climacteric. 2006;9:108–118. doi: 10.1080/13697130600647743. [DOI] [PubMed] [Google Scholar]
- 58.Klaiber EL, Broverman DM, Vogel W, et al. Relationships of serum estradiol levels, menopausal duration, and mood during hormonal replacement therapy. Psychoneuroendocrinology. 1997;22:549–558. doi: 10.1016/s0306-4530(97)00043-7. [DOI] [PubMed] [Google Scholar]
- 59.Genant HK, Lucas J, Weiss S, et al. Low-dose esterified estrogen therapy: effects on bone, plasma estradiol concentrations, endometrium, and lipid levels. Estratab/Osteoporosis Study Group. Arch Intern Med. 1997;157:2609–2615. doi: 10.1001/archinte.157.22.2609. [DOI] [PubMed] [Google Scholar]
- 60•.Villa P, Suriano R, Ricciardi L, et al. Low-dose estrogen and drospirenone combination: effects on glycoinsulinemic metabolism and other cardiovascular risk factors in healthy postmenopausal women. Fertil Steril. 2010 doi: 10.1016/j.fertnstert.2010.07.001. [Epub ahead of print]. A new study showing low-dose estrogen plus/drospirenone induced favorable changes in lipid profile improvement of vascular reactivity. [DOI] [PubMed] [Google Scholar]
- 61.Appt SE, Clarkson TB, Lees CJ, Anthony MS. Low dose estrogens inhibit coronary artery atherosclerosis in postmenopausal monkeys. Maturitas. 2006;55:187–194. doi: 10.1016/j.maturitas.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 62•.Zhan E, Keimig T, Xu J, et al. Dose-dependent cardiac effect of oestrogen replacement in mice postmyocardial infarction. Exp Physiol. 2008;93:982–993. doi: 10.1113/expphysiol.2008.042788. In mice with myocardial infarction, low dose of estradiol that restored plasma estrogen close to physiological levels tended to improve cardiac function and remodeling. At an increased dose, estradiol exacerbated cardiac fibrosis, hypertrophy, dysfunction and dilatation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63••.Meng X, Dai X, Liao T-D, et al. Dose-dependent toxic effects of high-dose estrogen on renal and cardiac injury in surgically postmenopausal mice. Life Sci. 2010 doi: 10.1016/j.lfs.2010.11.008. [Epub ahead of print]. A mouse study showing that excessive dose of estradiol that raises uterine weight beyond physiological levels adversely affects the kidney even before it damages the heart. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wakatsuki A, Okatani Y, Ikenoue N, Fukaya T. Effect of medroxyprogesterone acetate on endothelium-dependent vasodilation in postmenopausal women receiving estrogen. Circulation. 2001;104:1773–1778. doi: 10.1161/hc4001.097035. [DOI] [PubMed] [Google Scholar]
- 65.Adams MR, Register TC, Golden DL, et al. Medroxyprogesterone acetate antagonizes inhibitory effects of conjugated equine estrogens on coronary artery atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:217–221. doi: 10.1161/01.atv.17.1.217. [DOI] [PubMed] [Google Scholar]
- 66.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 67••.Miller VM, Black DM, Brinton EA, et al. Using basic science to design a clinical trial: baseline characteristics of women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS) J Cardiovasc Transl Res. 2009;2:228–239. doi: 10.1007/s12265-009-9104-y. An ongoing prospective, randomized, controlled trial designed to test the hypothesis that HRT when initiated early in menopause reduces progression of atherosclerosis. [DOI] [PMC free article] [PubMed] [Google Scholar]