Abstract
During acute lung injury and repair, leukocytes are thought to enter the lung primarily across alveolar capillaries and postcapillary venules. We hypothesized that leukocytes also migrate across pulmonary arterioles and venules, which serve as alternative sites for leukocyte influx into the lung during acute lung injury and repair. Lung sections from C57BL/6J mice up to 14 days after intratracheal bleomycin (3.33 U/kg) or saline instillation were assessed by light, fluorescence, confocal, and transmission electron microscopy for evidence of inflammatory cell sequestration and transmigration at these sites. After bleomycin treatment, large numbers of leukocytes (including neutrophils, eosinophils, and monocytes) were present in the vascular lumina and in perivascular interstitia of pulmonary arterioles and venules, as well as within the vascular walls. Leukocytes were observed within well-defined pathways in arteriolar walls and much less structured pathways in venular walls, apparently in the process of transmigration. Intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) were expressed at sites of leukocyte interaction with the luminal surface, especially in arterioles. Leukocytes appeared to exit from the vessels near collagen fibers into the perivascular interstitium. Results indicate that leukocytes can directly migrate across arteriolar and venular walls into the perivascular interstitium, which may represent an important but under-recognized pathway for leukocyte influx into the lung during injury and repair.
Inflammation of the lung is common to virtually all acute and chronic lung disorders.1–11 Although inflammation is a natural host response to infection and injury, excessive or unresolved inflammatory processes can play a role in cell injury and tissue remodeling with severe pathological consequences. For example, leukocytes such as neutrophils, eosinophils, and monocytes have been implicated as mediators of lung and vascular injury,12–17 although the extent to which inflammatory cells and mediators directly cause lung cell injury or modulate development of fibrosis remains controversial.18
Under normal conditions, granulocytes marginating in the lung microcirculation serve as first responders to infectious or injurious insults. In response to inflammatory stimuli and up-regulation of adhesive molecules, inflammatory cells sequester at sites along vascular luminal walls and adhere to the endothelium before migrating into tissue.19 We use the term leukocyte sequestration to describe the process by which leukocytes “accumulate in inflamed lungs in preparation for migration” (as defined for neutrophils by Doerschuk20). Leukocyte influx into the tissue of most organs occurs mainly across the postcapillary venules of the systemic circulatory system.21–24 However, leukocytes within the pulmonary circulation migrate into lung parenchymal tissue predominantly via alveolar capillaries and to a lesser extent the postcapillary venules.20,22,25–33
Although leukocyte transmigration at the alveolar-capillary level has been widely studied, little is really known about the routes or mechanisms of leukocyte accumulation within perivascular spaces. Nonetheless, marked leukocyte accumulation within perivascular interstitia is known to occur under pulmonary pathological conditions such as acute lung injury.34–36 We hypothesized that leukocytes can emigrate from larger, muscular pulmonary venules and arterioles directly into surrounding perivascular interstitia during acute injury and repair. Although others have suggested that it is unlikely that leukocytes migrate across muscular pulmonary vasculature,37,38 a few studies specifically report detection of leukocyte migration across larger pulmonary vessels.39–41 (These studies are, however, limited to a single model of airway antigen challenge.)
To investigate our hypothesis, we used the intratracheal bleomycin mouse model of acute lung injury and repair, which is associated with epithelial and endothelial cell injury42 and an intense inflammatory response including extensive accumulation of inflammatory cells within areas of perivascular interstitium.34–36,43 The present study provides clear histological and ultrastructural evidence that during bleomycin-induced lung injury and repair, sequestered leukocytes along the lumina of arterioles and venules can migrate directly across the muscular vessel walls into the contiguous perivascular interstitium. To our knowledge, leukocyte migration across small muscular vascular walls has not been previously reported in acute lung injury and repair. The present study is the first to describe and compare morphological features of arteriolar and venular leukocyte transmigration in a model of acute lung injury. We think that arterioles and venules may be under-recognized sites for leukocyte influx into lung tissue and may serve as novel anatomical targets to modulate pulmonary inflammatory responses during acute lung injury.
Materials and Methods
Bleomycin Mouse Model of Acute Lung Injury and Repair
All protocols and experimental procedures received prior approval by the Institutional Animal Care and Use Committee of the National Institute of Environmental Health Sciences (NIEHS). Monthly sentinel surveillance was used to screen for a wide range of pathogens, including mouse hepatitis virus, Sendai virus, pneumonia virus of mice, CAR bacillus, mouse parvovirus 1 and 2, epizootic diarrhea of infant mice, mouse norovirus, Mycoplasma pulmonis, Helicobacter spp., fur mites, and pinworms. No pathogens were detected in sentinel mice during this study. All animal housing and procedures were done within the NIEHS animal facility, which is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care.
Bleomycin (Sigma-Aldrich, St. Louis, MO) was administered intratracheally to C57BL/6J mice, a strain known for sensitivity to bleomycin-induced lung toxicity.44–49 Briefly, healthy C57BL/6J female mice, 9 to 13 weeks old (The Jackson Laboratory, Bar Harbor, ME), were anesthetized by isoflurane (Halocarbon Products Corp., North Augusta, SC) inhalation (3%) and given an analgesic [buprenorphine (Reckitt & Colman Pharmaceuticals Inc, Richmond, VA), 0.1 mg/kg, i.p.] before intratracheal administration of either normal saline (Aqualite System, Lake Forest, IL) or bleomycin in normal saline diluent for a final dose of 3.33 U/kg. After mice were anesthetized with isoflurane, the ventral region of the neck was shaved and cleaned with both Triadine (Traid Disposables, Brookfield, WI) and 70% alcohol before a longitudinal incision was made to expose the trachea. Bleomycin sulfate in 40 μL sterile saline (along with a small bolus of air to facilitate delivery) was instilled into the lungs via a ball-tipped, 24-gauge feeding needle (VWR, Radnor, PA) inserted into the trachea just before the bronchial bifurcation. An equivalent volume of sterile saline and bolus of air was administered to mice in the control groups in the same manner. The incision was closed with synthetic, absorbable tissue adhesive (Tissumend II adhesive; Veterinary Products Laboratories, Phoenix, AZ). All mice were housed in pathogen-free conditions in microisolator cages and were fed standard chow and water per standard protocol within the NIEHS animal facility until euthanized.
Lung Tissue Preparation and Sectioning
After intratracheal administration of saline or bleomycin, mice from each group were euthanized with pentobarbital (80 mg/kg, i.p.) at days 2, 4, 7, 9, 11, and 14 (n = 12 for each time point; 6 controls and 6 bleomycin-treated). After exsanguination and perfusion, the heart and lungs were removed from the thoracic cavity en bloc to prepare the lungs for either paraffin-embedding and sectioning or cryosectioning. The intact lungs to be paraffin-embedded were fixed overnight via intratracheal instillation of 10% formalin at a pressure of 25 cm H2O and then fixed with 70% alcohol for 15 minutes. Paraffin-embedding and sectioning of the fixed lungs was performed with standard procedures by the NIEHS histology lab. For frozen sections, fresh lungs were intratracheally instilled with Sakura Tissue-Tek optimal cutting temperature compound (Sakura Finetek USA, Torrance, CA), 50% in saline at a pressure of approximately 25 cm H2O, and subsequently frozen in liquid nitrogen. To obtain the maximal cross-sectional area of each side of the whole lung, and thereby reveal each lobe, paraffin-embedded and frozen lungs were dissected through the cranial-caudal plane at the first bronchial bifurcation. The paraffin-embedded lung tissue was sectioned by the NIEHS histology lab into 5-μm sections. The frozen lung tissue was cryosectioned into 7-μm sections in our laboratory using a cryomicrotome (CM1850; Leica Microsystems, Nussolch, Germany) and were stored frozen until staining was performed.
For lung tissue preparation for transmission electron microscopy (TEM), control and bleomycin-treated mice at the day 9 time point were sacrificed and exsanguinated before the removal of the lungs en bloc from the thoracic cavity. The lungs were fixed by intratracheal instillation of 1% osmium tetroxide and dehydrated in a series of graded alcohols, followed by treatment with propylene glycol and embedding in polybed resin. Fixed lung specimens for TEM were thick-sectioned (0.8 μm), stained with Toluidine Blue (Fisher Scientific, Fair Lawn, NJ), and examined before areas were selected for thin sectioning (90 nm). The sample blocks for TEM were prepared by the NIEHS electron microscopy lab.
Histochemical and IHC Staining
For each mouse lung, paraffin-embedded tissue sections were stained by the NIEHS histology lab according to standard staining procedures. H&E (Surgipath Medical Industries, Inc., Richmond, IL) staining was used for morphological assessment and differentiation and Masson's trichrome blue staining (Polyscientific R&D Corporation, Bay Shore, NY) for collagen identification. Carbol chromotrope staining (Acros Organics, Geel, Belgium) was used to identify eosinophils within the lung tissue. Paraffin-embedded sections from each lung and some frozen sections were also stained by the NIEHS immunohistochemistry (IHC) lab for myeloperoxidase (MPO, DakoCytomation, Carpinteria, CA) to identify neutrophils; F4/80 (Santa Cruz Biotechnology, Santa Cruz, CA) to identify monocytes/macrophages; CD3 (Abcam, Cambridge, MA) to identify T lymphocytes; and Pax-5 (Santa Cruz Biotechnology) to identify B lymphocytes. These IHC stains were used on lung sections adjacent to those used for H&E staining to identify the types and relative amounts of leukocytes within the same arteriolar or venular regions of the lung tissue.
IHC staining of formalin-fixed, paraffin-embedded (FFPE) lung tissue was performed using an avidin-biotin peroxidase detection technique as described previously.50 Tissues were first deparaffinized in xylene and rehydrated through graded ethanol. Endogenous peroxidase was blocked by immersing slides in 3% H2O2 for 15 minutes. Heat-induced epitope retrieval from the lung tissue sections was performed in citrate buffer solution. After blocking with diluted nonimmunized serum, the tissue was incubated for 1 hour at room temperature with the following primary antibodies used for cell identification at optimal dilutions: rabbit polyclonal anti-human myeloperoxidase (MPO) at 1:3000; F4/80 at 1:10; CD3 at 1:250; and goat polyclonal Pax-5 antibody at 1:1000. Biotinylated secondary antibodies were then applied at a 1:500 dilution, followed by peroxidase-conjugated streptavidin reagent. The stain was visualized using 3-diaminobenzidine chromogen (DAB; DakoCytomation, Carpinteria, CA) and Harris hematoxylin for counterstaining. CD3 immunostaining was conducted on a Discovery XT automated system (Ventana Medical Systems, Tucson, AZ) using an OmniMap anti-rabbit detection kit (Ventana Medical Systems). For negative control tissue sections, a nonimmune normalized serum (Jackson ImmunoResearch, West Grove, PA) was applied at the same dilution as the respective monoclonal dilutions. Spleen tissue was used as a positive control for neutrophil and B-lymphocyte immunostaining, and thymus tissue was used as a positive control for T-lymphocyte immunostaining.
Multiple mechanisms involving various adhesion molecules that facilitate leukocyte adherence and entry into the lungs have been identified.20,51,52 However, because leukocyte migration is largely dependent on expression of adhesion molecules on endothelium such as intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1),53,54 immunostaining for ICAM-1 and VCAM-1 was performed. Briefly, cryosections were fixed with methanol with 1% H2O2 (Sigma-Aldrich) for 10 minutes at 4°C to block endogenous peroxidase activity, then were rinsed with phosphate-buffered saline (PBS), permeabilized with 0.8% Triton X-100 (Sigma-Aldrich) for 10 minutes at room temperature, and rinsed again with PBS. To reveal ICAM-1 and VCAM-1 expression, the sections were then immunostained with Alexa Fluor 488 anti-mouse CD54 and Alexa Fluor 647 anti-mouse CD106 (BioLegend, San Diego, CA) at 1:100 dilutions for 1 hour and blocked with buffer (CAS-Block; Invitrogen, Camarillo, CA). Lung sections stained with secondary-conjugated antibody (Alexa Fluor 488 Rat IgG2b, κ Isotype Ctrl antibody, and Alexa Fluor 647 Rat IgG2a, κ Isotype Ctrl antibody, respectively; BioLegend) alone served as negative controls. Cell nuclei were stained via blue nuclear counterstain, DAPI (Roche Diagnostics, Indianapolis, IN), according to the manufacturer's instructions.
CD18, a well-known cell surface marker for polymorphonuclear cells (PMNs) and monocytes, was used together with standard morphological characteristics to identify these cells during adhesion and transmigration.20,55 Briefly, lung cryosections were stained with rat-anti-mouse CD18 antibody (1:100; BioLegend) and goat-anti-rat IgG conjugated with Alexa Fluor 488 (1:300; Invitrogen, Carlsbad, CA) for identification of neutrophils or monocytes in the lung tissue. Type I collagen, which is commonly found in lung and vascular tissue and is known to increase during acute injury and repair,56,57 was identified using rabbit-anti-mouse collagen I antibody (1:100; Abcam) and goat-anti-rabbit IgG conjugated with Alexa Fluor 594 (1:300; Invitrogen). Negative controls were performed using secondary antibody alone. Cell nuclei were stained via DAPI nuclear counterstain (Roche Diagnostics). Immunostaining of nonfixed, frozen sections were conducted on the adjacent lung sections used for H&E or Masson's trichrome staining.
Stained tissue sections from both right and left lung lobes were evaluated for areas of inflammation. Small muscular arteries (<200 μm) or arterioles (typically <100 μm) were distinguished from small veins or venules of comparable size primarily by the appearance of continuous layers of smooth muscle (as opposed to discontinuous bundles of smooth and striated muscle in murine veins or venules) and the close proximity of arteries or arterioles to bronchi and bronchioles.58,59 Because the histological criteria for arterioles/small arteries and for venules/small veins reflect a continuum,60 here we refer to both arterioles and small arteries as arterioles, and to both venules and small veins as venules.
Fluorescent imaging was performed using an Axioplan 2 fluorescence microscope equipped with an AxioCam MRC digital color camera (Carl Zeiss, Oberkochen, Germany) or a LSM 510 confocal microscope (Carl Zeiss). Whole-lung images were obtained from H&E-stained sections using an Aperio ScanScope (Aperio Technologies, Vista, CA). TEM imaging was performed using a JEOL1010 transmission electron microscope (JEOL, Tokyo, Japan) through a 12-bit digital camera (Advanced Microscopy Techniques, Danvers, MA), equipped with a phosphor scintillator (2624 × 2624 pixel array, with pixels 24 μm2) and cooled to 5°C. TEM imaging was performed with assistance from staff of the microscopy and imaging core at the NIEHS (Research Triangle Park, NC) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Rockville, MD).
Results
Evidence of Leukocytes in Arterioles, Venules, and Perivascular Interstitium
Stained whole-lung imaging revealed the typical progressive and patchy pattern of inflammatory cell infiltration, consolidation, and fibrosis after bleomycin treatment (see Supplemental Figure S1 at http://ajp.amjpathol.org). Within 2 days of intratracheal instillation of bleomycin or saline, there was already clear evidence of a widespread, leukocyte infiltration in the bleomycin-treated lungs that was not evident in the saline-treated lungs (Figure 1). Leukocyte sequestration at arteriolar and venular luminal surfaces was present in regions typically associated with large numbers of perivascular leukocytes. Leukocyte sequestration appeared more prominent in arterioles than venules, although this finding varied somewhat because of the patchiness of the injury pattern (Figure 1). In the present study, pulmonary arterioles and venules associated with perivascular inflammatory cells typically ranged from 30 to 200 μm in diameter. Leukocyte sequestration in arterioles was markedly evident from day 2 to day 9 after bleomycin treatment (Figure 1, A, C, E, and G). Periarteriolar leukocytes were present as early as day 2 and were most intense by day 9. Sequestered leukocytes were still present at days 11 and 14, but at diminished levels. The number of perivenular leukocytes also progressively increased from day 2 to day 9 after bleomycin treatment (Figure 1, B, D, F, and H). Increased numbers of leukocytes were not found in or around arterioles or venules in the saline-treated controls (Figure 1, I and J).
Figure 1.
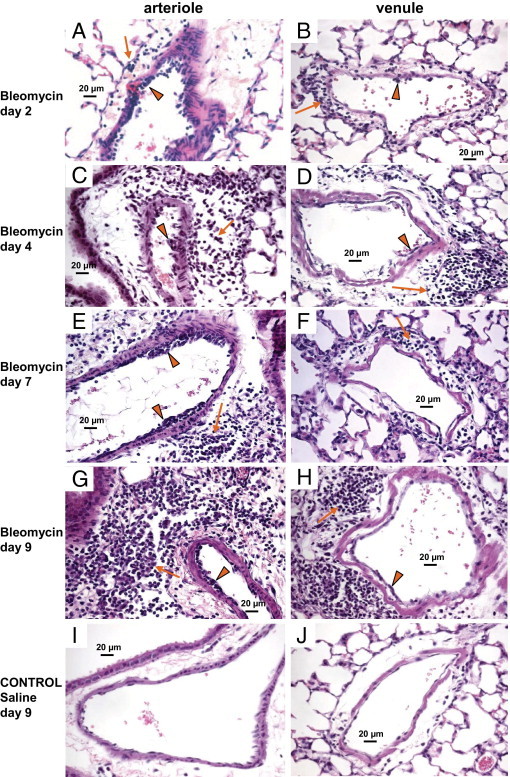
Evidence of inflammatory cell sequestration with perivascular inflammation in arterioles and venules. H&E-stained, paraffin-embedded lung sections reveal progressive inflammatory cell accumulation at the luminal surface (arrowheads) and in the perivascular interstitium (arrows) around pulmonary arterioles (A, C, E, and G) and venules (B, D, F, and H) from 2 to 9 days after intratracheal bleomycin. Inflammatory cells were more prominent on the luminal surface of pulmonary arterioles than pulmonary venules and were often sequestered in multiple layers along the luminal wall of arterioles. Significant inflammatory cell accumulation was present in the perivascular interstitium around both pulmonary arterioles and venules. For comparison, saline-treated control mouse lungs at day 9 are also shown (I and J). Scale bars = 20 μm.
The pattern of leukocyte sequestration and perivascular leukocyte accumulation differed between arterioles and venules, as revealed in lung sections that contained longitudinal sections of full-length arterioles and venules (Figure 2). In general, there was evidence of greater leukocyte sequestration in arterioles (Figure 2A) than in venules (Figure 2B) after bleomycin treatment. Leukocytes sequestered on the luminal surface of arterioles tended to form multiple cell layers or clusters (Figure 2C), whereas leukocytes sequestered within venules appeared in a more isolated, nonclustered fashion (Figure 2D). As thicker, muscular-walled arterioles (Figure 2C) became smaller in diameter (Figure 2E), there were fewer leukocytes in both the arteriolar lumen and the periarteriolar interstitium of the smaller arteriole (Figure 2E). In contrast, perivenular leukocytes were most prominent around smaller venules (Figure 2F), and the leukocytes diminished in number as the venules enlarged (Figure 2D). This association of sequestered leukocytes on the luminal surface with increased perivascular inflammatory cells was less consistent for venules than arterioles. These observations suggest that there may be additional sources of leukocytes in the interstitium of venules, compared with arterioles, including leukocytes derived from nonvenular sources such as perivenular lymphatics.61
Figure 2.
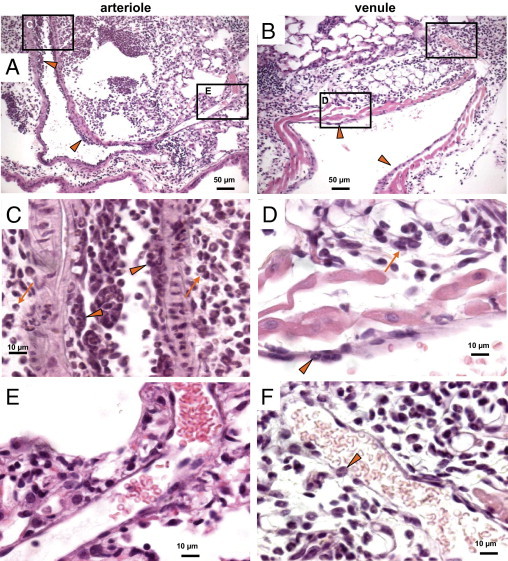
Pattern of vascular and perivascular inflammation depending on vessel size. H&E-stained, paraffin-embedded lung sections 7 to 9 days after bleomycin treatment reveal that inflammatory cell sequestration within vessel lumina (arrowheads) and accumulation in the adjacent perivascular interstitium are prominent in mid-sized arterioles but diminish as the arterioles become smaller (A, C, and E). The increased number of leukocytes on the arteriolar lumen and in the surrounding perivascular interstitium (arrows) diminishes as the vessels become smaller, correlating with the reduced numbers of leukocytes present within the perivascular interstitium around the smaller arterioles. In contrast, venules (B, D, and F) typically had fewer inflammatory cells sequestered on the lumen (arrowheads), compared with arterioles, and the smaller venules had more perivascular leukocytes (arrows) that tended to diminish as the venules enlarged (B, D, and F). Boxed areas in A and B correspond to the images at higher magnification in C/E and D/F, as labeled for each box. Scale bars: 50 μm (A and B); 10 μm (C–F).
Identification of Luminal and Perivascular Leukocytes of Pulmonary Arterioles and Venules
We identified leukocytes on the vascular luminal surface, within the vascular wall, and in the perivascular interstitium of arterioles and venules using various histological and IHC staining methods (Figure 3; see also Supplemental Figure S2 at http://ajp.amjpathol.org). For example, neutrophils were identified by their characteristic morphology (Figure 3, A and B) or by MPO staining (Figure 3, C and D). Monocytes/macrophages were identified with anti-F4/80 antibody (Figure 3, E and F). Eosinophils were identified with carbol chromotrope (Figure 3, G and H), and T and B lymphocytes were identified with anti-CD3 (Figure 3, I and J) and anti-Pax-5 (Figure 3, K and L), respectively. As already noted, leukocytes were observed more often around arterioles than venules, and this was especially true for granulocytes such as neutrophils (Figure 3, C and D) and eosinophils (Figure 3, G and H), as well as monocytes/macrophages (Figure 3, E and F). Notably, the venules revealed a predominance of T and B lymphocytes (Figure 3, J and L), compared with arterioles (Figure 3, I and K). Furthermore, lymphocytes were less common on the luminal surface of both arterioles and venules, suggesting that there are additional sources for lymphocytes in the perivascular interstitium, which might include capillaries within the interstitium. Saline-treated controls revealed no leukocyte transmigration or evidence of inflammation in the perivascular interstitium. Negative controls for the IHC staining and imaging in this experiment revealed no evidence of significant background staining, and tissues used as positive controls confirmed identification of the appropriate leukocytes through the immunostaining techniques (data not shown).
Figure 3.
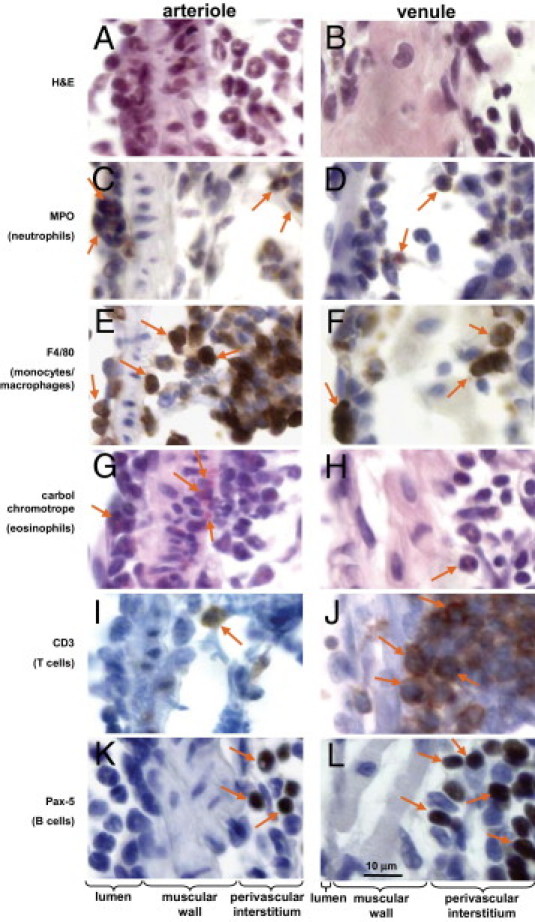
Identification of vascular and perivascular inflammatory and immune cells. Paraffin-embedded lung sections at day 9 after bleomycin treatment reveal inflammatory and immune cells in both pulmonary arterioles and venules. Inflammatory and immune cells were identified by standard H&E and IHC staining as follows: neutrophils by myeloperoxidase (MPO; C and D); monocytes/macrophages by F4/80 (E and F); eosinophils by carbol chromotrope (G and H); T lymphocytes by CD3 (I and J); and B lymphocytes by Pax-5 (K and L). In each row, arrows indicate the leukocyte cell type identified. At day 9 after bleomycin treatment, neutrophils were identified in both arterioles (A and C) and venules (B and D). In contrast, monocytes/macrophages and eosinophils were associated more with arterioles (E and G) than with venules (F and H). T and B lymphocytes were rarely present on the vascular lumen of either arterioles or venules; however, both T and B lymphocytes were commonly observed in the perivenular interstitium. For lower-power images corresponding to each of these photomicrographs, see Supplemental Figure S2 (available at http://ajp.amjpathol.org). Scale bar = 10 μm.
Evidence of Leukocyte Transmigration in Pulmonary Arterioles and Venules
The concomitance of sequestered leukocytes along the arteriolar and venular luminal walls with the increased numbers of leukocytes in the contiguous perivascular interstitia first suggested to us the possibility of transmigration (Figure 1). The H&E-stained, paraffin-embedded lung sections from day 9 after bleomycin treatment provide direct evidence of leukocyte migration across arteriolar walls (Figure 4, A and C). Under higher magnification, some leukocytes appeared to migrate through the arteriolar wall by modifying their shape, becoming elongated and very narrow as they extended into the muscular vessel wall (Figure 4, A inset, and C, insets 1 and 2). In contrast, leukocytes transmigrating venules did not appear to elongate their cell bodies and essentially retained their rounded shape (Figure 4, B and D). Notably, the arteriole at selected sites along the luminal wall appears to partially infold around leukocytes entering the vessel (Figure 5). The high-power H&E-stained lung cryosection under bright field (Figure 5B) or fluorescence (Figure 5C) imaging revealed a detailed view of the arteriolar luminal wall surrounding entering leukocytes. In contrast to arterioles, no infolded regions at points of leukocyte entry into venular walls were observed in the present study (Figure 5, D–F). Confocal imaging of adjacent frozen lung sections reveals ICAM-1 and VCAM-1 labeling at the point of contact of a leukocyte on the luminal surface of an arteriole (Figure 5G) and venule (Figure 5H). The ICAM-1 (green)/VCAM-1 (red) labeling (with yellow for coexpression) associated with leukocyte interaction appears more intense on the arteriolar endothelium than on the venular endothelium.
Figure 4.
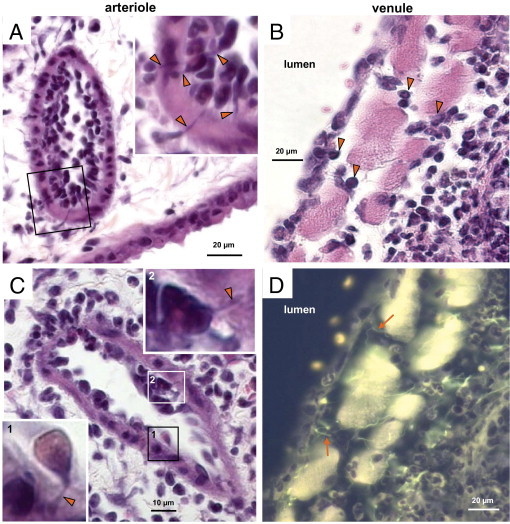
Evidence of leukocyte transmigration. H&E-stained paraffin-embedded lung sections of bleomycin-treated lungs at day 9 reveal leukocytes transmigrating the dense muscular wall of the arteriole with an occasional leukocyte dramatically elongating its cell body during transmigration. A and C: Images in insets show the corresponding boxed area at higher magnification; arrowheads indicate the marked elongation of the cell body. B: In contrast, leukocytes transmigrating venules typically appeared to retain a more rounded shape. D: The fluorescent image corresponding to B reveals further morphological details, including evidence of some multilobed granulocytes and the prominent collagen fibers (arrows) associated with transmigration pathways. For other examples of the presence of collagen fibers associated with influx/efflux sites, see fluorescent images in Figure 8, D and I. Scale bars: 20 μm (A, B, and D); 10 μm (C).
Figure 5.
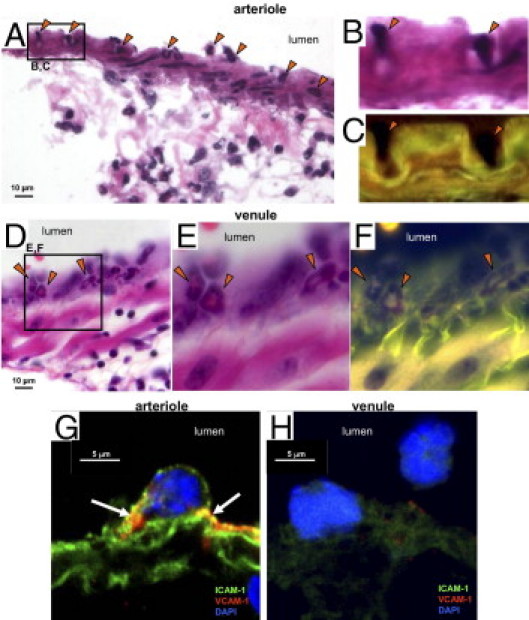
Evidence of vascular entry points by transmigrating leukocytes. A: A representative H&E-stained frozen section at day 9 after bleomycin treatment reveals luminal leukocytes (arrowheads) penetrating the arteriolar wall. B and C: In enlarged bright field (B) and fluorescent (C) images, the arteriolar wall appears to invaginate around leukocytes entering the arteriole wall (arrowheads). D: In contrast, leukocytes penetrating the walls of venules did not demonstrate distortion of the vessel wall (arrowheads) and the leukocytes appeared to retain a more rounded cell shape during transmigration. E and F: Enlarged bright field and fluorescent images corresponding to D. G and H: Confocal imaging of adjacent frozen lung sections reveals the pattern of ICAM-1 and VCAM-1 labeling at the point of contact of a leukocyte on the luminal surface of an arteriole (G) and venule (H). The ICAM-1 and VCAM-1 labeling observed in yellow (arrows) due to coexpression of the two markers appears more intense on the arteriolar luminal surface. Scale bars: 10 μm (A and D); 5 μm (G and H).
TEM imaging confirmed the typical pattern of transmigration by PMNs (Figure 6). First, PMNs appear to become surrounded by luminal endothelial cells in pulmonary arterioles (Figure 6C). As an example of the PMN transmigration, an eosinophil is shown with an elongated cell body within the muscular wall of the arteriole (Figure 6D), which is consistent with the light microscopic evidence of leukocyte transmigration (Figures 4 and 5). Ultrastructural imaging of leukocytes in the periarteriolar interstitium also revealed PMNs to be both granulated and degranulated (see Supplemental Figure S3, A and B, at http://ajp.amjpathol.org), confirming that PMN activation occurs during lung injury and repair.62 TEM imaging supports the light microscopic findings of a more lymphocyte-predominant influx around venules after bleomycin treatment (see Supplemental Figure S3, C and D, at http://ajp.amjpathol.org). Collagen fibrils are also present in the perivascular interstitium of both arterioles and venules (see Supplemental Figure S3, B and D, at http://ajp.amjpathol.org).
Figure 6.
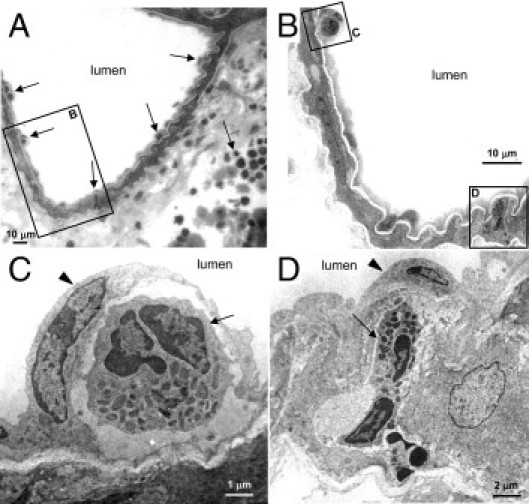
Ultrastructural evidence of leukocyte vascular entry and transmigration. TEM imaging of lung tissue from a mouse at day 9 after bleomycin treatment reveals some of the characteristics of leukocyte transmigration in arterioles. A: Leukocytes (arrows) are identified in the arteriolar lumen, arteriolar muscular wall, and periarteriolar interstitium. B: Enlargement of the boxed area in A reveals a granulocyte on the arteriolar lumen and a granulocyte transmigrating (boxes C and D, respectively, corresponding to panels C and D). C: An eosinophil (arrow) on the arteriolar lumen is surrounded by the arteriolar endothelial cell (arrowhead). D: An eosinophil (arrow) is shown during transmigration with an elongated cell body within the muscular arteriolar wall below the endothelial cell on the arteriolar lumen (arrowhead). These images are consistent with the light and confocal microscopic images of leukocyte transmigration. Scale bars: 10 μm (A and B); 2 μm (D); 1 μm (C).
In addition to leukocytes crossing the pulmonary arteriolar wall as single cells (Figure 4), leukocytes can also migrate as a group of cells in an apparent single-file fashion (Figure 7, A–C). A similar pattern of transmigrating leukocytes was observed for venules (data not shown). The transmigrating leukocytes in arterioles were typically PMNs (neutrophils and eosinophils), with some monocytes, and were CD18-positive (Figure 7, D and E). The CD18-positive leukocytes represented a majority of the cells transmigrating in single file (Figure 7, D and E). Confocal imaging of adjacent frozen lung sections revealed a pattern of ICAM-1 and VCAM-1 labeling on the arteriolar wall (Figure 7, F and G), with areas of coexpression of ICAM-1 and VCAM-1 at multiple sites of PMN attachment. Thus, PMN transmigration within larger pulmonary vessels appears consistent with patterns described for smaller postcapillary venules.53,54
Figure 7.
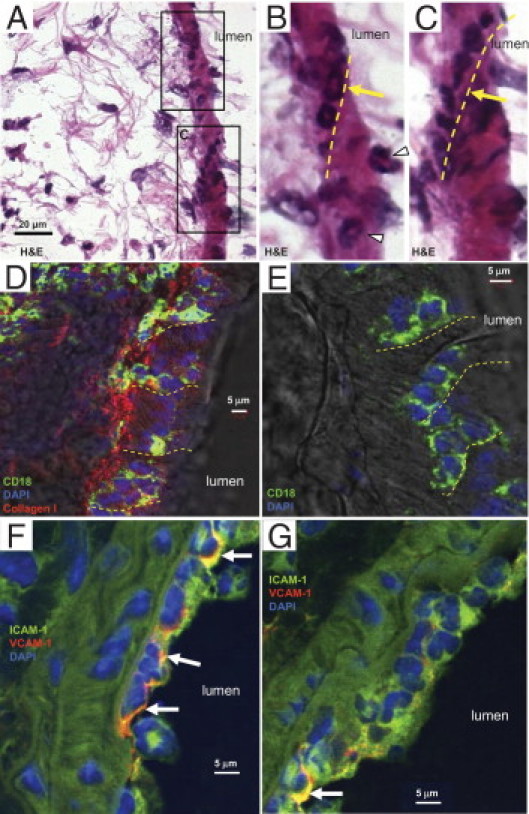
Patterns of leukocyte transmigration. A: H&E-stained frozen lung sections from day 9 after bleomycin treatment reveal groups of leukocytes crossing the arteriolar wall in a single-file manner (yellow arrows and dashed lines) or as single cells (arrowheads). Boxed areas in A are shown at higher magnification in B and C. D and E: Confocal imaging of adjacent frozen lung sections revealed most leukocytes to be transmigrating in single file and to be CD18 positive (green). The section, counterstained with DAPI (blue), was immunostained for collagen I (red), confirming its presence along the arteriole in the perivascular interstitium (D). F and G: Confocal imaging of adjacent frozen lung sections reveals the pattern of ICAM-1 and VCAM-1 labeling in an arteriolar wall. Note the multiple granulocytes on the luminal surface, together with many granulocytes and other leukocytes in the perivascular interstitium. ICAM-1 or VCAM-1 labeling is most intense on the luminal surface, where leukocytes are interacting with the endothelium; the yellow color (F, top arrow; G, bottom arrow) indicates areas of coexpression of both ICAM-1 and VCAM-1 (arrows). Scale bars: 20 μm (A); 5 μm (D–G).
Evidence for Localized Collagen Fibers Extending Near Leukocyte Exit Sites on Pulmonary Vascular Walls
Type I collagen was present in the periarteriolar interstitium in close physical proximity to transmigrating CD18-positive leukocytes in bleomycin-treated lungs (Figure 7D). Distinct collagen fibers56 were identified within the periarteriolar interstitium using adjacent Masson's trichrome- and or H&E-stained sections (Figure 8, A–E). The H&E-stained sections viewed by fluorescent microscopy revealed a markedly clear view of fibers between the perivascular interstitium and the surface of the arteriolar wall (Figure 8D). The fibers appear to extend between the perivascular interstitium and the vascular wall near leukocytes that are emerging into the interstitium. Collagen fibrils in the perivascular interstitium were identified by their characteristic ultrastructural morphology using TEM (see Supplemental Figure S3, B and D, at http://ajp.amjpathol.org). Although the collagen fibers were easily identified in the perivascular interstitium of pulmonary arterioles using H&E or Masson's trichrome staining (Figure 8, A–E), similar images of the perivenular interstitium were only weakly positive (Figure 8, F–J). Images of an arteriole and a venule from a saline-treated control revealed the presence of fibers extending between the vessel wall and the interstitium, but in the absence of leukocytes (see Supplemental Figure S4 at http://ajp.amjpathol.org). Collagen staining in the perivascular interstitium progressively increased for arterioles, with very dense staining by days 7 to 9 after bleomycin treatment; however, collagen staining in the perivascular interstitium of venules was modest at these time points, despite the presence of numerous leukocytes (see Supplemental Figure S5 at http://ajp.amjpathol.org). Thus, collagen fibers, which may be part of a pre-existing scaffold, appear near sites of transmigrating leukocytes emerging from vessel walls. Furthermore, collagen accumulation resulting in progressive fibrosis was more prominent in the perivascular interstitium of arterioles than of venules in bleomycin-treated murine lungs.
Figure 8.
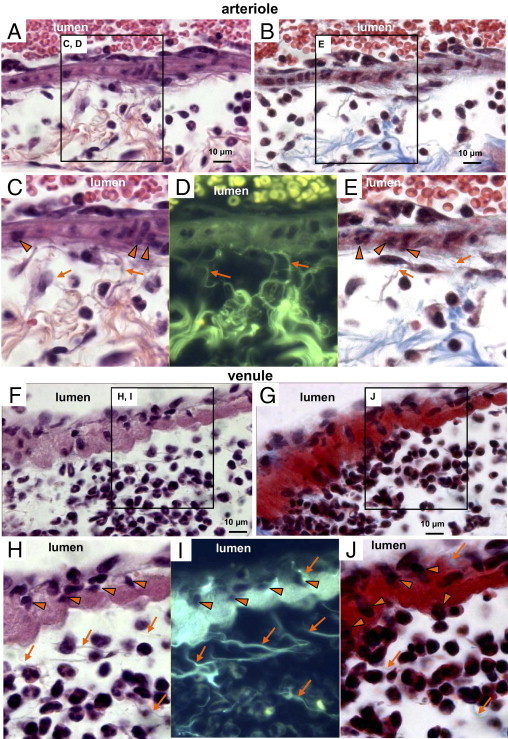
Evidence for collagen fibers near sites where leukocytes emerge from vascular walls. Paraffin-embedded lung sections at day 7 after bleomycin treatment reveal H&E- or Masson's trichrome-stained sections of a pulmonary arteriole (A–E) and a pulmonary venule (F–J). Arrowheads indicate transmigrating leukocytes and arrows indicate collagen fibrils (C–E and H–J). The collagen scaffold present in the periarteriolar interstitium (A and B), with enlarged images (C–E, corresponding to labeled boxed areas in A and B) revealing collagen fibers extending to the vascular wall near the sites where transmigrating leukocytes enter the periarteriolar interstitium (C–E, arrows). H&E-stained sections of venules (F) and the corresponding enlarged image (H) reveals transmigrating leukocytes (arrowheads, also in H–J; arrows indicate collagen fibers, H–J). In a fluorescent image (I) corresponding to H, the collagen fibers in the perivenular interstitium extend between the interstitium and the venular wall near the location of leukocytes. However, the Masson's trichrome staining for collagen in the perivenular interstitium was weak (G and J). For TEM images of lung sections from saline-treated controls revealing that baseline collagen-like fibers were present in the perivascular interstitium and were more prominent for arterioles than venules, see Supplemental Figure S3 (available at http://ajp.amjpathol.org). Scale bar = 10 μm.
Discussion
In the present study, we determined that large numbers of leukocytes were sequestered on vascular lumina of pulmonary arterioles and venules at sites where there was also an accumulation of inflammatory cells within the perivascular interstitium. At these sites, leukocytes were detected crossing the thick muscular layers of the pulmonary arterioles and entering the perivascular interstitium through well-defined pathways in the arterioles. Leukocytes were also observed to migrate across the walls of similar-sized venules, but through less discrete pathways than found in arterioles. Histologically, there was no evidence of arteritis or phlebitis in the bleomycin-exposed mice; rather, the apparent target for the transmigrating leukocytes was extravascular. Many leukocytes migrating across arteriolar walls were observed to have an altered shape, whereas leukocytes migrating across venular walls appeared to retain a more normal, rounded cell shape. Of note, the sites where leukocytes appeared to emerge from the arteriolar walls into the perivascular interstitium were typically associated with thin collagen fibers extending between the exterior vascular wall and the interstitium. Taken together, these data demonstrate that leukocyte transmigration occurs in both muscular arterioles and venules in response to bleomycin-induced lung injury at the same sites where there are extensive numbers of perivascular leukocytes. This pathway may represent an alternative and under-recognized route for leukocyte influx and efflux during lung injury and repair.
Perivascular inflammation is a characteristic finding in a variety of acute and chronic lung disorders, including bleomycin-induced pulmonary toxicity.34,63–66 It was the observation that leukocyte accumulation in the periarteriolar and perivenular interstitia after bleomycin treatment occurred at the same sites where leukocytes were sequestered in the lumina of contiguous arterioles and venules that first led us to hypothesize that transmigration of leukocytes might explain these findings. In the present study, arterioles and venules associated with areas of perivascular interstitia that contained significant numbers of inflammatory cells typically ranged from 30 to 200 μm in diameter. Previous assessments of the normal rodent pulmonary arterial circulation indicate that the structure of arteries with diameters of <300 μm are similar but not identical to those of humans.67 Using the intratracheal bleomycin mouse model, we observed a more intense leukocyte sequestration and perivascular inflammation in arterioles compared with venules of comparable size. The pattern of inflammation appeared to be at least in part dependent on the size of the arterioles, in that the inflammatory cells around arterioles decreased in numbers as the arterioles diminished in size. It is not clear why arterioles were observed to have more inflammatory cells than venules. One reason may relate to the possible differences in local tissue concentrations of the drug due to the intratracheal delivery method of bleomycin, because pulmonary arterioles are physically associated with the pulmonary airways but pulmonary venules are not. This underscores a potential limitation of the present study using intratracheal bleomycin: some of the patterns of the acute injury/response may possibly be unique to the mode of instillation of bleomycin in the present study and may not be directly applicable to all other models of lung injury and repair.
In the present study of bleomycin-induced lung injury and repair in mice, sequestered leukocytes in the lumina of arterioles and venules were able to directly migrate across vessel walls, even walls comprised of dense smooth muscle, into the contiguous perivascular interstitia. In reviewing the literature, we found only a few reports specifically noting leukocyte migration across larger pulmonary vessels, and these observations were limited to models of intratracheal antigen exposure in studies conducted some years ago (two in the 1990s, another more recently).39–41 Others who have assessed perivascular inflammation in response to intraperitoneal allergen exposure in mice reported that they did not observe transmigrating leukocytes in arterial walls and stated that it is unlikely that inflammatory cells migrate across the thick-walled arteries in the inflamed lung into the interstitium.37 Recent reviews indicate that leukocyte influx into the lung occurs across alveolar capillaries and postcapillary venules, without mention of alternative sites of migration across larger vessels such as arterioles.20,29,32,68,69
Intuitively, leukocyte migration across smaller vessels seems most reasonable, given that larger vessels such as arterioles have walls with more complex barriers, including tighter endothelial cell junctions and more compact medial smooth muscle.70 Nonetheless, the new findings in the present study clearly demonstrate that leukocyte transmigration in both arterioles and venules can occur during acute lung injury. These findings, together with the work of others using antigen exposure,39–41 support the premise that leukocytes transmigrate into the lung interstitium across both muscular arterioles and venules, serving as a potentially important alternative pathway for leukocyte influx into the lung.
Although we observed that leukocyte transmigration during bleomycin-induced injury appeared more commonly in muscular-walled arterioles than in similar-sized venules, there may be other explanations. For example, the apparent differences in observing transmigrating leukocytes may be that transition times for transmigration differ between arterioles and venules. If a leukocyte takes more time to migrate across the denser and more compact muscular wall of an arteriole than across the thinner, more loosely arranged venular wall, then there may appear to be more transmigrating leukocytes in arterioles at a given time. In addition, it is possible that leukocyte transmigration occurs more readily at earlier or different time points than were examined in the present study. The present study did not attempt to quantify the differences in inflammatory cell number by time point and location or to further explore underlying mechanisms. Clearly, future studies designed to assess specific differences in leukocyte quantities by subtypes, mechanisms, and transit times associated with transmigration across muscular arterioles and venules are warranted, both in the bleomycin model and in other models of acute lung injury and repair.
Leukocytes appear to use more well-defined pathways for transmigration in arterioles than in venules within the lung. Similarly, the apparent entry sites of leukocytes in pulmonary arterioles may have unique morphological features (Figure 5). Our ultrastructural finding of an endothelial cell on the arterial lumen entirely covering the entering leukocyte has also been also reported during leukocyte transmigration in a model of dermal inflammation.71 Leukocytes such as neutrophils appear to penetrate through the arteriolar wall barriers by markedly deforming their cell bodies into narrow elongated shapes. In contrast, many leukocytes within venular walls appear to follow a path of least resistance between small muscular bundles within the walls, retaining a more rounded cell shape (Figure 4, B and D). The leukocyte-endothelium interaction in larger pulmonary microvasculature appears to be associated with expression of both ICAM-1 and VCAM-1 (Figures 5 and 7), although expression appears stronger in arterioles than venules (Figure 5). In both pulmonary arterioles and venules, transmigrating cells were observed as either a single cell or as cells grouped in a single-file manner, similar to observations made by others for transepithelial cell transmigration of neutrophils in the lung72 and in systemic microvasculature in nonpulmonary models of the inflammatory response.71,73,74
Collagen fibers extend between the vascular walls and the perivascular interstitium and appear near the exit sites of leukocytes of both arterioles and venules. These fibers were more prominent around arterioles than venules in H&E- and Masson's trichrome-stained sections of both control and bleomycin-injured lungs. TEM and fluorescent microscopic imaging also confirmed the presence of collagen fibers in the perivascular interstitium, with more evidence of collagen fibers present in periarteriolar interstitium. These findings suggest the possibility that a collagen scaffold exists that may facilitate leukocyte migration directly in the perivascular interstitium. It is noteworthy that neutrophils, lymphocytes, and fibroblasts have previously been reported to use collagen scaffolding to facilitate their migration within the extracellular matrix.75–77 The ability of leukocytes to modulate the scaffold by targeted release of proteases likely controls the direction and intensity of leukocyte migration, although these mechanisms are still debated.78 Whether the collagen-based scaffold is built or remodeled during inflammation, thereby permitting neutrophils and other migrating cells to navigate across complex tissue structures and barriers, is unknown.79,80 Although we refer to exit sites for leukocytes on the vessel wall, these sites may also represent entry sites for perivascular leukocytes migrating toward the vascular lumen, as may occur during leukocyte efflux from the lung.
In summary, we report that leukocytes migrate across arteriolar and venular walls in the lung during bleomycin-induced lung injury and repair, and may be alternative sites of leukocyte influx and efflux to and from the perivascular interstitium of the lung. The presence of luminal and perivascular leukocytes appeared to be more common for mid-sized arterioles, diminishing as the arterioles branched and became smaller. In contrast, smaller venules appeared to have larger numbers of perivascular leukocytes, with numbers diminishing as the venules enlarged. The interaction of leukocytes with the endothelium is associated with more intense ICAM-1 and VCAM-1 expression in arterioles than venules. Pulmonary arterioles also revealed occasional infolding of the vascular walls at sites of leukocyte entry, a finding not observed with pulmonary venules in the present study. The transmigration pathways also appeared more well defined in arterioles than in venules. At the sites where transmigrating leukocytes entered the perivascular interstitium, there were often collagen fibers present that extended between the vascular wall and the perivascular interstitium, another finding more evident with arterioles than venules. Thus, the present study suggests that leukocytes migrate across pulmonary arterioles and venules into the perivascular interstitium during lung injury and repair. An improved understanding of these pathways, the conditions or mechanisms involved, and their clinical significance may provide new anatomical or molecular targets to modulate inflammatory or immune effector cell traffic in the lung.
Acknowledgments
We thank the staff of the National Institute of Environmental Health Sciences histology and IHC laboratories for their histochemical and IHC staining services and for preparation of lung tissue blocks for TEM imaging; we also thank the staff of the NIEHS microscopy core for confocal imaging. We thank the staff of the Eunice Kennedy Shriver National Institute of Child Health and Human Development microscopy and imaging laboratory for providing confocal and TEM equipment and services.
Footnotes
Supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Supplemental material for this article can be found at http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2011.02.047.
Supplementary data
Whole-lung imaging after bleomycin treatment. Whole-lung images were obtained at days 2 to 14 after bleomycin treatment from H&E-stained lung sections using an Aperio ScanScope (Aperio Technologies, Vista, CA). An increase in tissue density occurred as early as day 2, and a patchy distribution was evident as the consolidation progressed leading to marked fibrosis by day 14. For comparison, a saline-treated control lung from day 9 is also shown.
Identification of vascular and perivascular inflammatory and immune cells. These lower-power images correspond to each of the photomicrographs in Figure 3. Paraffin-embedded lung sections at day 9 after bleomycin treatment reveal inflammatory and immune cells in both pulmonary arterioles (A) and venules (B). Inflammatory and immune cells were identified by standard immunohistochemical staining as follows: neutrophils by myeloperoxidase (MPO; C and D); monocytes/macrophages by F4/80 (E and F); eosinophils by carbol chromotrope (G and H); T lymphocytes by CD3 (I and J); and B lymphocytes by Pax-5 (K and L). In each row, arrows indicate the leukocyte cell type identified. At day 9 after bleomycin treatment, neutrophils were identified in both arterioles (A and C) and venules (B and D). In contrast, monocytes/macrophages and eosinophils were associated more with arterioles (E and G) than venules (F and H). T and B lymphocytes were rarely present on the vascular lumen of either arterioles or venules; however, both T and B lymphocytes were increased in the perivenular interstitium.
Ultrastructural images of the perivascular interstitium of an arteriole and venule in the lung after bleomycin treatment. Images were obtained by TEM of lung sections at day 9 after bleomycin treatment revealing the presence of leukocytes and collagen fibrils in the perivascular interstitia. There is an abundance of granulocytes in the perivascular interstitium (A) and some are granulated but others are degranulated (B, thin arrows). Characteristic collagen fibrils are prominent within the periarteriolar interstitium (arrows). The perivenular interstitium (C) reveals cells with typical lymphocyte morphology. Collagen fibrils are seen (D, arrows), although less prominent than in the periarteriolar interstitium. These findings are consistent with the light microscopic findings (Figures 1–3 and 8).
Evidence for fibers between the vascular walls and interstitium in control mouse lungs. H&E-stained and Masson's trichrome-stained paraffin-embedded lung sections at day 9 after saline treatment reveal a pulmonary arteriole (A–C) and a pulmonary venule (D–F). Images of an arteriole are shown with H&E staining (A) and an adjacent section stained with Masson's trichrome stain (C). When the H&E image is viewed under fluorescent microscopy, the fibers in the periarteriolar interstitium (arrows) extend to the arteriolar wall. The adjacent section with Masson's trichrome staining indicates that these are collagen fibers (arrows). Similarly, the perivenular sections (D–F) reveal a few fibers present that also extend between the interstitium and venular wall, but the Masson's trichrome is very weakly positive. Lung sections from saline-treated controls reveal that collagen-like fibers (arrows) were present at baseline in the perivascular interstitium and were more prominent around arterioles.
Evidence of collagen deposition in the perivascular interstitium after bleomycin treatment. Masson's trichrome-stained paraffin-embedded lung sections are shown for days 2 to 9 after bleomycin treatment (A–H) and for day 9 after saline treatment (I and J), revealing a pulmonary arteriole (left column) and a pulmonary venule (right column) with surrounding interstitium in each section. The periarteriolar interstitium develops intense staining for collagen by day 7 and contains numerous leukocytes. In contrast, the perivenular interstitium reveals less intense staining for collagen despite leukocytes being present. Lung sections from a saline-treated control lung reveal minimal collagen staining at baseline for the interstitium surrounding an arteriole (I) and venule (J).
References
- 1.Johnson K.J., Ward P.A. Acute immunologic pulmonary alveolitis. J Clin Invest. 1974;54:349–357. doi: 10.1172/JCI107770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernardo J., Hunninghake G.W., Gadek J.E., Ferrans V.J., Crystal R.G. Acute hypersensitivity pneumonitis: serial changes in lung lymphocyte subpopulations after exposure to antigen. Am Rev Respir Dis. 1979;120:985–994. doi: 10.1164/arrd.1979.120.5.985. [DOI] [PubMed] [Google Scholar]
- 3.Staub N.C., Schultz E.L., Albertine K.H. Leucocytes and pulmonary microvascular injury. Ann N Y Acad Sci. 1982;384:332–343. doi: 10.1111/j.1749-6632.1982.tb21382.x. [DOI] [PubMed] [Google Scholar]
- 4.Crouch E. Pathobiology of pulmonary fibrosis. Am J Physiol. 1990;259:L159–L184. doi: 10.1152/ajplung.1990.259.4.L159. [DOI] [PubMed] [Google Scholar]
- 5.Sheppard M.N., Harrison N.K. New perspectives on basic mechanisms in lung disease: 1. Lung injury, inflammatory mediators, and fibroblast activation in fibrosing alveolitis. Thorax. 1992;47:1064–1074. doi: 10.1136/thx.47.12.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haley K.J., Sunday M.E., Wiggs B.R., Kozakewich H.P., Reilly J.J., Mentzer S.J., Sugarbaker D.J., Doerschuk C.M., Drazen J.M. Inflammatory cell distribution within and along asthmatic airways. Am J Respir Crit Care Med. 1998;158:565–572. doi: 10.1164/ajrccm.158.2.9705036. [DOI] [PubMed] [Google Scholar]
- 7.Tomashefski J.F., Jr Pulmonary pathology of acute respiratory distress syndrome. Clin Chest Med. 2000;21:435–466. doi: 10.1016/s0272-5231(05)70158-1. [DOI] [PubMed] [Google Scholar]
- 8.Tetley T.D. Inflammatory cells and chronic obstructive pulmonary disease. Curr Drug Targets Inflamm Allergy. 2005;4:607–618. doi: 10.2174/156801005774912824. [DOI] [PubMed] [Google Scholar]
- 9.Tirouvanziam R., Conrad C.K., Bottiglieri T., Herzenberg L.A., Moss R.B. High-dose oral N-acetylcysteine, a glutathione prodrug, modulates inflammation in cystic fibrosis. Proc Natl Acad Sci USA. 2006;103:4628–4633. doi: 10.1073/pnas.0511304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McClintock D., Zhuo H., Wickersham N., Matthay M.A., Ware L.B. Biomarkers of inflammation, coagulation and fibrinolysis predict mortality in acute lung injury. Crit Care. 2008;12:R41. doi: 10.1186/cc6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardison M.T., Galin F.S., Calderon C.E., Djekic U.V., Parker S.B., Wille K.M., Jackson P.L., Oster R.A., Young K.R., Blalock J.E., Gaggar A. The presence of a matrix-derived neutrophil chemoattractant in bronchiolitis obliterans syndrome after lung transplantation. J Immunol. 2009;182:4423–4431. doi: 10.4049/jimmunol.0802457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis W.B., Fells G.A., Sun X.H., Gadek J.E., Venet A., Crystal R.G. Eosinophil-mediated injury to lung parenchymal cells and interstitial matrix: A possible role for eosinophils in chronic inflammatory disorders of the lower respiratory tract. J Clin Invest. 1984;74:269–278. doi: 10.1172/JCI111411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donaldson K., Brown G.M., Brown D.M., Slight J., Maclaren W., Davis J.M.G. Characteristics of bronchoalveolar leukocytes from the lungs of rats in having 0.2–0.8 ppm of ozone. Inhalation Toxicology. 1993;5:149–164. [Google Scholar]
- 14.Brigham K.L., Meyrick B. Interactions of granulocytes with the lungs. Circ Res. 1984;54:623–635. doi: 10.1161/01.res.54.6.623. [DOI] [PubMed] [Google Scholar]
- 15.Strieter R.M. Mechanisms of pulmonary fibrosis: conference summary. Chest. 2001;120:77S–85S. doi: 10.1378/chest.120.1_suppl.s77-a. [DOI] [PubMed] [Google Scholar]
- 16.Ozyurt H., Söğüt S., Yildirim Z., Kart L., Iraz M., Armutçu F., Temel I., Ozen S., Uzun A., Akyol O. Inhibitory effect of caffeic acid phenethyl ester on bleomycine-induced lung fibrosis in rats. Clin Chim Acta. 2004;339:65–75. doi: 10.1016/j.cccn.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Wilson M.R., O'Dea K.P., Zhang D., Shearman A.D., van Rooijen N., Takata M. Role of lung-marginated monocytes in an in vivo mouse model of ventilator-induced lung injury. Am J Respir Crit Care Med. 2009;179:914–922. doi: 10.1164/rccm.200806-877OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakashima J.M., Hyde D.M., Giri S.N. Effects of a calmodulin inhibitor on bleomycin-induced lung inflammation in hamsters: Biochemical, morphometric, and bronchoalveolar lavage data. Am J Pathol. 1986;124:528–536. [PMC free article] [PubMed] [Google Scholar]
- 19.Brigham K.L., Meyrick B. Granulocyte-dependent injury of pulmonary endothelium: a case of miscommunication. Tissue Cell. 1984;16:137–155. doi: 10.1016/0040-8166(84)90039-9. [DOI] [PubMed] [Google Scholar]
- 20.Doerschuk C.M. Mechanisms of leukocyte sequestration in inflamed lungs. Microcirculation. 2001;8:71–88. [PubMed] [Google Scholar]
- 21.Cepinskas G., Noseworthy R., Kvietys P.R. Transendothelial neutrophil migration: Role of neutrophil-derived proteases and relationship to transendothelial protein movement. Circ Res. 1997;81:618–626. doi: 10.1161/01.res.81.4.618. [DOI] [PubMed] [Google Scholar]
- 22.Doerschuk C.M. Leukocyte trafficking in alveoli and airway passages. Respir Res. 2000;1:136–140. doi: 10.1186/rr24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller W.A. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24:327–334. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 24.Nourshargh S., Marelli-Berg F.M. Transmigration through venular walls: a key regulator of leukocyte phenotype and function. Trends Immunol. 2005;26:157–165. doi: 10.1016/j.it.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Shaw J.O. Leukocytes in chemotactic-fragment-induced lung inflammation: Vascular emigration and alveolar surface migration. Am J Pathol. 1980;101:283–302. [PMC free article] [PubMed] [Google Scholar]
- 26.Downey G.P., Worthen G.S., Henson P.M., Hyde D.M. Neutrophil sequestration and migration in localized pulmonary inflammation: Capillary localization and migration across the interalveolar septum. Am Rev Respir Dis. 1993;147:168–176. doi: 10.1164/ajrccm/147.1.168. [DOI] [PubMed] [Google Scholar]
- 27.Kuebler W.M., Kuhnle G.E., Groh J., Goetz A.E. Leukocyte kinetics in pulmonary microcirculation: intravital fluorescence microscopic study. J Appl Physiol. 1994;76:65–71. doi: 10.1152/jappl.1994.76.1.65. [DOI] [PubMed] [Google Scholar]
- 28.Burns A.R., Smith C.W., Walker D.C. Unique structural features that influence neutrophil emigration into the lung. Physiol Rev. 2003;83:309–336. doi: 10.1152/physrev.00023.2002. [DOI] [PubMed] [Google Scholar]
- 29.Wagner J.G., Roth R.A. Neutrophil migration mechanisms, with an emphasis on the pulmonary vasculature. Pharmacol Rev. 2000;52:349–374. [PubMed] [Google Scholar]
- 30.Kuhnle G.E., Kiefmann R., Sckell A., Kuebler W.M., Groh J., Goetz A.E. Leukocyte sequestration in pulmonary microvessels and lung injury following systemic complement activation in rabbits. J Vasc Res. 1999;36:289–298. doi: 10.1159/000025657. [DOI] [PubMed] [Google Scholar]
- 31.Albertine K.H., Wang L., Watanabe S., Marathe G.K., Zimmerman G.A., McIntyre T.M. Temporal correlation of measurements of airway hyperresponsiveness in ovalbumin-sensitized mice. Am J Physiol Lung Cell Mol Physiol. 2002;283:L219–L233. doi: 10.1152/ajplung.00324.2001. [DOI] [PubMed] [Google Scholar]
- 32.Reutershan J., Ley K. Bench-to-bedside review: acute respiratory distress syndrome—how neutrophils migrate into the lung. Crit Care. 2004;8:453–461. doi: 10.1186/cc2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aird W.C. Phenotypic heterogeneity of the endothelium: I: Structure, function, and mechanisms. Circ Res. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 34.Karmouty-Quintana H., Cannet C., Zurbruegg S., Blé F.X., Fozard J.R., Page C.P., Beckmann N. Bleomycin-induced lung injury assessed noninvasively and in spontaneously breathing rats by proton MRI. J Magn Reson Imaging. 2007;26:941–949. doi: 10.1002/jmri.21100. [DOI] [PubMed] [Google Scholar]
- 35.Moore B.B., Hogaboam C.M. Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;294:L152–L160. doi: 10.1152/ajplung.00313.2007. [DOI] [PubMed] [Google Scholar]
- 36.Saito F., Tasaka S., Inoue K., Miyamoto K., Nakano Y., Ogawa Y., Yamada W., Shiraishi Y., Hasegawa N., Fujishima S., Takano H., Ishizaka A. Role of interleukin-6 in bleomycin-induced lung inflammatory changes in mice. Am J Respir Cell Mol Biol. 2008;38:566–571. doi: 10.1165/rcmb.2007-0299OC. [DOI] [PubMed] [Google Scholar]
- 37.Singh B., Shinagawa K., Taube C., Gelfand E.W., Pabst R. Strain-specific differences in perivascular inflammation in lungs in two murine models of allergic airway inflammation. Clin Exp Immunol. 2005;141:223–229. doi: 10.1111/j.1365-2249.2005.02841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tschernig T., Janardhan K.S., Pabst R., Singh B. Lipopolysaccharide induced inflammation in the perivascular space in lungs. J Occup Med Toxicol. 2008;3:17. doi: 10.1186/1745-6673-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Curtis J.L., Warnock M.L., Arraj S.M., Kaltreider H.B. Histologic analysis of an immune response in the lung parenchyma of mice: Angiopathy accompanies inflammatory cell influx. Am J Pathol. 1990;137:689–699. [PMC free article] [PubMed] [Google Scholar]
- 40.Ichikawa S., Shiozawa Y., Uchino S. Immune cell migration through the arterial wall in the murine lung during a pulmonary inflammatory response. Arch Histol Cytol. 1996;59:87–96. doi: 10.1679/aohc.59.87. [DOI] [PubMed] [Google Scholar]
- 41.Schmiedl A., Tschernig T., Luhrmann A., Pabst R. Leukocyte infiltration of the periarterial space of the lung after allergen provocation in a rat asthma model. Pathobiology. 2005;72:308–315. doi: 10.1159/000091328. [DOI] [PubMed] [Google Scholar]
- 42.Zhang H., Lawson W.E., Polosukhin V.V., Pozzi A., Blackwell T.S., Litingtung Y., Chiang C. Inhibitor of differentiation 1 promotes endothelial survival in a bleomycin model of lung injury in mice. Am J Pathol. 2007;171:1113–1126. doi: 10.2353/ajpath.2007.070226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tokuda A., Itakura M., Onai N., Kimura H., Kuriyama T., Matsushima K. Pivotal role of CCR1-positive leukocytes in bleomycin-induced lung fibrosis in mice. J Immunol. 2000;164:2745–2751. doi: 10.4049/jimmunol.164.5.2745. [DOI] [PubMed] [Google Scholar]
- 44.Snider G.L., Celli B.R., Goldstein R.H., O'Brien J.J., Lucey E.C. Chronic interstitial pulmonary fibrosis produced in hamsters by endotracheal bleomycin: Lung volumes, volume-pressure relations, carbon monoxide uptake, and arterial blood gas studied. Am Rev Respir Dis. 1978;117:289–297. doi: 10.1164/arrd.1978.117.2.289. [DOI] [PubMed] [Google Scholar]
- 45.Snider G.L., Hayes J.A., Korthy A.L. Chronic interstitial pulmonary fibrosis produced in hamsters by endotracheal bleomycin: pathology and stereology. Am Rev Respir Dis. 1978;117:1099–1108. doi: 10.1164/arrd.1978.117.6.1099. [DOI] [PubMed] [Google Scholar]
- 46.Schrier D.J., Kunkel R.G., Phan S.H. The role of strain variation in murine bleomycin-induced pulmonary fibrosis. Am Rev Respir Dis. 1983;127:63–66. doi: 10.1164/arrd.1983.127.1.63. [DOI] [PubMed] [Google Scholar]
- 47.Lazo J.S., Hoyt D.G. The molecular basis of interstitial pulmonary fibrosis caused by antineoplastic agents. Cancer Treat Rev. 1990;17:165–167. doi: 10.1016/0305-7372(90)90042-e. [DOI] [PubMed] [Google Scholar]
- 48.Matute-Bello G., Frevert C.W., Martin T.R. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L379–L399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang H.M., Bodenstein M., Markstaller K. Overview of the pathology of three widely used animal models of acute lung injury. Eur Surg Res. 2008;40:305–316. doi: 10.1159/000121471. [DOI] [PubMed] [Google Scholar]
- 50.Cesta M.F., Ryman-Rasmussen J.P., Wallace D.G., Masinde T., Hurlburt G., Taylor A.J., Bonner J.C. Bacterial lipopolysaccharide enhances PDGF signaling and pulmonary fibrosis in rats exposed to carbon nanotubes. Am J Respir Cell Mol Biol. 2010;43:142–151. doi: 10.1165/rcmb.2009-0113OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuebler W.M., Borges J., Sckell A., Kuhnle G.E., Bergh K., Messmer K., Goetz A.E. Role of L-selectin in leukocyte sequestration in lung capillaries in a rabbit model of endotoxemia. Am J Respir Crit Care Med. 2000;161:36–43. doi: 10.1164/ajrccm.161.1.9901039. [DOI] [PubMed] [Google Scholar]
- 52.Sinikovic B., Larbig M., Hedrich H.J., Pabst R., Tschernig T. The numbers of leukocyte subsets in lung sections differ between intercellular adhesion molecule-1−/−, lymphocyte function-associated antigen-1−/− mice and intercellular adhesion molecule-1−/− mice after aerosol exposure to Haemophilus influenzae type-b. Virchows Arch. 2001;438:362–369. doi: 10.1007/s004280000384. [DOI] [PubMed] [Google Scholar]
- 53.Carman C.V., Springer T.A. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol. 2004;167:377–388. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cook-Mills J.M., Deem T.L. Active participation of endothelial cells in inflammation. J Leukoc Biol. 2005;77:487–495. doi: 10.1189/jlb.0904554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith C.W., Marlin S.D., Rothlein R., Toman C., Anderson D.C. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989;83:2008–2017. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fukuda Y., Ferrans V.J., Schoenberger C.I., Rennard S.I., Crystal R.G. Patterns of pulmonary structural remodeling after experimental paraquat toxicity: The morphogenesis of intraalveolar fibrosis. Am J Pathol. 1985;118:452–475. [PMC free article] [PubMed] [Google Scholar]
- 57.Teles-Grilo M.L., Leite-Almeida H., Martins dos Santos J., Oliveira C., Boaventura P., Grande N.R. Differential expression of collagens type I and type IV in lymphangiogenesis during the angiogenic process associated with bleomycin-induced pulmonary fibrosis in rat. Lymphology. 2005;38:130–135. [PubMed] [Google Scholar]
- 58.Shi W., Eidelman D.H., Michel R.P. Differential relaxant responses of pulmonary arteries and veins in lung explants of guinea pigs. J Appl Physiol. 1997;83:1476–1481. doi: 10.1152/jappl.1997.83.5.1476. [DOI] [PubMed] [Google Scholar]
- 59.Moreno L., Perez-Vizcaino F., Harrington L., Faro R., Sturton G., Barnes P.J., Mitchell J.A. Pharmacology of airways and vessels in lung slices in situ: role of endogenous dilator hormones. Respir Res. 2006;7:111. doi: 10.1186/1465-9921-7-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Samuelson D.A. Saunders Elsevier; St. Louis, MO: 2007. Textbook of Veterinary Histology. [Google Scholar]
- 61.Schraufnagel D.E., Agaram N.P., Faruqui A., Jain S., Jain L., Ridge K.M., Sznajder J.I. Pulmonary lymphatics and edema accumulation after brief lung injury. Am J Physiol Lung Cell Mol Physiol. 2003;284:L891–L897. doi: 10.1152/ajplung.00333.2002. [DOI] [PubMed] [Google Scholar]
- 62.Borregaard N., Cowland J.B. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89:3503–3521. [PubMed] [Google Scholar]
- 63.Taylor C.R., Sostman H.D., Gore J.C., Smith G.W. Proton relaxation times in bleomycin-induced lung injury. Invest Radiol. 1987;22:621–626. doi: 10.1097/00004424-198708000-00001. [DOI] [PubMed] [Google Scholar]
- 64.Mishra A., Doyle N.A., Martin W.J., II Bleomycin-mediated pulmonary toxicity: evidence for a p53-mediated response. Am J Respir Cell Mol Biol. 2000;22:543–549. doi: 10.1165/ajrcmb.22.5.3851. [DOI] [PubMed] [Google Scholar]
- 65.Atzori L., Chua F., Dunsmore S.E., Willis D., Barbarisi M., McAnulty R.J., Laurent G.J. Attenuation of bleomycin induced pulmonary fibrosis in mice using the heme oxygenase inhibitor Zn-deuteroporphyrin IX-2,4-bisethylene glycol. Thorax. 2004;59:217–223. doi: 10.1136/thx.2003.008979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carraway M.S., Suliman H.B., Kliment C., Welty-Wolf K.E., Oury T.D., Piantadosi C.A. Mitochondrial biogenesis in the pulmonary vasculature during inhalational lung injury and fibrosis. Antioxid Redox Signal. 2008;10:269–275. doi: 10.1089/ars.2007.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hislop A., Reid L. Normal structure and dimensions of the pulmonary arteries in the rat. J Anat. 1978;125:71–83. [PMC free article] [PubMed] [Google Scholar]
- 68.Aird W.C. Phenotypic heterogeneity of the endothelium: II: Representative vascular beds. Circ Res. 2007;100:174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 69.Ley K., Laudanna C., Cybulsky M.I., Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 70.Meyrick B., Reid L. Ultrastructural findings in lung biopsy material from children with congenital heart defects. Am J Pathol. 1980;101:527–542. [PMC free article] [PubMed] [Google Scholar]
- 71.Feng D., Nagy J.A., Pyne K., Dvorak H.F., Dvorak A.M. Neutrophils emigrate from venules by a transendothelial cell pathway in response to FMLP. J Exp Med. 1998;187:903–915. doi: 10.1084/jem.187.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zemans R.L., Colgan S.P., Downey G.P. Transepithelial migration of neutrophils: mechanisms and implications for acute lung injury. Am J Respir Cell Mol Biol. 2009;40:519–535. doi: 10.1165/rcmb.2008-0348TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Davis E.M., Trinkaus J.P. Significance of cell-to cell contacts for the directional movement of neural crest cells within a hydrated collagen lattice. J Embryol Exp Morphol. 1981;63:29–51. [PubMed] [Google Scholar]
- 74.Teddy J.M., Kulesa P.M. In vivo evidence for short- and long-range cell communication in cranial neural crest cells. Development. 2004;131:6141–6151. doi: 10.1242/dev.01534. [DOI] [PubMed] [Google Scholar]
- 75.Wolf K., Müller R., Borgmann S., Bröcker E.B., Friedl P. Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases. Blood. 2003;102:3262–3269. doi: 10.1182/blood-2002-12-3791. [DOI] [PubMed] [Google Scholar]
- 76.Wolf K., Alexander S., Schacht V., Coussens L.M., von Andrian U.H., van Rheenen J., Deryugina E., Friedl P. Collagen-based cell migration models in vitro and in vivo. Semin Cell Dev Biol. 2009;20:931–941. doi: 10.1016/j.semcdb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meshel A.S., Wei Q., Adelstein R.S., Sheetz M.P. Basic mechanism of three-dimensional collagen fibre transport by fibroblasts. Nat Cell Biol. 2005;7:157–164. doi: 10.1038/ncb1216. [DOI] [PubMed] [Google Scholar]
- 78.Korpos E., Wu C., Sorokin L. Multiple roles of the extracellular matrix in inflammation. Curr Pharm Des. 2009;15:1349–1357. doi: 10.2174/138161209787846685. [DOI] [PubMed] [Google Scholar]
- 79.Perez R.L., Roman J. Fibrin enhances the expression of IL-1 beta by human peripheral blood mononuclear cells: Implications in pulmonary inflammation. J Immunol. 1995;154:1879–1887. [PubMed] [Google Scholar]
- 80.Korpos E., Wu C., Song J., Hallmann R., Sorokin L. Role of the extracellular matrix in lymphocyte migration. Cell Tissue Res. 2010;339:47–57. doi: 10.1007/s00441-009-0853-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Whole-lung imaging after bleomycin treatment. Whole-lung images were obtained at days 2 to 14 after bleomycin treatment from H&E-stained lung sections using an Aperio ScanScope (Aperio Technologies, Vista, CA). An increase in tissue density occurred as early as day 2, and a patchy distribution was evident as the consolidation progressed leading to marked fibrosis by day 14. For comparison, a saline-treated control lung from day 9 is also shown.
Identification of vascular and perivascular inflammatory and immune cells. These lower-power images correspond to each of the photomicrographs in Figure 3. Paraffin-embedded lung sections at day 9 after bleomycin treatment reveal inflammatory and immune cells in both pulmonary arterioles (A) and venules (B). Inflammatory and immune cells were identified by standard immunohistochemical staining as follows: neutrophils by myeloperoxidase (MPO; C and D); monocytes/macrophages by F4/80 (E and F); eosinophils by carbol chromotrope (G and H); T lymphocytes by CD3 (I and J); and B lymphocytes by Pax-5 (K and L). In each row, arrows indicate the leukocyte cell type identified. At day 9 after bleomycin treatment, neutrophils were identified in both arterioles (A and C) and venules (B and D). In contrast, monocytes/macrophages and eosinophils were associated more with arterioles (E and G) than venules (F and H). T and B lymphocytes were rarely present on the vascular lumen of either arterioles or venules; however, both T and B lymphocytes were increased in the perivenular interstitium.
Ultrastructural images of the perivascular interstitium of an arteriole and venule in the lung after bleomycin treatment. Images were obtained by TEM of lung sections at day 9 after bleomycin treatment revealing the presence of leukocytes and collagen fibrils in the perivascular interstitia. There is an abundance of granulocytes in the perivascular interstitium (A) and some are granulated but others are degranulated (B, thin arrows). Characteristic collagen fibrils are prominent within the periarteriolar interstitium (arrows). The perivenular interstitium (C) reveals cells with typical lymphocyte morphology. Collagen fibrils are seen (D, arrows), although less prominent than in the periarteriolar interstitium. These findings are consistent with the light microscopic findings (Figures 1–3 and 8).
Evidence for fibers between the vascular walls and interstitium in control mouse lungs. H&E-stained and Masson's trichrome-stained paraffin-embedded lung sections at day 9 after saline treatment reveal a pulmonary arteriole (A–C) and a pulmonary venule (D–F). Images of an arteriole are shown with H&E staining (A) and an adjacent section stained with Masson's trichrome stain (C). When the H&E image is viewed under fluorescent microscopy, the fibers in the periarteriolar interstitium (arrows) extend to the arteriolar wall. The adjacent section with Masson's trichrome staining indicates that these are collagen fibers (arrows). Similarly, the perivenular sections (D–F) reveal a few fibers present that also extend between the interstitium and venular wall, but the Masson's trichrome is very weakly positive. Lung sections from saline-treated controls reveal that collagen-like fibers (arrows) were present at baseline in the perivascular interstitium and were more prominent around arterioles.
Evidence of collagen deposition in the perivascular interstitium after bleomycin treatment. Masson's trichrome-stained paraffin-embedded lung sections are shown for days 2 to 9 after bleomycin treatment (A–H) and for day 9 after saline treatment (I and J), revealing a pulmonary arteriole (left column) and a pulmonary venule (right column) with surrounding interstitium in each section. The periarteriolar interstitium develops intense staining for collagen by day 7 and contains numerous leukocytes. In contrast, the perivenular interstitium reveals less intense staining for collagen despite leukocytes being present. Lung sections from a saline-treated control lung reveal minimal collagen staining at baseline for the interstitium surrounding an arteriole (I) and venule (J).


