Abstract
Evidence is emerging for differential pathogenicity among Borrelia burgdorferi genotypes in the United States. By using two linked genotyping systems, ribosomal RNA intergenic spacer type (RST) and outer surface protein C (OspC), we studied the inflammatory potential of B. burgdorferi genotypes in cells and patients with erythema migrans or Lyme arthritis. When macrophages were stimulated with 10 isolates of each RST1, RST2, or RST3 strain, RST1 (OspC type A)–stimulated cells expressed significantly higher levels of IL-6, IL-8, chemokine ligand (CCL) 3, CCL4, tumor necrosis factor, and IL-1β, factors associated with innate immune responses. In peripheral blood mononuclear cells, RST1 strains again stimulated significantly higher levels of these mediators. Moreover, compared with RST2, RST1 isolates induced significantly more interferon (IFN)-α, IFN-γ, and CXCL10, which are needed for adaptive immune responses; however, OspC type I (RST3) approached RST1 (OspC type A) in stimulating these adaptive immune mediators. Similarly, serum samples from patients with erythema migrans who were infected with the RST1 genotype had significantly higher levels of almost all of these mediators, including exceptionally high levels of IFN-γ–inducible chemokines, CCL2, CXCL9, and CXCL10; and this pronounced inflammatory response was associated with more symptomatic infection. Differences among genotypes were not as great in patients with Lyme arthritis, but those infected with RST1 strains more often had antibiotic-refractory arthritis. Thus, the B. burgdorferi RST1 (OspC type A) genotype, followed by the RST3 (OspC type I) genotype, causes greater inflammation and more severe disease, establishing a link between spirochetal virulence and host inflammation.
Lyme disease, which is caused by the tickborne spirochete Borrelia burgdorferi, is the most common vectorborne disease in the United States and Europe.1 More than 20,000 cases are reported to the Centers for Disease Control and Prevention each year.2 The infection usually begins with an expanding skin lesion, erythema migrans (EM).1 Within days to weeks, B. burgdorferi in the northeastern United States may disseminate in blood, particularly to synovial tissue in joints. Months later, approximately 60% of untreated patients develop arthritis, most commonly affecting the knee.3,4 As with other manifestations of the disease, Lyme arthritis usually resolves with appropriate antibiotic therapy. However, in a few cases, synovitis persists for months or even several years after treatment with 2 to 3 months of oral and i.v. antibiotics, termed antibiotic-refractory Lyme arthritis.3,5,6
Infection with B. burgdorferi stimulates both innate and adaptive type 1 helper T-cell (TH1)–like immune responses, which are necessary for optimal spirochetal killing.7 The spirochete directly induces cells of monocyte lineage to secrete IL-6, IL-8, chemokine ligand (CCL) 3, CCL4, tumor necrosis factor (TNF), and IL-1β, which are typically involved in innate immune responses.8 In addition, spirochetes likely stimulate other cells, perhaps natural killer, natural killer T, or T cells, to secrete interferon (IFN)-γ, which causes cells of monocyte lineage to secrete the IFN-γ–inducible chemokines CXCL9 and CXCL10. These chemokines are strong chemoattractants for CD4+ and CD8+ T-effector cells, which are involved in TH1-like immune responses.9–11
Regional variations have been noted in the clinical manifestations and immune responses of patients with Lyme borreliosis in the United States and Europe. Early in the infection, B. burgdorferi, the sole agent of Lyme disease in the United States, is associated with faster expansion of EM lesions, more symptoms, and more frequent hematogenous dissemination compared with B. afzelii or B. garinii, the primary agent of the disease in Europe.1,8,12–16 In addition, B. burgdorferi is considerably more arthritogenic than B. afzelii, which usually remains localized to the skin; or B. garinii, which is particularly associated with neurological complications.1 Moreover, compared with EM lesions of B. afzelii–infected Austrian patients, those of B. burgdorferi–infected US patients had significantly higher mRNA levels of chemokines and cytokines associated with activation of macrophages,12 including chemoattractants for neutrophils (CXCL1), macrophages (CCL3, CCL4, IL-1β, and TNF), and TH1 cells (CXCL9 and CXCL10).17–19
Two genetically linked typing systems, one based on sequence variation of outer surface protein C (OspC) and the other on ribosomal RNA intergenic spacer type (RST), have been used to classify US B. burgdorferi strains.20–30 OspC typing divides B. burgdorferi strains into 21 genetically distinct types, 16 of which have been identified in the northeastern United States30,31; and RST divides B. burgdorferi into three groups.25 RST1 corresponds to OspC genotypes A and B; RST2 corresponds to OspC types F, H, K, and N; and RST3 corresponds to the remaining 10 OspC types, including D, E, G, and I.24,26,32,33 In the northeastern United States, infection with OspC type A (the most common RST1 strain) or OspC type K (the most common RST2 strain) accounts for approximately 60% of Lyme disease cases; and all of the OspC types within the RST1 and RST2 groups account for approximately 80% of cases.24,26,32 RST3, the least common type, is the most diverse, although infection with OspC type I accounts for approximately half of the RST3 cases.24,26,32 Because RST typing of isolates may miss differences within groups and because OspC typing may lead to small groups, more information for clinical correlations can be obtained from the use of both typing systems.
Evidence is emerging for differential pathogenicity among subtypes of B. burgdorferi in the United States.24–26,32–34 RST1 strains are more often detectable in blood in mice and humans,24,25,33–35 and they more frequently cause human antibiotic-refractory Lyme arthritis.32 However, the characteristics of the immune responses induced by different B. burgdorferi genotypes are incompletely described.
In this study, to determine whether strains of B. burgdorferi in the United States vary in inflammatory potential, macrophages and peripheral blood mononuclear cells (PBMCs) from healthy human donors were stimulated with a range of B. burgdorferi genotypes recovered from EM skin lesions of patients with Lyme disease; and cytokine and chemokine levels were measured in cell culture supernatants. In addition, levels of these inflammatory mediators were assessed in serum samples from patients with EM from whom the isolates were obtained and in joint fluid from patients with Lyme arthritis; the results were correlated with clinical outcomes. We found that the B. burgdorferi RST1 (OspC type A) genotype, followed by the RST3 (OspC type I) genotype, causes greater inflammation and more severe disease, establishing a link between spirochetal virulence and host inflammation.
Materials and Methods
Ethics Statement
All patients met the Centers for Disease Control and Prevention criteria for the diagnosis of Lyme disease.36,37 The study was approved by the Massachusetts General Hospital, Boston, Partners Human Research Committee, and all patients provided written informed consent.
Study Patients
During the summers of 1998 through 2001, we recovered 93 isolates of B. burgdorferi from biopsy samples of EM skin lesions in a study of US patients with Lyme disease from Rhode Island or Connecticut.26 In addition, serum samples that were obtained from these patients were used herein to determine protein levels of cytokines and chemokines. For clinical correlation, the patients were subdivided according to the number of symptoms associated with EM. Clinical observations were made by Nitin Damle, M.D., at the Rhode Island site and by Vijay Sikand, M.D., at the Connecticut site.
In addition, protein levels of cytokines and chemokines were assessed in joint fluid samples from the 17 patients who were treated with antibiotics, according to the guidelines of the Infectious Diseases Society of America; in these patients, the infecting strain could be determined by PCR.26 For clinical correlation, the patients were stratified into those with antibiotic-responsive arthritis, defined as the resolution of arthritis within 3 months after treatment with i.v. antibiotics for ≤4 weeks or oral antibiotics for ≤8 weeks; or antibiotic-refractory arthritis, defined as persistent joint swelling for ≥3 months after the start of treatment with i.v. antibiotics for ≥4 weeks or oral antibiotics for ≥8 weeks; or both.
Determination of B. burgdorferi RST and OspC Genotypes
In a previous study26 of genetic markers in patients with early Lyme disease, we identified the RST and OspC genotypes of isolates from the EM skin lesions of 90 of the patients seen from 1998 to 2001; 37 isolates were RST1 strains, 41 were RST2 strains, and 12 were RST3 strains. The OspC type of the isolates was determined using seminested PCR and sequencing techniques, and the RST was determined using nested PCR and restriction fragment length polymorphism. For this study, 30 isolates, 10 of each RST strain, were randomly selected from this larger group.
Because it has been almost impossible to culture B. burgdorferi from synovial fluid in patients with Lyme arthritis, the OspC and RST types could only be identified directly from small amounts of spirochetal DNA, sometimes present in joint fluid, using seminested PCR, sequencing, and restriction fragment length polymorphism analysis.32 Of 124 joint fluid samples obtained between 1975 and 2006, only 17 could be typed in patients who received recommended antibiotic regimens.32 For this study, 14 of these 17 samples were still available. Between 2006 and 2009, 39 additional joint fluid samples were collected, but it was possible to determine the OspC or RST type in only three of these samples. Thus, for this study, joint fluid samples were analyzed for cytokine and chemokine determinations in 17 patients, 14 from the previous study and 3 from more recent patients, who received recommended antibiotic regimens.
Preparation of Spirochetal Isolates
In preparation for cell culture experiments, each of the 30 low-passage (five or fewer) isolates was grown to mid- to late-log phase in complete Barbour-Stoenner-Kelly (BSK) medium (Sigma-Aldrich, St Louis, MO) containing 6% rabbit serum.38 Isolates were washed three times in PBS before assessing their numbers. Because it is difficult to count motile spirochetes reliably and because all 30 spirochetal cultures needed to be ready at the same time, the number of organisms in each culture was determined by OD. The concentration of organisms was adjusted to 1.25 × 108 spirochetes per milliliter for experiments with macrophages and to 2.5 × 108 spirochetes/mL for experiments with PBMCs, based on a carefully constructed standard curve, as previously described.8 The adjusted concentrations of organisms were confirmed by measuring the total protein concentration (DC Protein Assay; BioRad, Hercules, CA) of the isolates. This allowed for similar numbers of organisms of each isolate to be used in all experiments.
Macrophage Cell Culture
Human macrophages were differentiated from PBMCs obtained from three healthy donors by the Massachusetts General Hospital Blood-Component Laboratory. Before blood donation, donors were required to answer a questionnaire, provide a vaccination report, and undergo a physical examination to ensure their health. Blood samples were tested for markers of infectious diseases, including syphilis, hepatitis B virus, hepatitis C virus, HIV 1/2, human T-lymphotropic virus I/II, and West Nile virus, all of which were negative in our blood samples.
Macrophages were derived as previously described.8,39,40 Briefly, PBMCs from buffy coats were resuspended in RPMI 1640 medium (Invitrogen, Carlsbad, CA) containing 10% human serum (Mediatech, Manassas, VA) and seeded in flasks for 1 to 2 hours to allow attachment of monocytes. Detached cells were removed by washing, and adherent cells were allowed to differentiate into macrophages by 6-day culture in RPMI 1640 medium supplemented with 25% human serum, 2 mmol/L l-glutamine, 100 μg/mL streptomycin, and 100 U/mL penicillin at 37°C and 5% CO2. Before stimulation with borrelial isolates, adherent macrophages were washed three times, incubated in 5 mmol/L EDTA (Sigma-Aldrich) for 10 minutes, and removed by scraping. Cells were then washed and transferred to 96-well culture plates at 1 × 105 cells per well and deprived of serum and antibiotics for 12 hours to remove growth factors. Because the macrophages from frozen PBMC stocks exhibit a dampened inflammatory response, only fresh macrophages were used in this study. Macrophages derived in this manner are >95% pure.8 Preliminary experiments demonstrated that maximal expression of macrophage-derived cytokines and chemokines occurred within 48 hours at a multiplicity of infection of 25 organisms per cell (data not shown). These conditions were used in all macrophage experiments.
PBMC Cell Culture
Human PBMCs were obtained from three healthy donors by the Massachusetts General Hospital Blood-Component Laboratory. Different donors were used to obtain PBMCs versus macrophages, and the experiments with these two cell types were conducted independently. PBMCs were isolated from leukopaks after centrifugation in medium (Lymphocyte Separation Medium; MP Biomedicals, Solon, OH). Cells were then washed three times, resuspended in RPMI medium supplemented with 10% human serum and 2 mmol/L l-glutamine, and seeded into round-bottom 96-well plates at 2 × 105 cells per well at 37°C and 5% CO2. In an effort to exclude the possibility that B. burgdorferi strains altered the biological activity of PBMCs, global metabolic activity, cytotoxicity, and proliferation were assessed in these cells stimulated for 120 hours with each of the 30 isolates, as previously described for macrophages.8 In addition, preliminary experiments demonstrated that optimal expression of most cytokines and chemokines associated with the activation of innate immune cells occurred within 24 hours of stimulation, whereas induction of most IFN-inducible chemokines required 120 hours at a multiplicity of infection of 25 organisms per cell (data not shown). These conditions were used in subsequent PBMC experiments.
Detection of Cytokines and Chemokines from Normal Macrophages or PBMCs Stimulated with Patients' B. burgdorferi Isolates
Macrophages were cultured with each of the 10 isolates of B. burgdorferi RST1 strain (OspC type A), 10 isolates of RST2 strain (OspC type F, K, or N), or 10 isolates of RST3 strain (OspC type D, E, G, or I) at a multiplicity of infection of 25 for 48 hours in medium devoid of serum and antibiotics. All 30 isolates were tested with macrophages from each of the three healthy donors. PBMCs were cultured with the same 10 isolates of each B. burgdorferi RST type for 24 or 120 hours in culture medium devoid of antibiotics. As with macrophages, all 30 isolates were tested with PBMCs from each of the three healthy donors. The expression of macrophage-derived cytokines (ie, TNF, IL-1β, IL-6, and IL-10) and chemokines (ie, IL-8, CCL2, CCL3, CCL4, and CCL5) or PBMC-derived cytokines (ie, TNF, IL-1β, IL-6, IL-10, IFN-γ, and IFN-α) and chemokines (ie, IL-8, CCL2, CCL3, CCL4, CXCL9, and CXCL10) was assessed in culture supernatants (1:25) using two separate bead-based multiplex assays (Millipore, Billerica, MA) coupled with the Luminex-200 System Analyzer (Luminex, Austin, TX), as recommended by the manufacturer. The mean fluorescence intensity was converted to pg/mL by Upstate Beadview software (Millipore). The results for each chemokine and cytokine from all three macrophage experiments or all three PBMC experiments were averaged for analysis. This minimized host variation and allowed for a true assessment of differences among RST groups.
Detection of Cytokines and Chemokines in Patient Serum and Joint Fluid Samples
Serum samples, obtained from EM skin biopsy specimens, were available from the same 28 of the 30 patients from whom Borrelia isolates were recovered: 8 were from B. burgdorferi RST1 strain, 10 were from RST2 strain, and 10 were from RST3 strain. Joint fluid samples were available from the 17 patients with antibiotic-responsive or antibiotic-refractory Lyme arthritis in whom the RST and OspC subtype of the infecting strain could be determined. The levels of cytokines (IL-6, IL-10, TNF, IL-1β, and IFN-γ) and chemokines (IL-8, CCL2, CCL3, CCL4, CCL5, CXCL9, and CXCL-10) were assessed in all serum samples at the same time using bead-based multiplex assays (Millipore). Similarly, the levels of these factors and IFN-α were determined in all joint fluid samples at the same time using separate multiplex assays. Because of limited amounts of serum and joint fluid, cytokine and chemokine levels in these samples were determined once.
Statistical Analysis
Differences between groups of borrelial isolates in cell culture experiments, between patient groups according to the number of symptoms, or between patient groups according to antibiotic-responsive or antibiotic-refractory course of arthritis were assessed using the Mann-Whitney rank-sum test. This nonparametric test was selected because of the differences in the number and distribution of samples in each comparison group, which is common in studies using human samples. Statistical analyses were conducted using Sigma Stat version 3.0.1 software (SPSS, Chicago, IL). P < 0.05 was considered statistically significant; all P values are two tailed.
Results
Cytokine and Chemokine Secretion by Normal Human Macrophages Stimulated with B. burgdorferi Genotypes Isolated from Patients with EM
To test the inflammatory potential of B. burgdorferi isolates in cells of the innate immune lineage, macrophages from three healthy human donors were each stimulated with each of the 30 B. burgdorferi isolates from patients with EM, 10 each of the three RST genotypes. During these experiments, two isolates of the RST1 genotype were contaminated; and the values from those samples were discarded. Only a small variation in the levels of the nine cytokines and chemokines was detected among the cells from three donors, as shown for stimulation with RST1 isolates (Figure 1A); therefore, the results from the donors were averaged before comparing differences among the B. burgdorferi genotypes (Figure 1B).
Figure 1.
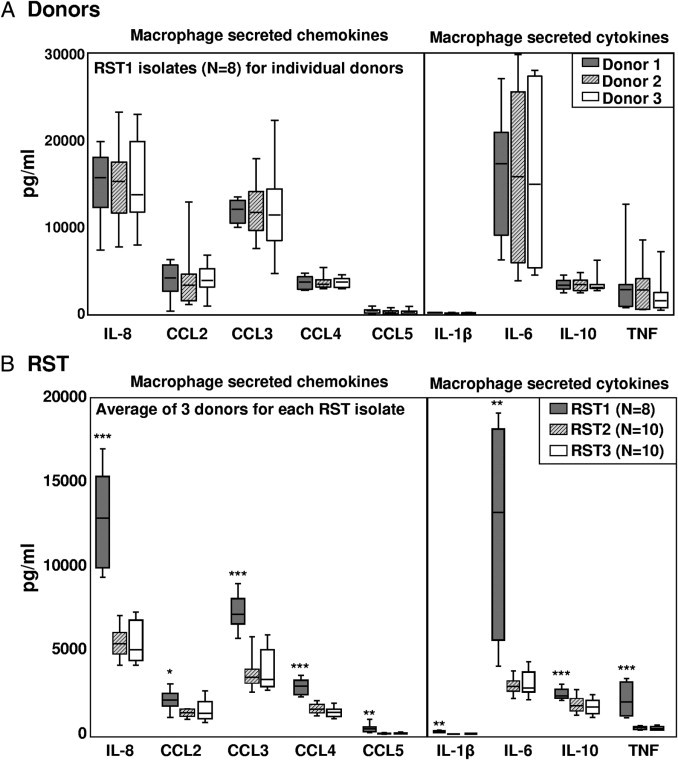
Inflammatory potential of the three B. burgdorferi RST genotypes in macrophage cultures. Monocyte-derived macrophages from three normal human donors were each stimulated with 28 B. burgdorferi isolates (8 RST1, 10 RST2, and 10 RST3) at a multiplicity of infection of 25 for 48 hours. Protein expression of five chemokines and four cytokines in culture supernatants was assessed simultaneously in one complete experiment using multiplex assays. A: Results are shown individually for each donor. B: Results from all three donors were averaged for comparison of the three RST groups. The box represents 25th to 75th percentiles; lines outside the box, 5th to 95th percentiles; and horizontal line in the box, median. For the comparison of RST1 with RST2 or RST3, *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001. Differences between RST2 and RST3 strains were not statistically significant.
All 28 isolates (8 RST1, 10 RST2, and 10 RST3) induced macrophages to secrete the macrophage-associated cytokines (ie, IL-1β, IL-6, IL-10, and TNF) and chemokines (ie, IL-8, CCL2, CCL3, CCL4, and CCL5) tested herein (Figure 1B). Compared with RST2 or RST3 strains, RST1 isolates stimulated the secretion of significantly higher levels of each of these inflammatory mediators. No statistically significant differences in cytokine and chemokine secretion were observed between macrophages stimulated with RST2 or RST3 isolates. The most highly expressed cytokine was IL-6 (median, 13,223 pg/mL), and the most highly expressed chemokines were IL-8 (median, 12,040 pg/mL) and CCL3 (median, 7294 pg/mL). Within the RST1 group, there were no clear trends regarding differences in inflammatory potential among the eight individual isolates. Unstimulated cells secreted only low levels of IL-8, CCL2, and CCL3; and undetectable levels of the other cytokines and chemokines.
The 28 isolates could also be classified into eight OspC subtypes. All of the RST1 isolates were OspC type A; seven of the RST2 isolates were OspC type K, two were type N, and one was type F; and five of the RST3 isolates were OspC type I, two each were type E or G, and one was type D (Figure 2). This distribution of B. burgdorferi genotypes is consistent with the frequencies of these subtypes in the northeastern United States.24,26,32 OspC type A (RST1) isolates induced macrophages to secrete significantly higher levels of all macrophage-associated cytokines and chemokines tested herein compared with OspC type K (RST2) isolates or OspC type I (RST3) isolates (Figure 2; see also Supplemental Table S1 at http://ajp.amjpathol.org). However, among the OspC types corresponding to RST2, OspC type K appeared to be slightly more inflammatory; and among the OspC types corresponding to RST3, OspC type I tended to be the most inflammatory. Because of small numbers, these differences within the RST2 and RST3 groups were not statistically significant. Thus, B. burgdorferi OspC type A (RST1) isolates induced macrophages to secrete significantly higher levels of cytokines and chemokines associated with innate immune responses, particularly IL-6, IL-8, and CCL3, than other B. burgdorferi genotypes.
Figure 2.
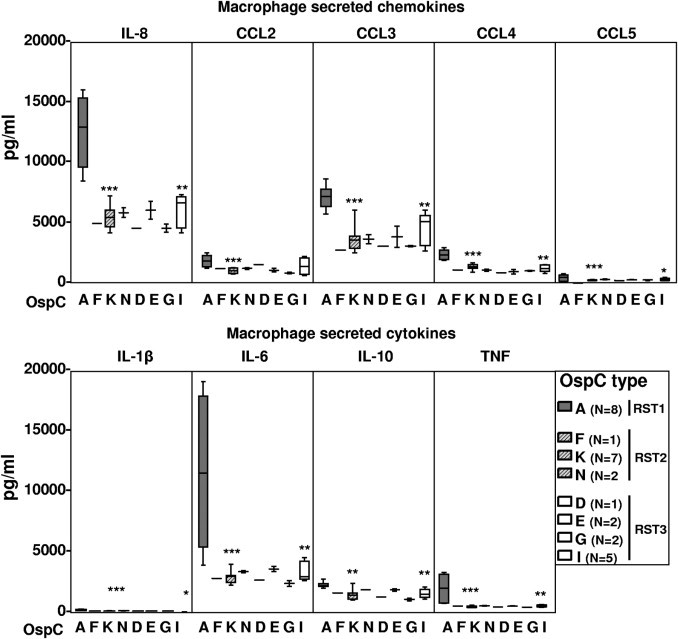
Inflammatory potential of eight B. burgdorferi OspC genotypes in macrophage cultures. The 28 B. burgdorferi isolates used in experiments in Figure 1 were subdivided according to their OspC type. The box represents 25th to 75th percentiles; lines outside the box, 5th to 95th percentiles; and horizontal line in the box, median. For the comparison of OspC type A with type K or I, *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001. There were no significant differences between OspC type K and OspC type I strains.
Cytokine and Chemokine Secretion by Normal Human PBMCs Stimulated with B. burgdorferi Genotypes Isolated from Patients with EM
Next, we tested the inflammatory potential of the B. burgdorferi isolates with PBMCs, a mixed cell sample that includes cells involved in innate and adaptive immune responses. For this purpose, PBMCs from three healthy human donors were stimulated with 30 isolates from patients with EM, 10 each of the three RST genotypes; and cell supernatants were collected at 24 hours and 5 days. Previous experiments with PBMCs showed that 24-hour stimulation was optimal for cytokine and chemokine secretion associated with direct activation of innate immune cells by B. burgdorferi, whereas 5 days was optimal for expression of cytokines and chemokines that require interaction between innate and adaptive immune cells.41 In addition, we found no significant differences in the ability of B. burgdorferi genotypes to alter the global metabolic activity, cytotoxicity, or proliferation of PBMCs (data not shown), which was consistent with recently published findings in macrophages.8 These results indicated that potential differences in the inflammatory capacity of strains could not be attributed to the ability of strains to alter global changes in cells' biological activity.
Consistent with the results in macrophages, PBMCs stimulated with RST1 isolates for 24 hours secreted significantly higher levels of IL-6, IL-8, CCL3, and CCL4 than RST2 or RST3 isolates (Figure 3A), reflecting the activation of cells of innate immune lineage. In addition, RST1 strains stimulated significantly higher levels of IFN-α and CXCL10 than RST2 strains (Figure 3A). At 5 days, all isolates induced high levels of IFN-γ and CXCL9, indicating that longer stimulation allowed for recruitment and activation of cells that link innate and adaptive responses (Figure 3B). Although RST1 strains still tended to stimulate the highest levels of IFN-γ and IFN-γ–inducible chemokines CXCL9 and CXCL10, the differences among RSTs were less pronounced at 5 days because RST3 strains approached RST1 strains in stimulating these mediators. No significant differences were detected between RST2 and RST3 strains.
Figure 3.
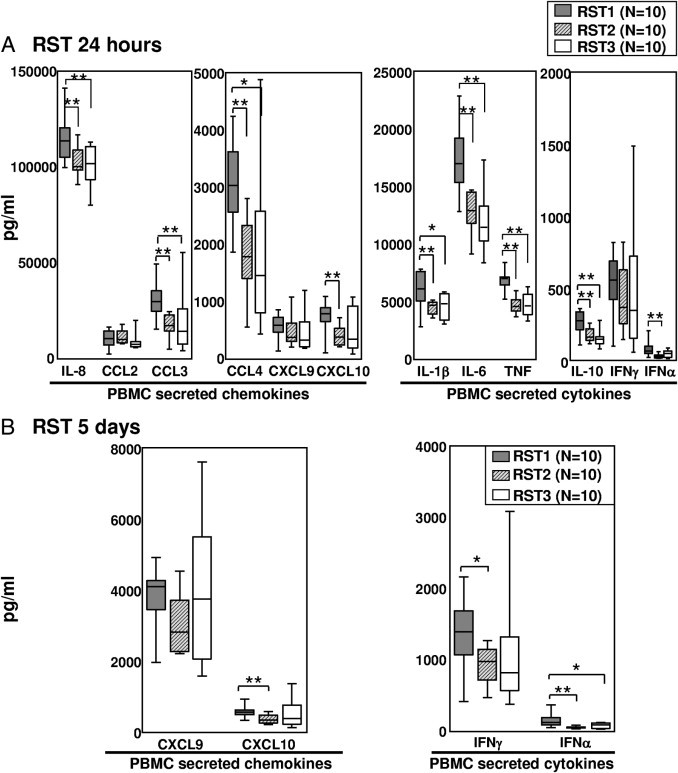
Inflammatory potential of three B. burgdorferi RST genotypes in PBMC cultures. PBMCs from three healthy donors were stimulated with 30 B. burgdorferi isolates, 10 of each RST strain, at a multiplicity of infection of 25 for 24 hours (A) or 5 days (B). Protein expression of six chemokines and six cytokines in culture supernatants was assessed simultaneously in one complete experiment using multiplex assays. All 30 isolates were tested with PBMCs from each of the three healthy donors. The results from all three donors were averaged for comparison of the three RST groups. The box represents 25th to 75th percentiles; lines outside the box, 5th to 95th percentiles; and horizontal line in the box, median. For the comparison of RST1 with RST2 or RST3, *P ≤ 0.05 and **P ≤ 0.01. There were no significant differences between RST2 and RST3 strains.
Similar findings were observed when the results were analyzed according to the three most common OspC types. All 10 RST1 strains were OspC type A, whereas seven of the RST2 strains were OspC type K, and five of the RST3 strains were OspC type I. Again, OspC type A (RST1) was the most inflammatory genotype. At 24 hours, OspC type A–stimulated PBMCs secreted significantly higher levels of most of the chemokines and cytokines compared with OspC type K (RST2)–stimulated cells (Figure 4; see also Supplemental Table S2 at http://ajp.amjpathol.org). However, OspC type I isolates (an RST3 strain) approached OspC type A isolates in inducing the secretion of certain cytokines and chemokines, particularly IFN-α, IFN-γ, CXCL9, and CXCL10; this trend became more apparent at 5 days. Thus, OspC type I strains within the RST3 genotype were similar to OspC type A (RST1) strains in their ability to induce the part of the inflammatory response needed for the induction of TH1-like adaptive immune responses.
Figure 4.
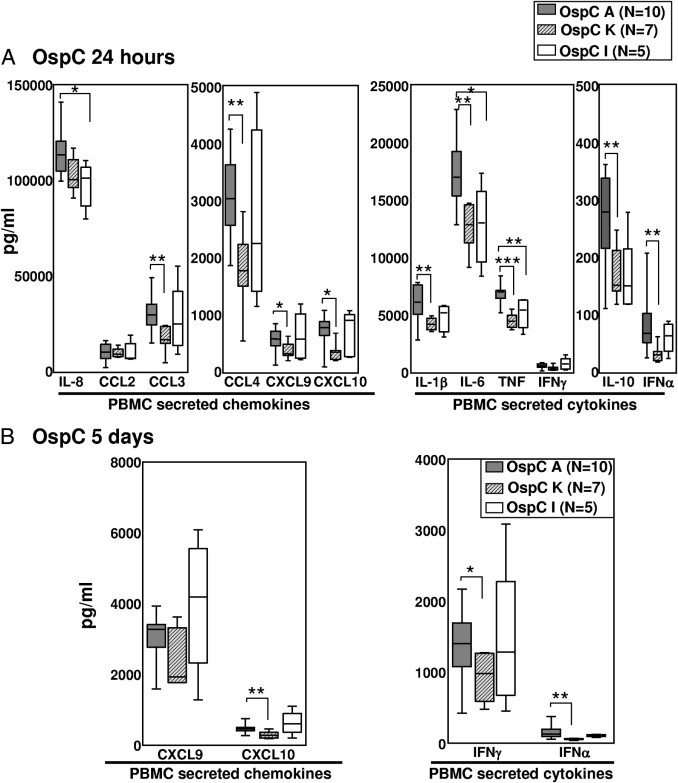
Inflammatory potential of eight B. burgdorferi OspC genotypes in PBMC cultures. The 30 B. burgdorferi isolates used in experiments in Figure 3 were subdivided according to their OspC type. Levels of cytokines and chemokines were compared between 10 OspC type A isolates, seven OspC type K isolates, and five OspC type I isolates after 24 hours (A) or 5 days (B) of stimulation. The box represents 25th to 75th percentiles; lines outside the box, 5th to 95th percentiles; and horizontal line in the box, median. For the comparison of OspC type A with type K or I, *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001.
Cytokine and Chemokine Levels in Serum Samples from Patients with EM
To examine the inflammatory potential of the B. burgdorferi genotypes in vivo, cytokine and chemokine levels were assessed in serum samples from the 28 patients with EM whose isolates were used for the macrophage experiments. These samples were obtained a median of 3 days after the onset of illness. Consistent with the in vitro responses of B. burgdorferi–stimulated macrophages and PBMCs, the levels of IL-8, CCL2, CCL3, CCL4, IL-1β, and TNF were significantly higher in serum samples of patients infected with the RST1 genotype compared with those infected with the RST2 or RST3 genotype (Figure 5A). The levels of these inflammatory mediators in healthy control subjects were low or undetectable (data not shown). Consistent with in vitro responses of B. burgdorferi–stimulated PBMCs, infection with the RST1 genotype was also associated with significantly higher levels of IFN-γ and exceptionally high levels of the IFN-γ–inducible chemokines CCL2, CXCL9, and CXCL10 (Figure 5A). When the patients with EM were stratified according to infection with the most common OspC types (A, K, and I), OspC type A (RST1) strains tended to be the most inflammatory, although all three of these OspC types induced high levels of CCL2, CXCL9, and CXCL10. Moreover, OspC type I (RST3) strains sometimes approached or even exceeded the inflammatory potential of OspC type A strains (Figure 5B; see also Supplemental Table S3 at http://ajp.amjpathol.org). Thus, in serum samples, the TH1-associated chemokines and cytokines were the predominant inflammatory mediators, whereas in cultured cells, factors associated with the innate immune response were induced at the highest levels.
Figure 5.
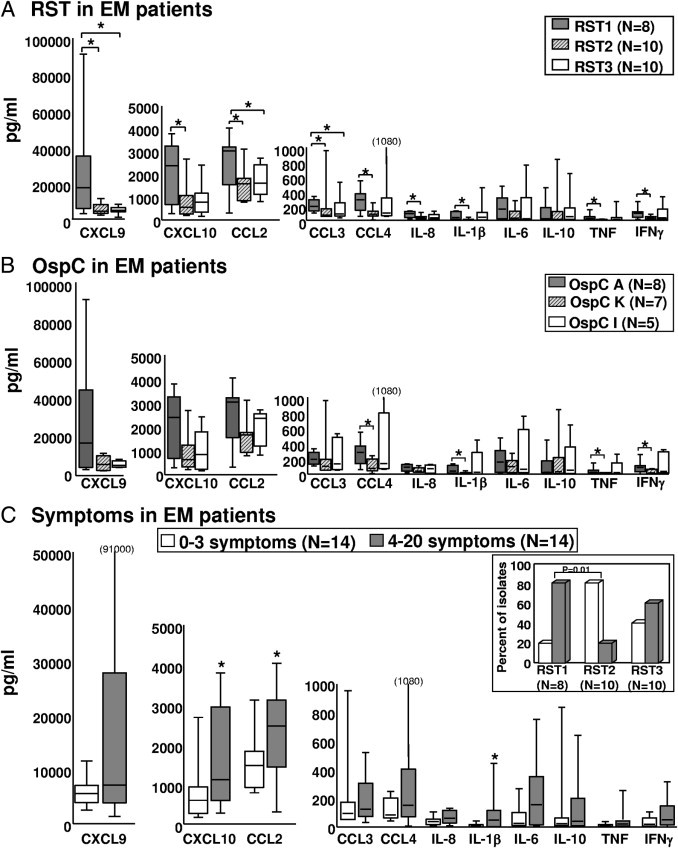
Cytokine and chemokine levels in serum samples from patients with EM. Serum samples were available from 28 patients with EM from whom the isolates were obtained. Protein expression of cytokines and chemokines was determined using multiplex assays from all serum samples in one complete experiment. Patient serum samples were subdivided according to the RST type of the infecting strain (8 RST1, 10 RST2, and 10 RST3) (A) or the three most common OspC types (B). C: Patient samples were subdivided into two equally sized groups based on the number of symptoms. Inset: The percentage of isolates in each RST group associated with either 0 to 3 or 4 to 20 symptoms. The box represents 25th to 75th percentiles; lines outside the box, 5th to 95th percentiles; and horizontal line in the box, median. *P ≤ 0.05.
The clinical characteristics of the 28 patients are shown in Table 1. The size (median, 10 cm) and duration (median, 3 days) of EM skin lesions were similar among the three RST patient groups. Similarly, the frequency of hematogenous dissemination, as determined by PCR positivity in blood or multiple EM lesions, did not differ significantly among the three groups; and positive or negative PCR results in blood did not correlate with the levels of chemokines and cytokines. However, patients infected with RST1 (OspC type A) strains had significantly more symptoms (median, 8.5), such as headache, stiff neck, fever, myalgias, or arthralgias, compared with RST2-infected patients (OspC types F, K, and N) (median, 1.5) (P < 0.05; Figure 5C, inset). Among RST3-infected patients, the number of associated symptoms was more variable; the five patients with OspC type I (RST3) infection had a median of 6.0 symptoms. Thus, during the first days of illness, OspC type A (RST1)–infected patients were usually the most symptomatic, followed by those infected with OspC type I (RST3) strains.
Table 1.
Clinical Findings in Patients with EM According to Isolate Genotype
| Species and genotype | Total isolates | EM |
No. of patients with hematogenous dissemination⁎ | No. of symptoms [median (range)]† | |
|---|---|---|---|---|---|
| Median size (cm) | Median duration (days) | ||||
| B. burgdorferi | 30 | 10 | 3 | 15 | 5.0 (0–20) |
| RST1 | 10 | 10 | 3 | 6 | 8.5 (3–11) |
| OspC type A | 10 | 10 | 3 | 6 | 8.5 (3–11) |
| RST2 | 10 | 12 | 4 | 6 | 1.5 (0–20) |
| OspC type F | 1 | 18 | 3 | 0 | 2.0 (2) |
| OspC type N | 2 | 14 | 6 | 1 | 0.5 (0–1) |
| OspC type K | 7 | 12 | 3 | 5 | 3.0 (0–20) |
| RST3 | 10 | 10 | 3 | 3 | 6.5 (0–10) |
| OspC type D | 1 | 8 | 7 | 1 | 0.0 (0) |
| OspC type E | 2 | 10 | 4 | 1 | 9.0 (8–10) |
| OspC type G | 2 | 12 | 4 | 0 | 4.5 (0–9) |
| OspC type I | 5 | 9 | 3 | 1 | 6.0 (2–9) |
Hematogenous dissemination in patients with EM was based on a positive PCR result in blood or multiple EM lesions.
The number of symptoms in patients with RST1 infection was significantly greater than in patients with RST2 infection (P = 0.01).
To investigate the relationship between the number of associated symptoms and the inflammatory immune response, the levels of cytokines (ie, IL-1β, IL-6, IL-10, TNF, and IFN-γ) and chemokines (ie, IL-8, CCL2, CCL3, CCL4, CXCL9, and CXCL10) in serum were compared among the 28 patients subdivided into two equally sized groups: those with zero to three symptoms and those with four symptoms or greater. In both groups, IFN-γ–inducible chemokines (ie, CCL2, CXCL9, and CXCL10) were most highly expressed, reflecting the predominant TH1 response early in the disease. However, patients with four symptoms or greater had significantly higher levels of IL-1β, CCL2, and CXCL10; and they tended to have higher levels of other inflammatory cytokines and chemokines (Figure 5C). Thus, a more pronounced inflammatory response, characterized by particularly high levels of IFN-γ–inducible chemokines, was associated with more symptomatic infection.
Cytokine and Chemokine Levels in Joint Fluid of Patients with Antibiotic-Responsive or Antibiotic-Refractory Lyme Arthritis
Although it has rarely been possible to culture B. burgdorferi from joint fluid in patients with Lyme arthritis,42,43 we have sometimes been able to determine the infecting strain directly from that site by PCR, primarily from samples obtained years ago before the use of antibiotics for the treatment of Lyme disease.32 Of 153 joint fluid samples collected during a 35-year period, only 20 could be typed in patients who received recommended antibiotic regimens; 17 were available for this study. By definition, all 17 patients had positive PCR results for B. burgdorferi DNA in joint fluid. Although there were examples of all three RSTs among the 17 patient samples, the number with a given OspC type was small, including only three with OspC type A and none with OspC type I.
Of the 17 patients, those infected with RST1 strains had significantly higher levels of IFN-γ than those infected with RST2 or RST3 strains (Figure 6A). In addition, RST1-infected patients had higher levels of TNF, IL-1β, and CCL2 than RST3-infected patients, although this group lacked patients infected with OspC type I. Thus, the differences among genotypes were not as great in patients with Lyme arthritis, a late disease manifestation, as in those with EM early in the infection.
Figure 6.
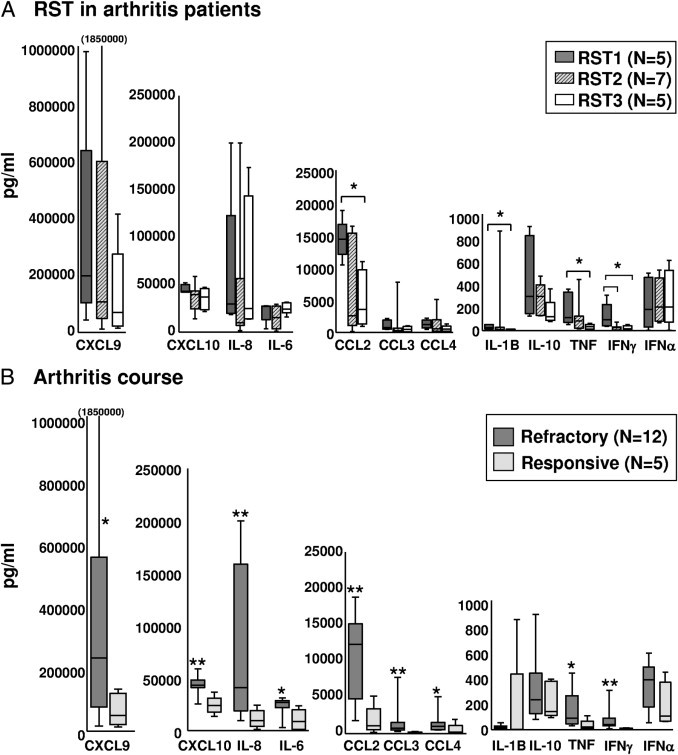
Cytokine and chemokine levels in joint fluid of patients with antibiotic-responsive or antibiotic-refractory Lyme arthritis. Joint fluid samples were available from 12 patients with antibiotic-refractory and five patients with antibiotic-responsive arthritis in whom the RST type of the infecting strain could be determined. Of the 17 patients, five were infected with RST1 strains (three with OspC type A and two with type B), seven were infected with RST2 strains (four with OspC type K, two with type H, and one with type N), and five were infected with RST3 strains (two with OspC type G, one with type J, and, in two, only the RST could be determined). Cytokine and chemokine levels were compared according to the RST type of the infecting strain (A) and antibiotic-refractory and antibiotic-responsive arthritis (B). All cytokine and chemokine levels in each of the 17 joint fluid samples were determined by multiplex assays in one complete experiment. The box represents 25th to 75th percentiles; lines outside the box, 5th to 95th percentiles; and horizontal line in the box, median. For the comparison of RST1 with RST2 or RST3, *P ≤ 0.05. For the comparison of refractory with responsive arthritis, *P ≤ 0.05 and **P ≤ 0.01.
Of the 17 patients, 12 had antibiotic-refractory arthritis and 5 had antibiotic-responsive arthritis. When the course of their arthritis was stratified by B. burgdorferi genotype, all five patients with RST1 infection had antibiotic-refractory arthritis compared with four of seven patients with RST2 and three of five patients with RST3 infection. However, the trend toward a greater frequency of RST1 infection in antibiotic-refractory patients was not statistically significant. Regardless of the infecting genotype, patients with antibiotic-refractory arthritis had significantly higher levels of almost all cytokines and chemokines tested, particularly the IFN-γ–inducible chemokines (ie, CCL2, CXCL9, and CXCL10), compared with patients with antibiotic-responsive arthritis (Figure 6B). Thus, in patients with Lyme arthritis, there was a trend toward a more pronounced inflammatory response in those infected with RST1 strains, but high inflammatory responses were found in those with antibiotic-refractory arthritis, regardless of the infecting strain.
Discussion
In this study, we evaluated the inflammatory potential of B. burgdorferi isolates in cultures of macrophages and PBMCs and in B. burgdorferi–infected patients with EM or Lyme arthritis. Several approaches were taken to standardize testing and validate the results. First, we tested 8 to 10 isolates of each of the three RSTs, including eight OspC types that cause Lyme disease in the northeastern United States.24,26,32 Second, in an effort to hold host factors constant, we tested all 28 isolates with fresh macrophages or PBMCs from each of the three healthy donors. Third, we used bead-based multiplex assays to simultaneously measure protein expression of all chemokines and cytokines from each complete experiment. Fourth, we compared the cytokine and chemokine results from cell culture studies with those obtained using serum samples from the same patients with EM from whom the isolates were obtained. Finally, we assessed inflammatory mediators in joint fluid from patients with Lyme arthritis, a late disease manifestation, in whom it was possible to determine the infecting strain by PCR. However, some of the less common OspC types were not examined, including OspC type B (RST1) and OspC type H (RST2), which appear to be more invasive strains.24 Although a more detailed analysis of B. burgdorferi genotypes is possible using techniques such as multilocus PCR electrospray mass spectrometry,44 the information gained from less common strains of B. burgdorferi is limited by lack of clinical correlations. Thus, the current study allows a comparison of more common B. burgdorferi genotypes but not all of the OspC subtypes.
With both culture systems, RST1 (OspC type A) isolates were more inflammatory than RST2 or RST3 isolates, but these differences in inflammatory potential among B. burgdorferi genotypes were most apparent with macrophages. Consistent with a previous study,8 we showed that B. burgdorferi, particularly RST1 strains, stimulated macrophages from normal human donors to secrete high levels of TNF, IL-1β, IL-6, IL-8, CCL3, and CCL4, cytokines and chemokines that are important in the recruitment and activation of neutrophils, monocytes, and macrophages in innate immune responses.9–11 At 24 hours, RST1-stimulated PBMCs also secreted greater amounts of these macrophage-associated cytokines and chemokines. Moreover, RST1-stimulated PBMCs expressed higher levels of IFN-α and CXCL10 than RST2-stimulated cells. Other investigators45 identified IFN-α–producing populations in B. burgdorferi–stimulated PBMCs as plasmacytoid dendritic cells and CD14+CD11c+ dendritic cells, which are also involved in innate immune responses.
By 5 days, PBMCs stimulated with OspC type A (RST1) isolates also secreted the highest levels of IFN-γ and IFN-γ–inducible chemokines (ie, CXCL9 and CXCL10), but OspC type I (RST3) strains approached OspC type A (RST1) strains in inducing these mediators. B. burgdorferi stimulates cells of monocyte lineage directly to secrete CCL4, whereas stimulation of intermediate cell types, probably natural killer, natural killer T, and T cells, is necessary for secretion of IFN-γ; this induces macrophages to secrete CXCL9 and CXCL10,41 thereby linking innate and TH1-like adaptive immune responses.10 Other investigators24 reported that OspC types A and I were more often detectable in blood, suggesting that these two types were particularly virulent. Thus, the B. burgdorferi OspC type A (RST1) genotype induced PBMCs to secrete the highest levels of cytokines and chemokines involved in innate and adaptive TH1-like immune responses, whereas the OspC type I (RST3) genotype approached OspC type A strains in stimulating the part of the inflammatory response needed for adaptive immune responses.
The greater inflammatory potential of B. burgdorferi RST1 (OspC type A) isolates in cell cultures was also observed in serum samples from patients with EM from whom the isolates were obtained. Compared with RST2- or RST3-infected patients, serum samples of RST1-infected patients contained significantly higher levels of a range of cytokines and chemokines involved in innate and adaptive immunity, but high levels of IFN-γ, CCL2, CXCL9, and CXCL10 were the most prominent. When analyzed by OspC type, infection with OspC type I was close to OspC type A in inflammatory potential. Consistent with these findings, previous studies of EM lesions, which were not available for study herein, showed that IFN-γ, IL-6, and IL-10 were the predominant cytokines17,19 and that CXCL9 and CXCL10 were the most highly expressed chemokines.12,18
In addition to the greater inflammatory response, RST1-infected patients with EM had more symptoms, including headache, stiff neck, fever, myalgias, and arthralgias, followed closely by OspC type I (RST3)-infected patients. Although significant differences among genotypes in the frequency of hematogenous dissemination were not observed in our study, a previous analysis24 of 422 EM skin and blood isolates found that OspC type A and I strains were more often detected in blood. Finally, in patients with EM who were seen before the cause of Lyme disease was known and before this infection was treated with antibiotics, those with more symptoms early in the infection were more likely to develop subsequent arthritis.4 Thus, in the first days of illness, infection with the RST1 (OspC type A) genotype, followed by infection with the RST3 (OspC type I) genotype, was associated with greater inflammation, more severe symptoms, more frequent hematogenous dissemination, and, probably, more common progression to late-stage disease.
In a previous study,32 patients with antibiotic-refractory Lyme arthritis were more often infected with RST1 strains; this trend was seen in the current patients. Moreover, we showed herein that RST1-infected patients had higher levels of IFN-γ in joint fluid than RST2- or RST3-infected patients, but differences among genotypes were less pronounced than those seen in serum early in the infection. Instead, consistent with previous experience,46 the current patients with antibiotic-refractory arthritis had significantly higher levels of almost all cytokines and chemokines measured herein compared with patients with antibiotic-responsive arthritis; this difference between patients with refractory or responsive arthritis was seen regardless of the infecting strain. Thus, infection with other inflammatory strains of B. burgdorferi, not just RST1 strains, and several host factors, such as HLA type,5 likely interact to produce the marked joint inflammation that is necessary for the development of antibiotic-refractory arthritis.
However, persistent arthritis after oral and i.v. antibiotic therapy does not seem to result from spirochetal persistence. After such therapy, PCR results for B. burgdorferi DNA in joint fluid are typically negative; they were uniformly negative in synovial tissue obtained at synovectomy months after the completion of oral and i.v. antibiotics.47 We postulate that highly inflammatory responses, such as those induced by RST1 infection, may set the stage for infection-induced autoimmunity in affected joints,3,32 leading to proliferative synovitis that may persist for months to several years after spirochetal killing with antibiotic therapy.
In summary, evidence continues to grow regarding differences among B. burgdorferi genotypes in the northeastern United States. In the enzootic infection, RST1 strains have higher transmission efficiency from ticks to mice than other strains,48 which may give them a selective advantage. Interestingly, RST1 (OspC type A) strains, which appear to be a recently evolved clonal lineage, may have been introduced into the northeastern United States <200 years ago and may be an important factor in the emergence of the Lyme disease epidemic in the northeastern United States in the late 20th century.49,50 This study shows that B. burgdorferi OspC type A (the most common RST1 genotype), followed by OspC type I (the most common RST3 genotype), causes greater inflammation and more severe disease, establishing a link between spirochetal virulence and host inflammation. Furthermore, these findings help to explain the markedly symptomatic early infection and occurrence of antibiotic-refractory Lyme arthritis late in the disease in the northeastern United States.
Acknowledgments
We thank Dr. Nitin Damle and Dr. Vijay Sikand for obtaining the skin biopsy and serum samples from patients with EM, Gail McHugh for help with samples, Dr. Lisa Glickstein for review of the manuscript, and Colleen Squires for help with preparation of the manuscript.
Footnotes
Supported by a grant from the National Institutes of Health (AR-20358); the English, Bonter, Mitchell Foundation; the Lyme/Arthritis Research Fund at Massachusetts General Hospital; the Eshe Fund; the Walter J. and Lille A. Berbecker Foundation for the study of Lyme disease (K.S.); and the Arthritis Foundation (K.S.).
Supplemental material for this article can be found at http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2011.02.018.
Supplementary data
References
- 1.Steere A.C. Lyme disease. N Engl J Med. 2001;345:115–125. doi: 10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
- 2.Bacon R.M., Kugeler K.J., Mead P.S. Surveillance for Lyme disease: United States, 1992–2006. MMWR Surveill Summ. 2008;57:1–9. [PubMed] [Google Scholar]
- 3.Steere A.C., Glickstein L. Elucidation of Lyme arthritis. Nat Rev Immunol. 2004;4:143–152. doi: 10.1038/nri1267. [DOI] [PubMed] [Google Scholar]
- 4.Steere A.C., Schoen R.T., Taylor E. The clinical evolution of Lyme arthritis. Ann Intern Med. 1987;107:725–731. doi: 10.7326/0003-4819-107-5-725. [DOI] [PubMed] [Google Scholar]
- 5.Steere A.C., Klitz W., Drouin E.E., Falk B.A., Kwok W.W., Nepom G.T., Baxter-Lowe L.A. Antibiotic-refractory Lyme arthritis is associated with HLA-DR molecules that bind a Borrelia burgdorferi peptide. J Exp Med. 2006;203:961–971. doi: 10.1084/jem.20052471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tory H.O., Zurakowski D., Sundel R.P. Outcomes of children treated for Lyme arthritis: results of a large pediatric cohort. J Rheumatol. 2010;37:1049–1055. doi: 10.3899/jrheum.090711. [DOI] [PubMed] [Google Scholar]
- 7.Steere A.C., Coburn J., Glickstein L. The emergence of Lyme disease. J Clin Invest. 2004;113:1093–1101. doi: 10.1172/JCI21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strle K., Drouin E.E., Shen S., El Khoury J., McHugh G., Ruzic-Sabljic E., Strle F., Steere A.C. Borrelia burgdorferi stimulates macrophages to secrete higher levels of cytokines and chemokines than Borrelia afzelii or Borrelia garinii. J Infect Dis. 2009;200:1936–1943. doi: 10.1086/648091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bromley S.K., Mempel T.R., Luster A.D. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat Immunol. 2008;9:970–980. doi: 10.1038/ni.f.213. [DOI] [PubMed] [Google Scholar]
- 10.Luster A.D. The role of chemokines in linking innate and adaptive immunity. Curr Opin Immunol. 2002;14:129–135. doi: 10.1016/s0952-7915(01)00308-9. [DOI] [PubMed] [Google Scholar]
- 11.Viola A., Luster A.D. Chemokines and their receptors: drug targets in immunity and inflammation. Annu Rev Pharmacol Toxicol. 2008;48:171–197. doi: 10.1146/annurev.pharmtox.48.121806.154841. [DOI] [PubMed] [Google Scholar]
- 12.Jones K.L., Muellegger R.R., Means T.K., Lee M., Glickstein L.J., Damle N., Sikand V.K., Luster A.D., Steere A.C. Higher mRNA levels of chemokines and cytokines associated with macrophage activation in erythema migrans skin lesions in patients from the United States than in patients from Austria with Lyme borreliosis. Clin Infect Dis. 2008;46:85–92. doi: 10.1086/524022. [DOI] [PubMed] [Google Scholar]
- 13.Carlsson S.A., Granlund H., Jansson C., Nyman D., Wahlberg P. Characteristics of erythema migrans in Borrelia afzelii and Borrelia garinii infections. Scand J Infect Dis. 2003;35:31–33. doi: 10.1080/0036554021000026978. [DOI] [PubMed] [Google Scholar]
- 14.Logar M., Ruzic-Sabljic E., Maraspin V., Lotric-Furlan S., Cimperman J., Jurca T., Strle F. Comparison of erythema migrans caused by Borrelia afzelii and Borrelia garinii. Infection. 2004;32:15–19. doi: 10.1007/s15010-004-3042-z. [DOI] [PubMed] [Google Scholar]
- 15.Strle F., Nadelman R.B., Cimperman J., Nowakowski J., Picken R.N., Schwartz I., Maraspin V., Aguero-Rosenfeld M.E., Varde S., Lotric-Furlan S., Wormser G.P. Comparison of culture-confirmed erythema migrans caused by Borrelia burgdorferi sensu stricto in New York state and by Borrelia afzelii in Slovenia. Ann Intern Med. 1999;130:32–36. doi: 10.7326/0003-4819-130-1-199901050-00006. [DOI] [PubMed] [Google Scholar]
- 16.Wormser G.P., McKenna D., Carlin J., Nadelman R.B., Cavaliere L.F., Holmgren D., Byrne D.W., Nowakowski J. Brief communication: hematogenous dissemination in early Lyme disease. Ann Intern Med. 2005;142:751–755. doi: 10.7326/0003-4819-142-9-200505030-00011. [DOI] [PubMed] [Google Scholar]
- 17.Muellegger R.R., McHugh G., Ruthazer R., Binder B., Kerl H., Steere A.C. Differential expression of cytokine mRNA in skin specimens from patients with erythema migrans or acrodermatitis chronica atrophicans. J Invest Derm. 2000;115:1115–1123. doi: 10.1046/j.1523-1747.2000.00198.x. [DOI] [PubMed] [Google Scholar]
- 18.Muellegger R.R., Means T.K., Shin J.J., Lee M., Jones K.L., Glickstein L.J., Luster A.D., Steere A.C. Chemokine signatures in the skin disorders of Lyme borreliosis in Europe: predominance of CXCL9 and CXCL10 in erythema migrans and acrodermatitis and CXCL13 in lymphocytoma. Infect Immun. 2007;75:4621–4628. doi: 10.1128/IAI.00263-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salazar J.C., Pope C.D., Sellati T.J., Feder H.M.J., Kiely T.G., Dardick K.R., Buckman R.L., Moore M.W., Caimano M.J., Pope J.G., Krause P.J., Radolf J.D. Coevolution of markers of innate and adaptive immunity in skin and peripheral blood of patients with erythema migrans. J Immunol. 2003;171:2660–2670. doi: 10.4049/jimmunol.171.5.2660. [DOI] [PubMed] [Google Scholar]
- 20.Wang G., van Dam A.P., Schwartz I., Dankert J. Molecular typing of Borrelia burgdorferi sensu lato: taxonomic, epidemiological, and clinical implications. Clin Microbiol Rev. 1999;12:633–653. doi: 10.1128/cmr.12.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alghaferi M.Y., Anderson J.M., Park J., Auwaerter P.G., Aucott J.N., Norris D.E., Dumler J.S. Borrelia burgdorferi ospC heterogeneity among human and murine isolates from a defined region of northern Maryland and southern Pennsylvania: lack of correlation with invasive and noninvasive genotypes. J Clin Microbiol. 2005;43:1879–1884. doi: 10.1128/JCM.43.4.1879-1884.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bunikis J., Garpmo U., Tsao J., Berglund J., Fish D., Barbour A.G. Sequence typing reveals extensive strain diversity of the Lyme borreliosis agents Borrelia burgdorferi in North America and Borrelia afzelii in Europe. Microbiology. 2004;150:1741–1755. doi: 10.1099/mic.0.26944-0. [DOI] [PubMed] [Google Scholar]
- 23.Hanincova K., Liveris D., Sandigursky S., Wormser G.P., Schwartz I. Borrelia burgdorferi sensu stricto is clonal in patients with early Lyme borreliosis. Appl Environ Microbiol. 2008;74:5008–5014. doi: 10.1128/AEM.00479-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wormser G.P., Brisson D., Liveris D., Hanincova K., Sandigursky S., Nowakowski J., Nadelman R.B., Ludin S., Schwartz I. Borrelia burgdorferi genotype predicts the capacity for hematogenous dissemination during early Lyme disease. J Infect Dis. 2008;198:1358–1364. doi: 10.1086/592279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wormser G.P., Liveris D., Nowakowski J., Nadelman R.B., Cavaliere L.F., McKenna D., Holmgren D., Schwartz I. Association of specific subtypes of Borrelia burgdorferi with hematogenous dissemination in early Lyme disease. J Infect Dis. 1999;180:720–725. doi: 10.1086/314922. [DOI] [PubMed] [Google Scholar]
- 26.Jones K.L., Glickstein L.J., Damle N., Sikand V.K., McHugh G., Steere A.C. Borrelia burgdorferi genetic markers and disseminated disease in patients with early Lyme disease. J Clin Microbiol. 2006;44:4407–4413. doi: 10.1128/JCM.01077-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lagal V., Postic D., Ruzic-Sabljic E., Baranton G. Genetic diversity among Borrelia strains determined by single-strand conformation polymorphism analysis of the ospC gene and its association with invasiveness. J Clin Microbiol. 2003;41:5059–5065. doi: 10.1128/JCM.41.11.5059-5065.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liveris D., Varde S., Iyer R., Koenig S., Bittker S., Cooper D., McKenna D., Nowakowski J., Nadelman R.B., Wormser G.P., Schwartz I. Genetic diversity of Borrelia burgdorferi in Lyme disease patients as determined by culture versus direct PCR with clinical specimens. J Clin Microbiol. 1999;37:565–569. doi: 10.1128/jcm.37.3.565-569.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ojaimi C., Mulay V., Liveris D., Iyer R., Schwartz I. Comparative transcriptional profiling of Borrelia burgdorferi clinical isolates differing in capacities for hematogenous dissemination. Infect Immun. 2005;73:6791–6802. doi: 10.1128/IAI.73.10.6791-6802.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seinost G., Dykhuizen D.E., Dattwyler R.J., Golde W.T., Dunn J.J., Wang I.N., Wormser G.P., Schriefer M.E., Luft B.J. Four clones of Borrelia burgdorferi sensu stricto cause invasive infection in humans. Infect Immun. 1999;67:3518–3524. doi: 10.1128/iai.67.7.3518-3524.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang I.N., Dykhuizen D.E., Qiu W., Dunn J.J., Bosler E.M., Luft B.J. Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics. 1999;151:15–30. doi: 10.1093/genetics/151.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones K.L., McHugh G.A., Glickstein L.J., Steere A.C. Analysis of Borrelia burgdorferi genotypes in patients with Lyme arthritis: high frequency of ribosomal RNA intergenic spacer type 1 strains in antibiotic-refractory arthritis. Arthritis Rheum. 2009;60:2174–2182. doi: 10.1002/art.24812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang G., Ojaimi C., Wu H., Saksenberg V., Iyer R., Liveris D., McClain S.A., Wormser G.P., Schwartz I. Disease severity in a murine model of Lyme borreliosis is associated with the genotype of the infecting Borrelia burgdorferi sensu stricto strain. J Infect Dis. 2002;186:782–791. doi: 10.1086/343043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang G., Ojaimi C., Iyer R., Saksenberg V., McClain S.A., Wormser G.P., Schwartz I. Impact of genotypic variation of Borrelia burgdorferi sensu stricto on kinetics of dissemination and severity of disease in C3H/HeJ mice. Infect Immun. 2001;69:4303–4312. doi: 10.1128/IAI.69.7.4303-4312.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dykhuizen D.E., Brisson D., Sandigursky S., Wormser G.P., Nowakowski J., Nadelman R.B., Schwartz I. The propensity of different Borrelia burgdorferi sensu stricto genotypes to cause disseminated infections in humans. Am J Trop Med Hyg. 2008;78:806–810. [PMC free article] [PubMed] [Google Scholar]
- 36.CDC Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep. 1995;44:590–591. [PubMed] [Google Scholar]
- 37.Wharton M., Chorba T.L., Vogt R.L., Morse D.L., Buehler J.W. Case definitions for public health surveillance. MMWR Recomm Rep. 1990;39:1–43. [PubMed] [Google Scholar]
- 38.Barbour A.G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 39.el Khoury J., Thomas C.A., Loike J.D., Hickman S.E., Cao L., Silverstein S.C. Macrophages adhere to glucose-modified basement membrane collagen IV via their scavenger receptors. J Biol Chem. 1994;269:10197–10200. [PubMed] [Google Scholar]
- 40.Hickman S.E., el Khoury J., Greenberg S., Schieren I., Silverstein S.C. P2Z adenosine triphosphate receptor activity in cultured human monocyte-derived macrophages. Blood. 1994;84:2452–2456. [PubMed] [Google Scholar]
- 41.Shin J.J., Strle K., Glickstein L.J., Luster A.D., Steere A.C. Borrelia burgdorferi stimulation of chemokine secretion by cells of monocyte lineage in patients with Lyme arthritis. Arthritis Res Ther. 2010;12:R168. doi: 10.1186/ar3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidli J., Hunziker T., Moesli P., Schaad U.B. Cultivation of Borrelia burgdorferi from joint fluid three months after treatment of facial palsy due to Lyme borreliosis. J Infect Dis. 1988;158:905–906. doi: 10.1093/infdis/158.4.905. [DOI] [PubMed] [Google Scholar]
- 43.Snydman D.R., Schenkein D.P., Berardi V.P., Lastavica C.C., Pariser K.M. Borrelia burgdorferi in joint fluid in chronic Lyme arthritis. Ann Intern Med. 1986;104:798–800. doi: 10.7326/0003-4819-104-6-798. [DOI] [PubMed] [Google Scholar]
- 44.Crowder C.D., Matthews H.E., Schutzer S., Rounds M.A., Luft B.J., Nolte O., Campbell S.R., Phillipson C.A., Li F., Sampath R., Ecker D.J., Eshoo M.W. Genotypic variation and mixtures of Lyme Borrelia in Ixodes ticks from North America and Europe. PLoS One. 2010;5:e10650. doi: 10.1371/journal.pone.0010650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petzke M.M., Brooks A., Krupna M.A., Mordue D., Schwartz I. Recognition of Borrelia burgdorferi, the Lyme disease spirochete, by TLR7 and TLR9 induces a type I IFN response by human immune cells. J Immunol. 2009;183:5279–5292. doi: 10.4049/jimmunol.0901390. [DOI] [PubMed] [Google Scholar]
- 46.Shin J.J., Glickstein L.J., Steere A.C. High levels of inflammatory chemokines and cytokines in joint fluid and synovial tissue throughout the course of antibiotic-refractory Lyme arthritis. Arthritis Rheum. 2007;56:1325–1335. doi: 10.1002/art.22441. [DOI] [PubMed] [Google Scholar]
- 47.Steere A.C., Angelis S.M. Therapy for Lyme arthritis: strategies for the treatment of antibiotic-refractory arthritis. Arthritis Rheum. 2006;54:3079–3086. doi: 10.1002/art.22131. [DOI] [PubMed] [Google Scholar]
- 48.Derdakova M., Dudioak V., Brei B., Brownstein J.S., Schwartz I., Fish D. Interaction and transmission of two Borrelia burgdorferi sensu stricto strains in a tick-rodent maintenance system. Appl Environ Microbiol. 2004;70:6783–6788. doi: 10.1128/AEM.70.11.6783-6788.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoen A.G., Margos G., Bent S.J., Diuk-Wasser M.A., Barbour A., Kurtenbach K., Fish D. Phylogeography of Borrelia burgdorferi in the eastern United States reflects multiple independent Lyme disease emergence events. Proc Natl Acad Sci U S A. 2009;106:15013–15018. doi: 10.1073/pnas.0903810106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Margos G., Gatewood A.G., Aanensen D.M., Hanincova K., Terekhova D., Vollmer S.A., Cornet M., Piesman J., Donaghy M., Bormane A., Hurn M.A., Feil E.J., Fish D., Casjens S., Wormser G.P., Schwartz I., Kurtenbach K. MLST of housekeeping genes captures geographic population structure and suggests a European origin of Borrelia burgdorferi. Proc Natl Acad Sci U S A. 2008;105:8730–8735. doi: 10.1073/pnas.0800323105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


