Abstract
Prior clinical findings have indicated a potential lack of naltrexone efficacy among African Americans with alcohol dependence. However, no definitive conclusions have been drawn due to the relatively small numbers of African Americans in most alcohol treatment trials. The purpose of this study was to examine alcohol and naltrexone effects on healthy African American individuals in a laboratory environment. Non-alcohol dependent social drinking adults of African descent (n = 43) were recruited for participation. After consenting and completing the baseline assessment, they participated in four separate alcohol challenge sessions each separated by at least 10 days. During each of the sessions, subjects were administered alcohol or sham drinks, after pretreatment with either naltrexone (50mg/day) or placebo in a double-blind fashion. The order of the four sessions was randomly assigned. During each session, breath alcohol levels and subjective responses were measured. Results indicate an alcohol effect among these subjects for subjective responses, but no naltrexone effect. Similar to the apparent lack of clinical efficacy findings, naltrexone does not appear to impact alcohol effects in African American social drinkers. Future studies should investigate African American populations with heavy drinking as well as alcohol-dependent subjects in order to strengthen the parallels to clinical findings.
Introduction
Despite the efficacy of naltrexone demonstrated in clinical trials, there is significant variability in response among individuals prescribed the medication. While some of this variability is due to differences in adherence and obtaining a functionally significant dose,1,2 there remain intra-individual differences in patient response that have not yet been accounted for or predicted. If it were possible to isolate variables that could predict a greater likelihood of positive response to the medication, it would be possible to use the medication with greater certainty and efficiency. Thus, a critical question remains: under which circumstances and for which patients will naltrexone have the greatest benefit?
The role of the mu-opioid receptor in alcoholism
Preclinical and animal studies have also shown that opiate antagonists such as naltrexone (NTX) reduce alcohol preference,3–6 particularly in strains bred for excessive alcohol drinking,7 and following environmental stressors that elicit excessive alcohol drinking.8 Furthermore, clinical studies have demonstrated that NTX may reduce the reinforcing or pleasurable effects of alcohol in social drinkers,9 and alcohol dependent subjects who slip and sample alcohol.7,10 Together, the clinical and preclinical data suggest that opioid antagonism may have important pharmacological effects to reduce the reinforcing effects of alcohol and a return to clinically significant drinking. However, recent analysis of the large data set from the COMBINE trial has shown no NTX efficacy for African American (AA) participants in that trial.11 As such, examining NTX’s effect in a human laboratory study focusing on AA subjects may help to elucidate the potential role of NTX in alcohol dependence treatment for AA individuals.
Human Laboratory Studies
King et al. found that NTX blunts the subjective stimulation or “high” experienced after alcohol consumption in individuals at high risk for developing alcohol dependence (HR) relative to individuals at low risk of developing alcohol dependence (LR) as measured by the Biphasic Alcohol Effects Scale.12 These investigators also demonstrated that a single dose of NTX led to a greater release of the stress hormones ACTH and cortisol in HR individuals as compared to LR individuals.13 Similarly, McCaul et al. demonstrated a dose dependent NTX effect on dampening of “liking” and “best effects” in non-alcohol dependent individuals compared to placebo after an acute alcohol challenge.14 They did not find effects of NTX on drunkenness or intoxication, suggesting that the effect was a blunting of the reward/reinforcement of alcohol use. They subsequently replicated this work and additionally demonstrated a NTX blunting of ACTH release after an alcohol challenge.15 These data complement our own center’s experience that demonstrate a blunting of the “high” associated with drinking among alcohol dependent subjects treated with NTX compared to placebo.16 Ray and Hutchinson have also demonstrated that NTX can blunt alcohol’s effects on stimulation, positive mood, craving, and enjoyment.17 Ray and Hutchinson also found an interaction between the A+118G allele variant and NTX in response to an alcohol challenge.18
Prior human laboratory studies such as those described above have focused almost exclusively on European Americans. In addition to the focus on European Americans, many of these studies have recruited convenience samples from University populations, often resulting in a relatively youthful sample. Percentages of European Americans in a sample of recent laboratory studies have ranged from 75 to 84%, and the mean ages in the same studies ranged from 22 to 24.17
A single prior study of subjective alcohol responses in African Americans found a link between alcohol response and risk of increased drinking behavior and alcohol-related problems.19 That study focused on development of latent growth models and did not report the specifics of subjective effects in response to alcohol. As such, additional studies on the subjective and objective effects of alcohol in African Americans are needed.
In summary, while NTX has demonstrated efficacy in numerous clinical trials, a significant proportion of subjects fail to respond to NTX. The lack of efficacy in some subjects has in part led to the low utilization of NTX in general clinical care. As such, this study sought to initially examine what effect NTX had on breath alcohol levels as well as on the subjective effects of alcohol in African Americans. We hypothesized that NTX would blunt the subjective high from alcohol as seen in prior NTX human laboratory studies that had samples of primarily European ancestry. In more general terms, we hoped this research would was to add to our fundamental clinical knowledge regarding the role of the opioid neurotransmitter system in reward and alcohol consumption. In addition, we hoped to extend work from this center and others on the efficacy of NTX to questions directly related to individual factors such as race.
Materials and Methods
Subjects
Subjects were male and female social drinkers age 21 or older who reported drinking no more than an average of 21 drinks/week with no more than 2 binge drinking episodes per week, and who were of African descent by self report. Subjects were excluded if they met DSM-IV criteria for lifetime dependence on any substance other than nicotine; tested positive on the urine drug screen for opioids, cocaine, marijuana, or amphetamine at the screening visit; met current or lifetime DSM-IV criteria for bipolar affective disorder, schizophrenia, or any psychotic disorder; evidenced the presence of unstable or serious medical illness; or were found to need treatment with any psychotropic medication (antidepressant, antipsychotic, benzodiazepine, or mood stabilizing medication) in the opinion of the principal investigator. In addition, pre-menopausal female subjects who were pregnant, nursing, or not using a reliable method of contraception were excluded, as were insulin-dependent diabetics, as well as individuals with any medical or psychological condition that could jeopardize the individual’s safe participation in the trial as determined by the principal investigator. The study was approved by the University of Pennsylvania’s Institutional Review Board and all subjects provided written informed consent.
Procedures
Screening
Screening was typically accomplished during 1–2 visits. The screening visit focused on establishing eligibility for the trial, and included assessments to determine eligibility. Urine and blood specimens were collected during this time. If a subject’s urine drug screen results were positive for cocaine, opiates, benzodiazepines, or marijuana, the subject was informed and was not allowed to participate in the alcohol challenge sessions.
Alcohol Challenge Sessions
We employed a within-subject crossover design with each subject receiving all 4 challenge sessions (Table 1). The order of the four challenge sessions was randomly assigned for each subject. Two days prior to and the morning of each challenge session, the subject took NTX or placebo.
Table 1.
Study Design
| NALTREXONE | |||
|---|---|---|---|
| NTX (50 mg/day) |
Placebo | ||
| ALCOHOL | Alcohol (0.8 g/kg) | Alcohol+ NTX | Alcohol + Placebo |
| Sham | Sham + NTX | Sham + Placebo |
Medication was supplied in a blinded fashion by the pharmacy in blister packets for each session. Medication was prepared by the Research Pharmacy of the University of Pennsylvania. Prior to each session, subjects received 50mg/day of NTX or placebo. As such, each subject had two courses of NTX and two of placebo. As subjects received NTX for a non-indicated use, the protocol was under an Investigational New Drug Application (IND) for NTX (#44337).
Subjects were asked to remain abstinent during the three-day period leading up to each session. If subjects had a positive breathalyzer or self-reported drinking during this three-day window, they had to reschedule that session. Subjects were not allowed to reschedule a session more than twice without being withdrawn from the study. The alcohol exposure sessions were conducted at the Treatment Research Center of the University of Pennsylvania.
At approximately 12 pm, testing sessions began with questionnaires and breathalyzer. At approximately 12:45 pm, each subject was presented with the ethanol drink (or placebo) prepared to provide 0.8g/kg of alcohol. This amount of alcohol is equivalent to 3–5 standard drinks for an average person. The ethanol drink was prepared with 190 proof alcohol prepared to 11% volume mixed with fruit juice of the subject’s choice. The placebo drink was prepared by using fruit juice equal to the volume of the ethanol drink and floating a small amount of the 190 proof alcohol on the drink surface to simulate an ethanol drink. A research assistant not involved in the testing sessions prepared the drinks.
The beverage was divided into two equal portions and consumed over a 30-minute period. The first 10 minutes were used to consume the first drink, followed by a 10-minute rest period and then the second drink was consumed in the last 10-minute period. The subject was then allowed to read or watch videos while having questionnaires, heart rate, and breathalyzer readings conducted at 15, 45, 105 and 165 minutes after the completion of drinking. Smoking and drinking caffeinated beverages was not allowed between 60 minutes prior to the session and the completion of the 165-minute assessment. Subjects were provided with snacks throughout the challenge sessions.
Participants were strongly encouraged to arrange alternative transportation from the clinic after each alcohol challenge session. Subjects who were not driving themselves from the sessions were allowed to leave the center once their BAC was lower than 0.08. Subjects who were driving from the sessions were not allowed to leave until their BAC was 0.02 or lower. In either case, subjects were not permitted to leave the center with any obvious signs of intoxication.
All subjects received $95 and were offered two public transportation tokens at the successful completion of each of the four alcohol challenge sessions. Thus total monetary compensation was $380 for completion of all four sessions.
Materials
During screening we used the MINI International Neuropsychiatric Interview (MINI)20 modules for mania, psychosis, panic disorder, generalized anxiety disorder (GAD), post-traumatic stress disorder (PTSD), and alcohol abuse/dependence; The Patient Health Questionnaire-9 (PHQ9) for depression;21 and the Family History Interview for Substance and Mood Disorders22 to assess family history of psychopathology and psychoactive substance abuse or dependence in first- and second-degree relatives. The Time-Line Follow-Back (TLFB) was used to assess drinking behavior (percent days abstinent from all substances, percent heavy drinking days), and time spent in controlled environments, such as prison.23 The Obsessive-Compulsive Drinking Scale to Measure Alcohol Craving (OCDS)24–26 was used to measure craving. The Self Rating of the Effects of Alcohol was used to measure the euphoria and consequences of alcohol use. The Fagerstrom Scale27,28 was used to measure physical dependence to nicotine. Chemistry labs were drawn to monitor for adverse events and to confirm that subjects met study inclusion criteria. Female subjects received a pregnancy test at study entry. EMIT urine toxicology was used to detect the following drugs: opiates, cocaine, benzodiazepines, and marijuana. Breathalyzer readings were recorded at each study visit.
During study sessions, the Biphasic Alcohol Effects Scale (BAES)29 was used to measure of the sedative and stimulated effects of alcohol consumption. The Subjective High Assessment Scale (SHAS) was used to assess the level of euphoria experienced after ingestion of alcohol in contrast to sedation and intoxication. A modified (37 item) version of the Profile of Mood States (POMS)30 was used to examine alcohol effects on mood.
Data Analyses
For each response, within-session summaries were calculated for each of the four sessions, and these four repeated measures were analyzed using linear mixed effects models, using PROC MIXED in SAS.31 These models comprised an intercept term, a binary factor representing alcohol condition (ALC vs. SHAM), a binary factor representing NTX condition (NTX vs. PLA), and factors representing sequence and carryover effects. We tested for one-period carryover, where the distributions of responses in a given session could depend on the treatment conditions for the previous session. The models also included a random effect for subject, and a random term for residual error. The effects of primary interest were the alcohol and NTX main effects and their interaction. The models also included some covariates, typically a session-baseline measure of the session response.
Results
Demographics
All subjects were of self-reported African descent. Approximately half of the subjects were female (54.3%). The mean age was 35.3 years (SD ± 13.0), which is considerably older than the mean age of subjects in prior studies of this type.18 Subjects reported an average of 3.3 (SD ± 5.1) drinks in the past week, and an average of 2.1 (SD ± 4.7) binges in the past three months. Family history of alcohol and/or other drug dependence was relatively common, ranging from 45.7% of subjects reporting alcohol dependence in a first degree family member, to 17.4% reporting cocaine dependence in a first degree relative.
Breathalyzer Results
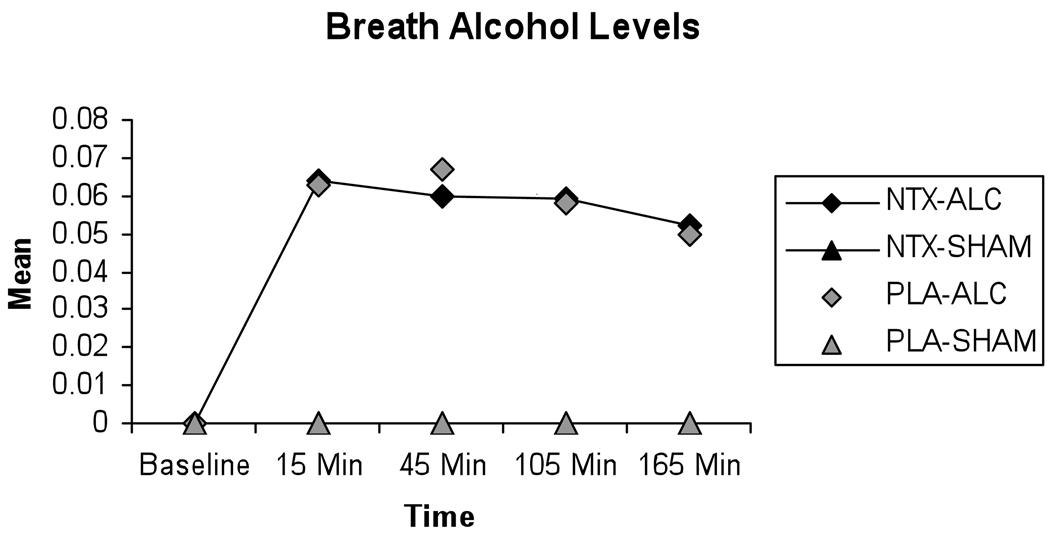
Results from the breathalyzer indicate that all subjects reached an elevated BrAC within 15 minutes after drinking during sessions where they drank alcohol (Figure 1), and that elevation was sustained through the 165 minute timepoint. In contrast, during those sessions where subjects received sham drink, no elevation in BrAC was seen. For the sham sessions, the session averages of the breathalyzer readings ranged from 0.0000 to 0.0025, with a mean value of 0.0002 and standard deviation of 0.0003. In the alcohol sessions, the session averages of the breathalyzer readings ranged from 0.0075 to 0.0925, with a mean value of 0.0565 and standard deviation of 0.0141. A formal mixed effects model test confirmed that alcohol levels were significantly higher in alcohol sessions than in sham sessions: F(1, 91)=1177.25, p<.0001. Thus, the alcohol and sham sessions were clearly differentiated with respect to alcohol levels. There was no difference in BrAC levels for men and women t(27) = −1.45, p = .16.
Figure 1. Breath Alcohol levels.
Mean breath alcohol levels for each combination of medication (Naltrexone or Placebo) and alcohol (Alcohol or Sham), as measured across study session. Baseline indicates pre-drinking breath alcohol level, the remaining timepoints indicate post-drinking breath alcohol levels.
Subjective Outcomes
Stimulation Effects
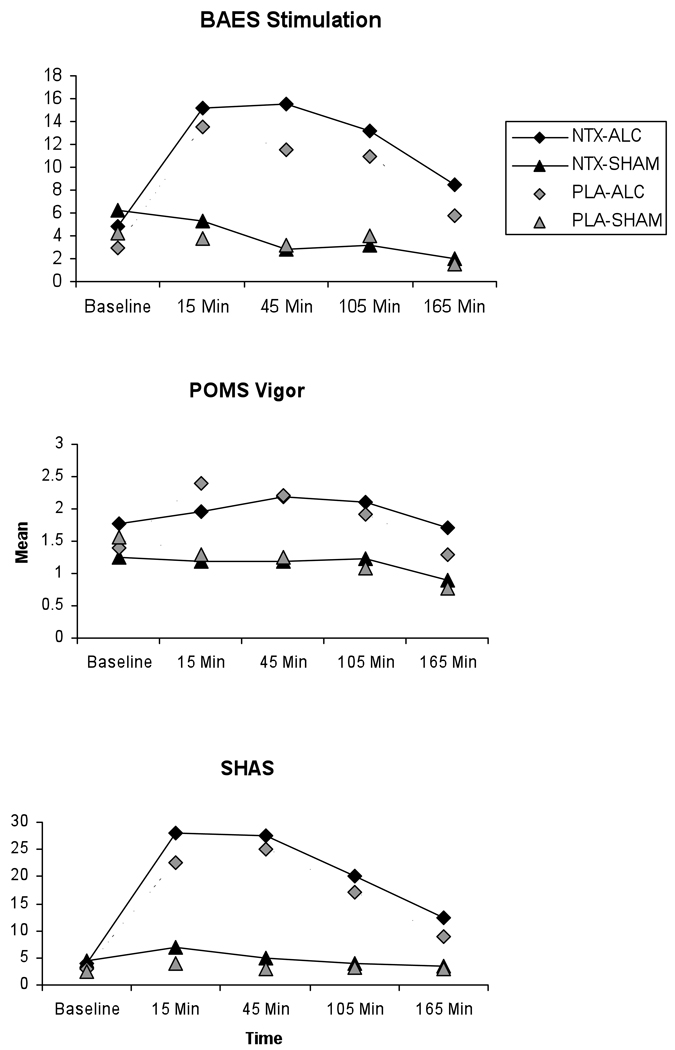
The BAES results for “stimulation” indicate that subjects reported more stimulation during alcohol sessions that during sham sessions, with stimulation peaking midway through the session F(1,89) = 89.46, p < .0001 (Figure 2). However, there was no NTX effect or NTX by alcohol interaction for Stimulation, F(1,89) = 0.06, p = .81 and F(1,89) = .2, p = .65, respectively.
Figure 2. Stimulation Measures.
Mean stimulation levels as measured by the Biphasic Alcohol Effects Scale (BAES) Stimulation subscale, the Profile of Mood States (POMS) Vigor subscale, and the Subjective High from Alcohol Scale (SHAS) for each combination of medication (Naltrexone or Placebo) and alcohol (Alcohol or Sham), as measured across study session. Baseline indicates pre-drinking stimulation level for each measure, the remaining timepoints indicate post-drinking stimulation levels for each measure.
POMS results for “Vigor”, the closest analog to the BAES stimulation, reveal a similar pattern F(1,90) = 22.67, p < .0001 (Figure 2). However, there was again no NTX effect or NTX by alcohol interaction for Vigor, F(1,90) = 0.08, p = .78 and F(1,90) = 2.31, p = .13, respectively. The Subjective High from Alcohol Scale (SHAS) again shows the same pattern, with the alcohol effect being significant F(1,90) = 122.49, p < .0001, as well as an almost significant NTX alcohol interaction F(1,90) = 3.66, p = .059 (Figure 2). Again, NTX appears to have no effect alone, F(1,90) =1.37, p = .24.
Sedation Effects
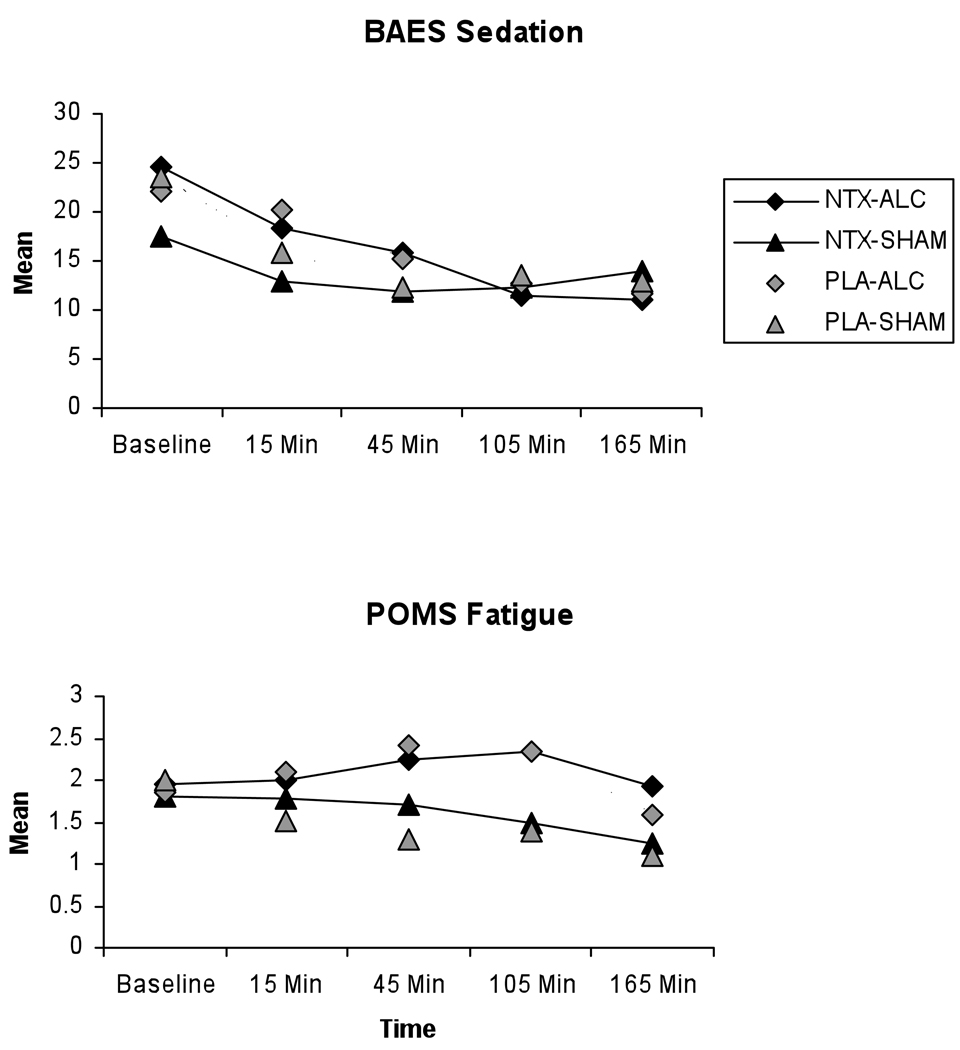
In contrast to the typical pattern shown for stimulation, sedation measures reveal an atypical pattern, with sedation level decreasing from baseline through the session (Figure 3).The BAES results for “sedation” have non-significant outcomes for both alcohol F(1, 89) = 3.60, p = .06, and alcohol by NTX F(1,89) = 0.39, p = .54. In contrast, the POMS fatigue by session shows a significant difference between the alcohol and sham drinking sessions, F(1,90) =12.74, p = .0006, but no NTX effect (Figure 3).
Figure 3. Sedation Measures.
Mean sedation levels as measured by the Biphasic Alcohol Effects Scale (BAES) Sedation subscale and the Profile of Mood States (POMS) Fatigue subscale for each combination of medication (Naltrexone or Placebo) and alcohol (Alcohol or Sham), as measured across study session. Baseline indicates pre-drinking sedation level for each measure, the remaining timepoints indicate post-drinking sedation levels for each measure.
Discussion
We believe our study adds to the body of knowledge on NTX for the treatment of alcohol dependence, as well as to the body of knowledge on human laboratory studies of alcohol self-administration.
As hypothesized, we found main effects for alcohol in all subjective measures of stimulation. We further found that NTX did not impact subjective outcomes or BrAC readings with the exception of the SHAS where there was an almost significant interaction of NTX and ALC. Although our stimulation results look similar to those found in other alcohol challenge studies, we had hypothesized, based on prior findings, that NTX would blunt the subjective high from alcohol as shown by the SHAS. However, what we actually saw during the ALC sessions was a slight elevation in stimulation in the NTX condition as compared to placebo. We interpret this finding as part of the overall lack of NTX effect in this sample and not as an indication that NTX is actually increasing alcohol stimulation effects in these subjects. Our sedation results suggest that subjects had elevated sedation levels at baseline in the sessions, and that sedation decreased during the sessions.
The strengths of this study include the entirely African American subject population, which has not been previously studied in such controlled NTX human laboratory studies. In addition, the age range for this study is larger than prior human laboratory studies of non-dependent subjects. As such, this study extends the findings of prior human laboratory alcohol self-administration studies in normal controls to both broader racial and age groups than previously studied.
Although we did not account for body water when administering ethanol doses, we found no BrAC differences between men and women. It is also important to note that our subjects drank considerably less than the inclusion criteria cutoff amounts of 21 drinks/week and 2 binge drinking episodes/week. As such, these results may have limited applicability to alcohol dependent populations.
In sum, this study demonstrated alcohol effects, but little NTX effect. This finding parallels that in the clinical literature.11 As such, although NTX treatment for AA subjects should in no way be abandoned, future work may need to examine other potential alcohol dependence medications for use in AA populations. In addition, future work could shift to investigating genes that occur with enough frequency in both European and African Americans to allow for studies using both races and thus broader applicability of findings.32
Acknowledgments
Funding for this study was provided by grant P60-DA-005186 from the National Institute on Drug Abuse, Bethesda, MD (Dr. O’Brien).
Footnotes
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- 1.Pettinati HM, Volpicelli JR, Pierce JD, Jr, O'Brien CP. Improving naltrexone response, an intervention for medical practitioners to enhance medication compliance in alcohol dependent patients. Journal of Addictive Diseases. 2000;19:71–83. doi: 10.1300/J069v19n01_06. [DOI] [PubMed] [Google Scholar]
- 2.McCaul ME, Wand GS, Sullivan J, Mumford G, Quigley J. Beta-naltrexol level predicts alcohol relapse. Alcoholism, Clinical and Experimental Research. 1997;21:32A. [Google Scholar]
- 3.Kornet M, Goosen C, van Ree JM. Opioid Modulation of Alcohol Intake in Monkeys by Low Doses of Naltrexone and Morphine. Annals of the New York Academy of Sciences. 1991;469:471. doi: 10.1111/j.1749-6632.1992.tb26002.x. [DOI] [PubMed] [Google Scholar]
- 4.van Ree JM, Kornet M, Goosen C. Neuropeptides and alcohol addiction in monkeys. EXS. 1994;71:165–174. doi: 10.1007/978-3-0348-7330-7_17. [DOI] [PubMed] [Google Scholar]
- 5.Ulm RR, Volpicelli JR, Volpicelli LA. Opiates and alcohol self-administration in animals. Journal of Clinical Psychiatry. 1995;56:5–14. [PubMed] [Google Scholar]
- 6.Hubbell CL, Czirr SA, Hunter GA, Beaman CM, LeCann NC, Reid LD. Consumption of ethanol solution is potentiated by morphine and attenuated by naloxone persistently across repeated daily administrations. Alcohol. 1986;3:39–54. doi: 10.1016/0741-8329(86)90070-4. [DOI] [PubMed] [Google Scholar]
- 7.Volpicelli JR, Davis MA, Olgin JE. Naltrexone blocks the post-shock increase of ethanol consumption. Life Science. 1986;38:841–847. doi: 10.1016/0024-3205(86)90601-6. [DOI] [PubMed] [Google Scholar]
- 8.Froehlich JC, Harts J, Lumeng L, Li T-K. Naloxone attenuates voluntary ethanol intake in rats selectively bred for high ethanol preference. Pharmacology Biochemistry and Behavior. 1990;35:385–390. doi: 10.1016/0091-3057(90)90174-g. [DOI] [PubMed] [Google Scholar]
- 9.Swift RM, Whelihan W, Kuznetsov O, Buongiorno G, Hsuing H. Naltrexone-induced alterations in human ethanol intoxication. American Journal of Psychiatry. 1994;151:1463–1467. doi: 10.1176/ajp.151.10.1463. [DOI] [PubMed] [Google Scholar]
- 10.O'Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence, a controlled study. Archives of General Psychiatry. 1992;49:881–887. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- 11.Ray LA, Oslin DW. Naltrexone for the treatment of alcohol dependence among African Americans: results from the COMBINE Study. Drug & Alcohol Dependence. 2009;105:256–258. doi: 10.1016/j.drugalcdep.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King AC, Volpicelli JR, Frazer A, O'Brien CP. Effect of naltrexone on subjective alcohol response in subjects at high and low risk for future alcohol dependence. Psychopharmacology. 1997;129:15–22. doi: 10.1007/s002130050156. [DOI] [PubMed] [Google Scholar]
- 13.King A, Schluger J, Gunduz M, et al. Hypothalamic-pituitary-adrenocorticaI (HPA) axis response and biotransformation of oral naltrexone, Preliminary examination of relationship to family history of alcoholism. Neuropsychopharmacology. 1992;26:778–788. doi: 10.1016/S0893-133X(01)00416-X. [DOI] [PubMed] [Google Scholar]
- 14.McCaul ME, Wand GS, Eissenberg T, Rohde CA, Cheskin LJ. Naltrexone alters subjective and psychomotor responses to alcohol in heavy drinking subjects. Neuropsychopharmacology. 2000;22:480–492. doi: 10.1016/S0893-133X(99)00147-5. [DOI] [PubMed] [Google Scholar]
- 15.McCaul ME, Wand GS, Stauffer R, Lee SM, Rohde CA. Naltrexone dampens ethanol-induced cardiovascular and hypothalamic- pituitary-adrenal axis activation. Neuropsychopharmacology. 2001;25:537–547. doi: 10.1016/S0893-133X(01)00241-X. [DOI] [PubMed] [Google Scholar]
- 16.Volpicelli JR, Watson NT, King AC, Sherman CE, O'Brien CP. Effect of naltrexone on alcohol "high" in alcoholics. American Journal of Psychiatry. 1995;152:613–615. doi: 10.1176/ajp.152.4.613. [DOI] [PubMed] [Google Scholar]
- 17.Ray LA, Hutchison KE. Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response, a double-blind placebo-controlled study. Arch Gen Psychiatry. 2007;64:1069–1077. doi: 10.1001/archpsyc.64.9.1069. [DOI] [PubMed] [Google Scholar]
- 18.Ray LA, Hutchison KE. A polymorphism of the mu-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcohol Clin Exp Res. 2004;28:1789. doi: 10.1097/01.alc.0000148114.34000.b9. [DOI] [PubMed] [Google Scholar]
- 19.Pedersen SL, McCarthy DM. An examination of subjective response to alcohol in African Americans. J Stud Alcohol Drugs. 2009;70:288–295. doi: 10.15288/jsad.2009.70.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheehan DV, Lecrubier Y, Sheehan K, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I), The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- 21.Kroenke K, Spitzer RL, Williams JB. The PHQ-9, validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andreasen NC, Rice J, Endicott J, Reich T, Coryell W. The family history approach to diagnosis: how useful is it? Arch. Gen. Psychiatry. 1986;43:421–429. doi: 10.1001/archpsyc.1986.01800050019002. [DOI] [PubMed] [Google Scholar]
- 23.Sobell LC, Sobell MB. Timeline follow-back, a technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring Alcohol Consumption. Totowa, NJ: Humana Press Inc.; 1992. pp. 41–65. [Google Scholar]
- 24.Roberts JS, Anton RF, Latham PK, Moak DH. Factor structure and predictive validity of the Obsessive Compulsive Drinking Scale. Alcoholism, Clinical & Experimental Research. 1999;23:1484–1491. [PubMed] [Google Scholar]
- 25.Bohn MJ, Barton BA, Barron KE. Psychometric properties and validity of the obsessive-compulsive drinking scale. Alcoholism, Clinical & Experimental Research. 1996;20:817–823. doi: 10.1111/j.1530-0277.1996.tb05257.x. [DOI] [PubMed] [Google Scholar]
- 26.Kranzler HR, Mulgrew CL, Modesto-Lowe V, Burleson JA. Validity of the Obsessive Compulsive Drinking Scale (OCDS), does craving predict drinking behavior? Alcoholism, Clinical & Experimental Research. 1999;23:108–114. [PubMed] [Google Scholar]
- 27.Fagerstrom KO, Schneider NG. Measuring nicotine dependence, a review of the Fagerstrom Tolerance Questionnaire. Journal of Behavioral Medicine. 1989;12:159–182. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]
- 28.Fagerstrom KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addictive Behaviors. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- 29.Martin C, Earlywine M, Musty R, Perrine M, Swift R. Development and validation of the biphasic alcohol effects scale. Alcoholism, Clinical and Experimental Research. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 30.McNair D, Lorr M, Droppelman L. EITS Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 31.Littell RC, Pendergast J, Natarajan R. Modeling covariance structure in the analysis of repeated measures data. Statistics in Medicine. 2000;19:1793–1819. doi: 10.1002/1097-0258(20000715)19:13<1793::aid-sim482>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 32.Wang JC, Grucza R, Cruchaga C, et al. Genetic variation in the CHRNA5 gene affects mRNA levels and is associated with risk for alcohol dependence. Mol Psychiatry. 2009;14:501–510. doi: 10.1038/mp.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]