Abstract
CD4 Th are critical for orchestrating adaptive immune responses. The expression of the transcription factor GATA3 (GATA-binding protein 3) is up-regulated or down-regulated during Th2 or Th1 cell differentiation, respectively. Furthermore, GATA3 is responsible for induction of Th2 differentiation and represses Th1 differentiation. In this review, we present an updated view on the molecular mechanisms through which GATA3 regulates Th1/Th2 differentiation. During Th2 cell differentiation, GATA3 directly binds to the Th2 cytokine gene locus at several regions and regulates expression. On the other hand, GATA3 inhibits Th1 cell differentiation by preventing up-regulation of IL-12 receptor β2 and STAT4 (signal transducer and activator of transcription 4) and neutralization of Runx3 (runt-related transcription factor 3) function through protein–protein interaction. GATA3 may also directly act on the Ifng gene. In summary, GATA3 serves as a transcriptional activator or repressor through direct action on transcriptional machinery and/or affecting chromatin remodeling at many critical loci encoding cytokines, cytokine receptors, signaling molecules as well as transcription factors that are involved in the regulation of Th1 and Th2 differentiation.
Keywords: cytokine, epigenetic modification, gene regulation
Introduction
Naive CD4 T cells have the potential to differentiate into several alternative cell types of which the most intensively studied are Th1 and Th2 cells (1). When innate immune cells recognize invasion of intracellular pathogens such as protozoa, bacteria or viruses, naive CD4 T cells differentiate into Th1 cells, which secrete IFN-γ, IL-2 and tumor necrosis factor-β. Th1 cells activate macrophages and CD8 T cells leading, under the right set of circumstances, to pathogen eradication.
On the other hand, when innate immune cells recognize extracellular parasites such as helminths, the naive CD4 T cells differentiate into Th2 cells, which secrete IL-4, IL-5 and IL-13. Th2 cells activate B cells to induce immunoglobulin class switching and epithelial cells to enhance their mucus production; Th2 cells also recruit mast cells and eosinophils to infection sites to aid in the clearance of parasites. Inappropriate activation of Th1 or Th2 cells to self-antigens or to harmless foreign antigens may cause autoimmune or allergic diseases. Thus, understanding the molecular mechanisms underlying the differentiation of naive CD4 T cells into Th1 or Th2 cells is of considerable importance.
Th1/Th2 differentiation is regulated by the cytokine milieu at the time of TCR engagement. This milieu is created under the influence of the particular pathogens as well as the dose of antigens and the genetic background of the host (2, 3). When naive CD4 T cells are activated through their TCR together with IL-12- or IL-4-mediated signaling, these cells differentiate into Th1 or Th2 cells, respectively. IL-12-stimulated CD4 T cells up-regulate the expression of the transcription factor T-bet (T-box expressed in T cells) and acquire their capability to produce IFN-γ; activated CD4 T cells that receive IL-4 signaling up-regulate GATA3 (GATA-binding protein 3) and become capable of producing Th2 cytokines. Indeed, cytokine-mediated up-regulation of these lineage-specific transcription factors determines CD4 T-cell fate.
Not only is GATA3 indispensable for Th2 differentiation and Th2 cytokine production, it is also essential for inhibition of Th1 differentiation and IFN-γ production. Here, we discuss the role of GATA3 in regulating Th1 and Th2 differentiation and the molecular mechanisms involved.
Functions of GATA3 in regulating Th2 cell differentiation
GATA3 expression is necessary for the development of CD4 single-positive (SP) cells in the thymus (4, 5). It continues to be expressed in naive CD4 T cells at a basal level. When naive CD4 T cells are activated under Th1- or Th2-skewing conditions, GATA3 is either down-regulated or up-regulated. GATA3 up-regulation is induced by IL-4–STAT6-mediated signaling (6, 7). A low dose but not high dose of antigen stimulation through TCR results in IL-4-independent GATA3 up-regulation and IL-4 production (8). Enforced expression of GATA3 has also been reported to up-regulate endogenous GATA3 expression (9). GATA3 is regarded as the master regulator to induce Th2 differentiation (10, 11) since enforced GATA3 expression induces Th2 differentiation even when the cells are cultured under Th1-skewing conditions (12) and GATA3-deficient ‘Th2’ cells fail to produce IL-4, IL-5 and IL-13 (13, 14).
It has been reported that histone modifications, such as histone H3 lysine 4 (H3K4) methylation and H3K14 acetylation, are induced at the Th2 cytokine gene locus (which includes the genes for IL-4, IL-13 and IL-5) during Th2 differentiation (15). These histone modifications change the chromatin structure so that the modified regions become accessible to transcription factors and are associated with DNase I hypersensitive (HS) sites. Chromatin remodeling at the Th2 cytokine gene locus is necessary for efficient expression of IL-4, IL-5 and IL-13 in Th2 cells and it has been proposed that GATA3 regulates chromatin remodeling.
GATA3 has been reported to bind to several regulatory elements at the Th2 cytokine gene locus (Fig. 1) including conserved non-coding sequence (CNS)-1, HSVa, the conserved GATA response element (CGRE), the Il5 promoter and HSII in intron 2 of the Il4 gene (16–20).
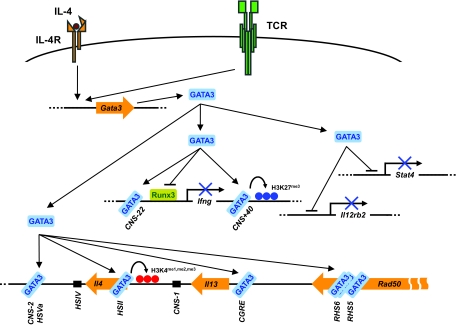
Fig. 1.
Molecular mechanisms of GATA3-mediated regulation of Th1 and Th2 cell differentiation. GATA3 expression can be induced by TCR and/or IL-4-mediated signaling. GATA3 induces Th2 cytokine expression through its direct binding to the Il4/Il13 gene locus at several sites including HSII, HSVa, CGRE as well as RHS5 and RHS6 in the LCR. GATA3 can remodel the chromatin structure of this gene locus such as induction of H3K4 methylation; GATA3 may also directly act on the Il13 gene to induce transcription. GATA3 inhibits Th1 cell differentiation by at least three distinct mechanisms: inhibition of IL-12Rβ2 and STAT4 expression, suppression of Runx3-mediated IFN-γ production and silencing the Ifng gene by adding H3K27me3 suppressive marks. Our GATA3 ChIPseq data suggest that GATA3 directly binds to Stat4, Il12rb2 and Ifng loci (24).
CNS-1, which includes two HS sites, HSS1 and HSS2, is located at the intergenic region of the Il4 and Il13 genes (21). A mobility shift assay showed that GATA3 binds to HSS2 (16, 17). In mice from which the 0.5-kbp genomic DNA segment containing the CNS-1 region was deleted, isolated CD4 T cells that were cultured under Th2-skewing conditions had diminished numbers of IL-4-producing cells and the mean fluorescence intensity of the IL-4 producers was lower (22). These mutant mice also produced less IL-4 in vivo (22). Interestingly, bone marrow-derived mast cells from these CNS-1−/− mice produced normal levels of IL-4, consistent with the observation that in mast cells, HS sites were not found in CNS-1 (23). Although the CNS-1 region appears to be critical for IL-4 production in Th2 cells, our genome-wide GATA3 ChIPseq (chromatin immunoprecipitation followed by high-throughput sequencing) data did not reveal significant GATA3 binding to CNS-1 (24), suggesting that this region recruits other critical transcription factors that promote IL-4 production in Th2 cells.
HSVa is located 5-kbp downstream of the 3′ end of the Il4 coding region; its DNase I HS is induced in Th2 cells upon re-stimulation (18). Both GATA3 and NFAT1 (nuclear factor of activated T cells 1) bind to HSVa in Th2 but not Th1 clones (18). ‘Th2’ cells generated from mice in which a 3.7-kbp region including HSVa and HSV (CNS-2) was deleted had reduced IL-4 production (25). Our GATA3 ChIPseq data confirmed GATA3 binding to HSVa (24), implying that HSVa is an important regulatory element through which GATA3 induces IL-4 production in stimulated Th2 cells.
CGRE (a 71-bp sequence) is located 1.6-kbp upstream of the Il13 gene and contains four putative GATA-binding sequences conserved across species (19). GATA3 binding to CGRE is confirmed by our GATA3 ChIPseq analysis. Since CGRE corresponds to the 5′ edge of the region of histone hyperacetylation in the Th2 cytokine gene locus and to the site to which RNA polymerase II and CBP/p300, containing histone acetyltransferase activity, bind (19), it may play an important role in Il13 transcription and in chromatin remodeling at the Il13 locus. Actually Th2 cells generated from CGRE-deleted mice have diminished IL-13 but not IL-4 and IL-5 production (20).
Our GATA3 ChIPseq data revealed a strong binding of GATA3 to the HSII site located at intron 2 of the Il4 gene (24). Previously, we have reported that activated STAT5 binds to HSII and that STAT5 is important for the maintenance of DNA accessibility at this region in Th2 cells (26, 27). Most recently, Kubo et al. have reported that deletion of a 1.4-kbp genomic DNA region including HSII resulted in a diminution of IL-4 but not IL-13 production (20). This implies that the HSII site is a critical element for regulating IL-4 but not IL-13 production. Strong H3K4 trimethylation (H3K4me3) was observed at HSII in Th2 but not Th1 cells (28), suggesting that GATA3 and STAT5 may collaborate to remodel the chromatin at this region. Indeed, our unpublished data showed that H3K4me1, me2 and me3 at HSII site is reduced in Gata3-deleted cells cultured under Th2-skewing conditions.
GATA3 also strongly binds to three sites in the locus control region (LCR) of the Th2 cytokine locus within the Rad50 gene: one at Rad50 HS5 (RHS5; RAD50-O) and two at RHS6 (RAD50-A and RAD50-B) (24). This LCR plays an important role in regulating the expression of Th2 cytokines (29–31). Whereas deletion of RHS7 caused only a partial reduction of IL-4 and IL-13 but not IL-5 expression (32), T-cell-specific additional deletion of RHS4 and RHS5 abolished the expression of all three cytokines and resulted in an altered pattern of chromatin modification at the Th2 cytokine locus (33). Although it has been shown by conventional ChIP that GATA3 binds to RHS7 (34), our genome-wide analysis only showed a weak binding. Binding of GATA3 to the LCR region may be critical for DNA looping and thus Th2 cytokine transcription.
Besides regulating chromatin remodeling, GATA3 may induce Il5 and Il13 transcription by direct binding to the promoters of these cytokine genes (10,35–37). Whereas GATA3 deletion during Th2 differentiation abolished the expression of all Th2 cytokines, GATA3 deletion in established Th2 cells resulted in only a modest reduction in IL-4 production but had a major impact on the expression of both IL-5 and IL-13 (14). This result suggests that GATA3 binding to the Il5 and Il13 promoters may directly regulate the transcription of these cytokine mRNAs.
Given the heritable nature of chromatin modifications, once an active chromatin status of Th2 cytokine genes has been achieved, GATA3 may not be necessary to maintain such an active structure (38) or the epigenetic marks might create a buffer period during which GATA3 activity is temporarily dispensable (39). For the expression of IL-5 or IL-13, however, GATA3 is always required presumably because it is a critical component of transcriptional machinery. Therefore, GATA3 may have at least two functions in regulating Th2 cytokine gene expression: direct activation of transcription and chromatin remodeling.
Although direct binding of GATA3 to the Th2 cytokine gene locus appears to be a major mechanism through which GATA3 induces Th2 differentiation, GATA3 is also critical for regulating other Th2-related genes, some of which may also be involved in the expression of Th2 cytokines. For example, GATA3 induces expression of another transcription factor, Dec2, which in turn induces JunB, which acts on the Il4 promoter (40). Our unpublished data also suggest that GATA3 regulates the expression of many novel Th2-specific transcription factors whose functions need to be further studied. Furthermore, GATA3 expression together with STAT5 activation is critical for the expression of Th2-specific cytokine receptors including T1/ST2 (41), whose ligand, IL-33, plays a critical role in the initiation and amplification of Th2 responses (42).
Thus, the functions of GATA3 in promoting Th2 differentiation go far beyond its regulation of Th2 cytokines; it is likely that a sophisticated GATA3-containing transcriptional network involving positive and negative feedback as well as feed-forward loops is responsible for full Th2 cell differentiation.
Mechanisms through which GATA3 suppresses Th1 cell differentiation
GATA3 not only promotes Th2 cell differentiation but also inhibits Th1 cell differentiation (12,43–45). Ectopic GATA3 expression in developing Th1 cells inhibits IFN-γ production while inducing IL-4. Although IL-4 is known to inhibit IFN-γ production, inhibition of IFN-γ production by GATA3 is not dependent on IL-4 since such inhibition was still observed in the absence of IL-4, established either by the addition of anti-IL-4 during the priming culture or using Il4−/− CD4 T cells (12, 43). How does GATA3 inhibit IFN-γ production? So far, several different mechanisms have been reported (12, 44, 45).
GATA3 suppresses IL-12–STAT4 signaling, which is a well-known pathway for Th1 cell differentiation (46, 47). IL-12Rβ2 is undetectable in naive CD4 T cells; its expression is induced by T-cell activation and further up-regulated by IL-12–STAT4 signaling (48). When CD4 T cells receive IL-12 signals during their activation, they differentiate into Th1 cells. Ectopic GATA3 expression in these developing cells inhibits expression of IL-12Rβ2 mRNA (12).
In such GATA3-expressing cells, the reduced IFN-γ production (which is linked to a failure of T-bet up-regulation) could be explained by a failure to respond to IL-12 because of the lack of IL-12Rβ2 expression (12). However, enforced GATA3 expression inhibited IFN-γ production even in developing Th1 cells in which IL-12Rβ2 expression was restored by CD2 promoter-driven expression. This result indicates that GATA3-mediated IFN-γ inhibition cannot be simply explained by suppression of IL-12Rβ2 expression (44). Indeed, in addition to suppressing IL-12Rβ2 expression, GATA3 also represses the expression of STAT4, which is normally highly expressed in Th1 cells compared with Th2 cells (44). Since STAT4 is a major downstream molecule in the IL-12 signaling pathway and plays a critical role in inducing Th1 cells, suppression of STAT4 expression by enforced GATA3 expression explains the observation that enforced IL-12Rβ2 expression fails to restore Th1 differentiation suppressed by GATA3.
Therefore, IFN-γ production suppressed by enforced GATA3 expression under Th1-skewing conditions is rescued only when both STAT4 and IL-12Rβ2 expression are restored. Indeed, GATA3 deletion during Th2 differentiation results in up-regulation of both STAT4 and IL-12Rβ2, although up-regulation of Il12rb2 mRNA is only partial suggesting that Th1-related factors are necessary for optimal IL-12Rβ2 expression (45).
Another mechanism through which GATA3 inhibits Th1 cell differentiation is by blocking Runx3-mediated IFN-γ production. Deletion of GATA3 in CD4 T cells during Th2 cell differentiation not only decreased IL-4 production but also induced IFN-γ production, despite the culture of these cells under Th2-skewing conditions, which include anti-IFN-γ and anti-IL-12 (14). This result suggests that GATA3 expression in developing Th2 cells actively suppresses IFN-γ production that is independent of IL-12 and IFN-γ—two cytokines known to be critical for inducing Th1 differentiation.
We have recently verified the finding that IFN-γ production can be IL-12 and IFN-γ independent by using GATA3-deficient naive CD4 T cells (45). As mentioned earlier, GATA3 is necessary for the development of CD4 SP cells in the thymus (4, 5) and GATA3 is required for the induction of ThPOK, which is a critical factor for CD4 T cell lineage commitment (49). To avoid defective CD4 T cell development in the thymus, a conditional GATA3 knockout mouse strain, Gata3fl/fl dLck-Cre, was established by breeding Gata3fl/fl mice to mice carrying transgenic Cre, whose expression is controlled by a distal Lck promoter, which allows GATA3 expression during CD4 T cell development but then deletes it (50). As expected, both thymic and peripheral T cells in these mice display normal phenotypes as judged by cell number and the expression of multiple cell surface markers (45).
Naive Gata3fl/fl dLck-Cre CD4 T cells cultured under Th2-skewing conditions in vitro produce IFN-γ. Similarly, instead of inducing Th2 responses in normal mice, parasite infection of these conditional knockout mice elicited a Th1-like response as indicated by IFN-γ production. IFN-γ production by ‘Th2’ cells with a double knockout of GATA3 plus T-bet was comparable to that of ‘Th2’ cells with a single knockout of GATA3, indicating that IL-12- and IFN-γ-independent IFN-γ production in GATA3-deficient ‘Th2’ cells is independent of T-bet, the master regulator for inducing Th1 cell differentiation (51, 52).
Runx3 has been reported to regulate IFN-γ production in both CD4 and CD8 T cells (53, 54). Interestingly, IFN-γ production in GATA3-deficient ‘Th2’ cells was diminished by additional disruption of Runx3, suggesting that Runx3 is responsible for IFN-γ- and IL-12-independent IFN-γ production.
Runx3 has been reported to bind to the Ifng promoter (55). Our ChIPseq analysis showed that, in addition to binding to the Ifng promoter, Runx complexes are bound to multiple sites across the Ifng gene locus, all of which are either located at HS sites or CNS regions, with some of the binding sites overlapping with T-bet binding (45, 56). These results imply that Runx3 can induce IFN-γ either by itself or by collaborating with T-bet family members, including T-bet and Eomes, to induce full levels of IFN-γ production. Further experiments suggest that the relative amounts of GATA3 and Runx3 determine the production of IFN-γ. Since GATA3 binds to Runx3 (45), GATA3 inhibition of Runx3-mediated IFN-γ production may result from the interaction of these two transcription factors during Th2 cell differentiation.
Thus, published data summarized above suggest that GATA3 can prevent Th1 differentiation by blocking IL-12Rβ2 and STAT4 up-regulation and by neutralizing the capacity of Runx3 to induce IFN-γ production. Our GATA3 ChIPseq data indicate that GATA3 can also directly bind to the Ifng gene, including CNS +40, a site ∼40 kbp downstream of the Ifng transcription start site and that it affects histone modification patterns near this binding site (24). Similarly, GATA3 may play a role in silencing T-bet expression through chromatin remodeling after its direct binding to the Tbx21 locus. Therefore, GATA3 suppression of Th1 cell differentiation may occur by directly blocking transcription of Th1-specific genes, such as Il12rb2, Stat4, Tbx21 and Ifng (Fig. 1) (24), and/or by blocking the functions of Th1-related proteins, including Runx3 and possibly T-bet as well (57).
Conclusions
When naive CD4 T cells are activated through their TCR and IL-4 receptors, they acquire the capacity to efficiently produce Th2 cytokines as a result of GATA3 up-regulation. GATA3 binds to several regulatory elements in the Th2 cytokine loci and is involved in the chromatin remodeling and/or direct transcriptional activation. GATA3 not only promotes Th2 differentiation but also inhibits Th1 differentiation. GATA3 prevents Th1 differentiation by blocking the IL-12 signaling pathway, neutralizing the function of Runx3 and directly silencing the Ifng gene.
The important regulatory elements to which GATA3 binds, such as CGRE, HSII, HSVa and LCR, can also recruit other transcription factors including STAT5 and NFAT. Therefore, it is likely that GATA3 regulates chromatin remodeling and gene expression in concert with other transcription factors, co-activators and/or co-repressors. The importance of binding of GATA3 to specific sites at any given gene locus in regulating gene expression remains to be tested by inducing point mutations at the GATA3-binding sites rather than by deletion of a larger DNA fragment containing a GATA3-binding site.
In some cases, through protein–protein interactions, GATA3 can be indirectly recruited to a site where there is no GATA3-binding sequence. For example the, Ifng gene locus consists of many regulatory elements across ∼140 kb DNA (1, 44, 55) and in Th2 cells, GATA3 binds to GATA sequences in distal regions of the Ifng gene, such as CNS −22 and CNS +40, but it does not bind to the Ifng promoter (24). GATA3 binding at the Ifng promoter detected in Th1 cells may result from its interaction with Runx3 and/or T-bet.
GATA3 expression varies in different cell types. Depending on the existence and/or abundance of co-factors or repressors, different amounts of GATA3 may be required for gene induction or suppression. For example, GATA3 is expressed at low levels in Th1 cells, but even when expressed at such low levels, it is able to cause cells to acquire IL-4-producing capacity in the presence of high-level STAT5 signaling. On the other hand, although GATA3 is highly expressed in CD4−CD8− (double-negative) thymocytes and binds to the Il13 gene in these cells (24), it fails to induce IL-13 expression. Therefore, identifying the co-factors of GATA3 in specific cell types becomes critical for understanding GATA3-mediated gene regulation. To complicate this even more, GATA3-regulated genes that are cell type specific may collaborate with GATA3 itself in regulating secondary GATA3 targets.
Thus, genome-wide assessment of GATA3-binding sites, and investigation of GATA3-interacting proteins as well as genes that are regulated by GATA3 in different cell types will be necessary to fully understand the biology of GATA3 and its detailed function in regulating Th cell differentiation and T-cell development in general.
Funding
National Institutes of Health (1ZIAAI000493-24).
Acknowledgments
Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA.
References
- 1.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu. Rev. Immunol. 2010;28:445. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu. Rev. Immunol. 1997;15:297. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 3.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 4.Pai SY, Truitt ML, Ting CN, Leiden JM, Glimcher LH, Ho IC. Critical roles for transcription factor GATA-3 in thymocyte development. Immunity. 2003;19:863. doi: 10.1016/s1074-7613(03)00328-5. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez-Hoyos G, Anderson MK, Wang C, Rothenberg EV, Alberola-Ila J. GATA-3 expression is controlled by TCR signals and regulates CD4/CD8 differentiation. Immunity. 2003;19:83. doi: 10.1016/s1074-7613(03)00176-6. [DOI] [PubMed] [Google Scholar]
- 6.Kurata H, Lee HJ, O'Garra A, Arai N. Ectopic expression of activated Stat6 induces the expression of Th2-specific cytokines and transcription factors in developing Th1 cells. Immunity. 1999;11:677. doi: 10.1016/s1074-7613(00)80142-9. [DOI] [PubMed] [Google Scholar]
- 7.Zhu J, Guo L, Watson CJ, Hu-Li J, Paul WE. Stat6 is necessary and sufficient for IL-4's role in Th2 differentiation and cell expansion. J. Immunol. 2001;166:7276. doi: 10.4049/jimmunol.166.12.7276. [DOI] [PubMed] [Google Scholar]
- 8.Yamane H, Zhu J, Paul WE. Independent roles for IL-2 and GATA-3 in stimulating naive CD4+ T cells to generate a Th2-inducing cytokine environment. J. Exp. Med. 2005;202:793. doi: 10.1084/jem.20051304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ouyang W, Lohning M, Gao Z, et al. Stat6-independent GATA-3 autoactivation directs IL-4-independent Th2 development and commitment. Immunity. 2000;12:27. doi: 10.1016/s1074-7613(00)80156-9. [DOI] [PubMed] [Google Scholar]
- 10.Zhang DH, Cohn L, Ray P, Bottomly K, Ray A. Transcription factor GATA-3 is differentially expressed in murine Th1 and Th2 cells and controls Th2-specific expression of the interleukin-5 gene. J. Biol. Chem. 1997;272:21597. doi: 10.1074/jbc.272.34.21597. [DOI] [PubMed] [Google Scholar]
- 11.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 12.Ouyang W, Ranganath SH, Weindel K, et al. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity. 1998;9:745. doi: 10.1016/s1074-7613(00)80671-8. [DOI] [PubMed] [Google Scholar]
- 13.Pai SY, Truitt ML, Ho IC. GATA-3 deficiency abrogates the development and maintenance of T helper type 2 cells. Proc. Natl Acad. Sci. U S A. 2004;101:1993. doi: 10.1073/pnas.0308697100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu J, Min B, Hu-Li J, et al. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat. Immunol. 2004;5:1157. doi: 10.1038/ni1128. [DOI] [PubMed] [Google Scholar]
- 15.Ansel KM, Djuretic I, Tanasa B, Rao A. Regulation of Th2 differentiation and Il4 locus accessibility. Annu. Rev. Immunol. 2006;24:607. doi: 10.1146/annurev.immunol.23.021704.115821. [DOI] [PubMed] [Google Scholar]
- 16.Takemoto N, Kamogawa Y, Jun Lee H, et al. Cutting edge: chromatin remodeling at the IL-4/IL-13 intergenic regulatory region for Th2-specific cytokine gene cluster. J. Immunol. 2000;165:6687. doi: 10.4049/jimmunol.165.12.6687. [DOI] [PubMed] [Google Scholar]
- 17.Takemoto N, Arai K, Miyatake S. Cutting edge: the differential involvement of the N-finger of GATA-3 in chromatin remodeling and transactivation during Th2 development. J. Immunol. 2002;169:4103. doi: 10.4049/jimmunol.169.8.4103. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal S, Avni O, Rao A. Cell-type-restricted binding of the transcription factor NFAT to a distal IL-4 enhancer in vivo. Immunity. 2000;12:643. doi: 10.1016/s1074-7613(00)80215-0. [DOI] [PubMed] [Google Scholar]
- 19.Yamashita M, Ukai-Tadenuma M, Kimura M, et al. Identification of a conserved GATA3 response element upstream proximal from the interleukin-13 gene locus. J. Biol. Chem. 2002;277:42399. doi: 10.1074/jbc.M205876200. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka S, Motomura Y, Suzuki Y, et al. The enhancer HS2 critically regulates GATA-3-mediated Il4 transcription in T(H)2 cells. Nat. Immunol. 2011;12:77. doi: 10.1038/ni.1966. [DOI] [PubMed] [Google Scholar]
- 21.Takemoto N, Koyano-Nakagawa N, Yokota T, Arai N, Miyatake S, Arai K. Th2-specific DNase I-hypersensitive sites in the murine IL-13 and IL-4 intergenic region. Int. Immunol. 1998;10:1981. doi: 10.1093/intimm/10.12.1981. [DOI] [PubMed] [Google Scholar]
- 22.Mohrs M, Blankespoor CM, Wang ZE, et al. Deletion of a coordinate regulator of type 2 cytokine expression in mice. Nat. Immunol. 2001;2:842. doi: 10.1038/ni0901-842. [DOI] [PubMed] [Google Scholar]
- 23.Monticelli S, Lee DU, Nardone J, Bolton DL, Rao A. Chromatin-based regulation of cytokine transcription in Th2 cells and mast cells. Int. Immunol. 2005;17:1513. doi: 10.1093/intimm/dxh329. [DOI] [PubMed] [Google Scholar]
- 24.Wei G, Abraham B, Yagi R, et al. Genome-wide analyses of GATA3-mediated gene regulation in distinct T cell types. Immunity. 2011 doi: 10.1016/j.immuni.2011.08.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solymar DC, Agarwal S, Bassing CH, Alt FW, Rao A. A 3' enhancer in the IL-4 gene regulates cytokine production by Th2 cells and mast cells. Immunity. 2002;17:41. doi: 10.1016/s1074-7613(02)00334-5. [DOI] [PubMed] [Google Scholar]
- 26.Zhu J, Cote-Sierra J, Guo L, Paul WE. Stat5 activation plays a critical role in Th2 differentiation. Immunity. 2003;19:739. doi: 10.1016/s1074-7613(03)00292-9. [DOI] [PubMed] [Google Scholar]
- 27.Cote-Sierra J, Foucras G, Guo L, et al. Interleukin 2 plays a central role in Th2 differentiation. Proc. Natl Acad. Sci. U S A. 2004;101:3880. doi: 10.1073/pnas.0400339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei G, Wei L, Zhu J, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee GR, Fields PE, Griffin TJ, Flavell RA. Regulation of the Th2 cytokine locus by a locus control region. Immunity. 2003;19:145. doi: 10.1016/s1074-7613(03)00179-1. [DOI] [PubMed] [Google Scholar]
- 30.Fields PE, Lee GR, Kim ST, Bartsevich VV, Flavell RA. Th2-specific chromatin remodeling and enhancer activity in the Th2 cytokine locus control region. Immunity. 2004;21:865. doi: 10.1016/j.immuni.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 31.Lee DU, Rao A. Molecular analysis of a locus control region in the T helper 2 cytokine gene cluster: a target for STAT6 but not GATA3. Proc. Natl Acad. Sci. U S A. 2004;101:16010. doi: 10.1073/pnas.0407031101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee GR, Spilianakis CG, Flavell RA. Hypersensitive site 7 of the TH2 locus control region is essential for expressing TH2 cytokine genes and for long-range intrachromosomal interactions. Nat. Immunol. 2005;6:42. doi: 10.1038/ni1148. [DOI] [PubMed] [Google Scholar]
- 33.Koh BH, Hwang SS, Kim JY, et al. Th2 LCR is essential for regulation of Th2 cytokine genes and for pathogenesis of allergic asthma. Proc. Natl Acad. Sci. U S A. 2010;107:10614. doi: 10.1073/pnas.1005383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spilianakis CG, Flavell RA. Long-range intrachromosomal interactions in the T helper type 2 cytokine locus. Nat. Immunol. 2004;5:1017. doi: 10.1038/ni1115. [DOI] [PubMed] [Google Scholar]
- 35.Lee HJ, O'Garra A, Arai K, Arai N. Characterization of cis-regulatory elements and nuclear factors conferring Th2-specific expression of the IL-5 gene: a role for a GATA-binding protein. J. Immunol. 1998;160:2343. [PubMed] [Google Scholar]
- 36.Schwenger GT, Fournier R, Kok CC, Mordvinov VA, Yeoman D, Sanderson CJ. GATA-3 has dual regulatory functions in human interleukin-5 transcription. J. Biol. Chem. 2001;276:48502. doi: 10.1074/jbc.M107836200. [DOI] [PubMed] [Google Scholar]
- 37.Kishikawa H, Sun J, Choi A, Miaw SC, Ho IC. The cell type-specific expression of the murine IL-13 gene is regulated by GATA-3. J. Immunol. 2001;167:4414. doi: 10.4049/jimmunol.167.8.4414. [DOI] [PubMed] [Google Scholar]
- 38.Yamashita M, Shinnakasu R, Nigo Y, et al. Interleukin (IL)-4-independent maintenance of histone modification of the IL-4 gene loci in memory Th2 cells. J. Biol. Chem. 2004;279:39454. doi: 10.1074/jbc.M405989200. [DOI] [PubMed] [Google Scholar]
- 39.Yamashita M, Hirahara K, Shinnakasu R, et al. Crucial role of MLL for the maintenance of memory T helper type 2 cell responses. Immunity. 2006;24:611. doi: 10.1016/j.immuni.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 40.Yang XO, Angkasekwinai P, Zhu J, et al. Requirement for the basic helix-loop-helix transcription factor Dec2 in initial TH2 lineage commitment. Nat. Immunol. 2009;10:1260. doi: 10.1038/ni.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo L, Wei G, Zhu J, et al. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc. Natl Acad. Sci. U S A. 2009;106:13463. doi: 10.1073/pnas.0906988106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat. Rev. Immunol. 2010;10:225. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferber IA, Lee HJ, Zonin F, et al. GATA-3 significantly downregulates IFN-gamma production from developing Th1 cells in addition to inducing IL-4 and IL-5 levels. Clin. Immunol. 1999;91:134. doi: 10.1006/clim.1999.4718. [DOI] [PubMed] [Google Scholar]
- 44.Usui T, Nishikomori R, Kitani A, Strober W. GATA-3 suppresses Th1 development by downregulation of Stat4 and not through effects on IL-12Rbeta2 chain or T-bet. Immunity. 2003;18:415. doi: 10.1016/s1074-7613(03)00057-8. [DOI] [PubMed] [Google Scholar]
- 45.Yagi R, Junttila IS, Wei G, et al. The transcription factor GATA3 actively represses RUNX3 protein-regulated production of interferon-gamma. Immunity. 2010;32:507. doi: 10.1016/j.immuni.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaplan MH, Sun YL, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- 47.Thierfelder WE, van Deursen JM, Yamamoto K, et al. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 1996;382:171. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- 48.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J. Exp. Med. 1997;185:817. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang L, Wildt KF, Zhu J, et al. Distinct functions for the transcription factors GATA-3 and ThPOK during intrathymic differentiation of CD4(+) T cells. Nat. Immunol. 2008;9:1122. doi: 10.1038/ni.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang DJ, Wang Q, Wei J, et al. Selective expression of the Cre recombinase in late-stage thymocytes using the distal promoter of the Lck gene. J. Immunol. 2005;174:6725. doi: 10.4049/jimmunol.174.11.6725. [DOI] [PubMed] [Google Scholar]
- 51.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 52.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 53.Cruz-Guilloty F, Pipkin ME, Djuretic IM, et al. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. J. Exp. Med. 2009;206:51. doi: 10.1084/jem.20081242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kohu K, Ohmori H, Wong WF, et al. The Runx3 transcription factor augments Th1 and down-modulates Th2 phenotypes by interacting with and attenuating GATA3. J. Immunol. 2009;183:7817. doi: 10.4049/jimmunol.0802527. [DOI] [PubMed] [Google Scholar]
- 55.Djuretic IM, Levanon D, Negreanu V, Groner Y, Rao A, Ansel KM. Transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Nat. Immunol. 2007;8:145. doi: 10.1038/ni1424. [DOI] [PubMed] [Google Scholar]
- 56.Schoenborn JR, Dorschner MO, Sekimata M, et al. Comprehensive epigenetic profiling identifies multiple distal regulatory elements directing transcription of the gene encoding interferon-gamma. Nat. Immunol. 2007;8:732. doi: 10.1038/ni1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hwang ES, Szabo SJ, Schwartzberg PL, Glimcher LH. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science. 2005;307:430. doi: 10.1126/science.1103336. [DOI] [PubMed] [Google Scholar]