Abstract
The peripheral B cell compartment in mice and humans is maintained by continuous production of transitional B cells in the bone marrow (BM). In other species however, including rabbits, B lymphopoiesis in the BM abates early in life and it is unclear how the peripheral B cell compartment is maintained. We identified transitional B cells in rabbits and classified them into T1 (CD24hiCD21lo) and T2 (CD24hiCD21+) B cell subsets. By neutralizing BAFF in vivo, we found an arrest in peripheral B cell development at the T1 B cell stage. Surprisingly, T1 B cells were present in GALT, blood and spleen of adult rabbits, long after B lymphopoiesis was arrested. T1 B cells were distinct from their counterparts in other species because they are proliferating and the Ig genes are somatically diversified. We designate these newly described cells as T1d B cells and propose a model in which they develop in GALT, self-renew, and continuously differentiate into mature B cells, and thereby maintain peripheral B cell homeostasis in adults in the absence of B lymphopoiesis.
Introduction
During B cell development, immature B cells in the BM, designated transitional B cells, exit the BM and migrate to the spleen where they develop into mature B cells (1, 2). Transitional B cells are identified by several cell surface markers expressed on newly formed B cells in the BM. One such marker, CD24, is expressed at high levels on both human and murine transitional B cells and is downregulated on mature B cells (3, 4). Using CD24 and AA4 (C1qR), several subsets of transitional B cells have been identified in mice. Loder et al. (2) classified the CD24hi transitional B cells into two stages: transitional type 1 (T1) and type 2 (T2) based on the differential expression of CD21, CD23 and IgD. Using AA4, a type I transmembrane protein, Allman et al. (5) identified 3 populations of transitional B cells: AA4+CD23-IgMhi (T1), AA4+CD23+IgMhi (T2), and AA4+CD23-IgMlo (T3). Adoptive transfer experiments revealed that T1 cells give rise to T2 and mature B cells (2) and Schiemann et al. (6) demonstrated that this maturation is dependent on B cell activating factor (BAFF). T1 B cells are found in the BM, blood and spleen, while T2 B cells are restricted to spleen (2). Transitional B cell subsets exhibit distinct functional characteristics. For example, T2 B cells proliferate upon BCR crosslinking, whereas T1 B cells die (7). Transitional B cells in humans are largely described as a single subset that is CD10+ (8) or CD24++CD38++ (4), although some investigators have classified these cells as T1-like and T2-like based on the differential expression of CD24 and CD38 (9) or IgD and CD38 (10). Recently, Suryani et al. (11), using CD21 as a marker, identified two transitional B cell subsets (CD21lo and CD21hi) in peripheral blood and demonstrated that the CD21lo subset is the precursor to the CD21hi B cells.
Transitional B cells mark a crucial link between immature BM B cells and mature peripheral B cells. While many studies of peripheral B cell development have been performed in mice and humans, essentially no such studies are available in rabbits or other mammals that utilize gut-associated lymphoid tissue (GALT) for B cell expansion and somatic diversification of Ig genes (12-17). The mechanism by which B cells undergo proliferative expansion in GALT is not known. Further, in rabbits, and likely in other species, B lymphopoiesis in primary lymphoid organs abates early in life (18, 19) and it is unclear how the peripheral B cell compartment is maintained in the absence of ongoing B-lymphopoiesis. Weill and Reynaud (14) proposed that the GALT-derived B cells in these species might serve as transitional-like B cells.
In this study, we used several cross-reactive antibodies to identify transitional B cells (T1 and T2) in rabbit. Using anti-CD24 and anti-CD21 mAb, we identified transitional B cell subsets in blood, spleen, and GALT of adult rabbits, long after the arrest of B lymphopoiesis in the BM. Using soluble decoy receptors that inhibited cell-cell and cell-cytokine interactions, we identified several signals required for the proliferative expansion of B cells in GALT. We describe a model for peripheral B cell development and maintenance in which proliferating and somatically diversified transitional B cells in adults develop in GALT, continuously differentiate into mature B cells, and thereby maintain peripheral B cell homeostasis.
Materials and Methods
Rabbits and reagents
Rabbits were from the colony maintained by K. L. Knight at Loyola University Chicago. Adult rabbits used in this study ranged from 4 months to 2 years of age. All studies were reviewed and approved by the Institutional Animal Care and Use Committee of Loyola University Chicago, Maywood, IL.
We tested commercially available antibodies for cross-reactivity to rabbit B lineage cells. A list of Abs that cross-reacted and were used in this study are shown in table I. Rabbit-specific Abs and secondary reagents were as follows: anti-IgM (clone 367; BD Bioscience, San Jose, CA), anti-IgA (clone 102; BD Biosciences), anti-rabbit L chain (KLK stock), anti-MHC class II (clone 2C4; BD Biosciences), FITC anti-rabbit C3 (Southern Biotechnology, Birmingham, AL), Dylight 649 goat Fab anti-mouse IgG and streptavidin PE/APC (Jackson ImmunoResearch, Westgrove, PA). Rabbit rBAFF (20) was biotinylated using NHS-LC biotin (Pierce Biochemicals, Rockford, IL).
Table I.
Cross-reactive antibodies used for flow cytometry and immunohistology
| Antibody | Specificity | Clone | Vendor |
|---|---|---|---|
| CD10 | Human | CB-CALLA | eBiosciences, Inc. San Diego, CA |
| CD20 | Human | B9E9 | Santa Cruz Biotechnology, Inc., Santa Cruz, CA Immunotech, Marseille Cedax, France |
| CD21 | Human | BL13 | Immunotech |
| CD23 | Human | 9P25 | Immunotech |
| CD24 | Mouse | M1/169 | eBiosciences, Inc. BD Biosciences, San Jose, CA |
| CD38 | Human | IB6 | Miltenyi Biotech Inc., Auburn, CA Also kindly provided by Dr. Malavasi, University of Turin, Italy |
| CD62L | Human | LAM-1 | Kindly provided by Dr. Tedder, Duke University, Durham, NC |
| CD90 | Human | 5E10 | BD Biosciences |
| Ki-67 | Human | B56 | BD Biosciences |
Cobra venom factor (CVF) was obtained from Calbiochem, San Diego, CA.
Recombinant adenovirus
Adenoviral (Ad) constructs expressing TACI-Ig (extracellular portion of human TACI fused to human Fcγ) and mouse Fcγ (as control) were kindly provided by Dr. Tong Zhou (University of Alabama at Birmingham, Birmingham, AL). Rabbit CTLA4-Ig (kindly provided by Dr. David Dichek, UCSF) was subcloned into CMV-shuttle vector (Invitrogen, Carlsbad, CA). For constructing CD40-Ig, the extracellular portion of human CD40 was PCR-amplified from Raji cDNA (OS HuCD40XhoI 5’-actcgagaccatggttcgtctgcctctgcag-3’ and AS HuCD40BamH1 5’-tggatc cccgatcctggggaccacagacaac3’) and cloned into the CMV-shuttle vector in frame with rabbit Fcγ. Similarly, the extracellular portion of rabbit CR2 was PCR-amplified from appendix cDNA (OSrCD21Xho 5’-actcgaggccgccaccatgggcgccgcg-3’ and ASrCD21Bam 5’-tggatcccccttcattgcaagaaatgtt-3’) and cloned into the CMV-shuttle vector in frame with rabbit Fcγ. Following homologous recombination and integration of CTLA4-Ig, CD40-Ig or CR2-Ig into the adenoviral genome, we selected recombinant clones (Invitrogen) and adenoviral constructs were transfected into QBI-293A cells (Qbiogene, Carlsbad, CA). Viral particles were purified on cesium chloride gradients, titered, (Qbiogene), and stored at -80°C. Recombinant viral particles (1010 in 0.3ml PBS) were injected i.p. into rabbit pups within 48 h of birth and the rabbits were sacrificed 7-10 d later.
Flow cytometry and immunohistochemistry
For analysis of transitional B cells, multicolor flow cytometry (3, 4 or 5 color) was performed by gating on CD24hi cells that were either CD21lo/CD21+ or IgMlo/IgM+. All flow cytometry data were acquired with FACSCanto or FACSCantoII or FACSAria (BD Biosciences), gated on live lymphocyte-sized cells on the basis of forward and side scatter, and analyzed using FlowJo software (Tree star, Ashland, OR). All FACs plots using fluorescent reagents are depicted on a logarithmic scale except where indicated. For flow cytometric analysis of commensal bacteria, luminal contents were flushed from rabbit appendix with 1X PBS, 5% FCS (FACs buffer), and after debris removal bacteria were pelleted and resuspended in buffer. Bacteria (25-50μl) (representing a pellet of ~1-2 mm3) were stained with the appropriate Ab and analyzed as described above. For immunohistochemistry, acetone-fixed cryosections (7-8μm) were blocked with goat serum and then stained with primary Ab (Table 1) and indirect reagents: Cy2- or Cy3-conjugated streptavidin and Cy2- or Dylight 549-conjugated goat (Fab) anti-mouse IgG (Jackson ImmunoResearch). Slides were viewed under a Leica DM IRB microscope (Leica Microsystems, Bannockburn, IL) and images captured using the MagnaFire 2.1C digital camera system (Optronics, Goleta, CA); grayscale images were edited using ImageJ software (National Institutes of Health, Bethesda, MD). The germ-free (GF) appendix tissues used for immunohistochemistry were obtained from rabbits previously described (21).
Nucleotide sequence analysis of VH genes
IgH VDJ genes from genomic DNA of single sorted splenic and appendix T1 B cells from two a2/a3 heterozygous rabbits (2.8 yrs and 4 mo of age) and one a3/a3 homozygous rabbit (1.8 yrs of age) were amplified by nested-PCR. First-round PCR was performed using pan VH primers: forward 5’-ctctggcacaggagctc-3’, reverse 5’-agttgagtaggaagagaga-3’. Aliquots (2μl) of first-round PCR products were used as template for the second-round PCR using primers: forward 5’-cactcaccatggagact -3’, reverse 5’-gagttggcaaggactcac-3’. Products from the second-round PCR were directly sequenced, and the frequency of VH mutations was determined by comparing sequences to germline VH genes.
Immunization and ELISA
To determine the efficacy of CTLA4-Ig in vivo, rabbits neonatally injected with Ad-CTLA4-Ig were re-injected with Ad-CTLA4-Ig at 2 and 5 weeks of age and 3 days after the 2 week injection, they received 0.5mg BGG in CFA (s.c.). After the 5 week injection of Ad-CTLA4-Ig, rabbits received a secondary immunization of 0.5mg BGG in IFA. Serum was harvested 7 d after the primary and 10 d after the secondary immunization and anti-BGG IgM and IgG levels were determined by ELISA using anti-rabbit IgM (clone 367; BD Biosciences) or anti-rabbit IgG (clone 359; BD Biosciences) coated microtiter plates. The ELISA was developed with goat anti-rabbit H&L chain-HRP (Jackson ImmunoResearch) plus ABTS (Sigma, St. Louis, MO) as substrate. The relative levels of serum IgM and IgG in Ad-CTLA4-Ig-treated and control PBS-treated rabbits were determined from a linear portion of the dilution curves.
Results
Identification of transitional B cell subsets
To identify B cell subpopulations in adult rabbits, we stained B cells from different tissues with antibodies used to delineate immature B cell subsets in mice and humans. Because human CD24 and its murine homologue, heat-stable antigen, are expressed early in B cell development on both BM B cell progenitors and transitional B cells, but are downregulated on mature B cells (2-4, 22), we tested if anti-CD24 can be used to identify transitional B cells in rabbits. Using anti-CD21 and anti-CD24 mAbs, we identified two subsets of CD24hi B cells in the spleen (CD21lo and CD21+), which we henceforth refer to as T1 and T2 B cells, respectively, and a CD24-CD21+ subset, designated mature (M) B cells (Fig 1A, upper). T1 B cells were IgMlo CD62Llo, while both T2 and mature B cells had higher levels of CD21, surface IgM, and CD62L expression (Fig 1A, upper). CD23 was expressed at similar levels on both T1 and T2 B cells (Fig 1A, upper) and thus did not serve as a useful marker to distinguish between these B cell subsets. To determine if these transitional B cells share features with human transitional B cells, which are broadly defined as CD24hiCD38hiCD10+CD20hi (23), we analyzed the CD24hi cells for these markers and found that the T1 B cells were CD10loCD38lo, while the T2 B cells were CD10hi and CD38hi ( Fig 1A, upper). Interestingly, CD20 was expressed on essentially all of the T1 and T2 cells but not on the mature B cells, and thus serves as a unique marker to identify transitional B cells (Fig 1A, lower). Further, T1 and T2 B cells expressed high levels of CD90 (Fig 1A, lower), a phenotype shared with rat immature B cells (24). Unlike in spleen, we observed only a single subset of CD24hi cells in the peripheral blood (PB) and these had a lower expression of CD21 compared to mature B cells (Fig 1B). These cells were IgMloCD62Llo (Fig 1B), suggesting that PB contains only a T1-like population of transitional B cells. We observed a similar T1-like CD21loCD24hi subset in GALT [appendix (Apx), sacculus rotundus (SR), Peyer’s patch (PP) and mesenteric lymph node (MLN)] (Fig 1C). Taken together, these results demonstrate that in adult rabbits, immature B cells can be phenotypically delineated into two transitional B cell subsets, T1 and T2. The frequencies of these cells in different tissues are shown in Table II.
Figure 1. Flow cytometric identification of transitional B cell subsets.
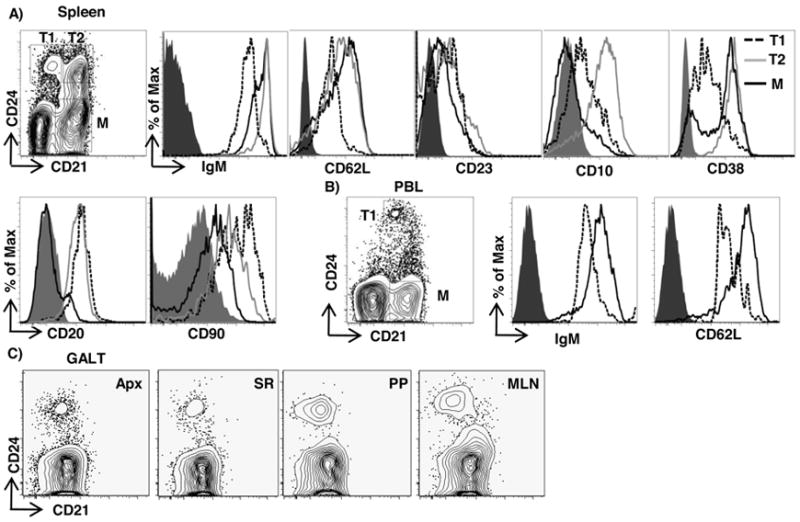
A) Staining of splenic B cells for T1 (CD24hiCD21lo) (dashed), T2 (CD24hiCD21+) (gray) and mature (M) (CD24lo/-CD21+) (black) for IgM, CD62L, CD23, CD10, CD38, CD20 and CD90. B) Staining of peripheral blood (PB) T1 (dashed) and M (black) for IgM and CD62L. C) IgM+ B cells from appendix (Apx), sacculus rotundus (SR), Peyer’s patches (PP) and mesenteric lymph nodes (MLN) stained for CD24 and CD21. The gray filled histograms in A and B represent staining with an appropriate isotype control mAb. The plots are representative of staining obtained from at least 3 rabbits.
Table II.
Frequency of transitional B cells in adult rabbit tissues
| Tissuea | T1b | T2b |
|---|---|---|
| Spleen (11) | 7.2 ± 2 | 13.6 ± 2.3 |
| Blood (6) | 2.9 ± 0.9 | ND |
| Appendix (6) | 2 ± 0.4 | ND |
| Sacculus rotundus (3) | 3.8 ± 0.9 | ND |
| Mesentric lymph node (4) | 3.7 ± 0.9 | ND |
| Peyer’s patch (2) | 9.8 ± 3.8 | ND |
The number in parentheses indicates the number of rabbits analyzed
Indicates the percent of total B cells and ± represents the SEM
T1 = CD24hiCD21loCD62LloCD10loCD38lo
T2 = CD24hiCD21+CD62L+CD10hiCD38hi
ND = Not detectable
Functional analysis of transitional B cells
In vivo, murine transitional B cells require BAFF for maturation into B cells, and in the absence of BAFF, peripheral B cell development is blocked at the T1 stage (6). We investigated the role of BAFF in rabbit peripheral B cell development by neutralizing BAFF in vivo. Newborns were injected with a soluble decoy receptor (TACI-Ig) and we found, a dramatic decrease in splenic T2 and mature B cells, while the T1 B cell population remained intact (Fig 2A, upper right). Similarly, in the appendix, the mature B cell population was eliminated by neutralization of BAFF, but the CD24hi transitional B cell population was not reduced, and instead appeared to accumulate (Fig 2A, lower right). These data indicate that T2 and mature B cells, but not T1 B cells require BAFF for their survival and/or maintenance. Because T1 cells are the earliest B cell precursors in the periphery, we focused our studies on T1 B cells.
Figure 2. Functional analysis of transitional B cells.
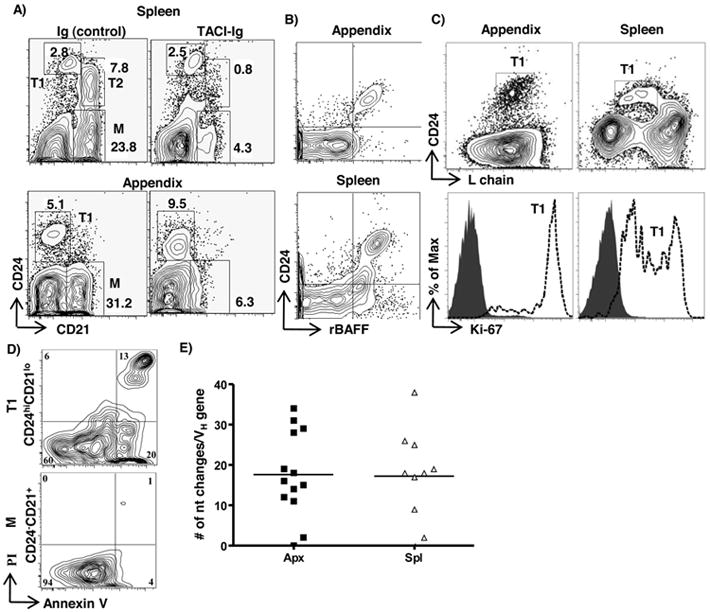
A) Flow cytometric analysis of appendix and spleen cells from TACI-Ig-treated and control (Ig)-treated rabbits stained with anti-CD21 and anti-CD24 mAb. Flow cytometric analysis of appendix and spleen cells from conventional rabbits stained with B) Anti-CD24 and recombinant soluble BAFF (rBAFF) and C) Upper: anti-CD24 and anti-L chain; lower: anti-Ki67 (open histograms) of T1 cells (from upper diagram). Shaded histogram = isotype control. D) Flow cytometric analysis of sorted splenic T1(CD24hiCD21lo) (upper) and mature B cells (CD24lo/-CD21+) (lower) stained with Annexin V and propidium iodide (PI) after 12-15 hrs in culture with anti-Ig (10μg/ml) [goat (F(ab’) anti-rabbit IgG (H+L); Jackson ImmunoResearch Laboratories], E) Somatic diversification of VH regions of PCR-amplified VDJ genes from splenic (spl) and appendix (apx) T1 B cells. The horizontal bar represents average number of nucleotide changes/VH gene (excluding D and J regions); each dot represents one VH gene sequence. Sequences obtained from three adult rabbits are shown. Data in B) to D) are representative of 2-3 independent experiments. Data in A) are representative of two control and three TACI-Ig treated rabbits.
Recombinant soluble BAFF (rBAFF) binds to most freshly isolated murine B cells (25), but not to most B cells in rabbit due to occupied receptors (20). Instead, in rabbits, rBAFF binds to a small subset of IgMlo cells in spleen, appendix and PB, which we previously described as putative transitional B cells (20). Here, we show that these BAFF-binding cells are CD24+ B cells (Fig 2B) and that all T1 B cells in the spleen and appendix are Ki-67+ (Fig 2C), indicating that they are proliferating.
To further characterize T1 B cells, we tested how they responded to anti-Ig stimulation in vitro. Following anti-Ig treatment of sorted splenic T1 and mature B cells, T1 cells underwent apoptosis, while the mature B cells did not (Fig 2D). We also tested if the Ig genes in T1 B cells were somatically diversified. Murine transitional B cells are constantly replenished from the BM and consequently have unmutated Ig genes even in adults (26). Because new B cells are not made in BM of adult rabbits (18, 19), we predicted that the T1 B cells would be diversified. We isolated T1 B cells from spleen and appendix of adult rabbits and PCR-amplified and sequenced the Ig VDJ genes. As expected, we found the IgH genes had undergone somatic diversification (Fig 2E and S1), suggesting that the B cells had been through a GC-like reaction and were not recent emigrants from the BM.
Tissue localization of transitional B cells
To localize transitional B cells in tissues, we performed immunohistology on tissue sections. Because anti-CD24 did not stain frozen tissue sections effectively, we used anti-CD20, which binds all CD24+ B cells both in the spleen (Fig 1A, lower) and appendix (Fig 3A, upper), but does not bind mature B cells or non-B cells (Fig 3A, lower). By using anti-CD23 to label the follicular zone, we found that CD20+ transitional B cells in spleen were located near the margins of the follicles and also in the red pulp (Fig 3B). These data are similar to the localization of splenic transitional B cells in mouse (2). We also identified T1 B cells (CD24hiCD21loIgMloCD62Llo) in the BM of young rabbits (Fig 4A) and a CD24loCD21lo/- population that we thought might include proB and preB cells (Arrow in Fig 4A). To test if proB and preB cells are CD24+, we stained BM cells for MHC II and cytoplasmic IgM (19) and found that proB cells and also cells in the preB & B cell gate (presumably preB cells) were CD24+ (Fig 4B). In addition to early B cell progenitors, the CD24loCD21- population might include other lineages such as CD24+ stromal cells and common lymphoid progenitors (27). In contrast to young rabbits, we found few, if any, CD24hi T1 B cells in the BM of adult rabbits (Fig 4C). This finding is consistent with the absence of B lineage precursors (proB and preB) in the BM of adult rabbits (19).
Figure 3. Tissue localization of CD20+ transitional B cells.
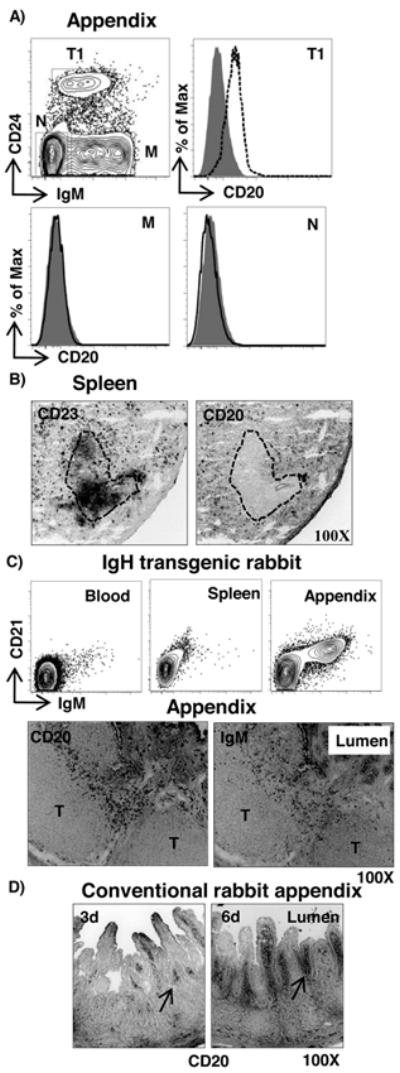
A) Flow cytometric staining of appendix B cells from a neonatal rabbit (1 wk of age) for T1 (CD24hiIgMlo) (dashed), M (CD24-IgM+) (black) and non-B cells (N) (IgM-CD24-) (black) for CD20. Shaded histogram = isotype control. B) Immunohistological staining of spleen (6-week old) section for CD23 (follicular B cells) and CD20 (transitional B cells). The dotted line represents a B cell follicle. C) Flow cytometric and immunohistological analyses of tissues from an IgH transgenic rabbit stained for IgM and CD21 (upper), and CD20 and IgM (lower), respectively. D) Staining for CD20 in appendix from a conventional 3 & 6-day-old rabbit. Magnification = 100X.
Figure 4. Flow cytometric analysis of T1 B cells in BM.
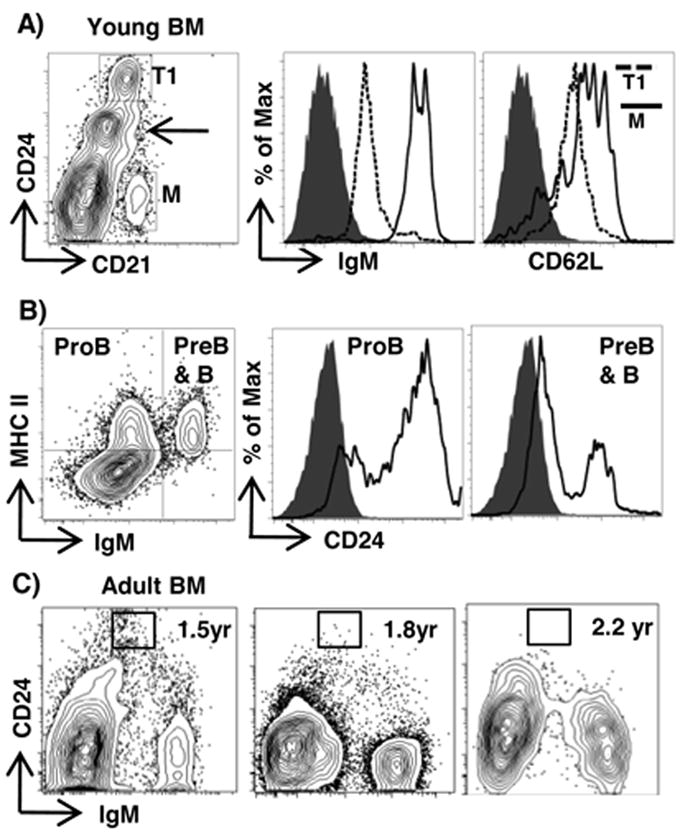
A) BM cells from a young rabbit (8 weeks-of-age) stained for CD21 and CD24, with histograms of IgM and CD62L staining of cells in T1 (dashed) and M (black) cell gates. Shaded histogram = isotype control. B) BM cells from a young rabbit (8 weeks-of-age) stained for IgM, MHC II (left) and CD24, with CD24 histograms of cells in proB and preB & B cell gates (center and right). Shaded histogram = isotype control. C) BM cells from adult rabbits aged 1.5 to 2.2 years stained for CD24 and IgM. All plots are representative of staining obtained from at least 3 rabbits.
During the time of ongoing B lymphopoiesis in young rabbits, B cells leave the BM and migrate to GALT where they undergo extensive proliferative expansion and somatic diversification of Ig genes (28, 29). To localize transitional B cells in GALT during this process, we examined the appendix from an IgH transgenic (Tg) rabbit. These IgH Tg rabbits are deficient in B cells early in ontogeny, but IgM+ B cells accumulate gradually first in the appendix and SR and later in PP, MLN, blood and spleen (30). The delayed temporal appearance of B cells in these rabbits offered an opportunity to study the early stages of peripheral B cell development. We examined one IgH Tg rabbit that lacked IgM+ B cells in the periphery (PB and spleen), but had a few B cells in GALT (Fig 3C). We found that these IgM+ B cells in the appendix were CD21lo (Fig 3C, right), and CD20+ (Fig 3C, lower), suggestive of a transitional B cell phenotype. The CD20+ B cells were scattered, predominantly in the domes and villi of underdeveloped B cell follicles located between large T cell areas (Fig 3C, lower). Similarly, we identified transitional B cells in the dome and villous regions of appendix from conventional neonatal rabbits (3&6 days of age) (Fig 3D). Similar to neonates, we found CD20+ cells located in the villous regions of the appendix of a 4 week old rabbit (data not shown). We conclude that during development, the transitional B cells migrate to the domes and villi of the appendix prior to differentiating into follicular B cells. The unique and close proximity of GALT transitional B cells to the intestinal lumen suggests to us that these B cells may interact with commensal bacteria or bacterial-derived products that promote B cell activation and maturation in GALT.
Role of complement in the proliferative expansion of appendix B cells
During the early stages of peripheral B cell development in rabbits, GALT serves as a site for B cell expansion and Ig diversification (12). We previously demonstrated that commensal bacteria in the intestinal lumen are required to stimulate B cell proliferation and Ig diversification in GALT (21, 31). Intestinal bacteria may contribute to these processes by regulating the expression and secretion of various bacterial- and host-derived stimulatory molecules. In a germ-free appendix, we found no C3 deposition, whereas in conventional appendices, C3 was readily identified in the B cell follicles (Fig 5A). These results indicate that C3 expression/localization in the appendix is regulated by commensal bacteria. Further, by flow cytometry, we found luminal bacteria were coated with C3 and IgA, and the IgA+ bacteria appeared to have a greater deposition of C3 on the surface compared to IgA- bacteria (Fig 5B). These findings prompted us to investigate if complement plays a role in promoting the proliferative expansion of B cells in GALT. To inhibit signaling via complement receptor 2 (CR2/CD21), we injected newborn rabbits with a recombinant adenovirus (rAd) expressing soluble CD21 (CD21-Ig) and analyzed the appendix by immunohistochemistry after 7-10 days. Upon CD21-Ig treatment, we found little to no Ki-67 expression in the appendix (Fig 5C), indicating that signaling through CD21 is required for B cell proliferation in GALT. Additionally, we depleted C3 in vivo by i.p. injection of Cobra venom factor (CVF) (0.5mg/kg body wt) 24 and 48 h after birth. Rabbits were sacrificed at 6-7 d of age and we found, similar to CD21-Ig treatment, B cell proliferation in the appendix was inhibited (Fig 5C). We conclude that complement is required for B cell proliferation in GALT.
Figure 5. Identification of molecules required for proliferative expansion of B cells in GALT.
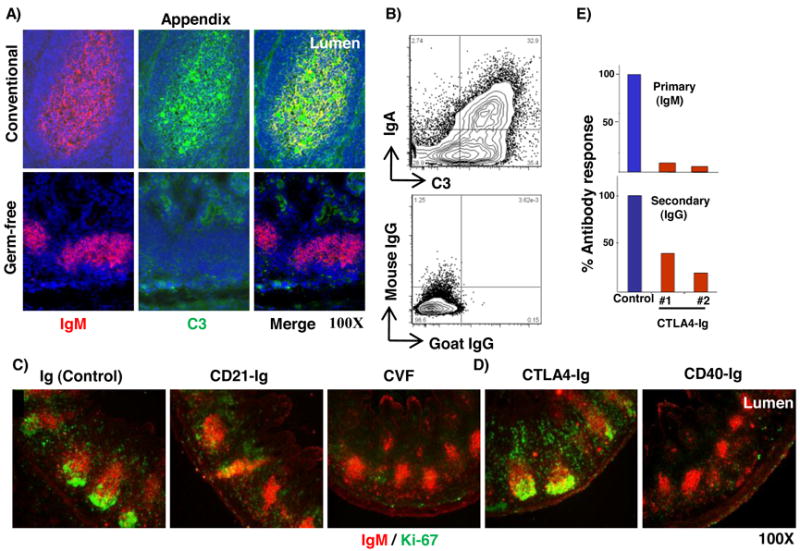
A) Immunofluorescent staining for IgM and C3 in appendix sections from conventional 4 wk old rabbit (upper) and 4 wk old rabbit with a germ-free appendix (lower). B) Flow cytometric analysis of IgA- and C3- stained intestinal commensal bacteria from 4 wk old rabbit. Plots are representative of two independent experiments. C) & D) Immunofluorescent staining of appendix sections for IgM and Ki-67 following treatment of newborn rabbits with adenovirus (Ad) expressing soluble receptors; Ig (negative control), CD21 (CR2-Ig), cobra venom factor (CVF), CTLA4 (CTLA4-Ig) and CD40 (CD40-Ig). Data are representative of 3 or more Ad- or CVF-treated rabbits. E) Bar graph showing the primary anti-BGG (IgM) (upper) and secondary anti-BGG (IgG) (lower) response, compared to the anti-BGG response from an age-matched littermate control (=100%) as determined by ELISA. #1 and #2 represent data from two rAdCTLA4-Ig treated rabbits. Magnification = 100X.
Co-stimulatory molecules required for the proliferative expansion of B cells in GALT
To determine if T-cell help is required for the proliferative expansion of B cells, we inhibited T cell activation by injecting newborn rabbits with a rAd expressing soluble CTLA4 (CTLA4-Ig) (32). After 7-10 days, we analyzed the appendix by immunohistochemistry for the presence of follicles with proliferating (Ki-67+) B cells and found that CTLA4-Ig did not inhibit B cell proliferation (Fig 5D). To confirm that the absence of a phenotype in the CTLA4-Ig injected rabbits was not due to insufficient or non-functional CTLA4-Ig, we immunized two rabbits with a T-dependent antigen (BGG) and found, as expected, a dramatic reduction in both primary IgM and secondary IgG (anti-BGG) Ab titers (Fig 5E), indicating that CTLA4-Ig was functional in vivo. To determine if CD40-CD40L interaction is required for the proliferative expansion of B cells in the appendix, we injected newborn rabbits with rAd expressing soluble CD40 (CD40-Ig) and found that it inhibited B cell proliferation in the appendix (Fig 5D). We conclude that activation of T cells via the B7-CD28 pathway is not required for the proliferative expansion of B cells in GALT whereas CD40-CD40L interaction is required.
Discussion
Studies in mice and humans indicate that transitional B cells play a key role in the peripheral stages of B cell development (4). However, in rabbits and other species (such as sheep, pigs and cattle) that use GALT to develop their B cell repertoire (12-17), essentially nothing is known about transitional-like B cells and their development. Due to the paucity of rabbit specific antibodies, we used cross-reactive antibodies to identify subsets of rabbit B cells. In general, the phenotype of transitional B cells in rabbit was more similar to transitional B cells in human than in mice. Using anti-CD24 and anti-CD21 mAbs, we identified two subsets of CD24hi transitional B cells: T1 and T2 (CD21lo=T1; CD21+=T2), as described recently in humans (11). Anti-CD23, which distinguishes T1 (CD23-) and T2 (CD23+) B cells (2, 5) in mice, but not humans (11), also did not distinguish rabbit T1 and T2 B cells. Like in humans, CD10 and CD38 can be used to identify transitional B cells. Another marker CD20, is a pan human B cell marker and is expressed at high levels on transitional B cells (10, 23). Interestingly in rabbits, CD20 is expressed only on transitional B cells; if CD20 is expressed on mature B cells, it is at a level below the limits of detection. As with all the other cross-reactive antibodies used in this study, we cannot be certain that the antigens recognized by the antibodies are the rabbit homologues of the respective mouse and human proteins. Nevertheless, these cross-reactive antibodies provide a means to identify B cell subsets.
Functionally, transitional B cells in rabbits exhibit similarities with their counterparts in other species. Neutralization of BAFF in neonatal rabbits arrested B cell development at the T1 stage, confirming that transitional B cells like in mice depend on BAFF for their development (33). Consistent with results in mice and humans (3, 10), T1 B cells underwent apoptosis upon anti-Ig stimulation. T1 B cells in rabbit also exhibited some unique characteristics. For example essentially all T1 B cells in adult rabbits were proliferating in vivo and had somatically diversified Ig genes. A diversified repertoire indicates that these cells had undergone a GC-like reaction and were not recent emigrants from the BM. The presence of diversified T1 B cells in adults, long after the arrest of B lymphopoiesis, suggests that T1 B cells are maintained in the periphery, possibly because they are long-lived and/or self-renewing.
B cell maturation in the mouse proceeds in a T1→T2→M pathway (2). It remains to be determined if rabbit T1 B cells give rise to T2 and mature B cells in a BAFF-dependent manner. Mature and transitional (T1 and T2) B cell subsets were readily detected in the spleen of neonatal rabbits, with transitional B cells being present at the frequencies similar to those in adults. In contrast, in neonatal mice, B cells in the periphery are mostly HSAhi immature/T1 B cells, and reach adult levels in the spleen (5-10% of all B cells) only after 6-8 weeks of age (2, 3, 26). Because B lymphopoiesis occurs only early in life, rabbits may have evolved a strategy to rapidly differentiate their immature B cells and generate a functional B cell compartment before the arrest of B lymphopoiesis.
During development, B cells leave the BM and migrate to GALT, where they expand in numbers and somatically diversify the Ig genes (29). Using an IgH Tg rabbit, which was B cell deficient at birth and in which few B cells accumulated over a span of several months (30), we found that the first B cells to appear in GALT were CD20+ transitional B cells. These cells were localized predominantly in the domes and villi. A similar distribution of transitional B cells was found in conventional neonatal rabbits, suggesting that CD20+ transitional B cells first migrate to the domes and villous regions of GALT before differentiating into mature follicular B cells.
What could be the significance of this unique pattern of localization of transitional B cells in the domes and villi of GALT? B cells in the domes are in close proximity to M cells, which are known to translocate bacteria and sample luminal antigens (34). The T1 B cells in the domes and villi may interact directly with commensal bacteria or with bacterial-derived products and promote further differentiation of transitional B cells into mature B cells. In support of this idea, rabbits, which had either limited and no microbiota in the appendix had reduced numbers of peripheral B cells (31). In germ-free mice, the number of mature B cells is strongly reduced; the number of T2 B cells was reportedly normal, indicating that commensal bacteria are required for the development of transitional B cells into mature B cells (2).
Following the appearance of T1 B cells in the appendix, organized follicles with proliferating B cells begin to form. These follicular B cells likely arise from the incoming T1 B cells and expand to form GC-like structures, where they somatically diversify the Ig genes. We think that these processes occur in an antigen- and T cell-independent manner because interference of B7-CD28 costimulation by CTLA4Ig did not inhibit B cell proliferation. However CD40-CD40L interactions are required for B cell proliferation. Although the source of CD40L would be expected to be activated T cells, we predict that one or several cell types, such as DCs, macrophages, NK cells, and epithelial cells, all of which can express CD40L is the source of CD40L (35). We predict this because we found CD40L transcripts and protein localized throughout the appendix tissue, rather than limited to the T cell areas (Yeramilli and Knight, unpublished data).
By inhibiting the interaction of CD21 and its ligand (CD21L), and also by depleting C3, we found that complement is required for B cell expansion in GALT. Many commensal bacteria are coated with C3 and IgA, and it may be that immune complexes of IgA and microbial antigens are trapped by follicular dendritic cells (FDCs) and presented to B cells, resulting in stimulation through crosslinking of BCR and its coreceptor, CD21. This possibility is suggested by the presence of C3 in the appendix follicles which could be due to deposition of complement C3-containing fragments on FDCs and/or B cells.
Transitional B cells in adult rabbits: Implications for peripheral B cell maintenance
We designate the transitional B cells in adult rabbits as T1d B cells because the Ig genes are somatically diversified. These T1d cells are likely generated in GALT during the first few weeks of life, a time during which the Ig genes of essentially all B cells somatically diversify (12, 18). Following the arrest of B lymphopoiesis a few weeks after birth, the T1d B cells, which are proliferating, are likely maintained by self-renewal. In the absence of new B cell formation in the BM after 2-4 months of age, we propose that it is the self-renewing T1d B cells that are responsible for maintaining the peripheral B cell compartment. We think the self-renewing T1d B cells in adults continuously develop into mature B cells in a BAFF-dependent manner, and thereby maintain B cell homeostasis, although we cannot rule out the possibility that a few B cells are generated in the BM in adults under conditions such as infection and inflammation (36-38). Moreover, the BAFF receptors of most mature B cells are occupied by endogenous BAFF (20), and we think that this chronic occupancy of BAFF-receptor(s) allows them to remain long-lived by providing a tonic and/or survival signal (20). Additionally, IL7II, a novel isoform of IL-7 may provide a survival signal to mature B cells (39). Thus, we think that together T1d B cells and long-lived mature B cells regulate peripheral B cell homeostasis in adult rabbits.
Model of T1d B cell development and maintenance
Based on our current and previous findings, we propose a model (Fig 6A) in which CD24hi immature B cells exit the BM early in ontogeny as transitional (T1) B cells (IgMloCD21loCD62Llo) and traffic to GALT (appendix) where they enter the domes and villi (Figure 6A, inset). Here, (after birth) they interact with commensal bacteria or bacterial-derived products, such as superantigens (40) and become activated. Following activation, T1 B cells proliferate and somatically diversify the Ig genes to become T1d B cells. The follicular B cells, which early in ontogeny are likely derived from BAFF-stimulated BM-T1 (or T1 B cells in GALT) expand in a B7-CD28 independent, CD40-CD40L and CD21-CD21L dependent manner to form GC-like structures. We propose that the follicular B cells as well as T1d B cells leave the appendix and seed other peripheral tissues and upon BAFF stimulation, the T1d B cells give rise to mature B cells. It may well be that some of the BM-derived T1 B cells also directly traffic from BM to other sites, such as spleen, and develop into mature B cells. We propose that in adults, in the absence of newly-formed B cells from the BM, the T1d B cells that are presumably maintained through self-renewal, continually develop into mature B cells and thus maintain peripheral B cell homeostasis (Fig 6B, left). Mature B cells have occupied BAFF-receptors (20) and we propose that BAFF provides tonic signaling to these B cells and thereby also contributes to B cell homeostasis (Fig 6B, right).
Figure 6. Model of T1d B cell development and maintenance.
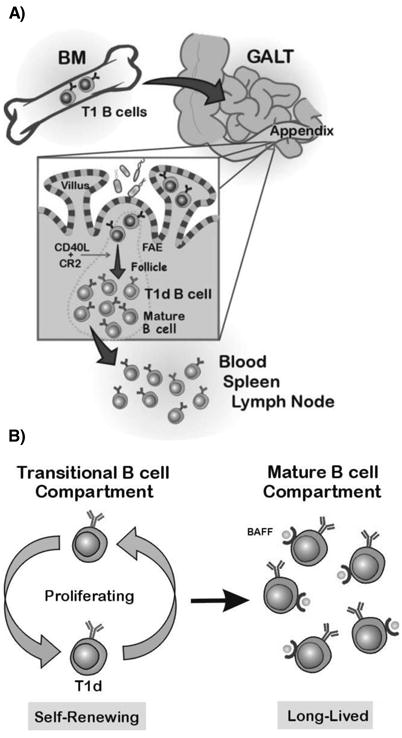
A) Development of T1d B cells. T1 B cells leave the BM and enter GALT (appendix) through the HEVs and traffic to the domes and villi, where they are stimulated by BAFF and commensal bacteria or bacterial-derived products. The activated T1 B cells proliferate and somatically diversify the Ig genes to become T1d B cells. The follicular B cells, derived from BAFF stimulated BM-T1 or GALT T1 B cells undergo a proliferative expansion to form organized follicles in a CR2-CR2L and CD40-CD40L dependent manner. After undergoing the GALT GC-like reaction, mature and T1d B cells enter the circulation where T1d B cells further differentiate into mature B cells. Some of the GALT-derived T1d B cells traffic to the spleen and differentiate into T2 and mature B cell subsets. Alternatively, some T1 B cells from the BM may directly traffic to the spleen and develop into mature B cells (not shown). B) Maintenance of peripheral B cells. In adult rabbits, in the absence of ongoing lymphopoiesis, the B cell compartment is maintained by the proliferating T1d B cells, which self-renew and continually differentiate into mature B cells. Additionally, the BAFF receptor(s) on mature B cells in the periphery are bound by endogenous BAFF and this chronic engagement of BAFF receptors may provide a tonic/survival signal for the B cells to remain long-lived.
Our model leads one to ask the question how and where T1d B cells mature into B cells; how this is regulated; how are T1d B cells maintained; and if T1d cells participated in germinal center reactions, as evidenced by somatic diversification of the Ig genes, then how do they remain transitional-like B cells that proliferate and yet die in response to BCR stimulation? As reviewed by Alitheen et al (41), different species have distinct strategies to maintain the B cell compartment, and rabbits may have evolved a new B cell type for this purpose. Alternatively, T1d-like B cells may be present in most mammals, but have not yet been identified. The finding that essentially all T1d B cells are proliferating may suggest that they are constantly being stimulated, perhaps because they have a restricted repertoire to specific bacterial and/or self-antigens and that continuous stimulation by these antigens promotes self-renewal, thereby maintaining a constant source of transitional B cells in the absence of ongoing B lymphopoiesis. Experiments to address these questions will elucidate some of the mechanism(s) by which rabbits and presumably other species develop and maintain their B cell compartment.
In summary, our study is the first to characterize transitional B cells in rabbit and by extension, in mammals that use GALT to develop their B cell repertoire. Remarkably, transitional B cells are maintained in the periphery of adult rabbits, when there is no evidence for ongoing lymphopoiesis in the BM. The finding(s) that these cells have a diversified repertoire and are undergoing proliferation confirms that these cells are not newly-made, and instead, leads to the idea that the T1d B cells are maintained by self-renewal and are responsible for maintaining the B cell compartment in the absence of detectable B lymphopoiesis. Many of the markers expressed on rabbit transitional B cells are also found on human transitional B cells, suggesting that rabbits can be used as a model to study human B cell biology. Finally, we suggest that similar to rabbits, subsets of human transitional B cells may contribute to the maintenance of peripheral B cells as B lymphopoiesis in the BM decreases in the elderly.
Supplementary Material
Acknowledgments
We thank Shi-Kang Zhai for performing the cloning and sequence analysis of Ig genes. We acknowledge the help of Patricia Simms in the FACS Core Facility at Loyola University Chicago (Maywood, IL).
Abbreviations used
- Apx
Appendix
- BM
Bone marrow
- BAFF
B cell activating factor
- CHO
Chinese hamster ovary
- CR2
Complement receptor 2
- CVF
Cobra venom factor
- GC
germinal center
- MLN
mesenteric lymph node
- PP
Peyer’s patch
- PB
peripheral blood
- SR
sacculus rotundus
- Tg
transgenic
- TACI
Transmembrane activator calcium modulator and cyclophilin ligand interactor
Footnotes
This work was supported by National Institutes of Health grants AI050260 and AI068390 (to K.L.K.).
References
- 1.Carsetti R, Kohler G, Lamers MC. Transitional B cells are the target of negative selection in the B cell compartment. J Exp Med. 1995;181:2129–2140. doi: 10.1084/jem.181.6.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loder F, Mutschler B, Ray RJ, Paige CJ, Sideras P, Torres R, Lamers MC, Carsetti R. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J Exp Med. 1999;190:75–89. doi: 10.1084/jem.190.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allman DM, Ferguson SE, Cancro MP. Peripheral B cell maturation. I. Immature peripheral B cells in adults are heat-stable antigenhi and exhibit unique signaling characteristics. J Immunol. 1992;149:2533–2540. [PubMed] [Google Scholar]
- 4.Carsetti R, Rosado MM, Wardmann H. Peripheral development of B cells in mouse and man. Immunol Rev. 2004;197:179–191. doi: 10.1111/j.0105-2896.2004.0109.x. [DOI] [PubMed] [Google Scholar]
- 5.Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol. 2001;167:6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 6.Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew E, Scott ML. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 7.Petro JB, Gerstein RM, Lowe J, Carter RS, Shinners N, Khan WN. Transitional type 1 and 2 B lymphocyte subsets are differentially responsive to antigen receptor signaling. J Biol Chem. 2002;277:48009–48019. doi: 10.1074/jbc.M200305200. [DOI] [PubMed] [Google Scholar]
- 8.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 9.Marie-Cardine A, Divay F, Dutot I, Green A, Perdrix A, Boyer O, Contentin N, Tilly H, Tron F, Vannier JP, Jacquot S. Transitional B cells in humans: characterization and insight from B lymphocyte reconstitution after hematopoietic stem cell transplantation. Clin Immunol. 2008;127:14–25. doi: 10.1016/j.clim.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Sims GP, Ettinger R, Shirota Y, Yarboro CH, Illei GG, Lipsky PE. Identification and characterization of circulating human transitional B cells. Blood. 2005;105:4390–4398. doi: 10.1182/blood-2004-11-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suryani S, Fulcher DA, Santner-Nanan B, Nanan R, Wong M, Shaw PJ, Gibson J, Williams A, Tangye SG. Differential expression of CD21 identifies developmentally and functionally distinct subsets of human transitional B cells. Blood. 2010;115:519–529. doi: 10.1182/blood-2009-07-234799. [DOI] [PubMed] [Google Scholar]
- 12.Vajdy M, Sethupathi P, Knight KL. Dependence of antibody somatic diversification on gut-associated lymphoid tissue in rabbits. J Immunol. 1998;160:2725–2729. [PubMed] [Google Scholar]
- 13.Weinstein PD, Anderson AO, Mage RG. Rabbit IgH sequences in appendix germinal centers: VH diversification by gene conversion-like and hypermutation mechanisms. Immunity. 1994;1:647–659. doi: 10.1016/1074-7613(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 14.Weill JC, Reynaud CA. Do developing B cells need antigen? J Exp Med. 2005;201:7–9. doi: 10.1084/jem.20042111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer-Bahlburg A, Andrews SF, Yu KO, Porcelli SA, Rawlings DJ. Characterization of a late transitional B cell population highly sensitive to BAFF-mediated homeostatic proliferation. J Exp Med. 2008;205:155–168. doi: 10.1084/jem.20071088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parng CL, Hansal S, Goldsby RA, Osborne BA. Gene conversion contributes to Ig light chain diversity in cattle. J Immunol. 1996;157:5478–5486. [PubMed] [Google Scholar]
- 17.Butler JE, Sun J, Weber P, Navarro P, Francis D. Antibody repertoire development in fetal and newborn piglets, III. Colonization of the gastrointestinal tract selectively diversifies the preimmune repertoire in mucosal lymphoid tissues. Immunology. 2000;100:119–130. doi: 10.1046/j.1365-2567.2000.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crane MA, Kingzette M, Knight KL. Evidence for limited B-lymphopoiesis in adult rabbits. J Exp Med. 1996;183:2119–2121. doi: 10.1084/jem.183.5.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jasper PJ, Zhai SK, Kalis SL, Kingzette M, Knight KL. B lymphocyte development in rabbit: progenitor B cells and waning of B lymphopoiesis. J Immunol. 2003;171:6372–6380. doi: 10.4049/jimmunol.171.12.6372. [DOI] [PubMed] [Google Scholar]
- 20.Yeramilli VA, Knight KL. Requirement for BAFF and APRIL during B cell development in GALT. J Immunol. 2010;184:5527–5536. doi: 10.4049/jimmunol.1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhee KJ, Sethupathi P, Driks A, Lanning DK, Knight KL. Role of commensal bacteria in development of gut-associated lymphoid tissues and preimmune antibody repertoire. J Immunol. 2004;172:1118–1124. doi: 10.4049/jimmunol.172.2.1118. [DOI] [PubMed] [Google Scholar]
- 22.Duperray C, Boiron JM, Boucheix C, Cantaloube JF, Lavabre-Bertrand T, Attal M, Brochier J, Maraninchi D, Bataille R, Klein B. The CD24 antigen discriminates between pre-B and B cells in human bone marrow. J Immunol. 1990;145:3678–3683. [PubMed] [Google Scholar]
- 23.Cuss AK, Avery DT, Cannons JL, Yu LJ, Nichols KE, Shaw PJ, Tangye SG. Expansion of functionally immature transitional B cells is associated with human-immunodeficient states characterized by impaired humoral immunity. J Immunol. 2006;176:1506–1516. doi: 10.4049/jimmunol.176.3.1506. [DOI] [PubMed] [Google Scholar]
- 24.Kroese FG, de Boer NK, de Boer T, Nieuwenhuis P, Kantor AB, Deenen GJ. Identification and kinetics of two recently bone marrow-derived B cell populations in peripheral lymphoid tissues. Cell Immunol. 1995;162:185–193. doi: 10.1006/cimm.1995.1068. [DOI] [PubMed] [Google Scholar]
- 25.Thompson JS, Bixler SA, Qian F, Vora K, Scott ML, Cachero TG, Hession C, Schneider P, Sizing ID, Mullen C, Strauch K, Zafari M, Benjamin CD, Tschopp J, Browning JL, Ambrose C. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. 2001;293:2108–2111. doi: 10.1126/science.1061965. [DOI] [PubMed] [Google Scholar]
- 26.Allman DM, Ferguson SE, Lentz VM, Cancro MP. Peripheral B cell maturation. II. Heat-stable antigen(hi) splenic B cells are an immature developmental intermediate in the production of long-lived marrow-derived B cells. J Immunol. 1993;151:4431–4444. [PubMed] [Google Scholar]
- 27.Israel E, Kapelushnik J, Yermiahu T, Levi I, Yaniv I, Shpilberg O, Shubinsky G. Expression of CD24 on CD19- CD79a+ early B-cell progenitors in human bone marrow. Cell Immunol. 2005;236:171–178. doi: 10.1016/j.cellimm.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 28.Knight KL, Winstead CR. Generation of antibody diversity in rabbits. Curr Opin Immunol. 1997;9:228–232. doi: 10.1016/s0952-7915(97)80140-9. [DOI] [PubMed] [Google Scholar]
- 29.Knight KL, Winstead CR. B lymphocyte development in the rabbit. Int Rev Immunol. 1997;15:129–163. doi: 10.3109/08830189709068174. [DOI] [PubMed] [Google Scholar]
- 30.Jasper PJ, Rhee KJ, Kalis SL, Sethupathi P, Yam PC, Zhai SK, Knight KL. B lymphocyte deficiency in IgH-transgenic rabbits. Eur J Immunol. 2007;37:2290–2299. doi: 10.1002/eji.200737191. [DOI] [PubMed] [Google Scholar]
- 31.Lanning D, Sethupathi P, Rhee KJ, Zhai SK, Knight KL. Intestinal microflora and diversification of the rabbit antibody repertoire. J Immunol. 2000;165:2012–2019. doi: 10.4049/jimmunol.165.4.2012. [DOI] [PubMed] [Google Scholar]
- 32.Linsley PS, Wallace PM, Johnson J, Gibson MG, Greene JL, Ledbetter JA, Singh C, Tepper MA. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science. 1992;257:792–795. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 33.Gross JA, Dillon SR, Mudri S, Johnston J, Littau A, Roque R, Rixon M, Schou O, Foley KP, Haugen H, McMillen S, Waggie K, Schreckhise RW, Shoemaker K, Vu T, Moore M, Grossman A, Clegg CH. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease. impaired B cell maturation in mice lacking BLyS. Immunity. 2001;15:289–302. doi: 10.1016/s1074-7613(01)00183-2. [DOI] [PubMed] [Google Scholar]
- 34.Miller H, Zhang J, Kuolee R, Patel GB, Chen W. Intestinal M cells: the fallible sentinels? World J Gastroenterol. 2007;13:1477–1486. doi: 10.3748/wjg.v13.i10.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schonbeck U, Libby P. The CD40/CD154 receptor/ligand dyad. Cell Mol Life Sci. 2001;58:4–43. doi: 10.1007/PL00000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagaoka H, Gonzalez-Aseguinolaza G, Tsuji M, Nussenzweig MC. Immunization and infection change the number of recombination activating gene (RAG)-expressing B cells in the periphery by altering immature lymphocyte production. J Exp Med. 2000;191:2113–2120. doi: 10.1084/jem.191.12.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalis SL, Zhai SK, Yam PC, Witte PL, Knight KL. Suppression of B lymphopoiesis at a lymphoid progenitor stage in adult rabbits. Int Immunol. 2007;19:801–811. doi: 10.1093/intimm/dxm048. [DOI] [PubMed] [Google Scholar]
- 38.Sehgal D, Schiaffella E, Anderson AO, Mage RG. Analyses of single B cells by polymerase chain reaction reveal rearranged VH with germline sequences in spleens of immunized adult rabbits: implications for B cell repertoire maintenance and renewal. J Immunol. 1998;161:5347–5356. [PubMed] [Google Scholar]
- 39.Siewe BT, Kalis SL, Esteves PJ, Zhou T, Knight KL. A novel functional rabbit IL-7 isoform. Dev Comp Immunol. 2010;34:828–836. doi: 10.1016/j.dci.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Severson KM, Mallozzi M, Driks A, Knight KL. B cell development in GALT: role of bacterial superantigen-like molecules. J Immunol. 2010;184:6782–6789. doi: 10.4049/jimmunol.1000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alitheen NB, McClure S, McCullagh P. B-cell development: one problem, multiple solutions. Immunol Cell Biol. 2010;88:445–450. doi: 10.1038/icb.2009.119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


