Abstract
Background
Exhaled breath studies suggest that humans exhale fine particles during tidal breathing, but little is known of their physical origin in the respiratory system during health or disease.
Methods
Particles generated by 3 healthy and 16 human rhinovirus (HRV)-infected subjects were counted using an optical particle counter with nominal diameter-size bins ranging between 0.3 and 10 μm. Data were collected from HRV-infected subjects during tidal breathing. In addition, data from healthy subjects were collected during coughs, swallows, tidal breathing, and breathing to total lung capacity (TLC) and residual volume (RV). Using general additive models, we graphed exhaled particle concentration versus airflow during exhalation. Exhaled particles were collected from expired air on gelatin filters and analyzed for HRV via quantitative PCR.
Results
HRV-infected subjects exhaled from 0.1 to 7200 particles per liter of exhaled air during tidal breathing (geometric mean = 32 part/L). A small fraction (24%) of subjects exhaled most (81%) of the particles measured and 82% of particles detected were 0.300–0.499 μm. Minute ventilation, maximum airflow during exhalation, and forced expiratory volume 1 second (FEV1 % predicted) were positively correlated with particle production. No human rhinovirus was detected in exhaled breath samples. Three healthy subjects exhaled less than 100 particles per liter of exhaled air during tidal breathing and increased particle concentrations more with exhalation to RV than with coughing, swallowing, or rapid exhalation.
Conclusions
Submicron particles were detected in the exhaled breath of healthy and HRV-infected subjects. Particle concentrations were correlated with airflow during the first half of exhalation, and peaked at the end of exhalation, indicating both lower and upper airways as particle sources. The effect of breathing maneuver suggested a major contribution from lower airways, probably the result of opening collapsed small airways and alveoli.
Key words: exhaled breath, aerosols, particles, human rhinovirus
Introduction
Studies of exhaled breath suggest that humans generate fine particles during tidal breathing but little is known of their origin in the respiratory system. Older studies of exhaled breath primarily detected particles larger than 1 μm due to less sensitive techniques, including counting particles in photographs of coughs and sneezes,(1) culturing of indicator bacteria exhaled and impacted on plates,(2) and counting slides or filters of exhaled dye droplets under a microscope.(2,3) In these studies, particles were rarely detected in breath exhaled during tidal breathing, but were detected during coughs and sneezes. As more recent studies of healthy subjects have shown, particles are also produced during tidal breathing, and approximately 98% of these particles are under 1 μm.(4–7) Particle concentrations reported in these studies span several logs: one study measured particle concentrations between 14 and 3230 particles per liter,(6) and another reported concentrations between 20 and 400 particles per liter.(8) In our previous study of subjects infected with influenza, we found that they produced 67 to 8500 particles per liter of air and that 87% of the particles were under 1 μm.(9)
Droplets can be generated by shear forces produced by air flow acting on the airway lining fluid and entraining particles composed of mucus, surfactant, and pathogens,(10,11) especially during cough.(12) It has also been hypothesized that droplets are produced from the destabilization of the lining fluid during the reopening of collapsed small airways and alveoli during breathing.(6) Recently, another study found that exhaled particle concentrations increased 4- to 18-fold when inhaling deeply and rapidly after a deep exhalation, hypothesizing that the opening of airways and alveoli blocked by fluid during inhalation is a significant source of particles.(13) Identifying the origin of these particles is important when interpreting studies of exhaled breath biomarkers, including cytokines,(14–19) metals,(20–22) and pathogens such as viruses(9,23) and bacteria.(24)
This report describes a study of exhaled particles in the setting of human rhinovirus (HRV) experimental infection, and includes adjacent experiments on the effect of breathing maneuvers on exhaled particle number and size distribution in healthy subjects.
Materials and Methods
Exhaled breath particles from HRV-infected subjects
Location and subject recruitment
Subjects were recruited from a study population at the University of Wisconsin Madison where they were investigating differences in outcomes for asthmatics and nonasthmatics experimentally infected with HRV. Nineteen of the 38 subjects (asthmatic and nonasthmatic) recruited for the parent study participated in our exhaled breath study. At screening, subjects underwent a physical examination, allergy skin prick testing, blood draw for RV16-neutralizing antibody, a urine pregnancy test, and pulmonary function tests before and after inhalation of albuterol. Details of the outcomes study have been published.(25) Protocols were reviewed and approved by the Harvard School of Public Health and the University of Wisconsin Institutional Review Boards for Human Subject Protection. Informed consent was obtained from all patients after the study goals and procedures were explained in detail.
Exhaled breath collection
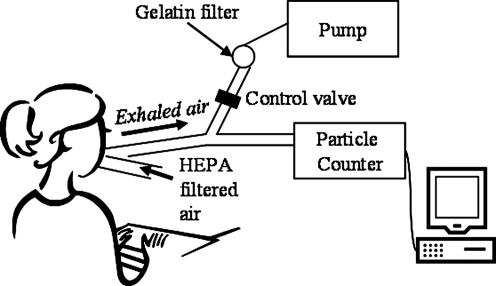
Exhaled breath measurements were conducted on the fifth day following HRV inoculation. The collection system was a prototype of the Exhalair (Pulmatrix Inc., Lexington, MA), and consisted of a mouthpiece, particle counter, airflow meter, collection filter, and computer control system. Another Exhalair prototype was previously used to measure exhaled breath aerosols in healthy subjects(6) and the Exhalair was used to measure particles in influenza infected subjects.(9) Particle counts, relative humidity, and temperature were recorded every 0.33 sec with a Climet CI-550 (Climet Instruments Company, Redlands, CA) optical particle counter. Particle counts were stored cumulatively in six channels with the following nominal diameters: >0.3, >0.5, >1, >3, >5, and >10 μm. Airflow measurements were recorded every 0.004 sec. The dead space between the mouthpiece and the particle counter was approximately 0.14 L and generated a time lag of 0.292 sec between airflow measurements and particle detection. Airflow and particle measurements were lined up by starting the first recorded airflow measurement at 0.292 sec. Figure 1 shows a schematic of a subject at the sampling setup.
FIG. 1.
Schematic of volunteer breathing into exhaled breath collection system. Subject inhaled HEPA-filtered room air and exhaled into (1) an optical particle counter, or (2) a gelatin filter for subsequent human rhinovirus analysis. For both branches the tubing diameter was 22 mm and the total tubing length was 36.8 cm.
Subjects were instructed to breathe normally through a mouthpiece during sampling and to stop the test if they had to sneeze, cough, or otherwise interrupt regular tidal breathing. If subjects stopped for any reason the sampling was repeated. Each subject breathed into the particle counter for 3 min while wearing a nose clip and without removing his/her mouth from the sampling mouthpiece. Minute ventilation was calculated by summing the total breath volume exhaled and dividing by the total sampling time.
Ambient particle washout time from the lungs was estimated by analysis of a graph of particle concentration versus time and selecting the time period it took for concentrations to drop to constant levels. After the ambient particle washout time, each subject's particle concentration and airflow graphs were scrutinized for peaks indicating leaks or deviations from tidal breathing. Leaks were identified when particle concentrations increased to levels similar to those observed during the ambient particle washout time (exhaled breath particle concentrations were generally several orders of magnitude lower) and airflow patterns deviated from those observed during tidal breathing. Data from these time periods were eliminated from the regular tidal breathing analysis. Other criteria for eliminating data were particle concentration peaks caused by respiratory events thought to be swallows or deep breaths. These irregular breathing patterns were detected by looking for abnormal inhalation–exhalation airflow cycles and particle counts. Once the tidal breathing data was quality checked for respiratory events other than tidal breathing, particle concentrations were graphed during inhalation and exhalation to check for leaks, assuming particles should only be measured during exhalation.
Human rhinovirus quantification
After the particle measurements were made, exhaled breath particles were collected on single-use 37-mm gelatin filters (Sartorius, Goettingen, Germany), previously used to sample airborne human influenza viruses.(26) A Medo pump (model VP0625, Medo USA, Hanover Park, IL) maintained airflow at 28.3 Lpm. Subjects were instructed to wear a nose clip and breathe for 20 min through the mouthpiece while all exhaled particles were collected on the filter. After collection, gelatin filters were shipped on dry ice to the University of Massachusetts Lowell and stored at −80°C until analyzed.
In the laboratory, gelatin filters were dissolved at room temperature in 800 μL of nuclease-free water (Promega, Madison, WI) and split into 400-μL aliquots. HRV RNA was extracted from the aliquots using Trizol-chloroform extraction, reverse transcription, and quantitative PCR protocols as previously described,(27) and the RNA obtained was suspended in a final volume of 20 μL. We could detect a minimum of 24 virus particles per PCR well, and the overall limit of detection was 192 virus particles per gelatin filter.
Exhaled breath particle measurements in healthy volunteers
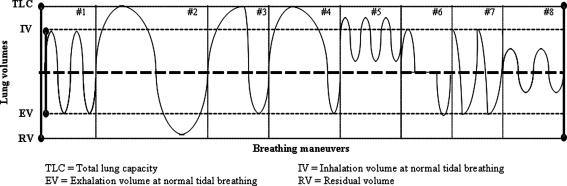
Three healthy volunteers from the University of Massachusetts Lowell community were recruited to study the effect of breathing maneuvers on total exhaled breath particle concentrations. Subjects breathed into the Exhalair system, which measured particles using an optical particle counter and recorded measurements in four channels with the following nominal diameters: 0.300–0.499, 0.500–0.999, 1.000–4.999, and >5.000 μm, and the Exhalair software calculated particle concentrations. Subjects breathed through a mouthpiece while wearing a nasal clip to prevent ambient air leakage. Following a washout period during which subjects inhaled and exhaled deeply to remove residual environmental particles in the lungs, subjects were instructed to perform the breathing maneuvers pictured in Figure 2.
FIG. 2.
Schematic of lung volumes attained while performing breathing maneuvers, where #1: tidal breathing, #2: TLC to RV with slow exhalation, #3: TLC to tidal volume with fast exhalation, #4: TLC to tidal volume with slow exhalation, #5: high volume tidal breath (panting), #6: swallowing, #7: coughing, #8: short breaths.
Data and statistical analysis
Exhaled breath particle concentrations from the healthy volunteer study were plotted over time using Excel (Microsoft Corporation, Redmond, WA). For the HRV infection study, Excel was used to compute average airflows, as well as inhaled and exhaled volumes. Particle concentrations were computed by dividing the particle counts recorded every 0.3 sec by the inhalation/exhalation volume. For time increments that contained both inhalation and exhalation values (end or beginning of a breath), only exhalation volumes were calculated and it was assumed that all particles counted in this time originated during exhalation. Particle and airflow data files were analyzed using macros written in Visual Basic 6.3 (Microsoft Corporation) for Excel. Regression analyses were performed using R software version 2.4.1 (The R Foundation for Statistical Computing, Vienna, Austria). Generalized additive models (GAM)(28) with a negative binomial link were used to evaluate the relationship between particle concentration and airflow for each subject. GAMs allow the relationship between the covariates and the response to follow a smooth curve. This curve is fit with a spline which lets the data determine its shape.(28) The negative binomial link allows for the model to fit data which is overdispersed relative to a Poisson distribution (i.e., mean less than the variance), as well as allowing analysis of data sets with a large proportion of zeros which cannot be approximated by a lognormal distribution.(29) The GAM models were fitted using R software with the mgcv library developed by Simon Wood.(30) Smoothed plots of particles versus exhaled volume and airflow were generated for each subject using Lowess smoothers (locally weighted polynomial regression) in R.
Results
Exhaled breath particles from HRV-infected subjects
Summary statistics
A total of 19 subjects were enrolled in the HRV exhaled breath study: 7 asthmatics (37%) and 12 nonasthmatics; 79% of subjects were female and 90% were under the age of 25. Complete exhaled breath particle count data were obtained from 17 of the 19 subjects enrolled. Data collected from two subjects were eliminated because of incorrect nose clip use, which contaminated the exhaled breath particle counts with environmental aerosols. Incorrect nose clip use was detected by looking for an irregular airflow pattern over time. Gelatin filters for HRV analysis were collected from 18 of the 19 subjects (16 of the 17 with particle data).
Exhaled breath particle counts
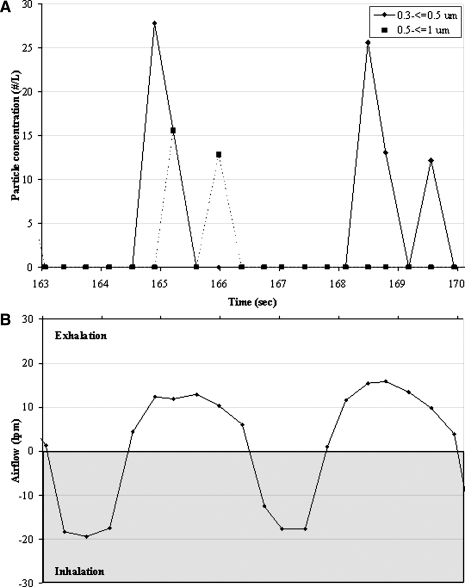
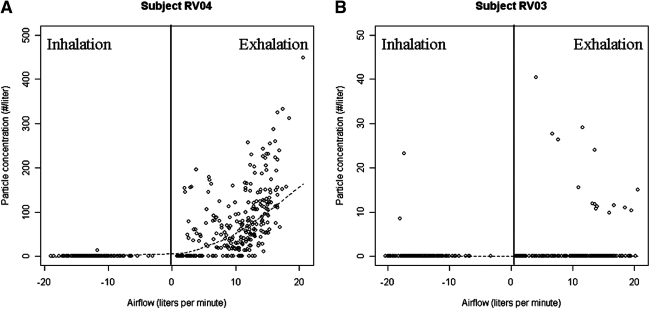
Figure 3A presents a typical particle concentration profile over two tidal breaths for one subject, and Figure 3B presents the corresponding airflow measured simultaneously. Particle concentrations during tidal breathing ranged between 0.2 and 7200 particles per liter [geometric mean (GM) = 32 particles per liter] in this study population. Minute ventilation ranged between 5.4 and 9.1 Lpm (GM = 6.8 Lpm), ambient particle washout time between 5 and 115 sec (GM = 34 sec), breathing frequency between 9 and 29 breaths/min (GM = 17 breaths/min), and maximum exhaled airflow from 14 to 21 Lpm (GM = 17 Lpm). On average, 16% of particle data was eliminated due to leaks or particle concentration peaks due to deviations from tidal breathing. Figure 4 shows an example of the graphs used to check for ambient air leakage during tidal breath sampling. Figure 4A and B shows a high (HHPs) and low particle producer (LPPs), respectively, where each point on the graph represents a breath. HPPs were defined as those that exhaled ≥500 particles per liter of air, based on a classification proposed by Edwards et al.(6) During exhalation, the mouthpiece was under positive pressure, and thus leakage contamination could only have occurred during inhalation. As is shown in the graphs, particles in inspired air were rarely recorded, and thus ambient air contamination was not present. In the rare instance that particles were recorded, the levels were orders of magnitude lower than ambient concentrations. These graphs are representative of the data used for all subjects.
FIG. 3.
Particle concentrations and airflow measured over two breaths from one HRV infected subject. (A) 0.3 to <0.5 μm and 0.5 to ≤1 μm particle concentration exhaled over time. (B) Airflow measurements over time. Each dot represents one measurement.
FIG. 4.
Particles exhaled versus airflow during normal breathing from two subjects infected with human rhinovirus during normal breathing. (A) Concentration of 0.3 to ≤0.5 μm particles versus airflow for a high particle producer, and (B) concentration of 0.3 to ≤0.5 μm particles versus airflow for a low particle producer. High particle producers were defined as those that exhaled ≥500 particles per liter of air. Regression lines were calculated using a Lowess smoother. Note: y-scale in figures not equal between subjects. Each point on the graph represents one breath.
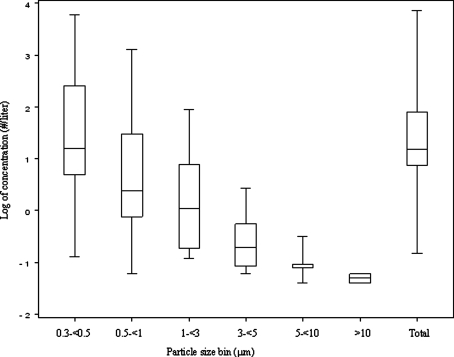
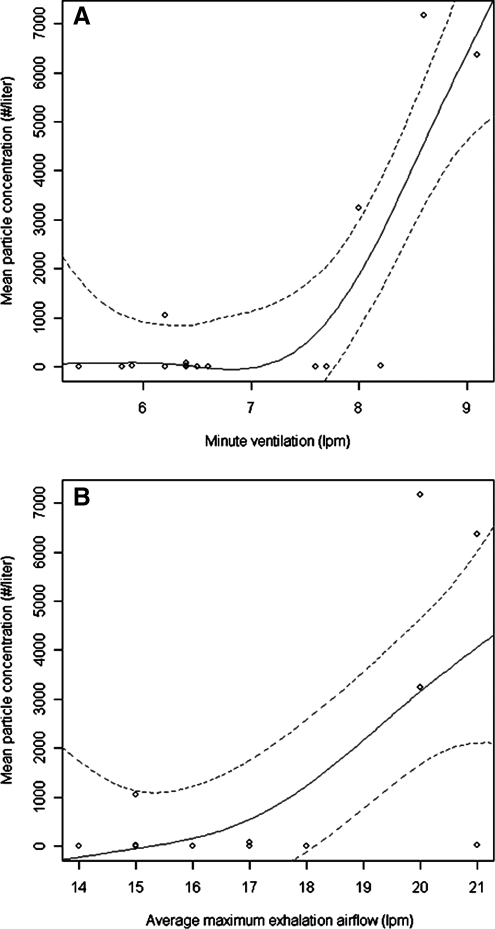
Figure 5 presents a box plot of the particle concentrations measured in each particle size bin across all subjects after ambient particle washout. Over 82% of particles exhaled were measured in the 0.300–0.499 μm bin and particles were rarely detected above 3 μm. Four of the 16 subjects were HPPs and their characteristics are presented in Table 1. Minute ventilation, maximum airflow during exhalation and FEV1 % predicted were significantly associated with high particle production. Figure 6A and B presents regression results showing constant particle concentrations at lower minute ventilation (<8 Lpm) and flow (<19 Lpm) values, and high particle concentrations at higher values.
FIG. 5.
Average exhaled breath particle concentrations by size bin and total across all HRV infected subjects (n = 17).
Table 1.
Characteristics of High and Low Particle Producers in HRV-Infected Population
| High particle producersa | Low particle producers | |
|---|---|---|
| Geometric mean particle concentration (#/L) | 3500 | 7.4 |
| Number of subjects | 4 | 13 |
| Female | 75% | 85% |
| Asthmatic | 50% | 38% |
| Age <20 years | 0% | 15% |
| 20–25 years | 75% | 85% |
| >25 years | 25% | 0% |
| Respiratory characteristicsb | ||
| Minute ventilation (lpm) | 8 (1.3)* | 7 (0.8) |
| Ambient particle washout time (sec) | 37 (42) | 44 (27) |
| Maximum airflow during exhalation (lpm) | 19 (2.7)* | 16 (1.9) |
| Breath frequency (#/min) | 20 (6.4) | 16 (4.6) |
| FEV1c | 4.1 (0.4) | 3.6 (0.5) |
| FEV1c % predicted | 109 (10.6)* | 92.7 (10.7) |
High particle producers defined as subjects that exhale ≥500 part/L during tidal breathing.
Averages for each characteristic. Values in parenthesis are 1 standard deviation.
FEV1 = maximum amount of air that can be exhaled in 1 second.
Statistically significant differences evaluated by calculating a relative risk for categorical variables and a Student's t-test for continuous variables (p < 0.05).
FIG. 6.
Concentration of exhaled breath particles ≤10 μm versus (A) minute ventilation and (B) maximum exhalation airflow for all HRV-infected subjects during tidal breathing (n = 17). Univariate regression lines and 95% confidence intervals were calculated using generalized additive models.
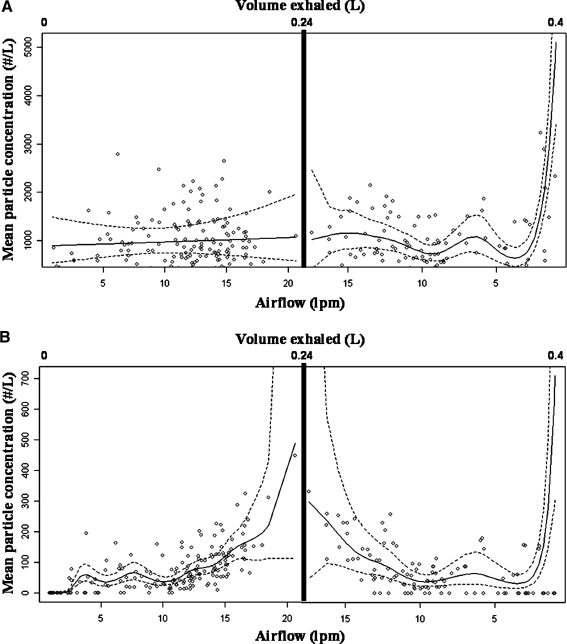
Figure 7A shows a graph of the regression model results superimposed on the particle concentration data for particles 0.300–0.499 μm over exhaled volume and airflow for one HPP. In order to model particle concentrations over the entire course of exhalation, two smooth functions were fitted to each subject, splitting the exhalation data at the maximum airflow. Particle concentrations increased as the airflow increased from 0 to ∼15 Lpm, then leveled as airflow continued to increase, and increased again as airflow declined at the end of the breath. This pattern was followed by all four HPPs independent of the amount of air exhaled. Figure 7B shows 0.500–0.999 μm particles behaved similarly: as airflow increased, particles increased, particle concentrations peaked at the maximum airflow, and dropped as airflow decreased. There was a second rise in particle production at the end of the breath, but it was not as pronounced for 0.500–0.999 μm particles as for the smaller particles. For LPPs, the Lowess smoother regression lines centered around zero. We observed almost identical trends in all 13 LPPs.
FIG. 7.
Concentration of (A) 0.3 to <0.5 μm and (B) 0.5 to <1 μm exhaled breath particles versus airflow from one high particle producer in HRV study. Regression lines and 95% confidence intervals were calculated using two generalized additive models, pre- and postmaximum airflow peak. Note: y-scale in figures not equal.
Human rhinovirus RNA on gelatin filters
Human rhinovirus infection was confirmed for all subjects via nasal lavage; all demonstrated productive viral infection. Nevertheless, all samples of exhaled breath particles collected on gelatin filters were negative for human rhinovirus RNA, indicating that the number of viruses collected was lower than the method limit of detection (192 virus copies per gelatin filter) or not present in the particles we collected. The HRV samples included as positive controls were all detected.
Exhaled breath particle measurements in healthy volunteers
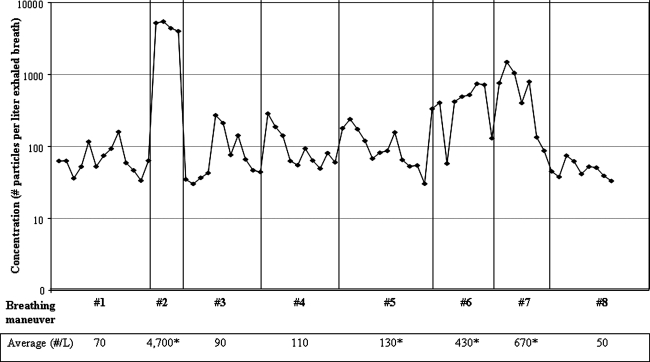
We sampled two males and one female; all were nonasthmatic nonsmokers, with ages 33 to 55. Particle generation rates during tidal breathing averaged between 43 and 70 particles/liter between subjects. Figure 8 shows particle concentrations measured in one subject while following the breathing maneuvers pictured in Figure 2. Exhaled breath particle concentrations from all three subjects followed similar trends: slight increases in particle production during maneuvers such as panting and swallowing, increases up to 10-fold during coughs, and a 10- to 70-fold increase when the subject exhaled to residual volume (RV) prior to inhalation to total lung capacity (TLC).
FIG. 8.
Exhaled breath particle concentrations measured from one healthy subject during breathing maneuvers #1 through #8 pictured in Figure 2. Each dot represents one breath. The table presents the average particle concentrations measured over a number of breaths for each maneuver. An * indicates particle concentrations that were significantly higher to those measured during tidal breathing.
Discussion
In this two-part study of exhaled breath particles, we found that in healthy volunteers a 70-fold increase in particle concentration occurred when subjects exhaled to RV after inhaling to TLC, compared to tidal breathing (Fig. 8). These increased particle concentrations were observed over a number of consecutive TLC to RV breaths. Other breathing maneuvers evaluated, including panting, swallowing, and coughing, also increased particle concentrations but to a lesser extent (maximum 10-fold). Rapid exhalation from TLC to end tidal volume only modestly increased particle concentrations. Johnson and Morawska(13) reported that subjects who exhaled deeply increased particle production four- to sixfold. The difference in measurements is likely due to the lung volume achieved prior to exhaling; in the Johnson study subjects inhaled to normal end tidal lung volume prior to exhaling to RV, while in our study subjects inhaled to TLC prior to exhaling to RV. It has been hypothesized that the source of particles is the opening of the small airways during inhalation,(6,8,13) which is consistent with results from both studies.
Exhaled breath particle measurements in HRV infected subjects were restricted to tidal breathing. Subjects exhaled between 0.2 and 7200 particles per liter of exhaled air (GM = 32 part/L) and 24% of the subjects exhaled over 81% of the particles detected. These subjects were classified as HPPs and exhaled between 1100 and 7200 particles per liter of air (GM = 3500 part/L). LPPs exhaled between 0.1 and 80 total particles per liter (GM = 7.4 part/L). For LPPs, 78% of the particles were detected in the 0.300–0.499 μm size range, and for HPPs this percentage increased to 82%. These numbers agree with data reported in other exhaled breath studies.(4–7) Fairchild and Stampfer(4) observed particles between 0.1 and 3 μm exhaled during nose and mouth breathing, and reported a geometric mean concentration of 230 particles per liter during tidal breathing averaged over five study subjects. Over 98% of the particles they measured were under 1 μm. No individual particle production information was given in the Fairchild study, but the high geometric mean concentration and their graph of data suggests that at least one of the five subjects was an HPP.(4) Papineni et al.(5) measured particles between 0.3 and 8.0 μm during mouth breathing and nose breathing, and found that over 84% of particles exhaled were under 1 μm. Differences between nose breathing and mouth breathing were small, but nose breathing produced the lowest number of particles.(5) Edwards et al.(6) measured particles between 0.15 and 0.5 μm exhaled during mouth breathing. They did not report a geometric mean, but most particles were measured between 0.15 to 0.199 μm and 54% of subjects were HPPs. In our previous study of influenza subjects, 50% were HPPs.(9)
Significant predictors of high particle production included minute ventilation (MV), maximum airflow measured during exhalation (Q), and FEV1 expressed as % predicted (Table 1). MV and Q are linearly correlated (correlation = 0.96), but MV predicted particle concentration the best (R2MV = 0.74 vs. R2Airflow = 0.43). Breath frequency did not predict high particle production (p-value = 0.20) nor was it correlated with MV (correlation = 0.26). Because minute ventilation equals the product of breath frequency and tidal volume, we concluded that high particle concentrations are correlated with higher tidal volumes and thus a greater probability of opening closed structures as well as corresponding higher expiratory flow rates. Although the proportion of asthmatics was higher among HPPs (50%) compared to LPPs (38%), we did not have the statistical power to identify an association between exhaled particles and asthma. Larger studies of HPPs are necessary to determine whether asthma plays a role in particle production, particularly during HRV infection.
In infected HPPs, we detected particles at all exhalation volumes during tidal breathing, indicating that some particles may originate in the upper airways or the mouth. Particle concentrations in the 0.300–0.499 μm size fraction (Fig. 7A) were positively correlated with airflow at the beginning of the breath, but continued increasing at the end of exhalation even as airflow decreased to almost zero. Particle concentrations for particles between 0.5 and 1 μm (Fig. 7B) had a better correlation with airflow compared to the 0.300–0.499 μm particles (Fig. 7A), and although the 0.500–0.999 μm particle concentrations increased slightly at the end of the breath, the trend was not as marked as with 0.300–0.4999 μm particles. These trends were observed in all HPPs and indicate two sources of particles: one related to airflow and the second related to the location of the measured breath (and thus the anatomic location of where the aerosols were originally generated).
The peak in particle concentration at the end of an exhalation supports the hypothesis that the particles measured at the end of the exhaled breath are generated during the previous inhalation by the reopening of collapsed small airways or alveoli which destabilize the lung fluid lining and create droplets.(6) Further support for this hypothesis is the significant positive relationship we found between minute ventilation and exhaled particle concentrations (Fig. 6A). Minute ventilation can increase with increasing tidal volume or increased breath frequency.(31) In our study, breath frequency was not related to particle concentration or correlated to minute ventilation (correlation = 0.26); thus, HPPs likely breathed with larger tidal volumes. High particle concentrations were a likely a product of increased alveolar ventilation, either by opening more airways during inhalation or by exhaling more alveolar air. Larger tidal and minute volumes have also been associated with an increased volume of collected exhaled breath condensate.(32) In LPPs, particle concentrations were mostly nondetectable.
Results from this study contradict previous studies that state that particles are not produced in the upper airways during mouth breathing.(2,8) One probable reason is that older studies of exhaled breath particles used techniques that could only measure particles larger than 1 μm, such as counting slides or filters of exhaled dyed droplets under a microscope.(2,3) Large droplet saliva contamination is unlikely because our apparatus transported exhaled breath from the mouthpiece to the particle counter through a 0.75-m long and 22-mm diameter corrugated tube, likely settling any saliva droplets. Another explanation is that our subjects had a respiratory infection, whereas in the older studies only healthy individuals were studied. It is possible that the mucus present due to infection decreased the airway cross-sectional area, thus increasing the shear velocity enough to create aerosols. Studies of mucus properties have shown that changes in the mucus depth and the subjacent more aqueous phase as well as viscosity modify the velocity at which mucus can be destabilized, allowing particle formation at velocities of 5 m/sec(11) and that particles can be generated from crests of waves that are formed by mucus piling up as air passes through the airways.(10) It is possible that particle production in healthy HPPs and those with viral infection occurs via different mechanisms. The importance of HPPs in this context is that they may be superspreaders: “those infrequently encountered persons with high values of cough and/or sneeze frequency, elevated pathogen concentration in respiratory fluid, and/or increased respirable aerosol volume per expiratory event such that their pathogen emission rate is much higher than average.”(33) If the small airways of these high particle producers were infected with a virus—human rhinoviruses are between 24 and 30 nm in diameter(34,35) and influenza viruses range between 80 and 120 nm in diameter(36)—they would be more likely to generate infectious aerosols. Larger studies of infected and noninfected subjects over time are needed to determine if infection and resulting mucus hypersecretion plays a role in particle production.
None of the exhaled breath filters were positive for human rhinovirus RNA, likely due to our limit of detection (192 virus particles/gelatin filter). Assuming no other particle losses through the system prior to particle collection, and a respiratory rate of 7 Lpm over the 20-min sampling time, this limit corresponds to approximately two virus particles per liter of exhaled air or 576 virus particles per hour. A previous study reported potential infectious quanta generation rates for human rhinovirus between 0.6 and 7.8 h−1, where an infectious quantum is the infectious dose needed to initiate disease and a quantum can contain a number of virus copies.(37) Adding breathing maneuvers such as cough might also increase the probability of detecting HRV. A study by Huynh et al.(23) detected human rhinovirus and parainfluenza virus via PCR from infected patients who coughed and breathed through masks made with electret. In contrast to our study, virus detection in the Huynh study may have been due to an increase in particle generation during coughs or the capture of larger particles that our sampling device excluded.
Several studies have addressed the limitations associated with using optical particle counters to correctly size and count particles, as the refractive index of the OPC calibration latex particles is different from that of ambient particles.(38,39) Measurements made with the OPC can overestimate or underestimate by up to a factor of 2, and depend on particle size and shape, as well as particle composition and location of solids within a liquid droplet when measuring particles in solution.(38) For ambient aerosols this error appears to be greatest for particles around 0.6 μm and is negligible for particles larger than 1.35 μm.(39) The OPC we used for this study was calibrated by the manufacturer with latex particles and its measurements were likely affected by these errors. The data were analyzed as collected because of the unknown refractive index of dried out exhaled breath particles as well as the low number of particles found larger than 0.5 μm. The analysis of the origin of the particles from the respiratory tract remains unaffected.
The collection system had some limitations. The tubing connecting the mouthpiece to the sampler and particle counter was long enough to eliminate by sedimentation many particles larger than 5 μm. Another limitation was the difficulty in making sure subjects maintained a seal when breathing through the mouthpiece during the 20-min collection period. Leaks at the mouthpiece and failure to use the nose clip were detected by presence of high particle counts approaching ambient levels. Leaks occurring during exhalation were detected because the OPC pump pulled at a flow that was greater than the subject's exhalation flow, thus directing the contaminated room air to the OPC. Although it seems unlikely that leaks would only have occurred during inhalation and gone undetected, it is theoretically possible that such leaks, if they existed, would have mimicked particle generation by the lung and that such leaks would have been greater during rapid inhalation. An improved exhaled breath collection system should have a mouthpiece or collection system that collects aerosols both by nose and mouth breathing, does not restrict breath airflow, prevents leaks, and is comfortable. It should also minimize breath condensation and particle deposition.
Future studies should include subjects with a variety of lung diseases [e.g., asthma and chronic obstructive pulmonary disease (COPD)], and more varied demographic characteristics such as increasing age in order to determine other predictors of particle production. Elderly subjects and those with COPD usually have reduced lung compliance and thus increased likelihood of airway closure. Studies of infectious disease transmission and exhaled breath particles should focus on diseases of the lower respiratory tract with a higher infectious particle generation rate or on infections that cause cough such as influenza. For studies of exhaled breath biomarkers, modifying breathing patterns to include low lung volumes will likely increase particle production and thus detection sensitivity.
In conclusion, we found that healthy subjects generated 10- to 70-fold higher exhaled particle concentrations when taking deep breaths and exhaling to residual volume, compared to concentrations generated during tidal breathing, supporting the hypothesis that fine particles are produced during the opening of collapsed small airways and alveoli. In HRV-infected subjects a small fraction (24%) of subjects exhaled most (>81%) of the particles measured, and were labeled HPPs. High particle production was significantly associated with larger minute ventilation, maximum airflow during exhalation, and FEV1 % predicted.
In HPPs with HRV infection, the pattern of exhaled particles versus airflow and versus exhaled volume during tidal breathing followed similar curves: increasing with airflow at the beginning of the breath and peaking at the end of the breath when the airflow was low. These results indicate that particles originate both in the upper and lower airways. In contrast, exhaled particle concentrations generated by low particle producers were mostly undetectable. The exhaled breath particles collected on gelatin filters were negative for HRV RNA in HRV-infected subjects, but our limit of detection was too high to exclude the possibility that clinically relevant virus concentrations were present.
Acknowledgments
This work has received financial support from the National Institutes of Health (grants NIH HL07118 and NIH AI061884) and the U.S. Centers for Disease Control and Prevention (cooperative grant #1U01CI000446-01). Although the NIH and CDC have sponsored this project, they neither endorse nor reject the findings of this research.
Author Disclosure Statement
No conflicts of interest exist.
References
- 1.Jennison MW. Aerobiology Publication. Washington, DC: American Association for the Advancement of Science; Atomizing of Mouth And Nose Secretions into the Air as Revealed by High-Speed Photography; p. 106. [Google Scholar]
- 2.Duguid J. The numbers and the sites of origin of the droplets expelled during expiratory activities. Edinburgh Med J. 1945;52:385–401. [PMC free article] [PubMed] [Google Scholar]
- 3.Loudon RG. Roberts RM. Droplet expulsion from the respiratory tract. Am Rev Respir Dis. 1967;95:435–442. doi: 10.1164/arrd.1967.95.3.435. [DOI] [PubMed] [Google Scholar]
- 4.Fairchild CI. Stampfer JF. Particle concentration in exhaled breath. Am Ind Hyg Assoc J. 1987;48:948–949. doi: 10.1080/15298668791385868. [DOI] [PubMed] [Google Scholar]
- 5.Papineni RS. Rosenthal FS. The size distribution of droplets in the exhaled breath of healthy human subjects. J Aerosol Med. 1997;10:105–116. doi: 10.1089/jam.1997.10.105. [DOI] [PubMed] [Google Scholar]
- 6.Edwards DA. Man JC. Brand P. Katstra JP. Sommerer K. Stone HA. Nardell E. Scheuch G. Inhaling to mitigate exhaled bioaerosols. Proc Natl Acad Sci USA. 2004;101:17383–17388. doi: 10.1073/pnas.0408159101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morawska L. Johnson GR. Ristovski ZD. Hargreaves H. Mengersen K. Corbett S. Chao CYH. Li Y. Katoshevski Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J Aerosol Sci. 2008;40:256–269. [Google Scholar]
- 8.Gebhart J, et al. The human lung as aerosol particle generator. J Aerosol Med. 1988;1:196–197. [Google Scholar]
- 9.Fabian P. McDevitt JJ. DeHaan WH. Fung ROP. Cowling BJ. Chan KH. Leung GM. Milton DK. Influenza virus in human exhaled breath: an observational study. PLoS ONE. 2008;3:e2691. doi: 10.1371/journal.pone.0002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King M. Brock G. Lundell C. Clearance of mucus by simulated cough. J Appl Physiol. 1985;58:1776–1782. doi: 10.1152/jappl.1985.58.6.1776. [DOI] [PubMed] [Google Scholar]
- 11.Moriarty JA. Grotberg JB. Flow-induced instabilities of a mucus-serous bilayer. J Fluid Mech. 1999;397:1–22. [Google Scholar]
- 12.Leith D, et al. In: Handbook of Physiology, The Respiratory System. 1. Cough , editor; Macklem MJ, editor. III. Bethesda, MD: American Physiological Society; 1986. pp. 315–336. Section 3. [Google Scholar]
- 13.Johnson GR. Morawska L. The mechanism of breath aerosol formation. J Aerosol Med Pulm Drug Deliv. 2009;22:229–237. doi: 10.1089/jamp.2008.0720. [DOI] [PubMed] [Google Scholar]
- 14.Shahid SK. Kharitonov SA. Wilson NM. Bush A. Barnes PJ. Increased interleukin-4 and decreased interferon-gamma in exhaled breath condensate of children with asthma. Am J Respir Crit Care Med. 2002;165:1290–1293. doi: 10.1164/rccm.2108082. [DOI] [PubMed] [Google Scholar]
- 15.Garey KW. Newhauser MM. Robbins RA. Danziger LH. Rubinstein I. Markers of inflammation in exhaled breath condensate of young healthy smokers. Chest. 2004;125:22–26. doi: 10.1378/chest.125.1.22. [DOI] [PubMed] [Google Scholar]
- 16.Rosias PP. Dompeling E. Dentener MA. Pennings HJ. Hendriks HJ. Van Iersel MP. Jöbsis Q. Childhood asthma: exhaled markers of airway inflammation, asthma control score, and lung function tests. Pediatr Pulmonol. 2004;38:107–114. doi: 10.1002/ppul.20056. [DOI] [PubMed] [Google Scholar]
- 17.Carpagnano GE. Resta O. Foschino-Barbaro MP. Gramiccioni E. Carpagnano F. Interleukin-6 is increased in breath condensate of patients with non-small cell lung cancer. Int J Biol Markers. 2002;17:141–145. doi: 10.1177/172460080201700211. [DOI] [PubMed] [Google Scholar]
- 18.Leung TF. Wong GW. Ko FW. Lam CW. Fok TF. Increased macrophage-derived chemokine in exhaled breath condensate and plasma from children with asthma. Clin Exp Allergy. 2004;34:786–791. doi: 10.1111/j.1365-2222.2004.1951.x. [DOI] [PubMed] [Google Scholar]
- 19.Rosias P. Robroeks C. Hendriks J. Dompeling E. Jöbsis Q. Exhaled breath condensate: a space odessey, where no one has gone before. Eur Respir J. 2004;24:189–190. doi: 10.1183/09031936.04.00025404. ; author reply 190. [DOI] [PubMed] [Google Scholar]
- 20.Broding HC. Michalke B. Göen T. Drexler H. Comparison between exhaled breath condensate analysis as a marker for cobalt and tungsten exposure and biomonitoring in workers of a hard metal alloy processing plant. Int Arch Occup Environ Health. 2009;82:565–573. doi: 10.1007/s00420-008-0390-5. [DOI] [PubMed] [Google Scholar]
- 21.Goldoni M. Caglieri A. Corradi M. Poli D. Rusca M. Carbognani P. Mutti A. Chromium in exhaled breath condensate and pulmonary tissue of non-small cell lung cancer patients. Int Arch Occup Environ Health. 2008;81:487–493. doi: 10.1007/s00420-007-0242-8. [DOI] [PubMed] [Google Scholar]
- 22.Mutti A. Corradi M. Goldoni M. Vettori MV. Bernard A. Apostoli P. Exhaled metallic elements and serum pneumoproteins in asymptomatic smokers and patients with COPD or asthma. Chest. 2006;129:1288–1297. doi: 10.1378/chest.129.5.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huynh KN. Oliver BG. Stelzer S. Rawlinson WD. Tovey ER. A new method for sampling and detection of exhaled respiratory virus aerosols. Clin Infect Dis. 2008;46:93–95. doi: 10.1086/523000. [DOI] [PubMed] [Google Scholar]
- 24.Fennelly KP. Martyny JW. Fulton KE. Orme IM. Cave DM. Heifets LB. Cough-generated aerosols of Mycobacterium tuberculosis: a new method to study infectiousness. Am J Respir Crit Care Med. 2004;169:604–609. doi: 10.1164/rccm.200308-1101OC. [DOI] [PubMed] [Google Scholar]
- 25.DeMore JP. Weisshaar EH. Vrtis RF. Swenson CA. Evans MD. Morin A. Hazel E. Bork JA. Kakumanu S. Sorkness R. Busse WW. Gern JE. Similar colds in subjects with allergic asthma and nonatopic subjects after inoculation with rhinovirus-16. J Allergy Clin Immunol. 2009;124:245–52. doi: 10.1016/j.jaci.2009.05.030. , 252 e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fabian P. McDevitt JJ. Houseman EA. Milton DK. Airborne influenza virus detection with four aerosol samplers using molecular and infectivity assays: considerations for a new infectious virus aerosol sampler. Indoor Air. 2009;19:433–441. doi: 10.1111/j.1600-0668.2009.00609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fabian P. McDevitt JJ. Lee WM. Houseman EA. Milton DK. An optimized method to detect influenza virus and human rhinovirus from exhaled breath and the airborne environment. J Environ Monit. 2009;11:314–317. doi: 10.1039/b813520g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hastie T. Tibshirani R. Generalized Additive Models. New York: CRC; 1990. [DOI] [PubMed] [Google Scholar]
- 29.Houseman EA. Coull BA. Shine JP. A nonstationary negative binomial time series with time-dependent covariates: Enterococcus counts in Boston Harbor. J Am Stat Assoc. 2006;101:1365–1376. [Google Scholar]
- 30.Wood SN. Modeling and smoothing parameter estimation with multiple quadratic penalties. JR Stat Soc (B) 2000;62:413–428. [Google Scholar]
- 31.West B. Respiratory Physiology—The Essentials. 5th. Baltimore, MD: Williams & Wilkins; 1995. p. 193. [Google Scholar]
- 32.Liu J. Thomas PS. Relationship between exhaled breath condensate volume and measurements of lung volumes. Respiration. 2007;74:142–145. doi: 10.1159/000094238. [DOI] [PubMed] [Google Scholar]
- 33.Nicas M. Nazaroff W. Hubbard A. Toward understanding the risk of secondary airborne infection: emission of respirable pathogens. J Occup Environ Hyg. 2005;2:143–153. doi: 10.1080/15459620590918466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacDowell AL. Bacharier LB. Infectious triggers of asthma. Immunol Allergy Clin North Am. 2005;25:45–66. doi: 10.1016/j.iac.2004.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pitkaranta A. Hayden FG. Rhinoviruses: important respiratory pathogens. Ann Med. 1998;30:529–537. doi: 10.3109/07853899709002600. [DOI] [PubMed] [Google Scholar]
- 36.Stanley WM. The size of influenza virus. J Exp Med. 1944;79:267–283. doi: 10.1084/jem.79.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rudnick SN. Milton DK. Risk of indoor airborne infection transmission estimated from carbon dioxide concentration. Indoor Air. 2003;13:237–245. doi: 10.1034/j.1600-0668.2003.00189.x. [DOI] [PubMed] [Google Scholar]
- 38.Pinnick RG. Pendleton JD. Videen G. Response Characteristics of the Particle Measuring Systems Active Scattering Aerosol Spectrometer Probes. London: Taylor & Francis; 2000. pp. 334–352. [Google Scholar]
- 39.Liu Y. Daum PH. The effect of refractive index on size distributions and light scattering coefficients derived from optical particle counters. J Aerosol Sci. 2000;31:945–957. [Google Scholar]