SUMMARY
We previously found that lenses lacking the Acvr1 gene, which encodes a bone morphogenetic protein (BMP) receptor, had abnormal proliferation and cell death in epithelial and cortical fiber cells. We tested whether the tumor suppressor protein p53 (encoded by Trp53) affected this phenotype. Acvr1 conditional knockout (Acvr1CKO) mouse fiber cells had increased numbers of nuclei that stained for p53 phosphorylated on serine 15, an indicator of p53 stabilization and activation. Deletion of Trp53 rescued the Acvr1CKO cell death phenotype in embryos and reduced Acvr1-dependent apoptosis in postnatal lenses. However, deletion of Trp53 alone increased the number of fiber cells that failed to withdraw from the cell cycle. Trp53CKO and Acvr1;Trp53DCKO (double conditional knockout), but not Acvr1CKO, lenses developed abnormal collections of cells at the posterior of the lens that resembled posterior subcapsular cataracts. Cells from human posterior subcapsular cataracts had morphological and molecular characteristics similar to the cells at the posterior of mouse lenses lacking Trp53. In Trp53CKO lenses, cells in the posterior plaques did not proliferate but, in Acvr1;Trp53DCKO lenses, many cells in the posterior plaques continued to proliferate, eventually forming vascularized tumor-like masses at the posterior of the lens. We conclude that p53 protects the lens against posterior subcapsular cataract formation by suppressing the proliferation of fiber cells and promoting the death of any fiber cells that enter the cell cycle. Acvr1 acts as a tumor suppressor in the lens. Enhancing p53 function in the lens could contribute to the prevention of steroid- and radiation-induced posterior subcapsular cataracts.
INTRODUCTION
The lens is an epithelial tissue that grows throughout life. The anterior surface of the lens consists of a simple cuboidal epithelium. Cells located at the periphery of the epithelium, near the lens equator, proliferate throughout life. Following division, they withdraw from the cell cycle, move posteriorly, and terminally differentiate into fiber cells. Fiber cells elongate, extending from the anterior to the posterior pole, and make up the bulk of the lens tissue. Its unique spatial organization makes the lens a valuable model in which to study the mechanisms that control the switch between cell proliferation and withdrawal from the cell cycle during terminal differentiation (Zhang et al., 1998). Previous studies showed that perturbation of the tightly controlled cell cycle kinetics in the lens by inactivation of the retinoblastoma gene (Rb) caused p53-mediated cell death (Morgenbesser et al., 1994; Pan and Griep, 1995; Liu and Zacksenhaus, 2000).
Cataracts occurring later in life are by far the leading cause of blindness worldwide (West, 2007; Brian and Taylor, 2001). Posterior subcapsular cataracts (PSCs) are one of the three main types of age-related cataracts, although the mechanisms of PSC formation have rarely been studied and remain poorly understood. It has long been thought that PSCs might occur as a result of abnormal proliferation and migration of epithelial cells or the failure of proper terminal differentiation of fiber cells (Streeten and Eshaghian, 1978; Eshaghian and Streeten, 1980).
Conditional inactivation of Acvr1 (also known as Alk2), a gene encoding a bone morphogenetic protein (BMP) receptor, causes aberrant mouse fiber cell proliferation and apoptosis (Rajagopal et al., 2008). To determine the role of p53 in Acvr1-mediated fiber cell death, we conditionally deleted Trp53 (which encodes p53) in the mouse lens. Inactivation of Trp53 caused a small number of lens fiber cells to fail to exit the cell cycle. Trp53 conditional knockout (Trp53CKO) lenses developed epithelioid plaques, which resembled PSCs, at their posterior pole, suggesting that p53 normally protects the lens from this type of cataract. Deleting Trp53 and Acvr1 showed that most Acvr1-dependent cell death was mediated by p53. The posterior epithelioid plaques that formed in these lenses transformed into tumor-like clusters, indicating that Acvr1 acts as a tumor suppressor in the lens.
RESULTS
Increased cell death in Acvr1CKO lenses is largely p53 dependent
We previously reported that conditional deletion of the BMP receptor Acvr1 from developing lenses increased cell death in lens epithelial and cortical fiber cells (Rajagopal et al., 2008). Previous studies showed that ablation of the Rb gene or inactivation of Rb protein in the lens prevented fiber cells from exiting the cell cycle and increased apoptosis (Griep et al., 1993; Morgenbesser et al., 1994). In these circumstances, cell death was reduced or eliminated by the removal of Trp53 or inactivation of its gene product. We generated Acvr1;Trp53 double conditional knockout (Acvr1;Trp53DCKO) lenses to determine whether p53 is required for the increased cell death caused by the absence of Acvr1 signaling.
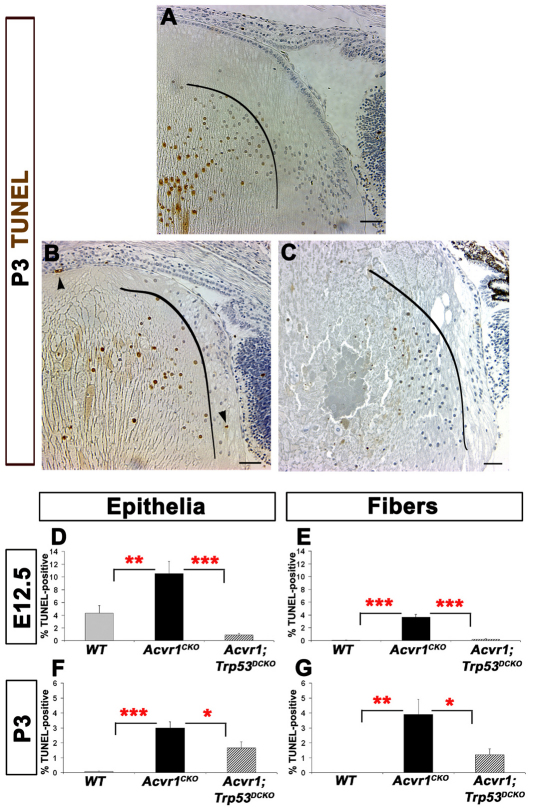
In Acvr1WT (wild-type) lenses at postnatal day 3 (P3), TUNEL-positive nuclei were seen only in fiber cells deeper in the lens, which were undergoing the normal process of denucleation (Fig. 1A) (Bassnett and Mataic, 1997). Deletion of Acvr1 increased cell death in cortical fiber cells, as shown previously (Fig. 1B) (Rajagopal et al., 2008). Sections of Acvr1;Trp53DCKO lenses showed normal denucleation of mature fiber cells, but few TUNEL-positive cortical fiber cells (Fig. 1C). Quantification of the TUNEL-labeling index in Acvr1CKO lenses revealed significantly more apoptosis than in Acvr1WT epithelial and fiber cells at embryonic day 12.5 (E12.5) and P3 (Fig. 1D–G). At E12.5, deletion of Trp53 in Acvr1CKO lenses reduced apoptosis in epithelial cells to below the level seen in wild-type lenses and nearly eliminated the apoptosis caused by deletion of Acvr1 in fiber cells (Fig. 1D,E). Apoptosis was not detected in wild-type fiber cells at P3, but cell death increased significantly after deletion of Acvr1 (Fig. 1F). This increase was reduced by more than two-thirds by deletion of Trp53 (Fig. 1G).
Fig. 1.
Increased apoptosis in the absence of Acvr1 is p53 dependent. The TUNEL labeling index was determined in lens epithelial and fiber cells of wild-type, Acvr1CKO and Acvr1;Trp53DCKO lenses. (A) A representative image of a TUNEL-stained Acvr1WT lens at P3. Central fibers normally denucleate, serving as a positive control for the TUNEL staining. The curved black line indicates the boundary between elongating fiber cells and deeper fiber cells undergoing the normal process of denucleation. TUNEL-positive, peripheral cortical fiber cells were rarely detected in P3 lenses. (B) Representative image of an Acvr1CKO lens at P3 showing a TUNEL-positive cortical fiber cell nucleus (upper arrowhead) and a TUNEL-positive epithelial cell (lower arrowhead). As in our previous study (Rajagopal et al., 2008), only elliptical fiber cell nuclei were counted to determine the number of TUNEL-positive cells. Rounded nuclei inside the curved line are deeper in the lens and are undergoing the normal denucleation process. Conditional deletion of Acvr1 significantly increased the TUNEL index of epithelial cells at E12.5. (C) Representative image of an Acvr1;Trp53DCKO double knockout lens at P3. TUNEL-positive mature fiber cells undergoing the normal process of denucleation are to the left of the curved line. In Acvr1;Trp53DCKO lenses, TUNEL-positive superficial fiber cells were rarely detected. (D,E) Deletion of Trp53 reduced the Acvr1-induced apoptosis in E12.5 epithelial (D) and fiber (E) cells. (F,G) A significantly lower percentage of TUNEL-positive epithelial (F) and fiber (G) cells was detected at P3 when Acvr1 and Trp53 were deleted together, compared WITH Acvr1 alone. *P<0.05; **P<0.01; ***P<0.001. Scale bars: 50 μm.
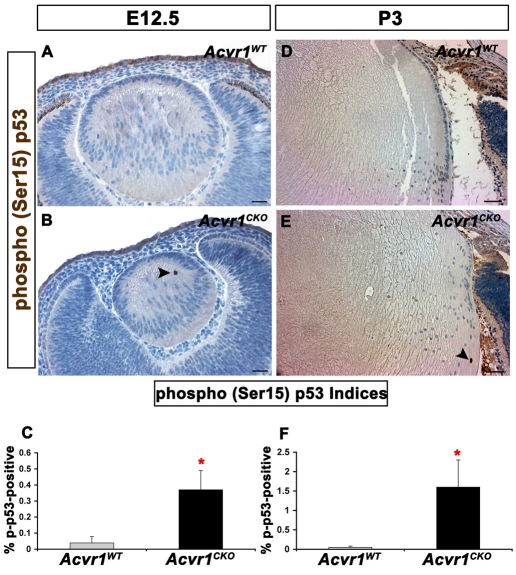
Under normal conditions, p53 is rapidly degraded in a ubiquitin-dependent manner (Haupt et al., 1997). After DNA damage or oncogenic stress, p53 can be stabilized by phosphorylation of one or more amino acids, reducing protein degradation and making p53 available to activate p53-responsive genes (Shieh et al., 1997). To determine whether this mechanism of p53 stabilization is activated in lens cells that fail to withdraw from the cell cycle, we stained E12.5 and P3 lenses for p53 phosphorylated at Ser15 (p-p53; Fig. 2). Nuclei stained for p-p53 were rarely seen in wild-type lenses at either age, but were significantly more abundant after deletion of Acvr1 (Fig. 2A–F).
Fig. 2.
Acvr1CKO lenses have increased phosphorylation of p53 at Ser15. Acvr1WT and Acvr1CKO lenses were stained for phosphorylated (p)-p53. (A) A representative image of an E12.5 Acvr1WT lens with no detectable p-p53 staining. (B) p-p53-positive fiber cell nuclei were detected in Acvr1CKO lenses at E12.5 (arrowhead). (C) The percentage of p-p53-positive fiber cells was significantly higher in Acvr1CKO lenses than in Acvr1WT lenses at E12.5. (D) A representative Acvr1WT lens with no detectable p-p53-positive cells at P3. (E) An Acvr1CKO lens with a p-p53-positive fiber cell nucleus at P3 (arrowhead). (F) At P3, significantly more p-p53-positive fiber cells were detected in Acvr1CKO lenses than in Acvr1WT lenses. *P<0.05. Scale bars: 20 μm (A,B), 50 μm (D,E).
p53 promotes fiber cell exit from the cell cycle and protects against posterior subcapsular plaque formation
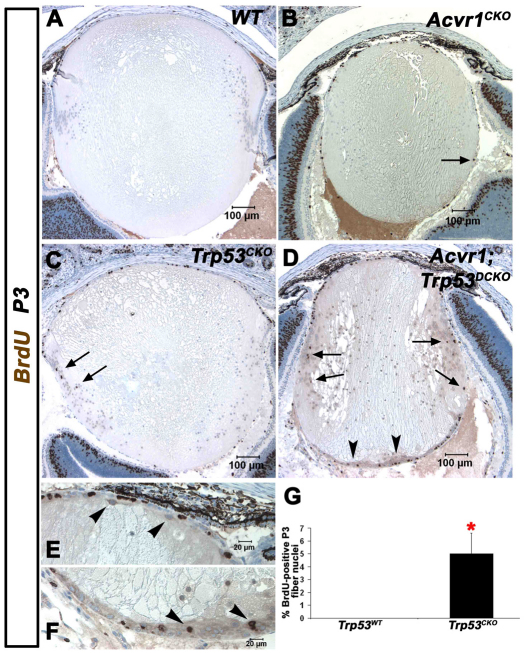
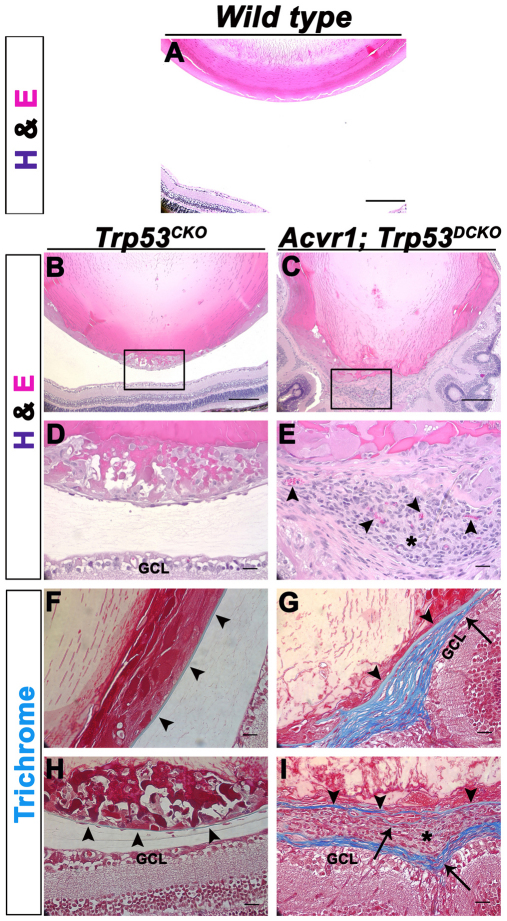
As a control for the double knockouts and to determine whether p53 plays any role in normal murine lens development, we generated Trp53CKO lenses. Trp53CKO lenses showed disorganization of the bow region of the cortical fiber cells and a small number of Trp53CKO fiber cell nuclei were BrdU-positive at P3 (Fig. 3C,G). This phenotype was exacerbated in Acvr1;Trp53DCKO lenses at P3, which were small and misshapen, with vacuoles in the deeper cortical fiber cells (Fig. 3D). The double knockout lenses also showed plaques containing BrdU-positive cells at the posterior of the fiber mass. At higher magnification, a small number of flattened, epithelioid cells were present at the anterior surface of the fiber mass, just beneath the epithelium (Fig. 3E). Compared with Trp53CKO lenses, a larger number of epithelioid cells were present along the posterior lens capsule and at the posterior pole, where they formed a multicellular aggregate (Fig. 3F).
Fig. 3.
Acvr1;Trp53DCKO lenses develop posterior subcapsular plaques of actively proliferating cells. Lenses were injected with BrdU at P3 to label cells in S-phase of the cell cycle. (A) The normal morphology of wild-type lenses. BrdU incorporation is restricted to the anterior epithelium. (B) Acvr1CKO lenses are smaller and have cortical fiber cells that incorporate BrdU (arrow). (C) Trp53CKO lenses have aberrant fiber cells that incorporate BrdU (arrows). (D) Acvr1;Trp53DCKO lenses have numerous BrdU-positive fiber cell nuclei (arrows), more than either Acvr1CKO or Trp53CKO lenses, and form posterior subcapsular plaques of epithelioid cells, some of which are BrdU positive (arrowheads). (E) Acvr1;Trp53DCKO lenses have ectopic, spindle-shaped cells beneath the anterior epithelium (arrowheads). (F) Ectopic cells at the posterior of Acvr1;Trp53DCKO lenses (arrowheads). (G) The percentage of BrdU-positive cortical fiber cell nuclei was significantly higher in Trp53CKO lenses than in Trp53WT lenses. *P<0.05.
During excessive activation of the Ras signaling pathway, p53 suppresses cell proliferation by increasing the expression of the cyclin-dependent kinase inhibitor p21CIP1/WAF1. We were unable to detect p21CIP1/WAF1 protein in the wild-type lens by western blotting using several antibodies. Reverse-transcriptase PCR (RT-PCR) analysis revealed that the level of p21CIP1/WAF1 transcripts was low and did not decrease after deletion of Trp53, suggesting that p53 does not normally suppress fiber cell proliferation by increasing the level of p21CIP1/WAF1 in the lens (not shown).
p53 is dispensable for most aspects of the fiber cell terminal differentiation program
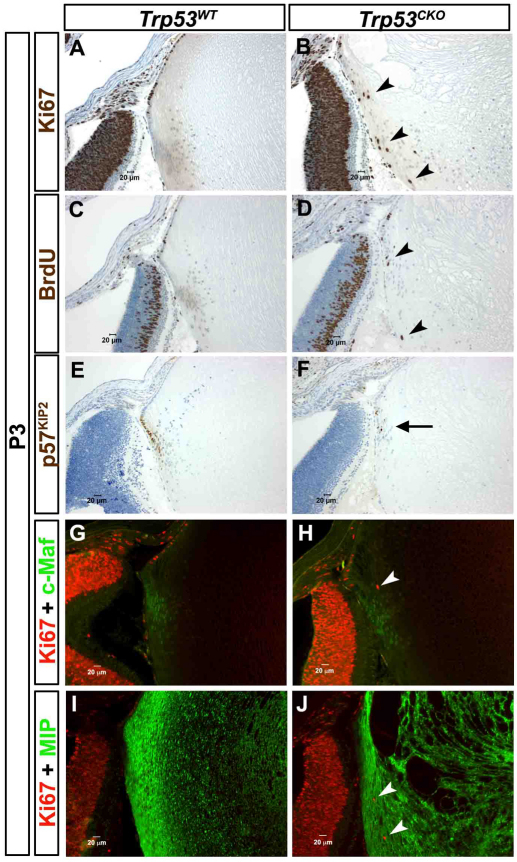
Withdrawal from the cell cycle is a key feature of fiber cell differentiation. Because some Trp53CKO fiber cells continued to incorporate BrdU (Fig. 3C, Fig. 4C,D), we tested whether other aspects of the fiber cell differentiation program were altered in Trp53-knockout lenses. We first confirmed the proliferation defect in Trp53CKO lenses by staining for Ki67, which labels any cells that are actively cycling, and p57KIP2, a cyclin-dependent kinase inhibitor that accumulates in the nuclei of differentiating fiber cells and is required for lens cell cycle exit during terminal differentiation (Zhang et al., 1998). As seen with BrdU, Ki67-stained nuclei were restricted to the epithelium in Trp53WT lenses (Fig. 4A), but were present in many fiber cells in Trp53CKO lenses (Fig. 4B,D). Conversely, p57KIP2 was first expressed near the lens equator and was strongly localized to all of the nuclei of peripheral lens fiber cells in Trp53WT lenses (Fig. 4E). In Trp53CKO lenses, many superficial fiber cell nuclei lacked detectable p57KIP2 expression, consistent with the continued proliferation of a subset of fiber cells (Fig. 4F). During their differentiation, fiber cells express the transcription factor Maf (Ring et al., 2000) and the abundant membrane protein MIP (Broekhuyse et al., 1976). Trp53CKO fiber cells expressed both Maf and MIP, similar to wild-type fibers, suggesting that p53 is dispensable for these aspects of the fiber cell terminal differentiation program (Fig. 4G–J).
Fig. 4.
A subset of Trp53CKO fiber cells fail to exit the cell cycle, but express fiber-cell-specific markers. P3 lenses were stained with antibodies that label markers of cell proliferation, cell cycle exit and lens fiber cell differentiation. (A) Labeling for Ki67, a marker for actively cycling cells, is restricted to the anterior epithelium of Trp53WT lenses. (B) In Trp53CKO lenses, a few cortical fiber cells are Ki67 positive (arrowheads). (C) BrdU is only incorporated into anterior epithelial cells in Trp53WT lenses. (D) A few Trp53CKO fiber cells are BrdU positive (arrowheads). (E) p57KIP2, a marker of cell cycle exit, is strongly expressed in all nuclei in the lens equator of Trp53WT lenses. (F) Most cells in Trp53CKO lenses have nuclei that are stained with antibody to p57KIP2, but a few nuclei are p57KIP2 negative (arrow). (G) Ki67 labeling is lost near the equator, where the fiber-specific transcription factor, Maf (c-Maf) is first expressed. (H) Fiber cells in Trp53CKO lenses express Maf, although some are also Ki67 positive (arrowhead). (I) MIP, a major component of fiber cell membranes, is strongly expressed throughout the fiber zone of Trp53WT lenses. (J) MIP is strongly expressed in Trp53CKO fiber cells, even when they express Ki67 (arrowheads).
Cells in the posterior subcapsular plaques of Trp53CKO lenses have fiber cell characteristics
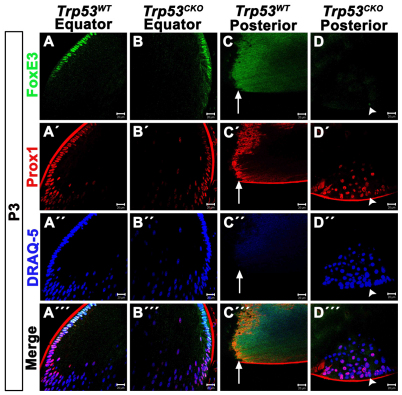
By P3, most Trp53CKO lenses developed posterior subcapsular plaques containing epithelioid cells (Fig. 5D″), whereas littermate Trp53WT lenses did not show these abnormalities (Fig. 5C″). Although cycling fiber cells in Trp53CKO lenses expressed fiber-cell-specific markers, it was unclear whether the epithelioid cells expressed fiber-cell-specific or epithelial-cell-specific markers. To test this, we performed double immunolabeling for the epithelial transcription factor FoxE3 and the fiber-cell-specific transcription factor Prox1. FoxE3 labeling was restricted to the nuclei of the epithelial cells in Trp53WT and Trp53CKO lenses (Fig. 5A,B). Prox1 was first expressed in epithelial cells at the lens equator and expression continued in all fiber cells in the lens bow region of lenses with and without Trp53 (Fig. 5A′,B′). A region of overlap was present in the peripheral epithelium in which cells coexpressed FoxE3 and Prox1. Once passed the equator, no fiber cells were FoxE3 positive (Fig. 5A″′,B″′). No nuclei were detected at the posterior pole of Trp53WT lenses (Fig. 5C″). The nuclei in the epithelioid plaques of Trp53CKO lenses were all Prox1 positive, although the cells stained at different intensities (Fig. 5D′). FoxE3-positive cells were rarely detected (Fig. 5D, arrowhead) and, like the superficial fiber cells, these cells also expressed Prox1 (Fig. 5D″′, arrowhead). These results indicate that, although they have an epithelial morphology, cells within the posterior subcapsular plaques of Trp53CKO lenses are fiber cells of abnormal morphology.
Fig. 5.
Epithelioid cells that constitute the posterior plaques in Trp53CKO lenses express markers of fiber cell terminal differentiation. Trp53WT and Trp53CKO P3 lenses were double stained with FoxE3 and Prox1. (A) FoxE3 is expressed in anterior epithelial cells, but is lost near the lens equator in Trp53WT lenses. (A′) Prox1 is first expressed near the lens equator and is strongly expressed in all fiber cell nuclei in Trp53WT lenses. (A″) The dye DRAQ-5 stains all lens nuclei and allows for visualization of the lens ‘bow’ region. (A′″) Merged image of a Trp53WT lens. (B) As in Trp53WT lenses, FoxE3 labels anterior epithelial cells in Trp53CKO lenses. (B′) Prox1 is normally expressed in differentiating lens fiber cells in Trp53CKO lenses. (B″) DRAQ-5 shows the abnormal appearance of the ‘bow’ region in a Trp53CKO lens. (B′″) Merged image of a Trp53CKO lens. (C) Image of the posterior pole of the same Trp53WT lens as in A. The image is taken at the suture and the laser intensity is purposefully increased to show the fiber zone and suture. No FoxE3-positive nuclei are detected. (C′) Similarly, no Prox1-positive nuclei are present at the posterior pole; non-specific staining is present along the sutures. The anti-mouse secondary antibody used to detect Prox1 non-specifically labels the lens capsule. (C″) Staining with DRAQ-5 shows that there are no cell nuclei at the posterior pole of the Trp53WT lens. (C′″) Merged image of the posterior pole of the Trp53WT lens. Arrows in C-C′″ indicate the posterior lens suture. (D) The posterior pole of the same Trp53CKO lens as in B has a posterior subcapsular plaque. Most epithelioid cells within this plaque do not express FoxE3; a lone cell displays weak FoxE3 labeling (arrowhead). (D′) By contrast, all cells that make up epithelioid plaques express Prox1, although some cells show weak expression. The cell that is FoxE3 positive is also Prox1 positive (arrowhead). (D″) Labeling with DRAQ-5 shows the posterior subcapsular plaque in a Trp53CKO lens. Arrowhead indicates the location of the lone FoxE3-positive cell detected in this section. (D′″) Merged image of the posterior subcapsular plaque in a Trp53CKO lens. Arrowhead indicates the location of the lone FoxE3-positive cell detected in this section. Scale bars: 20 μm.
Trp53CKO mouse PSCs have characteristics similar to human PSCs
We obtained an eye that was removed from a patient with severe diabetic retinopathy and neovascular glaucoma. The lens from this patient had a PSC (Fig. 6). The morphological characteristics of cells at the posterior of this lens were similar to published descriptions of human PSCs (Streeten and Eshaghian, 1978; Eshaghian and Streeten, 1980) and resembled the PSCs in Trp53CKO mouse lenses. Large eosinophilic ‘balloon’ cells filled the posterior fiber mass and epithelioid cells adhered to the posterior capsule in the human eye (Fig. 6D). This contrasted with the absence of epithelial cells and the regular organization of thin fiber cells in a non-cataractous human lens (Fig. 6C). No Ki67-positive nuclei were detected in the PSC, or in the anterior lens epithelium of either the cataractous or control lens, consistent with the generally low rate of cell proliferation in the adult human lens (Fig. 6E). As in the PSCs in Trp53CKO mouse lenses, the nuclei of the balloon and epithelioid cells in the human PSC stained with antibodies to the fiber-cell-specific transcription factors Prox1 and Maf (Fig. 6G,H).
Fig. 6.
The appearance of cells in a human PSC is similar to those in Trp53CKO mouse lenses. (A) The anterior surface of a normal adult human lens is bounded by the thick anterior capsule and a thin layer of lens epithelial cells (arrows). Beneath the epithelium are layers of well-organized superficial fiber cells. (B) The anterior surface of an adult human lens with a PSC appears similar to the normal lens in A. (C) The posterior surface of the normal lens has a thinner capsule than does the anterior of the lens. Beneath the capsule are layers of superficial fiber cells. (D) The posterior of the lens with a PSC has a layer of globular ‘balloon’ cells between the capsule and the well-organized, deeper fiber cells. A layer of epithelioid cells is present on the inner surface of the capsule (arrowheads). (E) No Ki67-labeled nuclei were detected in balloon or epithelioid cells at the posterior of the lens that had PSCs. The epitope unmasking method used in the staining procedure extracted the cytoplasm from the ‘balloon’ cells, causing them to appear as ‘ghosts’. No Ki67-positive nuclei were detected in the anterior epithelium from this lens (not shown). (F) Several nuclei in the corneal epithelium from the eye with a PSC were Ki67 positive, indicating that the staining procedure worked as expected. (G) The nuclei of balloon (arrow) and epithelioid (arrowhead) cells in the PSC stained with an antibody to the fiber-cell-specific marker Prox1. Some of the empty ‘ghosts’ resulting from the epitope unmasking method are labeled with an asterisk. (H) The nuclei of balloon (arrow) and epithelioid (arrowhead) cells in the PSC stained with an antibody to the fiber-cell-specific marker Maf.
Acvr1 acts as a tumor suppressor in the lens
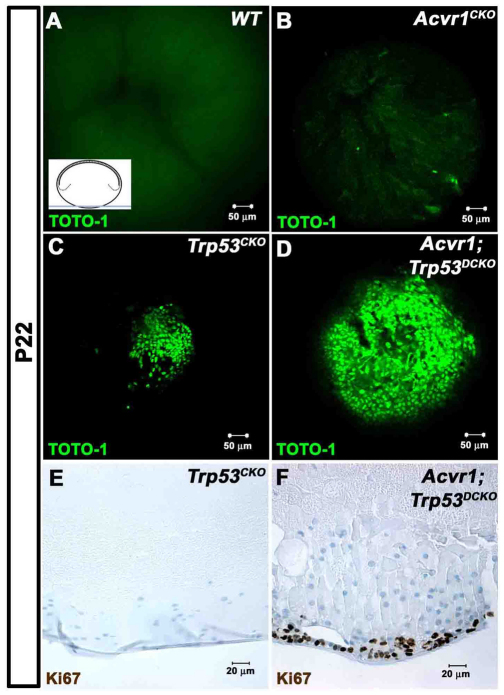
To better understand the origins of the posterior plaques that develop in Trp53CKO and Acvr1;Trp53DCKO lenses, we viewed the posterior pole of whole P22 lenses on an inverted confocal microscope (Fig. 7). Nuclei were stained with the fluorescent nucleic acid dye TOTO-1 and viewed as shown in the inset in Fig. 7A. TOTO-1 also stained RNA in the lens fiber cell cytoplasm, providing information about the orientation of the lenses. Wild-type lenses showed the normal organization of the ‘Y’ suture at the posterior of the fiber mass, with no evidence of nuclei, as expected for this plane of section (Fig. 7A). In lenses lacking Acvr1, a few scattered nuclei could be seen near the posterior ends of the fiber cells, but no cellular plaques were present (Fig. 7B). In Trp53CKO lenses, the nuclei of the cells in the posterior plaques obscured the sutures at the posterior pole of the lens and a few scattered nuclei were visible adjacent to the posterior plaque (Fig. 7C). Lenses lacking both Acvr1 and Trp53 had large confluent masses of cells at their posterior pole (Fig. 7D). We tested to see whether the cells in the posterior plaques in the Trp53CKO and Acvr1;Trp53DCKO lenses continued to proliferate. Cells in plaques from Trp53CKO lenses were not stained with Ki67, indicating that they were not cycling (Fig. 7E). However, many cells in the plaques in Acvr1;Trp53DCKO lenses were Ki67 positive (Fig. 7F).
Fig. 7.
Loss of Acvr1 leads to proliferation in posterior subcapsular plaques, forming ‘lens tumors’. P22 lenses were stained with the nucleic acid stain TOTO-1 and their posterior poles were examined using a confocal microscope. The inset in A shows the plane of section and region of the lens that was viewed. (A) No nuclei were seen in the posterior poles of Acvr1WT lenses. Staining of RNA in the fiber cell cytoplasm shows the posterior lens sutures. (B) A few scattered nuclei were evident near the posterior surface of Acvr1CKO lenses. (C) A small accumulation of nuclei was present at the posterior pole of Trp53CKO lenses. (D) A large mass of cells was present at the posterior poles of Acvr1;Trp53DCKO lenses. (E) Antibody to Ki67 showed that cells in the posterior subcapsular plaques of Trp53CKO lenses were not in the cell cycle. (F) Antibody to Ki67 showed that cells in the posterior subcapsular plaques of Acvr1;Trp53DCKO lenses were still in the cell cycle.
Cells in the plaques at the posterior of Acvr1;Trp53DCKO lenses have fiber-cell-specific characteristics
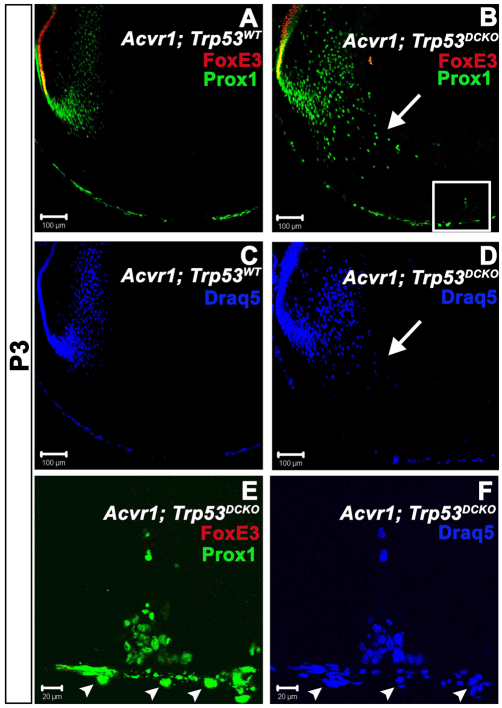
As shown above, cells that seemed to be epithelioid within the posterior plaques of Trp53CKO lenses were not proliferating and expressed markers of fiber cell terminal differentiation. Because cells within plaques of Acvr1;Trp53DCKO lenses continued to proliferate, we tested their state of differentiation by double staining with antibodies to FoxE3 and Prox1. At P3, wild-type and Acvr1;Trp53DCKO lenses displayed the expected staining pattern for each marker at their equators (Fig. 8A,B). Like Trp53CKO lenses, the bow regions of Acvr1;Trp53DCKO lenses had abnormal architecture, with nuclei scattered towards the anterior and posterior of the fiber mass, instead of in a tight arc (Fig. 8D, arrow). To more closely examine the cells at the posterior of the lens, we collected z-stacks using the confocal microscope (boxed area in Fig. 8B). Projection of this z-stack showed that all cells within the posterior masses in Acvr1;Trp53DCKO lenses were Prox1 positive and FoxE3 negative (Fig. 8E). Taken together, these data show that cells lacking Acvr1 and Trp53 acquire the molecular characteristics of fiber cells, while continuing to proliferate.
Fig. 8.
Cells in the posterior subcapsular plaques in Acvr1;Trp53DCKO lenses express the fiber-specific transcription factor Prox1. (A) In wild-type lenses at P3, the epithelial-specific transcription factor FoxE3 labels the nuclei of all anterior epithelial cells, but was lost in the transition zone when epithelial cells began to differentiate into fiber cells. The fiber-specific transcription factor Prox1 was first expressed in the transition zone and was detected in all differentiated fiber cells that retained nuclei. (B) FoxE3 and Prox1 had normal expression patterns in Acvr1;Trp53DCKO lenses. There was an overlap in the expression patterns for FoxE3 and Prox1 in the transition zone (yellow) in both Acvr1;Trp53WT (A) and Acvr1;Trp53DCKO (B) lenses. However, the bow region in Acvr1;Trp53DCKO lenses was disorganized, with Prox1-positive nuclei scattered in the posterior of the lens (arrow). Boxed region is shown in E and F. (C) Staining Acvr1;Trp53 lenses with DRAQ-5. (D) Staining Acvr1;Trp53DCKO lenses with DRAQ-5. Arrow indicates the disorganized bow region. (E,F) A projection of z-stacks from the boxed region in B. Cells making up the posterior subcapsular plaques in Acvr1;Trp53DCKO lenses at P4 were Prox1 positive and FoxE3 negative. The nuclei of cells of the tunica vasculosa lentis (TVL) were also stained for Prox1 (arrowheads). These could be distinguished from fiber cell nuclei because they lay on the opposite side of the lens capsule.
Adult Acvr1;Trp53DCKO lenses develop ocular ‘tumors’
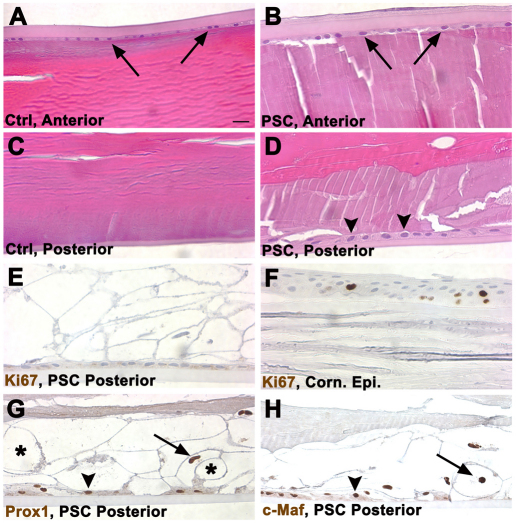
Because Acvr1 seemed to have the properties of a tumor suppressor in the lens, we collected 4-month-old wild-type, Trp53CKO and Acvr1;Trp53DCKO lenses to determine whether tumor-like structures formed as the lenses aged. As expected, Trp53CKO lenses had posterior subcapsular plaques not seen in wild-type lenses (Fig. 9A,B,D). Cells within the plaques were nucleated, swollen and vacuolated, with eosinophilic cytoplasm, resembling the ‘balloon’ cells typically seen in PSCs. Acvr1;Trp53DCKO lenses formed large growths at the posterior of the lens that filled the vitreous cavity between the lens and inner limiting membrane of the retina (Fig. 9C,E,I). These tumor-like masses were vascularized, as demonstrated by the abundant presence of erythrocytes (Fig. 9E, arrowheads). Cells within the masses of Acvr1;Trp53DCKO lenses were small and densely compacted, with basophilic cytoplasm. The cell mass was surrounded by layers of material that resembled fibrous connective tissue.
Fig. 9.
Adult Acvr1;Trp53DCKO lenses form vascularized, tumor-like structures at their posterior poles. 4-month-old Trp53CKO and Acvr1;Trp53DCKO lenses were sectioned and stained with hematoxylin and eosin (A–E) or Masson trichrome (F–I). (A) The posterior region of a wild-type lens and the adjacent retina. (B) Overview of the posterior half of a Trp53CKO lens. (C) Overview of the posterior of an Acvr1;Trp53DCKO lens. (D) Magnified image of the boxed area in B, showing a large posterior subcapsular-like plaque and normal separation between the lens and the ganglion cell layer (GCL) of the retina. (E) Magnified image of the boxed area in C, showing a vascularized (arrowheads) tumor-like structure (asterisk) at the posterior pole of the Acvr1;Trp53DCKO lens. (F) Section from the same Trp53CKO lens in B stained with Masson trichrome, which stains the lens capsule blue (arrowheads). (G) Section from the same Acvr1;Trp53DCKO lens in C. The original lens capsule is stained blue (arrowheads). However, towards the posterior of the lens the capsule seems to splay (arrow), forming a multilayered connective tissue. (H) The posterior capsule of the Trp53CKO lens was thin, but remained intact, despite the presence of the posterior subcapsular plaque. Arrowheads indicate the location of the posterior lens capsule. (I) In the posterior of the Acvr1;Trp53DCKO lens, the original lens capsule (arrowheads) was bordered by a multilayered connective tissue (arrows). The outer surface of this connective tissue separated the tumor-like growth (asterisk) from the GCL of the retina, encapsulating the ‘tumor’. Strands of connective tissue within the tumor also stained blue, suggesting that the collagen of the lens capsule had been ‘invaded’ by the tumor cells (arrow). Scale bars: 200 μm (A–C), 20 μm (D–I).
Masson trichrome, a stain for collagenous connective tissues, stained the thin capsule blue in Trp53CKO lenses (Fig. 9F,H, arrowheads). In Acvr1;Trp53DCKO lenses the lens capsule splayed out immediately posterior to the lens equator, forming a multilayered connective tissue with cells embedded in the matrix (Fig. 9G). The multilayered extension of the capsule extended around and within the tumor-like growths at the lens posterior pole (Fig. 9H). From these images, it was not possible to determine whether the capsule ruptured and reformed around the tumorous mass, or whether the tumor cells invaded the capsule and promoted invasion of the vascular system.
DISCUSSION
p53 promotes Acvr1-dependent apoptosis and contributes to cell cycle withdrawal during fiber cell differentiation
Deletion of Trp53 in the lens rescued cell death in lenses lacking Acvr1 and prevented a small percentage of fiber cells from withdrawing from the cell cycle. It has long been appreciated that p53 is responsible for much of the cell death that occurs when lens fiber cells fail to withdraw from the cell cycle (Morgenbesser et al., 1994; Pan and Griep, 1995; Fromm and Overbeek, 1997). Our observations further show that, along with BMP and fibroblast growth factor (FGF) signaling (Faber et al., 2001; Faber et al., 2002; Garcia et al., 2005; Zhao et al., 2008), p53 promotes fiber cell terminal differentiation. This observation is consistent with previous studies that showed that expression of wild-type p53 promotes terminal differentiation in a variety of cell culture models (Almog and Rotter, 1997).
Measurements in our laboratory found no BrdU-positive fiber cells at P3 when the growth factor receptor gene Tgfbr2 was deleted using the LeCre transgene (see Methods), suggesting that deletion of Trp53, not simply LeCre expression, was the cause of the excess proliferation (Claudia M. Garcia, unpublished results). A previous study found that deletion of Trp53 led to the accumulation of proliferating cells in the lens fiber compartment (Fromm and Overbeek, 1997). This occurred in lenses overexpressing a truncated version of the SV40 large T antigen, which inactivates Rb, thereby promoting excessive proliferation. These authors reported that they did not detect BrdU incorporation in fiber cells lacking Trp53. This might be due to the relatively small number of BrdU-positive fiber cells in adult Trp53-null lenses. We showed that, after E12.5, the effects of Acvr1 on lens cell proliferation are mediated by Smad proteins (Rajagopal et al., 2008). p53 interacts with Smad proteins to enhance transforming growth factor-β (TGFβ) signaling, raising the possibility that p53-Smad interactions control the withdrawal of fiber cells from the cell cycle (Cordenonsi et al., 2007; Atfi and Baron, 2008; Wilkinson et al., 2008). Further studies are needed to identify the molecular links between p53, the BMP and FGF signaling pathways, and cell cycle withdrawal during fiber cell terminal differentiation.
The cyclin-dependent kinase inhibitor p21CIP1/WAF1 is a well-known transcriptional target of p53 and suppresses cell proliferation after oncogene expression. Expression of p21CIP1/WAF1 increases in the lens during the excessive proliferation induced by expression of the Rb-binding fragment of the large T antigen in lens fiber cells, suggesting that p53 is activated under these conditions (Fromm and Overbeek, 1997). Germline deletion of Cdkn1a, the gene encoding p21CIP1/WAF1, did not impair normal development or cell cycle withdrawal during terminal differentiation (Brugarolas et al., 1995; Deng et al., 1995). However, the levels of p21CIP1/WAF1 protein and transcripts were too low to be detected in the normal lens, suggesting that p53-stimulated p21CIP1/WAF1 expression does not contribute to cell cycle withdrawal in lens fiber cells.
Apoptosis is activated when prospective fiber cells fail to withdraw from the cell cycle (Morgenbesser et al., 1994; Pan and Griep, 1995; Zhang et al., 1998; Wigle et al., 1999; Rajagopal et al., 2008; Zhao et al., 2008; Wiley et al., 2010). Conversely, deletion of Maf or Sox1, which prevents fiber cell differentiation but does not lead to increased proliferation in the fiber cell compartment, was not associated with increased apoptosis (Nishiguchi et al., 1998; Ring et al., 2000). When Rb is deleted or inactivated, p53-dependent cell death is mediated by excessive E2F activation, leading to ARF-dependent inhibition of Mdm2, a pathway not associated with p53 phosphorylation (Liu and Zacksenhaus, 2000). We found that, in lenses in which Rb is functional, aberrant entry of fiber cells into the cell cycle leads to phosphorylation and activation of p53 by an unknown mechanism. Proliferation-induced cell death in prospective fiber cells is not obviously associated with DNA damage, excessive growth factor signaling, or any of the other pathways known to lead to p53 stabilization (Levine et al., 2006). Therefore, apoptosis in cells escaping from terminal differentiation might be controlled by an unknown mechanism of p53 activation. Cell death in this setting might be relevant to cancer, because cells that fail to withdraw from the cell cycle during their terminal differentiation might later progress to tumor cells if not eliminated by p53.
PSC formation and the role of p53
PSCs are a type of age-related cataract that also occur commonly in diabetics and in patients treated with immunosuppressive steroids or therapeutic radiation (Worgul et al., 1976; Greiner and Chylack, 1979; Beebe, 2003). Although some of the external causes of PSC formation are known from clinical and epidemiologic studies, the cellular mechanisms responsible for PSC formation are less well understood.
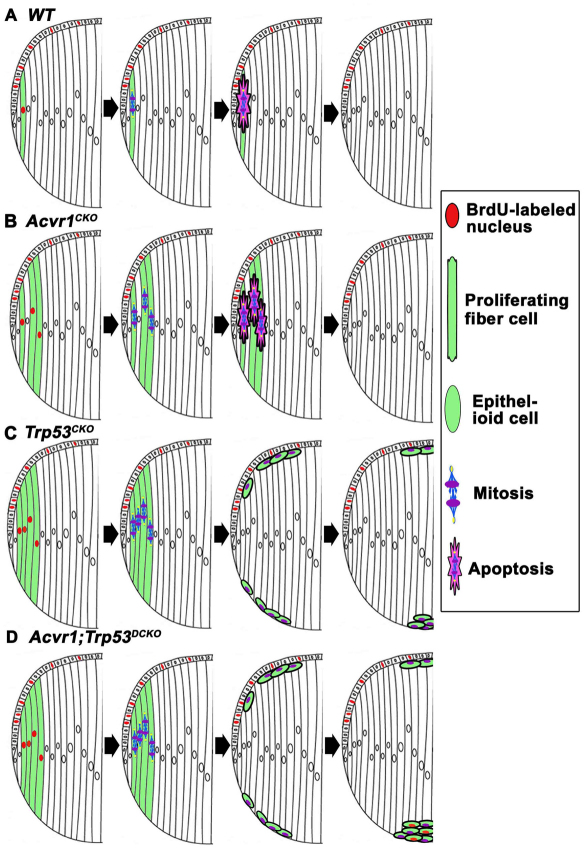
We can imagine PSCs arising by two general mechanisms. Epithelial cells might fail to differentiate into fiber cells at the lens equator. The resulting ‘epithelioid’ cells migrate along the inner surface of the posterior capsule, accumulate near the posterior pole of the lens and scatter light, resulting in cataract formation (Streeten and Eshaghian, 1978; Eshaghian and Streeten, 1980). Alternatively, a small fraction of the elongating fiber cells might fail to exit the cell cycle, undergo mitosis, and escape ‘execution’ by p53. Because they are long and thin, mitosis would occur perpendicularly to the long axis of the cells, dividing them into basal daughter cells, which maintain contact with the posterior capsule, and anterior daughter cells that retain their apical adherens junctions, but have no contact with the posterior capsule (Fig. 10). The posterior daughter cells, having lost their apical adherens junctions, would round up on the posterior capsule and be carried by adjacent, elongating fiber cells to the posterior sutures. At the sutures, the aberrant fiber cells would remain attached to the capsule, whereas normal fiber cells would separate from the capsule and be incorporated into the fiber mass. Over time, as an increased number of aberrant cells were ‘deposited’ at the sutures, a cataract would form. Our data show that the aberrant cells that accumulated in posterior subcapsular plaques in Trp53CKO, Acvr1;Trp53DCKO and human lenses had the characteristics of fiber cells (Prox1- and/or Maf-positive). These results suggest that the epithelioid cells observed in the mouse and in human lenses in this study did not arise from the failure of lens epithelial cells to undergo fiber cell differentiation. Instead, they support the view that failure to exit the cell cycle led to the accumulation of misshapen fiber-derived cells at the posterior pole of the lens.
Fig. 10.
A diagram illustrating the potential roles of p53 and Acvr1 in the formation of PSCs. (A) In wild-type lenses, the occasional fiber cells that fail to withdraw from the cell cycle are eliminated by apoptosis in a largely p53-dependent manner. (B) In Acvr1CKO lenses, a few fiber cells fail to exit from the cell cycle. These abnormal cells are eliminated by p53-dependent and p53-independent mechanisms. (C) In Trp53CKO lenses, an increased percentage of fiber cells continue to proliferate. Owing to the absence of p53, some of these cells are not removed by apoptosis. If these cells divide, one of the daughter cells will remain attached to its neighbors at the anterior surface of the fiber mass and the other will adhere to the posterior capsule. Cells remaining adherent to the capsule round up and move to the posterior pole, where they form a plaque of epithelioid cells. Acvr1 signaling prevents these cells from proliferating. Cells that accumulate beneath the lens epithelium can degenerate or be phagocytosed by epithelial cells. (D) In Acvr1;Trp53DCKO lenses, a substantial percentage of fiber cells proliferate, do not get eliminated by p53-mediated apoptosis and accumulate at the posterior pole of the lens. Because Acvr1 is absent, these cells continue to proliferate, leading to the formation of a large, subcapsular mass that might later form a vascularized tumor.
The lens exists in a hypoxic environment and hypoxia inhibits the ability of p53 to kill damaged cells (Achison and Hupp, 2003; Li et al., 2004; Holekamp et al., 2006; Shui et al., 2006). This suggests a mechanism by which fiber cells that aberrantly enter the cell cycle escape from p53-mediated apoptosis. Enhancing p53-mediated apoptosis might protect against the formation of PSCs.
In Trp53CKO and Acvr1;Trp53DCKO lenses, a subset of fiber cells failed to withdraw from the cell cycle and posterior subcapsular plaques formed that were similar to those observed in human PSCs. By contrast, lenses lacking Acvr1 showed a significant increase in BrdU-positive cortical fiber cells, aberrant organization of the lens bow and scattered nuclei near the posterior pole (Rajagopal et al., 2008), but subcapsular plaques did not form. This observation, combined with the known function of p53 in triggering apoptosis, led us to propose the model illustrated in Fig. 10.
In normal development, we have consistently detected a small number of phospho-histone-H3-positive (data not shown) and BrdU-positive nuclei in the fiber cell compartment of wild-type lenses (Garcia et al., 2005; Rajagopal et al., 2008). On the basis of previous studies and the results reported here, p53 assures the elimination of these aberrant fiber cells (Fig. 10A). Our data show that p53 also eliminates the increased number of fiber cells that proliferate in Acvr1CKO lenses (Fig. 10B). However, if p53 is absent or non-functional, the progeny of these mitotic fiber cells will survive. Some of the daughter cells will localize to the posterior pole, leading to the formation of PSCs (Fig. 10C). Some of the progeny will end up at the apical ends of the fiber cells, beneath the lens epithelium. Here, they might undergo anoikis by p53-independent apoptosis [because they are no longer in contact with their basal lamina (the lens capsule)], or be phagocytosed by epithelial cells (Fig. 10C). Finally, in the case of the Acvr1;Trp53DCKO lenses, more fiber cells will divide and there will be no p53 to eliminate the resulting aberrant fiber cells. The surviving cells will accumulate at the posterior pole of the lens. Because these cells are deficient in BMP signaling and do not respond to the signals promoting cell cycle withdrawal, they continue to proliferate, forming tumor-like masses at the posterior pole.
Therapeutic irradiation of the head or eye often leads to the formation of PSCs. X-ray treatment leads to excessive and aberrant cell proliferation at the lens equator (Von Sallmann et al., 1953; Von Sallmann et al., 1955) and blocking cell proliferation prevents X-ray-induced cataracts in vivo (Hayden et al., 1980). These observations raise the possibility that aberrant lens cell proliferation after X-irradiation leads to the formation of PSCs.
The use of systemic or ocular steroids also causes PSC formation. The past several years have seen a substantial increase in the use of intraocular steroid injections to treat patients with macular edema secondary to diabetic retinopathy or the neovascular form of age-related macular degeneration, increasing the incidence of PSCs (Gillies et al., 2005; Jonas et al., 2005; Jonas, 2006; Thompson, 2006). It will be important to know whether treatments that induce PSC formation, like X-irradiation or steroid therapy, prevent fiber cells from withdrawing from the cell cycle, inhibit the function of p53, or both. If p53 normally prevents the formation of PSCs, PSC formation after X-irradiation or steroid exposure might be inhibited by methods that stabilize and activate p53. Such therapies are under investigation to promote apoptosis in tumors (Chen et al., 2010). The use of these approaches to enhance p53 activity in eyes that receive therapeutic steroids or X-rays might inhibit PSC formation, preserving vision and minimizing surgery.
Deletion of Trp53 reveals that Acvr1 behaves as a tumor suppressor in the lens
Cells in the subcapsular plaques in lenses lacking Trp53 were not stained with an antibody to Ki67, indicating that they were not actively cycling. By contrast, many of the cells in the larger subcapsular masses in Acvr1;Trp53DCKO lenses were Ki67 positive. These observations suggest that, in cells that escape p53-mediated apoptosis, Acvr1 signaling suppresses proliferation. Concurrent loss of Acvr1 releases these cells from the ‘brake’ that Acvr1 signaling provides, leading to the formation of the large, proliferating, tumor-like masses seen in the double knockout lenses. As these lenses aged, the masses continued to grow in size, leading to the formation of vascularized tumors. Tumor formation in the lens has been described previously, but only in response to the overexpression of a viral oncoprotein in transgenic animals: in these animals the entire lens was transformed into a tumor-like structure that spread from the ruptured lens capsule (Mahon et al., 1987; Nakamura et al., 1989). In Acvr1;Trp53DCKO lenses, the tumor seemed to be derived from the small proportion of fiber cells that failed to withdraw from the cell cycle, could not be arrested by Acvr1 signaling and were protected from apoptosis by the loss of p53.
The BMP receptor BMPR1A is a well-characterized tumor suppressor. Inheritance of one non-functional allele of this protein often leads to the formation of hereditary juvenile colon polyps (Howe et al., 2001; Zhou et al., 2001). Hereditary cancer syndromes resulting from ACVR1 loss-of-function mutations are less well known. However, a recent study reported that 4% of colon tumors showed loss of ACVR1 (Jung et al., 2009). Müllerian inhibiting substance, which signals through Acvr1, is reported to be a gonadal tumor suppressor (Belville et al., 2005; Zhan et al., 2006). Together with our results, these findings suggest that Acvr1 functions as a tumor suppressor in tissues in which it transduces growth-inhibitory signals.
METHODS
Mice and genotyping
All animals were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and with the approval of the Animal Studies Committee of the Washington University School of Medicine. Mice expressing Cre recombinase under the control of the Pax6 P0 promoter/enhancer (LeCre) were described previously (Ashery-Padan et al., 2000; Rajagopal et al., 2008). Mice that were homozygous floxed, one of which was Cre positive, were mated to generate 50% Cre-positive [conditional knockout (CKO)] and 50% Cre-negative [wild type (WT)] offspring. Cre-positive animals were always mated to Cre-negative animals, assuring that Cre-positive offspring inherited only one copy of the LeCre transgene (example: Trp53fx/fx; LeCre−/− × Trp53fx/fx; LeCre+/−). Mouse genomic DNA from toe or embryonic tail tissue was extracted using the HotSHOT method (Truett et al., 2000). Amplification conditions were selected according to the Universal PCR protocol (Stratman et al., 2003). Primers for genotyping mice carrying the Cre transgene or the floxed alleles used in this study (Acvr1fx(exon7) and Trp53fx(exons2-10)) were described previously (Jonkers et al., 2001; Dudas et al., 2004; Rajagopal et al., 2008).
Histology, immunohistochemistry and immunofluorescence
Embryos or P3 lenses were fixed in 10% neutral-buffered formalin overnight at room temperature (RT). For standard immunohistochemical and immunofluorescent analysis, samples were processed and embedded in paraffin and sectioned at 4 μm. For morphological studies, sections were stained with hematoxylin and eosin (Surgipath, Richmond, IL) or with Masson trichrome to label connective tissue. For antibody staining, sections were deparaffinized and rehydrated. Endogenous peroxidase activity was inactivated with 3% H2O2 in methanol for 30 minutes at RT for those samples that would be treated for horseradish peroxidase (HRP). Epitope retrieval was performed in 0.01 M citrate buffer (pH 6.0) either at 100°C for 20 minutes using a water bath or in a Decloaking Chamber (Biocare Medical, Walnut Creek, CA) for 3 minutes. Slides were then incubated in blocking solution containing 20% inactivated normal donkey serum for 30 minutes at RT followed by incubation in primary antibodies overnight at 4°C, then for 1 hour at RT either with Alexa-Fluor-488-, 543- or 633-conjugated anti-mouse or anti-rabbit secondary antibodies (Invitrogen, Carlsbad, CA) at a dilution of 1:1000 or with biotinylated secondary antibodies (Vector Laboratories, Burlingame, CA). Slides incubated with biotinylated secondary antibodies were treated with the ABC-peroxidase reagent from the Vectastain Elite ABC kit (Vector Laboratories) followed by treatment with diaminobenzidine (DAB) (Sigma, St Louis, MO) and H2O2, and counterstaining with hematoxylin (Surgipath).
For confocal immunofluorescence, P3 lenses were embedded in 4% agarose and allowed to set at 4°C. Thick sections (120 μm) were cut using a vibrating tissue slicer (Electron Microscopy Sciences, Hatfield, PA). Lens sections were blocked in 5% normal goat serum, 0.5% Triton X-100 and 0.03% sodium azide for 1 hour at RT and incubated with primary antibodies overnight at 4°C. After rinsing, sections were incubated with fluorescent-labeled secondary antibodies for 1 hour at RT and counterstained with DRAQ-5 (Biostatus, Shepshed, Leicestershire, UK), a vital, fluorescent DNA dye. Sections were mounted in VECTASHIELD (Vector Laboratories) mixed with PBS at a 1:1 ratio and mounted on glass coverslips.
The primary antibodies used in this study were: rabbit anti-phosphorylated (Ser 15)-p53 (#sc-101762, Santa Cruz Biotechnology, Santa Cruz, CA) at 1:200, mouse anti-human Ki67 (#556003, BD Pharmingen, San Diego, CA) at 1:400, rabbit anti-human p57KIP2 (#8298, Santa Cruz) at 1:1000, rabbit anti-Maf (#sc-7866, Santa Cruz) at 1:500, rabbit anti-MIP (kindly provided by Alan Shiels, Washington University, St Louis, MO) at 1:1000, rabbit anti-FoxE3 (a gift from Peter Carlsson, Goteborg University, Goteborg, Sweden) at 1:1000, and mouse anti-Prox1 (#MAB5652, Chemicon International, Temecula, CA) at 1:1000.
P22 whole lenses were fixed as described above and permeabilized in 0.5% Triton X-100 and 0.03% sodium azide in 1× PBS for 1 hour at RT. Whole lenses were then stained with either TOTO-1 (1:10,000; Invitrogen) or DRAQ-5 for 1 hour at RT.
Human lenses
Paraffin sections from formalin-fixed human eyes were provided by the Pathology Service at Barnes-Jewish Hospital. One eye was removed owing to severe diabetic retinopathy and neovascular glaucoma. This patient also had PSCs. The other lens analyzed was from an eye with no reported lens pathology. The sections were stained with hematoxylin and eosin or subjected to epitope retrieval and treated with the same antibodies used for the mouse tissues (against Ki67, Prox1, or Maf), which were detected using the peroxidase-anti-peroxidase method (Vector Laboratories).
BrdU and TUNEL staining
Pregnant female mice were injected with 50 mg/kg of a mixture of 10 mM BrdU (Roche, Indianapolis, IN) and 1 mM 5-fluoro-5′-deoxyuridine (Sigma), and were sacrificed after 1 hour. A monoclonal anti-BrdU antibody (Dako, Carpinteria, CA) was used at 1:250 with a Vectastain Elite Mouse IgG ABC kit. Sections were counterstained with hematoxylin. Terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate nick end-labeling (TUNEL) was performed with an Apoptag kit (Chemicon). The deparaffinized slides were treated with 3% H2O2 in methanol for 30 minutes, followed by proteinase K treatment (20 μg/ml) for 15 minutes. Slides were incubated with TdT enzyme in equilibration buffer for 1 hour at 37°C. The reaction was terminated with wash buffer provided by the manufacturer for 10 minutes at RT. Anti-digoxigenin-peroxidase conjugate was added for 30 minutes at RT, followed by DAB and H202 treatment. Slides were counterstained with hematoxylin.
TRANSLATIONAL IMPACT.
Clinical issue
During the development of an eye, a layer of epithelial cells on the anterior side of the lens populates and then maintains the internal lens tissue by regulated terminal differentiation into elongated lens fiber cells, which occupy the bulk of the central space, stretching from the anterior to the posterior of the lens. Interruption of normal lens cell terminal differentiation is hypothesized to be the cause of posterior subcapsular cataracts (PSCs), a type of age-related cataract that is especially common in diabetics and in patients receiving immunosuppressive steroids or therapeutic radiation. Understanding how PSC formation is regulated could lead to therapies to prevent this disease, especially in cases in which the timing and nature of the cataractogenic insult are known.
Results
This study explores the role of the tumor suppressor protein p53 in the generation of PSCs. Using a mouse model in which p53 can be conditionally deleted in the lens, the authors show that loss of p53 causes increased cell division in the normally quiescent fiber cells, and the formation of PSCs. p53-negative cataractous cells do not proliferate and are morphologically and biochemically similar to those in a human PSC. The results suggest that p53 normally functions in the lens to prevent the formation of PSCs by triggering the death of aberrantly proliferating fiber cells. These data can also be linked to previous studies showing that mouse lenses lacking the bone morphogenetic protein receptor Acvr1 have increased epithelial and cortical fiber cell death. The authors demonstrate that this death requires the presence of the p53 protein. Mice lacking both Acvr1 and p53 in their lenses have far less apoptosis than Acvr1 mutant animals and, strikingly, their fiber cells do not withdraw from cycle as normal, but continue to proliferate, eventually forming vascularized tumors at the posterior of the lens. Therefore, in the absence of p53, Acvr1 can act as a tumor suppressor.
Implications and future directions
This study shows that p53 is required for proper lens development and is likely to play a role in preventing PSC formation. Therefore, the PSCs that occur frequently in diabetics, and after radiation or steroid therapy, might be prevented by pharmacologically increasing p53 activity in the lens during the period of cataractogenic insult. The study also identified a novel role for Acvr1 as a tumor suppressor in the lens. ACVR1 is mutated in a subset of colon tumors, suggesting that it might act as a tumor suppressor in other tissues. Future studies of the function of ACVR1 as a tumor suppressor could lead to enhanced diagnosis and treatment of some cancers.
Imaging
Brightfield and fluorescent images of lens sections were taken using Olympus BX60 (Olympus, Melville, NY) and Olympus BX51 microscopes, respectively, and images collected with a Spot camera (Diagnostic Instruments, Sterling Heights, MI). Lens thick sections and whole lenses were visualized using a Zeiss 510 confocal microscope (Carl Zeiss, Thornwood, NY).
Statistical tests
Student’s t-test was employed to determine statistical significance. For groups with multiple samples, the Bonferroni correction was applied. Statistical tests were performed in Excel or using GraphPad InStat, Version 3.05 (GraphPad Software, San Diego, CA). Error bars are ± s.e.m.
Acknowledgments
The authors are indebted to Ruth Ashery-Padan for providing the LeCre mice, Peter Carlsson for the anti-FoxE3 antibody, Alan Shiels for the anti-MIP antibody, Belinda McMahan and Jean Jones for their assistance with histology and immunohistochemistry, Chenghua Wu for genotyping, and George Harocopos for providing the tissue sections from human eyes. This work was supported by NIH grant EY04853, Department of Ophthalmology and Visual Sciences Core Grant EY002687, an unrestricted grant from Research to Prevent Blindness, and NHLBI 2 T32 HL007873, a Ruth L. Kirschstein National Research Service Award to L.A.W. from the Developmental Cardiology and Pulmonary Training Program at Washington University.
Footnotes
COMPETING INTERESTS
The authors declare that they do not have any competing or financial interests.
AUTHOR CONTRIBUTIONS
L.A.W., R.R. and D.C.B. conceived and designed the experiments, and wrote the paper. L.A.W., R.R. and L.K.D. performed the experiments and analyzed the data.
REFERENCES
- Achison M., Hupp T. R. (2003). Hypoxia attenuates the p53 response to cellular damage. Oncogene 22, 3431–3440 [DOI] [PubMed] [Google Scholar]
- Almog N., Rotter V. (1997). Involvement of p53 in cell differentiation and development. Biochim. Biophys. Acta. 1333, F1–F27 [DOI] [PubMed] [Google Scholar]
- Ashery-Padan R., Marquardt T., Zhou X., Gruss P. (2000). Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 14, 2701–2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atfi A., Baron R. (2008). p53 brings a new twist to the Smad signaling network. Sci. Signal. 1, pe33. [DOI] [PubMed] [Google Scholar]
- Bassnett S., Mataic D. (1997). Chromatin degradation in differentiating fiber cells of the eye lens. J. Cell. Biol. 137, 37–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe D. (2003). Lens. In Adler’s Physiology of the Eye (ed. Kaufman P. L., Alm A.), pp. 117–158 St Louis, MO: Mosby [Google Scholar]
- Belville C., Jamin S. P., Picard J. Y., Josso N., di Clemente N. (2005). Role of type I receptors for anti-Mullerian hormone in the SMAT-1 Sertoli cell line. Oncogene 24, 4984–4992 [DOI] [PubMed] [Google Scholar]
- Brian G., Taylor H. (2001). Cataract blindness – challenges for the 21st century. Bull. World Health Organ. 79, 249–256 [PMC free article] [PubMed] [Google Scholar]
- Broekhuyse R. M., Kuhlmann E. D., Stols A. L. (1976). Lens membranes II. Isolation and characterization of the main intrinsic polypeptide (MIP) of bovine lens fiber membranes. Exp. Eye Res. 23, 365–371 [DOI] [PubMed] [Google Scholar]
- Brugarolas J., Chandrasekaran C., Gordon J. I., Beach D., Jacks T., Hannon G. J. (1995). Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature 377, 552–557 [DOI] [PubMed] [Google Scholar]
- Chen F., Wang W., El-Deiry W. S. (2010). Current strategies to target p53 in cancer. Biochem. Pharmacol. 80, 724–730 [DOI] [PubMed] [Google Scholar]
- Cordenonsi M., Montagner M., Adorno M., Zacchigna L., Martello G., Mamidi A., Soligo S., Dupont S., Piccolo S. (2007). Integration of TGF-beta and Ras/MAPK signaling through p53 phosphorylation. Science 315, 840–843 [DOI] [PubMed] [Google Scholar]
- Deng C., Zhang P., Harper J. W., Elledge S. J., Leder P. (1995). Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 82, 675–684 [DOI] [PubMed] [Google Scholar]
- Dudas M., Sridurongrit S., Nagy A., Okazaki K., Kaartinen V. (2004). Craniofacial defects in mice lacking BMP type I receptor Alk2 in neural crest cells. Mech. Dev. 121, 173–182 [DOI] [PubMed] [Google Scholar]
- Eshaghian J., Streeten B. W. (1980). Human posterior subcapsular cataract. An ultrastructural study of the posteriorly migrating cells. Arch. Ophthalmol. 98, 134–143 [DOI] [PubMed] [Google Scholar]
- Faber S. C., Dimanlig P., Makarenkova H. P., Shirke S., Ko K., Lang R. A. (2001). Fgf receptor signaling plays a role in lens induction. Development 128, 4425–4438 [DOI] [PubMed] [Google Scholar]
- Faber S. C., Robinson M. L., Makarenkova H. P., Lang R. A. (2002). Bmp signaling is required for development of primary lens fiber cells. Development 129, 3727–3737 [DOI] [PubMed] [Google Scholar]
- Fromm L., Overbeek P. A. (1997). Inhibition of cell death by lens-specific overexpression of bcl-2 in transgenic mice. Dev. Genet. 20, 276–287 [DOI] [PubMed] [Google Scholar]
- Garcia C. M., Yu K., Zhao H., Ashery-Padan R., Ornitz D. M., Robinson M. L., Beebe D. C. (2005). Signaling through FGF receptor-2 is required for lens cell survival and for withdrawal from the cell cycle during lens fiber cell differentiation. Dev. Dyn. 233, 516–527 [DOI] [PubMed] [Google Scholar]
- Gillies M. C., Kuzniarz M., Craig J., Ball M., Luo W., Simpson J. M. (2005). Intravitreal triamcinolone-induced elevated intraocular pressure is associated with the development of posterior subcapsular cataract. Ophthalmology 112, 139–143 [DOI] [PubMed] [Google Scholar]
- Greiner J. V., Chylack L. T., Jr (1979). Posterior subcapsular cataracts: histopathologic study of steroid-associated cataracts. Arch. Ophthalmol. 97, 135–144 [DOI] [PubMed] [Google Scholar]
- Griep A. E., Herber R., Jeon S., Lohse J. K., Dubielzig R. R., Lambert P. F. (1993). Tumorigenicity by human papillomavirus type 16 E6 and E7 in transgenic mice correlates with alterations in epithelial cell growth and differentiation. J. Virol. 67, 1373–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt Y., Maya R., Kazaz A., Oren M. (1997). Mdm2 promotes the rapid degradation of p53. Nature 387, 296–299 [DOI] [PubMed] [Google Scholar]
- Hayden J. H., Rothstein H., Worgul B. V., Merriam G. R., Jr (1980). Hypophysectomy exerts a radioprotective effect on frog lens. Experientia 36, 116–118 [DOI] [PubMed] [Google Scholar]
- Holekamp N. M., Shui Y.-B, Beebe D. (2006). Lower intraocular oxygen tension in diabetic patients: possible contribution to decreased incidence of nuclear sclerotic cataract. Am J. Ophthalmol. 141, 1027–1032 [DOI] [PubMed] [Google Scholar]
- Howe J. R., Bair J. L., Sayed M. G., Anderson M. E., Mitros F. A., Petersen G. M., Velculescu V. E., Traverso G., Vogelstein B. (2001). Germline mutations of the gene encoding bone morphogenetic protein receptor 1A in juvenile polyposis. Nat. Genet. 28, 184–187 [DOI] [PubMed] [Google Scholar]
- Jonas J. B. (2006). Intravitreal triamcinolone acetonide: a change in a paradigm. Ophthalmic Res. 38, 218–245 [DOI] [PubMed] [Google Scholar]
- Jonas J. B., Degenring R., Vossmerbauemer U., Kamppeter B. (2005). Frequency of cataract surgery after intravitreal injection of high-dosage triamcinolone acetonide. Eur. J. Ophthalmol. 15, 462–464 [DOI] [PubMed] [Google Scholar]
- Jonkers J., Meuwissen R., van der Gulden H., Peterse H., van der Valk M., Berns A. (2001). Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat. Genet. 29, 418–425 [DOI] [PubMed] [Google Scholar]
- Jung B., Gomez J., Chau E., Cabral J., Lee J. K., Anselm A., Slowik P., Ream-Robinson D., Messer K., Sporn J., et al. (2009). Activin signaling in microsatellite stable colon cancers is disrupted by a combination of genetic and epigenetic mechanisms. PloS ONE 4, e8308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J., Hu W., Feng Z. (2006). The P53 pathway: what questions remain to be explored? Cell Death Differ. 13, 1027–1036 [DOI] [PubMed] [Google Scholar]
- Li J., Zhang X., Sejas D. P., Bagby G. C., Pang Q. (2004). Hypoxia-induced nucleophosmin protects cell death through inhibition of p53. J. Biol. Chem. 279, 41275–41279 [DOI] [PubMed] [Google Scholar]
- Liu Y., Zacksenhaus E. (2000). E2F1 mediates ectopic proliferation and stage-specific p53-dependent apoptosis but not aberrant differentiation in the ocular lens of Rb deficient fetuses. Oncogene 19, 6065–6073 [DOI] [PubMed] [Google Scholar]
- Mahon K. A., Chepelinsky A. B., Khillan J. S., Overbeek P. A., Piatigorsky J., Westphal H. (1987). Oncogenesis of the lens in transgenic mice. Science 235, 1622–1628 [DOI] [PubMed] [Google Scholar]
- Morgenbesser S. D., Williams B. O., Jacks T., DePinho R. A. (1994). p53-dependent apoptosis produced by Rb-deficiency in the developing mouse lens. Nature 371, 72–74 [DOI] [PubMed] [Google Scholar]
- Nakamura T., Mahon K. A., Miskin R., Dey A., Kuwabara T., Westphal H. (1989). Differentiation and oncogenesis: phenotypically distinct lens tumors in transgenic mice. New Biol. 1, 193–204 [PubMed] [Google Scholar]
- Nishiguchi S., Wood H., Kondoh H., Lovell-Badge R., Episkopou V. (1998). Sox1 directly regulates the gamma-crystallin genes and is essential for lens development in mice. Genes Dev. 12, 776–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H., Griep A. E. (1995). Temporally distinct patterns of p53-dependent and p53-independent apoptosis during mouse lens development. Genes Dev. 9, 2157–2169 [DOI] [PubMed] [Google Scholar]
- Rajagopal R., Dattilo L., Kaartinen V., Deng C., Umans L., Zwijsen A., Roberts A., Bottinger E., Beebe D. C. (2008). The functions of the type I BMP receptor, Acvr1 (Alk2), in lens development: cell proliferation, terminal differentiation and survival. Invest. Ophthalmol. Vis. Sci. 49, 4953–4960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring B. Z., Cordes S. P., Overbeek P. A., Barsh G. S. (2000). Regulation of mouse lens fiber cell development and differentiation by the Maf gene. Development 127, 307–317 [DOI] [PubMed] [Google Scholar]
- Shieh S. Y., Ikeda M., Taya Y., Prives C. (1997). DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell 91, 325–334 [DOI] [PubMed] [Google Scholar]
- Shui Y.-B, Fu J.-J, Garcia C., Dattilo L. K., Rajagopal R., McMillan S., Mak G., Holekamp N. M., Lewis A., Beebe D. C. (2006). Oxygen distribution in the rabbit eye and oxygen consumption by the lens. Invest. Ophthalmol. Vis. Sci. 47, 1571–1580 [DOI] [PubMed] [Google Scholar]
- Stratman J. L., Barnes W. M., Simon T. C. (2003). Universal PCR genotyping assay that achieves single copy sensitivity with any primer pair. Transgenic Res. 12, 521–522 [DOI] [PubMed] [Google Scholar]
- Streeten B. W., Eshaghian J. (1978). Human posterior subcapsular cataract. A gross and flat preparation study. Arch. Ophthalmol. 96, 1653–1658 [DOI] [PubMed] [Google Scholar]
- Thompson J. T. (2006). Cataract formation and other complications of intravitreal triamcinolone for macular edema. Am J. Ophthalmol. 141, 629–637 [DOI] [PubMed] [Google Scholar]
- Truett G. E., Heeger P., Mynatt R. L., Truett A. A., Walker J. A., Warman M. L. (2000). Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). Biotechniques 29, 52–54 [DOI] [PubMed] [Google Scholar]
- Von Sallmann L., Munoz C. M., Drungis A. (1953). Effects of beta irradiation on the rabbit lens. A. M. A. Arch. Ophthalmol. 50, 727–736 [DOI] [PubMed] [Google Scholar]
- Von Sallmann L., Tobias C. A., Anger H. O., Welch C., Kimura S. F., Munoz C. M., Drungis A. (1955). Effects of high-energy particles, X-rays, and aging on lens epithelium. A. M. A. Arch. Ophthalmol. 54, 489–514 [DOI] [PubMed] [Google Scholar]
- West S. (2007). Epidemiology of cataract: accomplishments over 25 years and future directions. Ophthalmic Epidemiol. 14, 173–178 [DOI] [PubMed] [Google Scholar]
- Wigle J. T., Chowdhury K., Gruss P., Oliver G. (1999). Prox1 function is crucial for mouse lens-fibre elongation. Nat. Genet. 21, 318–322 [DOI] [PubMed] [Google Scholar]
- Wiley L. A., Dattilo L. K., Kang K. B., Giovannini M., Beebe D. C. (2010). The tumor suppressor merlin is required for cell cycle exit, terminal differentiation, and cell polarity in the developing murine lens. Invest. Ophthalmol. Vis. Sci. 51, 3611–3618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D. S., Tsai W. W., Schumacher M. A., Barton M. C. (2008). Chromatin-bound p53 anchors activated Smads and the mSin3A corepressor to confer transforming-growth-factor-beta-mediated transcription repression. Mol. Cell Biol. 28, 1988–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worgul B. V., Merriam G. R., Szechter A., Srinivasan D. (1976). Lens epithelium and radiation cataract. I. Preliminary studies. Arch. Ophthalmol. 94, 996–999 [DOI] [PubMed] [Google Scholar]
- Zhan Y., Fujino A., MacLaughlin D. T., Manganaro T. F., Szotek P. P., Arango N. A., Teixeira J., Donahoe P. K. (2006). Mullerian inhibiting substance regulates its receptor/SMAD signaling and causes mesenchymal transition of the coelomic epithelial cells early in Mullerian duct regression. Development 133, 2359–2369 [DOI] [PubMed] [Google Scholar]
- Zhang P., Wong C., DePinho R. A., Harper J. W., Elledge S. J. (1998). Cooperation between the Cdk inhibitors p27(KIP1) and p57(KIP2) in the control of tissue growth and development. Genes Dev. 12, 3162–3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Yang T., Madakashira B. P., Thiels C. A., Bechtle C. A., Garcia C. M., Zhang H., Yu K., Ornitz D. M., Beebe D. C., et al. (2008). Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev. Biol. 318, 276–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X. P., Woodford-Richens K., Lehtonen R., Kurose K., Aldred M., Hampel H., Launonen V., Virta S., Pilarski R., Salovaara R., et al. (2001). Germline mutations in BMPR1A/ALK3 cause a subset of cases of juvenile polyposis syndrome and of Cowden and Bannayan-Riley-Ruvalcaba syndromes. Am J. Hum. Genet. 69, 704–711 [DOI] [PMC free article] [PubMed] [Google Scholar]