Abstract
Alterations in the structure of the corpus callosum (CC) have been observed in schizophrenia. Offspring of schizophrenia parents have 10–15 times higher risk for developing schizophrenia. We examined CC volume in offspring at genetic high risk (HR) subjects. Since the sub regions of CC are topographically mapped to cortical brain regions, we hypothesized that HR subjects may show a decrement in total volume and differential volume decreases in sub-regions of CC. The offspring of schizophrenia parents (HR; n=70; 36 males) and healthy volunteers with no family or personal history of psychotic disorders (HC; n=73; 37 males) matched for age, gender and education were selected for the study. Magnetic resonance images were collected using a GE 1.5T scanner and processed using Freesurfer image analysis software. The CC was divided into five neuroanatomically based partitions. The volume of total CC and the five sub-regions were measured blind to clinical information. Covarying intra cranial volume, HR subjects had significantly reduced total CC, more prominently observed in the anterior splenium. An age-related increase in CC volume was found in the anterior and posterior splenium of healthy controls but not in HR subjects. The volume reduction was greater in male than female HR subjects. The volume reduction in the CC may reflect a reduction in axonal fibers crossing the hemispheres and/or myelination between the left and right temporo-parietal cortices. The absence of an age-related volume increase suggests an abnormal developmental trajectory that may underlie susceptibility to schizophrenia.
Keywords: Corpus Callosum, Schizophrenia, High-Risk Offspring, MRI, Brain Regions
1. Introduction
The corpus callosum (CC) is the main white matter tract connecting the two cerebral hemispheres, and consists of approximately 200–350 million fibers in humans (Aboitiz et al., 1992a; Aboitiz et al., 1992b). The CC communicates perceptual, cognitive, mnemonic, learned, and volitional information between the two brain hemispheres (Bogen et al., 1965) and plays a pivotal role in integration of information. The fiber connections between corresponding hemispheric regions are topographically arranged across the callosum (de Lacoste et al., 1985). Studying rhesus monkeys, Pandya and colleagues (Karol et al., 1970; Pandya et al., 1971; Barbas and Pandya., 1984) showed that, anteriorly, the rostrum and genu connect fibers originating from the prefrontal premotor and precentral regions, the isthmus and the anterior splenium connect the fibers generated at the temporal regions, while the parietal and insulo-opercular regions are connected by the anterior and mid-splenium (Pandya and Vignolo., 1969; Barbas and Pandya., 1989). The posterior splenium is a conduit for fibers emanating from the bilateral occipital lobes (Barbas and Pandya., 1989; Keshavan et al., 2002; Brambilla et al, 2005). The volume of the CC reflects the number and/or myelination of interhemispheric axons (Chance et al., 2006; Chance et al., 2008); thus, the volumetric abnormalities of CC or the sub-regions could imply altered connectivity between the hemispheres.
Several studies have shown that CC volume is abnormal in schizophrenia (SZ) (Casanova et al., 1990; Tibbo et al., 1998; Gruzelier 1999; Kubicki et al., 2005). Early CC studies in SZ were mainly done post mortem and showed volume increases in some regions (e.g., genu) and/or decreases in others (e.g., isthmus) (Rosenthal and Bigelow., 1972; Bigelow et al., 1983; Highley et al., 1999). The reduced CC volume in SZ was correlated with altered minicolumn spacing in topographically corresponding cortical regions (Chance et al., 2006; 2008). The volume reductions in the CC could reflect altered laterality (minicolumn spacing) leading to impaired interhemispheric integration of information and in turn to the positive symptoms of SZ (Nasrallah et al., 1986; Woodruff et al., 1995; Hoffman and McGlashan., 1998; Crow, 1998). Nasrallah et al. (1986) proposed that abnormal interhemispheric connectivity mediated by the CC may underlie several Schneiderian first rank symptoms. An artificial neural network computer simulation model of corticocortical connectivity designed by Hoffman and McGlashan., (1998) suggested that CC abnormalities could explain some of the positive symptoms of SZ such as auditory hallucinations. The language dysfunction, hallucinatory symptoms and thought disorder in schizophrenia could be related to callosal alterations which may impair inter-hemispheric integration of verbal information (Mohr et al., 2000; Endrass et al., 2002; Barnett et al., 2005; 2007) and prosody (Leitman et al., 2007). Callosal volume may also correlate with severity of auditory verbal hallucinations (Rossell et al., 2001). Abnormalities of callosal metabolism and neurotransmission may also contribute to the altered inter-hemispheric transfer of information across the CC (Aydin et al., 2008).
A positive correlation was found between delusions and regional thinning of the CC by Woodruff et al. (1993). Finally, diffusion tensor imaging (DTI) studies (Kubicki et al., 2005; Miyata et al., 2007) and functional magnetic resonance imaging studies (Schlosser et al., 2007) have shown SZ subjects to exhibit reduced connectivity. In sum, as Crow has proposed, trans-callosal misconnectivity may be one of the key deficits in SZ (Crow, 1998).
Studies using magnetic resonance imaging (MRI) in SZ have found reductions of the anterior part of the CC (genu) or the posterior (splenium) (reviewed in Shenton., 2001). This review of structural findings in schizophrenia found that among 27 MRI studies of the CC, 17 (63%) reported reductions in the CC, while 10 (37%) reported no reductions in CC volume (Shenton, 2001). An early meta-analysis of 11 MRI studies revealed that the CC was significantly smaller in patients with schizophrenia (Woodruff et al., 1995). A meta-analysis done on studies after 1995 by Arnone et al, (2008) examined 28 studies of the CC and found that CC regional volumes were reduced in SZ in comparison to healthy controls. This effect was larger in first-episode patients. Our group previously examined the CC in first-episode SZ and found that patients had a smaller CC, anterior genu, anterior body, isthmus and anterior splenium in comparison to healthy controls; the age-related increase found in controls was not seen in patients (Keshavan et al., 2002). A recent study (Venkatasubramanian et al., 2010) examined the CC in antipsychotic naïve schizophrenia subjects and normal controls. They found that the surface area of the CC was significantly reduced in the genu, body and isthmus in patients.
This suggests that SZ is associated with callosal changes that may mirror alterations in structural integrity of frontal and parieto-temporal cortical regions and that these changes may reflect alterations in their developmental trajectories.
Since structural alterations may reflect genetic liability to schizophrenia, CC regions altered in patients may also be altered in their relatives, albeit to a lesser extent. Detection of subtle alterations in relatives warrants statistically powerful studies on populations at high genetic predisposition (first-degree relatives) during the age of peak vulnerability to schizophrenia. Several studies have looked at CC volume in subjects at high risk for SZ. Structural alterations of the CC have also been thought to contribute to negative symptoms in those at ultra-high-risk for schizophrenia (Barnett at al., 2005).
In the present study, we examined the CC volume in a relatively large sample of 70 offspring of SZ parents and 73 healthy controls. We defined high risk as those subjects who had one parent with a diagnosis of SZ or schizoaffective disorder (SZA) based on DSM-IV criteria. Thus, high risk subjects in our study were at familial high risk and not clinical high risk (CHR) which requires the presence of specific subsyndromal psychotic symptoms (Seidman et al., 2010).
We hypothesized that the corpus callosum in HR subjects would have significantly smaller total volume and smaller subregional volumes (i.e., genu and splenium) similar to those observed in SZ and also reduced age-related increases.
2. METHODS
2.1. Participants
Subjects were recruited from the Western Psychiatric Institute and Clinic, Pittsburgh, and consisted of racially diverse adolescents, 70 offspring of schizophrenia probands (HR subjects) and 73 healthy controls (HC). Within the HR group, 28 offspring had one parent with SZA and 42 offspring had one parent with SZ. Of these, 23 fathers had a diagnosis of SZ/SZA, while 43 mothers had a diagnosis of SZ/SZA. The parents of the HR subjects were diagnosed as per DSM-IV criteria on diagnostic interviews (Structured Clinical Interview for DSM-IV; SCID) conducted by a trained clinician and a “best estimate” consensus diagnosis. The SZ, SZA parents were assessed for all Axis I disorders covered in the SCID, and no parent was screened out due to co-morbidity. Table 1 gives the parental diagnosis. For those ill relatives who were unwilling or unable to do a face-to-face interview, a thorough history of the relative’s illness (including specific symptoms indicative of an Axis I disorder psychotic, depressive, manic, drug and alcohol use--and the onset and chronology of such symptoms) was obtained through direct interview with a family member(s). No identifying information, however, was collected for non-consenting family members. For HR and HC subjects, the Structured Clinical Interview for DSM-IV Disorders and the Behavioral Disorders (ADHD, Conduct Disorder, Oppositional Defiant Disorder) and Separation Anxiety Disorder supplements from the Kiddie-SADS-PL (Present and Lifetime Version) were used to ascertain diagnoses.
Table 1.
Demographic Information of Subject Populations
| Variable | Offspring (N = 70) | Control (N = 74) | |
|---|---|---|---|
| M (SD)/N (%) | M (SD)/N (%) | Pb | |
| Age | 16.30 (3.40) | 16.62 (3.65) | 0.601 |
| Male | 38 (54%) | 24 (38%) | 0.082 |
| Caucasian | 34 (49%) | 41 (65%) | 0.080 |
| Education (years) | 9.72 (3.33) | 10.18 (3.29) | 0.432 |
Fisher’s exact test or independent t-test, two-tailed, for significant differences between high risk participants and healthy controls.
Offspring were recruited by approaching patients and through advertisements. Subjects were excluded for mental retardation as described in the DSM IV, lifetime evidence of a psychotic disorder, exposure to antipsychotic medications, recent history (within the past month) of a substance use disorder, a significant neurological or unstable medical condition. Age- and gender-matched healthy controls were recruited from the same community neighborhoods as HR subjects. All subjects, and parents for minors, signed an informed consent; Participants younger than 18 provided assent. The study was approved by the University of Pittsburgh’s Institutional Review Board.
2.2 Image acquisition
MRI scans were obtained on subjects using a GE 1.5-Tesla whole body scanner (GE Medical Systems, Milwaukee, Wisconsin). The detailed scanning protocol has been described in an earlier publication (Bhojraj et al. (2009). Briefly, the scans were three-dimensional spoiled gradient recall (SPGR), acquired in a steady-state pulse sequence (124 coronal slices, 1.5 mm thickness, TE=5 ms, TR=25 ms, acquisition matrix=256×192, FOV=24 cm, flip angle=40°). Images with motion artifacts were not included in the study.
2.3 Partitions of the CC
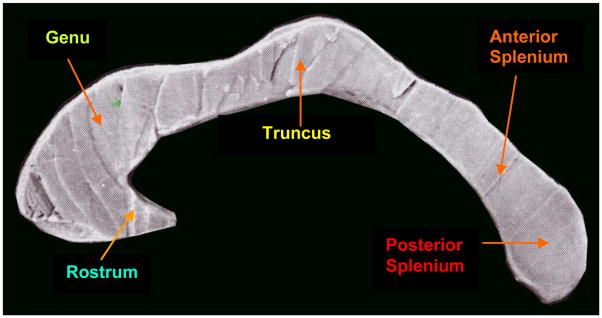
Several partitioning schemes have been adopted for dividing the CC into three-seven sub-regions (Witelson, 1989; Rumsey et al., 1996; Jancke et al., 1997; Bachmann et al., 2003; Teipel et al., 2004; Keshavan et al., 2007). Based on microscopic evidence from myelin-staining studies done by Pandya and colleagues (Pandya and Vignolo, 1969; Karol, et al., 1970; Pandya et al., 1971; Barbas and Pandya, 1984; Barbas and Pandya, 1989), our earlier work (Keshavan et al., 2002) and that of Fischl et al. (2004), we chose a five-subdivision partition, namely the rostrum, genu, truncus, anterior splenium, and posterior splenium.
2.4. Morphometry of the CC volume using FreeSurfer
Volume of the CC was defined by including voxels in both hemispheres consisting of fibers that run horizontally from the mid sagittal plane until the point laterally when the fibers change direction (fan out into the corona radiata) within the hemispheres. The volume of the CC was the sum total of the 3D voxels defined within this region.
FreeSurfer uses a functional classification such as posterior (which is roughly equivalent of the posterior splenium), mid-posterior (anterior splenium), central (truncus/isthmus), mid-anterior (genu), and anterior (rostrum); see Fig 1.
Fig. 1.
Corpus callosum morphology
2.4.1. Rationale for CC parcellation scheme
In the present study, we chose automated morphometric analyses for specific reasons. Intersubject variability and random error inherent in manual techniques are minimized by automated methods (FreeSurfer). The possibility of systematic error in automated approaches can be detected and corrected by rigorous manual editing, such as that followed in this study.
2.5 Image analysis
2.5.1. Semi-automated morphometric analysis using FreeSurfer
We used Freesurfer (FS) 4.0.5 (64-bit version) (Massachusetts General Hospital, running on Linux for morphometric analysis. Freesurfer (FS), a semi-automated brain image morphometric software package, has been used to study the brain morphology of several illnesses including schizophrenia (Fischl et al., 1999; Dale et al., 1999; Fischl et al., 2004; Desikan et al., 2006; van der Kouwe et al., 2008). FS has three automated stages (Fischl et al., 2004), each followed by manual image editing by an experienced neuroimage analyst (AF).
At the first stage, FS performs motion correction, intensity correction, normalization and skull stripping. Motion correction may be defined as a process where multiple acquisitions are available for a single subject. The two T1 volumes are spatially registered and averaged together into a single, more accurate representation. In this processing step, multiple scans from each subject are registered using the first scan as the template, and a single averaged, motion-corrected volume for each subject is generated as output. The motion-corrected volume undergoes several intensity-normalization steps, along with a transformation to Talairach space. The intensity-corrected T1 volume is fed into an mri_watershed, which strips off the skull and any remaining background noise and generates the brainmask volume. In our study, we had only single acquisitions of T1 images. The resultant brain was manually edited to remove non-brain matter.
At the second stage, the stripped brain is subjected to gray-white segmentation. Each slice is visually examined for errors in segmentation. The pial surface, which denotes the boundary between gray matter and cerebrospinal fluid (CSF), is carefully examined and manually corrected using FreeSurfer’s own editing toolbox. The edited brain surface is saved. The interior portions with demarcated regions of interest (ROIs) are also carefully examined by an experienced neuroimage analyst (AF). Errors to ROIs are corrected by importing the ROI into the manual morphometric program 3D Slicer and making corrections. The corrected ROI is then saved. Thereafter, stage 2 is repeated with the saved parameters. Stage 3 uses an atlas-based algorithm and parcellates brain regions based on gyral anatomical landmarks and surface area and thickness measurements. Each stage is robust to anatomical variability, including ventricular enlargement typically associated with schizophrenia.
2.5. Statistical analysis
We used SPSS version 17 as our statistical tool of analysis. Structural measures and age were normally distributed [Shapiro-Wilk’s test (W statistic, p > 0.1)]. Multivariate analyses of covariance (MANCOVAs) and analyses of covariance (ANCOVAs) were used with gender, age, and intracranial volume (ICV) as covariates. We also used a quadratic model to examine the effect of age on callosal volume.
The groups did not differ in the ICV [mean HC = 1416925 mm3 SD ± 207767.2; mean HR = 1387015 mm3 SD ± 178013. 9 (p = 0.73)]. Total CC volume was initially compared between study groups using ANCOVA. To control for multiple comparisons, we performed GLM MANCOVA with each of the CC subregions as the dependent variables, study group and gender as categorical predictors, and ICV as covariate. Correlations with age were computed using partial correlations with ICV and age at the time of scan as covariates. The correlations were performed only for those subregions showing volumetric deficits. The two-tailed p values of the correlations were corrected for multiple comparisons using the Bonferroni correction to achieve an overall alpha error rate of 0.05.
3. Results
3.1 Group effects
An initial ANCOVA, covarying for age and ICV, showed that the controls had an average total volume of 3094.88 mm3 (SD =±436.07); for HR subjects, it was 2916.98 mm3 (SD = ± 490.5) (p < 0.03). Standardized mean differences were calculated using Cohen’s d statistic. Cohen’s d statistic for the significance of the mean difference was = 0.4390, indicating that the difference in the volume of the CC between the two groups is significant.
An omnibus MANCOVA was performed using CC subregional volumes as the dependent variables, study group as the independent variable and controlling for gender, age and ICV. The MANCOVA showed an overall effect of study group [Wilk’s lambda=0.91, partial eta-squared= 0.080, F (5,133)=2.31, overall model p=0.047], suggesting HR subjects had smaller volumes compared with controls. We also found a group × gender interaction, with males in the HR group showing larger reductions [F(5,133)=3.07, p=0.012].
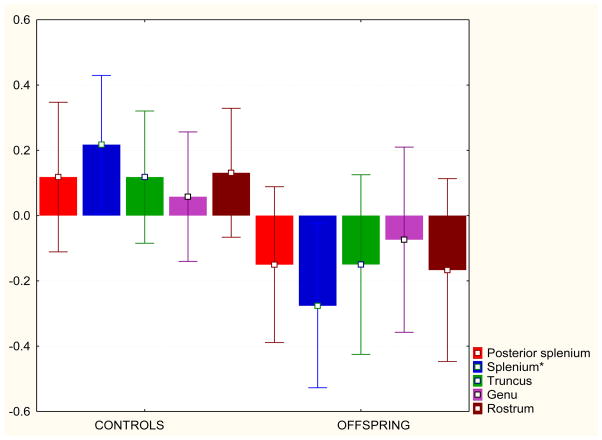
The between-subjects ANCOVA for the individual subregions showed that the mid-posterior subregion (splenium) showed significantly greater volume in controls than in HR subjects (p=0.006). The rostrum showed a difference between the groups at a trend level (p=0.06) (see Table 2 and Fig. 2).
Table 2.
| Corpus callosum subregions | |||||
|---|---|---|---|---|---|
| Subdivisions | Volume in mm3 | df | F | p | |
| Controls | Offspring | ||||
| Posterior splenium | 846.32 | 803.34 | 143 | 2.707 | 0.102 |
| Anterior splenium | 464.7 | 416.8 | 143 | 9.163 | 0.006 |
| Truncus | 490.5 | 460.5 | 143 | 1.923 | 0.168 |
| Genu | 453.5 | 437.03 | 143 | 0.493 | 0.484 |
| Rostrum | 844.6 | 800.28 | 143 | 3.601 | 0.06 |
Fig. 2.
Corpus callosum subregional volumes were z-transformed for the pooled group of controls and offspring. The z scores for these volumes are plotted on the y axis. The bars denote 95% CI
3.2. CC and age
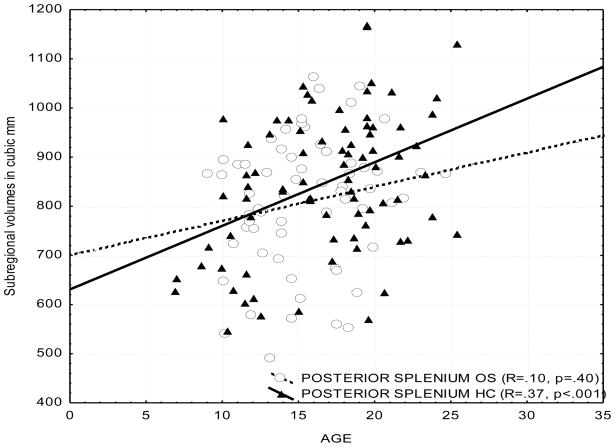
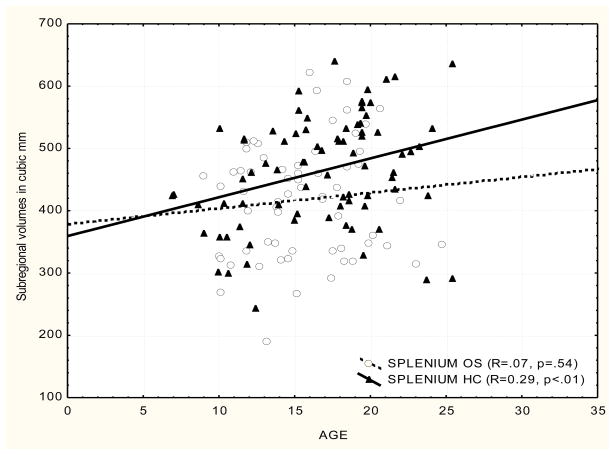
We used groupwise partial correlations controlling for ICV to examine the effect of age on the volume of the CC. To control the experiment-wise error rate, partial correlations were limited to regions showing volumetric variations on the MANCOVA. A total of four correlations (posterior and mid-posterior subregions for healthy control and offspring groups) were performed, with each thresholded at p=0.05; hence, the acceptable p value was less than or equal to 0.05/4=0.0125.
We found that the posterior and mid-posterior CC (splenium) volume correlated positively with age in healthy controls. We secondarily explored correlations between age and other subregions. The correlations noted for the posterior and mid-posterior volumes were not found in the HR subjects in any of the subregions of the CC. Across-group comparisons of these correlations were performed for those significant in at least one group. The posterior splenium-age correlation was greater in controls than in HR subjects (p=0.093, two tailed). The mid-posterior-age correlation was not significantly different across groups. The results are consistent with our earlier work showing that first episode SZ patients did not show an increase of CC size with age (Keshavan et al., 2002), although the present study used a different methodology.
3.2.1. Quadratic model for CC volume and age
The relationship of CC volume and age was examined using a quadratic model. Age was incorporated as the independent (i.e., age squared) and ICV as a control variable. We ran a regression with CC volume as the dependent variable. The results are in keeping with what we found earlier:
Posterior splenium:
for healthy controls R=0.42 (p=0.001); for HR subjects (p>0.1)
Anterior splenium:
for healthy controls R=0.33 (p=0.020); for HR subjects (p>0.1)
3.3 Callosal volume and gender
Using GLM MANCOVA, we examined the callosal volume in male and female HR subjects. In comparison with male control subjects, male HR subjects showed volume deficits in the mid-posterior splenium (p < 0.003) and trend level reductions in the genu (p < 0.07) and the truncus (p < 0.08). Female HR subjects did not differ from healthy controls in the volume of the CC.
4. Discussion
The study of the CC in HR subjects yields insights into the developmental processes preceding onset of psychosis. A previous study (Venkatasubramanian et al., 2010) examined the CC area in antipsychotic-naïve SZ patients in the mid-sagittal slice and used intracranial area, sex and age as covariates. That study found a significant reduction in the genu [SZ (122.1 ± 24.1); HC (136.6 ± 30.1) mm2, p<0.006] and the body (truncus) [SZ (186.9 ± 33.0) HC (206.0 ± 34.6) mm2, p<0.004] of the CC. Walterfang et al. (2008) examined a different aspect of the CC in subjects at ultra-high risk for SZ. They had two ultra-high risk groups – one psychotic and another nonpsychotic. They looked at total CC area, length, bending angle and mean thickness. They did not find differences in CC area (t=−11, p=0.91), CC length (t=1.34, p=0.18), bending angle (t=1.00, p=0.32), and mean thickness (t=−0.41, p=0.68). The ultra-high risk group (psychotic), however, had significantly smaller regional callosal thickness in the anterior and posterior genu area (p<0.05). The investigators also found that mean thickness of the anterior genu was predictive of transition to psychosis in the ultra-high risk group.
A study by Sismanlar et al. (2010) examined CC volume in young offspring of SZ patients. However, they defined CC volume as the mid-sagittal slice with a slice thickness of 5 mm and a gap of 1.5 mm. They found CC volume in HR subjects to be 3.284 ± 0.116 mm3 and that in healthy controls to be 3.353 ± 0.065 mm3 (p < 0.016). Our study examined the 3D volume of the CC using FreeSurfer. We used a cross-sectional design and showed that HR subjects have significantly smaller total CC volumes than healthy controls, which is comparable to the findings of Sismanalar et al.(2010): total CC volume in healthy controls=3094.88 mm3 (SD=436.07); for HR subjects, it was 2916.98 mm3 (SD=490.5) (p<0.03)]. In addition, our study found volume reductions in the splenium and also a trend level decrement in the rostrum of the CC. Since the study of Walterfang et al. found a decrement in the thickness in the anterior genu in subjects at ultra-high risk, the question remains: Is the volumetric abnormality centered in the genu or splenium or both in the preclinical phase of SZ?
Job et al. (2005) showed that progressive reductions may occur for a period of 3 years before the onset of frank psychosis, suggesting that findings in a HR group may represent a progressive process in an adolescent neurodevelopmental phase. The observed reductions in the present study may be the beginning of a cascade of changes that culminate in frank psychosis in the regions shown in our previous publication (Keshavan et al 2002). Therefore, are volumetric abnormalities in the CC predictors of future psychosis?
The volume decrement in the total CC and specifically in the splenium is consistent with studies examining the morphology and connectivity of the CC in SZ (Walterfang et al., 2008; Rotarska-Jagiela et al., 2008). Studies have shown that the development of the CC proceeds caudorostrally, and the genu matures later than the splenium (Lebel et al., 2008). This also could reflect altered myelination that begins at the splenium and moves to the genu. The anterior splenium of the CC is generally considered as the conduit for inter-hemispheric transmission between bilateral temporo-parietal regions that are part of the heteromodal association cortices (HAC). The HAC has been shown to be morphologically abnormal in schizophrenia (Buchanan et al., 2004) and in offspring at risk for SZ (Bhojraj et al., 2009).
Although we did not measure myelination directly, the alterations in CC volume, especially in the splenium, may be related to aberrations in developmental myelination. Recent studies have shown that myelination (Lebel et al., 2008) begins in the late fetal stage and continues until the third decade in healthy people. Our findings of CC reductions and lack of age-related increases may reflect a derailment in myelination leading to an aberrant development trajectory. However, CC volumes may not necessarily be related to myelination alone; the changes could also be related to changes in axon thickness or numbers.
Taken together, these results show that the development of the CC may be altered in HR subjects. Whether these changes happen prenatally or postnatally is unclear. The CC serves a mini “homunculus” that may mirror neurodevelopmental trajectories of cortical brain regions. Studies have shown that genetic expression of axonal guidance is significantly altered in mice models of SZ (Fatemi et al., 2005) and that offspring of these mice showed thinning of the CC (Fatemi et al. 2008). The normal progression of CC development seen during this period is not seen in HR subjects (Pujol et al., 1993). However, this observation needs to be viewed with caution because of the cross-sectional design of these data. We are also unable to define the timing of callosal changes, i.e. whether they are primary, secondary or occur in concert with gray matter changes. Longitudinal studies of CC morphology such as the study by Mitelman et al. (2009) are crucial to elucidating the relationship of this commissure and the etiopathogenesis of schizophrenia. In measuring callosal size, thickness, volume and shape, race could be a potential confounder. One study found differences between Caucasian, Asian Japanese and Asian Indian populations in the volume of the CC (Gupta et al., 2009).
4.1. Limitations of the study
To our knowledge, this is the first study that used FreeSurfer volumetry of the CC in subjects at genetic high risk for SZ. Therefore, the results we obtained are not directly comparable to those achieved in other CC studies such as those of Walterfang et al. (2008), Venkatasubramanian et al. (2010) or Keshavan et al. (2002), which used other methods of image analysis. However, the volumes of the CC in our study were comparable with those in the study of Sismanalar et al. (2010).
A second limitation concerns normalization and standardization of brain volumes for comparison across groups. According to Bermudez and Zatorre (2001), one aspect of CC morphometry that has surely been a source of conceptual confusion and contributed to inconsistent results and interpretation is that of normalization or standardization for overall brain size/volume. It centers on the non-isometric, geometric relationship between an area and a volume. Because of the incommensurate increase in the volume of an object over the cross-sectional area of that object, the value of this ratio is reduced disproportionately as head size increases, regardless of sex. Since males in general have larger heads, and assuming a reasonably good correlation between CC mid-sagittal area and brain size, there exists the risk of introducing a systematic bias in favor of the smaller headed females. In an attempt to correct for the area/volume relationship, some studies have raised the volume measurement to the power of 2/3. Barring this correction, the only way to avoid this basic geometric problem when using a ratio strategy is to have the normalization variable also be two-dimensional, in other words, an area (such as a sagittal cerebral area). In the present study, the brains were mapped into standardized stereotaxic space using Talairach co-ordinates as an initial step of normalization. Thereafter, we calculated CC volume (3-dimensional) and used a normalization variable, namely intra cranial volume (3-dimensional). We acknowledge the statistical limitations associated with this correction.
Fig. 3.
Scatterplot of CC subregional volumes and age
Fig. 4.
Scatterplot of CC subregional volumes and age
Acknowledgments
The research reported was supported by grants from the National Institute of Mental Health, MH064023 (MSK) and MH045203 (MSK), and a NARSAD Established Investigator award (MSK) We are grateful to Diana Mermon, M.S., for her assistance in clinical assessments and recruitment.
Footnotes
Disclosure/conflict of interest statement
This research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain Research. 1992;598(1–2):143–153. doi: 10.1016/0006-8993(92)90178-c. [DOI] [PubMed] [Google Scholar]
- Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Individual differences in brain asymmetries and fiber composition in the human corpus callosum. Brain Research. 1992;598 (1–2):154–161. doi: 10.1016/0006-8993(92)90179-d. [DOI] [PubMed] [Google Scholar]
- Arnone D, McIntosh AM, Tan GM, Ebmeier KP. Meta-analysis of magnetic resonance imaging studies of the corpus callosum in schizophrenia. Schizophrenia Research. 2008;101 (1–3):124–132. doi: 10.1016/j.schres.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Aydin K, Ucok A, Guler J. Altered metabolic integrity of corpus callosum among individuals at ultra high risk of schizophrenia and first-episode patients. Biological Psychiatry. 2008;64 (9):750–757. doi: 10.1016/j.biopsych.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Bachmann S, Pantel J, Flender A, Bottmer C, Essig M, Schroder J. Corpus callosum in first-episode patients with schizophrenia--a magnetic resonance imaging study. Psychological Medicine. 2003;33 (6):1019–1027. doi: 10.1017/s0033291703008043. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Topography of commissural fibers of the prefrontal cortex in the rhesus monkey. Experimental Brain Research. 1984;55 (1):187–191. doi: 10.1007/BF00240516. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. Journal of Comparative Neurology. 1989;286 (3):353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- Barnett KJ, Corballis MC, Kirk IJ. Symmetry of callosal information transfer in schizophrenia: a preliminary study. Schizophrenia Research. 2005;74 (2–3):171–178. doi: 10.1016/j.schres.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Barnett KJ, Kirk IJ, Corballis MC. Bilateral disadvantage: lack of interhemispheric cooperation in schizophrenia. Consciousness and Cognition. 2007;16 (2):436–444. doi: 10.1016/j.concog.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Bermudez P, Zatorre RJ. Sexual dimorphism in the corpus callosum: methodological considerations in MRI morphometry. Neuroimage. 2001;13 (6 Pt 1):1121–1130. doi: 10.1006/nimg.2001.0772. [DOI] [PubMed] [Google Scholar]
- Bhojraj TS, Francis AN, Rajarethinam R, Eack S, Kulkarni S, Prasad KM, Montrose DM, Dworakowski D, Diwadkar V, Keshavan MS. Verbal fluency deficits and altered lateralization of language brain areas in individuals genetically predisposed to schizophrenia. Schizophrenia Research. 2009;115 (2–3):202–208. doi: 10.1016/j.schres.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigelow LB, Nasrallah HA, Rauscher FP. Corpus callosum thickness in chronic schizophrenia. British Journal of Psychiatry. 1983;142:284–287. doi: 10.1192/bjp.142.3.284. [DOI] [PubMed] [Google Scholar]
- Bogen JE, Fisher ED, Vogel PJ. Cerebral commissurotomy. A second case report. JAMA. 1965;194 (12):1328–1329. [PubMed] [Google Scholar]
- Brambilla P, Cerini R, Gasparini A, Versace A, Andreone N, Vittorini E, Barbui C, Pelizza L, Nose M, Barlocco L, Marrella G, Gregis M, Tournikioti K, David AS, Keshavan MS, Tansella M. Investigation of corpus callosum in schizophrenia with diffusion imaging. Schizophrenia Researcj. 2005;79 (2–3):201–210. doi: 10.1016/j.schres.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Francis A, Arango C, Miller K, Lefkowitz DM, McMahon RP, Barta PE, Pearlson GD. Morphometric assessment of the heteromodal association cortex in schizophrenia. American Journal of Psychiatry. 2004;161 (2):322–331. doi: 10.1176/appi.ajp.161.2.322. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Sanders RD, Goldberg TE, Bigelow LB, Christison G, Torrey EF, Weinberger DR. Morphometry of the corpus callosum in monozygotic twins discordant for schizophrenia: a magnetic resonance imaging study. Journal of Neurology, Neurosurgery, and Psychiatry. 1990;53 (5):416–421. doi: 10.1136/jnnp.53.5.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance SA, Casanova MF, Switala AE, Crow TJ. Minicolumnar structure in Heschl’s gyrus and planum temporale: Asymmetries in relation to sex and callosal fiber number. Neuroscience. 2006;143 (4):1041–1050. doi: 10.1016/j.neuroscience.2006.08.057. [DOI] [PubMed] [Google Scholar]
- Chance SA, Casanova MF, Switala AE, Crow TJ. Auditory cortex asymmetry, altered minicolumn spacing and absence of ageing effects in schizophrenia. Brain. 2008;131(Pt 12):3178–3192. doi: 10.1093/brain/awn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow TJ. Schizophrenia as a transcallosal misconnection syndrome. Schizophrenia Research. 1998;30 (2):111–114. doi: 10.1016/s0920-9964(97)00139-4. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9 (2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- de Lacoste MC, Kirkpatrick JB, Ross ED. Topography of the human corpus callosum. Journal of Neuropathology and Experimental Neurology. 1985;44 (6):578–591. doi: 10.1097/00005072-198511000-00004. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31 (3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Endrass T, Mohr B, Rockstroh B. Reduced interhemispheric transmission in schizophrenia patients: evidence from event-related potentials. Neuroscience Letters. 2002;320 (1–2):57–60. doi: 10.1016/s0304-3940(02)00032-0. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Pearce DA, Brooks AI, Sidwell RW. Prenatal viral infection in mouse causes differential expression of genes in brains of mouse progeny: a potential animal model for schizophrenia and autism. Synapse. 2005;57 (2):91–99. doi: 10.1002/syn.20162. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Huang H, Oishi K, Mori S, Smee DF, Pearce DA, Winter C, Sohr R, Juckel G. Maternal infection leads to abnormal gene regulation and brain atrophy in mouse offspring: implications for genesis of neurodevelopmental disorders. Schizophrenia Research. 2008;99 (1–3):56–70. doi: 10.1016/j.schres.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping. 1999;8 (4):272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004;14 (1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Gruzelier JH. Functional neuropsychophysiological asymmetry in schizophrenia: a review and reorientation. Schizophrenia Bulletin. 1999;25 (1):91–120. doi: 10.1093/oxfordjournals.schbul.a033370. [DOI] [PubMed] [Google Scholar]
- Gupta T, Singh B, Kapoor K, Gupta M, Kochhar S. Normative data of corpus callosal morphology in a North-West Indian population- an autopsy and MRI study. JNMA: Journal of the Nepalese Medical Association. 2009;48 (173):46–51. [PubMed] [Google Scholar]
- Highley JR, Esiri MM, McDonald B, Cortina-Borja M, Herron BM, Crow TJ. The size and fibre composition of the corpus callosum with respect to gender and schizophrenia: a post-mortem study. Brain. 1999;122 (Pt 1):99–110. doi: 10.1093/brain/122.1.99. [DOI] [PubMed] [Google Scholar]
- Hoffman RE, McGlashan TH. Reduced corticocortical connectivity can induce speech perception pathology and hallucinated ‘voices’. Schizophrenia Research. 1998;30 (2):137–141. doi: 10.1016/s0920-9964(97)00142-4. [DOI] [PubMed] [Google Scholar]
- Jancke L, Staiger JF, Schlaug G, Huang Y, Steinmetz H. The relationship between corpus callosum size and forebrain volume. Cerebral Cortex. 1997;7 (1):48–56. doi: 10.1093/cercor/7.1.48. [DOI] [PubMed] [Google Scholar]
- Job DE, Whalley HC, Johnstone EC, Lawrie SM. Grey matter changes over time in high risk subjects developing schizophrenia. Neuroimage. 2005;25(4):1023–1030. doi: 10.1016/j.neuroimage.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Karol EA, Heilbronn D, Pandya D. The termination of callosal fibers in the rhesus monkey. Transactions of the American Neurological Association. 1970;95:269–271. [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, Harenski K, Rosenberg DR, Sweeney JA, Pettegrew JW. Abnormalities of the corpus callosum in first episode, treatment naive schizophrenia. Journal of Neurology, Neurosurgery, and Psychiatry. 2002;72(6):757–760. doi: 10.1136/jnnp.72.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Prasad KM, Pearlson G. Are brain structural abnormalities useful as endophenotypes in schizophrenia? International Review of Psychiatry. 2007;19 (4):397–406. doi: 10.1080/09540260701486233. [DOI] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, McCarley RW, Shenton ME. The application of DTI to investigate white matter abnormalities in schizophrenia. Annals of the New York Academy of Sciences. 2005;1064:134–148. doi: 10.1196/annals.1340.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40 (3):1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Leitman DI, Hoptman MJ, Foxe JJ, Saccente E, Wylie GR, Nierenberg J, Jalbrzikowski M, Lim KO, Javitt DC. The neural substrates of impaired prosodic detection in schizophrenia and its sensorial antecedents. American Journal of Psychiatry. 2007;164 (3):474–482. doi: 10.1176/ajp.2007.164.3.474. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Nikiforova YK, Canfield EL, Hazlett EA, Brickman AM, Shihabuddin L, Buchsbaum MS. A longitudinal study of the corpus callosum in chronic schizophrenia. Schizophrenia Research. 2009;114 (1–3):144–153. doi: 10.1016/j.schres.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata J, Hirao K, Namiki C, Fukuyama H, Okada T, Miki Y, Hayashi T, Murai T. Interfrontal commissural abnormality in schizophrenia: tractography-assisted callosal parcellation. Schizophrenia Research. 2007;97 (1–3):236–241. doi: 10.1016/j.schres.2007.07.032. [DOI] [PubMed] [Google Scholar]
- Mohr B, Pulvermuller F, Cohen R, Rockstroh B. Interhemispheric cooperation during word processing: evidence for callosal transfer dysfunction in schizophrenic patients. Schizophrenia Research. 2000;46 (2–3):231–239. doi: 10.1016/s0920-9964(00)00020-7. [DOI] [PubMed] [Google Scholar]
- Nasrallah HA, Andreasen NC, Coffman JA, Olson SC, Dunn VD, Ehrhardt JC, Chapman SM. A controlled magnetic resonance imaging study of corpus callosum thickness in schizophrenia. Biological Psychiatry. 1986;21 (3):274–282. doi: 10.1016/0006-3223(86)90048-x. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Karol EA, Heilbronn D. The topographical distribution of interhemispheric projections in the corpus callosum of the rhesus monkey. Brain Research. 1971;32 (1):31–43. doi: 10.1016/0006-8993(71)90153-3. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Vignolo LA. Interhemispheric projections of the parietal lobe in the rhesus monkey. Brain Research. 1969;15 (1):49–65. doi: 10.1016/0006-8993(69)90309-6. [DOI] [PubMed] [Google Scholar]
- Pujol J, Vendrell P, Junque C, Marti-Vilalta JL, Capdevila A. When does human brain development end? Evidence of corpus callosum growth up to adulthood. Annals of Neurology. 1993;34(1):71–75. doi: 10.1002/ana.410340113. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, Bigelow LB. Quantitative brain measurements in chronic schizophrenia. British Journal of Psychiatry. 1972;121 (562):259–264. doi: 10.1192/bjp.121.3.259. [DOI] [PubMed] [Google Scholar]
- Rossell SL, Shapleske J, Fukuda R, Woodruff PW, Simmons A, David AS. Corpus callosum area and functioning in schizophrenic patients with auditory--verbal hallucinations. Schizophrenia Research. 2001;50 (1–2):9–17. doi: 10.1016/s0920-9964(00)00070-0. [DOI] [PubMed] [Google Scholar]
- Rotarska-Jagiela A, Schonmeyer R, Oertel V, Haenschel C, Vogeley K, Linden DE. The corpus callosum in schizophrenia-volume and connectivity changes affect specific regions. Neuroimage. 2008;39 (4):1522–1532. doi: 10.1016/j.neuroimage.2007.10.063. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Casanova M, Mannheim GB, Patronas N, De Vaughn N, Hamburger SD, Aquino T. Corpus callosum morphology, as measured with MRI, in dyslexic men. Biological Psychiatry. 1996;39 (9):769–775. doi: 10.1016/0006-3223(95)00225-1. [DOI] [PubMed] [Google Scholar]
- Schlosser R, Wagner G, Kohler S, Sauer H. [Schizophrenia as a disconnection syndrome. Studies with functional magnetic resonance imaging and structural equation modeling] Radiologe. 2005;45(2):137–140. 142–133. doi: 10.1007/s00117-004-1160-3. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Giuliano AJ, Meyer EC, Addington J, Cadenhead KS, Cannon TD, McGlashan TH, Perkins DO, Tsuang MT, Walker EF, Woods SW, Bearden CE, Christensen BK, Hawkins K, Heaton R, Keefe RS, Heinssen R, Cornblatt BA North American Prodrome Longitudinal Study (NAPLS) Group. Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Archives of General Psychiatry. 2010;67 (6):578–588. doi: 10.1001/archgenpsychiatry.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Yolken RH. Novel alpha7 nicotinic receptor isoforms and deficient cholinergic transcription in schizophrenia. Genes, Brain, and Behavior. 2008;7 (1):37–45. doi: 10.1111/j.1601-183X.2007.00323.x. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophrenia Research. 2001;49 (1–2):1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sişmanlar SG, Anik Y, Coşkun A, Ağaoğlu B, Karakaya I, Yavuz CI. The volumetric differences of the fronto-temporal region in young offspring of schizophrenic patients. European Child and Adolescent Psychiatry. 2010;19 (2):151–157. doi: 10.1007/s00787-009-0052-5. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Alexander GE, Schapiro MB, Moller HJ, Rapoport SI, Hampel H. Age-related cortical grey matter reductions in non-demented Down’s syndrome adults determined by MRI with voxel-based morphometry. Brain. 2004;127 (Pt 4):811–824. doi: 10.1093/brain/awh101. [DOI] [PubMed] [Google Scholar]
- Tibbo P, Nopoulos P, Arndt S, Andreasen NC. Corpus callosum shape and size in male patients with schizophrenia. Biological Psychiatry. 1998;44 (6):405–412. doi: 10.1016/s0006-3223(98)00096-1. [DOI] [PubMed] [Google Scholar]
- van der Kouwe AJ, Benner T, Salat DH, Fischl B. Brain morphometry with multiecho MPRAGE. Neuroimage. 2008;40 (2):559–569. doi: 10.1016/j.neuroimage.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatasubramanian G, Jayakumar PN, Reddy VV, Reddy US, Gangadhar BN, Keshavan MS. Corpus callosum deficits in antipsychotic-naïve schizophrenia: evidence for neurodevelopmental pathogenesis. Psychiatry Research: Neuroimaging. 2010;182 (2):141–145. doi: 10.1016/j.pscychresns.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Walterfang M, Yung A, Wood AG, Reutens DC, Phillips L, Wood SJ, Chen J, Velakoulis D, McGorry PD, Pantelis C. Corpus callosum shape alterations in individuals prior to the onset of psychosis. Schizophrenia Research. 2008;103 (1–3):1–10. doi: 10.1016/j.schres.2008.04.042. [DOI] [PubMed] [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112 (Pt 3):799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Woodruff PW, Pearlson GD, Geer MJ, Barta PE, Chilcoat HD. A computerized magnetic resonance imaging study of corpus callosum morphology in schizophrenia. Psychological Medicine. 1993;23 (1):45–56. doi: 10.1017/s0033291700038836. [DOI] [PubMed] [Google Scholar]
- Woodruff PW, McManus IC, David AS. Meta-analysis of corpus callosum size in schizophrenia. Journal of Neurology, Neurosurgery, and Psychiatry. 1995;58 (4):457–461. doi: 10.1136/jnnp.58.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]