Abstract
We have purified three chromatographically distinct human enzyme activities from HeLa cells, that are capable of converting bleomycin-treated DNA into a substrate for E. coli DNA polymerase I. The bleomycin-treated DNA substrate used in this study has been characterized via a 32P-postlabeling assay and shown to contain strand breaks with 3'-phosphoglycolate termini as greater than 95% of the detectable dose-dependent lesions. The purified HeLa cell enzymes were shown to be capable of removing 3'-phosphoglycolates from this substrate. Also 3'-phosphoglycolate removal and nucleotide incorporation were enzyme dependent. In addition, all three Hela cell enzymes have been determined to possess Class II AP endonuclease activity. The enzymes lack 3'----5' exonuclease activity and are, therefore, dissimilar to exonuclease III--an E. coli enzyme that can remove 3'-phosphoglycolate.
Full text
PDF

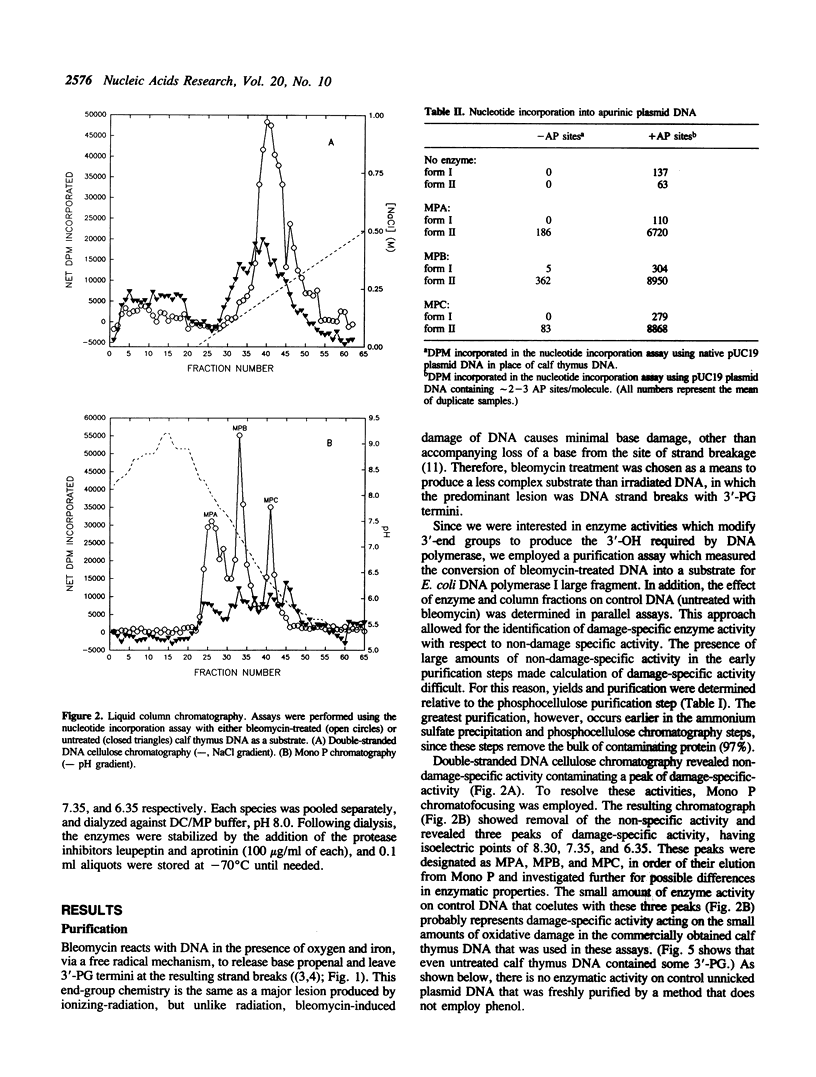
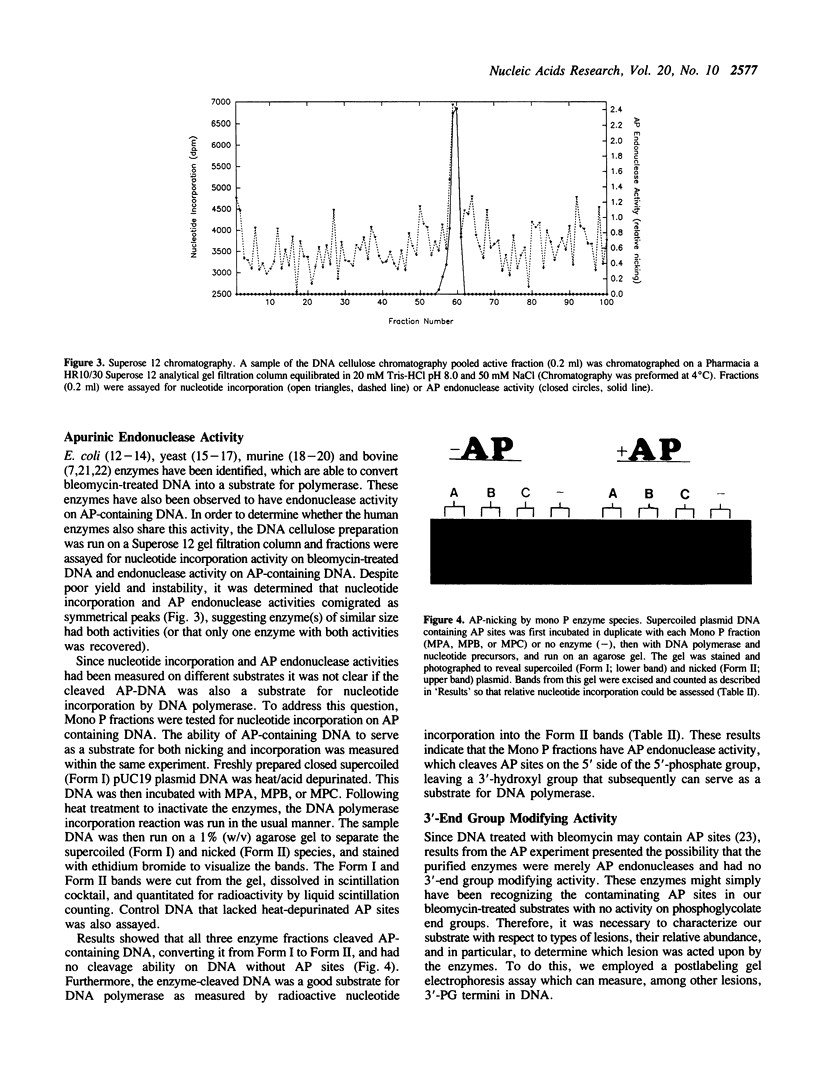
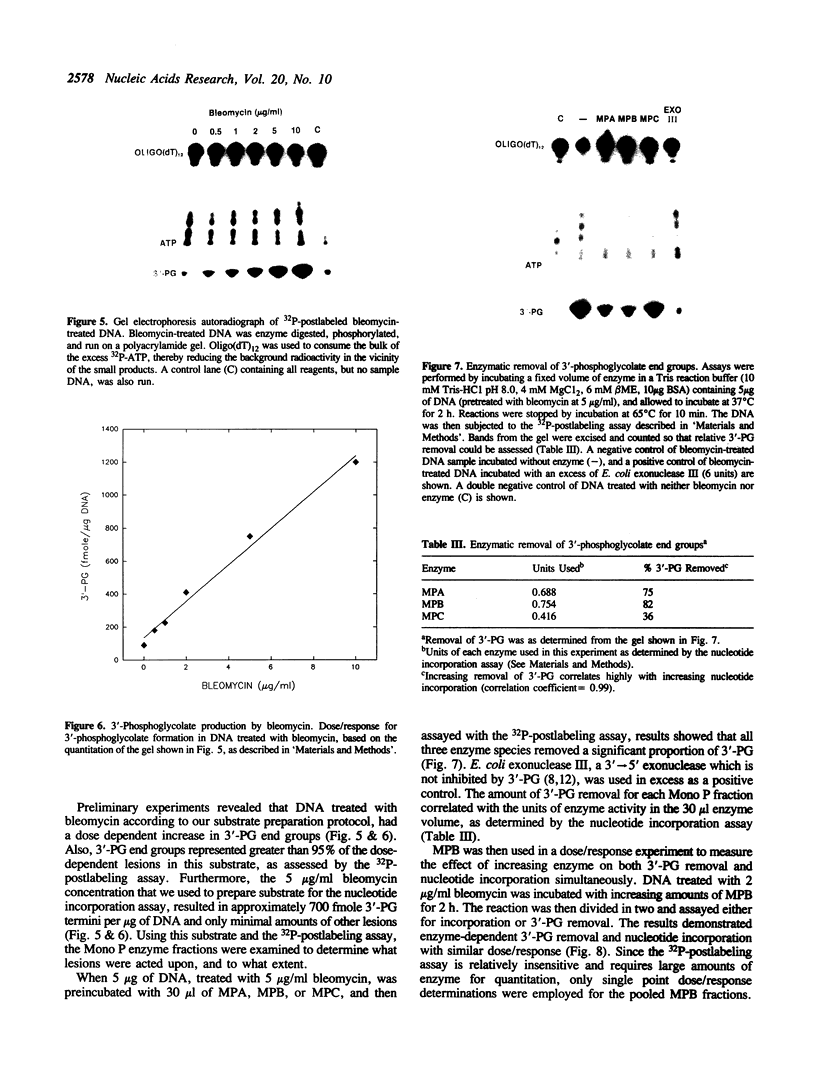
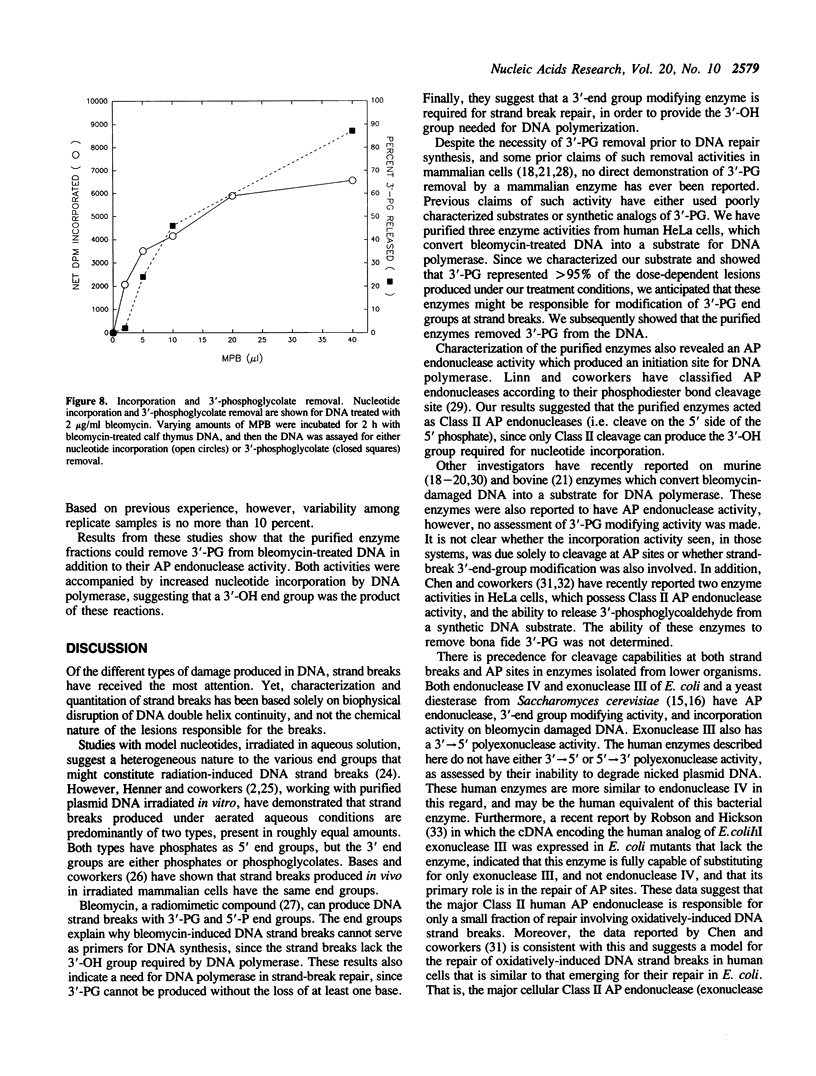

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bases R., Hamori I., Piazza L., Maio J., Mendez F. DNA base and strand damage in X-irradiated monkey CV-1 cells: influence of pretreatment using small doses of radiation. Int J Radiat Biol. 1990 Jul;58(1):35–54. doi: 10.1080/09553009014551421. [DOI] [PubMed] [Google Scholar]
- Bernelot-Moens C., Demple B. Multiple DNA repair activities for 3'-deoxyribose fragments in Escherichia coli. Nucleic Acids Res. 1989 Jan 25;17(2):587–600. doi: 10.1093/nar/17.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen D. S., Herman T., Demple B. Two distinct human DNA diesterases that hydrolyze 3'-blocking deoxyribose fragments from oxidized DNA. Nucleic Acids Res. 1991 Nov 11;19(21):5907–5914. doi: 10.1093/nar/19.21.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B., Herman T., Chen D. S. Cloning and expression of APE, the cDNA encoding the major human apurinic endonuclease: definition of a family of DNA repair enzymes. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11450–11454. doi: 10.1073/pnas.88.24.11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski E., Aruoma O. I., Dizdaroglu M., Halliwell B. Bleomycin-dependent damage to the bases in DNA is a minor side reaction. Biochemistry. 1991 Mar 5;30(9):2444–2448. doi: 10.1021/bi00223a021. [DOI] [PubMed] [Google Scholar]
- Giloni L., Takeshita M., Johnson F., Iden C., Grollman A. P. Bleomycin-induced strand-scission of DNA. Mechanism of deoxyribose cleavage. J Biol Chem. 1981 Aug 25;256(16):8608–8615. [PubMed] [Google Scholar]
- Hagensee M. E., Moses R. E. Bleomycin-treated DNA is specifically cleaved only by endonuclease IV in E. coli. Biochim Biophys Acta. 1990 Jan 30;1048(1):19–23. doi: 10.1016/0167-4781(90)90016-u. [DOI] [PubMed] [Google Scholar]
- Henner W. D., Grunberg S. M., Haseltine W. A. Sites and structure of gamma radiation-induced DNA strand breaks. J Biol Chem. 1982 Oct 10;257(19):11750–11754. [PubMed] [Google Scholar]
- Henner W. D., Kiker N. P., Jorgensen T. J., Munck J. N. Purification and amino-terminal amino acid sequence of an apurinic/apyrimidinic endonuclease from calf thymus. Nucleic Acids Res. 1987 Jul 24;15(14):5529–5544. doi: 10.1093/nar/15.14.5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henner W. D., Rodriguez L. O., Hecht S. M., Haseltine W. A. gamma Ray induced deoxyribonucleic acid strand breaks. 3' Glycolate termini. J Biol Chem. 1983 Jan 25;258(2):711–713. [PubMed] [Google Scholar]
- Johnson A. W., Demple B. Yeast DNA 3'-repair diesterase is the major cellular apurinic/apyrimidinic endonuclease: substrate specificity and kinetics. J Biol Chem. 1988 Dec 5;263(34):18017–18022. [PubMed] [Google Scholar]
- Johnson A. W., Demple B. Yeast DNA diesterase for 3'-fragments of deoxyribose: purification and physical properties of a repair enzyme for oxidative DNA damage. J Biol Chem. 1988 Dec 5;263(34):18009–18016. [PubMed] [Google Scholar]
- Murugesan N., Xu C., Ehrenfeld G. M., Sugiyama H., Kilkuskie R. E., Rodriguez L. O., Chang L. H., Hecht S. M. Analysis of products formed during bleomycin-mediated DNA degradation. Biochemistry. 1985 Oct 8;24(21):5735–5744. doi: 10.1021/bi00342a008. [DOI] [PubMed] [Google Scholar]
- Niwa O., Moses R. E. Synthesis by DNA polymerase I on bleomycin-treated deoxyribonucleic acid: a requirement for exonuclease III. Biochemistry. 1981 Jan 20;20(2):238–244. doi: 10.1021/bi00505a002. [DOI] [PubMed] [Google Scholar]
- Pawlak A. L., Kotecki M., Ignatowicz R. Increased frequency of chromatid breaks in lymphocytes of heterozygotes of ataxia telangiectasia after in vitro treatment with caffeine. Mutat Res. 1990 Jun;230(2):197–204. doi: 10.1016/0027-5107(90)90057-b. [DOI] [PubMed] [Google Scholar]
- Povirk L. F., Austin M. J. Genotoxicity of bleomycin. Mutat Res. 1991 Mar;257(2):127–143. doi: 10.1016/0165-1110(91)90022-n. [DOI] [PubMed] [Google Scholar]
- Ramotar D., Popoff S. C., Gralla E. B., Demple B. Cellular role of yeast Apn1 apurinic endonuclease/3'-diesterase: repair of oxidative and alkylation DNA damage and control of spontaneous mutation. Mol Cell Biol. 1991 Sep;11(9):4537–4544. doi: 10.1128/mcb.11.9.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson C. N., Hickson I. D. Isolation of cDNA clones encoding a human apurinic/apyrimidinic endonuclease that corrects DNA repair and mutagenesis defects in E. coli xth (exonuclease III) mutants. Nucleic Acids Res. 1991 Oct 25;19(20):5519–5523. doi: 10.1093/nar/19.20.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson C. N., Milne A. M., Pappin D. J., Hickson I. D. Isolation of cDNA clones encoding an enzyme from bovine cells that repairs oxidative DNA damage in vitro: homology with bacterial repair enzymes. Nucleic Acids Res. 1991 Mar 11;19(5):1087–1092. doi: 10.1093/nar/19.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson B. J., Chang C. N., Grollman A. P., Henner W. D. Mechanism of DNA cleavage and substrate recognition by a bovine apurinic endonuclease. Biochemistry. 1989 May 2;28(9):3894–3901. doi: 10.1021/bi00435a040. [DOI] [PubMed] [Google Scholar]
- Seki S., Akiyama K., Watanabe S., Hatsushika M., Ikeda S., Tsutsui K. cDNA and deduced amino acid sequence of a mouse DNA repair enzyme (APEX nuclease) with significant homology to Escherichia coli exonuclease III. J Biol Chem. 1991 Nov 5;266(31):20797–20802. [PubMed] [Google Scholar]
- Seki S., Ikeda S., Tsutui K., Teraoka H. Repair of X-ray-induced single-strand breaks by a cell-free system. Carcinogenesis. 1990 Jul;11(7):1213–1216. doi: 10.1093/carcin/11.7.1213. [DOI] [PubMed] [Google Scholar]
- Seki S., Ikeda S., Watanabe S., Hatsushika M., Tsutsui K., Akiyama K., Zhang B. A mouse DNA repair enzyme (APEX nuclease) having exonuclease and apurinic/apyrimidinic endonuclease activities: purification and characterization. Biochim Biophys Acta. 1991 Aug 9;1079(1):57–64. doi: 10.1016/0167-4838(91)90024-t. [DOI] [PubMed] [Google Scholar]
- Seki S., Oda T. An exonuclease possibly involved in the initiation of repair of bleomycin-damaged DNA in mouse ascites sarcoma cells. Carcinogenesis. 1988 Dec;9(12):2239–2244. doi: 10.1093/carcin/9.12.2239. [DOI] [PubMed] [Google Scholar]
- Steighner R. J., Povirk L. F. Effect of in vitro cleavage of apurinic/apyrimidinic sites on bleomycin-induced mutagenesis of repackaged lambda phage. Mutat Res. 1990 Feb;240(2):93–100. doi: 10.1016/0165-1218(90)90012-q. [DOI] [PubMed] [Google Scholar]
- Téoule R. Radiation-induced DNA damage and its repair. Int J Radiat Biol Relat Stud Phys Chem Med. 1987 Apr;51(4):573–589. doi: 10.1080/09553008414552111. [DOI] [PubMed] [Google Scholar]
- Weinfeld M., Liuzzi M., Paterson M. C. Response of phage T4 polynucleotide kinase toward dinucleotides containing apurinic sites: design of a 32P-postlabeling assay for apurinic sites in DNA. Biochemistry. 1990 Feb 20;29(7):1737–1743. doi: 10.1021/bi00459a011. [DOI] [PubMed] [Google Scholar]
- Weinfeld M., Soderlind K. J. 32P-postlabeling detection of radiation-induced DNA damage: identification and estimation of thymine glycols and phosphoglycolate termini. Biochemistry. 1991 Jan 29;30(4):1091–1097. doi: 10.1021/bi00218a031. [DOI] [PubMed] [Google Scholar]