Abstract
Sideritis raeseri spp. raeseri Boiss. & Heldr., known as “mountain tea,” has been widely used in the Mediterranean region as a spice and in folk medicine as a very popular decoction because of its anti-inflammatory, carminative, analgesic, antitussive, stomachic, and antimicrobial properties. The study was aimed to investigate the effects of an ethanol extract of S. raeseri on intestinal activity. Air-dried and powdered aerial parts were extracted with 96% ethanol. The rat ileum preparations were incubated in Tyrode's solution gassed (95% O2/5% CO2) at 37°C. The ethanol extract of S. raeseri (0.03–0.3 mg/mL) relaxed spontaneous contractions in isolated rat ileum, similar to that produced by papaverine. The plant extract in a concentration-dependent manner (0.015–0.15 mg/mL) significantly inhibited the contractile response to acetylcholine (P<.01). Atropine inhibited the response to acetylcholine. A similar relaxation-inducing effect of the S. raeseri extract was observed on the precontracted ileum by histamine and barium chloride. Plant extract (0.03–0.3 mg/mL) significantly shifted the histamine concentration–response curve to the right and down (P<.01). The S. raeseri extract (0.03–0.3 mg/mL) significantly inhibited the contractions induced by barium chloride (P<.01). The results show that the ethanol extract of S. raeseri can produce inhibition of the the spontaneous rat ileum contractions and contractions induced by different spasmogens. These data indicate that S. raeseri acts as a spasmolytic on intestinal smooth muscle, which justifies its use in gastrointestinal disorders.
Key Words: ethanol extract, ileum, rat, Sideritis raeseri
Introduction
Sideritis raeseri Boiss. & Heldr. spp. raeseri belongs to the genus Sideritis L. (Family Lamiaceae), which consists mostly of perennial herbs with a slightly woody base, often densely hairy. Sideritis L. comprises approximately 150 species of annuals and perennials distributed throughout North Africa, the Iberian Peninsula, the Mediterranean countries, and the Middle East region.1 Aerial flowering parts of plants from this genus are known as “mountain tea,” which is widely used in Mediterranean folk medicine as a very popular tea because of its anti-inflammatory,2–4 carminative, analgesic, antitussive, stomachic, and antimicrobial properties.5,6 Anticataract,7 immunomodulating,8 anti–human immunodeficiency virus replication, antifeedant, anti-ulcerogenic, analgesic, antihypoglycemic,9 and antioxidative10 activities were also reported. In Serbian folk medicine, S. raeseri is used as a herbal tea in the treatment of inflammations, gastrointestinal disorders, and coughs and as a tonic, while extracts are used as a component of dietary supplements for anemia. It is mostly imported from the former Yugoslav Republic of Macedonia and Albania.
The essential oils and extracts of Sideritis species have been the subject of many recent studies, but their physiological effects on the motility of intestinal smooth muscles have not been established yet. The present study was aimed to investigate the effects of an ethanol extract of S. raeseri spp. raeseri Boiss. & Heldr. on the contractile responses of isolated rat ileum.
Materials and Methods
Animals
In this study the male Wistar albino rats (weighing 200–250 g) that were used were obtained from the Animal Research Center of the Medical Faculty, University of Niš, Niš, Serbia. The animals were housed in stainless steel cages under standard laboratory conditions. These animals were maintained at 20–24°C with a 12-hour light–dark cycle at least 1 week before the experiment. All animals had free access to food and water. All experimental procedures with animals were in compliance with the European Council Directive of November 24, 1986 (86/609/EEC).
Plant material
The aerial parts of cultivated S. raeseri spp. raeseri were collected in the phase of full flowering, from the experimental field at the Institute for Medicinal Plants Research in Pančevo, Serbia. The upper 20 cm of the plants was harvested and open-air-dried in the shade. Air-dried and powdered aerial parts were extracted with 96% ethanol in a Soxhlet apparatus. The extracts were filtered and evaporated in a vacuum to dryness.
Drugs
Acetylcholine chloride, histamine dihydrochloride, and atropine sulfate were obtained from Sigma Chemical Co. (St. Louis, MO, USA), and papaverine hydrochloride was obtained from Merck (Darmstadt, Germany). All drugs were dissolved in distilled water.
Isolated tissue experiments
The ileum portions were isolated out, and mesenteries were cleaned off. Preparations 2 cm long were mounted in 10-mL tissue baths containing Tyrode's solution maintained at 37°C and aerated with a mixture of 5% carbon dioxide in oxygen. The Tyrode's solution was composed of 136.89 mM NaCl, 2.68 mM KCl, 1.05 mM MgCl2, 1.80 mM CaCl2, 0.42 mM NaH2PO4, 11.90 mM NaHCO3, and 5.5 mM glucose. The fragments were stretched to a sufficient tension and equilibrated for at least 30 minutes before experiments were started. The change of intestinal contractility was recorded using a TSZ-04-E Spell Iso system (Experimetria Ltd., Budapest, Hungary).
After each assay, tissues were washed with fresh Tyrode's solution and equilibrated for around 10 minutes. Rat ileum exhibits spontaneous rhythmic contractions. The isolated ileum had been treated with extract in cumulative concentrations. Papaverine was used as a positive control. Agonists such as acetylcholine, histamine hydrochloride, and barium chloride were cumulatively added to the bath in the absence and presence of S. raeseri extract (0.015–0.15 mg/mL). The relaxation of intestinal preparations precontracted with acetylcholine, histamine, and barium ions was expressed as a percentage of the control response mediated by agonist.
Statistical analysis
The results were expressed as mean±SD values of six determinations. Statistical evaluation was performed using Student's t test. A probability value of P<.05 was considered to be significant. The mean effective concentration (EC50), that is, the concentration that elicited 50% of maximal response, was established by regression analysis.
Results
Effects of the extract on spontaneous contractions of isolated rat ileum
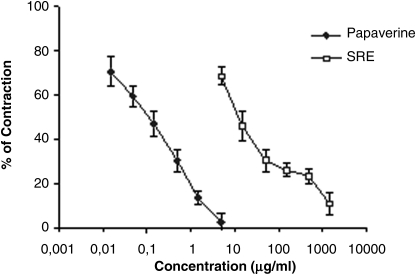
Figure 1 shows the effect of the S. raeseri extract on spontaneous contractions of rat ileum. The extract in a concentration-dependent manner inhibited contractility of the intestine. The EC50 value for the S. raeseri extract-induced relaxation was 15.05±0.97 mg/mL. Papaverine (0.015–5 μg/mL) also relaxed rat ileum in a concentration-dependent manner.
FIG. 1.
Inhibitory effects of the ethanol extract of S. raeseri (SRE) and papaverine on spontaneous contractions in isolated rat ileum. Data are mean±SEM values.
Effects of the extract on acetylcholine-evoked contraction of isolated rat ileum
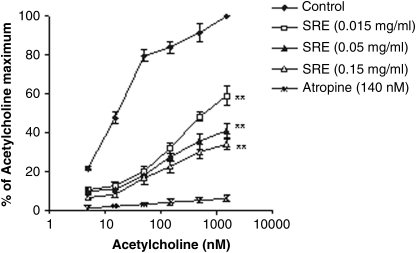
The extract of S. raeseri (0.03–0.3 mg/mL) concentration-dependently inhibited the contraction induced with acetylcholine (P<.01), with an EC50 value of 764.01±64.23 nM (the EC50 value of acetylcholine was 17.95±0.97 nM) (Fig. 2). Atropine (140 nM) inhibited the response to acetylcholine.
FIG. 2.
Comparison of dose–response curves of acetylcholine in the absence and presence of SRE in isolated rat ileum. Data are mean±SEM values. **P<.01 versus contractions induced in the presence of stimulator alone.
Effects of the extract on histamine-evoked contraction of isolated rat ileum
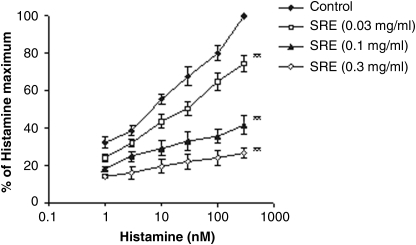
Figure 3 shows the histamine-dependent contraction curve of rat ileum in the absence and presence of the extract of S. raeseri. Plant extract (0.03–0.3 mg/mL) significantly shifted the histamine concentration–response curve to the right and down (P<.01). The EC50 value of histamine (6.14±0.58 nM) was affected by the extract of S. raeseri (EC50=383.01±18.25 nM).
FIG. 3.
Comparison of dose–response curves of histamine in the absence and presence of SRE in isolated rat ileum. Data are mean±SEM values. **P<.01 versus contractions induced in the presence of stimulator alone.
Effects of the extract on barium chloride-evoked contraction of isolated rat ileum
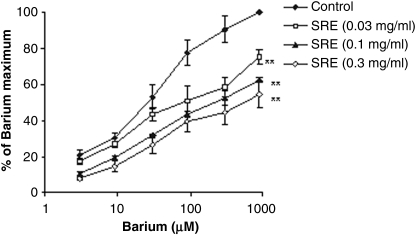
The S. raeseri extract at a concentration of 0.03–0.3 mg/mL inhibited the contractions induced by barium chloride, in a concentration-dependent manner (Fig. 4). The concentration–response curves of barium chloride in the presence of S. raeseri extract were significantly shifted downward (P<.01). The EC50 value of barium ion (23.11±1.47 μM) was changed by the extract of S. raeseri (EC50=488.30±28.36 μM).
FIG. 4.
Comparison of dose–response curves of barium ions in the absence and presence of SRE in isolated rat ileum. Data are mean±SEM values. **P<.01 versus contractions induced in the presence of stimulator alone.
Discussion
The spasmolytic effects of the ethanol extract of S. raeseri were studied for scientific evaluation of its potential medicinal uses. The present data showed that the extract caused inhibition of spontaneous contraction, similar to the spasmolytic agent papaverine. The spasmolytic effect of the extract of S. raeseri was concentration-dependent and reversible after washing, suggesting that this inhibition was not due to damage of the intestine by the extract. These results are in good agreement with the traditional uses of this genus.
The rhythmic contractions of smooth muscle cells depend on an endogenous pacemaker driven by the cytosolic calcium (Ca2+) oscillator that is responsible for the periodic release of Ca2+ from the endoplasmic reticulum. The periodic pulses of Ca2+ often cause membrane depolarization.11 Action potential is generated at the peak of depolarization and constitutes influx of calcium ions through calcium channels.12–14
We observed that the extract of S. raeseri decreased contractions induced by acetylcholine, histamine, and barium chloride. The interactions of acetylcholine and histamine with muscarinic and histamine receptors, respectively, cause depolarization and contraction of intestinal smooth muscle. Also, Walsh and Singer12 showed that action potentials could be elicited when barium ions were present in high concentrations in extracellular fluid. Barium ions depolarize the cell membrane and open the voltage-dependent calcium channels, resulting in a calcium influx.15 It is possible that the extract of S. raeseri contains some compounds that interfere with calcium channel activity or with release of calcium ions from intracellular stores.
Phytochemical studies on samples of the genus Sideritis have shown that these plants are rich in flavones and diterpenoids.16 Actually, flavone glycosides, in particular, 8-hydroxyflavone glycosides, are a chemotaxonomic characteristic for some sections of the genus Sideritis. Several flavones, such as the 7-O-glycosides of 8-hydroxyflavones (isoscutellarein, chryseriol) and apigenin, have been identified from S. raeseri.17,18 These compounds, isolated from Sideritis spp., have been shown to possess biological properties.19 The aglycone apigenin exhibited antispasmodic activities in isolated ileum, mainly caused by blockade of the calcium influx.20
The spasmolytic effects of other plants of the Lamiaceae family have also been reported. The essential oil of Melissa officinalis and its constituent citral inhibited intestinal contractions induced by acetylcholine, histamine, and KCl.21 It is well known that some terpenoids can act as spasmolytic agents. Terpenoid pulegone and essential oil of Calamintha glandulosa (Family Lamiaceae) also relaxed spontaneous and K+-induced contraction of rat ileum.22
In conclusion, the present results show that the ethanol extract of S. raeseri spp. raeseri Boiss. & Heldr. can inhibit spontaneous ileum contractions and contractions induced by acetylcholine, histamine, and barium ions. All the above findings are in agreement with their usage in traditional medicine. Based on our results, S. raeseri may be phytotherapeutically used, after full pharmacological and toxicological evaluation, as an alternative drug to synthetic spasmolytic agents.
Acknowledgments
The authors are grateful for the financial support of the Ministry of Sciences and Technological Development of the Republic of Serbia (grant III 46013) and the project Training and Research in Environmental Health in the Balkans D43 TW00641 supported by the National Institutes of Health/Fogarty International Center, USA.
Author Disclosure Statement
No competing interests exist.
References
- 1.Barber JC. Francisco-Ortega J. Santos-Guerra A. Turner KG. Hansen RK. Origin of Macaronesian Sideritis L. (Lamioideae, Lamiaceae) inferred from nuclear and chloroplast sequence datasets. Mol Phylogenet Evol. 2002;23:293–306. doi: 10.1016/s1055-7903(02)00018-0. [DOI] [PubMed] [Google Scholar]
- 2.Obón de Castro C. Rivera-Núñez D. Phanerogamarum Monographiae. Vol. 21. J. Cramer; Berlin: 1994. A Taxonomic Revision of the Section Sideritis (Genus Sideritis) (Labiatae) [Google Scholar]
- 3.Godoy A. De Las Heras B. Vivas M. Villar A. Antiinflammatory properties of a lipid fraction obtained from Sideritis javalambrensis. Biol Pharm Bull. 2000;23:1193–1197. doi: 10.1248/bpb.23.1193. [DOI] [PubMed] [Google Scholar]
- 4.Aboutabl E. Nassar M. Elsakhawy F. Maklad Y. Osman A. El-Khrisy E. Phytochemical and pharmacological studies on Sideritis taurica Stephan ex Wild. J Ethnopharmacol. 2002;82:177–184. doi: 10.1016/s0378-8741(02)00172-1. [DOI] [PubMed] [Google Scholar]
- 5.Ozcan M. Chalchat J. Akgul A. Essential oil composition of Turkish mountain tea (Sideritis spp.) Food Chem. 2001;75:459–463. [Google Scholar]
- 6.Gabrieli C. Kefalas P. Kokkalou E. Antioxidant activity of flavonoids from Sideritis raeseri. J Ethnopharmacol. 2005;96:423–428. doi: 10.1016/j.jep.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 7.Tomas-Barberan F. Lopez-Gomex C. Villar A. Tomas-Lorente F. Inhibition of lens aldose reductase by Labiatae flavonoids. Planta Med. 1988;52:239–240. [PubMed] [Google Scholar]
- 8.Navarro A. de Las Heras B. Villar A. Immunomodulating properties of diterpene andalusol. Planta Med. 2000;66:289–291. doi: 10.1055/s-2000-8567. [DOI] [PubMed] [Google Scholar]
- 9.Piozzi F. Bruno M. Rosselli S. Maggio A. The diterpenoids from the genus Sideritis. Studies Nat Products Chem. 2006;33:493–540. [Google Scholar]
- 10.Armata M. Gabrieli C. Termentzi A. Zervou M. Kokkalou E. Constituents of Sideritis syriatica ssp. syriaca (Lamiaceae) and antioxidant activity. Food Chem. 2008;111:179–186. [Google Scholar]
- 11.Berridge M. Smooth muscle cell calcium activation mechanisms. J Physiol. 2008;586:5047–5061. doi: 10.1113/jphysiol.2008.160440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh J. Singer J. Calcium action potentials in single freshly isolated smooth muscle cells. Am J Physiol. 1980;239:C162–C174. doi: 10.1152/ajpcell.1980.239.5.C162. [DOI] [PubMed] [Google Scholar]
- 13.Brading A. How do drugs initiate contraction in smooth muscle? Trends Pharmacol Sci. 1981;2:262–265. [Google Scholar]
- 14.Gilani A. Shah A. Ghayur M. Majeed K. Pharmacological basis for the use of tumeric in gastrointestinal and respiratory disorders. Life Sci. 2005;76:3089–3105. doi: 10.1016/j.lfs.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 15.Karaki H. Satake N. Shibata S. Mechanism of barium-induced contraction in the vascular smooth muscle of rabbit aorta. Br J Pharmacol. 1986;88:821–826. doi: 10.1111/j.1476-5381.1986.tb16255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sagdic O. Aksoy A. Ozkan G. Ekici L. Albayrak S. Biological activities of the extracts of two endemic Sideritis species in Turkey. Innov Food Sci Emerg Technol. 2008;9:80–84. [Google Scholar]
- 17.Janeska B. Stefova M. Alipieva K. Assay of flavonoid aglycones from the species of genus Sideritis (Lamiaceae) from Macedonia with HPLC-UV DAD. Acta Pharm. 2007;57:371–377. doi: 10.2478/v10007-007-0030-8. [DOI] [PubMed] [Google Scholar]
- 18.Gabrieli C. Kokkalou E. A glucosylated acylflavone from Sideritis raeseri. Phytochemistry. 1990;29:681–683. [Google Scholar]
- 19.Ghoumari H. Benajiba M. Azmani A. García-Granados A. Martínez A. Parra A. Rivas F. Socorro O. ent-Kauranoid derivates from Sideritis moorei. Phytochemistry. 2005;66:1492–1498. doi: 10.1016/j.phytochem.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 20.Lemmens-Gruber R. Marchart E. Rawnduzi P. Engel N. Benedek B. Kopp B. Investigation of the spasmolytic activity of the flavonoid fraction of Achillea millefolium s.l. on isolated guinea-pig ilea. Arzneimittelforschung. 2006;56:582–588. doi: 10.1055/s-0031-1296755. [DOI] [PubMed] [Google Scholar]
- 21.Sandraei H. Ghannadi A. Malekshahi K. Relaxant effect of essential oil of Melissa officinalis and citral on rat ileum contractions. Fitoterapia. 2003;74:445–452. doi: 10.1016/s0367-326x(03)00109-6. [DOI] [PubMed] [Google Scholar]
- 22.Brankovic S. Kitic D. Radenkovic M. Veljkovic S. Golubovic T. Calcium blocking activity as a mechanism of the spasmolytic effect the essential oil of Calamintha glandulosa Silic on isolated rat ileum. Gen Physiol Biophys. 2009;28:172–176. [PubMed] [Google Scholar]