Abstract
There is growing interest in the relationship between gestational weight gain (GWG) and long-term maternal and child outcomes, yet little is known about the accuracy of long-term maternal recall of GWG. Our objective was to assess the accuracy of maternal recall of GWG at 4–12 years postpartum (mean, 8 years) compared with medical-record documented GWG, and compare recalled GWG to documented GWG with respect to their associations with adverse pregnancy outcomes including small for gestational age (SGA) birth, preterm birth, cesarean delivery, and postpartum weight retention (PPWR) (n = 503). Adequacy of recalled and documented GWG was assessed according to the 2009 Institute of Medicine (IOM) guidelines. We observed moderate agreement between documented and maternal self-reported GWG as continuous variables (r = 0.63, P < 0.01). When recalled GWG was used to categorize women, 45, 53, and 20% of women with inadequate, adequate, and excessive documented GWG were misclassified, respectively. When comparing models fitted with documented or recalled GWG, there were no meaningful differences in associations between inadequate GWG and SGA birth (odds ratio 2.2 (95% confidence interval: 1.3, 3.7) vs. 2.1 (1.2, 3.8), respectively) or excessive GWG and PPWR (2.5 (1.6, 3.9) vs. 2.5 (1.5, 4.0), respectively). However, the use of recalled GWG attenuated associations between inadequate GWG and PPWR (documented: 0.5 (0.3, 0.9) vs. recalled GWG: 1.3 (0.7, 2.3)) and excessive GWG and preterm birth (documented: 2.5 (1.4, 4.5) vs. recalled GWG: 1.5 (0.9, 2.7)). Our data suggest a varying degree of bias when using recalled GWG to study selected adverse outcomes.
INTRODUCTION
Maternal gestational weight gain (GWG) is an important predictor of short-term maternal and infant health outcomes (1), but less research is available on long-term consequences of inadequate and excessive weight gain. Some data suggest that excessive GWG is associated with postpartum weight retention (PPWR) and subsequent obesity in the short (up to 3 months postpartum) (2–6), intermediate (3 months–3 years) (6–8) and long term (up to 21 years postpartum) (9–12). In addition, a weak association between excessive GWG and breast cancer has been reported (13). Researchers have also proposed biologically plausible mechanisms linking excessive GWG to maternal metabolic and cardiovascular diseases later in life (14,15). GWG has also been associated with obesity among children 3–14 years of age (16–19), childhood neurodevelopment (20), and cancer risk (13,21–23). However, gaps in knowledge relating GWG to long-term child and maternal outcomes remain. In fact, in their recently published report, the Institute of Medicine (IOM) called for more research relating GWG to long-term outcomes for mothers and children in order to inform future evidence-based guidelines on GWG recommendations (1).
Studies of long-term consequences of GWG may be retrospective in design, relying on maternal recall of GWG data to reduce cost and time. Six months to 2.5 years after delivery, maternal recall of GWG and self-reported delivery weight have been observed to be highly correlated with that documented in the medical record (24,25). Nevertheless, little is known about the accuracy of maternal recall of GWG many years postpartum. Quantifying the degree of misclassification in recalled GWG may inform investigators about the desirability of using recalled GWG in epidemiologic research. At the same time, it would provide necessary validation data for sensitivity analyses to account for measurement error in exposure-disease relationships (26,27). Our objectives were to assess the accuracy of maternal recall of GWG at 4–12 years after delivery and examine the impact of reporting error on associations between GWG and risk for adverse outcomes.
METHODS AND PROCEDURES
The Women and Infant Study of Healthy Hearts (WISH) is a cohort study of cardiovascular risk factors assessed among women 4–12 years after the delivery of singleton infants who were either small for gestational age (SGA, <10th percentile for based on hospital nomograms), preterm (<37 weeks gestation), or term non-SGA births. Eligible women were those who gave birth from 1997 to 2002 at Magee-Womens Hospital in Pittsburgh, PA who did not have preeclampsia or prepregnancy hypertension or diabetes. Of the 4,908 eligible women identified via a hospital electronic birth registry, 1,569 (32%) were located via mail or phone and were screened. A total of 817 women (52.1%) declined participation, and an additional 50 women were ineligible due to being currently pregnant or reporting that they had preeclampsia or chronic hypertension before the index pregnancy. Of the women screened, 702 (45%) provided informed consent and enrolled (318 term non-SGA births, 196 term SGA births, and 188 preterm births). The 702 enrolled women were more likely to be African American (28.6% vs. 24.4%, P = 0.02) and were slightly older (37.3 vs. 36.8 years, P < 0.01) compared to eligible women. The institutional review board of the University of Pittsburgh approved this study.
Of the 702 women enrolled, 683 had data on self-reported GWG. Of these, 180 were missing information on documented GWG (n = 128 were missing pregravid weight, n = 37 were missing delivery weight, and n = 15 were missing both pregravid and delivery weight). The final analytic sample was 503. Women included in the study sample were similar to women excluded on a variety of sociodemographic, weight, and pregnancy characteristics (data not shown). However, women with missing documented GWG were more likely to have had a preterm birth (43% vs. 21%) than women included in the study sample.
Variables of interest
Self-reported GWG was assessed at 4–12 years after the target pregnancy with the question, “What was your weight gain during this pregnancy?” Medical records for each woman’s target pregnancy were reviewed to obtain pregnancy, labor, and delivery data, including weight data. Prepregnancy weight, recalled at the first prenatal visit, and admission weight at delivery (either measured or self-reported) were abstracted from medical records. Documented GWG was calculated by subtracting the prepregnancy weight from weight at delivery admission. At the WISH study visit, height and current weight were measured. Prepregnancy BMI (recalled pregravid weight (kg)/measured height (m)2) and current BMI (measured weight (kg)/measured height (m)2) were categorized as underweight (BMI <18.5), normal weight (18.5–24.9), overweight (25.0–29.9), or obese (≥30.0). PPWR was classified as current weight ≥5 kg over prepregnancy weight.
We defined adequacy of GWG as a ratio of observed GWG to expected (recommended) GWG at the gestational age of delivery multiplied by 100, as described previously (28–30). Expected GWG was defined as 100% of the 2009 IOM recommendations at the gestational age of delivery (1). Percent of weight gain recommendations met were classified as inadequate, adequate, or excessive based on ranges of IOM-recommended weight gains (1,28,29). Because prior studies of self-reported weight have shown that rounding weights to zero or five increases reporting error, (31,32) we evaluated for digit preference of zero, five, or other. At the WISH participant visit, data on race/ethnicity (non-Hispanic black, other); current marital status (married or marriage-like, unmarried); current maternal education (less than high school, high school, some college, college graduate), current insurance (medicaid or medicare, private, none); current annual household income (<$20,000; $20,000–$50,000; $50,000–$100,000; >$100,000); current smoking (yes, no); current parity (one; two; three; or more live births), and parity at target pregnancy (1, 2, ≥3 live births) were ascertained. Additional variables collected from medical record review of the target pregnancy included: birth weight; gestational length; route of delivery; breastfeeding (any/none); smoking during pregnancy (yes/no); marital status at delivery (married, unmarried); education at delivery (less than high school, high school, some college, college graduate); and insurance at delivery (insurance, public assistance).
Statistical methods
Correlation analysis between self-reported GWG and documented GWG was carried out using Pearson correlation. A paired t-test was used to determine if recalled GWG varied significantly from documented GWG. The scatter between recalled and documented GWG and of the residuals of the linear regression between recalled GWG and documented GWG were analyzed graphically. Distribution of reporting error, categorized as underreporting by >2.27 kg (5 lb), reporting within 2.27 kg, and overreporting by >2.27 kg, was assessed using χ2-tests and ANOVA.
The effect of reporting error on IOM adequacy of GWG categories was tested by calculating GWG adequacy categories first using documented and then using recalled GWG. Agreement was assessed using the κ statistic (33).
We assessed the impact of reporting error on associations between GWG and maternal and infant outcomes (SGA, preterm birth, cesarean delivery, and maternal PPWR) by comparing adjusted odds ratios generated from multivariable logistic regression models where GWG adequacy was based on documented GWG with models where GWG adequacy was based on recalled GWG. GWG was classified as inadequate, adequate, or excessive based on ranges of IOM-recommended weight gains and entered into the models as indicator variables with adequate GWG serving as the referent (1,28,29). Potential confounders, selected a priori, were age at target pregnancy, race/ethnicity, parity at target pregnancy, smoking during target pregnancy, marital status at delivery, education at delivery, insurance at delivery, prepregnancy BMI, and time since delivery. Effect modification by prepregnancy BMI and time since delivery were assessed using a likelihood ratio test (α = 0.05). Analyses were performed with SAS (version 9.2; SAS Institute, Cary, NC).
Results
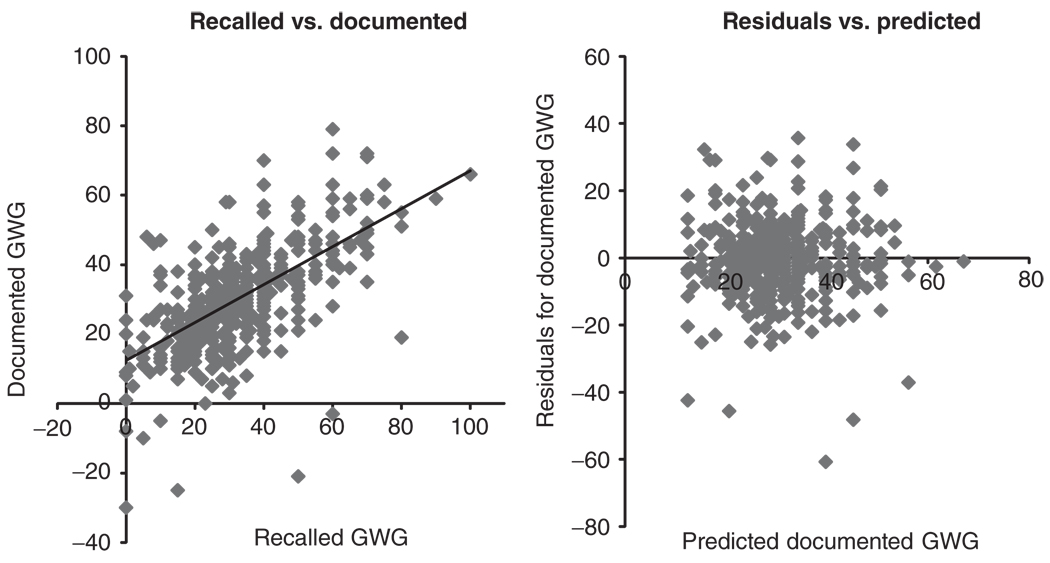
On average, women tended to overreport their GWG by 1.0 kg (range −19 to 32 kg; interquartile range −2 to 4 kg.). Self-reported GWG and documented GWG were moderately correlated (r = 0.63, P < 0.01). Nevertheless, there was a systematic bias between recalled and documented GWG. Not only was the mean of the raw differences between recalled and documented GWG significantly different from zero (P < 0.01), but the equation predicting documented GWG had an intercept of 12.4 and slope = 0.55 (Figure 1). These data suggest that recalled GWG tended to overestimate documented GWG. Despite the systematic bias, the scatter of the residuals plotted by predicted GWG exhibits uniformity of error (Figure 1).
Figure 1.
Graphical analysis of validity. Left panel: plot of recalled GWG by documented GWG; regression equation: documented GWG = 12.43 + 0.55* recalled GWG; evidence of systematic bias. Right panel: plot of residuals after linear regression of recalled GWG on documented GWG by predicted documented gestational weight gain (GWG). Approximately equal scatter around zero represents uniformity of error.
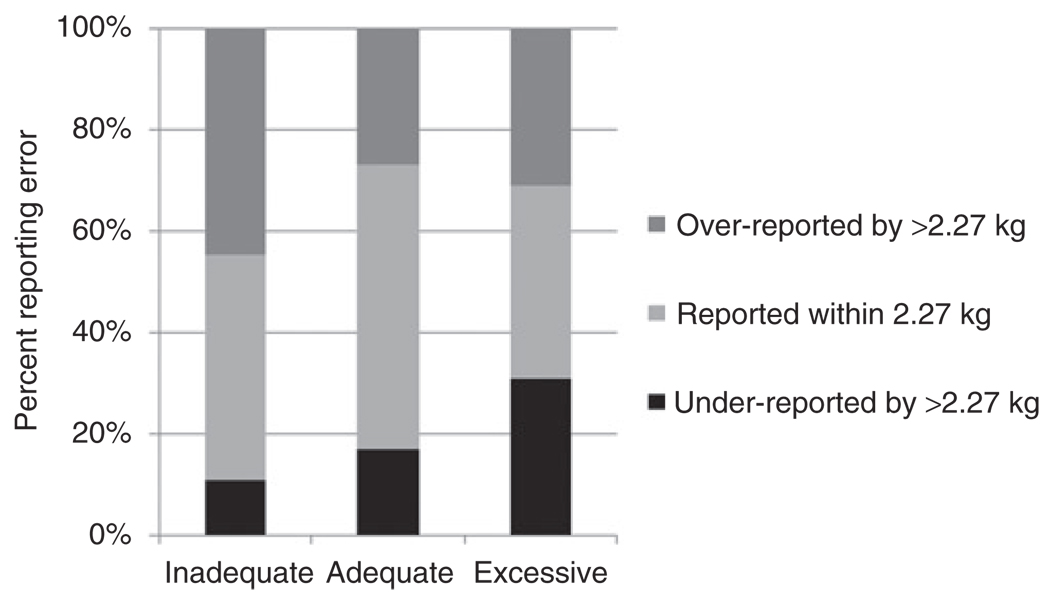
Self-reported GWG was within 2.27 kg (5 lb) of the documented GWG in 45% of the sample population; 33% overreported their GWG by more than 2.27 kg, and 22% underreported their weight by >2.27 kg (Table 1). The distribution of reporting error was significantly different across adequacy of GWG categories. On average, women who gained an inadequate amount of weight were more likely to overreport by >2.27 kg, whereas women who gained excessively were more likely to underreport (Figure 2). Non-Hispanic black women were more likely to underreport their GWG by >2.27 kg, whereas women with less than a high school education, unmarried women, and current smokers were more likely to overreport (Table 1). Women whose target pregnancy was within 8 years of recall, women reporting a GWG ending in zero or five, and women who were obese at WISH enrollment were also more likely to overreport (P = 0.049, 0.047, and 0.01, respectively). Reporting error did not differ by prepregnancy BMI.
Table 1.
Distribution of reporting error comparing self-report and documented gestational weight gain, stratified by covariates
| GWG misreportinga (kg) | ||||
|---|---|---|---|---|
| Under-report by more than 2.27 (n = 112) n (%) or mean (s.d.) |
Report within 2.27 (n = 225) n (%) or mean (s.d.) |
Over-report by more than 2.27 (n = 166) n (%) or mean (s.d.) |
P value | |
| Maternal characteristics at target pregnancy | ||||
| Age (years) | 28 ± 7 | 30 ± 6 | 28 ± 7 | <0.01 |
| Race | ||||
| Non-Hispanic black | 43 (33) | 43 (33) | 46 (35) | |
| Other | 69 (19) | 182 (49) | 120 (32) | <0.01 |
| Marital status | ||||
| Married or marriage-like | 49 (17) | 149 (52) | 88 (31) | |
| Other | 63 (29) | 76 (35) | 78 (36) | <0.01 |
| Maternal education | ||||
| <High school | 15 (28) | 16 (30) | 22 (42) | |
| High school | 39 (25) | 67 (43) | 50 (32) | |
| Some college | 22 (23) | 37 (39) | 37 (39) | |
| College degree | 31 (17) | 100 (55) | 52 (28) | 0.03 |
| Insurance | ||||
| Insurance | 58 (17) | 166 (50) | 111 (33) | |
| Public assistance | 54 (32) | 59 (35) | 55 (33) | <0.01 |
| Parity | ||||
| 1 | 57 (21) | 129 (47) | 89 (32) | |
| 2 | 33 (23) | 63 (44) | 46 (32) | |
| 3+ | 22 (26) | 33 (38) | 31 (36) | 0.71 |
| Breastfeed | ||||
| Yes | 57 (20) | 134 (47) | 96 (33) | |
| No | 55 (25) | 91 (42) | 70 (32) | 0.31 |
| Smoke | ||||
| Yes | 32 (28) | 41 (36) | 41 (36) | |
| No | 79 (20) | 184 (47) | 125 (32) | 0.07 |
| Prepregnancy BMI | ||||
| Underweight/normal weight | 75 (22) | 164 (48) | 100 (30) | |
| Overweight | 24 (22) | 44 (40) | 43 (39) | |
| Obese | 13 (25) | 17 (32) | 23 (43) | 0.10 |
| Adequacy of gestational weight gain | ||||
| Inadequate | 12 (11) | 49 (45) | 49 (45) | |
| Adequate | 26 (17) | 86 (56) | 42 (27) | |
| Excessive | 74 (31) | 90 (38) | 75 (31) | <0.01 |
| Current maternal characteristics | ||||
| Age (years) | 37 ± 8 | 39 ± 7 | 36 ± 7 | <0.01 |
| Current income | ||||
| <$20,000 | 36 (33) | 34 (31) | 39 (36) | |
| $20,000–$50,000 | 27 (24) | 46 (41) | 40 (35) | |
| $50,000–$100,000 | 31 (19) | 82 (51) | 48 (30) | |
| $100,000+ | 14 (14) | 51 (52) | 33 (34) | <0.01 |
| Ever smoker | ||||
| Yes | 66 (27) | 90 (37) | 87 (36) | |
| No | 46 (18) | 135 (52) | 79 (30) | <0.01 |
| Current BMI | ||||
| Underweight/normal weight | 41 (17) | 124 (51) | 77 (32) | |
| Overweight | 37 (25) | 65 (44) | 46 (31) | |
| Obese | 34 (30) | 36 (32) | 43 (38) | 0.01 |
| Years since delivery | ||||
| <8 | 41 (17) | 111 (47) | 83 (35) | |
| ≥8 | 70 (27) | 112 (43) | 81 (31) | 0.049 |
| Reported gestational weight gain last digit | ||||
| 0 | 53 (21) | 108 (43) | 92 (36) | |
| 5 | 34 (23) | 59 (41) | 52 (36) | |
| Other | 25 (24) | 58 (55) | 22 (21) | 0.047 |
| Outcomes | ||||
| Route of delivery target pregnancy | ||||
| C-section delivery | 17 (21) | 36 (44) | 29 (35) | |
| Vaginal delivery | 95 (23) | 189 (45) | 137 (33) | 0.87 |
| Preterm target pregnancy | ||||
| Yes | 29 (28) | 43 (41) | 33 (31) | |
| No | 83 (21) | 182 (46) | 133 (33) | 0.33 |
| SGA target pregnancy | ||||
| Yes | 29 (20) | 69 (48) | 45 (31) | |
| No | 83 (23) | 156 (43) | 121 (34) | 0.59 |
| Postpartum weight retention | ||||
| Yes | 69 (28) | 93 (38) | 82 (34) | |
| No | 43 (17) | 132(51) | 84 (32) | <0.01 |
Misreporting = reported GWG-documented GWG.
Figure 2.
Distribution of reporting error by adequacy of gestational weight gain categories.
Although the prevalence of inadequate, adequate, and excessive GWG was similar when using documented GWG (22, 31, and 48%, respectively) and recalled GWG (22, 26, and 52%, respectively), there was only fair agreement in these categories (κ = 0.43 (95% CI: 0.37, 0.49) (Table 2). Indeed, the use of recalled GWG misclassified 36% of women. Women who gained excessively were less likely to be misclassified based on self-report (80% agreement); only about half of women who gained inadequately (55%) and adequately (47%) were correctly categorized based on self-report.
Table 2.
Agreement between IOM adequacy of gestational weight gain categorization
| Categorization based on documented gestational weight gain |
Categorization based on self-reported gestational weight gain, N (%) |
Total | ||
|---|---|---|---|---|
| Inadequate | Adequate | Excessive | ||
| Inadequate | 61 (55) | 28 (25) | 21 (19) | 110 (100) |
| Adequate | 32 (21) | 72 (47) | 50 (32) | 154 (100) |
| Excessive | 16 (7) | 32 (13) | 191 (80) | 239 (100) |
| Total | 109 (22) | 132 (26) | 262 (52) | |
When comparing models fitted with documented or recalled GWG, there were no meaningful differences in associations between inadequate GWG and SGA birth (odds ratio 2.2 (95% confidence interval: 1.3, 3.7) vs. 2.1 (1.2, 3.8), respectively) or excessive GWG and PPWR (2.5 (1.6, 3.9) vs. 2.5 (1.5, 4.0), respectively) (Table 3). However, the use of recalled GWG attenuated associations between inadequate GWG and PPWR (documented: 0.5 (0.3, 0.9) vs. recalled GWG: 1.3 (0.7, 2.3)) and excessive GWG and preterm birth (documented: 2.5 (1.4, 4.5) vs. recalled GWG: 1.5 (0.9, 2.7)). Cesarean delivery was not associated with documented or recalled GWG. Neither prepregnancy BMI nor time from delivery modified the associations between GWG and any of the selected adverse outcomes.
Table 3.
Associations between documented and recalled gestational weight gain and birth outcomes
| SGA | Preterm birth | Cesarean delivery | Postpartum weight retention |
||||||
|---|---|---|---|---|---|---|---|---|---|
| AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | ||
| Documented gestational weight gaina | Inadequate | 2.2 | 1.3, 3.7 | 1.1 | 0.5, 2.4 | 1.4 | 0.7, 3.0 | 0.5 | 0.3, 0.9 |
| Adequate | Ref. | Ref. | Ref. | Ref. | |||||
| Excessive | 0.8 | 0.5, 1.2 | 2.5 | 1.4, 4.5 | 1.6 | 0.9, 3.1 | 2.5 | 1.6, 3.9 | |
| Recalled gestational weight gaina | Inadequate | 2.1 | 1.2, 3.8 | 0.9 | 0.4, 1.8 | 2.0 | 0.9, 4.3 | 1.3 | 0.7, 2.3 |
| Adequate | Ref. | Ref. | Ref. | Ref. | |||||
| Excessive | 0.7 | 0.5, 1.2 | 1.5 | 0.9, 2.7 | 1.8 | 0.9, 3.5 | 2.5 | 1.5, 4.0 | |
AOR, adjusted odds ratio; CI, confidence interval; SGA, small for gestational age.
Models adjusted for age at target pregnancy, race/ethnicity, parity at target pregnancy, smoking during pregnancy, education at delivery, insurance status at delivery, marital status at delivery, and time since delivery; N = 438.
Discussion
To our knowledge, this is the first study to assess the impact of reporting error on adequacy of GWG categorization using the 2009 IOM GWG guidelines, and only the second to assess the accuracy of self-reported GWG >2.5 years after delivery. Our results suggest that a substantial proportion of women are misclassified into adequacy of GWG categories when GWG is ascertained by maternal recall an average of 8 years after delivery. When GWG was studied continuously, we observed a systematic bias, whereby recalled GWG tended to overestimate documented GWG. When GWG was categorized, over 30% of women were misclassified. Despite this systematic error, the magnitude and direction of bias in point estimates relating GWG to SGA were not considerably different; however, the estimates relating excessive GWG to an increased risk of preterm birth and inadequate GWG to a lower risk of PPWR were substantially attenuated and no longer significant when recalled GWG was used to classify weight gain into IOM adequacy categories.
Our results confirm those by Schieve et al., who assessed the accuracy of self-reported delivery weight among 3,518 respondents to the 1988 National Maternal and Infant Health Survey at 6 to 31 months postpartum (25). Overall, the 1988 NMIHS data suggested underreporting of recalled delivery weight by 1.28 kg. When categorizing GWG, 30–40% of women were misclassified. Like us, Schieve et al. observed that women gaining the greatest amounts of weight are more likely to underreport their GWG, whereas women gaining the least amount are more likely to overreport GWG. In our study and Schieve et al., reporting error was greater among women who were nonwhite, less educated and unmarried. However, while Schieve et al. observed that reporting error increased with increasing pregravid BMI, we found this to be the case with current postpartum BMI only. Differences in our studies may be due to the study of weight at delivery vs. GWG and difference in population characteristics.
Two other studies assessed the accuracy of maternal recall of GWG using correlation analysis. In a study reporting the accuracy of GWG 32 years after delivery, maternal recall was modestly correlated with documented GWG (r = 0.42), which was similar to our finding (34). Other studies are required to confirm if reporting error is similar a decade or more postpartum. In a study of maternal recall in the short-term (mean 6.6 months following delivery), Biro et al. reported that adolescents reliably recall weight gain during pregnancy (r = 0.99) (24). Thirty-four of 40 subjects recalled their GWG within 0.91 kg of that recorded in the medical record.
Despite our finding that 30% of women were misclassified based on self-reported GWG, substantial bias was only noted when studying preterm birth and PPWR. There was no bias for SGA in using recalled GWG. Similar to the results of Schieve et al., in the majority of our analyses we found less pronounced associations of GWG with birth outcomes when GWG was ascertained by self-report.
Measured GWG in our study was calculated using weight at admission to delivery (which may have been either measured or self-reported by the mother) and prepregnancy weight (which was self-reported). This method of ascertainment introduces a potential for bias into the calculation of total GWG. Data suggest that both self-reported delivery weight and recalled pregravid weight are underreported on average, but may vary widely among individuals (25,35,36). Future research including physical measures of both pregravid and delivery weights are warranted to determine the true accuracy of recalled compared with documented GWG.
This analysis was limited to women who agreed to enroll in the WISH study and had complete data for the analyses. Women excluded from these analyses were more likely to deliver preterm infants than women with missing data. Each of these factors introduces potential for selection bias. These results also may not be generalizeable to other populations due to the recruitment of a convenience sample of women delivering SGA, preterm, or uncomplicated births at a single, high-risk referral hospital in Pittsburgh, PA. The outcomes we studied in relation to GWG were limited and did not include a measure of large-for-gestational age births, childhood obesity, or other outcomes potentially related to GWG (1). We were also limited in our ability to determine whether PPWR represented actual gestational weight retained or postpartum weight gained.
As investigators pursue research to fill the gap in knowledge regarding long-term consequences of GWG for mothers and offspring, our study’s findings highlight the potential for misclassification of GWG if relying on mother’s recall many years postpartum. Nevertheless, our regression equation between recalled and documented GWG may be used to correct estimates of recalled GWG in future studies with similar populations. Moreover, with the recent publication of automated methods to account for the impact of misclassification on study results (26,27), epidemiologists can use recalled GWG and other imperfectly measured exposures, covariates, and outcomes to study key questions in the perinatal literature. Our data and others like it (25) that estimate the degree of misreporting will be essential for informing such sensitivity analyses.
ACKNOWLEDGMENTS
The WISH study was funded by NIH grant R01 HL076532. L.M.B. was supported by NIH grants R01 HD056999 and K01 MH074092.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Institute of Medicine (Committee to Reexamine IOM Pregnancy Weight Guidelines FaNBaBoCYaF. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: National Academies Press; 2009. [Google Scholar]
- 2.Muscati SK, Gray-Donald K, Koski KG. Timing of weight gain during pregnancy: promoting fetal growth and minimizing maternal weight retention. Int J Obes Relat Metab Disord. 1996;20:526–532. [PubMed] [Google Scholar]
- 3.Stevens-Simon C, McAnarney ER. Adolescent pregnancy. Gestational weight gain and maternal and infant outcomes. Am J Dis Child. 1992;146:1359–1364. doi: 10.1001/archpedi.1992.02160230117031. [DOI] [PubMed] [Google Scholar]
- 4.Scholl TO, Hediger ML, Schall JI, Ances IG, Smith WK. Gestational weight gain, pregnancy outcome, and postpartum weight retention. Obstet Gynecol. 1995;86:423–427. doi: 10.1016/0029-7844(95)00190-3. [DOI] [PubMed] [Google Scholar]
- 5.Luke B, Hediger ML, Scholl TO. Point of diminishing returns: when does gestational weight gain cease benefiting birthweight and begin adding to maternal obesity? J Matern Fetal Med. 1996;5:168–173. doi: 10.1002/(SICI)1520-6661(199607/08)5:4<168::AID-MFM2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 6.Walker L, Freeland-Graves JH, Milani T, et al. Weight and behavioral and psychosocial factors among ethnically diverse, low-income women after childbirth: II. Trends and correlates. Women Health. 2004;40:19–34. doi: 10.1300/J013v40n02_02. [DOI] [PubMed] [Google Scholar]
- 7.Ohlin A, Rössner S. Maternal body weight development after pregnancy. Int J Obes. 1990;14:159–173. [PubMed] [Google Scholar]
- 8.Soltani H, Fraser RB. A longitudinal study of maternal anthropometric changes in normal weight, overweight and obese women during pregnancy and postpartum. Br J Nutr. 2000;84:95–101. doi: 10.1017/s0007114500001276. [DOI] [PubMed] [Google Scholar]
- 9.Callaway LK, Prins JB, Chang AM, McIntyre HD. The prevalence and impact of overweight and obesity in an Australian obstetric population. Med J Aust. 2006;184:56–59. doi: 10.5694/j.1326-5377.2006.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 10.Linné Y, Dye L, Barkeling B, Rössner S. Weight development over time in parous women–the SPAWN study–15 years follow-up. Int J Obes Relat Metab Disord. 2003;27:1516–1522. doi: 10.1038/sj.ijo.0802441. [DOI] [PubMed] [Google Scholar]
- 11.Rooney BL, Schauberger CW, Mathiason MA. Impact of perinatal weight change on long-term obesity and obesity-related illnesses. Obstet Gynecol. 2005;106:1349–1356. doi: 10.1097/01.AOG.0000185480.09068.4a. [DOI] [PubMed] [Google Scholar]
- 12.Amorim AR, Rössner S, Neovius M, Lourenço PM, Linné Y. Does excess pregnancy weight gain constitute a major risk for increasing long-term BMI? Obesity (Silver Spring) 2007;15:1278–1286. doi: 10.1038/oby.2007.149. [DOI] [PubMed] [Google Scholar]
- 13.Kinnunen TI, Luoto R, Gissler M, Hemminki E, Hilakivi-Clarke L. Pregnancy weight gain and breast cancer risk. BMC Womens Health. 2004;4:7. doi: 10.1186/1472-6874-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation. 2008;117:1658–1667. doi: 10.1161/CIRCULATIONAHA.107.739714. [DOI] [PubMed] [Google Scholar]
- 16.Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol. 2007;196:322.e1–322.e8. doi: 10.1016/j.ajog.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oken E, Rifas-Shiman SL, Field AE, Frazier AL, Gillman MW. Maternal gestational weight gain and offspring weight in adolescence. Obstet Gynecol. 2008;112:999–1006. doi: 10.1097/AOG.0b013e31818a5d50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreira P, Padez C, Mourão-Carvalhal I, Rosado V. Maternal weight gain during pregnancy and overweight in Portuguese children. Int J Obes (Lond) 2007;31:608–614. doi: 10.1038/sj.ijo.0803582. [DOI] [PubMed] [Google Scholar]
- 19.Wrotniak BH, Shults J, Butts S, Stettler N. Gestational weight gain and risk of overweight in the offspring at age 7 y in a multicenter, multiethnic cohort study. Am J Clin Nutr. 2008;87:1818–1824. doi: 10.1093/ajcn/87.6.1818. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez A, Miettunen J, Henriksen TB, et al. Maternal adiposity prior to pregnancy is associated with ADHD symptoms in offspring: evidence from three prospective pregnancy cohorts. Int J Obes (Lond) 2008;32:550–557. doi: 10.1038/sj.ijo.0803741. [DOI] [PubMed] [Google Scholar]
- 21.Hjalgrim LL, Westergaard T, Rostgaard K, et al. Birth weight as a risk factor for childhood leukemia: a meta-analysis of 18 epidemiologic studies. Am J Epidemiol. 2003;158:724–735. doi: 10.1093/aje/kwg210. [DOI] [PubMed] [Google Scholar]
- 22.Michels KB, Trichopoulos D, Robins JM, et al. Birthweight as a risk factor for breast cancer. Lancet. 1996;348:1542–1546. doi: 10.1016/S0140-6736(96)03102-9. [DOI] [PubMed] [Google Scholar]
- 23.Ahlgren M, Wohlfahrt J, Olsen LW, Sørensen TI, Melbye M. Birth weight and risk of cancer. Cancer. 2007;110:412–419. doi: 10.1002/cncr.22773. [DOI] [PubMed] [Google Scholar]
- 24.Biro FM, Wiley-Kroner B, Whitsett D. Perceived and measured weight changes during adolescent pregnancy. J Pediatr Adolesc Gynecol. 1999;12:31–32. doi: 10.1016/S1083-3188(00)86618-8. [DOI] [PubMed] [Google Scholar]
- 25.Schieve LA, Perry GS, Cogswell ME, et al. Validity of self-reported pregnancy delivery weight: an analysis of the 1988 National Maternal and Infant Health Survey. NMIHS Collaborative Working Group. Am J Epidemiol. 1999;150:947–956. doi: 10.1093/oxfordjournals.aje.a010103. [DOI] [PubMed] [Google Scholar]
- 26.Lash TL, Fox MP, Thwin SS, et al. Using probabilistic corrections to account for abstractor agreement in medical record reviews. Am J Epidemiol. 2007;165:1454–1461. doi: 10.1093/aje/kwm034. [DOI] [PubMed] [Google Scholar]
- 27.Fox MP, Lash TL, Greenland S. A method to automate probabilistic sensitivity analyses of misclassified binary variables. Int J Epidemiol. 2005;34:1370–1376. doi: 10.1093/ije/dyi184. [DOI] [PubMed] [Google Scholar]
- 28.Bodnar LM, Siega-Riz AM, Arab L, Chantala K, McDonald T. Predictors of pregnancy and postpartum haemoglobin concentrations in low-income women. Public Health Nutr. 2004;7:701–711. doi: 10.1079/phn2004597. [DOI] [PubMed] [Google Scholar]
- 29.Siega-Riz AM, Adair LS, Hobel CJ. Maternal underweight status and inadequate rate of weight gain during the third trimester of pregnancy increases the risk of preterm delivery. J Nutr. 1996;126:146–153. doi: 10.1093/jn/126.1.146. [DOI] [PubMed] [Google Scholar]
- 30.Bodnar LM, Siega-Riz AM, Simhan HN, Himes KP, Abrams B. Severe obesity, gestational weight gain, and adverse birth outcomes. Am J Clin Nutr. 2010;91:1642–1648. doi: 10.3945/ajcn.2009.29008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart AL. The reliability and validity of self-reported weight and height. J Chronic Dis. 1982;35:295–309. doi: 10.1016/0021-9681(82)90085-6. [DOI] [PubMed] [Google Scholar]
- 32.Rowland ML. Self-reported weight and height. Am J Clin Nutr. 1990;52:1125–1133. doi: 10.1093/ajcn/52.6.1125. [DOI] [PubMed] [Google Scholar]
- 33.Lachin JM. The role of measurement reliability in clinical trials. Clin Trials. 2004;1:553–566. doi: 10.1191/1740774504cn057oa. [DOI] [PubMed] [Google Scholar]
- 34.Tomeo CA, Rich-Edwards JW, Michels KB, et al. Reproducibility and validity of maternal recall of pregnancy-related events. Epidemiology. 1999;10:774–777. [PubMed] [Google Scholar]
- 35.Yu SM, Nagey DA. Validity of self-reported pregravid weight. Ann Epidemiol. 1992;2:715–721. doi: 10.1016/1047-2797(92)90016-j. [DOI] [PubMed] [Google Scholar]
- 36.Stevens-Simon C, McAnarney ER, Coulter MP. How accurately do pregnant adolescents estimate their weight prior to pregnancy? J Adolesc Health Care. 1986;7:250–254. doi: 10.1016/s0197-0070(86)80017-1. [DOI] [PubMed] [Google Scholar]