Abstract
Simple, mild, and efficient conditions are reported for a Pd0-catalyzed Heck reaction that delivers high yields and selectivity for (E)-styrenyl products using electronically non-biased olefin substrates bearing a range of useful functionality. Preliminary mechanistic studies demonstrate that the σ-donating DMA solvent is crucial for high selectivity. Further studies suggest that the catalyst distinguishes between β-hydrogens based on their relative hydridic character, which is in contrast to previously reported PdII-catalyzed oxidative reaction conditions.
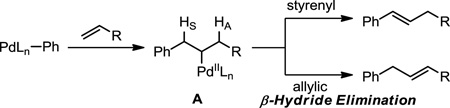
The Heck reaction, or palladium-catalyzed substitution of a vinylic C-H bond for an arene, is a transformation utilized extensively in synthesis despite significant limitations.1 Arguably, the most restrictive limitation is the required use of an electronically biased olefin, such as a styrene or α,β-unsaturated carbonyl, in order to deliver a single arylated alkene isomer. In the absence of substrate bias, a mixture of olefin products arises under both typical Pd0-catalyzed, and most oxidative PdII-catalyzed,2 Heck reaction conditions. This mixture of products likely results from PdII-σ-alkyl intermediate A undergoing indiscriminate β-hydride elimination with either HS or HA (eq 1).3 Additionally, there exists no generally applicable set of Heck conditions; parameters typically must be optimized for a specific desired reaction.1b
 |
(1) |
Recently, we reported a PdII-catalyzed oxidative Heck reaction capable of delivering a variety of (E)-styrenyl products from electronically non-biased terminal alkene substrates, where the high selectivity observed was attributed to catalyst control (eq 2).4 Specifically, preliminary mechanistic studies suggested that the unusually high selectivity resulted from a cationic palladium catalyst stabilized by a strongly σ-donating ligand. It appears that, when undergoing β-hydride elimination from A, this metal center is capable of distinguishing between HA and HS based on relative C-H bond strength. This surprising result, which suggests that the hydrogen leaving in the selectivity-determining step may have partial radical character, has engaged our interest in this fundamental organometallic process.
While the aforementioned report significantly improved the substrate scope of the oxidative Heck reaction, it requires catalyst synthesis, a three-fold excess of the arene organometallic, and a catalytic amount of copper salts to proceed with high yield and excellent selectivity. Additionally, these conditions are incompatible with some common functional groups, such as carboxylic acids and nitriles. Guided by our previous mechanistic studies, we report herein the development of a complementary and operationally simple Pd0-catalyzed Heck reaction using electronically unbiased terminal alkenes, which allows for the introduction of diverse functional groups and is highly selective for (E)-styrenyl products.
 |
(2) |
In order to achieve high selectivity, it was hypothesized that mimicking the electrophilic nature of the proposed PdII-σ-alkyl intermediate A capable of distinguishing between β-hydrogens would be essential, and that the elevated temperatures typically required for oxidative addition should be avoided. As such, it was envisioned that aryl diazonium tetrafluoroborates, pioneered by Matsuda, would be well-suited as the oxidant in light of both their tendency to undergo facile oxidative addition5 and the non-coordinating nature of the BF4 counterion of the resultant aryl-PdII complex.6 An initial experiment utilizing this oxidant resulted in a modest yield and relatively low selectivity (Table 1, entry 1).7
Table 1.
Optimization of the Heck reaction
 | ||||||
|---|---|---|---|---|---|---|
| entry | x | y | time | % conversiona | % yielda | selectivitya,b |
| 1c | 5 | 1.5 | 16 h | >99 | 68.4 | 3.8 |
| 2 | 5 | 1.5 | 16 h | >99 | 43.3 | 6.8 |
| 3 | 5 | 1.5 | 15 min | >99 | 62.6 | 7.1 |
| 4 | 3 | 1.5 | 15 min | >99 | 86.2 | 7.5 |
| 5 | 3 | 1.1 | 15 min | >99 | >99 | 10.7 |
| 6 | 0 | 1.1 | 15 min | 3.6 | 0 | - |
| 7d | 3 | 1.1 | 15 min | >99 | 20 | 0.2:1 |
| 8e | 3 | 1.1 | 15 min | 98.1 | 15 | 0.3:1 |
Conversion and yield calculated by comparing starting material and product peak integration to integration of internal standard using corrected GC analysis. Yields refers to the sum of all product isomers.
Selectivity refers to the ratio of (E)-styrene to all other isomers.
12.5 mol % liPr carbine added.
MeOH used as solvent.
MeCN used as solvent.
A control experiment (entry 2) excluding the N-heterocyclic carbene ligand delivered higher selectivity, prompting the elimination of this additive in future experiments. A significant disparity between consumption of substrate and product yield dictated the careful monitoring of this ratio over time, revealing reaction completion after only 15 minutes and further consumption of the product if the reaction mixture is not quenched (entries 2 vs 3).8 Decreased catalyst and arene loadings resulted in improved yield and selectivity (entries 4 and 5), while entry 6 confirms that palladium is required. Of particular note, the use of MeOH or MeCN, solvents commonly employed in Matsuda-Heck reactions,5 results in very poor selectivity (entries 7 and 8).
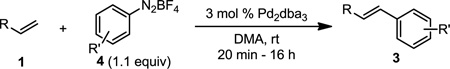
The optimized conditions were evaluated for compatibility with a variety of substrates that performed well under oxidative conditions.4 For example, substrates bearing an ester (Table 2, entry 1), a ketone (entry 2) and a silyl ether (entry 3) all proceed with excellent yield and selectivity. A free homoallylic alcohol (entry 4) was compatible and, more surprisingly, an allylic acetate is an excellent substrate that does not undergo oxidative addition under these conditions (entry 5).9 A doubly protected allylic amine is a good substrate,10 as is a distal free alcohol (entries 6 and 7). The PdII-catalyzed conditions are incompatible with carboxylic acids,4 but gratifyingly, 3h and 3i were prepared in good to excellent yield using the present system. Of note, the heteroatom-free substrate dodecene gives excellent results, providing strong evidence that the selectivity observed under these conditions is catalyst-controlled rather than dependent on substrate chelation (entry 10).11 A substrate bearing a nitrile (also incompatible with oxidative conditions) is arylated with either a phenyl group or an arene bearing a methyl ester (entries 11 and 12). A free 1,2-diol reacts cleanly when installing a phenyl group, but the yield suffers when installing an arene with more steric bulk (cf. entries 13 and 14). Free phenols and aryl chlorides are compatible with these conditions (entries 15 and 16), and submission of a free allylic alcohol gives the desired product, but the reaction proceeds more slowly and the yield is diminished due to the formation of a ketone by-product (entry 17).12 Challenging nitro- and iodo-substituted arenes are also compatible under the conditions described, providing valuable handles for further functionalization (entries 18 and 19).13 The reaction proceeds rapidly and in high yield using α-methylstyrene as the substrate, but unfortunately a mixture of olefin isomers is observed (entries 20 and 21).14 Finally, a highly enantiomerically enriched substrate, that may be susceptible to racemization, suffers no erosion of enantiomeric excess (entry 22).
Table 2.
Scope of the Heck reaction.
 | |||
|---|---|---|---|
| entry | product | time | % yielda |
| 1 | 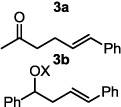 |
20 min | 97b |
| 2 | 20 min | 89 | |
| 3 | X = TBS 3c | 16 h | 87 |
| 4 | X = H 3d | 1.5 h | 72 |
| 5 | 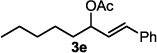 |
16 h | 87c |
| 6 | 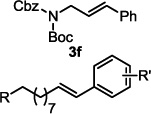 |
16 h | 96 |
| 7 | R = CH2OH, R' = H 3g | 40 min | 77b |
| 8 | R = CO2H, R' = H 3h | 2 h | 95b |
| 9 | R = CO2H, R' = 3,5-dimethoxy 3i | 2 h | 69b |
| 10 | 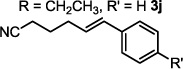 |
20 min | 77b |
| 11 | R' = H 3k | 3 h | 96 |
| 12 | 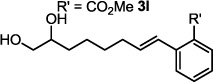 |
3 h | 98 |
| 13 | R' = H 3m | 2 h | 83 |
| 14 | 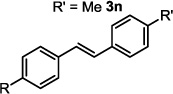 |
2 h | 66c |
| 15 | R = OH, R' = H 3o | 1.5 h | 98 |
| 16 | R = CI, R' = OMe 3p | 1.5 h | 98 |
| 17 | 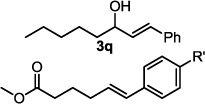 |
1.6 h | 55c,d |
| 18 | R' = NO2 3r | 20 min | 97 |
| 19 | 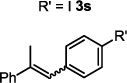 |
20 min | 66 |
| 20 | R' = F 3t | 2 h | 99c,e |
| 21 | R' = OMe 3u | 2 h | 99c,e |
| 22 | 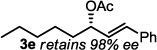 |
16 h | 81 |
Yields are averages of two experiments performed on a 0.5 mmol scale, and are the sum of all product isomers. The selectivity for (E)-styrene >20:1 unless otherwise noted.
Selectivity for (E)-styrene was 10:1.
Using 5 mol % Pd2dba3 and 1.5 equiv ArN2BF4.
Isolated 44% keton product.
Obtained ~1:1 mixture of olefin isomers.
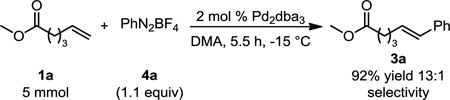 |
(3) |
While evaluating the scope of this transformation, it was observed that the reaction is highly exothermic raising concerns that the selectivity of a reaction performed on larger scale may suffer. Therefore, on 5 mmol scale, 1a was subjected to the conditions described to prepare 3a, except that the catalyst loading was decreased to 2 mol % and the reaction was performed at −15 °C (eq 3). Under these conditions, 3a was obtained in comparable yield and improved selectivity for the (E)-styrenyl product after 5.5 h.
Having established that the active catalyst described in this report indeed exhibits a unique preference for (E)-styrenyl products, we wished to gain an understanding of how, exactly, the catalyst imparts selectivity. The mechanistic origin of selectivity under oxidative conditions was previously determined by submitting β,γ-unsaturated ester 1t to PdII-catalyzed conditions using a variety of electronically disparate arene sources; an experiment designed to probe the selectivity-determining β-hydride elimination step where a significant substituent effect was observed.4 Interestingly, the product distribution measured under Pd0-catalyzed conditions reveals no clear trend, with styrene products favored in similar ratios regardless of the electronic nature of the arene (Table 3).15
Table 3.
Product distribution using a β,γ-unsaturated ester.
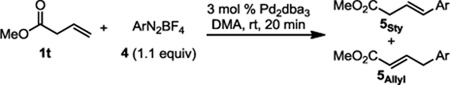 | |||
|---|---|---|---|
| Ar | 5Sty/5Allyl | Ar | 5Sty/5Allyl |
| 4-H | 6.03 | 4-CO2Me | 7.69 |
| 4-NO2 | 6.11 | 4-Br | 6.47 |
| 4-OMe | 7.87 | 4-F | 6.12 |
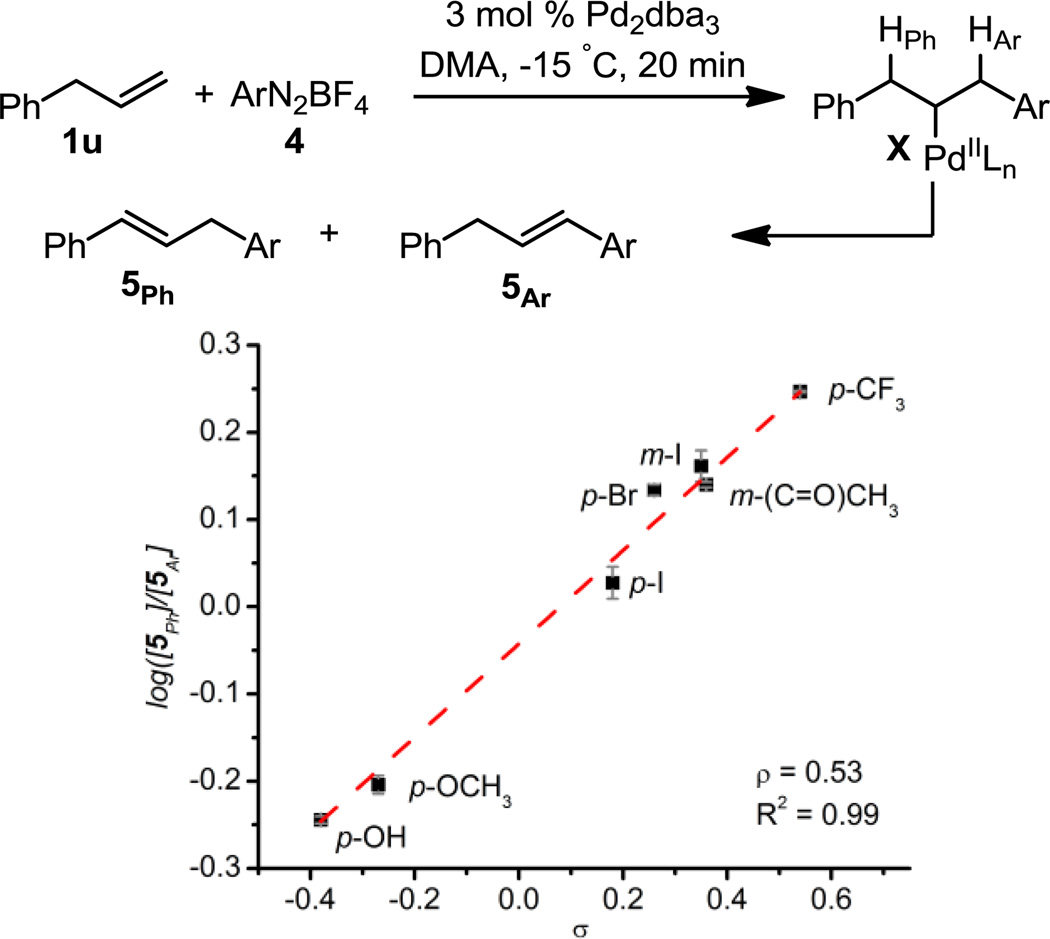
A more sensitive mechanistic experiment was designed, whereby allyl benzene was submitted to the optimal conditions with a variety of electronically disparate aryl diazonium salts. In this product-partitioning experiment, the catalyst must distinguish between two benzylic hydrogens, which presumably differ only by virtue of the arenes immediately adjacent (Figure 1). The linear free energy relationship observed suggests that the origin of selectivity under these conditions is related to the ability of the metal center in intermediate X to distinguish between β-hydrogens as a function of their relative hydridic nature.16,17 Therefore, submission of electron-rich diazonium salts results in relatively more 5Ar, due to the greater ability of the newly installed arene to stabilize partial positive charge developing during β-hydride elimination. Consistent with this proposal, submission of electron-deficient aryl diazonium salts results in relatively more 5Ph, due to destabilization of partial positive charge by the newly installed arene. These results contrast markedly with those observed under oxidative PdII catalysis,4 suggesting an interesting mechanistic complementarity along with the more intuitive counterpart arising from the differing oxidation states of the two precatalysts.
Figure 1.
Hammett plot.
In conclusion, we have developed simple and efficient conditions for a Pd0-catalyzed Heck reaction that delivers high selectivity for (E)-styrenyl products in the absence of substrate bias. This reaction is compatible with a greater range of functional groups than the related PdII system and utilizes a commercially-available catalyst. Additionally, the reaction requires no base, elevated temperatures, nor additional oxidant. For most substrates evaluated, the reaction is completed rapidly, but the rate is retarded when using substrates with allylic coordinating groups. Some functional groups are incompatible,10 but it is reasonably easy to predict these, providing another advantage over other Heck protocols, which can require optimization for each substrate. Initial mechanistic experiments suggest that the selectivity observed is highly sensitive to the identity of the solvent. A linear free energy relationship probing product distribution as a function of the electronic nature of the introduced arene suggests that this catalyst selects between β-hydrogens based on their hydridic nature, which is in contrast with the PdII system previously reported. This reactivity manifold has inspired further experiments designed to probe the factors that dictate selectivity in β-hydride elimination from related organometallic intermediates. Investigations in this pursuit are currently underway in our laboratory.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (NIGMS RO1 GM3540). We are grateful to Johnson Matthey for the gift of various Pd salts.
Footnotes
Supporting Information Available: Optimization data, experimental procedures, and characterization data. This material is available free of charge via the Internet at http://pubs.acs.org
REFERENCES
- 1.For reviews of the Heck reaction see: (a) for information regarding substrate scope Heck R. F. Org. React. 1982;27:345–390. (b) For updated scope information and brief mechanistic discussion, see Meijere A, Meyer FE. Angew. Chem., Int. Ed. 1994;33:2379–2411. (c) For mechanistic details, see Beletskaya IP, Cheprakov AV. Chem. Rev. 2000;100:3009–3066. doi: 10.1021/cr9903048. (d) For advances using aryl chloride and aryl bromide oxidants, see Whitcombe NJ, Hii KK, Gibson SE. Tetrahedron. 2001;57:7449–7476. (e) For the use of 1,1-disubstituted olefins as substrates, see Kondolff I, Doucet H, Santelli M. Tetrahedron Lett. 2003;44:8487–8491. (f) For advances in asymmetric Heck methodology, see Dounay AB, Overman LE. Chem. Rev. 2003;103:2945–2964. doi: 10.1021/cr020039h.
- 2.For examples of the oxidative Heck reaction, see: Du X, Suguro M, Hirabayashi K, Mori A, Nishikata T, Hagiwara N, Kawata T, Okeda T, Wang HF, Fugami K, Kosugi M. Org. Lett. 2001;3:3313–3316. doi: 10.1021/ol016529y. Parrish JP, Jung YC, Shin SI, Jung KW. J. Org. Chem. 2002;67:7127–7130. doi: 10.1021/jo020159p. Jung YC, Mishra RK, Yoon CH, Jung KW. Org. Lett. 2003;5:2231–2234. doi: 10.1021/ol034458s. Andappan MMS, Nilsson P, von Schenck H, Larhed MJ. J. Org. Chem. 2004;69:5212–5218. doi: 10.1021/jo049434t. Yoo KS, Yoon CH, Jung KW. J. Am. Chem. Soc. 2006;128:16384–16393. doi: 10.1021/ja063710z. Jia C, Piao D, Oyamada J, Lu W, Kitamura T, Fujiwara Y. Science. 2000;287:1992–1995. doi: 10.1126/science.287.5460.1992. Zhang Y-H, Shi B-F, Yu J-Q. J. Am. Chem. Soc. 2009;131:5072–5074. doi: 10.1021/ja900327e. Zhang X, Fan S, He C-Y, Wan X, Min Q-Q, Yang J, Jiang Z-X. J. Am. Chem. Soc. 2010;132:4506–4507. doi: 10.1021/ja908434e.
- 3.Additional byproducts can arise from Heck insertion in the opposite orientation resulting in terminal olefin products, and internal olefin isomeric products arising from Pd-H migration. Z-olefin isomers may also be observed.
- 4.Werner EW, Sigman MS. J. Am. Chem. Soc. 2010;132:13981–13983. doi: 10.1021/ja1060998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.For seminal work, see: Kikukawa K, Nagira K, Wada F, Matsuda T. Tetrahedron. 1981;37:31–36. For reviews of the use of aryl-diazonium salts in Pd-catalyzed cross coupling reactions, see: Roglans A, Pla-Quintana A, Moreno-Manas M. Chem. Rev. 2006;106:4622–4643. doi: 10.1021/cr0509861. Taylor JG, Moro AV, Correia CRD. Eur. J. Org. Chem. 2011:1403–1428. For a mechanistic study of the Matsuda-Heck reaction, see: Sabino AA, Machado AHL, Correia CRD, Eberlin MN. Angew. Chem., Int. Ed. 2004;43:2514–2518. doi: 10.1002/anie.200353076. For a recent example utilizing in situ-prepared aryldiazonium salts, see Callonnec FL, Fouquet E, Felpin F-X. Org. Lett. 2011 doi: 10.1021/ol200752x. ASAP.
- 6.The pKA of the conjugate acid provides a commonly used estimation of the coordinative abilities of counterions in catalysis; the pKA of HBF4 is -5.
- 7.Throughout the paper, when isomers are detected they arise either from migratory insertion in the opposite direction to give terminal olefin products, or from olefin migration via non-selective β-hydride elimination. (Z)-Olefin isomers were not observed.
- 8.A minor byproduct arises from a subsequent Heck reaction of the resultant styrene utilizing the remainder of the aryldiazonium salt. The desired product is also believed to be oligomerized in the reaction mixture overtime, likely due to the equivalent of HBF4 generated in the reaction. See supporting information for details.
- 9.Allylic acetates are known to be displaced by Pd0 to form π-allyl complexes. For a review, see: Trost BM. Angew. Chem. Int. Ed. 1989;28:1173.
- 10.More basic nitrogen functionality, such as a trialkyl amine-containing substrate, was incompatible with these conditions. Other incompatible functionality included quinoline and indole arenes. See supporting information for further details.
- 11.For examples of chelation-controlled Heck reactions see: Filippini L, Gusmeroli M, Riva R. Tetrahedron Lett. 1993;34:1643–1646. Kang SK, Lee HW, Jang SB, Kim TH, Pyun SJ. J. Org. Chem. 1996;61:2604–2605. doi: 10.1021/jo951923t. Olofsson K, Sahlin H, Larhead M, Hallberg A. J. Org. Chem. 2001;66:544–549. doi: 10.1021/jo001416y. Buezo ND, Rosa JC, Priego J, Alonso I, Carretoero JC. Chem. Eur. J. 2001;7:3890–3900. doi: 10.1002/1521-3765(20010917)7:18<3890::aid-chem3890>3.0.co;2-y. Delcamp JH, Brucks AP, White MC. J. Am. Chem. Soc. 2008;130:11270–11271. doi: 10.1021/ja804120r.
-
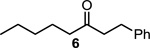
12.Ketones like 6 are usually the major product observed using free allylic alcohols under Heck conditions; see above references.
- 13.It is important to note that while most aryl diazonium salts submitted to this reaction performed well, there are several examples of synthetically inaccessible aryl diazonium salts. See supporting information for details.
- 14.See supporting information for details.
- 15.Interestingly, allylic products 5Allyl were typically favored under oxidative Heck conditions, while they were the minor products under these conditions regardless of arene. See supporting information for details.
- 16.For examples of Hammett analyses of other Heck reactions see: Benhaddou R, Czernecki S, Ville G, Zegar A. Organometallics. 1988;7:2435–2439. Fristrup P, Le Quement S, Tanner D, Norrby PO. Organometallics. 2004;23:6160–6165.
- 17.The modest slope of the Hammett plot may explain why no trend was apparent when attempting to construct a similar Hammett plot using a β,γ-unsaturated ester as the substrate. It is likely that the hydridic nature of benzylic hydrogens and those α to an ester are significantly different.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.