Abstract
Diseases of intestinal inflammation, including Crohn’s disease, ulcerative colitis and necrotizing enterocolitis, cause substantial acute and chronic disability in a large proportion of the population. Crohn’s disease and ulcerative colitis, which are collectively referred to as inflammatory bowel disease (IBD), lead to recurrent episodes of intestinal dysfunction and systemic illness, whereas necrotizing enterocolitis is characterized by the development of dramatic and all too often fatal intestinal necrosis in infants. To determine the molecular underpinnings of these disorders, investigators have explored a variety of animal models that vary widely in their complexity. These experimental systems include the invertebrate nematode Caenorhabditis elegans, the more complex invertebrate Drosophila melanogaster, and vertebrate systems including mice, rats and other mammals. This review explores the experimental models that are used to mimic and evaluate the pathogenic mechanisms leading to these diseases of intestinal inflammation. We then highlight, as an example, how the use of different experimental models that focus on the role of Toll-like receptor 4 (TLR4) signaling in the gut has revealed important distinctions between the pathogenesis of IBD and necrotizing enterocolitis. Specifically, TLR4-mediated signaling plays a protective role in the development of Crohn’s disease and ulcerative colitis, whereas this signaling pathway plays a causative role in the development of necrotizing enterocolitis in the newborn small intestine by adversely affecting intestinal injury and repair mechanisms.
Introduction: the burden of intestinal inflammation
Diseases of intestinal inflammation represent major causes of morbidity, resulting in pain and suffering in 1- to 2-million individuals in the United States per year (Garthright et al., 1988; Kappelman et al., 2008; Schnabl et al., 2008). The overall financial burden of intestinal inflammatory diseases – which for the purposes of this review include inflammatory bowel disease (IBD; which encompasses Crohn’s disease and ulcerative colitis) and necrotizing enterocolitis (NEC) – is estimated at about US$23 billion per year, which represents the combined costs of treatment, hospitalization and lost productivity (Garthright et al., 1988; Holman et al., 2006; Kappelman et al., 2008). Despite relatively slow initial progress in our understanding of the pathogenesis and treatment of intestinal inflammatory diseases, researchers have made significant advances in the past decade towards uncovering the etiological underpinnings of these disorders. In this Commentary, we explore the role of biological models including worms, flies and mammals in elucidating the pathogenesis of intestinal inflammatory diseases. Although neither a worm nor a fly can ever truly develop IBD or NEC in the same manner as a human, research using these biological systems has resulted in definitive advances in our understanding of intestinal inflammation in humans. We first briefly examine the three clinical conditions that make up the intestinal inflammatory diseases, and then evaluate recent progress towards understanding them from studies performed in biological systems ranging from simple invertebrates to complex mammalian models.
Clinical overview: what are intestinal inflammatory diseases?
For the purpose of this review, the intestinal diseases that we consider are IBD – which includes the related but distinct conditions of ulcerative colitis and Crohn’s disease – and NEC, a major cause of death and disability in premature infants. IBD represents a significant cause of acute and chronic morbidity in patients owing to symptoms including abdominal pain, food intolerance, rectal bleeding and, in severe cases, intestinal perforation and intra-abdominal infection (Xavier and Podolsky, 2007). Most patients with IBD are diagnosed between the ages of 15 and 35, and more than 10% of new cases occur in patients that are less than 18 years of age (Clayburgh et al., 2004; Kappelman et al., 2008). Given the young age of presentation of these chronic illnesses, and the fortunate fact that they are rarely, if ever, immediately fatal, the subsequent lifetime morbidity is substantial (Park and Bass, 2010). From a histopathological viewpoint, both ulcerative colitis and Crohn’s disease characteristically show features of chronic mucosal damage (i.e. epithelial disruption, glandular atrophy and architectural distortion). Crohn’s disease differs from ulcerative colitis in that it is typically characterized by the presence of granulomas, skip lesions or primarily small intestinal involvement, although ∼10% of cases have a diagnosis that is not clearly Crohn’s or colitis (Mizoguchi and Mizoguchi, 2010; Mokrowiecka et al., 2010). Treatment of both Crohn’s disease and ulcerative colitis currently involves the use of immunosuppressive agents, careful dietary management and, for progressive or severe disease, surgery. Most individuals with the disease can achieve a reasonable quality of life through a combination of various therapies but, along with their caregivers, most would readily agree that more effective therapies are desperately needed (Vucelic, 2009; Ha and Kornbluth, 2010).
Whereas Crohn’s disease and ulcerative colitis affect older children and adults, NEC is a devastating disease that predominantly affects premature neonates (Caplan, 2008; Sodhi et al., 2008). NEC is characterized by inflammation and necrosis of the small and large intestine of the premature infant and can rapidly progress to systemic sepsis, multisystem organ failure and death within 24 hours (Gribar et al., 2008). The typical patient with NEC is a tiny baby who is relatively stable in the neonatal intensive care unit, but who develops sudden feeding intolerance and then, by 24 hours, is either dying or dead from dramatic and overwhelming sepsis (Holman et al., 1989; Holman et al., 1997). In many cases, laparotomy reveals extensive necrosis of portions of the intestines (Hackam et al., 2005). The incidence of NEC is currently 0.9–2.4 per 1000 live births, and NEC is by far the leading cause of mortality from gastrointestinal disease in premature neonates (Llanos et al., 2002; Hackam et al., 2005). Treatment of NEC involves fluid resuscitation, broad-spectrum antimicrobial agents and surgical resection of the dead or dying intestine. The overall survival of infants with NEC is 20–50%, which is essentially unchanged since NEC was initially described over 30 years ago (Mizrahi et al., 1965; Blakely et al., 2005; Schnabl et al., 2008). The fact that current therapy for both IBD and NEC is both nonspecific and inadequate (two features that are probably co-dependent) strongly indicates that an improved understanding of the nature of these diseases is urgently required.
This review evaluates the role of various experimental systems in our understanding of IBD and NEC. Although both of these diseases are characterized by inflammation of the intestine, it is important to emphasize that they are two distinct clinical entities, with different etiologies and different molecular mechanisms. NEC affects premature or early newborn infants, is characterized by transmural inflammation and intestinal necrosis, and is fatal in many cases. By contrast, IBD affects older individuals, is associated either with inflammation that is confined to the mucosa (ulcerative colitis) or can be transmural (Crohn’s disease), but never leads to NEC; in cases in which IBD causes death, it is rarely caused directly by the disease, but rather results from secondary effects, including the nutritional and potentially malignant effects of long-standing chronic intestinal inflammation. In view of these differences, we do not intend to provide a unifying hypothesis to explain their pathogenesis; rather, we describe how applying the scientific method using various biological systems can expand our understanding of the underlying molecular mechanisms responsible for their pathology.
What are the important research questions?
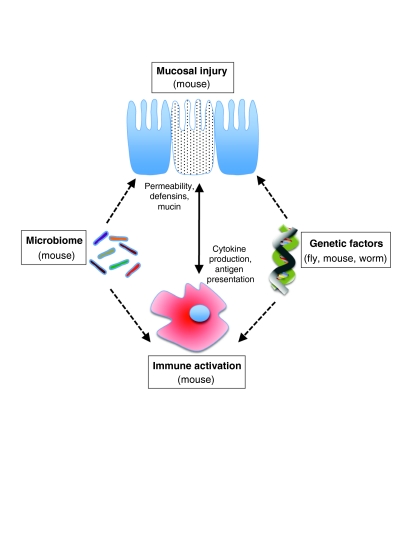
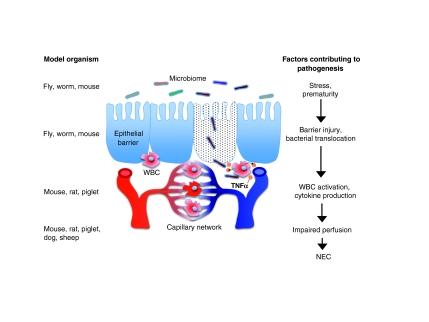
Because our understanding of the mechanisms that lead to the development of IBD and NEC is incomplete, management strategies remain only partially effective in many patients. Substantial strides have been made in advancing our understanding of the pathogenesis of these diseases (for reviews, see Xavier and Podolsky, 2007; Afrazi et al., 2010), but many fundamental questions still remain largely unanswered, which has left a large gap between the therapies that we have and the therapies that our patients need. Such fundamental questions are expectedly different with respect to IBD and NEC, which further underscores the distinct nature of the two diseases. In the IBD field, key areas of research include improving our understanding of: how the host genome regulates disease susceptibility; how interactions between the host microbiome and host immune system contribute to disease induction; and how cellular and molecular events at the epithelial barrier interact with the host immune system to regulate disease severity and progression. In understanding the pathogenesis of NEC, major issues to investigate include: identifying the specific biological features that render the premature infant particularly susceptible to the development of NEC; understanding how the host microbiome interacts with innate immune receptors on intestinal epithelium and host leukocytes to regulate mucosal injury, repair and regeneration; and how alterations in the intestinal microvasculature of the premature infant lead to the impaired perfusion that culminates in intestinal necrosis that characterizes NEC. The answers to these questions can be addressed at least in part through the thoughtful application of various experimental models (Figs 1, 2), as reviewed below.
Fig. 1.
Factors involved in IBD pathogenesis, and the application of model organisms for investigating them. IBD is thought to involve interactions between genetic factors, the microbiome and effector pathways to result in mucosal injury (indicated by the dotted cell) and immune-cell activation. Various animal models can be used to dissect these interactions; those useful for studying each contributing factor are noted in parentheses in each case. See main text for further details.
Fig. 2.
Factors contributing to NEC, and the application of model organisms to study them. NEC develops in the setting of prematurity and systemic stress, resulting in microbial activation in the intestinal mucosa, bacterial translocation, systemic immune activation and impaired intestinal perfusion. To investigate the factors that contribute to the pathology of NEC (as illustrated and also listed on the right), various model organisms can be used (listed on the left). See the main text for further details. WBC, white blood cell.
Various model systems contribute to research on intestinal inflammatory diseases
The intestinal mucosal barrier must accomplish a seemingly impossible task. It must protect against a potentially harmful external environment while concomitantly providing a means for nutrient absorption and waste excretion. To accomplish these ostensibly disparate goals, the barrier must have exquisite solute selectivity, must recognize and respond to potential microbial pathogens that are present within its indigenous commensal microbial flora, and must have the capacity to heal and self-renew (Madara, 1990; Rowlands et al., 1999; Turner, 2009). The architectural integrity and impermeability of the epithelial barrier is maintained through a highly regulated network of inter-epithelial tight junctions, intermediate junctions and desmosomes, and is covered by a hydrostatic layer of mucin (Arrieta et al., 2006; Marchiando et al., 2010). Current dogma indicates that loss of structural integrity – as can result from direct toxicity or impaired development – results in the transfer of lumenal antigens into the submucosa, where they are encountered by immune cells that elicit an inflammatory response (MacDonald and Monteleone, 2006; Turner, 2009). This inflammatory response compounds the initial injury and initiates a cycle of further mucosal disruption (Hackam et al., 2005; Bruewer et al., 2006). Understanding the molecular and cellular components of the intestinal barrier, and gaining clues into the processes by which mucosal disruption occurs in the pathogenesis of IBD and NEC, is no simple task, in part owing to the large variety of different types of cells (epithelial, immune, mesenchymal and endothelial) that must interact to maintain homeostasis. To define the nature of these interactions during both health and disease, investigators have turned to a wide array of model systems. We now consider the essential features of several different model systems and how their study has contributed to our understanding of the pathophysiology of intestinal inflammation. We then consider, as an example, how the use of different experimental models that focus on the role of the innate immune receptor Toll-like receptor 4 (TLR4) has revealed important distinctions between the pathogenesis of IBD, during which TLR4 signaling plays a protective role by maintaining bacterial-enterocyte homeostasis, and NEC, during which TLR4 activation in the newborn small intestine plays a causative role in disease by adversely affecting intestinal injury and repair mechanisms.
Insights from worm models
The worm has emerged as a powerful tool for advancing our understanding of the mechanisms of intestinal inflammation in humans. This small hermaphroditic animal, the nematode Caenorhabditis elegans, which grows to only 1 mm in length, harbors an intestine composed of a 20-cell monolayer (Brenner, 1974; Alper, 2010; Kormish et al., 2010). The unique features of this highly organized intestinal epithelium, coupled with the ability to quickly generate genetically modified nematodes in the lab, provide significant potential advantages for studying intestinal inflammation, compared with the use of more complex model systems (McGhee, 2007). The genome of C. elegans has been completely sequenced and discovered to share significant homology with those of mammals (Pujol et al., 2001; Stein et al., 2003; Haerty et al., 2008). The worm has a short lifespan and is transparent at all stages of development, which allows for visualization of all cells by differential interference contrast microscopy (Aboobaker and Blaxter, 2000). It is noteworthy that bacteria-enterocyte interactions can be studied with relative ease using the worm, by taking advantage of the fact that the worm survives on a diet of bacteria, allowing for replacement of this normal food source with the bacterial species of choice (Darby, 2005).
Several recent studies performed in worms have contributed to our understanding of the pathogenesis of NEC. Specifically, Luke et al. identified that a family of serine and cysteine protease inhibitors called serpins plays a role in the protection of intestinal epithelial cells from necrosis by regulating the intracellular proteolytic pathways that would otherwise lead to enterocyte death (Luke et al., 2007). The ability to rapidly manipulate the worm genome (Markaki and Tavernarakis, 2010), combined with the visual identification of each of the intestinal epithelial cells in the living worm (Achilleos et al., 2010), greatly facilitated these studies, highlighting the advantages of the worm as a model in which to study NEC (Silverman et al., 2009). In related work, studies in C. elegans have led to the discovery of specific genes that are important in the regulation of enterocyte apoptosis (Chinnadurai et al., 2008; Lu et al., 2009; Kang and Avery, 2010), the process of programmed cell death that is known to be increased in the development of ulcerative colitis (Araki et al., 2010), Crohn’s disease (Van den Brande et al., 2007) and NEC (Leaphart et al., 2007a). Together, these studies support the idea that C. elegans represents a useful tool for studying mechanisms of intestinal inflammation that are relevant to humans. However, the overall applicability of worms for the study of these diseases is limited owing to the lack of immune and circulatory systems that are comparable to those in higher organisms (see Figs 1, 2). Therefore, studies in worms might be most useful for applications such as high-throughput screening for potential therapeutic targets that are conserved between worms, mice and humans (Gosai et al., 2010), as well as for using gene-silencing approaches to explore the biological role of candidate genes that have been identified in human studies – two areas that are much more time-consuming and costly to carry out in mammalian models compared with in C. elegans.
Insights from fly studies
The fruit fly Drosophila melanogaster has been demonstrated to be a useful model system in which to study mechanisms of intestinal homeostasis and disease, in part owing to the fact that it has several anatomical features that are similar to the human intestine. The intestinal epithelium of the fly contains proliferating and differentiated epithelial cells that are similar to those of the vertebrate intestine (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006). This is important in terms of understanding the factors that regulate IBD and NEC, because both of these diseases have been shown to be associated with alterations in enterocyte proliferation (Sodhi et al., 2010; Steinbrecher et al., 2008; Lee et al., 2010). In terms of the relevance of fly studies to our understanding of intestinal inflammation in mammals, it is noteworthy that several of the pathways that regulate intestinal stem cell signaling in flies are common to those present in mouse and human enterocytes, including the JAK-STAT pathway (Suzuki et al., 2001; Koon et al., 2006; Jiang et al., 2009; Liu, W. et al., 2010).
Studies in Drosophila have been instrumental in advancing our understanding of the mechanisms that regulate cell death in the intestine. In both NEC and IBD, the intestinal epithelium undergoes a marked loss of enterocytes through apoptosis, which leads to defects in the intestinal barrier that allows for the transmucosal passage of bacteria and activation of the host immune system (Leaphart et al., 2007a; Van den Brande et al., 2007; Araki et al., 2010). The pathways that regulate enterocyte apoptosis remain incompletely understood, in part because of the time and effort required to generate genetically mutant animals with specific alterations in apoptotic regulatory molecules. Studies performed in the fly can provide at least some clues as to the processes involved. For instance, Biteau et al. showed that JNK signaling plays key roles in regeneration of the fly intestine by directing the proliferation of intestinal stem cells in a process that requires Delta and Notch signaling (Biteau et al., 2008). Furthermore, Apidianakis et al. recently demonstrated that, when flies were administered the human opportunistic pathogen Pseudomonas aeruginosa, activation of JNK in the intestine led to a loss of enterocytes via apoptosis, resulting in compensatory increased proliferation that was dependent upon the appropriate activation of the cell cycle regulatory protein Ras1 (Apidianakis et al., 2009). Amcheslavsky and colleagues recently administered the epithelial toxins dextran sulfate sodium (DSS) and bleomycin to flies that were expressing proapoptotic proteins, and demonstrated that the induction of enterocyte apoptosis itself might serve as a signal for the generation of mature enterocytes from precursors in the intestine (Amcheslavsky et al., 2009). Similarly, Terhzaz et al. showed that, in response to oxidative stress – which is known to play an important role in enterocyte apoptosis in NEC (Baregamian et al., 2009) and IBD (You et al., 2009) – epithelial cells in the fly undergo apoptosis that requires the activation of cell-specific inositol (1,4,5)-trisphosphate 3-kinase (Terhzaz et al., 2010). Neisch et al. recently demonstrated that regulation of epithelial apoptosis in the fly requires the small GTPase Rho1 and its effects on JNK signaling (Neisch et al., 2010) – two pathways that are linked to both IBD (Segain et al., 2003; Shkoda et al., 2007) and NEC (Cetin et al., 2007). The fly has also been used to determine the pathways that lead to the regulation of mitogen-activated protein kinase (MAPK) signaling within the intestinal epithelium (Park et al., 2009), an important pathway in the regulation of intestinal homeostasis in mammals (Wei and Feng, 2010). These and other elegant studies of the signaling pathways that regulate intestinal cell survival and death in flies do not require immediate translation to mammals to have value. Rather, it is by harnessing the power of fly genetics that components of disease-relevant signaling pathways and cellular processes in the intestine – an organ that the fly shares in part with the human – can be determined. Only through further analysis in mammalian models can the role of such molecules in IBD and NEC be determined (Yamamoto, 2010).
In addition to providing insight into mechanisms of intestinal epithelial injury and repair, fly studies have uncovered innate-immune regulatory pathways with surprising and important relevance to IBD and NEC. Landmark studies performed in Drosophila identified the molecule Toll, which was initially found to play a central role in antifungal responses (Lemaitre et al., 1996). More recently, Toll signaling in the fly has been shown to play a role in the regulation of gut homeostasis through influencing the balance between microbe-induced epithelial cell damage and stem cell repair (Buchon et al., 2009). In mammals, the first Toll-like receptor that was identified (TLR4) has been shown to recognize endotoxin through MD2 (Poltorak et al., 1998). Studies performed in mice carrying mutations in Tlr4 or related genes have shed light on the role of these innate immune receptors in the pathogenesis of both IBD and NEC, as we later describe in detail.
So, what can we learn from fly studies that will advance our understanding of IBD and NEC? From a purely clinical viewpoint, it is difficult to grasp how studies performed in an insect that has little overlap in the vascular, lymphatic, hematopoietic and microbial features that are relevant to IBD and NEC in humans could have direct relevance to these diseases (see Figs 1, 2). However, the purpose of fly studies is not to model the intestinal disease processes (although there is some evidence, as discussed above, that this is possible), nor to evaluate the efficacy of potential drug therapies. Rather, fly studies afford the unparalleled benefit of allowing for large-scale determination of the role of individual disease-associated genes on the function of the whole organism (Hou, 2010; Lindquist et al., 2011). Future studies in this area can therefore employ large-scale screens for genes that affect intestinal cell proliferation, differentiation and function, and will offer exciting insights into disease processes in humans that are relevant to both NEC and IBD.
Insights from studies in vertebrates
Studies performed in vertebrate models have substantially advanced our understanding of the pathogenesis of NEC and IBD, and are arguably the most commonly used experimental systems in the field of intestinal inflammation. Among the vertebrate animals studied, rodents are most common. The benefits of using rodent models for the study of NEC and IBD include the facts that the rodent intestinal epithelium has the same four cell types as humans (enterocyte, goblet, paneth and enteroendocrine), that relatively reproducible models of intestinal inflammation that mimic the human disease can be induced and, especially in the case of mice, that transgenic strains can be developed to examine specific molecules or pathways that regulate intestinal epithelial homeostasis and disease (Schmidt et al., 1988; Calvert and Pothier, 1990; Hall et al., 1994). We now consider vertebrate models of IBD followed by NEC, and then consider how relevant findings from these models apply to these diseases in humans, highlighting the role of TLR4 activation.
Vertebrate models of IBD
In general, vertebrate models for the study of IBD can be broadly grouped into three categories: those induced by defects in epithelial integrity and permeability, those induced by defects in innate immune cells, and those induced by defects in the adaptive immune system. Most of these models are based either on the administration of chemical agents that directly damage the intestinal epithelium, the transfer of immune cells that activate the immune system of the host, or the manipulation of genes that regulate epithelial integrity or immune function.
Defects in epithelial integrity are typically induced either through the instillation of an epithelial toxin – such as DSS, trinitrobenzene sulfonate (TNBS) or oxazolone (Wirtz et al., 2007) – or through the generation of transgenic mice that lack key molecules that are required for maintenance of an intact epithelial barrier, as observed in the dominant-negative N-cadherin transgenic strain, the multidrug resistance protein 1 (Mdr1)-knockout mouse or the SAMP1/YitFc (Samp) strain (Uhlig and Powrie, 2009). Each of these models has advantages and disadvantages. For instance, the DSS-induced colitis model is simple, reproducible and allows for the study of epithelial repair mechanisms, but lacks some of the important clinical features that are seen in human IBD, such as a lack of transmural inflammation and the involvement of the small intestine. By comparison, the induction of colitis with TNBS or oxazolone involves the intra-rectal instillation of these haptenating substances dissolved in ethanol, which leads to alterations in the microbiota (Boismenu and Chen, 2000). These changes in the host microbial flora typically increase the immunogenicity of the flora, which leads to the induction of colitis in a T helper (Th)-cell-dependent manner (Boismenu and Chen, 2000). The dominant-negative N-cadherin transgenic mouse model of intestinal inflammation expresses a dominant-negative mutant of the cell-adhesion molecule N-cadherin in intestinal epithelial cells, which results in severe intestinal inflammation in regions of epithelial disruption (Hermiston and Gordon, 1995). The MDR1-deficient mouse spontaneously develops intestinal inflammation through ill-defined impairments in enterocyte function (Panwala et al., 1998). Of note, MDR1 polymorphisms have been associated with ulcerative colitis in humans (Schwab et al., 2003). The Samp mouse suffers from inflammation in the terminal ileum, and this seems to involve activation of peroxisome proliferator-activated receptor-γ (PPARγ) (Matsumoto et al., 1998). The terminal ileal distribution of disease in this model renders it useful for the study of Crohn’s disease, which affects this region in humans. It is noteworthy that, with the exception of the DSS-colitis model, each of the above models has limitations that include high cost, variability in the incidence of intestinal inflammation and a lengthy period of time before intestinal inflammation develops.
Other models of intestinal inflammation involve defects in innate immune cell function. For instance, STAT3-deficient and A20-deficient mouse models both lack key negative regulators of immune cell activation, which results in spontaneous colitis (Takeda et al., 1999; Lee et al., 2000). Intestinal inflammation can also be experimentally induced as a result of defects in adaptive immune cells. These include: a model in which CD4+CD45RBhigh T cells are transferred to an immunodeficient mouse, resulting in transmural intestinal inflammation in the colon (Leach et al., 1996); interleukin-10 (IL-10)-deficient mice (Kuhn et al., 1993), which develop chronic enterocolitis owing to impaired suppression of Th1 cells, which involves IL-10; and mice lacking the T-cell receptor (TCR) α-chain, resulting in increased Th2 signaling and the subsequent development of intestinal inflammation (Mombaerts et al., 1993). As mentioned above, limitations of these models include the high cost associated with maintaining colonies of transgenic mice, as well as the time required to generate chimeric mice carrying donor T cells on an immunodeficient background.
Other vertebrate models have also been used for the study of colitis. In pigs, the administration of either DSS or TNBS results in the induction of colitis that is similar to that observed in mice and which is characterized by weight loss and increased intestinal permeability (Krauter et al., 2007; Kim et al., 2009). Colitis can also be induced experimentally in other vertebrate animals such as guinea pigs and rabbits (Reinshagen et al., 1994; Odashima et al., 2005), and dogs (Onderdonk, 1985; Shibata et al., 1993). The fact that the same regimen can induce the phenotype of intestinal inflammation in several different species supports the potential applicability of these models for the preclinical testing of novel therapeutic agents. Although the mouse is the animal model most commonly used, the use of larger animal models will be extremely important in terms of evaluating the efficacy of potential experimental drugs, particularly in terms of toxicity profiles, prior to clinical trials with a candidate therapeutic compound.
Scientists involved in the study of IBD have long debated which is the ‘best’ model for the study of the human disease. This is a challenge: the fact that there are so many models of IBD implies de facto that none is perfect. The answer to this issue is not necessarily to use the model that most closely mimics all features of clinical IBD, but to choose the model that is most appropriate for addressing a specific disease-relevant question in an experimental setting. For instance, to evaluate mechanisms of epithelial injury and repair, a model involving an epithelial toxin such as DSS can be extremely valuable, although extrapolation of the results from such studies to ulcerative colitis in humans might be somewhat difficult. Similarly, testing the role of the adaptive immune system or the mechanical properties of the intestinal epithelial barrier could be addressed using the cell transfer or transgenic mouse models described above. Because IBD pathogenesis involves the combined effects of host genetics, microbial factors and effector cells on both the intestine and leukocytes (Fig. 1), it is indeed difficult to conceive the development of a single model to address all of these features. Therefore, rather than seek to define the ‘best’ vertebrate model of IBD, we should value rather than criticize the marked heterogeneity and genetic diversity among currently available models.
Vertebrate models of NEC
In contradistinction to experimental IBD, NEC has not been found to occur spontaneously in any model, and must be induced experimentally. Animal models of NEC must exhibit important pathological and clinical features of the disease that are seen typically in humans. As shown in Fig. 2, NEC is characterized by the development of patchy areas of intestinal inflammation and necrosis, as well as marked evidence of systemic sepsis (Neu and Walker, 2011). Pathological evaluation reveals mucosal inflammation and ulceration, leukocyte infiltration and the presence of vascular thrombosis in the intestinal microcirculation (Mannoia et al., 2011; Neu and Walker, 2011). We and others have shown that the development of NEC involves an increased proinflammatory response to the microbial flora of the host under conditions of stress and premature birth, with associated bacterial translocation and the activation of macrophages in the lamina propria of the intestine (Grave et al., 2007; Dai et al., 2010; Khailova et al., 2010). The development of NEC is associated with impaired perfusion of the intestine, which is ultimately responsible for necrosis, but which can either precede or follow the initial inflammatory nidus (Richardson et al., 2010; Sodhi et al., 2010). To establish a model that has these important features of NEC, investigators have traditionally replicated the clinical scenario in various vertebrates, typically in rodents. NEC in humans occurs in the stressed, premature neonate on exposure to feeds, often after a hypoxic episode (Anand et al., 2007; Grave et al., 2007; Frost et al., 2008; Halpern and Dvorak, 2008). Thus, rodent models of NEC are induced through the administration of infant formula by gavage to premature or early postnatal mouse or rat pups, together with exposure to hypoxia, over a period of 3–4 days (Leaphart et al., 2007a; Richardson et al., 2010; Sodhi et al., 2010). In contrast to the wide variety of models used for studying IBD, most investigators that study NEC use the model described above, with slight variation from center to center. Although the incidence of NEC varies from laboratory to laboratory, most groups report an incidence of experimental NEC of 50–60%, as defined by histological features of inflammation and necrosis, an increase in the expression of mucosal proinflammatory factors [including interleukin-1 (IL-1), tumor necrosis factor-α (TNFα), inducible nitric oxide synthase (iNOS) and interferon-γ (IFNγ)] and vascular thrombosis (Nadler et al., 2000; Cetin et al., 2004; Feng and Besner, 2007; Lu et al., 2007; Halpern and Dvorak, 2008). Although wild-type mice and rats can be used for this model, it can also be induced in various mouse strains carrying mutations in important factors, including TLR4 (Jilling et al., 2006; Leaphart et al., 2007a), IL-18 (Halpern and Dvorak, 2008), heparin-binding epidermal growth factor (Radulescu et al., 2010) and IFNγ (Leaphart et al., 2007b).
In addition to studies performed in mice and rats, NEC can also be induced in other vertebrate animals, including sheep (Wolfs et al., 2009), rabbits (Choi et al., 2010), piglets (Sangild et al., 2006) and chickens (Park et al., 2008), using animal-specific variations of the protocol described above. Although induction of the disease in large animals might resemble the human disease more closely than that induced in mice, disadvantages of using a large animal model include the increased cost of animals and their housing, and the limited availability of transgenic strains. What are the limitations of the current animal models for NEC, and how can they be improved? An important point regarding the models described above is the variable degree of inflammation that is achieved, both within experimental groups (e.g. mouse to mouse) as well as in different species, a situation that is compounded by the small size of animals that must be used to replicate the clinical scenario of the preterm infant. Thus, any approach that increases the severity of the disease that is induced would be particularly useful, so that the effects of ‘anti-NEC’ strategies can be better appreciated and measured. Such approaches, which could be developed by obtaining an increased understanding of the molecular and cellular events that lead to the development of NEC, could involve either pharmacological or genetic manipulation in models of NEC. One area in which the application of experimental models has substantially advanced our understanding of the pathogenesis of NEC has been in the area of the role of the innate immune system – and particularly the innate immune receptor TLR4 – as described below.
Insights from vertebrate models of IBD and NEC: the role of TLR4 in pathogenesis
Studies performed using various animal models have identified important differences in the pathogenesis of IBD and NEC, particularly with respect to the role of TLR4. TLR4 seems to have a role in both diseases: several groups have demonstrated that TLR4 is expressed in the intestinal epithelium in both mice and humans (Abreu et al., 2001; Abreu et al., 2002; Melmed et al., 2003; Neal et al., 2006), and the development of both IBD and NEC is known to require intestinal colonization with gram-negative bacteria (which express the TLR4-activating molecule endotoxin) (Claud and Walker, 2008; Friswell et al., 2010). However, data from various studies indicate that TLR4 plays opposing roles in the pathogenesis of IBD versus NEC. Specifically, our group and the Caplan laboratory have shown that TLR4 activation in the intestine is required for the pathogenesis of NEC (Jilling et al., 2006; Leaphart et al., 2007a). At the mechanistic level, we demonstrated that TLR4 expression by enterocytes leads to increased mucosal injury owing to increased apoptosis, and a reduction in mucosal healing through inhibition of enterocyte proliferation (Sodhi et al., 2010) and migration (Leaphart et al., 2007a), overall indicating that TLR4 activation contributes to the pathogenesis of NEC. By contrast, studies by the laboratories of Medzhitov (Rakoff-Nahoum et al., 2004) and Abreu (Fukata et al., 2005) have shown that TLR4-mediated activation of nuclear factor-κB (NFκB) in the intestine is important for the maintenance of mucosal homeostasis. Furthermore, TLR4 activation has been shown to be important for epithelial repair following acute injury (Fukata et al., 2006). However, it should be noted that the protective role for TLR4 in the gut demonstrated by Abreu and Medzhitov is in contrast to other data that TLR4 activation in fact leads to intestinal injury and generally contributes to the pathogenesis of inflammation (Fort et al., 2005; Ungaro et al., 2009; Liu, Y. et al., 2010).
Overall, the general view in the field is that TLR4 signaling protects against the development of IBD yet contributes to the development of NEC. How can these seemingly opposite roles of TLR4 in intestinal inflammation be explained? We propose that TLR4 activation leads to intestinal injury and delayed repair in the pathogenesis of NEC in a specific context, namely the newborn small intestine. In support of this finding, we recently demonstrated that TLR4 activation by endotoxin leads to increased enterocyte apoptosis in the terminal ileum of newborn but not adult mice, and in the small intestine but not the colon of the newborn (Richardson et al., 2010; Sodhi et al., 2010), which we speculate is due to different patterns of expression or function of TLR4 in the intestine in different regions and in mice of different ages. Differences in study design between labs might also account for the differences in the assigned roles for TLR4 with respect to its contribution to versus protection from mucosal injury. Specifically, different studies vary in how they administer the model for NEC in mice, and a few have used DSS-induced colitis to make inferences regarding the role of TLR4 in the induction of both NEC and colitis (Fukata et al., 2005). Notably, a protective role for TLR4 in the intestinal epithelium has been determined using global Tlr4-knockout mice, in which TLR4 signaling is disrupted not only in the enterocytes, but also in all other cell types that are known to express TLR4, including T cells and myeloid cells (Iwasaki and Medzhitov, 2010). By contrast, other groups concluded that enterocyte-specific TLR4 signaling is important for the induction of intestinal injury from studies involving enterally administered adenoviral constructs that enabled expression, mainly in the small bowel mucosa, of inhibitory mutations in TLR4 (i.e. that silenced endogenous TLR4) in neonatal mice (Gribar et al., 2009). It can therefore be concluded that TLR4 activation in enterocytes leads to increased intestinal injury, whereas the protective effects attributed to TLR4 activation in the gut might reflect in part the mitigating effects induced by TLR4 activation in other cell types, including immune cells. Together, these findings indicate that the role of TLR4 in intestinal inflammation is related to various factors, including the effector cells involved (enterocytes versus immune cells), developmental factors (newborn versus adult) and the region of the intestine involved (small bowel versus large bowel).
Conclusions and future directions
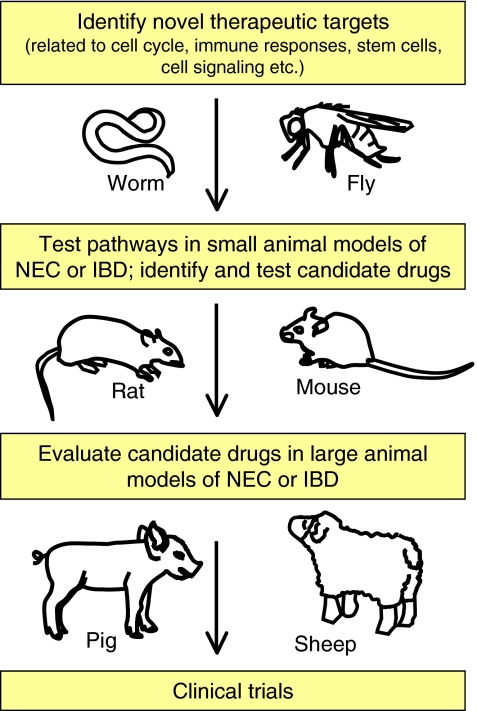
Current data demonstrate that insights into the mechanisms that lead to the development of NEC and IBD can be gained from studying different models, including worms, flies and vertebrates. Perhaps chief among the various pathways that have been found to be relevant in the pathogenesis of both NEC and IBD is TLR4 signaling, which is based in part on studies that have been performed in all three model systems and that revealed opposing roles for this pathway in the pathogenesis of these two diseases. Future studies will involve continuous refinements to available models to increase the frequency with which these diseases can be induced, thereby minimizing the number of animals needed for analysis. Future directions in the field that will serve to advance our understanding of both IBD and NEC might include high-throughput screening to identify, using small-interfering RNA- or microRNA-mediated silencing approaches in simple systems such as the fly and worm, candidate genes that regulate intestinal injury and homeostasis; the results of these studies could then be tested in more complex systems (see Fig. 3). Complex systems could involve the generation of mutant mice with inducible cell-specific mutations in key effector pathways, and the establishment of transgenic approaches in large animals. The overall goal of such approaches is to develop novel therapeutic agents that can be tested in small animal models of IBD and NEC, and subsequently in large animal models of these diseases, as a prerequisite for the design and implementation of clinical trials in humans. Through these efforts, an increased understanding of the pathogenesis of these important diseases could be achieved and, through the identification of novel therapies, the suffering of a large part of our population could be alleviated.
Fig. 3.
The application of model organisms for the design of novel therapies for IBD and NEC. Studies performed in worms and flies provide clues about the fundamental genetic factors and biological processes that regulate intestinal and immune function (see main text for details), which can then be followed up in rodent models of IBD and NEC and lead to the design of rational molecular strategies for therapy. These can then be tested in both small and large animal models of these diseases. Promising results at this stage might then lead to initiation of Phase 1 clinical trials in humans with NEC and IBD.
Acknowledgments
D.J.H. is supported by R01GM078238 and RO1DK08752 from the National Institutes of Health, and The Hartwell Foundation, Memphis, TN.
Footnotes
COMPETING INTERESTS
The authors declare that they do not have any competing or financial interests.
REFERENCES
- Aboobaker A. A., Blaxter M. L. (2000). Medical significance of Caenorhabditis elegans. Ann. Med. 32, 23–30 [DOI] [PubMed] [Google Scholar]
- Abreu M. T., Vora P., Faure E., Thomas L. S., Arnold E. T., Arditi M. (2001). Decreased expression of Toll-Like Receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J. Immunol. 167, 1609–1616 [DOI] [PubMed] [Google Scholar]
- Abreu M. T., Arnold E. T., Thomas L. S., Gonsky R., Zhou Y., Hu B., Arditi M. (2002). TLR4 and MD-2 expression is regulated by immune-mediated signals in human intestinal epithelial cells. J. Biol. Chem. 277, 20431–20437 [DOI] [PubMed] [Google Scholar]
- Achilleos A., Wehman A. M., Nance J. (2010). PAR-3 mediates the initial clustering and apical localization of junction and polarity proteins during C. elegans intestinal epithelial cell polarization. Development 137, 1833–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afrazi A., Sodhi C. P., Richardson W., Neal M., Good M., Siggers R., Hackam D. J. (2010). New insights into the pathogenesis and treatment of necrotizing enterocolitis: Toll-like receptors and beyond. Pediatr. Res. 69, 183–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper S. (2010). Model systems to the rescue: the relationship between aging and innate immunity. Commun. Integr. Biol. 3, 409–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amcheslavsky A., Jiang J., Ip Y. T. (2009). Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell 4, 49–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand R. J., Leaphart C. L., Mollen K. P., Hackam D. J. (2007). The role of the intestinal barrier in the pathogenesis of necrotizing enterocolitis. Shock 27, 124–133 [DOI] [PubMed] [Google Scholar]
- Apidianakis Y., Pitsouli C., Perrimon N., Rahme L. (2009). Synergy between bacterial infection and genetic predisposition in intestinal dysplasia. Proc. Natl. Acad. Sci. USA 106, 20883–20888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki Y., Mukaisyo K., Sugihara H., Fujiyama Y., Hattori T. (2010). Increased apoptosis and decreased proliferation of colonic epithelium in dextran sulfate sodium-induced colitis in mice. Oncol. Rep. 24, 869–874 [DOI] [PubMed] [Google Scholar]
- Arrieta M. C., Bistritz L., Meddings J. B. (2006). Alterations in intestinal permeability. Gut 55, 1512–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baregamian N., Song J., Bailey C. E., Papaconstantinou J., Evers B. M., Chung D. H. (2009). Tumor necrosis factor-alpha and apoptosis signal-regulating kinase 1 control reactive oxygen species release, mitochondrial autophagy, and c-Jun N-terminal kinase/p38 phosphorylation during necrotizing enterocolitis. Oxid. Med. Cell. Longev. 2, 297–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B., Hochmuth C. E., Jasper H. (2008). JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell 3, 442–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely M. L., Lally K. P., McDonald S., Brown R. L., Barnhart D. C., Ricketts R. R., Thompson W. R., Scherer L. R., Klein M. D., Letton R. W., et al. (2005). Postoperative outcomes of extremely low birth-weight infants with necrotizing enterocolitis or isolated intestinal perforation: a prospective cohort study by the NICHD Neonatal Research Network. Ann. Surg. 241, 984–989; discussion 989–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boismenu R., Chen Y. (2000). Insights from mouse models of colitis. J. Leukoc. Biol. 67, 267–278 [DOI] [PubMed] [Google Scholar]
- Brenner S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruewer M., Samarin S., Nusrat A. (2006). Inflammatory bowel disease and the apical junctional complex. Ann. N. Y. Acad. Sci. 1072, 242–252 [DOI] [PubMed] [Google Scholar]
- Buchon N., Broderick N. A., Poidevin M., Pradervand S., Lemaitre B. (2009). Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe 5, 200–211 [DOI] [PubMed] [Google Scholar]
- Calvert R., Pothier P. (1990). Migration of fetal intestinal intervillous cells in neonatal mice. Anat. Rec. 227, 199–206 [DOI] [PubMed] [Google Scholar]
- Caplan M. S. (2008). Neonatal necrotizing enterocolitis. Introduction. Semin. Perinatol. 32, 69. [DOI] [PubMed] [Google Scholar]
- Cetin S., Ford H. R., Sysko L. R., Agarwal C., Wang J., Neal M. D., Baty C., Apodaca G., Hackam D. J. (2004). Endotoxin inhibits intestinal epithelial restitution through activation of Rho-GTPase and increased focal adhesions. J. Biol. Chem. 279, 24592–24600 [DOI] [PubMed] [Google Scholar]
- Cetin S., Leaphart C. L., Li J., Ischenko I., Hayman M., Upperman J., Zamora R., Watkins S., Ford H. R., Wang J., et al. (2007). Nitric oxide inhibits enterocyte migration through activation of RhoA-GTPase in a SHP-2-dependent manner. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G1347–G1358 [DOI] [PubMed] [Google Scholar]
- Chinnadurai G., Vijayalingam S., Gibson S. B. (2008). BNIP3 subfamily BH3-only proteins: mitochondrial stress sensors in normal and pathological functions. Oncogene 27 Suppl. 1, S114–S127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. H., Kim I. O., Cheon J. E., Kim J. E., Kim E. K., Kim W. S., Yeon K. M. (2010). Doppler sonographic findings in an experimental rabbit model of necrotizing enterocolitis. J. Ultrasound Med. 29, 379–386 [DOI] [PubMed] [Google Scholar]
- Claud E. C., Walker W. A. (2008). Bacterial colonization, probiotics, and necrotizing enterocolitis. J. Clin. Gastroenterol. 42 Suppl. 2, S46–S52 [DOI] [PubMed] [Google Scholar]
- Clayburgh D. R., Rosen S., Witkowski E. D., Wang F., Blair S., Dudek S., Garcia J. G. N., Alverdy J. C., Turner J. R. (2004). A differentiation-dependent splice variant of Myosin Light Chain Kinase, MLCK1, regulates epithelial tight junction permeability. J. Biol. Chem. 279, 55506–55513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S., Sodhi C. P., Cetin S., Richardson W., Branca M., Neal M. D., Prindle T., Ma C., Shapiro R. A., Li B., et al. (2010). Extracellular high mobility group box1 (HMGB1) inhibits enterocyte migration via activation of toll like receptor 4 and increased cell-matrix adhesiveness. J. Biol. Chem. 285, 4995–5002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby C. (2005). Interactions with microbial pathogens. WormBook (ed. The C. elegans Research Community, Wormbook ), pp. 1–15 http://www.workbook.org [DOI] [PMC free article] [PubMed]
- Feng J., Besner G. E. (2007). Heparin-binding epidermal growth factor-like growth factor promotes enterocyte migration and proliferation in neonatal rats with necrotizing enterocolitis. J. Pediatr. Surg. 42, 214–220 [DOI] [PubMed] [Google Scholar]
- Fort M. M., Mozaffarian A., Stöver A. G., Correia J. S., Johnson D. A., Crane R. T., Ulevitch R. J., Persing D. H., Bielefeldt-Ohmann H., Probst P., et al. (2005). A synthetic TLR4 antagonist has anti-inflammatory effects in two murine models of inflammatory bowel disease. J. Immunol. 174, 6416–6423 [DOI] [PubMed] [Google Scholar]
- Friswell M., Campbell B., Rhodes J. (2010). The role of bacteria in the pathogenesis of inflammatory bowel disease. Gut Liver 4, 295–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost B. L., Jilling T., Caplan M. S. (2008). The importance of pro-inflammatory signaling in neonatal necrotizing enterocolitis. Semin. Perinatol. 32, 100–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M., Michelsen K. S., Eri R., Thomas L. S., Hu B., Lukasek K., Nast C. C., Lechago J., Xu R., Naiki Y., et al. (2005). Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G1055–G1065 [DOI] [PubMed] [Google Scholar]
- Fukata M., Chen A., Klepper A., Krishnareddy S., Vamadevan A. S., Thomas L. S., Xu R., Inoue H., Arditi M., Dannenberg A. J., et al. (2006). Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: role in proliferation and apoptosis in the intestine. Gastroenterology 131, 862–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthright W. E., Archer D. L., Kvenberg J. E. (1988). Estimates of incidence and costs of intestinal infectious diseases in the United States. Public Health Rep. 103, 107–115 [PMC free article] [PubMed] [Google Scholar]
- Gosai S. J., Kwak J. H., Luke C. J., Long O. S., King D. E., Kovatch K. J., Johnston P. A., Shun T. Y., Lazo J. S., Perlmutter D. H., et al. (2010). Automated high-content live animal drug screening using C. elegans expressing the aggregation prone serpin alpha1-antitrypsin Z. PLoS ONE 5, e15460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grave G. D., Nelson S. A., Walker W. A., Moss R. L., Dvorak B., Hamilton F. A., Higgins R., Raju T. N. (2007). New therapies and preventive approaches for necrotizing enterocolitis: report of a research planning workshop. Pediatr. Res. 62, 510–514 [DOI] [PubMed] [Google Scholar]
- Gribar S. C., Anand R., Sodhi C. P., Hackam D. J. (2008). The role of epithelial Toll-like receptor signaling in the pathogenesis of intestinal inflammation. J. Leukoc. Biol. 83, 493–498 [DOI] [PubMed] [Google Scholar]
- Gribar S. C., Sodhi C. P., Richardson W. M., Anand R. J., Gittes G. K., Branca M. F., Jakub A., Shi X. H., Shah S., Ozolek J. A., et al. (2009). Reciprocal expression and signaling of TLR4 and TLR9 in the pathogenesis and treatment of necrotizing enterocolitis. J. Immunol. 182, 636–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha C., Kornbluth A. (2010). Mucosal healing in inflammatory bowel disease: where do we stand? Curr. Gastroenterol. Rep. 12, 471–478 [DOI] [PubMed] [Google Scholar]
- Hackam D. J., Upperman J. S., Grishin A., Ford H. R. (2005). Disordered enterocyte signaling and intestinal barrier dysfunction in the pathogenesis of necrotizing enterocolitis. Semin. Pediatr. Surg. 14, 49–57 [DOI] [PubMed] [Google Scholar]
- Haerty W., Artieri C., Khezri N., Singh R. S., Gupta B. P. (2008). Comparative analysis of function and interaction of transcription factors in nematodes: extensive conservation of orthology coupled to rapid sequence evolution. BMC Genomics 9, 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall P. A., Coates P. J., Ansari B., Hopwood D. (1994). Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J. Cell Sci. 107, 3569–3577 [DOI] [PubMed] [Google Scholar]
- Halpern M. D., Dvorak B. (2008). Does abnormal bile acid metabolism contribute to NEC? Semin. Perinatol. 32, 114–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermiston M. L., Gordon J. I. (1995). Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science 270, 1203–1207 [DOI] [PubMed] [Google Scholar]
- Holman R., Stehr-Green J., Zelasky M. (1989). Necrotizing enterocolitis mortality in the United States. Am. J. Public Health 79, 987–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman R. C., Stoll B. J., Clarke M. J., Glass R. I. (1997). The epidemiology of necrotizing enterocolitis infant mortality in the United States. Am. J. Public Health 87, 2026–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman R. C., Stoll B. J., Curns A. T., Yorita K. L., Steiner C. A., Schonberger L. B. (2006). Necrotising enterocolitis hospitalisations among neonates in the United States. Paediatr. Perinat. Epidemiol. 20, 498–506 [DOI] [PubMed] [Google Scholar]
- Hou S. X. (2010). Intestinal stem cell asymmetric division in the Drosophila posterior midgut. J. Cell. Physiol. 224, 581–584 [DOI] [PubMed] [Google Scholar]
- Iwasaki A., Medzhitov R. (2010). Regulation of adaptive immunity by the innate immune system. Science 327, 291–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Patel P. H., Kohlmaier A., Grenley M. O., McEwen D. G., Edgar B. A. (2009). Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 137, 1343–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilling T., Simon D., Lu J., Meng F. J., Li D., Schy R., Thomson R. B., Soliman A., Arditi M., Caplan M. S. (2006). The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J. Immunol. 177, 3273–3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C., Avery L. (2010). Death-associated protein kinase (DAPK) and signal transduction: fine-tuning of autophagy in Caenorhabditis elegans homeostasis. FEBS J. 277, 66–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappelman M. D., Rifas-Shiman S. L., Porter C. Q., Ollendorf D. A., Sandler R. S., Galanko J. A., Finkelstein J. A. (2008). Direct health care costs of Crohn’s disease and ulcerative colitis in US children and adults. Gastroenterology 135, 1907–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khailova L., Mount Patrick S. K., Arganbright K. M., Halpern M. D., Kinouchi T., Dvorak B. (2010). Bifidobacterium bifidum reduces apoptosis in the intestinal epithelium in necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G1118–G1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. J., Kovacs-Nolan J., Yang C., Archbold T., Fan M. Z., Mine Y. (2009). L-cysteine supplementation attenuates local inflammation and restores gut homeostasis in a porcine model of colitis. Biochim. Biophys. Acta 1790, 1161–1169 [DOI] [PubMed] [Google Scholar]
- Koon H. W., Zhao D., Zhan Y., Rhee S. H., Moyer M. P., Pothoulakis C. (2006). Substance P stimulates cyclooxygenase-2 and prostaglandin E2 expression through JAK-STAT activation in human colonic epithelial cells. J. Immunol. 176, 5050–5059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kormish J. D., Gaudet J., McGhee J. D. (2010). Development of the C. elegans digestive tract. Curr. Opin. Genet. Dev. 20, 346–354 [DOI] [PubMed] [Google Scholar]
- Krauter E. M., Strong D. S., Brooks E. M., Linden D. R., Sharkey K. A., Mawe G. M. (2007). Changes in colonic motility and the electrophysiological properties of myenteric neurons persist following recovery from trinitrobenzene sulfonic acid colitis in the guinea pig. Neurogastroenterol. Motil. 19, 990–1000 [DOI] [PubMed] [Google Scholar]
- Kuhn R., Lohler J., Rennick D., Rajewsky K., Muller W. (1993). Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75, 263–274 [DOI] [PubMed] [Google Scholar]
- Leach M. W., Bean A. G., Mauze S., Coffman R. L., Powrie F. (1996). Inflammatory bowel disease in C.B-17 scid mice reconstituted with the CD45RBhigh subset of CD4+ T cells. Am. J. Pathol. 148, 1503–1515 [PMC free article] [PubMed] [Google Scholar]
- Leaphart C. L., Cavallo J. C., Gribar S. C., Cetin S., Li J., Branca M. F., Dubowski T. D., Sodhi C. P., Hackam D. J. (2007a). A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J. Immunol. 179, 4808–4820 [DOI] [PubMed] [Google Scholar]
- Leaphart C. L., Qureshi F., Cetin S., Li J., Dubowski T., Batey C., Beer-Stolz D., Guo F., Murray S. A., Hackam D. J. (2007b). Interferon-[gamma] inhibits intestinal restitution by preventing gap junction communication between enterocytes. Gastroenterology 132, 2395–2411 [DOI] [PubMed] [Google Scholar]
- Lee E. G., Boone D. L., Chai S., Libby S. L., Chien M., Lodolce J. P., Ma A. (2000). Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science 289, 2350–2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G., Goretsky T., Managlia E., Dirisina R., Singh A. P., Brown J. B., May R., Yang G. Y., Ragheb J. W., Evers B. M., et al. (2010). Phosphoinositide 3-kinase signaling mediates beta-catenin activation in intestinal epithelial stem and progenitor cells in colitis. Gastroenterology 139, 869–881.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B., Nicolas E., Michaut L., Reichhart J. M., Hoffmann J. A. (1996). The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86, 973–983 [DOI] [PubMed] [Google Scholar]
- Lindquist R. A., Ottina K. A., Wheeler D. B., Hsu P. P., Thoreen C. C., Guertin D. A., Ali S. M., Sengupta S., Shaul Y. D., Lamprecht M. R., et al. (2011). Genome-scale RNAi on living-cell microarrays identifies novel regulators of Drosophila melanogaster TORC1-S6K pathway signaling. Genome Res. 21, 433–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Singh S. R., Hou S. X. (2010). JAK-STAT is restrained by Notch to control cell proliferation of the Drosophila intestinal stem cells. J. Cell. Biochem. 109, 992–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhang Z., Wang L., Li J., Dong L., Yue W., Chen J., Sun X., Zhong L., Sun D. (2010). TLR4 monoclonal antibody blockade suppresses dextran-sulfate-sodium-induced colitis in mice. J. Gastroenterol. Hepatol. 25, 209–214 [DOI] [PubMed] [Google Scholar]
- Llanos A. R., Moss M. E., Pinzon M. C., Dye T., Sinkin R. A., Kendig J. W. (2002). Epidemiology of neonatal necrotising enterocolitis: a population-based study. Paediatr. Perinat. Epidemiol. 16, 342–349 [DOI] [PubMed] [Google Scholar]
- Lu J., Jilling T., Li D., Caplan M. S. (2007). Polyunsaturated fatty acid supplementation alters proinflammatory gene expression and reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Pediatr. Res. 61, 427–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu N., Yu X., He X., Zhou Z. (2009). Detecting apoptotic cells and monitoring their clearance in the nematode Caenorhabditis elegans. Methods Mol. Biol. 559, 357–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke C. J., Pak S. C., Askew Y. S., Naviglia T. L., Askew D. J., Nobar S. M., Vetica A. C., Long O. S., Watkins S. C., Stolz D. B., et al. (2007). An intracellular serpin regulates necrosis by inhibiting the induction and sequelae of lysosomal injury. Cell 130, 1108–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald T. T., Monteleone G. (2006). Overview of role of the immune system in the pathogenesis of inflammatory bowel disease. Adv. Exp. Med. Biol. 579, 98–107 [DOI] [PubMed] [Google Scholar]
- Madara J. L. (1990). Warner-Lambert/Parke-Davis Award lecture. Pathobiology of the intestinal epithelial barrier. Am. J. Pathol. 137, 1273–1281 [PMC free article] [PubMed] [Google Scholar]
- Mannoia K., Boskovic D. S., Slater L., Plank M. S., Angeles D. M., Gollin G. (2011). Necrotizing enterocolitis is associated with neonatal intestinal injury. J. Pediatr. Surg. 46, 81–85 [DOI] [PubMed] [Google Scholar]
- Marchiando A. M., Graham W. V., Turner J. R. (2010). Epithelial barriers in homeostasis and disease. Annu. Rev. Pathol. 5, 119–144 [DOI] [PubMed] [Google Scholar]
- Markaki M., Tavernarakis N. (2010). Modeling human diseases in Caenorhabditis elegans. Biotechnol. J. 5, 1261–1276 [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Okabe Y., Setoyama H., Takayama K., Ohtsuka J., Funahashi H., Imaoka A., Okada Y., Umesaki Y. (1998). Inflammatory bowel disease-like enteritis and caecitis in a senescence accelerated mouse P1/Yit strain. Gut 43, 71–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. D. (2007). The C. elegans intestine, WormBook (ed. The C. elegans Research Community, Wormbook ), pp. 1–36, http://www.wormbook.org
- Melmed G., Thomas L. S., Lee N., Tesfay S. Y., Lukasek K., Michelsen K. S., Zhou Y., Hu B., Arditi M., Abreu M. T. (2003). Human intestinal epithelial cells are broadly unresponsive to Toll-Like Receptor 2-Dependent bacterial ligands: implications for host-microbial interactions in the gut J. Immunol. 170, 1406–1415 [DOI] [PubMed] [Google Scholar]
- Micchelli C. A., Perrimon N. (2006). Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439, 475–479 [DOI] [PubMed] [Google Scholar]
- Mizoguchi A., Mizoguchi E. (2010). Animal models of IBD: linkage to human disease. Curr. Opin. Pharmacol. 10, 578–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi A., Barlow O., Berdon W., Blanc W. A., Silverman W. A. (1965). Necrotizing enterocolitis in premature infants. J. Pediatr. 66, 697–705 [DOI] [PubMed] [Google Scholar]
- Mokrowiecka A., Kumor A., Jakubczyk E., Pietruczuk M., Malecka-Panas E. (2010). The application of montreal classification in different clinical and serological IBD subtypes. Hepatogastroenterology 57, 787–793 [PubMed] [Google Scholar]
- Mombaerts P., Mizoguchi E., Grusby M. J., Glimcher L. H., Bhan A. K., Tonegawa S. (1993). Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell 75, 274–282 [DOI] [PubMed] [Google Scholar]
- Nadler E. P., Dickinson E., Knisely A., Zhang X. R., Boyle P., Beer-Stolz D., Watkins S. C., Ford H. R. (2000). Expression of inducible nitric oxide synthase and interleukin-12 in experimental necrotizing enterocolitis. J. Surg. Res. 92, 71–77 [DOI] [PubMed] [Google Scholar]
- Neal M. D., Leaphart C., Levy R., Prince J., Billiar T. R., Watkins S., Li J., Cetin S., Ford H., Schreiber A., et al. (2006). Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J. Immunol. 176, 3070–3079 [DOI] [PubMed] [Google Scholar]
- Neisch A. L., Speck O., Stronach B., Fehon R. G. (2010). Rho1 regulates apoptosis via activation of the JNK signaling pathway at the plasma membrane. J. Cell Biol. 189, 311–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu J., Walker W. A. (2011). Necrotizing enterocolitis. N. Engl. J. Med. 363, 255–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odashima M., Bamias G., Rivera-Nieves J., Linden J., Nast C. C., Moskaluk C. A., Marini M., Sugawara K., Kozaiwa K., Otaka M., et al. (2005). Activation of A2A adenosine receptor attenuates intestinal inflammation in animal models of inflammatory bowel disease. Gastroenterology 129, 26–33 [DOI] [PubMed] [Google Scholar]
- Ohlstein B., Spradling A. (2006). The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439, 470–474 [DOI] [PubMed] [Google Scholar]
- Onderdonk A. B. (1985). Experimental models for ulcerative colitis. Dig. Dis. Sci. 30, 40S–44S [DOI] [PubMed] [Google Scholar]
- Panwala C. M., Jones J. C., Viney J. L. (1998). A novel model of inflammatory bowel disease: mice deficient for the multiple drug resistance gene, mdr1a, spontaneously develop colitis. J. Immunol. 161, 5733–5744 [PubMed] [Google Scholar]
- Park J.-S., Kim Y.-S., Yoo M.-A. (2009). The role of p38b MAPK in age-related modulation of intestinal stem cell proliferation and differentiation in Drosophila. Aging 1, 637–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K. T., Bass D. (2010). Inflammatory bowel disease-attributable costs and cost-effective strategies in the United States: a review. Inflamm. Bowel Dis. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- Park S. S., Lillehoj H. S., Allen P. C., Park D. W., FitzCoy S., Bautista D. A., Lillehoje E. P. (2008). Immunopathology and cytokine responses in broiler chickens coinfected with Eimeria maxima and Clostridium perfringens with the use of an animal model of necrotic enteritis. Avian Dis. 52, 14–22 [DOI] [PubMed] [Google Scholar]
- Poltorak A., He X., Smirnova I., Liu M. Y., Van Huffel C., Du X., Birdwell D., Alejos E., Silva M., Galanos C., et al. (1998). Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 [DOI] [PubMed] [Google Scholar]
- Pujol N., Link E. M., Liu L. X., Kurz C. L., Alloing G., Tan M. W., Ray K. P., Solari R., Johnson C. D., Ewbank J. J. (2001). A reverse genetic analysis of components of the Toll signaling pathway in Caenorhabditis elegans. Curr. Biol. 11, 809–821 [DOI] [PubMed] [Google Scholar]
- Radulescu A., Zhang H. Y., Yu X., Olson J. K., Darbyshire A. K., Chen Y., Besner G. E. (2010). Heparin-binding epidermal growth factor-like growth factor overexpression in transgenic mice increases resistance to necrotizing enterocolitis. J. Pediatr. Surg. 45, 1933–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S., Paglino J., Eslami-Varzaneh F., Edberg S., Medzhitov R. (2004). Recognition of commensal microflora by Toll-Like receptors is required for intestinal homeostasis. Cell 118, 229–241 [DOI] [PubMed] [Google Scholar]
- Reinshagen M., Patel A., Sottili M., Nast C., Davis W., Mueller K., Eysselein V. (1994). Protective function of extrinsic sensory neurons in acute rabbit experimental colitis. Gastroenterology 106, 1208–1214 [DOI] [PubMed] [Google Scholar]
- Richardson W. M., Sodhi C. P., Russo A., Siggers R. H., Afrazi A., Gribar S. C., Neal M. D., Dai S., Prindle T. J., Branca M., et al. (2010). Nucleotide-binding oligomerization Domain-2 inhibits Toll Like Receptor-4 signaling in the intestinal epithelium. Gastroenterology 139, 904–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands B. J., Soong C. V., Gardiner K. R. (1999). The gastrointestinal tract as a barrier in sepsis. Br. Med. Bull. 55, 196–211 [DOI] [PubMed] [Google Scholar]
- Sangild P. T., Siggers R. H., Schmidt M., Elnif J., Bjornvad C. R., Thymann T., Grondahl M. L., Hansen A. K., Jensen S. K., Boye M. (2006). Diet- and colonization-dependent intestinal dysfunction predisposes to necrotizing enterocolitis in preterm pigs. Gastroenterology 130, 1776–1792 [DOI] [PubMed] [Google Scholar]
- Schmidt G. H., Winton D. J., Ponder B. A. (1988). Development of the pattern of cell renewal in the crypt-villus unit of chimaeric mouse small intestine. Development 103, 785–790 [DOI] [PubMed] [Google Scholar]
- Schnabl K. L., Van Aerde J. E., Thomson A. B., Clandinin M. T. (2008). Necrotizing enterocolitis: a multifactorial disease with no cure. World J. Gastroenterol. 14, 2142–2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab M., Schaeffeler E., Marx C., Fromm M. F., Kaskas B., Metzler J., Stange E., Herfarth H., Schoelmerich J., Gregor M., et al. (2003). Association between the C3435T MDR1 gene polymorphism and susceptibility for ulcerative colitis. Gastroenterology 124, 26–33 [DOI] [PubMed] [Google Scholar]
- Segain J. P., Raingeard de la Bletiere D., Sauzeau V., Bourreille A., Hilaret G., Cario-Toumaniantz C., Pacaud P., Galmiche J. P., Loirand G. (2003). Rho kinase blockade prevents inflammation via nuclear factor kappa B inhibition: evidence in Crohn’s disease and experimental colitis. Gastroenterology 124, 1180–1187 [DOI] [PubMed] [Google Scholar]
- Shibata Y., Taruishi M., Ashida T. (1993). Experimental ileitis in dogs and colitis in rats with trinitrobenzene sulfonic acid-colonoscopic and histopathologic studies. Gastroenterol. Jpn. 28, 518–527 [DOI] [PubMed] [Google Scholar]
- Shkoda A., Werner T., Daniel H., Gunckel M., Rogler G., Haller D. (2007). Differential protein expression profile in the intestinal epithelium from patients with inflammatory bowel disease. J. Proteome Res. 6, 1114–1125 [DOI] [PubMed] [Google Scholar]
- Silverman G. A., Luke C. J., Bhatia S. R., Long O. S., Vetica A. C., Perlmutter D. H., Pak S. C. (2009). Modeling molecular and cellular aspects of human disease using the nematode Caenorhabditis elegans. Pediatr. Res. 65, 10–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi C., Richardson W., Gribar S. C., Hackam D. J. (2008). The development of animal models for the study of necrotizing enterocolitis. Dis. Model. Mech. 1, 94–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi C. P., Shi X. H., Richardson W. M., Grant Z. S., Shapiro R. A., Prindle T. J., Branca M., Russo A., Gribar S. C., Ma C., et al. (2010). Toll-like Receptor-4 inhibits enterocyte proliferation via impaired beta-catenin signaling in necrotizing enterocolitis. Gastroenterology 138, 185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein L. D., Bao Z., Blasiar D., Blumenthal T., Brent M. R., Chen N., Chinwalla A., Clarke L., Clee C., Coghlan A., et al. (2003). The genome sequence of Caenorhabditis briggsae: a platform for comparative genomics. PLoS Biol. 1, E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrecher K. A., Harmel-Laws E., Sitcheran R., Baldwin A. S. (2008). Loss of epithelial RelA results in deregulated intestinal proliferative/apoptotic homeostasis and susceptibility to inflammation. J. Immunol. 180, 2588–2599 [DOI] [PubMed] [Google Scholar]
- Suzuki A., Hanada T., Mitsuyama K., Yoshida T., Kamizono S., Hoshino T., Kubo M., Yamashita A., Okabe M., Takeda K., et al. (2001). CIS3/SOCS3/SSI3 plays a negative regulatory role in STAT3 activation and intestinal inflammation. J. Exp. Med. 193, 471–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K., Clausen B. E., Kaisho T., Tsujimura T., Terada N., Forster I., Akira S. (1999). Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity 10, 39–49 [DOI] [PubMed] [Google Scholar]
- Terhzaz S., Finlayson A. J., Stirrat L., Yang J., Tricoire H., Woods D. J., Dow J. A., Davies S. A. (2010). Cell-specific inositol 1,4,5 trisphosphate 3-kinase mediates epithelial cell apoptosis in response to oxidative stress in Drosophila. Cell. Signal. 22, 737–748 [DOI] [PubMed] [Google Scholar]
- Turner J. R. (2009). Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809 [DOI] [PubMed] [Google Scholar]
- Uhlig H. H., Powrie F. (2009). Mouse models of intestinal inflammation as tools to understand the pathogenesis of inflammatory bowel disease. Eur J Immunol. 39, 2021–2026 [DOI] [PubMed] [Google Scholar]
- Ungaro R., Fukata M., Hsu D., Hernandez Y., Breglio K., Chen A., Xu R., Sotolongo J., Espana C., Zaias J., et al. (2009). A novel Toll-like receptor 4 antagonist antibody ameliorates inflammation but impairs mucosal healing in murine colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G1167–G1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Brande J. M., Koehler T. C., Zelinkova Z., Bennink R. J., te Velde A. A., ten Cate F. J., van Deventer S. J., Peppelenbosch M. P., Hommes D. W. (2007). Prediction of antitumour necrosis factor clinical efficacy by real-time visualisation of apoptosis in patients with Crohn’s disease. Gut 56, 509–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucelic B. (2009). Inflammatory bowel diseases: controversies in the use of diagnostic procedures. Dig. Dis. 27, 269–277 [DOI] [PubMed] [Google Scholar]
- Wei J., Feng J. (2010). Signaling pathways associated with inflammatory bowel disease. Recent Pat. Inflamm. Allergy Drug Discov. 4, 105–117 [DOI] [PubMed] [Google Scholar]
- Wirtz S., Neufert C., Weigmann B., Neurath M. F. (2007). Chemically induced mouse models of intestinal inflammation. Nature protocols 2, 541–546 [DOI] [PubMed] [Google Scholar]
- Wolfs T. G., Buurman W. A., Zoer B., Moonen R. M., Derikx J. P., Thuijls G., Villamor E., Gantert M., Garnier Y., Zimmermann L. J., et al. (2009). Endotoxin induced chorioamnionitis prevents intestinal development during gestation in fetal sheep. PLoS ONE 4, e5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier R. J., Podolsky D. K. (2007). Unravelling the pathogenesis of inflammatory bowel disease. Nature 448, 427–434 [DOI] [PubMed] [Google Scholar]
- Yamamoto M. T. (2010). Drosophila genetic resource and stock center; The National BioResource Project. Exp. Anim. 59, 125–138 [DOI] [PubMed] [Google Scholar]
- You Y., Fu J. J., Meng J., Huang G. D., Liu Y. H. (2009). Effect of N-acetylcysteine on the murine model of colitis induced by dextran sodium sulfate through up-regulating PON1 activity. Dig. Dis. Sci. 54, 1643–1650 [DOI] [PubMed] [Google Scholar]