This work demonstrates that clathrin-dependent endocytosis exists in plants. Moreover, it shows that clathrin function is required for polarity of PIN auxin transporters, auxin distribution, and associated developmental processes.
Abstract
Endocytosis is a crucial mechanism by which eukaryotic cells internalize extracellular and plasma membrane material, and it is required for a multitude of cellular and developmental processes in unicellular and multicellular organisms. In animals and yeast, the best characterized pathway for endocytosis depends on the function of the vesicle coat protein clathrin. Clathrin-mediated endocytosis has recently been demonstrated also in plant cells, but its physiological and developmental roles remain unclear. Here, we assessed the roles of the clathrin-mediated mechanism of endocytosis in plants by genetic means. We interfered with clathrin heavy chain (CHC) function through mutants and dominant-negative approaches in Arabidopsis thaliana and established tools to manipulate clathrin function in a cell type–specific manner. The chc2 single mutants and dominant-negative CHC1 (HUB) transgenic lines were defective in bulk endocytosis as well as in internalization of prominent plasma membrane proteins. Interference with clathrin-mediated endocytosis led to defects in constitutive endocytic recycling of PIN auxin transporters and their polar distribution in embryos and roots. Consistent with this, these lines had altered auxin distribution patterns and associated auxin transport-related phenotypes, such as aberrant embryo patterning, imperfect cotyledon specification, agravitropic growth, and impaired lateral root organogenesis. Together, these data demonstrate a fundamental role for clathrin function in cell polarity, growth, patterning, and organogenesis in plants.
INTRODUCTION
Endocytosis is the process by which fragments of the plasma membrane are pinched off to form membrane vesicles in the cytosol. Through this mechanism, the endocytic vesicles can incorporate cargo from the plasma membrane and extracellular space and reroute it to various subcellular compartments, including retargeting to the plasma membrane. In animals and yeast, endocytosis is an important mechanism to regulate protein abundance at the plasma membrane during signaling events and retargeting or degradation of membrane proteins (Mukherjee et al., 1997). In plants, the existence, physical feasibility, and physiological significance of endocytosis have been a matter of debate for decades, specifically due to the presence of a cell wall and high cellular turgor pressure (Cram, 1980; Robinson et al., 2008). Yet, in plants, endocytosis can be observed in many processes important for plant development, such as auxin transport (Geldner et al., 2001; Paciorek et al., 2005; Dhonukshe et al., 2008a), cytokinesis (Dhonukshe et al., 2006; Boutté et al., 2010), cell wall formation (Baluška et al., 2002), root hair morphogenesis (Takeda et al., 2008), pollen tube growth (Sousa et al., 2008; Zhao et al., 2010), self-incompatiblity responses (Ivanov and Gaude, 2009), responses to pathogens (Robatzek et al., 2006), abscisic acid responses (Sutter et al., 2007), brassinosteroid signaling (Russinova et al., 2004; Geldner et al., 2007), and responses to high boron levels (Takano et al., 2005, 2010).
During responses to pathogens, abscisic acid, and high boron levels, the abundance of specific proteins in the plasma membrane is downregulated through induction of their endocytosis (Takano et al., 2005, 2010; Robatzek et al., 2006; Sutter et al., 2007), whereas other signals, such as auxin, actively repress endocytosis (Paciorek et al., 2005; Robert et al., 2010). The significance of endocytosis for these regulations is beyond doubt; however, functional data remain scarce.
In animals and yeast, the selective budding of cargo proteins from cellular membranes involves predominantly the formation of clathrin-coated vesicles (CCVs). Fundamentally, CCV formation requires the assembly of a polyhedral cage composed of clathrin heteropolymers of heavy and light chains (Fotin et al., 2004). Such CCVs were found at the plasma membrane, trans-Golgi network, endosomes, and lysosomes, where they effect endocytosis, protein sorting, and degradation (Kirchhausen, 2000; Brodsky et al., 2001; McNiven and Thompson, 2006). The significance of clathrin is also apparent in other processes, such as establishment of polarity (Deborde et al., 2008), cytokinesis (Schweitzer et al., 2005), virus infection (Marsh and Helenius, 2006), and so on.
The genomes of flowering plants contain several essential genes coding for clathrin-related machinery, including clathrin light and heavy chains (Holstein, 2002). Moreover, clathrin can be found at the trans-Golgi network–related structures and plasma membrane in higher plants (Blackbourn and Jackson, 1996; Dhonukshe et al., 2007; Fujimoto et al., 2010; Van Damme et al., 2011). Thus, it seems that clathrin could also mediate protein sorting, degradation, and endocytosis in plants (Robinson et al., 2008). Moreover, pharmacological inhibitors of clathrin-mediated endocytosis in yeast and animals are also potent inhibitors of plant endocytosis (Dhonukshe et al., 2007; Boutté et al., 2010). As they also interfere with the interaction between cargo proteins and clathrin-recruiting adaptor protein complexes in plants, these drugs have been suggested to impede clathrin-mediated endocytosis (Ortiz-Zapater et al., 2006). The requirement of clathrin function for endocytosis was further demonstrated via dominant-negative approaches in plant cell suspensions (Tahara et al., 2007), protoplasts (Dhonukshe et al., 2007), and plants (Robert et al., 2010). However, the physiological and developmental importance of clathrin-mediated endocytosis awaits detailed characterization. Here, we present a genetic characterization of the in planta role of clathrin in endocytosis and auxin-mediated plant development.
RESULTS
Dominant-Negative Clathrin HUB Interferes with Bulk Endocytosis in Planta
The formation of a functional clathrin polyhedral involves the formation of complexes between clathrin heavy and light chains. Clathrin heavy chains interact with light chains through residues in their C terminus (Brodsky et al., 2001). Previously, overexpression of a C-terminal portion of clathrin heavy chain1 (HUB) had been shown in mammalian cells to act as a dominant-negative form of clathrin through competition for clathrin light chain binding (Liu et al., 1995, 1998). Also in plant systems, expression of a truncated plant clathrin heavy chain (CHC) was able to inhibit endocytosis (Dhonukshe et al., 2007; Tahara et al., 2007; Robert et al., 2010). We used transgenic Arabidopsis thaliana lines harboring a 4-hydroxytamoxifen-inducible RFP-HUB1 (INTAM>>RFP-HUB1) to investigate further the effects of HUB expression on endocytosis in plants.
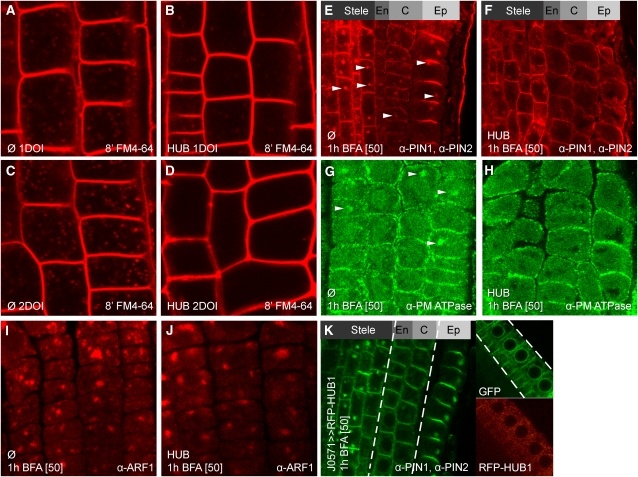
To monitor general endocytosis, we analyzed the intracellular accumulation of the endocytic tracer N-(3-triethylammoniumpropyl)-4-(4-diethylaminophenylhexatrienyl) pyridinium dibromide (FM4-64) (Jelínková et al., 2010). Twenty-four hours or 1 d of induction (DOI) of HUB expression significantly (P < 0.0001; Student’s t test) reduced intracellular labeling with FM4-64 in INTAM>>RFP-HUB1 compared with the driver control (Figures 1A and 1B; see Supplemental Figure 1A online). After 48 h or 2 DOI, the inhibitory effect of HUB expression on endocytosis was further enhanced (P < 0.0001; Student’s t test) (Figures 1C and 1D; see Supplemental Figure 1B online). These data show that genetic interference with CHC function inhibited general endocytotic events in planta.
Figure 1.
Requirement of Clathrin Function for Endocytosis.
(A) to (D) Uptake of endocytic tracer dye FM4-64 (2 μM) after 8 min in root meristem epidermal cells of the INTAM driver line ([A] and [C]) and INTAM>>RFP-HUB1 ([B] and [D]) induced for 1 ([A] and [B]) or 2 ([C] and [D]) d with 2 μM 4-hydroxytamoxifen.
(E) to (J) Immunolocalization of PIN1 and PIN2 (red signal; median section) ([E] and [F]), PM-ATPase (green signal; epidermis) ([G] and [H]), and ARF1 (red signal; epidermis) ([I] and [J]) after 1 h of BFA (50 μM) treatment on seedlings of the INTAM driver line ([E], [G], and [I]) and INTAM>>RFP-HUB1 ([F], [H], and [J]) germinated on 2 μM 4-hydroxytamoxifen. Arrowheads highlight BFA bodies.
(K) Immunolocalization of PIN1 and PIN2 in J0571>>RFP-HUB1 after 1 h of BFA (50 μM) treatment (median section).
En, endodermis; C, cortex; Ep, epidermis. Right-hand panels indicate J0571>>mGFP5-ER (green), and J0571>>RFP-HUB1 (red) expression in cortex and endodermis prior to immunolocalization.
To corroborate this observation, we investigated the effects on the intracellular accumulation of several transmembrane proteins. To visualize their endocytosis, we inhibited exocytosis using the fungal toxin, brefeldin A (BFA). This toxin not only inhibits trafficking from the endosomes to the plasma membrane, it also induces aggregation of endosomes into so-called BFA compartments (Geldner et al., 2001; Baluška et al., 2002; Grebe et al., 2003). In the driver line, BFA treatment caused an intracellular accumulation of the auxin transport proteins PINFORMED1 (PIN1), PIN2, plasma membrane H+-ATPase (PM ATPase), and the endosomal marker ADP ribosylation factor 1 (ARF1) in BFA compartments (Figures 1E, 1G, and 1I), consistent with previous observations (Geldner et al., 2001; Tanaka et al., 2009). By contrast, PIN1, PIN2, and PM ATPase no longer accumulated in BFA compartments after HUB induction (Figures 1F and 1H), whereas ARF1 did (Figures 1I and 1J), implying that HUB expression impaired internalization of proteins from the plasma membrane without affecting BFA compartment formation.
To exclude potential artifacts inherent to pharmacological treatments, we transactivated HUB expression in cortical and endodermal cells using a cell type–specific GAL4-expressing enhancer trap line (J0571>>RFP-HUB1) (Figure 1K; see Supplemental Figure 2 online). Following BFA treatment, PIN1 and PIN2 proteins accumulated in the BFA bodies in the stele and epidermis, whereas no BFA bodies were visible in the cortex and endodermis (Figure 1K), suggesting that the observed inhibition of endocytosis is cell autonomous and specific for HUB expression.
These results demonstrate that expression of a dominant-negative clathrin HUB interferes with the endocytosis of integral plasma membrane proteins in plants. It also provides a tool to manipulate endocytosis in specific cells and cell types. It should be noted that these experiments do not allow the exclusion of a role for clathrin in protein sorting and degradation.
Dominant-Negative Clathrin HUB Interferes with Different Aspects of Plant Development
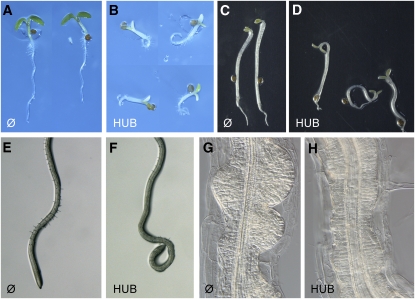
Next, we assessed the effects of impaired clathrin function caused by clathrin HUB induction on postembryonic plant development. Seedlings germinated under inductive conditions were strongly defective in seedling growth, with highly impaired root elongation, agravitropic root growth, and yellow/white, nonexpanded cotyledons (Figures 2A and 2B). Etiolated seedlings had agravitropic hypocotyls and open cotyledons (Figures 2C and 2D).
Figure 2.
Effect of HUB Induction on Seedling Growth.
(A) and (B) Seedlings grown for 3 d in the presence of 4-hydroxytamoxifen of the INTAM driver control (A) and INTAM>>RFP-HUB1 (B).
(C) and (D) Etiolated seedlings, germinated in the presence of 4-hydroxytamoxifen of the INTAM driver control (C) and INTAM>>RFP-HUB1 (D).
(E) and (F) Root growth of seedlings grown for 2 d on half-strength Murashige and Skoog medium followed by transfer to 4-hydroxytamoxifen for 2 d of INTAM driver control (E) and INTAM>>RFP-HUB1 (F).
(G) and (H) Auxin-induced lateral roots of INTAM driver control (G) and INTAM>>RFP-HUB1 (H), transferred for 2 d to 4-hydroxytamoxifen and 10 μM 1-naphthaleneacetic acid.
Moreover, after transfer from noninductive medium to medium supplemented with 4-hydroxytamoxifen, roots became agravitropic within 2 DOI (Figures 2E and 2F). Some of the observed phenotypic defects were difficult to attribute to a specific signaling process. However, the observed agravitropic growth was indicative of defects in auxin transport. Therefore, we tested specifically whether other auxin transport–dependent processes, such as lateral root formation (Benková et al., 2003; Geldner et al., 2004), were affected by interference with clathrin function. Seedlings were germinated on noninductive medium and transferred to auxin-containing inductive medium. Within 2 DOI, discrete lateral root primordia formed along the main root of control seedlings (Figure 2G). By contrast, HUB-expressing seedlings displayed a highly disorganized proliferation of pericycle cells, reminiscent of the effects of impaired polar auxin transport (Figure 2H) (Benková et al., 2003; Geldner et al., 2004). Together, these data show that clathrin function is required for auxin-dependent plant development and other processes.
Dominant-Negative Clathrin HUB Interferes with Auxin Distribution
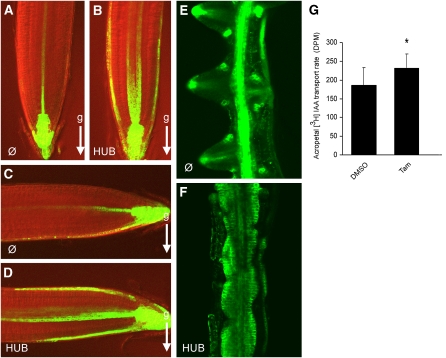
Because gravitropic growth and lateral root morphogenesis notoriously depend on dynamic auxin redistribution (Luschnig et al., 1998; Swarup et al., 2001; Benková et al., 2003; Geldner et al., 2004; Abas et al., 2006), we investigated the effect of clathrin HUB induction on auxin distribution with the auxin reporter DR5rev:green fluorescent protein (GFP; Friml et al., 2003). In wild-type meristems, DR5rev:GFP was highly active in columella and in stele tissues (Figure 3A). After gravistimulation, the DR5rev:GFP signal relocated asymmetrically to the gravistimulated side, indicating a shift in auxin distribution (Figure 3C) (Luschnig et al., 1998; Ottenschläger et al., 2003). By contrast, induction of clathrin HUB caused ectopic lateral DR5rev:GFP expression on both sides of the meristem, independently of gravistimulation (Figures 3B and 3D).
Figure 3.
Changes in Auxin Distribution Caused by HUB Induction.
(A) to (D) DR5rev:GFP activity in root tips of control (A) and INTAM>>RFP-HUB1 (B) after 24 h of induction with 4-hydroxytamoxifen. DR5rev:GFP activity in gravistimulated (5 h) root tips of control (C) and INTAM>>RFP-HUB1 (D) induced with 4-hydroxytamoxifen (24 h). Arrows (g) indicate gravity vector.
(E) and (F) DR5rev:GFP expression pattern in auxin-induced lateral roots of control (E) and INTAM>>RFP-HUB1 (F).
(G) Induction of HUB expression increases acropetal auxin transport in root meristems (26 h treatment with 2 μM 4-hydroxytamoxifen [Tam] or DMSO). Asterisk indicates P < 0.05; n = 9. Error bars are sd.
In auxin-induced lateral roots, the DR5rev:GFP activity showed a maximum in the tip of developing lateral roots (Figure 3E) (Benková et al., 2003; Geldner et al., 2004). However, this maximum could not be observed after RFP-HUB1 induction but was rather a signal continuum (Figure 3F). These data suggest that interference with clathrin function through HUB expression affects plant growth responses through its effects on auxin distribution.
Therefore, we tested the effect of HUB expression on auxin transport. To exclude potential artifacts due to changed root growth and morphology after long-term HUB induction, we used 1 DOI seedlings for auxin transport measurements, in which root morphology and PIN2 polarity was not visibly affected (see Supplemental Figure 3 online). We found that acropetal auxin transport was increased by ~25% after 1 DOI compared with the mock treatment (P < 0.05; Student’s t test) (Figure 3G). Given the prominent role of PIN proteins and their constitutive endocytic dynamics in auxin transport, it is likely that these effects are a consequence of altered PIN trafficking dynamics.
CHC Mutants Have Defects in Endocytosis
As we demonstrated that interference with clathrin function through induction of HUB strongly impairs endocytosis, we wondered whether endocytosis might also be affected in loss-of-function mutants defective in clathrin.
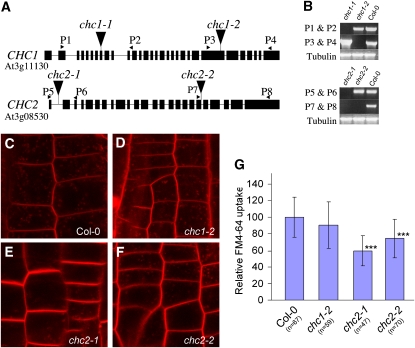
The Arabidopsis genome encodes three clathrin light chains (CLCs) and two CHCs. In each of the CHC genes, we identified homozygous T-DNA insertion lines, for which no full-length transcripts could be detected, indicating they represent knockout mutants (Figures 4A and 4B).
Figure 4.
Characterization of chc Mutant Alleles.
(A) Schematic representation of the intron (bar)-exon (black box) structure in CHC1 and CHC2. Arrowheads indicate the T-DNA insertion sites.
(B) RT-PCR from RNA extracts of the chc1 or chc2 single mutants and wild-type Columbia-0 (Col-0) plantlets. The positions of primers are shown in (A).
(C) to (F) Delay of FM4-64 uptake in chc2 mutants. Intracellular accumulation of FM4-64 is apparent within 6 min after incubation in 2 μM FM4-64 in the wild type (C) and chc1-2 mutants (D). By contrast, the intracellular signal is much weaker in chc2 alleles ([E] and [F]).
(G) Quantification of relative FM4-64 uptake. Error bars indicate sd. Asterisks indicate P < 0.0001 (Student’s t test); n = number of cells analyzed.
The high degree of sequence identity (>90%) between the two CHC gene products hints at functional redundancy between both CHC genes. Therefore, we tried to obtain double mutants. Assuming an average recombination frequency of 250 kb/centimorgan in Arabidopsis (Lukowitz et al., 2000), a recombination frequency of ~4% might be expected between both CHC genes (<900 kb apart). However, we found no double mutants nor plants that were homozygous for one mutation and heterozygous for the other in a large population of F2 plants (Table 1), suggesting that CHC1 and CHC2 genes are redundantly essential for the viability of gametophytes and/or zygotes. Nonetheless, we cannot rule out a lower recombination frequency between both genes.
Table 1.
Progeny of a Selfed chc1-1/CHC1; chc2-2/CHC2 Plant
| Genotype CHC1 CHC2 | No. Observeda | 4% Recombination (P Value = 0.455)b | 4% Recombination (P Value <0.062)c |
| WT WT | 0 | 0 | 0 |
| WT Het | 13 | 5 | 5 |
| WT Mut | 32 | 33 | 32 |
| Het WT | 10 | 5 | 5 |
| Het Het | 120 | 131 | 127 |
| Het Mut | 0 | 0 | 3 |
| Mut WT | 65 | 66 | 63 |
| Mut Het | 0 | 0 | 5 |
| Mut Mut | 0 | 0 | 0 |
| Total | 240 | 240 | 240 |
WT, wild-type plants; Het, heterozygotes; Mut, plants homozygous for indicated mutant alleles. P value was calculated by the χ2 test.
Genotypes of 240 progeny plants with the respective indicated genotypes were determined by PCR.
Expected number was calculated as follows: (1) 4% recombination ratio between the two genes, (2) the observed frequencies of genotypically homozygous chc2 mutants in control experiments with the selfed progeny of a chc2 heterozygous plant (12.8%, n = 180), and (3) either gametophytic lethality of chc1 chc2 double mutants or zygotic lethality of Het;Mut, Mut;Het, and Mut;Mut embryos.
Expected number was calculated with the same condition, except that the chc1 mutation does not affect viability of gametes and zygotes.
Previously, it was shown that CHC localizes at the plasma membrane and to trans-Golgi–related structures of root cells (Dhonukshe et al., 2007). We analyzed CHC subcellular localizations in the wild type and the respective chc mutants but could not find obvious differences, suggesting that CHC1 and CHC2 share subcellular localizations (see Supplemental Figure 4 online). Following the intracellular accumulation of the endocytic tracer dye FM4-64, we could find clear labeling of endosomes in the wild type and chc1-2 within 6 min (Figures 4C, 4D, and 4G). However, the intracellular accumulation of FM4-64 within the same time frame was reduced in two chc2 mutant alleles (chc2-1 and chc2-2) (Figures 4E to 4G), suggesting that the endocytosis rate was reduced in these lines, corroborating our observations with HUB induction. On the other hand, chc2 mutants showed no discernible defect in PIN1 internalization after 1 h of BFA treatment (see Supplemental Figure 5 online). This apparent discrepancy might be explained by a lower sensitivity of the BFA-based assay than that of the FM4-64 uptake assay. Alternatively, this could be indicative of a difference in endocytosis rate of PIN proteins compared with internalization of plasma membrane visualized by FM4-64. Together with our dominant-negative approach, these genetic data demonstrate that clathrin is functionally involved in endocytosis in planta. The absence of defects in chc1 and only partial defects in chc2 mutants suggest a functional redundancy between both CHC genes.
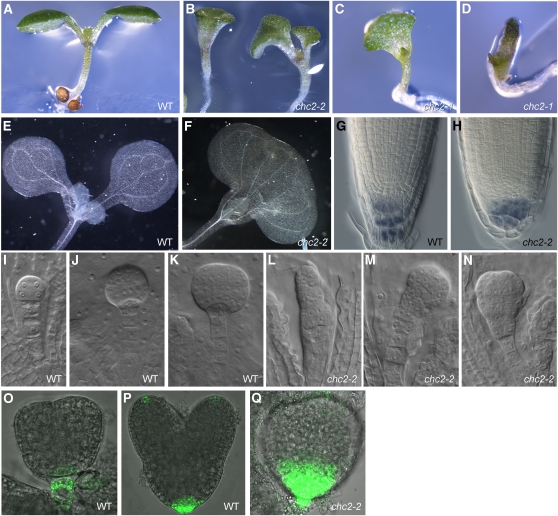
chc2 Mutants Are Defective in Embryonic and Postembryonic Development
Given the prominent morphological defects observed after HUB induction, we analyzed chc mutant development. At the seedling stage, no phenotypic alterations were obvious for chc1-1 and chc1-2 single mutants. However, in two independent mutant alleles of chc2, phenotypes reminiscent of mutants defective in polar auxin transport could be observed (Okada et al., 1991; Mayer et al., 1993; Benjamins et al., 2001; Friml et al., 2002b; Benková et al., 2003). Most prominent among them was the defect in cotyledon organogenesis in ~15 to 20% of seedlings, varying from stick-shaped and collar-shaped to trumpet-shaped cotyledons, as commonly seen in auxin transport mutants (Okada et al., 1991; Benková et al., 2003; Friml et al., 2003; Furutani et al., 2004) (Figures 5A to 5F, Table 2). Moreover, chc2 seedlings with such stick-shaped cotyledons also had other auxin transport-related phenotypes: for example, the columella was highly disorganized compared with that of the wild type (Figures 5G and 5H) (Sabatini et al., 1999; Friml et al., 2002b), and the auxin-induced formation of lateral roots was less pronounced (see Supplemental Figure 6 online) (Benková et al., 2003; Geldner et al., 2004). chc2-1/chc2-2 transheterozygotes exhibited identical developmental defects, demonstrating that the observed developmental defects are specific to chc2 mutation (see Supplemental Table 1 online).
Figure 5.
Patterning Defects of Seedlings and Embryos in the chc2 Mutants.
(A) to (D) Ten-day-old seedlings of the wild type (WT) (A) and chc2 mutants with trumpet-shaped cotyledons (B), collar-shaped cotyledons (C), and stick-shaped cotyledons (D).
(E) and (F) Vascular pattern in cotyledons of 10-d-old seedlings of the wild type (E) and monocotyledonous chc2 mutants (F).
(G) and (H) Lugol’s staining of primary root meristems of 10-d-old wild-type (G) and chc2 seedlings with stick-shaped cotyledons (H). Columella cells of these chc2 seedlings are highly disorganized.
(I) to (N) Embryonic development in the wild type ([I] to [K]) and chc2 mutants ([L] to [N]).
(O) to (Q) DR5rev:GFP expression pattern in wild-type ([O] and [P]) and chc2 (Q) embryos.
Table 2.
Frequencies of Abnormal Cotyledon Phenotypes
| Phenotype | chc2-1 | chc2-2 | chc1-2 | Wild Type |
| No fusion | 322 | 277 | 130 | 127 |
| Stick-shaped | 26 (7%) | 25 (7%) | 0 | 0 |
| Collar-shaped | 11 (3%) | 26 (7%) | 0 | 0 |
| Trumpet-shaped | 12 (3%) | 22 (6%) | 0 | 0 |
| Tricotyledon | 5 (1%) | 5 (1%) | 0 | 0 |
Percentage is in parentheses.
During embryogenesis, a subset of chc2 mutant embryos had strongly defective patterning from the globular stage onward (Figures 5I to 5N). Typically, aberrant cell division was discernible in the basal part of the mutant embryos, reminiscent of auxin transport mutants (Friml et al. 2003). Moreover, whereas wild-type embryos showed restricted DR5rev:GFP expression at the future root pole and tips of incipient cotyledons (Figures 5O and 5P) (Benková et al., 2003), mutant embryos had a broad DR5rev:GFP expression domain at their future root pole, while no expression could be observed at sites where discrete cotyledons are formed (Figure 5Q). Altogether, these findings imply that auxin transport is impaired in chc2 mutants.
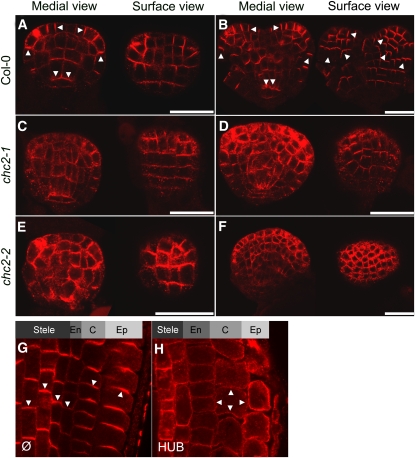
Clathrin Is Required for PIN Polarization
The subcellular polar localization of PIN proteins provides vectorial information to directional auxin transport (Wiśniewska et al., 2006). Recently, endocytosis has been proposed to be a prerequisite for the establishment of PIN polarity (Dhonukshe et al., 2008a). Hence, the observed defects in clathrin-dependent PIN endocytosis and auxin distribution prompted us to examine the polar localization of PIN proteins.
Consistent with the specific requirement of CHC2 during embryogenesis, as demonstrated by cotyledon-patterning defects and ectopic auxin accumulation, chc2 embryos showed defects in polar PIN1 localization. From the heart stage onward, PIN1 accumulated at the basal side of vascular precursor cells of wild-type embryos, while a polarity pointing toward the tips of cotyledons was observed in the epidermal cells (Figures 6A and 6B) (Benková et al., 2003). In chc2 embryos, such a bilateral localization of PIN1 was largely disrupted, with increased cytosolic background staining (Figures 6C to 6F).
Figure 6.
PIN Localization Defects Caused by Impaired CHC Function.
(A) to (F) Immunolocalization of PIN1 in globular stage embryos ([A], [C], and [E]) and in heart-stage embryos ([B], [D], and [F]) from wild-type Columbia-0 (Col-0) plants ([A] and [B]), chc2-1 plants ([C] and [D]), and chc2-2 plants ([E] and [F]). The left panels show medial view of embryos, and the right ones surface views of the same embryos in each panel.
(G) to (H) Immunolocalization of PIN1 (stele and endodermis) and PIN2 (cortex and epidermis) in roots from a INTAM driver line seedling (G) and a INTAM>>RFP-HUB1 seedling (H). Arrowheads indicate individual cellular PIN polarities. En, endodermis; C, cortex; Ep, epidermis.
To substantiate the effect on PIN polarity in a conditional situation, we examined the effects of RFP-HUB1 induction on PIN polarity in postembryonic roots. In the INTAM driver control roots, PIN1 localized to the basal (lower) side of vascular cells, whereas PIN2 localized apically (upper) in epidermal cells (Figure 6G). Induction of INTAM>>RFP-HUB1 during germination changed the polar localization of both PIN1 and PIN2 proteins into a more or less uniform apolar distribution at the plasma membrane of the cell types examined (Figure 6H). These results demonstrate that clathrin function is critical for the establishment of PIN polarity.
DISCUSSION
An Evolutionarily Conserved Clathrin-Dependent Mechanism Mediates Endocytosis in Plants
For most nonplant model organisms, the involvement of clathrin has been demonstrated in several membrane trafficking processes, most prominently in endocytosis (Seeger and Payne, 1992; Deborde et al., 2008; Kirchhausen, 2009). Although plant genomes encode molecular components of the clathrin-dependent trafficking machinery, including clathrin heavy and light chains as well as putative components of the clathrin adaptor protein machinery (Holstein, 2002; Ortiz-Zapater et al., 2006), the physiological relevance of this pathway in planta has remained unclear.
Here, we examined the consequences of genetic manipulation of clathrin function by either analyzing knockout lines for CHC genes or expressing dominant-negative CHC HUB in different tissues of the model plant Arabidopsis. Genetic interference with clathrin function not only inhibited trafficking of tested plasma membrane proteins but also impaired the uptake of the endocytic tracer dye FM4-64, highlighting the clathrin-dependent pathway as the dominant route for endocytosis in plants. These findings are consistent with observations in cultured plant cells (Tahara et al., 2007) and protoplasts (Dhonukshe et al., 2007). The functional requirement of clathrin for bulk endocytosis, the similar effects of pharmacological interference with clathrin adaptor function in different eukaryotes including plants (Ortiz-Zapater et al., 2006; Dhonukshe et al., 2007; Boutté et al., 2010), and sequence conservation of several other regulatory elements of clathrin-mediated endocytosis (Holstein, 2002) collectively suggest that the clathrin-mediated pathway represents an evolutionarily conserved mechanism for endocytosis among eukaryotes. Besides clathrin-dependent endocytosis, clathrin-independent modes of endocytosis have been found in animals (Howes et al., 2010), but it remains to be seen whether these pathways are also conserved in plants. Given the prominent effect of clathrin interference on endocytosis in plants, the other pathways, if operational in plants, are likely to have minor functions or be involved only in a subset of specific endocytic processes.
Clathrin Function Is Required for Auxin-Dependent Growth and Patterning
Clathrin function seems to be essential for plant life since lines lacking function of both CHC genes could not be recovered. Postembryonically, plants with impaired CHC function show a range of defects prominently including those indicative of defects in processes regulated by auxin.
The spatio-temporal distribution of the plant hormone auxin is of fundamental importance for plant growth and development (Vanneste and Friml, 2009). One main route for regulating local auxin accumulation is through active directional transport mediated by polarly localized PIN auxin transporters (Petrášek et al., 2006). Their subcellular localization is instructive for the direction of auxin flow (Wiśniewska et al., 2006) but is not static (Friml et al., 2002a, 2003; Benková et al., 2003; Abas et al., 2006), providing a flexible system for quickly redirecting the auxin flow. The molecular basis for dynamic relocation of PIN polarity is rapid cycling of PIN proteins between the plasma membrane and endosomal compartments (Geldner et al., 2001; Kleine-Vehn et al., 2008). We found that disruption of clathrin function impaired endocytosis of PIN proteins, causing altered patterns of auxin accumulation and increased auxin transport. Consequently, the chc loss-of-function mutants and dominant-negative CHC HUB-expressing plants exhibited various morphological defects, such as in embryonic patterning, in gravitropic growth, and in lateral root development. These phenotypes were reminiscent of defects in auxin transport as observed in gnom and multiple pin mutants and/or plants treated with auxin transport inhibitors (Friml et al., 2003; Benková et al., 2003; Geldner et al., 2004). The auxin-related phenotypes do not fully explain the defects caused by impaired clathrin-dependent endocytosis, probably because many other plasma membrane proteins depend on this pathway for endocytosis. Moreover, clathrin, besides its involvement in endocytosis, might also act in protein sorting and degradation; thus, part of the observed phenotypes might be attributed to defects in these processes. The dominant prevalence of auxin-related phenotypes is probably due to the strong impact of auxin distribution on plant growth and development and its profound dependence on constitutive endocytic recycling and retargeting of PIN auxin transporters.
Mechanistic Basis for Clathrin-Mediated Endocytosis in Regulating PIN Polarity
It is still not entirely clear what role PIN endocytic recycling has in auxin transport (Dhonukshe et al., 2008b). Besides a requirement for transcytosis-mediated changes in PIN polarity (Kleine-Vehn et al., 2008, 2009, 2010), it has been also proposed that PIN polarity is established by initial nonpolar secretion followed by PIN endocytosis and polar recycling (Dhonukshe et al., 2008a). The genetic interference with clathrin function resulted in strong defects in PIN internalization associated with a defect in PIN polarity both in embryos and in postembryonic roots. These data imply a role for clathrin-mediated endocytosis in the two-step mechanism of PIN polarity establishment. However, we cannot entirely rule out that interference of clathrin function also causes intracellular trafficking defects, independent of endocytosis but rather associated with altered polar secretion, as recently demonstrated in mammalian epithelial cells (Deborde et al., 2008).
An additional role of clathrin in polarity regulation might be related to the effect of auxin itself on clathrin-dependent PIN endocytosis (Paciorek et al., 2005; Robert et al., 2010). This mechanism accounts for a positive feedback loop of auxin on its own transport, but its role in PIN polarity establishment has not been demonstrated. Nonetheless, mathematical modeling suggests that feedback regulation of PIN endocytosis by extracellular auxin perception is capable of generating auxin-mediated cell and tissue polarity (Wabnik et al., 2010).
In conclusion, our results provide clear genetic evidence for the involvement of clathrin-dependent trafficking in the establishment of PIN polarity. We suggest the following updated model for generating and maintaining PIN polarity with clathrin-dependent PIN endocytosis integrated into a feedback circuit: (1) clathrin-dependent endocytosis, followed by polar recycling establishes polar PIN localization; (2) asymmetrically localized PIN proteins direct polar auxin transport to generate a local auxin maximum; and (3) transported auxin locally inhibits PIN endocytosis to stabilize PINs at the plasma membrane and reinforcing the PIN polarity.
Given the functional relevance of clathrin in PIN endocytosis, an important mechanistic question remains: How do PIN proteins get recruited into the clathrin-mediated pathway? In yeast and animals, cargo proteins bind to adaptors, causing conformational changes so that the cargo-adaptor complex can bind to the clathrin triskelion (Kirchhausen, 2009). Remarkably, pharmacological inhibition of Tyr motif–based sorting is effective for plant adaptin (Ortiz-Zapater et al., 2006) and for PIN internalization (Dhonukshe et al., 2007; Robert et al., 2010). Thus, it is tempting to speculate that a similar cargo-selecting mechanism may be involved in endocytosis of PIN proteins in planta, but this hypothesis awaits experimental proof.
METHODS
Plant Materials and Growth Conditions
For analysis of seedling phenotypes, growth condition of plants was described elsewhere (Tanaka et al., 2009). The transactivation line (J0571) and T-DNA insertion lines were obtained from the Nottingham Arabidopsis Stock Centre: chc1-1 (SALK_112213), chc1-2 (SALK_103252), chc2-1 (SALK_028826), and chc2-2 (SALK_042321) (Alonso et al., 2003). INTAM>>RFP-CHC1 (HUB) (Robert et al., 2010) and DR5rev:GFP (Friml et al., 2003) have been described previously. Genotypes of all insertion lines were confirmed by PCR and further analyzed by RT-PCR. The primers used for genotyping were 112213RP and 112213LP for chc1-1, P3 and 103252LP3 for chc1-2, 28826RP and 28826LP for chc2-1, and 042321LP2 and 042321RP3 for chc2-2 (see Supplemental Table 2 online). The primers used for RT-PCR were P1, P2, P3, P4, P5, P6, P7, and P8 (see Supplemental Table 2 online). The tubulin primers were as described (Semiarti et al., 2001).
Drug Treatment, Immunodetection, and Confocal Microscopy
Treatment with BFA (Molecular Probes), labeling with FM4-64 (Molecular Probes), and immunodetection were as described previously (Sauer et al., 2006) using the following antibody dilutions: anti-PIN1 (1:1000) (Paciorek et al., 2005), anti-PIN2 (1:1000) (Abas et al., 2006), anti-CHC (1:1000) (Dhonukshe et al., 2007), anti-PM-ATPase (1:1000) (Geldner et al., 2001), anti-ARF1 (1:1000) (Tanaka et al., 2009), anti-rabbit-Alexa488 (1:600) (Invitrogen), and anti-rabbit-Cy3 (1:600) (Sigma-Aldrich). Stock concentrations of 10 mM 4-hydroxytamoxifen (Sigma-Aldrich) were prepared in dimethylsulfoxide and diluted 5000-fold in half-strength Murashige and Skoog medium for inducing of INTAM:GAL4 expression. Confocal images were taken with SP2 (Leica), FV1000 (Olympus), or LSM5 Exciter (Zeiss) confocal microscopes.
Quantification of FM4-64 Uptake
The mean pixel intensity of the cytosolic side of cells, excluding the plasma membrane, and the mean pixel intensity of the adjacent plasma membrane was measured with ImageJ (http://rsbweb.nih.gov/ij/), and the quotients of values between the inside and the plasma membrane were calculated. The value of each genotype was standardized to the corresponding wild-type control to evaluate the percentage of endocytosis inhibition compared with a wild-type condition (Robert et al., 2010).
Phenotype Analysis
Three-day-old seedlings were transferred to media containing 5 μM 1-naphthaleneacetic acid. After 2 d of auxin treatment, roots were cleared (Malamy and Benfey, 1997) and analyzed with differential interference contrast (Olympus BX51). Vascular patterns in cotyledons of 10-d-old seedlings were observed by dark-field illumination (Leica MZ16). Prior to observation, the cotyledons were cleared as following. Chlorophyll was removed from cotyledons of 10-d-old seedlings by overnight incubation in ethanol (100%) followed by mounting on slides in lactic acid (70%) (Acros Organics). Starch granules in the root tips were stained with Lugol’s solution for 1 to 2 min and then mounted on slides with chloral hydrate and analyzed immediately with differential interference contrast (Olympus BX51).
Auxin Transport Assay
Acropetal auxin transport was measured in 8-d-old plants after the modified method of Rashotte et al. (2000). Twenty-four hours before the assay started, the seedlings were transferred on half-strength Murashige and Skoog plates supplemented with 2 μM 4-hydroxytamoxyfen (Sigma-Aldrich) or an equal amount of solvent (DMSO). Warm agar at 1.5% (v/v) was mixed with 200 nM 3-[5(n)-3H]IAA (26 Ci/mmol; Amersham), 100 nM cold indole-3-acetic acid (IAA), and DMSO at a final concentration of 0.2% (v/v). Narrow stem glass transfer pipettes were used to excise small cylinders out of the hardened agar mixture. Such agar cylinders were applied at the junction between the root and hypocotyl. The IAA transport was measured in vertically oriented plants after 18 h in the dark (to minimize IAA degradation) at 22°C. Subsequently, the first 1 cm of the root after the [3H]IAA source was discarded, and the remaining root segment was used for analysis. Each root sample was placed into a mixture of 10 mL scintillation fluid (Lumasafe plus; Luma-LSA) and 1 mL water. Radioactivity was measured for 10 min using a scintillation counter (model Tri-Carb 2800TR; Perkin-Elmer). The experiment was repeated independently three times.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: CLC1 (At2g40060), CLC2 (At2g20760), CLC3 (At3g51890), CHC1 (At3g11130), and CHC2 (At3g08530).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Quantification of FM4-64 Uptake after HUB Induction.
Supplemental Figure 2. HUB Transactivation in Cortex and Endodermis.
Supplemental Figure 3. Effect of 1 DOI HUB on PIN2 Polarity.
Supplemental Figure 4. CHC Subcellular Localization in Mutants and 1 DOI HUB.
Supplemental Figure 5. BFA Bodies in chc Mutants.
Supplemental Figure 6. Auxin-Induced Lateral Root Formation in chc2 Mutants.
Supplemental Table 1. Number of chc2-1/chc2-2 Transheterozygous Plants.
Supplemental Table 2. Primer Sequences for Genotyping and RT-PCR.
Acknowledgments
We thank Christian Luschnig (Institute for Applied Genetics and Cell Biology, Austria), David G. Robinson (Universität Heidelberg, Germany), Inhwan Hwang (Pohang University of Science and Technology, Korea), and Wolfgang Michalke (Universität Freiburg, Germany) for kindly sharing materials, the Nottingham Arabidopsis Stock Centre for seed stocks, the Laboratory for Radioisotopes Goettingen for technical support, Ellie Himschoot for technical assistance, Jürgen Kleine-Vehn for critical reading of the manuscript, and Martine De Cock for help in preparing it. This work was supported by the Odysseus program of Research Foundation-Flanders. S.K. and H.T. were recipients of postdoctoral fellowships of the Japan Society for the Promotion of Science. S.V. is grateful to the European Molecular Biology Organization for a long-term fellowship (ATLF 142-2007) and is a postdoctoral fellow of the Research Foundation-Flanders.
References
- Abas L., Benjamins R., Malenica N., Paciorek T., Wiśniewska J., Moulinier-Anzola J.C., Sieberer T., Friml J., Luschnig C. (2006). Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat. Cell Biol. 8: 249–256 Erratum. Nat. Cell Biol. 8: 424 [DOI] [PubMed] [Google Scholar]
- Alonso J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Baluška F., Hlavacka A., Šamaj J., Palme K., Robinson D.G., Matoh T., McCurdy D.W., Menzel D., Volkmann D. (2002). F-actin-dependent endocytosis of cell wall pectins in meristematic root cells. Insights from brefeldin A-induced compartments. Plant Physiol. 130: 422–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamins R., Quint A., Weijers D., Hooykaas P.J., Offringa R. (2001). The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development 128: 4057–4067 [DOI] [PubMed] [Google Scholar]
- Benková E., Michniewicz M., Sauer M., Teichmann T., Seifertová D., Jürgens G., Friml J. (2003). Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 [DOI] [PubMed] [Google Scholar]
- Blackbourn H.D., Jackson A.P. (1996). Plant clathrin heavy chain: Sequence analysis and restricted localisation in growing pollen tubes. J. Cell Sci. 109: 777–786 [DOI] [PubMed] [Google Scholar]
- Boutté Y., Frescatada-Rosa M., Men S., Chow C.M., Ebine K., Gustavsson A., Johansson L., Ueda T., Moore I., Jürgens G., Grebe M. (2010). Endocytosis restricts Arabidopsis KNOLLE syntaxin to the cell division plane during late cytokinesis. EMBO J. 29: 546–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky F.M., Chen C.Y., Knuehl C., Towler M.C., Wakeham D.E. (2001). Biological basket weaving: formation and function of clathrin-coated vesicles. Annu. Rev. Cell Dev. Biol. 17: 517–568 [DOI] [PubMed] [Google Scholar]
- Cram W.J. (1980). Pinocytosis in plants. New Phytol. 84: 1–17 [Google Scholar]
- Deborde S., Perret E., Gravotta D., Deora A., Salvarezza S., Schreiner R., Rodriguez-Boulan E. (2008). Clathrin is a key regulator of basolateral polarity. Nature 452: 719–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhonukshe P., Aniento F., Hwang I., Robinson D.G., Mravec J., Stierhof Y.D., Friml J. (2007). Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr. Biol. 17: 520–527 [DOI] [PubMed] [Google Scholar]
- Dhonukshe P., Baluska F., Schlicht M., Hlavacka A., Samaj J., Friml J., Gadella T.W., Jr (2006). Endocytosis of cell surface material mediates cell plate formation during plant cytokinesis. Dev. Cell 10: 137–150 [DOI] [PubMed] [Google Scholar]
- Dhonukshe P., et al. (2008b). Auxin transport inhibitors impair vesicle motility and actin cytoskeleton dynamics in diverse eukaryotes. Proc. Natl. Acad. Sci. USA 105: 4489–4494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhonukshe P., et al. (2008a). Generation of cell polarity in plants links endocytosis, auxin distribution and cell fate decisions. Nature 456: 962–966 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Fotin A., Cheng Y., Sliz P., Grigorieff N., Harrison S.C., Kirchhausen T., Walz T. (2004). Molecular model for a complete clathrin lattice from electron cryomicroscopy. Nature 432: 573–579 [DOI] [PubMed] [Google Scholar]
- Friml J., Benková E., Blilou I., Wiśniewska J., Hamann T., Ljung K., Woody S., Sandberg G., Scheres B., Jürgens G., Palme K. (2002b). AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell 108: 661–673 [DOI] [PubMed] [Google Scholar]
- Friml J., Vieten A., Sauer M., Weijers D., Schwarz H., Hamann T., Offringa R., Jürgens G. (2003). Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426: 147–153 [DOI] [PubMed] [Google Scholar]
- Friml J., Wiśniewska J., Benková E., Mendgen K., Palme K. (2002a). Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415: 806–809 [DOI] [PubMed] [Google Scholar]
- Fujimoto M., Arimura S., Ueda T., Takanashi H., Hayashi Y., Nakano A., Tsutsumi N. (2010). Arabidopsis dynamin-related proteins DRP2B and DRP1A participate together in clathrin-coated vesicle formation during endocytosis. Proc. Natl. Acad. Sci. USA 107: 6094–6099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furutani M., Vernoux T., Traas J., Kato T., Tasaka M., Aida M. (2004). PIN-FORMED1 and PINOID regulate boundary formation and cotyledon development in Arabidopsis embryogenesis. Development 131: 5021–5030 [DOI] [PubMed] [Google Scholar]
- Geldner N., Friml J., Stierhof Y.D., Jürgens G., Palme K. (2001). Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413: 425–428 [DOI] [PubMed] [Google Scholar]
- Geldner N., Hyman D.L., Wang X., Schumacher K., Chory J. (2007). Endosomal signaling of plant steroid receptor kinase BRI1. Genes Dev. 21: 1598–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldner N., Richter S., Vieten A., Marquardt S., Torres-Ruiz R.A., Mayer U., Jürgens G. (2004). Partial loss-of-function alleles reveal a role for GNOM in auxin transport-related, post-embryonic development of Arabidopsis. Development 131: 389–400 [DOI] [PubMed] [Google Scholar]
- Grebe M., Xu J., Möbius W., Ueda T., Nakano A., Geuze H.J., Rook M.B., Scheres B. (2003). Arabidopsis sterol endocytosis involves actin-mediated trafficking via ARA6-positive early endosomes. Curr. Biol. 13: 1378–1387 [DOI] [PubMed] [Google Scholar]
- Holstein S.E. (2002). Clathrin and plant endocytosis. Traffic 3: 614–620 [DOI] [PubMed] [Google Scholar]
- Howes M.T., Mayor S., Parton R.G. (2010). Molecules, mechanisms, and cellular roles of clathrin-independent endocytosis. Curr. Opin. Cell Biol. 22: 519–527 [DOI] [PubMed] [Google Scholar]
- Ivanov R., Gaude T. (2009). Endocytosis and endosomal regulation of the S-receptor kinase during the self-incompatibility response in Brassica oleracea. Plant Cell 21: 2107–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelínková A., Malínská K., Simon S., Kleine-Vehn J., Parezová M., Pejchar P., Kubeš M., Martinec J., Friml J., Zazímalová E., Petrásek J. (2010). Probing plant membranes with FM dyes: Tracking, dragging or blocking? Plant J. 61: 883–892 [DOI] [PubMed] [Google Scholar]
- Kirchhausen T. (2000). Clathrin. Annu. Rev. Biochem. 69: 699–727 [DOI] [PubMed] [Google Scholar]
- Kirchhausen T. (2009). Imaging endocytic clathrin structures in living cells. Trends Cell Biol. 19: 596–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine-Vehn J., Dhonukshe P., Sauer M., Brewer P.B., Wiśniewska J., Paciorek T., Benková E., Friml J. (2008). ARF GEF-dependent transcytosis and polar delivery of PIN auxin carriers in Arabidopsis. Curr. Biol. 18: 526–531 [DOI] [PubMed] [Google Scholar]
- Kleine-Vehn J., Ding Z., Jones A.R., Tasaka M., Morita M.T., Friml J. (2010). Gravity-induced PIN transcytosis for polarization of auxin fluxes in gravity-sensing root cells. Proc. Natl. Acad. Sci. USA 107: 22344–22349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine-Vehn J., Huang F., Naramoto S., Zhang J., Michniewicz M., Offringa R., Friml J. (2009). PIN auxin efflux carrier polarity is regulated by PINOID kinase-mediated recruitment into GNOM-independent trafficking in Arabidopsis. Plant Cell 21: 3839–3849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.H., Marks M.S., Brodsky F.M. (1998). A dominant-negative clathrin mutant differentially affects trafficking of molecules with distinct sorting motifs in the class II major histocompatibility complex (MHC) pathway. J. Cell Biol. 140: 1023–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.H., Wong M.L., Craik C.S., Brodsky F.M. (1995). Regulation of clathrin assembly and trimerization defined using recombinant triskelion hubs. Cell 83: 257–267 [DOI] [PubMed] [Google Scholar]
- Lukowitz W., Gillmor C.S., Scheible W.R. (2000). Positional cloning in Arabidopsis. Why it feels good to have a genome initiative working for you. Plant Physiol. 123: 795–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luschnig C., Gaxiola R.A., Grisafi P., Fink G.R. (1998). EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev. 12: 2175–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy J.E., Benfey P.N. (1997). Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124: 33–44 [DOI] [PubMed] [Google Scholar]
- Marsh M., Helenius A. (2006). Virus entry: Open sesame. Cell 124: 729–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer U., Büttner G., Jürgens G. (1993). Apical-basal pattern formation in the Arabidopsis embryo: Studies on the role of the gnom gene. Development 117: 149–162 [Google Scholar]
- McNiven M.A., Thompson H.M. (2006). Vesicle formation at the plasma membrane and trans-Golgi network: The same but different. Science 313: 1591–1594 [DOI] [PubMed] [Google Scholar]
- Mukherjee S., Ghosh R.N., Maxfield F.R. (1997). Endocytosis. Physiol. Rev. 77: 759–803 [DOI] [PubMed] [Google Scholar]
- Okada K., Ueda J., Komaki M.K., Bell C.J., Shimura Y. (1991). Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 3: 677–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Zapater E., Soriano-Ortega E., Marcote M.J., Ortiz-Masiá D., Aniento F. (2006). Trafficking of the human transferrin receptor in plant cells: Effects of tyrphostin A23 and brefeldin A. Plant J. 48: 757–770 [DOI] [PubMed] [Google Scholar]
- Ottenschläger I., Wolff P., Wolverton C., Bhalerao R.P., Sandberg G., Ishikawa H., Evans M., Palme K. (2003). Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc. Natl. Acad. Sci. USA 100: 2987–2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciorek T., Zazímalová E., Ruthardt N., Petrásek J., Stierhof Y.D., Kleine-Vehn J., Morris D.A., Emans N., Jürgens G., Geldner N., Friml J. (2005). Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435: 1251–1256 [DOI] [PubMed] [Google Scholar]
- Petrášek J., et al. (2006). PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 312: 914–918 [DOI] [PubMed] [Google Scholar]
- Rashotte A.M., Brady S.R., Reed R.C., Ante S.J., Muday G.K. (2000). Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol. 122: 481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robatzek S., Chinchilla D., Boller T. (2006). Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes Dev. 20: 537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert S., et al. (2010). ABP1 mediates auxin inhibition of clathrin-dependent endocytosis in Arabidopsis. Cell 143: 111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D.G., Jiang L., Schumacher K. (2008). The endosomal system of plants: charting new and familiar territories. Plant Physiol. 147: 1482–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russinova E., Borst J.W., Kwaaitaal M., Caño-Delgado A., Yin Y., Chory J., de Vries S.C. (2004). Heterodimerization and endocytosis of Arabidopsis brassinosteroid receptors BRI1 and AtSERK3 (BAK1). Plant Cell 16: 3216–3229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini S., Beis D., Wolkenfelt H., Murfett J., Guilfoyle T., Malamy J., Benfey P., Leyser O., Bechtold N., Weisbeek P., Scheres B. (1999). An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99: 463–472 [DOI] [PubMed] [Google Scholar]
- Sauer M., Balla J., Luschnig C., Wiśniewska J., Reinöhl V., Friml J., Benková E. (2006). Canalization of auxin flow by Aux/IAA-ARF-dependent feedback regulation of PIN polarity. Genes Dev. 20: 2902–2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer J.K., Burke E.E., Goodson H.V., D’Souza-Schorey C. (2005). Endocytosis resumes during late mitosis and is required for cytokinesis. J. Biol. Chem. 280: 41628–41635 [DOI] [PubMed] [Google Scholar]
- Seeger M., Payne G.S. (1992). A role for clathrin in the sorting of vacuolar proteins in the Golgi complex of yeast. EMBO J. 11: 2811–2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semiarti E., Ueno Y., Tsukaya H., Iwakawa H., Machida C., Machida Y. (2001). The ASYMMETRIC LEAVES2 gene of Arabidopsis thaliana regulates formation of a symmetric lamina, establishment of venation and repression of meristem-related homeobox genes in leaves. Development 128: 1771–1783 [DOI] [PubMed] [Google Scholar]
- Sousa E., Kost B., Malhó R. (2008). Arabidopsis phosphatidylinositol-4-monophosphate 5-kinase 4 regulates pollen tube growth and polarity by modulating membrane recycling. Plant Cell 20: 3050–3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter J.U., Sieben C., Hartel A., Eisenach C., Thiel G., Blatt M.R. (2007). Abscisic acid triggers the endocytosis of the Arabidopsis KAT1 K+ channel and its recycling to the plasma membrane. Curr. Biol. 17: 1396–1402 [DOI] [PubMed] [Google Scholar]
- Swarup R., Friml J., Marchant A., Ljung K., Sandberg G., Palme K., Bennett M. (2001). Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes Dev. 15: 2648–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara H., Yokota E., Igarashi H., Orii H., Yao M., Sonobe S., Hashimoto T., Hussey P.J., Shimmen T. (2007). Clathrin is involved in organization of mitotic spindle and phragmoplast as well as in endocytosis in tobacco cell cultures. Protoplasma 230: 1–11 [DOI] [PubMed] [Google Scholar]
- Takano J., Miwa K., Yuan L., von Wirén N., Fujiwara T. (2005). Endocytosis and degradation of BOR1, a boron transporter of Arabidopsis thaliana, regulated by boron availability. Proc. Natl. Acad. Sci. USA 102: 12276–12281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano J., Tanaka M., Toyoda A., Miwa K., Kasai K., Fuji K., Onouchi H., Naito S., Fujiwara T. (2010). Polar localization and degradation of Arabidopsis boron transporters through distinct trafficking pathways. Proc. Natl. Acad. Sci. USA 107: 5220–5225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S., Gapper C., Kaya H., Bell E., Kuchitsu K., Dolan L. (2008). Local positive feedback regulation determines cell shape in root hair cells. Science 319: 1241–1244 [DOI] [PubMed] [Google Scholar]
- Tanaka H., Kitakura S., De Rycke R., De Groodt R., Friml J. (2009). Fluorescence imaging-based screen identifies ARF GEF component of early endosomal trafficking. Curr. Biol. 19: 391–397 [DOI] [PubMed] [Google Scholar]
- Van Damme D., Gadeyne A., Vanstraelen M., Inzé D., Van Montagu M.C.E., De Jaeger G., Russinova E., Geelen D. (2011). Adaptin-like protein TPLATE and clathrin recruitment during plant somatic cytokinesis occurs via two distinct pathways. Proc. Natl. Acad. Sci. USA 108: 615–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S., Friml J. (2009). Auxin: A trigger for change in plant development. Cell 136: 1005–1016 [DOI] [PubMed] [Google Scholar]
- Wabnik K., Kleine-Vehn J., Balla J., Sauer M., Naramoto S., Reinöhl V., Merks R.M.H., Govaerts W., Friml J. (2010). Emergence of tissue polarization from synergy of intracellular and extracellular auxin signaling. Mol. Syst. Biol. 6: 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiśniewska J., Xu J., Seifertová D., Brewer P.B., Růžička K., Blilou I., Rouquié D., Benková E., Scheres B., Friml J. (2006). Polar PIN localization directs auxin flow in plants. Science 312: 883. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Yan A., Feijó J.A., Furutani M., Takenawa T., Hwang I., Fu Y., Yang Z. (2010). Phosphoinositides regulate clathrin-dependent endocytosis at the tip of pollen tubes in Arabidopsis and tobacco. Plant Cell 22: 4031–4044 [DOI] [PMC free article] [PubMed] [Google Scholar]