Abstract
Although studies have shown that physically active breast cancer survivors have lower all-cause mortality, the association between change in physical activity from before to after diagnosis and mortality is not clear. We examined associations among pre- and postdiagnosis physical activity, change in pre- to postdiagnosis physical activity, and all-cause and breast cancer–specific mortality in post-menopausal women. A longitudinal study of 4,643 women diagnosed with invasive breast cancer after entry into the Women’s Health Initiative study of postmenopausal women. Physical activity from recreation and walking was determined at baseline (prediagnosis) and after diagnosis (assessed at the 3 or 6 years post-baseline visit). Women participating in 9 MET-h/wk or more (~3 h/wk of fast walking) of physical activity before diagnosis had a lower all-cause mortality (HR = 0.61; 95% CI, 0.44–0.87; P = 0.01) compared with inactive women in multivariable adjusted analyses. Women participating in ≥9 or more MET-h/wk of physical activity after diagnosis had lower breast cancer mortality (HR = 0.61; 95% CI, 0.35–0.99; P = 0.049) and lower all-cause mortality (HR = 0.54; 95% CI, 0.38–0.79; P < 0.01). Women who increased or maintained physical activity of 9 or more MET-h/wk after diagnosis had lower all-cause mortality (HR = 0.67; 95% CI, 0.46–0.96) even if they were inactive before diagnosis. High levels of physical activity may improve survival in postmenopausal women with breast cancer, even among those reporting low physical activity prior to diagnosis. Women diagnosed with breast cancer should be encouraged to initiate and maintain a program of physical activity.
Introduction
In observational studies, physically active women have a substantially less risk of developing breast cancer compared with inactive women (1–3). In addition, physically active women at the time of breast cancer diagnosis have been reported to have lower all-cause mortality, with 2–3 h/wk of moderate-intensity physical activity associated with an approximate 50% less risk of death (4–9). However, the question of whether there is a survival benefit to increasing physical activity after a breast cancer diagnosis, especially among women who were inactive before diagnosis, has received limited attention. In the only previous study examining this issue, a nonsignificant survival benefit of increasing physical activity after a breast cancer diagnosis was suggested but the finding was limited by retrospective assessment of prediagnosis physical activity and a relatively small sample size (7).
Against this background, we examined the associations between pre- and postdiagnosis physical activity, and changes in pre- to postdiagnosis physical activity and mortality directly attributed to breast cancer and from all causes in a large cohort of postmenopausal women enrolled in the Women’s Health Initiative (WHI) who were diagnosed with invasive breast cancer.
Methods
Study population
The study population included 161,808 women enrolled in the WHI Observational Study (n = 93,646) or Clinical Trials (n = 61,132) between October 1993 and December 1998 from 40 U.S. clinical centers (10). Eligible women were between 50 and 79 years of age, postmenopausal, and planned to reside in the area for 3 years or more (10). Participants completed in-person visits at baseline and 3 and 6 years post-baseline. The institutional review boards at all institutions approved the protocol, and participants provided informed consent.
Participants for prediagnosis physical activity analyses
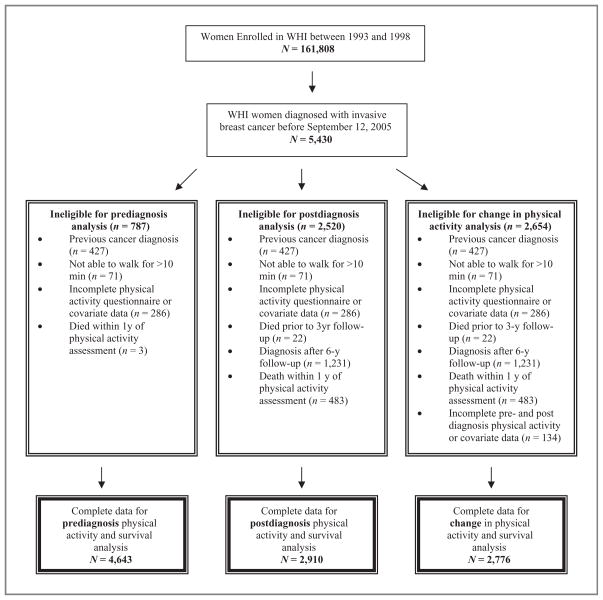
Eligibility criteria for the analyses of physical activity and mortality included (i) invasive breast cancer diagnosis before September 12, 2005 (n = 5,430); (ii) no previous cancer diagnosis (n = 5,003 of 5,430); (iii) ability to walk outside for more than 10 minutes without stopping (n = 4,932 of 5,003); (iv) complete physical activity and covariate data (n = 4,646 of 4,932); and (v) available health status or centrally adjudicated cancer and death outcomes (n = 4,646). To reduce the potential for confounding due to occult disease, women had to be alive for at least 1 year following baseline (prediagnosis) physical activity assessment (n = 4,643 of 4,646). Thus, 4,643 women (86% of 5,430 WHI women diagnosed with invasive breast cancer) were included in the analysis of prediagnosis (baseline measures) physical activity and mortality (Fig. 1)
Figure 1.
Eligibility criteria of WHI participants included in analysis of pre- and postdiagnosis physical activity and survival in women with breast cancer.
Participants for postdiagnosis physical activity analyses
Eligibility criteria for the postdiagnosis physical activity analyses included available postdiagnosis physical activity data collected at the visit closest to but after diagnosis, that is, at year 3 or year 6 (n = 2,910 of 4,643). Thus, 1,733 (37%) women were excluded from the postdiagnosis analysis for the following reasons: (i) death prior to the 3-year follow-up assessment and therefore no postdiagnosis physical activity data available (n = 19); (ii) a breast cancer diagnosis after the 6-year follow-up assessment and therefore less than 3 years of a follow-up time period to assess survival (n = 1,231, of which 17 died); or (iii) a death within 1 year of the postdiagnosis physical activity assessment (n = 483). These 1,733 excluded women did not differ from the 4,643 women included in the prediagnosis analyses or the 2,910 women included in the postdiagnosis analysis in terms of prediagnosis physical activity levels, disease stage, body mass index (BMI), or age (data not shown).
Participants for physical activity change (pre- to postdiagnosis) analyses
Eligibility criteria for the pre- to postdiagnosis change in physical activity analyses included complete pre- and postdiagnosis physical activity and covariate data. Complete data were available for 2,776 (95%) of the 2,910 women eligible for postdiagnosis analysis.
Exposure assessments
A standardized written protocol, centralized training of staff, and quality assurance visits by the coordinating center were used to ensure uniform administration of data collection. At baseline, participants completed self-administered health history questionnaires and a food frequency questionnaire (11). Height and weight were collected by using standardized methods.
Physical activity was assessed by questionnaire at baseline, year 3, and year 6. Participants were asked about walking outside the home for more than 10 minutes without stopping. Categories of frequency were never to 1 to 7 days/wk. Duration categories were less than 20 minutes, 20 to 39 minutes, 40 to 59 minutes, and 1 hour or more. Four speed categories were created: less than 2 mph (strolling), 2 to 3 mph (average/normal walking), 3 to 4 mph (fairly fast walking), and more than 4 mph (very fast walking).
To capture recreational physical activity, women were asked how often they currently exercised at vigorous levels (that increased heart rate and produced sweating) by checking categories on a Likert-type scale ranging from never to 1 to 5+ days/wk, and for how long they exercised at each session (less than 20 minutes, 20 to 39 minutes, 40 to 59 minutes, or 1 hour or more). Vigorous activities included aerobics, jogging, tennis, and swimming laps. Women were asked similar questions about moderate-intensity physical activities (including biking outdoors, exercise machine, calisthenics, easy swimming, and popular or folk dancing).
We imputed the midpoint value for ranges of frequency and duration of exercise sessions, and multiplied duration times frequency to create a variable “h/wk.” We assigned metabolic equivalent task (MET) values for walking (average, 3 METs; fast, 4 METs; and very fast 4.5 METs), moderate-intensity recreational (4 METs), and vigorous-intensity recreational (7 METs) activities (10). We then multiplied the MET level for the activity by h/wk to compute a moderate- to vigorous-intensity physical activity variable and moderate-intensity physical activity variable (MET-h/wk), both of which included walking (12).
Reproducibility and validation of the physical activity assessment
Among a random sample of 536 participants, second measures of all physical activity variables were ascertained approximately 10 weeks after baseline. The test-retest reliability (weighted κ) for the physical activity variables ranged from 0.53 to 0.72, and the intraclass correlation for the total physical activity variable was 0.77 (10). Validity of the physical activity questionnaire was also examined by comparing the questionnaire with accelerometer data (r = 0.73, and 100% sensitivity for meeting the physical activity guidelines; 13).
Ascertainment of breast cancer outcomes
Reported breast cancer outcomes were verified by centrally trained physician adjudicators at the clinical centers after medical record and pathology report review, and coded according to National Cancer Institute Surveillance, Epidemiology, and End Results guidelines (14). Vital status of participants was collected through clinical center follow-up of participants and surrogates. In addition, the National Death Index was systematically run on all participants at 2-year intervals. Cause of death was determined by medical record and death certificate review at the coordinating center.
Statistical analyses
The physical activity assessment at baseline was used for prediagnosis analyses. The 3- or 6-year physical activity assessment (whichever followed more closely the breast cancer diagnosis) was used for postdiagnosis analyses. Follow-up continued until death, loss to follow-up, or data close on September 12, 2005.
For physical activity, we created indicator variables for “no physical activity” (0 MET-h/wk), 3 MET-h/wk or less, 3.1 to 8.9 MET-h/wk, and 9 or more MET-h/wk. We chose these cutpoints for 2 reasons: (i) consistency with other published studies of physical activity and mortality, and (ii) recommended amounts of physical activity for overall health, for example, 9 MET-h/wk would be equivalent to 3 h/wk of moderate intensity. For change in physical activity, we collapsed the 2 middle categories so as to have 3 categories: inactive (0 MET-h/wk), insufficiently active (>0 to <9 MET-h/wk), and active (≥9 MET-h/wk of moderate-vigorous intensity physical activity). We then defined change in physical activity as (i) no change/inactive—remaining in the inactive or insufficiently active category before and after diagnosis; (ii) increase/active—inactive or insufficiently active before diagnosis, but increasing physical activity to 9+ MET-h/wk, or maintaining physical activity at 9+ MET-h/wk before and after diagnosis; and (3) decrease/inactive—decreasing physical activity categories from active to insufficiently or inactive category, or insufficiently active to inactive category.
We used stratified adjustment and Cox proportional hazards regression models to adjust for potential confounding variables. Two outcomes were defined: total mortality and breast cancer–specific mortality (with deaths from other causes censored). Covariates (based on P ≤ 0.1) included WHI study component (observational study vs. clinical trial), trial arm [e.g., diet intervention, calcium intervention, or hormone therapy intervention (estrogen alone vs. estrogen plus progestin vs. control)], age at enrollment, BMI at enrollment, use of hormone therapy prior to diagnosis, race/ethnicity, smoking status, alcohol use, daily kilocalorie intake, percentage of calories from fat, and servings per day of fruits and vegetables. We further adjusted postdiagnosis physical activity analyses for clinically relevant prognostic variables including disease stage, grade, estrogen receptor (ER) and progesterone receptor (PR) status, and HER2/neu status. For analyses of change in physical activity, we further adjusted for prediagnosis physical activity levels. Some covariates did not alter the pre- or postdiagnosis physical activity HR by more than 10% and therefore were not included: income, education, ever breastfed, hysterectomy status, first-degree relative with breast cancer, parity, age at first birth, number of mammograms in 5 years before study enrollment, age at menarche, and age at menopause. We tested for heterogeneity of trends in risk by using a 1 degree of freedom χ2 test. All analyses were carried out by SAS version 9 (SAS Institute Inc.).
Results
Among the 4,463 women included in this analysis, there were 350 deaths, 194 from breast cancer. The distribution of covariates according to category of prediagnosis physical activity (assessed at the WHI baseline visit) is shown in Table 1. Although BMI differed by category of physical activity, disease stage and hormone receptor status did not differ by category of physical activity.
Table 1.
Characteristics of WHI breast cancer survivors according to prediagnosis physical activity category (N = 4,643)
| Sports/recreational physical activity, MET-h/wk
|
||||
|---|---|---|---|---|
| 0 (n = 1,119) | >0–2.9 (n = 473) | 3–8.9 (n = 1,065) | ≥9 (n = 1,986) | |
| Age at enrollment, y | 63.9 ± 7.1 | 63.5 ± 6.8 | 63.4 ± 7.1 | 63.8 ± 7.1 |
| BMI at enrollment, kg/m2 | 30.2 ± 7.1 | 29.6 ± 6.0 | 28.1 ± 5.6 | 26.7 ± 5.1a,b |
| High school graduate, % | 94 | 96 | 95 | 97 |
| Race/ethnicity, % | ||||
| African American | 10 | 9 | 6 | 5 |
| Non-Hispanic white | 83 | 85 | 87 | 90 |
| Hispanic white | 3 | 4 | 4 | 2 |
| Other | 4 | 2 | 3 | 3 |
| Disease stage, % | ||||
| Local | 70 | 75 | 73 | 74 |
| Regional | 30 | 25 | 27 | 26 |
| ER status, % | ||||
| Positive | 73 | 77 | 77 | 76 |
| Negative | 16 | 15 | 14 | 13 |
| Unknown | 11 | 8 | 9 | 11 |
| HER2 status, % | ||||
| Positive | 14 | 10 | 12 | 11 |
| Negative | 48 | 54 | 51 | 47 |
| Unknown | 38 | 36 | 37 | 42 |
| Type 2 diabetes | 6 | 6 | 4 | 2a,b |
| Current smoker, % | 9 | 7 | 6 | 4a,b |
≥9 MET-h/wk significantly different from 0 MET-h/wk, P < 0.05.
≥9 MET-h/wk significantly different from >0–2.9 MET-h/wk, P < 0.05.
Physical activity before diagnosis
The average time from prediagnosis physical activity assessment to invasive breast cancer diagnosis was 4.1 ± 2.3 years. The average time from prediagnosis physical activity assessment to death or censor date was 8.0 ± 1.3 years. Higher levels of prediagnosis moderate- to vigorous-intensity physical activity, which included brisk walking, was associated with a 39% less risk of all-cause mortality (Ptrend = 0.0004; Table 2). Because the number of participants and deaths per physical activity category differed for moderate- to vigorous-intensity physical activity compared with only moderate-intensity physical activity, the hazard ratios were different for these 2 analyses (Table 2). Pre-diagnosis moderate-intensity physical activity was associated with a 42% less risk of all-cause mortality (Ptrend = 0.0003), and 40% less risk of breast cancer mortality (Ptrend = 0.014) even after adjusting for vigorous-intensity physical activity.
Table 2.
Associations between breast cancer outcomes and prediagnosis physical activity (N = 4,643)
| 0 MET-h/wk | >0–3.0 MET-h/wk | 3.1–8.9 MET-h/wk | 9+ MET-h/wk | Ptrend | |
|---|---|---|---|---|---|
| Moderate- to vigorous-intensity physical activity from walking and recreational activitiesa | |||||
| Total no. of deaths (n = 350) | 119 | 40 | 70 | 121 | |
| Age-adjusted HR (95% CI) | 1.00 | 0.79 (0.56–1.14) | 0.60 (0.45–0.81) | 0.55 (0.43–0.71) | 0.0001 |
| Multivariable-adjusted HR (95% CI) | 1.00 | 0.81 (0.56–1.16) | 0.67 (0.50–0.91) | 0.61 (0.47–0.81) | 0.0004 |
| No. of breast cancer deaths (n = 194) | 61 | 21 | 43 | 69 | |
| Age-adjusted HR (95% CI) | 1.00 | 0.81 (0.49–1.32) | 0.72 (0.49–1.07) | 0.61 (0.44–0.87) | 0.0054 |
| Multivariable-adjusted HR (95% CI) | 1.00 | 0.83 (0.51–1.37) | 0.82 (0.55–1.22) | 0.71 (0.49–1.03) | 0.073 |
| Moderate-intensity physical activity from walking and recreational activities, adjusted for vigorous-intensity physical activityb | |||||
| Total no. of deaths (n = 350) | 129 | 47 | 91 | 83 | |
| Age-adjusted HR (95% CI) | 1.00 | 0.79 (0.56–1.10) | 0.68 (0.52–0.89) | 0.52 (0.40–0.69) | 0.0001 |
| Multivariable-adjusted HR (95% CI) | 1.00 | 0.81 (0.58–1.13) | 0.75 (0.57–0.99) | 0.58 (0.44–0.78) | 0.0003 |
| No. of breast cancer deaths (n = 194) | 68 | 28 | 54 | 44 | |
| Age-adjusted HR (95% CI) | 1.00 | 0.87 (0.56–1.36) | 0.77 (0.54–1.10) | 0.54 (0.37–0.79) | 0.0014 |
| Multivariable-adjusted HR (95% CI) | 1.00 | 0.91 (0.58–1.41) | 0.87 (0.60–1.25) | 0.60 (0.40–0.90) | 0.014 |
NOTE: Physical activity assessed at baseline visit (i.e., prediagnosis). Adjusted for age, ethnicity, WHI study arm, previous hormone therapy use, BMI, diabetes, alcohol, smoke, total calories, percentage calories from fat, and servings of fruit and vegetables.
0 MET-h/wk, n = 1,119; >0–3.0 MET-h/wk, n = 473; 3.1–8.9 MET-h/wk, n = 1,065; 9+ MET-h/wk, n = 1,986.
0 MET-h/wk, n = 1,275; >0–3.0 MET-h/wk, n = 600; 3.1–8.9 MET-h/wk, n = 1,283; 9+ MET-h/wk, n = 1,485.
Physical activity after diagnosis
The average time from breast cancer diagnosis to postdiagnosis physical activity assessment was 1.8 ± 1.0 years. During a mean length of follow-up from postdiagnosis physical activity assessment to death or censor date of 3.3 ± 1.8 years, there were 186 deaths, with 86 from breast cancer. Women reporting moderate-to vigorous-intensity physical activity after a breast cancer diagnosis, which included brisk walking, showed a 46% less risk of all-cause mortality even after adjusting for prognostic variables (Ptrend = 0.0014) and a 39% less risk of breast cancer–specific mortality (Ptrend = 0.049; Table 3). When examining analyses of postdiagnosis moderate-intensity physical activity (including walking), adjusted for covariates and prognostic variables, a 38% less risk of all-cause mortality was observed (Ptrend = 0.020; Table 3).
Table 3.
Associations between breast cancer outcomes and postdiagnosis physical activity (N = 2,910)
| 0 MET-h/wk | >0–3.0 MET-h/wk | 3.1–8.9 MET-h/wk | 9+ MET-h/wk | Ptrend | |
|---|---|---|---|---|---|
| Moderate- to vigorous-intensity physical activity from walking and recreational activitiesa | |||||
| Total no. of deaths (n = 186) | 83 | 10 | 38 | 55 | |
| Age-adjusted HR (95% CI) | 1.00 | 0.42 (0.22–0.81) | 0.68 (0.46–1.00) | 0.48 (0.34–0.67) | 0.0001 |
| Multivariable-adjusted HR (95% CI) | 1.00 | 0.42 (0.21–0.82) | 0.72 (0.48–1.07) | 0.54 (0.38–0.79) | 0.0014 |
| No. of breast cancer deaths (n = 86) | 36 | 3 | 20 | 27 | |
| Age-adjusted HR (95% CI) | 1.00 | 0.28 (0.09–0.91) | 0.79 (0.46–1.36) | 0.51 (0.31–0.84) | 0.0077 |
| Multivariable-adjusted HR (95% CI) | 1.00 | 0.30 (0.09–0.99) | 0.77 (0.43–1.38) | 0.61 (0.35–0.99) | 0.049 |
| Moderate-intensity physical activity from walking and recreational activities, adjusted for vigorous-intensity physical activityb | |||||
| Total no. of deaths (n = 186) | 87 | 14 | 42 | 43 | |
| Age-adjusted HR (95% CI) | 1.00 | 0.48 (0.27–0.84) | 0.65 (0.45–0.94) | 0.52 (0.36–0.76) | 0.0006 |
| Multivariable-adjusted HR (95% CI) | 1.00 | 0.50 (0.28–0.89) | 0.70 (0.48–1.03) | 0.62 (0.41–0.93) | 0.020 |
| No. of breast cancer deaths (n = 86) | 39 | 5 | 22 | 20 | |
| Age-adjusted HR (95% CI) | 1.00 | 0.37 (0.15–0.94) | 0.71 (0.42–1.20) | 0.51 (0.30–0.87) | 0.014 |
| Multivariable-adjusted HR (95% CI) | 1.00 | 0.43 (0.16–1.12) | 0.74 (0.42–1.30) | 0.66 (0.36–1.21) | 0.18 |
NOTE: Physical activity assessed at visit closest to diagnosis (i.e., either year 3 or year 6). Adjusted for age, stage, ER, PR, grade, HER2, ethnicity, WHI study arm, previous hormone therapy use, time from diagnosis to physical activity assessment, BMI, diabetes, alcohol, smoke, total calories, and percentage calories from fat, and servings of fruit and vegetables.
0 MET-h/wk, n = 907; >0–3.0 MET-h/wk, n = 253; 3.1–8.9 MET-h/wk, n = 570; 9+ MET-h/wk, n = 1,180.
0 MET-h/wk, n = 1,008; >0–3.0 MET-h/wk, n = 332; 3.1–8.9 MET-h/wk, n = 696; 9+ MET-h/wk, n = 874.
Change in physical activity
Postdiagnosis physical activity, when compared with prediagnosis physical activity, was increased in 40%, unchanged in 35%, and decreased in 25% of women (Table 4). Compared with women who were inactive or insufficiently active before diagnosis, women who increased their physical activity level to 9 ore more MET-h/wk or maintained their physical activity at 9+ MET-h/wk had a multivariable-adjusted 33% less risk of all-cause mortality (HR = 0.67; 95% CI, 0.46–0.96; Table 4).
Table 4.
Associations between breast cancer outcomes and change in physical activity from before to after a breast cancer diagnosis
| Change in moderate- to vigorous-intensity physical activity, MET-h/wk (N = 2,776)
|
|||
|---|---|---|---|
| No change/inactive (n = 958) | Increase/active (n = 1,121) | Decrease/inactive (n = 697) | |
| Total no. of deaths (n = 168) | 46 | 69 | 53 |
| Age-adjusted HR (95% CI) | 1.00 | 0.59 (0.40–0.85) | 1.01 (0.71–1.45) |
| Multivariable-adjusted HR (95% CI) | 1.00 | 0.67 (0.46–0.96) | 1.06 (0.73–1.54) |
| No. of breast cancer deaths (n = 79) | 25 | 32 | 22 |
| Age-adjusted HR (95% CI) | 1.00 | 0.66 (0.39–1.11) | 0.93 (0.54–1.59) |
| Multivariable-adjusted HR (95% CI) | 1.00 | 0.91 (0.51–1.64) | 1.06 (0.59–1.88) |
NOTE: Adjusted for age, stage, ER, PR, grade, HER2, ethnicity, WHI study arm, previous hormone therapy use, time from diagnosis to physical activity assessment, BMI, diabetes, alcohol, smoke, total calories, and percentage calories from fat, and servings of fruit and vegetables.
When further dividing the “increase/active” category into two categories (i.e., women who increased their physical activity to ≥9 MET-h/wk and women who maintained their physical activity at 9+ MET-h/wk), a similar proportion of deaths was observed for both groups (4%) compared with 7% of deaths observed in both the “no change/inactive” group and “decrease/inactive” group. Furthermore, we observed a multivariable-adjusted 15% and 37% less risk of all-cause mortality (HR = 0.85; 95% CI, 0.48–1.01 and HR = 0.63; 95% CI, 0.40–0.97), respectively, for the “increase” group and the “maintained/active” group compared with the “no change/inactive” group (data not shown).
Stratified analyses of physical activity after diagnosis and death
In subgroup analyses, the association between physical activity and death appeared to be stronger in women with BMI less than 30 kg/m2, women with advanced stage of disease, and those with ER-positive tumors and tumors that were HER2/neu-negative (Table 5). However, none of the tests for interaction were significant; thus, these subgroup findings may be due to chance.
Table 5.
Associations between total deaths and postdiagnosis moderate- to vigorous-intensity physical activity stratified by demographic and prognostic variables
| Postdiagnosis moderate-vigorous intensity physical activity, MET-h/wk | ||
|---|---|---|
| 0 | >0 | |
| Full sample | ||
| Total deaths/total women | 83/907 | 103/2,003 |
| Multivariable HR (95% CI)2 | 1.00 | 0.58 (0.42–0.79) |
| BMI < 25 kg/m2 | ||
| Total deaths/total women | 20/201 | 44/780 |
| Multivariable HR (95% CI) | 1.00 | 0.49 (0.27–0.91) |
| BMI = 25–29.9 kg/m2 | ||
| Total deaths/total women | 28/305 | 27/696 |
| Multivariable HR (95% CI) | 1.00 | 0.43 (0.24–0.76) |
| BMI ≥ 30 kg/m2 | ||
| Total deaths/total women | 32/393 | 31/512 |
| Multivariable HR (95% CI) | 1.00 | 0.80 (0.45–1.41) |
| Stage I | ||
| Total deaths/total women | 43/654 | 60/1,535 |
| Multivariable HR (95% CI) | 1.00 | 0.65 (0.42–0.99) |
| Stage II–IIIA | ||
| Total Deaths/Total Women | 33/234 | 36/423 |
| Multivariable HR (95% CI) | 1.00 | 0.46 (0.27–0.78) |
| ER-negative | ||
| Total deaths/total women | 14/123 | 23/255 |
| Multivariable HR (95% CI) | 1.00 | 0.78 (0.35–1.73) |
| ER-positive | ||
| Total deaths/total women | 57/688 | 64/1,530 |
| Multivariable HR (95% CI) | 1.00 | 0.50 (0.34–0.74) |
| HER2-positive | ||
| Total deaths/total women | 7/97 | 9/202 |
| Multivariable HR (95% CI) | 1.00 | 0.71 (0.16–3.11) |
| HER2-negative | ||
| Total deaths/total women | 21/369 | 19/782 |
| Multivariable HR (95% CI) | 1.00 | 0.37 (0.19–0.75) |
NOTE: Physical activity assessed at visit closest to diagnosis (i.e., either year 3 or year 6). Adjusted for age, stage, ER, PR, grade, her2, ethnicity, WHI study arm, previous hormone therapy use, time from diagnosis to physical activity assessment, BMI, diabetes, alcohol, smoke, total calories, and percentage calories from fat, and servings of fruit and vegetables. P = 0.63, 0.13, 0.79, and 0.40 for interaction between BMI, stage, ER status, and her2 status, respectively.
Discussion
In a large cohort of postmenopausal women, those participating in moderate- to vigorous-intensity recreational physical activity, such as brisk walking, bicycling, and swimming, before or after a diagnosis of breast cancer experienced a lower risk of all-cause and breast cancer–specific mortality. Furthermore, women who increased or maintained physical activity at recommended levels of approximately 3 h/wk after diagnosis, including those who were inactive prior to diagnosis, experienced lower risk of all-cause mortality compared with women who remained inactive.
To our review, this study represents the largest single cohort analysis to prospectively evaluate relationships among pre- and postdiagnosis physical activity, change in physical activity, and all-cause mortality and breast-cancer specific mortality in postmenopausal women diagnosed breast cancer. After a diagnosis of breast cancer, women who reported participating in moderate- to vigorous-intensity physical activity for 9 or more MET-h/wk (~3 h/wk of brisk walking) experienced a 46% less risk of all-cause mortality and 39% less risk of breast cancer–specific mortality. Although severity of disease could influence whether a woman is able to be physically active after diagnosis, our analyses excluded women who rarely walked outside the house for 10 minutes or more without stopping and those who died within 1 year of the physical activity assessment. The former excludes women with severe comorbidities and the latter effectively excludes women receiving chemotherapy during their physical activity assessment. Moreover, a benefit for physical activity was seen regardless of the stage of disease at diagnosis.
Our results of an inverse association between physical activity and mortality are similar to other reports examining this relationship in breast cancer survivors (4–9). However, our study expands on previous research by examining change in physical activity from before to after a breast cancer diagnosis. Following diagnosis, women who increased their physical activity level to 9 ore more MET-h/wk experienced a 33% less risk of all-cause mortality compared with women who were inactive or insufficiently active both before and after diagnosis. This finding suggests that adopting an active lifestyle after a cancer diagnosis can improve prognosis.
We examined associations between physical activity and mortality stratified by 4 variables including BMI, stage, ER, and HER2 status. Subgroup analyses did reveal an association between physical activity and all-cause mortality among women diagnosed with ER-positive cancers; however, tests for interaction were nonsignificant and therefore our findings may be due to chance. However, the finding does provide some evidence that physical activity may modulate estrogen or other factors that influence hormone receptor function (15, 16). Other potential mediating factors include change in body fat, insulin, insulin-like growth factors, adipocytokines, or inflammation (17, 18). Randomized trials of exercise have shown a benefit of exercise on decreasing insulin and insulin-like growth factors in women treated for breast cancer (18).
Study strengths include the large sample size and the prospective assessment of physical activity before and after diagnosis. Additional strengths include the use of a validated physical activity assessment tool, geographic diversity of the cohort, centralized adjudicated outcomes, and comprehensive adjustment for relevant prognostic and lifestyle factors.
Study limitations include a focus on recreational physical activity rather than total physical activity that includes household and occupational activities; however, previous studies examining all types and intensities of physical activity and survival after a breast cancer diagnosis have observed the strongest associations for recreational physical activity and improved survival (7). For our postdiagnosis analyses, our sample size is smaller than the sample size for the prediagnosis analyses. Because enrollment into WHI occurred over many years, some women were diagnosed with breast cancer 6+ years after enrollment, thus not allowing us to capture enough follow-up time to examine survival analyses.
Other study limitations include an absence of information on systemic adjuvant therapy. However, we did adjust for stage and grade, and these variables have been shown to be correlated with adjuvant therapy. In the Health, Eating, Activity, and Lifestyle (HEAL) Study (7), the correlation between treatment and stage was r = 0.41, P < 0.0001. Also, 25% and 78% of HEAL women diagnosed with stages I and II/III disease, respectively, received chemotherapy, further supporting the use of stage as a proxy of treatment. In the HEAL analyses, after adjusting for stage, further adjustment for treatment did not change the hazard ratio (7). However, there is the possibility that women who are more active are better able to withstand and complete treatment. In fact, a post hoc finding from an exercise trial conducted by Courneya and colleagues showed that breast cancer survivors randomized to exercise versus control had a higher chemotherapy completion rate (89% vs. 84%, P = 0.03; ref. 19). Thus, one mechanism mediating the observed association between physical activity and improved survival may be improved adherence to adjuvant treatment. Thus, maintaining or increasing exercise during treatment is likely beneficial for completing therapy, and in turn improving prognosis. Randomized controlled trials of exercise on disease-free survival should also be conducted to confirm these findings.
In conclusion, moderate- to vigorous-intensity physical activity for 9 or more MET-h/wk before or after a breast cancer diagnosis may improve survival, even among women reporting low physical activity prior to diagnosis. Large-scale, randomized trials are needed to confirm these findings. In the meantime, women diagnosed with breast cancer should be made aware of the current evidence associating higher physical activity levels to lower risk of death due to breast cancer and all causes.
Acknowledgments
We thank the WHI participants for their ongoing dedication to this study.
Grant Support
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221.
Footnotes
Disclosure of Potential Conflicts of Interest
This article represents original unpublished material, except in abstract form, and does not contain any previously published material in the text, illustrations, or tables without proper reference citation.
References
- 1.Thune I, Ferberg A. Physical activity and cancer risk: dose-response and cancer, all sites and site specific. Med Science Sports Exerc. 2001;33:S530–50. doi: 10.1097/00005768-200106001-00025. [DOI] [PubMed] [Google Scholar]
- 2.McTiernan A, Kooperberg C, White E, Wilcox S, Coates R, Adams-Campbell LL, et al. Recreational physical activity and the risk of breast cancer in postmenopausal women: The Women’s Health Initiative Cohort Study. JAMA. 2003;290:1331–36. doi: 10.1001/jama.290.10.1331. [DOI] [PubMed] [Google Scholar]
- 3.Chlebowski RT, Ligibel JA. Lifestyle issues in breast cancer survivors. In: Harris JR, Lippman ME, Morrow M, Osborne CK, editors. Diseases of the breast. 4. Lippincott Williams & Wikins; Philadelphia, PA: 2010. pp. 676–82. [Google Scholar]
- 4.Holmes MD, Chen WY, Feskanich D, Holmes MD, Chen WY, Feskanich D. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–86. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 5.Holick CN, Newcomb PA, Trentham-Dietz A, Hampton JM, Bersch AJ, Passarelli MN, et al. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:379–86. doi: 10.1158/1055-9965.EPI-07-0771. [DOI] [PubMed] [Google Scholar]
- 6.Pierce JP, Stefanick ML, Flatt SW, Natarajan L, Sternfeld B, Madlensky L, et al. Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity. J Clin Oncol. 2007;25:2345–51. doi: 10.1200/JCO.2006.08.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irwin ML, Smith A, McTiernan A, et al. Association between pre- and post-diagnosis physical activity on mortality in breast cancer survivors: the Health, Eating, Activity, and Lifestyle (HEAL) study. J Clin Oncol. 2008;26:3958–64. doi: 10.1200/JCO.2007.15.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sternfeld B, Weltzien E, Quesenberry CP, Jr, Castillo AL, Kwan M, Slattery ML, et al. Physical activity and risk of recurrence and mortality in breast cancer survivors: findings from the LACE study. Cancer Epidemiol Biomarkers Prev. 2009;18:87–95. doi: 10.1158/1055-9965.EPI-08-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keegan TH, Milne RL, Andrulis IL, Chang ET, Sangaramoorthy M, Phillips KA, et al. Past recreational physical activity, body size, and all-cause mortality following breast cancer diagnosis: results from the Breast Cancer Family Registry. Breast Cancer Res Treat. 2010;123:531–42. doi: 10.1007/s10549-010-0774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Design of the Women’s Health Initiative clinical trial and observational study: the Women’s Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 11.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T, et al. (Measurement characteristics of the Women’s Health Inititative food frequency questionnaire. Ann Epidemiol. 1999;9:178–87. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 12.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr, Montoye HJ, Sallis JF, et al. Compendium of physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Johnson-Kozlow M, Rock CL, Gilpin EA, Hollenbach KA, Pierce JP. Validation of the WHI brief physical activity questionnaire among women diagnosed with breast cancer. Am J Health Behav. 2007;31:193–202. doi: 10.5555/ajhb.2007.31.2.193. [DOI] [PubMed] [Google Scholar]
- 14.US Dept of Health and Human Services, Public Health Service, National Institutes of Health. SEER Program: comparative staging guide for cancer. Version 1.1. Washington, DC: NIH Publication; 1993. pp. 93–3640. [Google Scholar]
- 15.McTiernan A, Ulrich C, Slate S, Potter J. Physical activity and cancer etiology: associations and mechanisms. Cancer Causes Control. 1998;9:487–509. doi: 10.1023/a:1008853601471. [DOI] [PubMed] [Google Scholar]
- 16.McTiernan A, Tworoger S, Ulrich C, Yasui Y, Irwin M, Rajan B, et al. Effect of exercise on serum estrogens in postmenopausal women: a 12-month randomized clinical trial. Cancer Res. 2004;64:2923–28. doi: 10.1158/0008-5472.can-03-3393. [DOI] [PubMed] [Google Scholar]
- 17.Irwin ML, McTiernan A, Bernstein L, Gilliland FD, Baumgartner R, Baumgartner K, et al. Relationship of obesity and physical activity with c-peptide, leptin, and insulin-like growth factors in breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2005;14:2881–8. doi: 10.1158/1055-9965.EPI-05-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irwin ML, Varma K, Alvarez-Reeves, Cadmus L, Wiley A, Chung GG, et al. Randomized controlled exercise trial on insulin and IGFs in breast cancer survivors: the Yale Exercise and Survivorship Study. Cancer Epidemiol Biomarkers Prev. 2009;18:306–13. doi: 10.1158/1055-9965.EPI-08-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Courneya K, Segal R, Mackey J, Gelmon K, Reid RD, Friedenreich CM, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007;25:4396–404. doi: 10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]