Abstract
Despite extensive research in the nanopore-sensing field, there is a paucity of experimental studies that investigate specific ion effects in confined spaces, such as in nanopores. Here, the effect of halogen anions on a simple bimolecular complexation reaction between monodisperse poly(ethylene glycol) (PEG) and α-hemolysin nanoscale pores have been investigated at the single-molecule level. The anions track the Hofmeister ranking according to their influence upon the on-rate constant. An inverse relationship was demonstrated for the off-rate and the solubility of PEG. The difference among anions spans several hundredfold. Halogen anions play a very significant role in the interaction of PEG with nanopores although, unlike K+, they do not bind to PEG. The specific effect appears dominated by a hydration-dehydration process where ions and PEG compete for water. Our findings provide what we believe to be novel insights into physicochemical mechanisms involved in single-molecule interactions with nanopores and are clearly relevant to more complicated chemical and biological processes involving a transient association of two or more molecules (e.g., reception, signal transduction, enzyme catalysis). It is anticipated that these findings will advance the development of devices with nanopore-based sensors for chemical and biological applications.
Introduction
Because ion-channel proteins are so finely tuned to respond to specific molecules, they serve as models for developing nanopore devices for biomolecular sensing. A single nanometer-scale pore formed by Staphylococcus aureus α-hemolysin (αHL) is one of the most promising biological structures for creating a single molecule detector and analyzer. However, further development of these strategies is impeded by several technological and scientific problems, including difficulties in stabilizing a nanopore-membrane complex over a period of time and insufficient understanding of physics that regulate the rate at which molecules enter into nanocavities and the energy of their interactions.
The salting-out effect is a very general phenomenon, in which the solubility of a solute in water is decreased when electrolyte is added. It occurs in cultured microorganisms, in aqueous dispersions of macromolecules and amino acids, in self-assembled amphiphilic structures, in the surface tension of water, and even simple gas molecules (1–8). Cations and anions affect salting-out processes with widely varied effectiveness. The ordering of ions in terms of their effectiveness is known as the Hofmeister series (9–11). In recent years, there has been an explosion in research articles tied to the Hofmeister series (11–14); however, despite this increase in attention, a molecular-level understanding of the Hofmeister series is still lacking. Moreover, to the best of our knowledge there are only four isolated publications related to nanopores: the influence of Hofmeister anion series on αHL channel formation in lipid bilayers (15); the anion-dependent gating of roflamycoin ion channels (16); and the specific guest-host complexation of the cyclic carbohydrate, γ-cyclodextrin, with adamantane carboxylate inside the pore of αHL channels (17); and the influence of alkali chlorides on αHL channel conductance (18).
Our recent studies with poly(ethylene glycol) (PEG) as an analyte (19,20) demonstrated that an increase in KCl concentration from 1 M to 4 M, leads to high-resolution recording of PEG/αHL nanopore interactions. The effect of salt concentration is linked with an ∼100-fold increase in the on- and off-rate constants of the process (21–24). It was suggested that salting-out was responsible for the change in the on-rate constant (22), and consequently for changes in the transition rate and the detection limit.
The goal of this study was:
-
1.
To investigate the influence of halides on a simple bimolecular complexation reaction between monodisperse poly(ethylene glycol) and α-hemolysin nanoscale pores;
-
2.
To reveal whether halogen anions have a specific effect on complex formation; and
-
3.
To disclose the possible mechanism of action.
To achieve this and to improve the sensitivity of nanopore sensors, we performed systematic studies of the kinetics of PEG/αHL channel interactions to examine the effect of halogen anions. The key finding is that the type of halogen anion strongly influenced PEG/αHL nanopore interactions. Remarkably, both on-rates and off-rates were affected. The anions track the direct and inverse Hofmeister series for the on-rate, and the off-rate and the solubility of PEG, respectively. The difference among anions spans several hundredfold. The specific effect appears dominated by hydration-dehydration processes where anions and PEG compete for water.
Materials and Methods
Wild-type S. aureus α-hemolysin (αHL) was purchased from Calbiochem (Madison, WI). 1,2-diphytanoyl-sn-glycero-3-phosphocholine (DPhPC) was purchased from Avanti Polar Lipids (Alabaster, AL). High quality (>99.99%) halides (KCl, KF, KBr, and KI) were purchased from Sigma (St. Louis, MO). Monodisperse poly(ethylene glycol), (molecular mass = 1294 g/mol; PEG), was purchased from Polypure (Oslo, Norway). The 2-amino-2-hydroxymethyl-1,3-propanediol (TRIS) and citric acid were from Schwarz/Mann Biotech (Cleveland, OH) and Fluka (Buchs, Switzerland), respectively.
Solvent-free planar bilayer lipid membranes, with a capacitance of 40 pF, were formed by the lipid monolayer apposition technique, using DPhPC in hexane (J. T. Baker, Phillipsburg, NJ) at 25 ± 1°C. Because it was recently shown that the elevated KCl concentrations considerably improve single molecule identification by unitary protein nanopores (19,20,22), in this study membrane-bathing solutions contained 4 M halide in 5 mM TRIS adjusted to pH 7.5 with citric acid.
PEG1294 was used as a representative analyte and added to the trans compartment of the experimental chamber. αHL was added from the cis side of the membrane in a concentration sufficient to form unitary protein nanopores in planar lipid membrane. If not mentioned otherwise, the applied potential was 40 mV. A positive current is defined by cation flow from trans to cis. Single αHL nanopore incorporation and measurement of molecular signature parameters (mean duration and amplitude of the blockage), transition rate, and kinetic constants of the PEG-nanopore interactions were done essentially as described by Rodrigues et al. (22). In short, the on-rate constant, kon, was defined as 1/(CPEG × τon). The characteristic time, τon, was obtained from the collected time intervals between the end of one blockade event and the onset of the next. The off-rate constant, koff, was defined as 1/τoff, where τoff is the characteristic time of PEG staying in the pore. The constant of PEG-αHL pore complex formation, Kf, was calculated by using the association (on-) kon and dissociation (off-) koff rate constants as kon/koff.
Experiments were done using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA) in voltage-clamp mode. Membrane potential was maintained using Ag/AgCl electrodes in 3 M KCl 2% agarose bridges assembled within standard 200-μL pipette tips. Currents were filtered by a low-pass eight-pole Bessel filter (Model 9002; Frequency Devices, Haverhill, MA) at 15 kHz and directly saved into computer memory with a sampling frequency of 50–250 kHz. Experiments employed dilute polymer solutions. The greatest PEG concentration used in bilayer experiments was well below the overlap concentration (∼13%) (25) and solubility for this PEG (see Results and Discussion). The limit of PEG solubility was estimated by the modified cloud-point method as described by Rodrigues et al. (22). Inaccuracy was <1%.
Results and Discussion
Conductance
First, we examined the conductance of single αHL channels surrounded by 4 M solutions of different halides. At the chosen pH (7.5), the channel was in a high conductance state at all transmembrane potentials from −200 mV to +200 mV in all solutions. Conductance-voltage curves of αHL channels in KF, KBr, and KI solutions were found to be slightly asymmetric (data not shown), similar to that in KCl (26). Such behavior probably results from the asymmetry in charge distribution between the channel openings and in the channel structure itself. The channel conductance was considerably higher in solutions of KCl, KBr, and KI compared with KF solution (more than one-and-a-half times) (Fig. 1). This finding appears to be in accordance with ionic conductivities of halides (27) because a strong correlation between ionic conductivity of halogen anions and ion channel conductance was established (Fig. 1).
Figure 1.
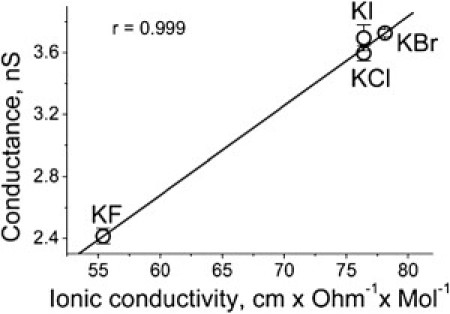
The correlation between the ionic conductivity of halogens and the αHL channel conductance (extrapolated to zero voltage) in 4 M halide solutions. Values of αHL channel conductance at zero voltage were obtained by fitting the experimental data points of G/V curves (obtained in the range of ±200 mV) with second-order polynomial function. (Data points) Means from at least three separate experiments ± SD. (Line) First-order regression fit of the data. The correlation coefficient is shown in the figure.
This result is consistent with earlier data for the channel obtained at low salt concentrations (28). In addition, we found that the ability of αHL to form ion channels was influenced considerably by the halogen ion species. It was highest in KCl (∼10 pM of αHL is sufficient to form a single channel in the planar lipid bilayer) and smallest in KF solution (where up to 1 nM of αHL must be added). In KBr- and KI-solutions, the channel-forming activity was of intermediate magnitude. The result with KCl, KBr, and KI is consistent with earlier observations that the rate of αHL channel formation is inversely proportional to the size of the anions (15). However, the small channel-forming activity in the presence of KF in the bathing solution was unexpected. This influence of F− needs further study.
Integral effect
Stable conductance of single αHL channels in all 4 M halide solutions was a prerequisite to using the channel as a sensor element. Using PEG1294 as an analyte, we found that the PEG effect is strongly influenced by anion type, and is voltage- and dose-dependent (Fig. 2). The maximal blocking effect was observed at 40 ± 10 mV. Such behavior is consistent with the findings of Rodrigues et al. (22) and indicates that molecules of nonionic PEG possess an acquired positive charge due to complex formation with K+ in all halide solutions.
Figure 2.
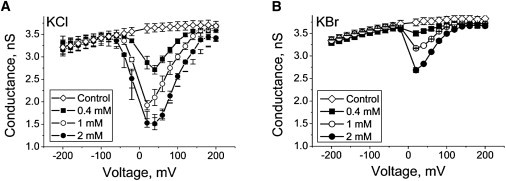
Effect of trans-side addition of PEG1294 to membrane-bathing solutions on channel conductance. (A) 4 M KCl; (B) 4 M KBr. The result of at least three independent experiments (mean ± SE) is presented in each case.
Single molecule events
As expected, high-resolution recordings of PEG/αHL interactions in the presence of different halides revealed a huge difference in the frequency and duration of well-defined current blockades (Fig. 3). Results obtained in KCl, KBr, and KI solutions indicate that the frequency of events is high in the presence of KCl, lower in the presence of KBr, and lowest in 4 M KI. The apparently lower frequency of PEG blockages in KF solution than in KI is delusive because there is a 40,000-fold difference in PEG concentration. It was not possible to use the same PEG concentration with KF-solutions due to much larger on-rate and much lower off-rate constants of the PEG/αHL interaction (see below). Interpolation of the KF results obtained at low PEG concentration (Fig. 3 D, 10 nM) to larger PEG concentrations projects much higher frequency values than seen in the presence of any other halides.
Figure 3.
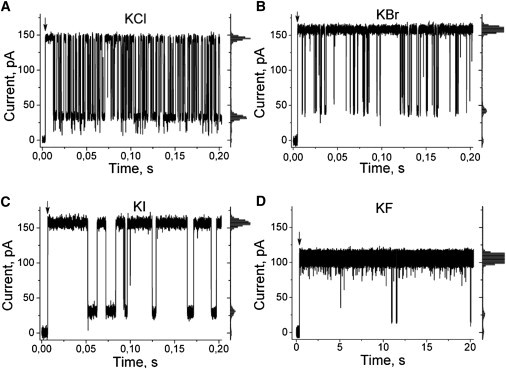
Typical traces of the ion current through single αHL channels in the presence of PEG1294 in the bath solution at a time resolution of 0.1 ms. (Arrows) Voltage shift from zero to 40 mV. Halide concentration was 4 M. Concentration of PEG was 400 μM (for A–C) and 10 nM (for panel D). Note the change in the timescale and PEG concentration from panels A–D. The respective all-point histograms are shown at the right of each record and used to give the mean value of blockage amplitudes. The effectiveness of such inhibition was very similar for all halides and comprised 73–80% of the maximal current.
Rate constants of PEG/αHL channel interaction
To determine the reason for this anion-specific effect, we analyzed the rate constants of the process by essentially the same method as described recently by Rodrigues et al. (22). We have demonstrated that the on-rate constant (measured at an optimal 40 mV) is strikingly dependent on type of halogen (Fig. 4 A, solid line). The largest on-rate constant was observed with KF in the bath and the smallest in the presence of KI. The difference among on-rate values exceeded two orders of magnitude. The effect of anions follows the Hofmeister series: F− > Cl− > Br− > I. The influence of anions on the off-rate constant follows the reverse Hofmeister series (Fig. 4 A, dashed line). The difference among off-rate values exceeded 20-fold, but was smaller than the difference among on-rate constant values. As a result, the formation constant of the PEG/αHL channel interaction follows the Hofmeister series (Fig. 4 B). Voltage dependencies of the kinetic constants in KF−, KBr−, and KI− solution resemble those in KCl that we published recently (22). The off- and on-rate constants show turnover behavior with differences up to four orders of magnitude (data not shown). Detailed analysis of the effects of transmembrane voltage on dynamics of PEG-αHL channel interactions deserves careful study. These results will be included in a future work.
Figure 4.
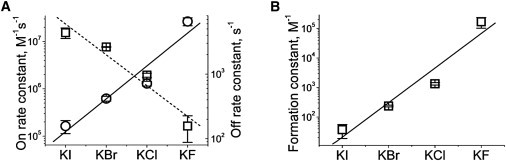
Specific ionic effect on the PEG1294/αHL pore interaction. The average on-rate (○, solid line), off-rate (□, dashed line) (A), and PEG/αHL pore complex formation constant (B) in 4 M solutions of different halides. (Data points) Means (± SD) of at least three single protein nanopores reconstituted in separate experiments with >5000 events as in Fig. 3. (Lines) Guide for the eye. The data presented in panel A were used to build the dependence shown in panel B.
PEG solubility in halide solutions
Salting-out was shown recently to affect the dependence of the on-rate upon KCl concentration (22). Therefore, we decided to verify whether salting-out is also responsible for the specific anion effects demonstrated above. The solubility of PEG in halide solutions was examined. The type of anion was found to exert a strong influence on PEG solubility. PEG solubility in 4 M KF (19 ± 2 μM) was more than two-orders-of-magnitude smaller than in 4 M KCl (3840 ± 390 μM). On the contrary, PEG solubility in 4 M KBr or 4 M KI was so large that it was not possible to determine precisely. To overcome this difficulty and obtain numerical values for all anions, we the carried out experiments with mixed (KF/KCl, KBr/KCl, and KI/KCl) solutions, keeping the total concentration at 4 M (Fig. 5).
Figure 5.
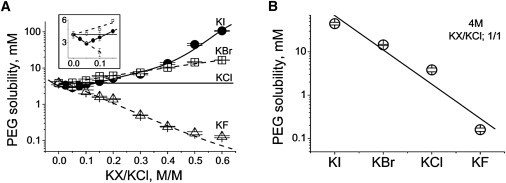
Solubility of PEG in 4 M halide solutions. (A) PEG solubility as a function of different molar fractions of KF, KBr, and KI in KCl solution. (Inset) The zone of the anomalous molar fraction effect for KI/KCl mixtures visualized at higher resolution. (B) Solubility of PEG at 1:1 (M/M) KX/KCl ratio, where X represents F−, Br−, or I−. (Lines) Guide for the eye. Total salt concentration was 4 M. (Data points) Means of 5–8 separate experiments ± SD.
Increasing the molar fraction of KF decreases PEG solubility, whereas increasing KBr monotonously increases it. PEG solubility as a function of KI concentration showed an anomalous molar fraction effect. KI decreased the solubility of PEG at small molar fractions, and started to increase only at a KI molar fraction larger than 0.15 (Fig. 5 A, inset). The reason for this effect has yet to be discovered.
Because PEG solubility in 4 M KBr and 4 M KI was practically impossible to determine, the values obtained at 1:1 molar ratios KX/KCl were used to compare with the kinetic constants of PEG/αHL pore interactions. A strong negative correlation exists between the on-rate constant and solubility, indicating the direct involvement of salting-out in this effect of halogen ions (Fig. 6 A). A positive correlation was established between the off-rate constant and PEG solubility (Fig. 6 B), reinforcing our conclusion that PEG salting-out dominates the specific effects of halogen ions on the on- and off-rates of PEG/αHL channel interactions.
Figure 6.
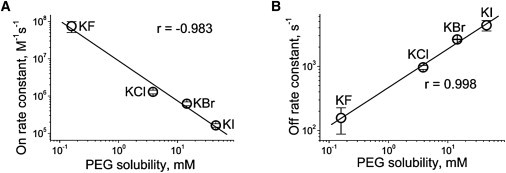
The on-rate (A) and off-rate (B) constants of PEG/αHL channel interaction as a function of PEG solubility. (Data points) Means of 5–8 separate experiments ± SD for solubility and at least three separate experiments for the rate constants. (Lines) First-order regression fits of the data. The correlation coefficient is shown in the figure.
Transition rate and detection limit
The PEG effect on αHL channel conductance is dose-dependent (Fig. 2). Recent studies (22–24,29) demonstrated that the event frequency (transition rate, 1/τon) is linearly related to the concentration of molecules of PEGs, cyclodextrin derivatives, and amino acids in KCl and NaCl solutions, thus providing a basis for quantifying these molecules. Here we have demonstrated that it is true for PEG in all halide solutions. In all cases the transition rate was directly proportional to analyte concentration (PEG in this case) with a slope of ∼1 (0.97 ± 0.06) (Fig. 7). This finding suggests that the partitioning of analyte into the αHL pore can be described by a first-order reaction. Assigning reasonably the detection limit as a background equivalent PEG concentration at 1-Hz event frequency, we have established that it equals 8.0, 2.0, 0.5, and 0.05 μM (PEG1294) at 4 M of KI, KBr, KCl, and KF, respectively (Fig. 7). It is evident that the sensitivity of αHL pores to PEG depends crucially on anion type and is more than two-orders-of-magnitude higher in KF than in KI solutions.
Figure 7.
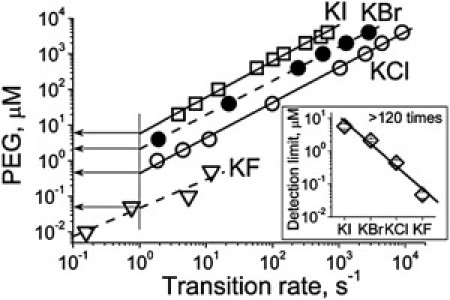
Transition rate and PEG concentration. The concentration of PEG1294 as a function of the transition rate in different halide solutions. (Lines) First-order regression fits of the data with a slope of 0.97 ± 0.06. (Data points) Means of at least three separate experiments. Standard deviations were omitted for clarity. (Horizontal arrows) Background equivalent PEG concentration at an event frequency of 1 Hz. (Inset) The background equivalent PEG concentration at different halide solutions. The number shown in the figure is the difference in values obtained in KI and KF solutions.
It is known that experimental parameters, including pH, temperature, ionic strength, and applied potential have considerable influence on the resolution and sensitivity of stochastic detection. We have shown here that it could be significantly improved by simply using an appropriate type of electrolyte.
The role of hydration
In this study, halides were used at the same high (4 M) concentration where the difference in electrostatic interactions between analyte molecules and nanopore channels is significantly limited. However, a huge difference in kinetic constants of PEG/αHL channel interactions was established, indicating the specific ion effect (Hofmeister) in confined spaces such as those of protein nanopores. The competition between ions and analyte for water is frequently noted as one possible reason for Hofmeister effects. To verify that this competition is responsible for the observed effect of halides on PEG/αHL channel interactions, a correlation analysis between the rate-constants and anion hydration was performed. Very strong correlations between the rate constants of PEG/αHL channel interaction and the hydration enthalpy of anions were demonstrated (Fig. 8). Higher hydration of the anion resulted in a higher probability of the interaction and the stronger stability of the complex.
Figure 8.
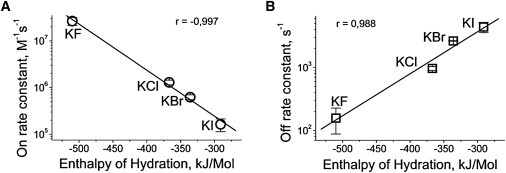
Rate constants of PEG/αHL channel interactions in halide solutions as function of hydration enthalpy of anions. Enthalpy values were taken from Marcus (27). The correlation coefficient is shown in the figure.
Higher hydration means that a large number of water molecules are bound to ions so that fewer of them are available to interact with cosolute (PEG, in this case). Because of this competition between ions and cosolute for water, it appears that ions exclude cosolutes from the bath to the protein nanopores which serve as harbors. Hence, the confined space presents more favorable conditions for PEG to compete with ions for water, indicating that the confined water is more accessible to PEG (and, probably, for other analytes (cosolute)). The existence of water that is accessible to nonionic analytes (confined water) accords with the presence of a hydration water layer at nanopore surfaces, where the presence of ions is severely restricted (30,31) and with remarkable decreases in the hydration number for ions confined in nanospaces (32).
Electroosmotic flow
All experiments presented in our study were made at high (4 M) salt concentrations. At first glance, a significant electroosmotic flow through αHL channel would not be expected under these conditions because it is known (26) that the cation-anion selectivity of αHL channel at high KCl concentrations is close to zero. This means that the channel is unselective and the transport number of cations and anions is equal to ∼0.5. On the other hand, it seems reasonable to assume that for this condition (nonselective channel), the water transported per ion might be equal to that in the primary hydration sphere. Thereby one would expect that despite a similar transport number between cations and anions, the net water transport will not equal zero, because the hydration numbers of the anions (4.97, 2.87, 2.30, and 1.67 (27) for F−, Cl−, Br−, and I−, respectively) are significantly different from the hydration number for K+ (3.33).
Moreover, the anions rank in Hofmeister series according to their hydration number. If this parameter dominated the kinetics of PEG/αHL channel interactions, the on-rate constant might be largest in KI and the lowest in KF solution, that is, exactly the opposite to the established dependence (Fig. 4 A). Ionic mobility is another important characteristic of ions that could influence the results. However, their ionic mobilities do not correspond to their rank in the Hofmeister series: F− has the lowest mobility (55.4 cm2 Ohm−1 Mol−1), whereas the mobilities of other three anions are close to each other (76.4–78.1 cm2 Ohm−1 Mol−1) (27). The foregoing reasons permit us to conclude that in 4 M potassium halide solutions, electroosmotic flow does not significantly affect the observed dynamics, if at all.
Concluding Remarks
In this work, we systematically investigated the interaction of the nonionic polymer, PEG, with a protein pore formed by αHL in planar lipid bilayers in solutions of different halides. We found that the type of anion has very strong influence on the rate constants of the process (the difference reaches several hundredfold). As a consequence, the transition rate and the detection limit of the nanopore-based sensor were correspondingly changed. All probed anions follow the Hofmeister ranking according to their influence on the on-rate constant (F− > Cl− > Br− > I−). An inverse relationship was demonstrated for the off-rate and the solubility of the analyte (F− < Cl− < Br− < I−). Therefore, a salting-out phenomenon is responsible for the anion-induced effect on single molecule detection with solitary protein nanopores. The specific effect appears dominated by hydration-dehydration (chemical) processes where ions and cosolutes compete for water.
We have shown that halogen ions play a very significant role in creating conditions for polymers to interact with the nanopores although K+ (not anions) binds with PEG (22,33,34). Anion influence may provide an explanation for why the residence time for PEG in KCl solution is ∼100-fold longer than expected from a theoretical model based on an electrostatic (Poisson-Nernst-Planck) approach (35). Hereby our findings provide what we believe to be previously undescribed insights into physicochemical mechanisms involved in single-molecule interactions with nanopores. The results will advance the development of devices with nanopore-based sensors for chemical and biological applications. Moreover, though our study deals with a simple first-order kinetic complexation reaction, our results are clearly relevant for more complicated chemical and biological processes, which involve a transient association of two or more molecules (e.g., reception, signal transduction, enzyme catalysis) where the hydration-dehydration reactions occur.
Acknowledgments
The authors thank Dr. Steven D. Aird (University of Maryland, University College Asia) for editing that improved the clarity of the manuscript.
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico, a Rede de Nanotecnologia Molecular e de Interfaces, and Instituto Nacional de Ciência e Tecnologia de Nanotecnologia Para Marcadores Integrados, Brazil.
References
- 1.Lo Nostro P., Ninham B.W., Baglioni P. Hofmeister effects in supramolecular and biological systems. Biophys. Chem. 2006;124:208–213. doi: 10.1016/j.bpc.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Koynova R., Brankov J., Tenchov B. Modulation of lipid phase behavior by kosmotropic and chaotropic solutes: Experiment and thermodynamic theory. Eur. Biophys. J. 1997;25:261–274. doi: 10.1007/s002490050038. [DOI] [PubMed] [Google Scholar]
- 3.Collins K.D., Washabaugh M.W. The Hofmeister effect and the behavior of water at interfaces. Q. Rev. Biophys. 1985;18:323–422. doi: 10.1017/s0033583500005369. [DOI] [PubMed] [Google Scholar]
- 4.Lo Nostro P., Ninham B.W., Baglioni P. Specific ion effects on the growth rates of Staphylococcus aureus and Pseudomonas aeruginosa. Phys. Biol. 2005;2:1–7. doi: 10.1088/1478-3967/2/1/001. [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Leon T., Santander-Ortega M.J., Bastos-Gonzalez D. Hofmeister effects in colloidal systems: influence of the surface nature. J. Phys. Chem. C. 2008;112:16060–16069. [Google Scholar]
- 6.Zhou H.X. Interactions of macromolecules with salt ions: an electrostatic theory for the Hofmeister effect. Proteins. 2005;61:69–78. doi: 10.1002/prot.20500. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y.J., Cremer P.S. Interactions between macromolecules and ions: the Hofmeister series. Curr. Opin. Chem. Biol. 2006;10:658–663. doi: 10.1016/j.cbpa.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 8.Jungwirth P., Tobias D.J. Specific ion effects at the air/water interface. Chem. Rev. 2006;106:1259–1281. doi: 10.1021/cr0403741. [DOI] [PubMed] [Google Scholar]
- 9.Hofmeister F. Doctrine of the effect of the salts. Second communication [Zur lehre von der wirkung der salze. Zweite mittheilung] Arch. Exp. Pathol. Pharmakol. 1888;24:247–260. [Google Scholar]
- 10.Kunz W., Lo Nostro P., Ninham B.W. The present state of affairs with Hofmeister effects. Curr. Opin. Colloid Interface Sci. 2004;9:1–18. [Google Scholar]
- 11.Zhang Y., Cremer P.S. Chemistry of Hofmeister anions and osmolytes. Annu. Rev. Phys. Chem. 2010;61:63–83. doi: 10.1146/annurev.physchem.59.032607.093635. [DOI] [PubMed] [Google Scholar]
- 12.Jungwirth P. Spiers Memorial Lecture. Ions at aqueous interfaces. Faraday Discuss. 2009;141:9–30. doi: 10.1039/b816684f. discussion 81–98. [DOI] [PubMed] [Google Scholar]
- 13.Freire M.G., Neves C.M.S.S., Coutinho J.A. 1H NMR and molecular dynamics evidence for an unexpected interaction on the origin of salting-in/salting-out phenomena. J. Phys. Chem. B. 2010;114:2004–2014. doi: 10.1021/jp9095634. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y.J., Cremer P.S. The inverse and direct Hofmeister series for lysozyme. Proc. Natl. Acad. Sci. USA. 2009;106:15249–15253. doi: 10.1073/pnas.0907616106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krasilnikov O.V., Sabirov R.Z., Tashmukhamedov B.A. The influence of ionic composition of the medium on the dynamics of staphylotoxin channel formation in BLM. Biologicheskie Membrany. 1986;3:1057–1061. [Google Scholar]
- 16.Grigorjev P.A., Bezrukov S.M. Hofmeister effect in ion transport: reversible binding of halide anions to the roflamycoin channel. Biophys. J. 1994;67:2265–2271. doi: 10.1016/S0006-3495(94)80711-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurnev P.A., Harries D., Bezrukov S.M. The dynamic side of the Hofmeister effect: a single-molecule nanopore study of specific complex formation. ChemPhysChem. 2009;10:1445–1449. doi: 10.1002/cphc.200900312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhattacharya S., Muzard J., Viasnoff V. Rectification of the current in α-hemolysin pore depends on the cation type: the alkali series probed by molecular dynamics simulations and experiments. J. Phys. Chem. C. 2011;115:4255–4264. doi: 10.1021/jp111441p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krasilnikov O.V., Rodrigues C.G., Bezrukov S.M. Single polymer molecules in a protein nanopore in the limit of a strong polymer-pore attraction. Phys. Rev. Lett. 2006;97:018301. doi: 10.1103/PhysRevLett.97.018301. [DOI] [PubMed] [Google Scholar]
- 20.Robertson J.W., Rodrigues C.G., Kasianowicz J.J. Single-molecule mass spectrometry in solution using a solitary nanopore. Proc. Natl. Acad. Sci. USA. 2007;104:8207–8211. doi: 10.1073/pnas.0611085104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orlik F., Schiffler B., Benz R. Anthrax toxin protective antigen: inhibition of channel function by chloroquine and related compounds and study of binding kinetics using the current noise analysis. Biophys. J. 2005;88:1715–1724. doi: 10.1529/biophysj.104.050336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodrigues C.G., Machado D.C., Krasilnikov O.V. Mechanism of KCl enhancement in detection of nonionic polymers by nanopore sensors. Biophys. J. 2008;95:5186–5192. doi: 10.1529/biophysj.108.140814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Q., Jayawardhana D.A., Guan X. Stochastic study of the effect of ionic strength on noncovalent interactions in protein pores. Biophys. J. 2008;94:1267–1275. doi: 10.1529/biophysj.107.117598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nestorovich E.M., Karginov V.A., Bezrukov S.M. Blockage of anthrax PA63 pore by a multicharged high-affinity toxin inhibitor. Biophys. J. 2010;99:134–143. doi: 10.1016/j.bpj.2010.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zitserman V.Y., Berezhkovskii A.M., Bezrukov S.M. Nonideality of polymer solutions in the pore and concentration-dependent partitioning. J. Chem. Phys. 2005;123:146101. doi: 10.1063/1.2052589. [DOI] [PubMed] [Google Scholar]
- 26.Krasilnikov O.V., Merzlyak P.G., Capistrano M.F. Protein electrostriction: a possibility of elastic deformation of the α-hemolysin channel by the applied field. Eur. Biophys. J. 2005;34:997–1006. doi: 10.1007/s00249-005-0485-9. [DOI] [PubMed] [Google Scholar]
- 27.Marcus Y. Marcel Dekker; New York: 1997. Ion Properties. [Google Scholar]
- 28.Krasilnikov O.V., Sabirov R.Z. Ion transport through channels formed in lipid bilayers by Staphylococcus aureus α-toxin. Gen. Physiol. Biophys. 1989;8:213–222. [PubMed] [Google Scholar]
- 29.Movileanu L., Cheley S., Bayley H. Partitioning of individual flexible polymers into a nanoscopic protein pore. Biophys. J. 2003;85:897–910. doi: 10.1016/S0006-3495(03)74529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bek S., Jakobsson E. Brownian dynamics study of a multiply-occupied cation channel: application to understanding permeation in potassium channels. Biophys. J. 1994;66:1028–1038. doi: 10.1016/S0006-3495(94)80884-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li S.C., Hoyles M., Chung S.H. Brownian dynamics study of ion transport in the vestibule of membrane channels. Biophys. J. 1998;74:37–47. doi: 10.1016/S0006-3495(98)77764-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaneko K., Ohba T., Iijima S. Nanospace molecular science and adsorption. Adsorption. 2005;11:21–28. [Google Scholar]
- 33.Okada T. Thermodynamic origin of selectivity in polyoxyethylene complexes with alkali cations. J. Chem. Soc. Chem. Commun. 1991;17:1209–1210. [Google Scholar]
- 34.Harris J.M. Introduction of biotechnical and biomedical applications of poly(ethylene glycol) In: Milton Harris J., editor. Poly(Ethylene Glycol) Chemistry: Biotechnical and Biomedical Applications. Springer; New York: 1992. pp. 1–14. [Google Scholar]
- 35.Reiner J.E., Kasianowicz J.J., Robertson J.W. Theory for polymer analysis using nanopore-based single-molecule mass spectrometry. Proc. Natl. Acad. Sci. USA. 2010;107:12080–12085. doi: 10.1073/pnas.1002194107. [DOI] [PMC free article] [PubMed] [Google Scholar]


