Abstract
Endometriosis, the growth of endometrial tissue outside the uterine cavity, is a common gynecological disorder affecting 10% to 15% of women in their reproductive years. Retrograde menstrual shedding containing endometrial stem/progenitor cells has been postulated to be involved in its pathogenesis. In this study, we identified putative endometriotic stem/progenitor cells by their colony-forming potential, self-renewal capacity, and multipotency. Purified epithelial and stromal cells isolated from ovarian endometriotic cysts formed large and small colony-forming units (CFUs) in clonogenic assay. The colony-forming activity of epithelial and stromal cells was found to differ greatly between autologous endometrium and ovarian endometrioma samples. The large CFUs could propagate more than the small CFUs. The endometriotic epithelial small CFUs expressed epithelial markers (epithelial cell adhesion molecule, cytokeratin, and α6 integrin); only occasional large CFUs expressed α6 integrin. Aside from the expression of fibroblast markers, stromal CFUs also expressed three somatic stem cell markers: sal-like 4, CD133, and Musashi-1. Endometriotic stromal cells derived from large CFUs could differentiate into four mesenchymal lineages when cultured in the respective inducing-media, as determined by histochemical staining and RT-PCR of lineage specific markers. These findings demonstrate that ovarian endometrioma contains a subset of cells displaying somatic stem cell properties.
Endometriosis is a pathological condition that involves adhesion, proliferation, and development of endometrial tissues in ectopic regions such as the ovary and the peritoneal cavity. The disease is a major health care problem, causing pain and infertility in 6% to 10% of women.1 Current available medical treatment can suppress the symptoms of endometriosis in many women, but in many cases the disease recurs after cessation of treatment. The pathogenesis of endometriosis has challenged gynecologists for decades, and not much is known about the development and characteristics of the cell types contributing to progression of the disease. Generally, there are three types of endometriosis found in the pelvis: ovarian endometriosis (ovarian endometrioma), peritoneal endometriosis, and deep endometriotic nodules. They represent three different entities differing in pathogenesis.2
Aside from contributing factors such as genetic, hormonal, and immunological influences, several theories have been proposed to explain the pathogenesis of endometriosis.3 The metaplasia theory involves spontaneous transformation of coelomic tissue into endometrium under unknown exogenous influences.4 This theory is based on an early developmental event in which the same precursor cells differentiate into both endometrial and peritoneal cells5 and has been proposed to be the etiology of ovarian endometrioma. The embryonic rest theory proposes that remnant Müllerian cells in the pelvic tissue after development of the Müllerian system are induced to differentiate into functioning endometriotic cells under certain conditions.6,7 Last, Sampson's transplantation theory suggests that endometriosis is formed by implantation and growth of endometrial tissue reaching the ectopic sites via retrograde menstruation.8 This theory is widely accepted, because the anatomical distribution of endometriotic implants in the pelvis is where reflux menstrual effluent is expected.
In recent years, evidence has emerged on the existence of endometrial stem/progenitor cells. Although no endometrial stem cell surface marker is available at present, a number of classic functional assays have identified endometrial stem cells.9 Of these, a subpopulation of cells in the human endometrium has been shown to be clonogenic,10 to undergo prolonged self-renewal,11 and to be multipotent.12 Side-population cells of the human endometrium have been isolated.13–15 In addition, a small percentage of quiescent cells thought to be stem/progenitor cells in the mouse endometrium,16 and in an endometrial breakdown/repair model,17 can be induced to proliferate on exposure to estrogen.
The existence of endometrial stem cells has led to the suggestion that certain gynecological diseases, including endometriosis, adenomyosis, and endometrial carcinoma, may be a consequence of abnormal proliferation of these stem cells.18 In this study, therefore, we hypothesized that cells with somatic stem cell properties are present in ovarian endometrioma. Our findings indicate that a small population of ovarian endometriotic cells exhibits colony-forming activity, self-renewal capacity, and multipotency. To determine any difference between cells in endometriosis and those from endometrium, we compared the colony-forming activity of the endometrium and ovarian endometrioma of the same patient, to avoid possible variation due to individual difference in genetic background. Significantly more clonogenic cells were detected from the endometrium, compared with the ovarian endometrioma, suggesting that the microenvironment in which these clonogenic cells reside may contribute to their distinct biological properties.
Materials and Methods
Human Tissue Samples
Three types of endometrial tissues were collected: i) ovarian endometrioma (ectopic endometrium), ii) endometrium from women with endometriosis (eutopic endometrium), and iii) endometrium from women without endometriosis (normal endometrium). Cyst walls of ovarian endometrioma (n = 50) were collected from women aged 20 to 50 years (mean ± SEM, 39.9 ± 1.0 years) undergoing ovarian cystectomy through laparoscopy or laparotomy (see Supplemental Table S1 at http://ajp.amjpathol.org). The eutopic endometrial samples of 10 of these women who were undergoing total abdominal hysterectomy and bilateral salpingo-oophorectomy (autologous samples) were also obtained and compared with the women's own ovarian endometrioma. Normal endometrial samples (n = 25) were collected from ovulating women aged 41 to 52 years (mean, 44.9 ± 0.5 years) undergoing hysterectomy for leiomyoma or adenomyosis. Only women who had not taken exogenous hormones for 3 months before surgery were included in this study. Informed written consent was obtained from each patient and ethical approval was obtained from the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster.
The stage of the menstrual cycle was categorized into proliferative (endometriotic/ectopic, n = 24; eutopic, n = 4; normal, n = 13) and secretory (endometriotic/ectopic, n = 26; eutopic, n = 6; normal, n = 12). We dated the samples based on the reported day of the menstrual cycle and histology examination by histopathologists. Well-established histological criteria for endometrial dating of the menstrual phase were used.19 Endometriosis was staged according to the 1996 revised classification of the American Society for Reproductive Medicine.20
Full-thickness endometrial tissue samples (with or without endometriosis) comprising myometrium (5-mm thick) or ovarian endometriotic cyst were collected in Dulbecco's modified Eagle's medium/Hams F-12 (DMEM/F-12; Invitrogen, Carlsbad, CA) containing 1% antibiotic solution (Gibco, Rockville, MD) and 5% fetal bovine serum (Gibco). The samples were stored at 4°C and processed within 2 to 16 hours.
Purification of Human Endometrial and Endometriotic Cells into Single-Cell Suspensions
Human endometrial and endometriotic tissues were digested to single-cell suspensions using 300 μg/mL collagenase type 3 and 40 μg/mL deoxyribonuclease (both from Worthington Biochemical, Lakewood, NJ) and mechanical digestion, as described previously.10 Red blood cells were removed using Ficoll-Paque (GE Healthcare, Pittsburgh, PA) density-gradient centrifugation. Purified epithelial cell suspensions were obtained by using magnetic anti-EpCAM antibody-coated Dynabeads (Clone Ber-EP4; Dynal Biotech, Oslo, Norway) specific to the epithelial cell surface antigen. Presence of EpCAM in ovarian endometrioma is shown in Figure 1A. Contaminating leukocytes among the stromal cells were further eliminated using anti-CD45 antibody-coated Dynabeads (Dynal Biotech).
Figure 1.
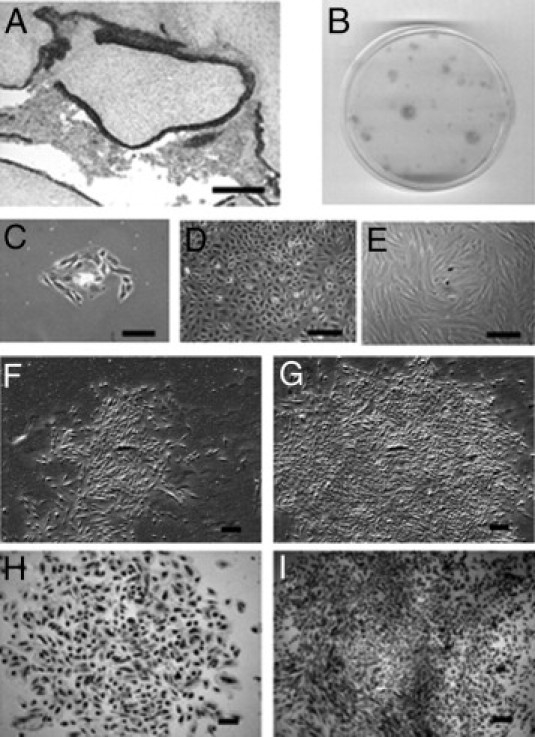
A: Representative IHC staining with epithelial marker EpCAM, specific for epithelial cells in ovarian endometrioma tissue. Formation of CFUs by ovarian endometriotic epithelial cells seeded at clonal density for 21 days in vitro. B: Plate showing variation of CFUs. C: Small cluster of endometriotic epithelial cells at day 4. D: Intercalated and honeycomb-shaped ovarian epithelial cells. E: Morphology of endometriotic epithelial cells at day 10. F–I: Small and large CFUs. Phase contrast photomicrograph of a typical epithelial small CFU (F) and large CFU (G). Limiting dilution assay with small (H) and large (I) CFUs. Hematoxylin stain (H and I). Scale bars = 200 μm.
Endometrial and Endometriotic Clonal Culture
For the assessment of colony-forming ability, endometrial (with or without endometriosis) and endometriotic epithelial and stromal cells were seeded in triplicate at a clonal density of 500 cells/cm2 into 60-mm Petri dishes (BD Discovery Labware, Bedford, MA) coated with gelatin (Sigma-Aldrich, St. Louis, MO) and cultured in DMEM-F12 medium (Invitrogen) containing 10% FBS (Gibco), 1% antibiotics (Gibco), and 2 mmol/L glutamine (Gibco), as described previously.10 Cells were incubated at 37°C in 5% CO2. Medium was changed every 7 days, and colonies formed were regularly monitored using an Eclipse TS100 inverted microscope (Nikon, Tokyo, Japan) to ensure that they were derived from single cells. Cultures were stopped at day 15 for endometrial samples and at up to day 21 for endometriotic samples. The cultures were stained with 1% Toluidine Blue (Sigma-Aldrich). Clones or colony-forming units (CFUs) consisting of >50 cells were counted to determine the cloning efficiency (CE) percentage,10 which was the number of colonies formed per seeded cell multiplied by 100.
In Vitro Serial Cloning and Limiting Dilution of Endometriotic Epithelial and Stromal Cells
To examine the self-renewal capacity, individual large and small endometriotic epithelial (n = 9) and stromal (n = 12) CFUs (an average of three small and large CFUs/patient sample) were harvested using cloning rings (Sigma-Aldrich) and 0.25% trypsin (Gibco) after 21 days in culture, reseeded onto another gelatin-coated Petri dish (BD Discovery Labware) at a density of 20 cells/cm2, and cultured for a further 21 days for the formation of secondary clones. This process continued until the cells could no longer form clones.
To ensure that the CFUs were derived from a single cell, limiting dilution assay was conducted for endometriotic epithelial cells (n = 3) and stromal cells (n = 3). Cells were diluted to 1 cell per 100 μL using serial dilution and dispensed 100 μL/well into three 96-well flat-bottom plates (Iwaki, Funabashi, Japan). All wells were examined under the microscope for the number of cells attached. Empty wells and wells with >1 cell were marked and disregarded; wells that contained a single cell were examined daily for their colony-forming activity for 21 days.
Antibodies
The following primary antibodies were used: mouse monoclonal smooth muscle actin (α-SMA, 1.8 μg/mL; DakoCytomation, Glostrup, Denmark), rat monoclonal CD49f (α6 integrin, 10 μg/mL; BD Pharmingen, San Diego, CA), mouse monoclonal epithelial antigen (EpCAM, 4.7 μg/mL; DakoCytomation), mouse monoclonal CD10 (20 μg/mL; Abcam, Cambridge, MA), rat monoclonal CD31 (10 μg/mL; BD Pharmingen), mouse monoclonal CD90 (10 μg/mL; BD Pharmingen), mouse monoclonal CD133 (0.5 μg/mL; Miltenyi Biotec, Bergisch-Gladbach, Germany), mouse monoclonal cytokeratin (2 μg/mL; DakoCytomation), rabbit polyclonal collagen II (2 μg/mL; Abcam), mouse monoclonal estrogen receptor α (ERα, 20 μg/mL; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit polyclonal estrogen receptor β (ERβ, 3.33 μg/mL; Abcam), mouse monoclonal musashi-1 (Msi1, 0.5 μg/mL; R&D Systems, Minneapolis, MN), rabbit polyclonal osteopontin (17 μg/mL; Abcam), rabbit polyclonal peroxisome proliferator-activated receptor-γ (PPARγ, 0.05 μg/mL; Cell Signaling Technology, Danvers, MA), and rabbit polyclonal sal-like 4 (SALL4, 0.5 μg/mL; Abcam). The corresponding biotinylated secondary antibodies were used: rabbit anti-mouse (6 μg/mL; DakoCytomation), goat anti-rat (7.5 μg/mL; Millipore–Chemicon, Temecula, CA), and goat anti-rabbit (3.8 μg/mL; DakoCytomation). Isotype-matched IgGs at concentrations identical to those of the primary antibodies were included in every staining experiment, to serve as the negative control. Positive controls for ER-α and ER-β were performed on the endometrial cell line Ishikawa (data not shown).
IHC
Endometriotic epithelial and stromal cells were seeded at 500 cells/cm2 onto 25-mm Thermanox coverslips (Nalge Nunc International, Naperville, IL) in six-well dishes and cultured in serum-supplemented medium as described above. After culturing for 21 days, the coverslips were washed with PBS and the cells were fixed with 10% formalin (Sigma-Aldrich) for 5 minutes before being treated with 0.3% hydrogen peroxide (Merck, Darmstadt, Germany) to quench endogenous peroxidase, followed by treatment with 10% blocking serum (Sigma-Aldrich) from the host species of the secondary antibody. Primary antibodies were diluted with 10% serum and incubated either for 1 hour at room temperature or overnight at 4°C. Coverslips were then washed and incubated with their corresponding secondary antibody for 1 hour, followed by ABC regent (Vector Laboratories, Burlingame, CA) for 30 minutes. Positive immunoreactivities were visualized after incubation with diaminobenzidine (Sigma-Aldrich) chromogen for 5 minutes, lightly counterstained with Mayer's hematoxylin (Merck) for 1 minute, washed with distilled H2O, and mounted on glass slides. Washing with PBS was conducted between each step, and all incubations were performed at room temperature unless otherwise specified. Sections were examined under an Axioskop microscope (Carl Zeiss, Oberkochen, Germany), and images were acquired with a Photometrics CoolSNAP charge-coupled device camera (Roper Scientific, Duluth, GA) using CoolSNAP version 1.1 software.
In Vitro Decidualization of Endometriotic Stromal Cells
To examine the differentiation potential, endometriotic stromal cells were seeded on 25-mm Thermanox coverslips in six-well dishes (Iwaki, 2 × 104 cells per coverslip) and grown until 70% confluency. Progesterone (100 ng/mL) or 0.1% ethanol vehicle (control) in Phenol-Red-free DMEM/F-12 (Invitrogen) containing 0.3% bovine serum albumin (Invitrogen) and 1% antibiotics (Gibco) was added to induce decidualization for 4 to 7 days. Morphology of the stromal cells was assessed and immunohistochemistry (IHC) was performed for expression of the decidualization marker, insulin growth factor binding protein-1 (IGFBP-1, 0.4 μg/mL; Santa Cruz Biotechnology).
In Vitro Differentiation of Endometriotic Stromal Cells
To assess the multipotency, protocols for in vitro differentiation were adapted from those of Pittenger et al.21 Large endometriotic stromal clones (n = 3, patient samples) were isolated with cloning rings (Sigma-Aldrich), treated with 0.25% trypsin (Invitrogen), pooled together in a 12-well culture plate (Iwaki), and then transferred to a CellStar 75-cm2 flask (Greiner Bio-One, Frickenhausen, Germany). The cells were grown until 70% to 85% confluency. Endometriotic stromal cells were then seeded at a density of 4.0 × 104 cells/well in six-well culture plates (Iwaki) and were cultured in either adipogenic, myogenic, or osteogenic differentiation media for up to 4 weeks. For chondrogenic differentiation, 3 × 105 cells were cultured as micropellets in centrifuge tubes containing chondrogenic differentiation medium for 4 weeks. Some endometriotic stromal cells were cultured in serum-containing medium, to serve as the undifferentiated control. To assess differentiation (Table 1), cells were histochemically stained with HCS LipidTOX neutral lipid stain (Invitrogen), alkaline phosphatase kit (Sigma-Aldrich), Alcian Blue (Sigma-Aldrich), Safranin O, or IHC using antibodies for PPARγ (Cell Signaling Technology), α-SMA (DakoCytomation), osteopontin (Abcam), and collagen type II (Abcam) for adipogenic, myogenic, osteogenic, and chondrogenic differentiation, respectively.
Table 1.
Induction for the Differentiation of Mesenchymal Lineages and the Detection of Specific Markers Using Histochemical Staining, IHC, and RT-PCR
| Lineage, staining, and gene | Direction | Primer sequence |
|---|---|---|
| Adipogenic lineage (staining: HCS LipidTOX neutral lipid stain, peroxisome proliferation activated receptor γ (PPARγ) | ||
| PPARG | Sense | 5′-GCTCCGTGGATCTCTCCGTAATG-3′ |
| Antisense | 5′-AATTGCCATGAGGGAGTTGGAAGG-3′ | |
| CEBPA | Sense | 5′-TCGACATCAGCGCCTACATC-3′ |
| Antisense | 5′-CTTGTCCACCGACTTCTTGG-3′ | |
| LPL | Sense | 5′-CAAAACTTGTGGCCGCCCTGTA-3′ |
| Antisense | 5′-GGGGACCCTCTGGTGAATGTGTGT-3′ | |
| Myogenic lineage [staining: alpha smooth muscle actin (α-SMA)] | ||
| ACTA2 | Sense | 5′-CCGGGAGAAAATGACTCAAA-3′ |
| Antisense | 5′-GCGTCCAGAGGCATAGAGAG-3′ | |
| CNN1 | Sense | 5′-CGTCGCATCGGCAACAACTTCAT-3′ |
| Antisense | 5′-ACCTTGTTTCCTTTCGTCTTCGC-3′ | |
| CALD1 | Sense | 5′-ACAGTCACCAAGTCCTACCAGAAGAATG-3′ |
| Antisense | 5′-CCTCCAGGGCGGCTGAAAGT-3′ | |
| Osteogenic lineage (staining: alkaline phosphatase osteopontin) | ||
| ATHS | Sense | 5′-TGGAGCTTCAGAAGCTCAACACCA-3′ |
| Antisense | 5′-ATCTCGTTGTCTGAGTACCAGTCC-3′ | |
| RUNX2 | Sense | 5′-CTCACTACCACACCTACCTGCCAC-3′ |
| Antisense | 5′-TCAATATGGTCGCCAAACAGATTC-3′ | |
| SPP1 | Sense | 5′-AGGAGGAGGCAGAGCACA-3′ |
| Antisense | 5′-CTGGTATGGCACAGGTGATG-3′ | |
| PTH1R | Sense | 5′-CCTCACCGTAGCTGTGCTCATCCT-3′ |
| Antisense | 5′-GCCCCTCCACCAGAATCCAGTAG-3′ | |
| Chondrogenic lineage (staining: 1% Alcian Blue, Safranin O, collagen type II) | ||
| COL2A1 | Sense | 5′-CACTCCTGGCACTGATGGTCCC-3′ |
| Antisense | 5′-CTTCTCCCTTCTCGCCGTTAGCAC-3′ | |
| COL10A | Sense | 5′-GGACACAATGGAGAGGCTGGC-3′ |
| Antisense | 5′-ATGACTGCTTGACCTGGTGGGC-3′ | |
| GAPDH | Sense | 5′-ACCACAGTCCATGCCATCAC-3′ |
| Antisense | 5′-TCCACCACCCTGTTGCTGTA-3′ | |
ACTA2 (encodes α-SMA protein): actin, alpha 2, smooth muscle; ATHS (previously ALPL): alkaline phosphatase: atherosclerosis susceptibility (lipoprotein associated); CALD1: caldesmon 1; CEBPA: CCAAT/enhancer binding protein (C/EBP), alpha; CNN1: calponin 1, basic, smooth muscle; ATHS: COL10A1: collagen, type X, alpha 1; COL2A1: collagen, type II, alpha 1; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; LPL: lipoprotein lipase; PPARG: peroxisome proliferator-activated receptor gamma; PTH1R: parathyroid hormone 1 receptor; RUNX2: runt-related transcription factor 2; SPP1 (previously OPN, osteopontin): secreted phosphoprotein 1.
Reverse transcription polymerase chain reaction was used to assess the expression of lineage-specific genes: PPARG, CEBPA, and LPL for adipocytes; ACTA2 (encoding α-SMA), CNN1 and CALD1 for myoblasts. ALPL (encoding alkaline phosphatase) RUNX2 SPP1 and PTH1R for osteoblasts; and COL2A1 and COL10A for chondrocytes. Full gene names and the primer sequences used are given in Table 1. The concentration used was 0.15 μmol/L. Total RNA was extracted using a Stratagene Absolutely RNA microprep kit (Agilent Technologies, La Jolla, CA) according to the manufacturer's protocol. The quality and quantity of the total RNA was checked by spectrophotometry. A first-strand cDNA synthesis kit (GE Healthcare, Piscataway, NJ) was used to synthesize the complementary DNA (cDNA). The resulting cDNA was subjected to RT-PCR in a 15-μL reaction mixture with the use of a Bio-Rad I cycler (Bio-Rad Laboratories, Hercules, CA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal control, and water as the no-template control. The amplification protocol was denaturation at 95°C for 5 minutes, followed by 35 cycles at 94°C for 1 minute, 55°C for 30 seconds, and 72°C for 1 minute, with a final extension at 72°C for 10 minutes. The amplified products were analyzed by 1.2% agarose gel electrophoresis (Invitrogen), and visualized with ethidium bromide staining in a gel-documentation system (Alpha Innotech, San Leandro, CA). The experiments were performed at least twice using three separate samples.
Statistical Analysis
All statistical analysis was performed using GraphPad Prism Software (version 4.01; GraphPad Software, San Diego, CA). Gaussian distribution was examined with D'Agostino-Pearson omnibus normality tests, and Kruskal-Wallis tests were conducted for multiple cloning efficiencies comparisons and Mann-Whitney U-tests for comparison between two groups. Data are reported as means ± SEM. A difference with P < 0.05 was considered statistically significant.
Results
Morphology of Endometriotic Epithelial and Stromal Clonogenic Cells
Purified endometriotic epithelial cells were separated from surrounding stromal cells using magnetic Dynabeads (Dynal Biotech) specific to the epithelial surface antigen, Ber-EP4 (Figure 1A). Epithelial cells attached onto the culture plate within 24 hours, and small clusters of six to eight cells were apparent by day 4 (Figure 1C). For some endometrioma samples, two distinct epithelial cell types were detected: a small portion were intercalated and honeycomb-shaped (Figure 1D), resembling the ovarian surface epithelial cells, but the majority were similar to the endometrial and endometriotic epithelial cells previously reported (Figure 1E).10,22 Under phase-contrast microscopy, epithelial small (Figure 1F) and large (Figure 1G) CFUs were observed by day 21. Histological examination of the ovarian endometriosis samples showed that the upper lining of the lesion might comprise a layer of ovarian surface epithelium.23 Because of their unique morphology, ovarian surface epithelial-like colonies were not included in the CE calculation.
Similarly, individual endometriotic stromal cells attached within 24 hours and small clusters of three to four flattened fibroblastic cells were observed by day 4 (Figure 2B). Of the attached stromal cells, a small portion of the initiated colonies (Figure 2C) were similar to those of the epithelial cells. Small (Figure 2E) and large (Figure 2F) stromal CFUs were observed by day 21. Endometriotic stromal cells were characteristically flat and spindle-shaped and were indistinguishable from those found in normal endometrial samples (Figure 2D). To confirm the endometrial origin of the stromal cells obtained from ovarian endometrioma, progesterone was added, to determine their ability to undergo in vitro decidualization, a characteristic of endometrial stromal cell remodeling. By day 4, some endometriotic stromal cells exhibited a characteristic change in morphology from bipolar fibroblasts to polygonal decidual cells and expressed the decidualization marker IGFBP-1 (see Supplemental Figure S1, A and B, at http://ajp.amjpathol.org).
Figure 2.
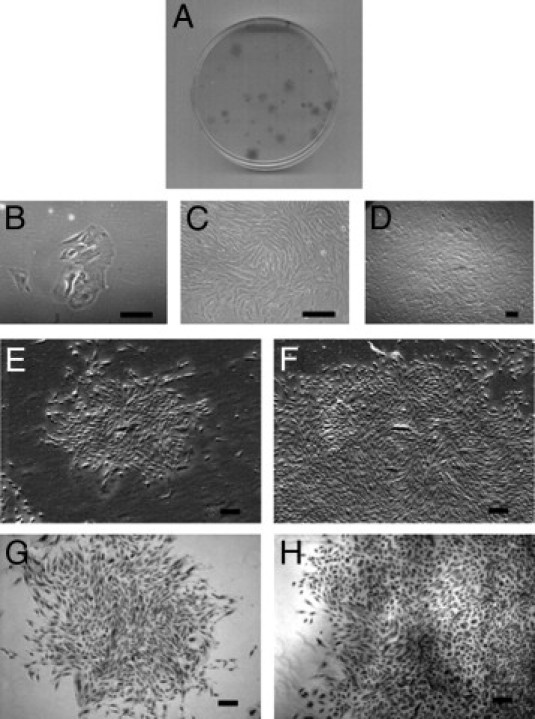
Formation of CFUs by ovarian endometriotic stromal cells seeded at clonal density for 21 days. A: Plate showing variation of CFUs. B: Attachment of endometriotic stromal cells at day 4. C and D: Morphology of endometriotic stromal cells at day 13 (C) and day 21 (D). E–H: Small and large CFUs. Phase contrast photomicrograph of typical stromal small CFU (E) and large CFU (F). Limiting dilution assay with small (G) and large (H) CFUs. Hematoxylin stain (G and H). Scale bars = 200 μm.
The categorization of colony size for endometriotic epithelial and stromal cells was based on a previous observation.10 Small CFUs were defined as comprising <4000 large loosely-packed cells and large CFUs as comprising >4000 cells with a dense center of tightly packed cells (Figures 1B and 2A). During the first week of culture, the growth rates for the two colony types were similar, with colonies generally comprising <100 cells after 7 days. Around day 13 to day 14, however, small CFUs stopped proliferation and maintained their size, but the growth of some colonies increased dramatically and formed large CFUs containing as many as 15,000 cells by day 21.
To ensure that the CFUs were derived from single cells, endometriotic epithelial and stromal cells were seeded onto 96-well plates using limiting dilution. Only wells that contained a single cell were regularly observed to determine the growth of the large and the small clones. Small and large CFUs from epithelial cells (Figure 1, H and I) and small and large CFUs from stromal cells (Figure 2, G and H) of ovarian endometrioma samples were detected. The experiment was repeated three times for each cell type.
Cellular Phenotype of Human Endometriotic Epithelial and Stromal Cells
Epithelial and stromal cells isolated from ovarian endometrioma were seeded on coverslips and the CFUs formed underwent IHC for cellular phenotypes assessment. Endometriotic epithelial small CFUs contained cells positive for three epithelial markers: epithelial cell adhesion molecule (EpCAM) (Figure 3A), cytokeratin (CK; Figure 3B), and α6 integrin (CD49f; Figure 3C). Occasionally, however, some endometriotic epithelial large CFUs were found to be immunoreactive only for α6 integrin (Figure 3D), being negative for EpCAM (Figure 3E) and CK (data not shown). Both types of epithelial CFUs were positive for ERα (Figure 3F) and ERβ (Figure 3G), but negative for the stromal markers studied (data not shown). Endometriotic stromal large and small CFUs were immunoreactive for fibroblast markers CD90 (Figure 3H) and CD10 (Figure 3I), and also for hormone receptors ERα (Figure 3K) and ERβ (Figure 3L).
Figure 3.
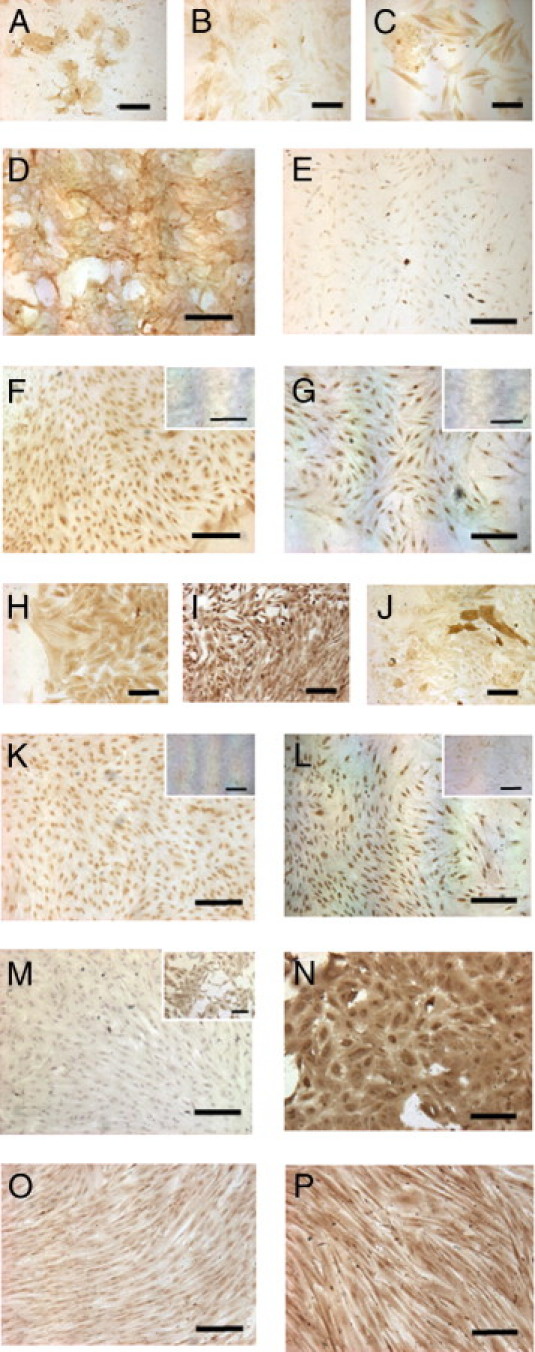
IHC phenotyping of CFUs initiated by endometriotic epithelial cells (A–G) and stromal cells (H–P). Epithelial markers are EpCAM (A), cytokeratin (B), and α6 integrin (C) on epithelial small CFUs and α6 integrin (D), EpCAM (E), and hormone receptors ERα (F) and ERβ (G) on epithelial large CFUs. Large stromal CFUs were immunostained with fibroblast markers CD90 (H) and CD10 (I), smooth muscle cell marker α-SMA (J), and hormone receptors ERα (K) and ERβ (L). Endothelial cell marker CD31; somatic stem cell markers are SALL4 (N), CD133 (O), and Msi1 (P). Note positive staining in small clusters of endometriotic stromal cells (M, inset). Isotype-matched negative controls are shown in the insets for ERα mouse IgG2a (F and G) and ERβ rabbit IgG (K and L). Scale bars = 200 μm.
Aside from the above markers, endometriotic stromal CFUs were examined for expression of markers for α-SMA, endothelial cells (CD31), and three somatic stem cell markers: sal-like 4 (SALL4), CD133, and Musashi-1 (Msi1). Occasional α-SMA-positive cells were found within both the large and small endometriotic stromal CFUs (Figure 3J). Immunoreactivities of CD31 were not detected in large or small endometriotic stromal CFUs (Figure 3M), but were present in small clusters of stromal cells (Figure 3M, inset). Of the three somatic stem cell markers, SALL4 (Figure 3N), CD133 (Figure 3O), and Msi1 (Figure 3P) had positive immunoreactivity in large and small endometriotic stromal CFUs. In normal endometrial stromal CFUs, however, the staining intensity of SALL4 and Msi1 were weak, and that of CD133 was negative, compared with endometriotic stromal clones (see Supplemental Figure S2 at http://ajp.amjpathol.org).
Clonogenicity of Human Endometriotic Epithelial and Stromal Cells
Cloning efficiencies were determined for both large and small CFUs. The total (large and small colonies) CEs for endometriotic epithelial and stromal cells from ovarian endometrioma are shown in Figure 4A. Overall, the CE of endometriotic epithelial cells was 0.09 ± 0.02% (n = 50), with 0.04 ± 0.01% for large CFUs and 0.05 ± 0.01% for small CFUs. There was no difference between the CEs of large and small endometriotic epithelial colonies (P = 0.70). The mean total CE for endometriotic stromal cells was 0.13 ± 0.02% (n = 50), with 0.06 ± 0.01% for large CFUs and 0.07 ± 0.01% for small CFUs. No difference was found for CE between large and small endometriotic stromal colonies (P = 0.55).
Figure 4.
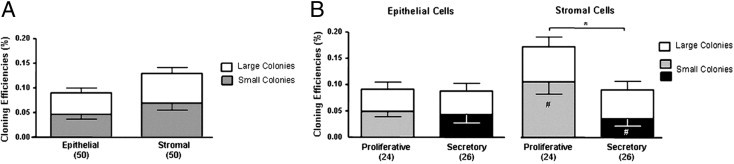
Ovarian endometrioma cloning efficiency (CE). A: CE of endometriotic epithelial and stromal cells. B: Effect of menstrual phase on CE of endometriotic epithelial and stromal cells. Bars represent total CE (sum of small and large CFUs), shown as means ± SEM. *P < 0.05, total stromal CE, proliferative versus secretory menstrual phase, and **P < 0.001, small stromal CE, proliferative versus secretory menstrual phase. Numbers in parentheses indicate sample size; white bars indicate large CFUs; gray and black indicate small CFUs.
To examine the change in colony-forming activity of endometriotic epithelial and stromal cells in the menstrual cycle, CEs were compared between the proliferative (n = 24) and the secretory phases (n = 26) (Figure 4B). There was no difference in epithelial cell clonogenicity between the proliferative (0.09 ± 0.02%) and the secretory phase (0.09 ± 0.02%) (P = 0.35). The CE of large CFUs was constant throughout the menstrual cycle, at 0.04 ± 0.01% in both phases.
For the stromal cells, the total CE for the proliferative phase (0.17 ± 0.04%) was significantly (P < 0.05) greater than that of the secretory phase (0.09 ± 0.02%). Significant difference (P < 0.001) was also observed between the CE of stromal small CFUs at the proliferative phase (0.11 ± 0.02%) and the secretory phase (0.03 ± 0.01%). The CE of the large CFUs was constant throughout the menstrual cycle (proliferative, 0.07 ± 0.02%; secretory, 0.05 ± 0.02%).
Clonogenicity of Human Epithelial and Stromal Cells from Autologous Endometrium and Ovarian Endometrioma
To compare the clonogenic activity between endometrial and endometriotic cells, the CEs of epithelial and stromal cells from matched (autologous) samples (ie, the endometrium and ovarian endometriotic cyst from the same women) were compared. Figure 5A shows the comparison from 10 women who underwent total abdominal hysterectomy and bilateral salpingo-oophorectomy. Overall, the total CE of epithelial cells was significantly different between the endometrial (0.19 ± 0.07%) and the endometriotic samples (0.06 ± 0.04%; P < 0.05) (Table 2). The proportion of large epithelial clones from endometrial samples (0.10 ± 0.05%) was significantly higher than that from endometriotic samples (0.03 ± 0.02%; P < 0.05). As for the stromal cells, the total CE was also significantly different between the two types of samples (endometrium, 0.53 ± 0.17%; endometriotic, 0.10 ± 0.02%; P < 0.05) (Table 2). These results indicate a major difference in the amount of clonogenic cells present in the endometrial and endometriotic tissues of the same patient (Table 2).
Figure 5.
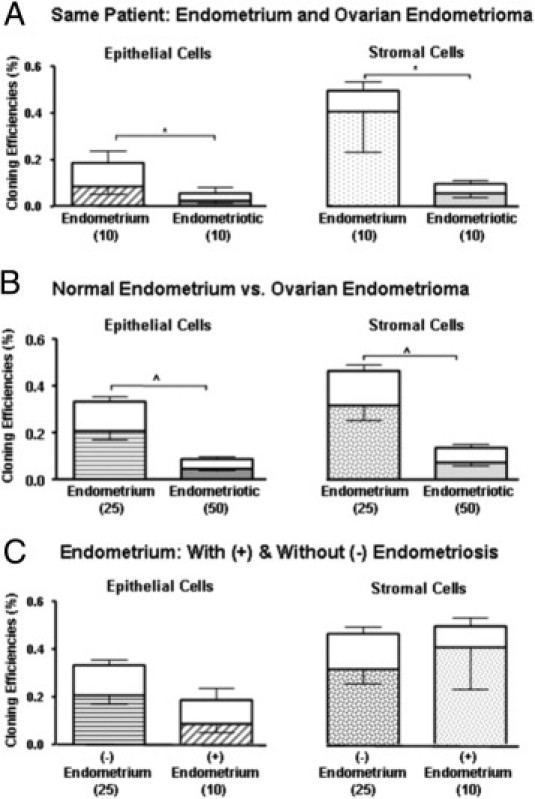
A: The CE of epithelial and stromal cells from endometrium and ovarian endometrioma of the same patient. B: CE of epithelial and stromal cells from normal endometrium and ovarian endometrioma. C: CE of endometrial epithelial and stromal cells from women with (+) and without (−) endometriosis. Results are shown as means ± SEM. *P < 0.05 and **P < 0.0001, total CE between endometrial groups. Numbers in parentheses indicate sample size. Bars represent total CE, as sum for large CFUs (open area) and small CFUs (hatched area).
Table 2.
Statistical Significance of Total Clonogenicity at Different Endometrial Sites
| Total clonogenicity | Normal vs endometriotic | Eutopic vs ectopic (same patient) | Eutopic endometrium: with vs without endometriosis |
|---|---|---|---|
| Epithelial cells | P < 0.0001 | P < 0.05 | P = 0.69 |
| Stromal cells | P < 0.0001 | P < 0.05 | P = 0.49 |
Clonogenicity of Human Epithelial and Stromal Cells between Normal Endometrium and Ovarian Endometrioma
The colony-forming activity of normal endometrium (women without endometriosis, n = 25) and ovarian endometrioma/endometriotic samples (n = 50) was also compared. The total CEs of epithelial cells from ovarian endometrioma biopsies (0.06 ± 0.01%) was significantly lower, compared with that of normal endometrium (0.33 ± 0.06%; P < 0.0001) (Figure 5B and Table 2). The establishment of epithelial endometriotic clones in culture was difficult, and viable epithelial cells were isolated from only 34 of 50 samples. Of the 16 samples with no clonogenic epithelial cells isolated, 12 samples were from the secretory phase; that is, the epithelial cells from nearly half of the secretory endometrioma used failed to form CFUs. There was no correlation between patient age and the total CE of the samples. As for the stromal cells (Figure 5B), there was also a significant difference between the CE of ovarian endometrioma biopsies (0.13 ± 0.03%) and that of the normal endometrium (0.46 ± 0.08%; P < 0.0001) (Table 2).
Clonogenicity of Human Epithelial and Stromal Cells between Endometrium from Women with and without Endometriosis
The colony-forming activity of endometrial samples with (n = 10) and without (n = 25) endometriosis was also compared. The CEs of epithelial and stromal cells of the two groups are presented in Figure 5C. There was no difference in the total clonogenicity of epithelial cells between endometrium without (0.33 ± 0.06%) and with (0.18 ± 0.07%) endometriosis (P = 0.68) (Table 2). No difference was found in the total clonogenicity for stromal cells (without endometriosis, 0.46 ± 0.08%; with endometriosis, 0.53 ± 0.02%; P = 0.49) (Table 2).
Propagation of Human Endometriotic Clonogenic Cells by Serial Cloning
The self-renewal capacity of large and small colonies from endometrioma biopsies was evaluated by serial cloning. The numbers for serial cloning of large and small CFUs from epithelial (n = 9) and stromal (n = 12) endometriotic cells before senescence of the cells are presented in Figure 6. Overall, large clones could propagate significantly more (epithelial, 2.60 ± 0.32; stromal, 3.10 ± 0.37) than could the small clones (epithelial, 0.94 ± 0.30; stromal, 1.03 ± 0.31) (P < 0.05). Serial cultivation of large CFUs produced large and small CFUs at later passages. Small CFUs, however, at all times produced no large clones and eventually failed to form clones with loosely dispersed cells attached on the culture dish. These results suggest that the large CFUs, but not the small CFUs, are endowed with a high capacity for self-renewal division (ie, generation of its own progeny).
Figure 6.
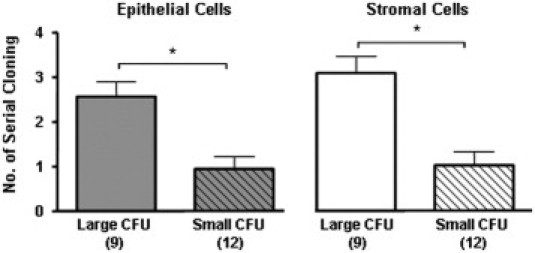
Self-renewal activity of endometriotic epithelial and stromal CFUs using serial cloning assay. Results are shown as means ± SEM. *P < 0.05, large versus small CFUs. Numbers in parentheses indicate sample size (average of three small and large CFUs per cell type per patient sample).
Differentiation of Human Endometriotic Stromal Cells to Mesenchymal Cell Lineages
The potential of large endometriotic stromal clones to differentiate toward the mesenchymal cell lineages was examined by cultivating the large endometriotic stromal clones in adipogenic, myogenic, osteogenic, or chondrogenic inducing medium for 4 weeks. Those cultured in adipogenic inducing medium developed vacuoles that stained positively with the fluorescence lipid stain (Figure 7A). Positive control staining with mouse adipose tissue demonstrated specificity of the lipid stain (Figure 7B). PPARγ, a major regulator of adipocyte development,24 was detected by IHC (Figure 7C) and RT-PCR. Another important transcription factor in adipogenesis, CEBPAα, was also detected. However, the mRNA of a key enzyme involving in the breakdown of triglycerides, lipoprotein lipase (LPL), was undetectable (Figure 7D).
Figure 7.
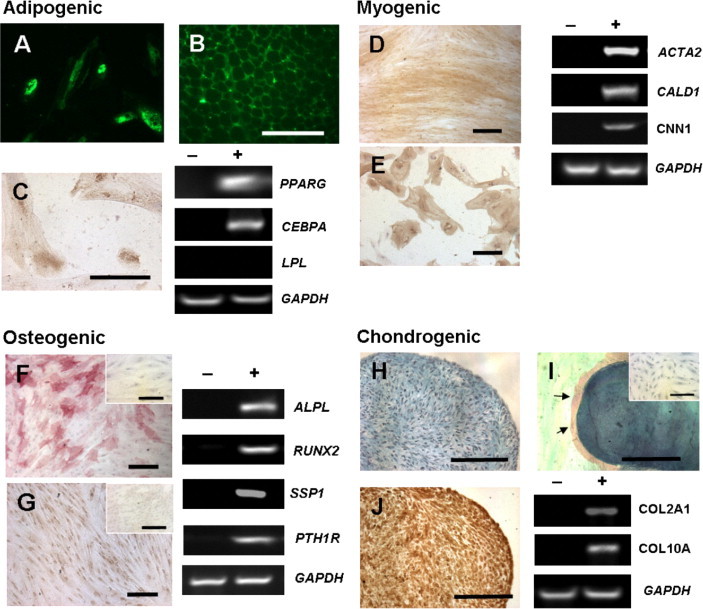
Multipotency of endometriotic stromal CFUs. In vitro differentiation of endometriotic stromal CFUs toward the adipogenic (A–C), myogenic (D–E), osteogenic (F–G), and chondrogenic (H–J) lineages was confirmed with histochemical staining, IHC, and RT-PCR by mRNA expression of specific lineage markers. A: Adipogenic differentiation with fluorescent lipid stain (green) on cells clonally derived from endometriotic stromal large CFUs. B: Positive staining on mouse adipose tissue. C: IHC staining of peroxisome proliferation activated receptor γ (PPARγ) and expression of PPARG, CEBPA (CCAAT/enhanced binding protein alpha) and LPL (lipoprotein lipase). Myogenic differentiation with α smooth muscle cell marker (α-SMA, brown) staining on cells clonally derived from endometriotic stromal large CFUs (D) and untreated endometriotic stromal cells (E). F and G: Expression of ACTA2 (encoding α-SMA), caldesmon (CALD1) and CNN1 (calponin 1). Osteogenic differentiation with alkaline phosphates (red) staining (F) and IHC staining of osteopontin (G) and expression of alkaline phosphates (ALPL), RUNX2 (runt-related transcription factor 2), SSP1 (secreted phosphoprotein 1, previously OPN, osteopontin), and parathyroid hormone 1 receptor (PTH1R). H–J: Chondrogenic differentiation shown in paraffin section of micropellet stained with Alcian Blue (H) and Safranin O (red, arrows) (I); IHC staining of collagen type II (J). Expression of COL2A1 (collagen, type II, alpha 1) and COL10A1 (collagen, type X, alpha 1). Cells cultured in control culture medium for 4 weeks and stained for lineage markers are shown in insets (F, G, and I) and as negative control (−) for RT-PCR analysis; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was the internal control. Results shown are from a single sample representative of three patients. Scale bars = 200 μm.
For cells cultured in myogenic inducing-medium, the densely packed fibroblasts were immunoreactive for α-SMA (Figure 7D), compared with the untreated mature endometriotic stromal cells (Figure 7E), which were only occasionally α-SMA-positive. The mRNA expression for α-SMA (ACTA2) was also detected. The regulatory contractile proteins, caldesmon (CALD1) and calponin 1 (CNN1) in these cells further indicated that they had fully differentiated to smooth muscle cells, because these proteins were expressed primarily at late stages of smooth muscle cell maturation.25
Cells cultured in osteogenic inducing-medium exhibited mRNA and positive staining for alkaline phosphatase (ALPL; Figure 7F) and osteopontin (SPP1; Figure 7G). The mRNA expression of RUNX2 and PTH1R which are molecules essential for bone formation was also detected.
For chondrogenic differentiation, endometriotic stromal cells were cultured as micropellets and showed staining of extracellular sulfated glycosaminoglycans with Alcian Blue (Figure 7H), sulfated proteoglycans with Safranin O (Figure 7I), and IHC stained with collagen type II (Figure 7J). The mRNA expression of the early and late chondrocyte marker collagen type II COL2A1 and type X COL10A further confirmed chondrogenic differentiation of these cells. The vast majority of the cells cultured in the control medium did not stain positive in the differentiation assays for any of the lineages studied. Overall, large endometriotic stromal CFUs could differentiate into the four mesenchymal cell lineages.
Discussion
The major finding of this study was the presence of a subset of ovarian endometriotic cells displaying properties of somatic stem/progenitor cells. This subset possessed colony-forming activity, underwent self-renewal in vitro, and was multipotent. Both epithelial and stromal progenitor cells were identified from the ovarian endometrioma, suggesting that they may contribute to development and progression of the disease. The significant difference in the CFU activity of autologous endometrium and ovarian endometrioma also points to the importance of the stem cell niche in the proliferation and regulation of putative somatic stem cells.
Ovarian Endometrioma Contains Cells with Colony-Forming Ability and Self-Renewal Capacity
This study has provided the first evidence of the existence of putative somatic stem/progenitor cells in ovarian endometriotic lesions. The colony-forming activity of normal human endometrial cells has been studied with the classic functional assay.10,26 Using the same approach, we found colony-forming activity in 0.09% of epithelial cells and in 0.13% of stromal cells from ovarian endometrioma. Two types of CFUs for epithelial and stromal cells were detected: small loosely-arranged colonies and large densely-packed colonies. Based on published results on normal endometrium, we hypothesize that the large CFUs are initiated by the putative stem/progenitor cells and the small CFUs by the transit amplifying cells. The overall amount of large stromal CFUs was more apparent when compared with that of the epithelial cells, although the difference did not reach significance. This finding correlates with the small amount of epithelial cells observed in the ovarian endometrioma samples (Figure 1A).27
We demonstrated a significant difference in the colony-forming activity of human endometriotic stromal cells from the proliferative and secretory phase of the menstrual cycle. Other studies have indicated that hormonal stimulation during the menstrual cycle may result in a prolific increase of endometriotic cysts.28 In the present study, a greater proportion of clonogenic stromal cells (due primarily to the increase in the number of small CFUs) were observed in the proliferative than the secretory phase. This finding suggests that the endometriotic lesions possess a hormone-dependent population of stromal transit amplifying cells and can readily proliferate and differentiate under estrogen stimulation during the proliferative phase. However, this differs from the endometriotic small epithelial CFUs, the number of which did not change throughout the cycle, thus suggesting that the endometriotic epithelial transit amplifying cells may not be responsive to estrogen. This notion is supported by reports of comparable expression of the proliferative marker Ki-67 between the proliferative and secretory phase in the epithelia of ovarian endometrioma.29,30 The number of epithelial and stromal large CFUs was relatively stable in the two phases studied, indicating that the number of putative stem/progenitor cells within the cysts remains constant throughout the menstrual cycle.
Our data also demonstrated that the large epithelial and stromal CFUs exhibited substantial self-renewal ability by producing secondary and tertiary clones, supporting the notion that they originated from putative somatic stem/progenitor cells. Small epithelial and stromal CFUs, however, were more readily exhausted and failed to form clones in vitro subsequently, consistent with their derivation from transit amplifying cells that differentiate progressively as they undergo several rounds of proliferation.
Cellular Phenotypes and Multipotency of Endometriotic Clones
All endometriotic epithelial small CFUs expressed epithelial markers EpCAM,31 (Figure 1A), α6 integrin,32 and CK,33 which are known to be expressed in the epithelial cells of ovarian endometrioma. EpCAM and CK failed to be expressed in the large epithelial CFUs, and α6 integrin staining was expressed in only two out of five samples. EpCAM is the surface molecule used for affinity purification of the epithelial cells. Thus, the lack of EpCAM immunoreactivities in the large CFUs may be due to a suboptimal culture condition that could not sustain certain epithelial characteristics (resulting in a loss of the molecule during in vitro proliferation) or may be a result of epithelial-to-stromal transition. The lack of CK and only occasional expression of α6 integrin further suggest that these epithelial markers are lost during culture, a phenomenon that has been reported for normal endometrial epithelial large CFUs.10 As for stromal cells, all of the endometriotic large and small CFUs were positive for CD9034 and CD10,35 confirming the fibroblastic origin of these cells. The occasional expression of α-SMA-positive cells among endometriotic stromal large and small CFUs suggests that some of the stromal cells could spontaneously differentiate into another lineage.
A recent report has suggested that human endometrial stem-like cells originate from bone marrow and that they display endothelial progenitor cell-like characteristics.14 Because bone marrow endothelial progenitor cells are involved in endometrial angiogenesis36 and give rise to uterine epithelial cells,37 it was proposed that endometrial stem cells residing in the perivascular region could be involved in ectopic implantation via retrograde menstruation.14 In the present study, however, expression of the endothelial marker CD31 was observed primarily in small clusters of endometriotic stromal cells, and not in cells of large or small CFUs. This observation indicates that the stromal clonogenic cells are not originated from endothelial progenitor cells.
Apart from common stromal surface markers, endometriotic stromal CFUs possess immunoreactivities of three somatic stem cell markers: SALL4, Msi1, and CD133. Differential expression of SALL4 and Msi-1 between endometrial and endometriotic tissue has been reported.38,39 SALL4 is involved in the preservation of pluripotency in embryonic stem cells.40 In the present study, endometriotic stromal CFUs expressed a higher level of SALL4, compared with normal endometrial CFU; weak staining of Msi1 was found in normal endometrial stromal CFUs, and the expression was more intense for endometriotic stromal CFUs. Endometriotic stromal CFUs also expressed CD133, a marker of mesenchymal stem cells 41 and cancer-initiating cells in several cancerous tissues,42 including ovarian43 and endometrial cancer.44 Given that endometriosis is accompanied in 10% to 15% of ovarian cancers45 and because previous study has shown the absence of CD133 in endometrial stromal CFUs,46 whether there is a link between CD133-positive stromal cells in endometriosis and cancer development remains to be determined. Overall, the detection of somatic stem cell markers in the endometriotic stromal CFUs supports the notion of a stem cell origin in the etiology of the disease.
Endometriosis is regarded as an estrogen-dependent disease.1 The expression of ERα and ERβ in the endometriotic large and small CFUs of epithelial and stromal cells confirmed the expression of ERs in cultured endometriotic cells, and may contribute to the pathophysiology of ovarian endometrioma.47–49 ERβ has been shown to regulate ERα expression of stromal endometriotic cells by affecting cell cycling and promoting proliferation.50 The role of steroids in the regulation of endometriotic stem cells is unknown. However, studies on mice have shown that proliferating endometrial epithelial cells do not express ERα, and that wound healing mechanisms other than estrogen may be involved in the regeneration of mouse endometrium.16,17
The multipotency of endometriotic stromal large CFUs was demonstrated by their ability to differentiate into the four mesenchymal lineages. The results support the notion that retrograde endometrial cells may originate from circulating bone marrow mesenchymal stem cells. Studies have shown that circulating bone marrow mesenchymal stem cells may be involved in endometrial regeneration, because bone-marrow–derived stem cells can populate the human endometrium of bone marrow transplant patients.51 In mice, bone-marrow–derived cells from male donors are involved in endometrial gland formation of female transplant recipients52 and in ectopic endometrial implants within the peritoneal cavity.37 Endometrial stromal cells isolated by the expression of perivascular markers [melanoma cell adhesion molecule (MCAM, CD146) and platelet-derived growth factor receptor β (PDGFRβ)] displayed a high colony-formation ability and differentiated into different mesenchymal lineages.11,12 Thus, these findings and our present observations suggest the possible contribution of bone-marrow–mesenchymal stem cells in the development of endometriosis.
Role of Endometrial Stem Cells in Endometriosis
We consider the origin of ovarian endometriotic cells to be the endometrium because i) endometriotic CFUs expressed markers of endometrial CFUs, ii) ovarian surface epithelial cells differ in morphology from endometrial epithelial cells, and iii) endometriotic stromal CFUs displayed a decidualization capacity on progesterone treatment. Retrograde menstruation occurs in approximately 90% of menstruating women,53 but only a small minority develop endometriosis. We propose that ovarian endometrioma may result from retrograde menstruation when the shed tissues consist of putative endometrial stem/progenitor cells. Although endometrial stem cells are postulated to reside within the basalis layer,9 there is evidence that fragments of the shed endometrial basalis are more often found in the menstrual blood of women with endometriosis than in that of healthy control subjects.54 We speculate that the retrograde menstrual tissues from normal women contain essentially terminally differentiated endometrial cells, which may implant at ectopic sites but develop only small endometriotic lesions, because their limited proliferative potential does not allow them to grow extensively before they are induced to detach at the next menses. For women with endometriosis, however, their endometrial stem/progenitor cells are more likely to be shed during menses. On exposure to an environment conducive to the formation of endometriosis (eg, an estrogen and cytokine environment), the stem/progenitor cells proliferate and differentiate, allowing the rapid growth of the ectopic tissues that prevents them from complete detachment at the next menstruation.
Differences in the endometrium between women with and without endometriosis are well reported. For example, the endometria of women with endometriosis have reduced apoptosis and altered gene expression,55,56 and is more likely to grow on peritoneal surfaces.57–59 Increased proliferation of endometrial cells in women with endometriosis has been reported,60 but was not supported in another study.61 Although we cannot find any significant difference in the endometrial stem/progenitor cells from women with and without endometriosis in the parameters studied, it is possible that differences exist in other parameters yet to be determined. We believe that putative endometriotic stem/progenitor cells may also be involved in the recurrence of endometriotic lesions after laparoscopic excision.62
At the turn of a new decade, the pathogenesis of endometriosis remains uncertain. This study has taken the first step in providing new insights into the role of putative somatic stem cells in endometriosis. Undoubtedly, a better understanding is needed of the cellular and molecular mechanisms involved in the regulation of these putative somatic stem cells.
Acknowledgments
We thank Ms. Joyce Yuen for the collection of tissue and the gynecologists at Queen Mary Hospital for the provision of laparoscopic ovarian cystectomy and hysterectomy tissue. We also thank Ms. Vicki Geall for proof reading the manuscript.
Footnotes
Supported by a grant from the General Research Fund (GRF), Research Grants Council (HKU 7822/09M to W.S.B.Y.).
Supplemental material for this article can be found at http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2011.02.025.
Supplementary data
In vitro decidualization of endometriotic stromal cells at day 4, immunostained with insulin growth factor binding protein-1 (IGFBP-1) for progesterone treatment (A) and vehicle control (B). Scale bar = 200 μm.
IHC phenotyping of endometrial stromal CFU with somatic stem cell marker Sall-4 (A), Msi1 (B), and CD133 (C). Scale bar = 200 μm.
References
- 1.Giudice L.C., Kao L.C. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 2.Nisolle M., Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril. 1997;68:585–596. doi: 10.1016/s0015-0282(97)00191-x. [DOI] [PubMed] [Google Scholar]
- 3.Vinatier D., Orazi G., Cosson M., Dufour P. Theories of endometriosis. Eur J Obstet Gynecol Reprod Biol. 2001;96:21–34. doi: 10.1016/s0301-2115(00)00405-x. [DOI] [PubMed] [Google Scholar]
- 4.Meyer R. Über eine adenomatöse Wucherung der Serosa in einer Bauchnarbe. Z Geburtsh Gynakol. 1903;49:32–41. [On an adenomatous proliferation of the serosa in an abdominal scar]. German. [Google Scholar]
- 5.Fujii S. Secondary Müllerian system and endometriosis. Am J Obstet Gynecol. 1991;165:219–225. doi: 10.1016/0002-9378(91)90255-p. [DOI] [PubMed] [Google Scholar]
- 6.von Recklinghausen F. Adenomyomas and cystadenomas of the wall of the uterus and tube: their origin as remnants of the Wolffian body. Wien Klin Wochenschr. 1896;8:530. [Google Scholar]
- 7.Russell W.W. Aberrant portions of the Müllerian duct found in an ovary. Bull Johns Hopkins Hosp. 1899;10:8–10. [Google Scholar]
- 8.Sampson J. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422–469. [Google Scholar]
- 9.Gargett C.E., Chan R.W., Schwab K.E. Endometrial stem cells. Curr Opin Obstet Gynecol. 2007;19:377–383. doi: 10.1097/GCO.0b013e328235a5c6. [DOI] [PubMed] [Google Scholar]
- 10.Chan R.W., Schwab K.E., Gargett C.E. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70:1738–1750. doi: 10.1095/biolreprod.103.024109. [DOI] [PubMed] [Google Scholar]
- 11.Gargett C.E., Schwab K.E., Zillwood R.M., Nguyen H.P., Wu D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009;80:1136–1145. doi: 10.1095/biolreprod.108.075226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwab K.E., Gargett C.E. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod. 2007;22:2903–2911. doi: 10.1093/humrep/dem265. [DOI] [PubMed] [Google Scholar]
- 13.Kato K., Yoshimoto M., Kato K., Adachi S., Yamayoshi A., Arima T., Asanoma K., Kyo S., Nakahata T., Wake N. Characterization of side-population cells in human normal endometrium. Hum Reprod. 2007;22:1214–1223. doi: 10.1093/humrep/del514. [DOI] [PubMed] [Google Scholar]
- 14.Masuda H, Matsuzaki Y, Hiratsu E, Ono M, Nagashima T, Kajitani T, Arase T, Oda H, Uchida H, Asada H, Ito M, Yoshimura Y, Maruyama T, Okano H: Stem cell-like properties of the endometrial side population: implication in endometrial regeneration. PLoS One, 5:e10387 [DOI] [PMC free article] [PubMed]
- 15.Cervelló I., Gil-Sanchis C., Mas A., Delgado-Rosas F., Martinez-Conejero J.A., Galan A., Martinez-Romero A., Martinez S., Navarro I., Ferro J., Horcajadas J.A., Esteban F.J., O'Connor J.E., Pellicer A., Simón C. Human endometrial side population cells exhibit genotypic, phenotypic and functional features of somatic stem cells. PLoS One. 2010;5:e10964. doi: 10.1371/journal.pone.0010964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan R.W., Gargett C.E. Identification of label-retaining cells in mouse endometrium. Stem Cells. 2006;24:1529–1538. doi: 10.1634/stemcells.2005-0411. [DOI] [PubMed] [Google Scholar]
- 17.Kaitu'u-Lino T.J., Ye L., Gargett C.E. Reepithelialization of the uterine surface arises from endometrial glands: evidence from a functional mouse model of breakdown and repair. Endocrinology. 2010;151:3386–3395. doi: 10.1210/en.2009-1334. [DOI] [PubMed] [Google Scholar]
- 18.Gargett C.E., Chan R.W., Schwab K.E. Hormone and growth factor signaling in endometrial renewal: role of stem/progenitor cells. Mol Cell Endocrinol. 2008;288:22–29. doi: 10.1016/j.mce.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 19.Noyes R.W., Hertig A.T., Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122:262–263. doi: 10.1016/s0002-9378(16)33500-1. [DOI] [PubMed] [Google Scholar]
- 20.Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817–821. doi: 10.1016/s0015-0282(97)81391-x. [DOI] [PubMed] [Google Scholar]
- 21.Pittenger M., Mackay A., Beck S., Jaiswal R., Douglas R., Mosca D., Morrman M., Simonett D., Craig S., Marshak D. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 22.Ryan I.P., Schriock E.D., Taylor R.N. Isolation, characterization, and comparison of human endometrial and endometriosis cells in vitro. J Clin Endocrinol Metab. 1994;78:642–649. doi: 10.1210/jcem.78.3.8126136. [DOI] [PubMed] [Google Scholar]
- 23.Okamura H., Katabuchi H., Nitta M., Ohtake H. Structural changes and cell properties of human ovarian surface epithelium in ovarian pathophysiology. Microsc Res and Tech. 2006;69:469–481. doi: 10.1002/jemt.20306. [DOI] [PubMed] [Google Scholar]
- 24.Tontonoz P., Hu E., Graves R.A., Budavari A.I., Spiegelman B.M. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 25.Frid M.G., Shekhonin B.V., Koteliansky V.E., Glukhova M.A. Phenotypic changes of human smooth muscle cells during development: late expression of heavy caldesmon and calponin. Dev Biol. 1992;153:185–193. doi: 10.1016/0012-1606(92)90104-o. [DOI] [PubMed] [Google Scholar]
- 26.Schwab K.E., Chan R.W., Gargett C.E. Stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil Steril. 2005;84(Suppl 2):1124–1130. doi: 10.1016/j.fertnstert.2005.02.056. [DOI] [PubMed] [Google Scholar]
- 27.Donnez J., Nisolle M., Gillet N., Smets M., Bassil S., Casanas-Roux F. Large ovarian endometriomas. Hum Reprod. 1996;11:641–646. doi: 10.1093/humrep/11.3.641. [DOI] [PubMed] [Google Scholar]
- 28.Bulun S.E., Cheng Y.H., Pavone M.E., Xue Q., Attar E., Trukhacheva E., Tokunaga H., Utsunomiya H., Yin P., Luo X., Lin Z., Imir G., Thung S., Su E.J., Kim J.J. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Med. 2010;28:36–43. doi: 10.1055/s-0029-1242991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki A., Horiuchi A., Oka K., Miyamoto T., Kashima H., Shiozawa T. Immunohistochemical detection of steroid receptor cofactors in ovarian endometriosis: involvement of down-regulated SRC-1 expression in the limited growth activity of the endometriotic epithelium. Virchows Arch. 2010;456:433–441. doi: 10.1007/s00428-010-0884-x. [DOI] [PubMed] [Google Scholar]
- 30.Nisolle M., Casanas-Roux F., Donnez J. Immunohistochemical analysis of proliferative activity and steroid receptor expression in peritoneal and ovarian endometriosis. Fertil Steril. 1997;68:912–919. doi: 10.1016/s0015-0282(97)00341-5. [DOI] [PubMed] [Google Scholar]
- 31.Nakayama K., Masuzawa H., Li S.F., Yoshikawa F., Toki T., Nikaido T., Silverberg S.G., Fujii S. Immunohistochemical analysis of the peritoneum adjacent to endometriotic lesions using antibodies for Ber-EP4 antigen, estrogen receptors, and progesterone receptors: implication of peritoneal metaplasia in the pathogenesis of endometriosis. Int J Gynecol Pathol. 1994;13:348–358. doi: 10.1097/00004347-199410000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Regidor P.A., Vogel C., Regidor M., Schindler A.E., Winterhager E. Expression pattern of integrin adhesion molecules in endometriosis and human endometrium. Hum Reprod Update. 1998;4:710–718. doi: 10.1093/humupd/4.5.710. [DOI] [PubMed] [Google Scholar]
- 33.Song I.O., Hong S.R., Huh Y., Yoo K.J., Koong M.K., Jun J.Y., Kang I.S. Expression of vimentin and cytokeratin in eutopic and ectopic endometrium of women with adenomyosis and ovarian endometrioma. Am J Reprod Immunol. 1998;40:26–31. doi: 10.1111/j.1600-0897.1998.tb00384.x. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez-Shaw S., Shorter S.C., Naish C.E., Barlow D.H., Starkey P.M. Isolation and purification of human endometrial stromal and glandular cells using immunomagnetic microspheres. Hum Reprod. 1992;7:156–161. doi: 10.1093/oxfordjournals.humrep.a137609. [DOI] [PubMed] [Google Scholar]
- 35.Sumathi V.P., McCluggage W.G. CD10 is useful in demonstrating endometrial stroma at ectopic sites and in confirming a diagnosis of endometriosis. J Clin Pathol. 2002;55:391–392. doi: 10.1136/jcp.55.5.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asahara T., Masuda H., Takahashi T., Kalka C., Pastore C., Silver M., Kearne M., Magner M., Isner J.M. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 37.Du H., Taylor H.S. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells. 2007;25:2082–2086. doi: 10.1634/stemcells.2006-0828. [DOI] [PubMed] [Google Scholar]
- 38.Forte A., Schettino M.T., Finicelli M., Cipollaro M., Colacurci N., Cobellis L., Galderisi U. Expression pattern of stemness-related genes in human endometrial and endometriotic tissues. Mol Med. 2009;15:392–401. doi: 10.2119/molmed.2009.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Götte M., Wolf M., Staebler A., Buchweitz O., Kelsch R., Schüring A.N., Kiesel L. Increased expression of the adult stem cell marker Musashi-1 in endometriosis and endometrial carcinoma. J Pathol. 2008;215:317–329. doi: 10.1002/path.2364. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J., Tam W.L., Tong G.Q., Wu Q., Chan H.Y., Soh B.S., Lou Y., Yang J., Ma Y., Chai L., Ng H.H., Lufkin T., Robson P., Lim B. Sall4 modulates embryonic stem cell pluripotency and early embryonic development by the transcriptional regulation of Pou5f1. Nat Cell Biol. 2006;8:1114–1123. doi: 10.1038/ncb1481. [DOI] [PubMed] [Google Scholar]
- 41.Tondreau T., Meuleman N., Delforge A., Dejeneffe M., Leroy R., Massy M., Mortier C., Bron D., Lagneaux L. Mesenchymal stem cells derived from CD133-positive cells in mobilized peripheral blood and cord blood: proliferation, Oct4 expression, and plasticity. Stem Cells. 2005;23:1105–1112. doi: 10.1634/stemcells.2004-0330. [DOI] [PubMed] [Google Scholar]
- 42.Wu Y., Wu P.Y. CD133 as a marker for cancer stem cells: progresses and concerns. Stem Cells Dev. 2009;18:1127–1134. doi: 10.1089/scd.2008.0338. [DOI] [PubMed] [Google Scholar]
- 43.Baba T., Convery P.A., Matsumura N., Whitaker R.S., Kondoh E., Perry T., Huang Z., Bentley R.C., Mori S., Fujii S., Marks J.R., Berchuck A., Murphy S.K. Epigenetic regulation of CD133 and tumorigenicity of CD133+ ovarian cancer cells. Oncogene. 2008;28:209–218. doi: 10.1038/onc.2008.374. [DOI] [PubMed] [Google Scholar]
- 44.Rutella S., Bonanno G., Procoli A., Mariotti A., Corallo M., Prisco M.G., Eramo A., Napoletano C., Gallo D., Perillo A., Nuti M., Pierelli L., Testa U., Scambia G., Ferrandina G. Cells with characteristics of cancer stem/progenitor cells express the CD133 antigen in human endometrial tumors. Clin Cancer Res. 2009;15:4299–4311. doi: 10.1158/1078-0432.CCR-08-1883. [DOI] [PubMed] [Google Scholar]
- 45.McMeekin D.S., Burger R.A., Manetta A., DiSaia P., Berman M.L. Endometrioid adenocarcinoma of the ovary and its relationship to endometriosis. Gynecol Oncol. 1995;59:81–86. doi: 10.1006/gyno.1995.1271. [DOI] [PubMed] [Google Scholar]
- 46.Schwab K.E., Hutchinson P., Gargett C.E. Identification of surface markers for prospective isolation of human endometrial stromal colony-forming cells. Hum Reprod. 2008;23:934–943. doi: 10.1093/humrep/den051. [DOI] [PubMed] [Google Scholar]
- 47.Brandenberger A.W., Lebovic D.I., Tee M.K., Ryan I.P., Tseng J.F., Jaffe R.B., Taylor R.N. Oestrogen receptor (ER)-alpha and ER-beta isoforms in normal endometrial and endometriosis-derived stromal cells. Mol Hum Reprod. 1999;5:651–655. doi: 10.1093/molehr/5.7.651. [DOI] [PubMed] [Google Scholar]
- 48.Matsuzaki S., Uehara S., Murakami T., Fujiwara J., Funato T., Okamura K. Quantitative analysis of estrogen receptor alpha and beta messenger ribonucleic acid levels in normal endometrium and ovarian endometriotic cysts using a real-time reverse transcription-polymerase chain reaction assay. Fertil Steril. 2000;74:753–759. doi: 10.1016/s0015-0282(00)00712-3. [Erratum appeared in Fertil Steril 2001, 75:231] [DOI] [PubMed] [Google Scholar]
- 49.Matsuzaki S., Murakami T., Uehara S., Canis M., Sasano H., Okamura K. Expression of estrogen receptor alpha and beta in peritoneal and ovarian endometriosis. Fertil Steril. 2001;75:1198–1205. doi: 10.1016/s0015-0282(01)01783-6. [DOI] [PubMed] [Google Scholar]
- 50.Trukhacheva E., Lin Z., Reierstad S., Cheng Y.H., Milad M., Bulun S.E. Estrogen receptor (ER) beta regulates ERalpha expression in stromal cells derived from ovarian endometriosis. J Clin Endocrinol Metab. 2009;94:615–622. doi: 10.1210/jc.2008-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taylor H.S. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA. 2004;292:81–85. doi: 10.1001/jama.292.1.81. [DOI] [PubMed] [Google Scholar]
- 52.Ikoma T., Kyo S., Maida Y., Ozaki S., Takakura M., Nakao S., Inoue M. Bone marrow-derived cells from male donors can compose endometrial glands in female transplant recipients. Am J Obstet Gynecol. 2009;201 doi: 10.1016/j.ajog.2009.07.026. 608.e1–608.e8. [DOI] [PubMed] [Google Scholar]
- 53.Halme J., Hammond M.G., Hulka J.F., Raj S.G., Talbert L.M. Retrograde menstruation in healthy women and in patients with mild endometriosis. Obstet Gynecol. 1984;64:151–154. [PubMed] [Google Scholar]
- 54.Leyendecker G., Herbertz M., Kunz G., Mall G. Endometriosis results from the dislocation of basal endometrium. Hum Reprod. 2002;17:2725–2736. doi: 10.1093/humrep/17.10.2725. [DOI] [PubMed] [Google Scholar]
- 55.Meresman G.F., Vighi S., Buquet R.A., Contreras-Ortiz O., Tesone M., Rumi L.S. Apoptosis and expression of Bcl-2 and Bax in eutopic endometrium from women with endometriosis. Fertil Steril. 2000;74:760–766. doi: 10.1016/s0015-0282(00)01522-3. [DOI] [PubMed] [Google Scholar]
- 56.Johnson M.C., Torres M., Alves A., Bacallao K., Fuentes A., Vega M., Boric M.A. Augmented cell survival in eutopic endometrium from women with endometriosis: expression of c-myc, TGF-beta1 and bax genes. Reprod Biol Endocrinol. 2005;3:45. doi: 10.1186/1477-7827-3-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jolicoeur C., Boutouil M., Drouin R., Paradis I., Lemay A., Akoum A. Increased expression of monocyte chemotactic protein-1 in the endometrium of women with endometriosis. Am J Pathol. 1998;152:125–133. [PMC free article] [PubMed] [Google Scholar]
- 58.Noble L.S., Takayama K., Zeitoun K.M., Putman J.M., Johns D.A., Hinshelwood M.M., Agarwal V.R., Zhao Y., Carr B.R., Bulun S.E. Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J Clin Endocrinol Metab. 1997;82:600–606. doi: 10.1210/jcem.82.2.3783. [DOI] [PubMed] [Google Scholar]
- 59.Leyendecker G., Kunz G., Noe M., Herbertz M., Mall G. Endometriosis: a dysfunction and disease of the archimetra. Hum Reprod Update. 1998;4:752–762. doi: 10.1093/humupd/4.5.752. [DOI] [PubMed] [Google Scholar]
- 60.Wingfield M., Macpherson A., Healy D.L., Rogers P.A. Cell proliferation is increased in the endometrium of women with endometriosis. Fertil Steril. 1995;64:340–346. doi: 10.1016/s0015-0282(16)57733-4. [DOI] [PubMed] [Google Scholar]
- 61.Jürgensen A., Mettler L., Volkov N.I., Parwaresch R. Proliferative activity of the endometrium throughout the menstrual cycle in infertile women with and without endometriosis. Fertil Steril. 1996;66:369–375. doi: 10.1016/s0015-0282(16)58502-1. [DOI] [PubMed] [Google Scholar]
- 62.Guo S.W. Recurrence of endometriosis and its control. Hum Reprod Update. 2009;15:441–461. doi: 10.1093/humupd/dmp007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In vitro decidualization of endometriotic stromal cells at day 4, immunostained with insulin growth factor binding protein-1 (IGFBP-1) for progesterone treatment (A) and vehicle control (B). Scale bar = 200 μm.
IHC phenotyping of endometrial stromal CFU with somatic stem cell marker Sall-4 (A), Msi1 (B), and CD133 (C). Scale bar = 200 μm.


