Abstract
Background
The optimal threshold for initiating HIV treatment is unclear.
Objective
To compare different thresholds for initiating HIV treatment.
Design
We used our validated computer simulation to weigh important harms from earlier initiation of antiretroviral therapy (toxicity, side effects, and resistance accumulation) against important benefits (decreased HIV-related mortality).
Data Sources
Veterans Aging Cohort Study (5742 HIV-infected patients and 11 484 matched uninfected controls) and published reports.
Target Population
Individuals with newly diagnosed chronic HIV infection and varying viral loads (10 000, 30 000, 100 000, and 300 000 copies/mL) and ages (30, 40, and 50 years).
Time Horizon
Unlimited.
Perspective
Societal.
Intervention
Alternative thresholds for initiating antiretroviral therapy (CD4 counts of 200, 350, and 500 cells/mm3).
Outcome Measures
Life-years and quality-adjusted life-years (QALYs).
Results of Base-Case Analysis
Although the simulation was biased against earlier treatment initiation because it used an upper-bound assumption for therapy-related toxicity, earlier treatment increased life expectancy and QALYs at age 30 years regardless of viral load (life expectancies with CD4 initiation thresholds of 500, 350, and 200 cells/mm3 were 18.2 years, 17.6 years, and 17.2 years, respectively, for a viral load of 10 000 copies/mL and 17.3 years, 15.9 years, and 14.5 years, respectively, for a viral load of 300 000 copies/mL), and increased life expectancies at age 40 years if viral loads were greater than 30 000 copies/mL (life expectancies were 12.5 years, 12.0 years, and 11.4 years, respectively, for a viral load of 300 000 copies/mL).
Results of Sensitivity Analysis
Findings favoring early treatment were generally robust.
Limitations
Results favoring later treatment may not be valid. The findings may not be generalizable to women.
Conclusions
This simulation suggests that earlier initiation of combination antiretroviral therapy is often favored compared with current recommendations.
The optimal timing for initiating HIV treatment has long been controversial. Soon after combination anti- retroviral therapies became available, the prevailing treatment ethos was “hit early, hit hard,” and patients often began receiving therapy immediately after HIV infection was diagnosed (1). However, as it became clear that toxicity and side effects from combination antiretroviral therapy were substantial (2–5) and that early treatment may hasten genotypic resistance (6–8), treatment guidelines increasingly have favored postponing therapy initiation (9, 10). Yet postponing therapy has its own disadvantages because it exposes HIV-infected patients to a greater risk for AIDS than is otherwise necessary (9–15) and may impair immune reconstitution (16). Guidelines once again may be in flux, trending toward earlier treatment initiation (17).
The controversy about when to initiate HIV treatment has persisted because it has been difficult to systematically and quantitatively weigh the benefits and harms of earlier treatment (17). Furthermore, because benefits and harms may be delayed (for example, the accrual of genotypic resistance) (8), they must be estimated over time horizons that exceed usual follow-up periods for clinical trials. Mathematical models offer the possibility of quantitatively weighing harms and benefits over long time horizons, and therefore have theoretical promise for informing this question. However, published mathematical models addressing this question have not considered toxicity, side effects, and accrual of resistance mutations, the primary risks of earlier treatment initiation (18–21). Furthermore, they have not considered how the balance of risks to benefits may vary with advancing age because of changes in antiretroviral toxicity or immune reconstitution. For these reasons, we have incorporated toxicity and side effects of combination antiretroviral therapy into our previously validated HIV computer simulation, which considers resistance mutations and their likelihood of causing premature antiretroviral failure (22–24). We then used the simulation to evaluate how these tradeoffs influence the optimal time to initiate therapy.
Methods
We first describe how we quantified the potentially harmful consequences of combination antiretroviral therapy and then discuss how we incorporated these estimates into our HIV simulation.
Estimating the Harmful Consequences of Combination Antiretroviral Therapy
We partitioned the harmful effect of combination antiretroviral therapy into 2 quantifiable categories: 1) a harmful effect on quantity of life due to toxicity and am-plified risks for common comorbid events (for example, myocardial infarction) (2–5), manifested by higher non–HIV-related mortality and 2) a harmful effect on quality of life due to side effects, manifested by decreased scores on a preference-based quality-of-life measure (25).
Estimating the Effect of Combination Antiretroviral Therapy on Non–HIV-Related Mortality
It is not possible to directly estimate the effect of combination antiretroviral therapy on non–HIV-related mortality because there has been no randomized, controlled trial with requisite statistical power. Large observational cohorts of HIV-infected patients have ample statistical power to address this question by comparing non–HIV-related mortality among individuals receiving with those not receiving combination antiretroviral therapy. However, confounding by treatment assignment may bias these results substantially, and the direction of this bias may be unclear.
We therefore conducted our own analysis of mortality among all HIV-infected individuals receiving care within the Veterans Health Administration nationwide, together with a sample of uninfected Veterans Health Administration controls, matched 2:1 by age, race, and site. This “virtual cohort” includes inpatients as well as outpatients and patients receiving subspecialty care as well as those receiving primary care. It was created by integrating data from pharmacy, laboratory, and administrative sources from 1997 through 2004. The creation of this “virtual cohort” is described in detail elsewhere (26) and has been shown to identify HIV-infected veterans with a sensitivity of 90% and a specificity of 99.9% (26).
We limited our analysis to HIV-infected individuals who were receiving combination antiretroviral therapy (≥3 drugs) and were at exceptionally low risk for HIV-related death. We then compared the mortality of HIV-infected patients who met these inclusion criteria with the mortality of their matched uninfected controls. Our rationale in making this comparison was that excess mortality observed in the HIV-infected group could be viewed as an upper limit for the non–HIV-related mortality attributable to combination antiretroviral therapy because it would reflect non–HIV-related mortality as well as other factors (for example, residual mortality attributable to HIV). We decided not to analyze only HIV-infected patients because we did not think that we could fully control for intention-to-treat bias and because we would not know the direction of this bias (that is, we would not know that the result was accurate or that it bounded the true value in a particular direction).
We selected individuals at low risk for death related to HIV by specifying a minimum threshold for their time-updated CD4 count (that is, individuals could contribute a particular time interval of observation to the analysis only if they remained above the CD4 threshold during that interval). We used a threshold of 500 cells/mm3 because it is extremely uncommon for individuals with higher CD4 counts to die of an AIDS-related cause (27). We also explored alternative analyses using thresholds based on minimum CD4 count rather than time-varying CD4 count (9), but this did not affect our results substantially.
We performed Cox proportional hazards models to compare the mortality of the HIV-infected patients with that of their matched uninfected controls, including the covariates age, sex, race, site of care, and presence or absence of the most common serious comorbid conditions in this sample (coronary artery disease, congestive heart failure, diabetes mellitus, hepatitis C, pancreatitis, peripheral vascular disease, pulmonary disease, stroke, pneumonia, and non-AIDS cancer) . Diagnoses were identified by using a previously validated algorithm based on International Classification of Diseases, ninth revision, administrative codes in the inpatient and outpatient settings (26). To avoid overadjusting for comorbid conditions, we required comorbidity diagnoses to have been made before combination antiretroviral therapy was initiated. We did not stratify combination antiretroviral therapy by type (for example, regimens with protease inhibitors versus regimens with nonnucleoside reverse transcriptase inhibitors) because this would have greatly limited our statistical power.
Because there appeared to be a substantially higher mortality hazard in the short term after the start of combination antiretroviral therapy (during the first 3 months) than over the long term, we considered this short-term hazard separately. We judged this high short-term hazard as probably due to confounding (that is, attributable to an acute clinical syndrome that may have prompted the initiation of care) rather than to combination antiretroviral therapy toxicity, and therefore we did not incorporate it into the simulation’s base-case analysis. However, we also explored alternative analyses in which we did attribute this high short-term hazard to toxicity, and this did not affect our results substantially.
Among 33 420 HIV-infected patients in the virtual cohort, 9633 (29%) had infrequent or missing CD4 counts (>1 year between successive CD4 measurements), and an additional 18 045 did not meet inclusion criteria (no combination antiretroviral therapy, or CD4 count always < 500 cells/mm3), leaving 5742 HIV-infected patients along with 11 484 controls (17 226 patients total) in our analysis. The mean age of patients and controls was 45.7 years, 98.0% were men, and 68.9% were nonwhite. We found that combination antiretroviral therapy was associated with a 3.4-fold increased long-term hazard of non–HIV-related mortality in unadjusted analyses (95% CI, 2.8-fold to 4.1-fold) and a 3.8-fold increased hazard in adjusted analyses (CI, 3.1-fold to 4.6-fold) (Table 1). Because this association is likely to encompass other factors besides toxicity of combination antiretroviral therapy, it is an upper-bound estimate—that is, the true magnitude of the effect of combination antiretroviral therapy is likely to be lower. We included this estimate in the simulation model with the understanding that it biases the model in a known, conservative direction (against showing a benefit from earlier treatment initiation).
Table 1.
Analysis To Estimate Upper Bound for Mortality from Therapy-Related Toxicity: Predictors of Mortality among HIV-Infected Veterans and Matched Uninfected Controls Nationwide in the Veterans Affairs Health System*
| Characteristic | Hazard Ratio (95% CI) | P Value | Adjusted Hazard Ratio (95% CI) | P Value |
|---|---|---|---|---|
| Exposure to combination antiretroviral therapy and HIV-infected† | 3.4 (2.8–4.1) | <0.001 | 3.8 (3.1–4.6) | <0.001 |
| Age (compared with <40 y) | ||||
| 40–49 y | 2.5 (2.2–2.9) | <0.001 | 2.3 (1.9–2.6) | <0.001 |
| 50–59 y | 4.2 (3.5–4.9) | <0.001 | 3.3 (2.8–4.0) | <0.001 |
| ≥60 y | 7.8 (6.5–9.3) | <0.001 | 5.4 (4.5–6.7) | <0.001 |
| Race/ethnicity (compared with white) | ||||
| African American | 0.9 (0.8–1.0) | 0.06 | 0.9 (0.8–1.0) | 0.10 |
| Hispanic | 0.7 (0.6–0.9) | 0.01 | 0.8 (0.6–1.0) | 0.05 |
| Comorbid condition | ||||
| Coronary artery disease | 2.1 (1.8–2.4) | <0.001 | 0.9 (0.7–1.0) | 0.07 |
| Congestive lheart failure | 4.9 (4.0–5.9) | <0.001 | 2.2 (1.8–2.8) | <0.001 |
| Diabetes melitus | 2.4 (2.1–2.8) | <0.001 | 1.5 (1.3–1.8) | <0.001 |
| Hepatitis C | 1.7 (1.3–2.1) | <0.001 | 1.7 (1.3–2.2) | <0.001 |
| Pancreatitis | 2.0 (1.6–2.6) | <0.001 | 1.7 (1.3–2.2) | <0.001 |
| Peripheral vascular disease | 3.0 (2.4–3.8) | <0.001 | 1.2 (0.9–1.6) | 0.19 |
| Pulmonary disease | 2.1 (1.8–2.4) | <0.001 | 1.4 (1.2–1.6) | <0.001 |
| Stroke | 3.3 (2.7–3.9) | <0.001 | 1.6 (1.3–2.0) | <0.001 |
| Pneumonia | 2.9 (2.4–3.5) | <0.001 | 1.7 (1.4–2.1) | <0.001 |
| Non-AIDS cancer | 3.6 (3.1–4.3) | <0.001 | 2.4 (1.9–2.9) | <0.001 |
Each HIV-infected participant contributed to the analysis only when he or she was receiving antiretroviral therapy and was at low risk for AIDS-related death (CD4 count ≥500 cells/mm3). This analysis excludes the first 3 months after individuals started antiretroviral therapy because this period was associated with a brief, high mortality rate that was probably attributable to the circumstances surrounding presentation for care, rather than to antiretroviral therapy.
This analysis does not allow us to disaggregate the effect of antiretroviral therapy from known and unknown effects of HIV and other factors. It may be interpreted as a “ceiling” estimate for the mortality effect of combination antiretroviral therapy.
Estimating the Effect of Combination Antiretroviral Therapy on Quality of Life
We estimated the effect of combination antiretroviral therapy on quality of life on the basis of the Veterans Aging Cohort Study (28), an 8-site prospectively consented study of HIV-infected and matched uninfected veterans receiving care that includes detailed surveys on symptom burden and quality of life. We compared the quality of life among individuals who did and did not have side effects from combination antiretroviral therapy, controlling for other covariates. On the basis of an analysis of 1864 patients (median age, 50 years; 97% men, 73% nonwhite) that is described in more detail elsewhere (25), we found that 66% of individuals receiving antiretroviral therapy reported side effects that they possibly or definitely attributed to antiretroviral drugs, and that the associated decrement in quality of life, or utility, was 0.08. Utility is a preference-weighted quality-of-life measure on a scale from 0 to 1, and this change in utility is clinically meaningful (for example, similar to the decrement in the utility of mild angina) but not overwhelming (for example, less than the decrement in the utility of moderate angina) (29).
Incorporating Harms and Benefits in Computer Simulation
We previously developed a computer simulation of HIV that represents the beneficial effect of combination antiretroviral therapy on CD4 count and viral load trajectories, and consequently on HIV-related mortality (22). It advances previous simulation efforts by explicitly modeling the biological processes that underlie the eventual decreased effectiveness of antiretroviral therapy (accumulation of genotypic resistance and poor adherence) and using them to estimate time to treatment failure.
Overview of Simulation
The computer model is a second-order Monte Carlo simulation, and it mimics a patient cohort in which each person is followed until death. It can assign otherwise similar patient cohorts to different treatment decisions (that is, starting combination antiretroviral therapy at a CD4 count of 350 cells/mm3 vs. starting therapy at a CD4 count of 500 cells/mm3) and compare the effect of this decision on designated outcomes. Because the simulation is probabilistic, it can represent much of the heterogeneity of actual patient populations (for example, clinical events, such as deaths, may or may not happen within any particular time interval, and their probability of occurrence is based on known predictors). Because of its long time horizon, the simulation can capture the aggregate effect of long-term exposure to toxicities, using the analytic machinery of the computer to “sum” this exposure over time. In other words, a younger person starting therapy may have exposure to toxicity from combination antiretroviral therapy for many decades, far longer than older patients starting this therapy; this differential toxicity exposure is reflected in survival. Concepts represented in the simulation fall into 2 broad categories: genetic characteristics of the HIV strain and clinical characteristics of the patient (Figure 1). The simulation predicts mortality related to HIV separately from mortality unrelated to HIV, and includes a toxicity parameter that can amplify the mortality attributable to non–HIV-related causes. The simulation was originally developed by using Decision Maker for Windows software (version beta 0.99.11.12a, University of Medicine and Dentistry of New Jersey), and has since been converted to the C programming language.
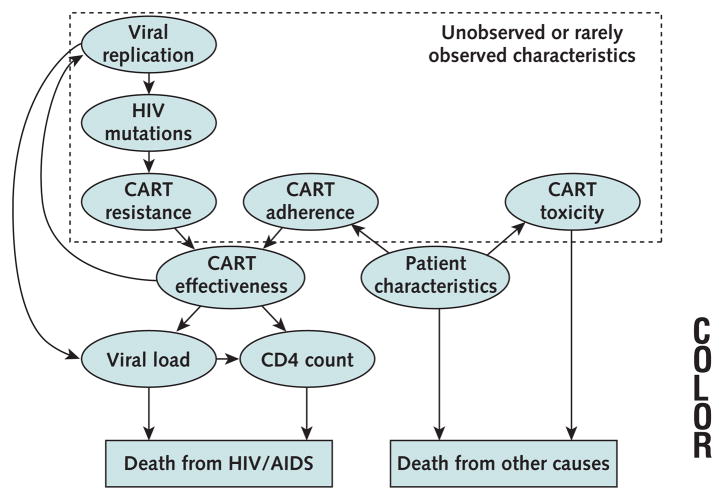
Figure 1. Selected constructs in computer simulation.
Constructs fall into 2 broad categories: genetic characteristics of the HIV strain and clinical characteristics of the patient. Genetic characteristics reflect the acquisition of mutations and affect clinical characteristics by altering the effectiveness of combination antiretroviral therapy (CART). Clinical characteristics affect the probability of dying of HIV-related or non–HIV-related causes. With the passing of time, combination antiret-roviral therapy may give rise to HIV mutations through selection pressures on viral replication. As resistance accrues, the viral replication rate increases, and this in turn increases the probability that subsequent mutations will develop. Adherence, viral resistance, and other patient characteristics together determine the level of effectiveness of combination therapies, as manifested by changes in CD4 count and viral load. The simulation does not merely extrapolate long-term mortality risks based on short-term mortality data but rather predicts long-term mortality risks based on the duration of the effectiveness of combination antiretroviral therapy and other factors, including toxicity. Although determinants of the effectiveness of combination antiretroviral therapy may be unobserved or rarely observed, they may have a profound effect on survival and quality of life.
Validation of Simulation
This validation of the simulation has been described elsewhere (22–24). It has closely reproduced Kaplan–Meier curves of time to treatment failure for the first 3 rounds of combination antiretroviral therapy among 3545 antiretro-viral-naive patients (22), and has closely reproduced a Kaplan–Meier curve of survival in the same cohort (22). It has yielded 3-year mortality estimates stratified by age, CD4 count, and viral load that were similar to those from a large (n = 12 574) patient cohort that was distinct from the derivation cohort (22, 30). The simulation replicated and explained clinically observed heterogeneity in the relationship of antiretroviral adherence to accumulation of resistance mutations (23). Finally, it predicted rates of accumulating resistance mutations that were observed among antiretroviral-naive patients in several patient samples, even though these data were not yet published at the time the model was calibrated (24).
In accord with clinical data, this simulation has previously estimated that the beneficial effect of combination antiretroviral therapy on HIV-related mortality is substantial, increasing life expectancy by 5 to 20 years depending on clinical and behavioral characteristics (22). For the current study, we used the same parameter estimates (other than age, viral load, and CD4 count) that resulted in the best goodness of fit during model calibration and that are described elsewhere (23). For example, the current study assumes a baseline adherence of 75% of antiretroviral doses taken as directed. This is the estimate that resulted in the best model calibration (22), and it is also consistent with the 95% CI from a meta-analysis of antiretroviral adherence (24).
Scenarios Evaluated
We evaluated successive cohorts of individuals with newly diagnosed chronic HIV infection (that is, not primary infection), each starting with a CD4 count of 500 cells/mm3, and considering alternative thresholds for treatment initiation of 500, 350, and 200 cells/mm3. We considered ages of 30, 40, and 50 years and baseline viral loads of 10 000, 30 000, 100 000, and 300 000 copies/mL. The outcomes we evaluated were mean life expectancy and mean quality-adjusted life-years (QALYs). We compared these treatment thresholds (rather than using the simulation to evaluate a wider range of possibilities) to simplify comparisons with current guidelines and previous studies. We performed sensitivity analyses by varying each parameter in our simulation across its plausible range and by estimating the minimum toxicity necessary to influence treatment initiation decisions.
Role of the Funding Source
The funding source had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Results
Even after the harmful consequences of combination antiretroviral therapy were incorporated into our model, earlier treatment was still favored for many scenarios that we evaluated.
Life Expectancy
Although our analyses were biased against earlier treatment because of our upper-bound estimate for treatment toxicity, we found that earlier treatment initiation improved life expectancy for many of the scenarios we evaluated (Table 2). This benefit occurred even though earlier therapy hastened the accumulation of resistance mutations and reduced future drug options (Figure 2).
Table 2.
Computer Simulation Estimates of Life Expectancy and Quality-Adjusted Life-Years by Treatment Initiation Threshold, Age, and Viral Load*
| Life-Years (QALYs)
|
|||
|---|---|---|---|
| Viral Load, copies/mL | Starting Therapy at CD4 Count of 200 cells/mm3 | Starting Therapy at CD4 Count of 350 cells/mm3 | Starting Therapy at CD4 Count of 500 cells/mm3 |
|
Age 30 y
| |||
| 10 000 | 17.2 (15.1) | 17.6 (15.5) | 18.2 (15.9) |
|
| |||
| 30 000 | 16.7 (14.5) | 17.3 (15.1) | 18.0 (15.8) |
|
| |||
| 100 000 | 15.3 (13.3) | 16.3 (14.2) | 17.5 (15.3) |
|
| |||
| 300 000 | 14.5 (12.5) | 15.9 (13.7) | 17.3 (15.1) |
|
Age 40 y
| |||
| 10 000 | 13.6 (12.1) | 13.3 (11.7) | 12.9 (11.4) |
|
| |||
| 30 000 | 12.9 (11.4) | 12.9 (11.4) | 12.8 (11.3) |
|
| |||
| 100 000 | 12.1 (10.6) | 12.4 (10.9) | 12.6 (11.1) |
|
| |||
| 300 000 | 11.4 (9.9) | 12.0 (10.5) | 12.5 (10.9) |
|
Age 50 y
| |||
| 10 000 | 10.4 (9.3) | 9.8 (8.8) | 9.2 (8.1) |
|
| |||
| 30 000 | 9.9 (8.9) | 9.6 (8.5) | 9.2 (8.1) |
|
| |||
| 100 000 | 9.4 (8.4) | 9.3 (8.3) | 9.0 (8.0) |
|
| |||
| 300 000 | 9.0 (8.0) | 9.0 (8.0) | 9.0 (7.9) |
The simulation uses a very pessimistic (upper-bound) toxicity assumption, and therefore estimates are lower than probable values. Boldface numbers signify the most favored threshold. The pessimistic toxicity assumptions mute the effect of baseline viral load on life expectancy because of the high competing risks for non–HIV-related death, particularly with older age and earlier combination anti-retroviral therapy initiation. QALY = quality-adjusted life-year.
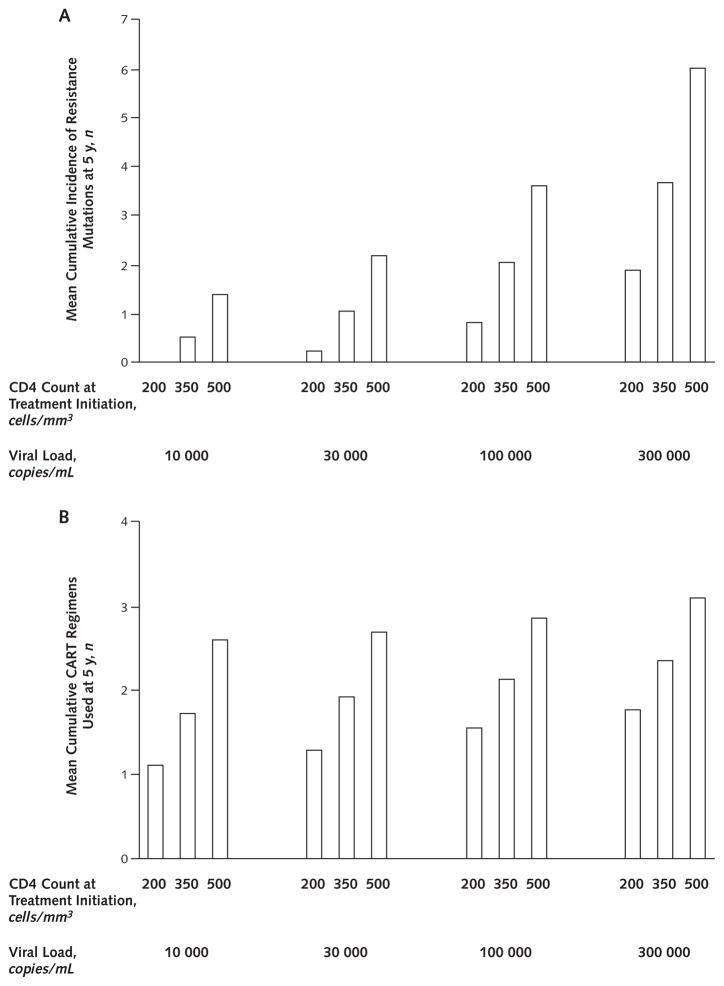
Figure 2. Cumulative incidence of resistance mutations (A) and combination antiretroviral therapy (CART) regimens (B) after 5 years, by treatment initiation threshold and viral load.
Earlier therapy leads to more resistance mutations and to more regimens being used. Resistance mutations are defined as any mutation that may give rise to antiretroviral resistance in any drug class. A change in regimen is defined as a change in 2 or more antiretroviral drugs. We show results for age 40 years; results for other age groups did not differ greatly.
For 30-year-olds, earlier initiation of combination antiretroviral therapy was always preferred regardless of viral load because the harms (that is, increase in non–HIV-related mortality from toxicity, increase in accumulation of resistance mutations, and decrease in future drug options) were small compared with the benefit (that is, decrease in HIV-related mortality from the effect of therapy on the natural history of HIV). For 40-year-olds, therapy was associated with a greater increase in non–HIV-related mortality compared with that seen for 30-year-olds. For this reason, earlier therapy initiation increased life expectancy only among individuals in the higher viral load strata, who had more to gain from earlier treatment initiation. The harm from earlier therapy initiation was highest in 50-year- olds, and therefore later treatment initiation was generally preferred.
Quality-Adjusted Life Expectancy
Results from analyses that used QALY as an outcome measures were generally similar to results from analyses that used life expectancy as an outcome measure (Table 2). They favored earlier treatment initiation for 30-year-olds, earlier treatment initiation for 40-year-olds with higher viral loads, and later treatment for 50-year-olds.
Sensitivity Analyses
Findings in favor of earlier treatment were generally robust. To favor later treatment thresholds, therapy-related toxicity would need to be far higher than our upper-bound estimate (Appendix Figure 1, available at www.annals.org). When we varied each model parameter across its plausible range, earlier treatment remained favorable except in unlikely combinations of circumstances in which the upper-bound toxicity assumption was paired with another particularly strong assumption opposing earlier treatment (Appendix Figure 2, available at www.annals.org).
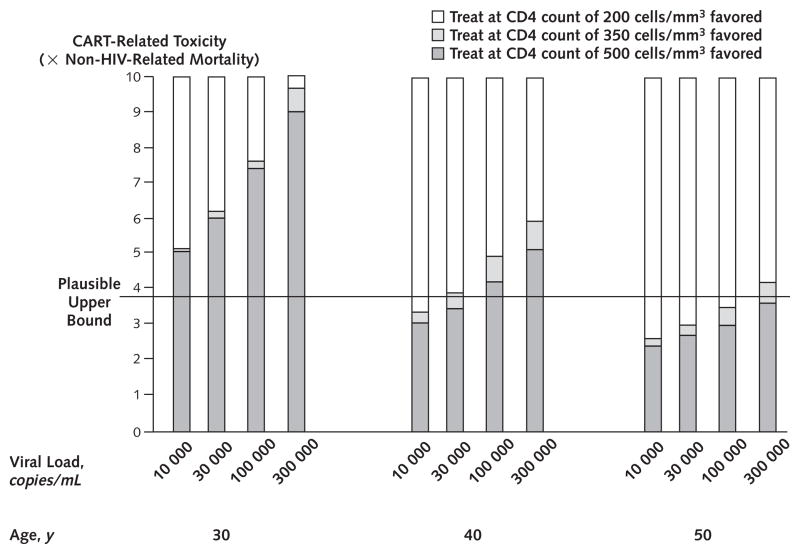
Appendix Figure 1.
Quantity of therapy-related toxicity required to favor earlier rather than later initiation of combination antiretroviral therapy (CART).
In each bar, as the hazard ratio of therapy-related toxicities increase, the different fills indicate the preferred treatment strategy, stratified by age and viral load. Therapy-related toxicity is manifested by greater non–HIV-related mortality. The upper bound used in our base-case analyses (3.8 × non–HIV-related mortality) favors starting therapy at a CD4 count of 500 cells/mm3 at age 30 years and at age 40 years if the viral load is greater than 30 000 copies/mL. If toxicity were below 2.4 × non–HIV-related mortality, starting therapy at a CD4 count of 500 cells/mm3 would be preferred for all ages and viral loads examined.
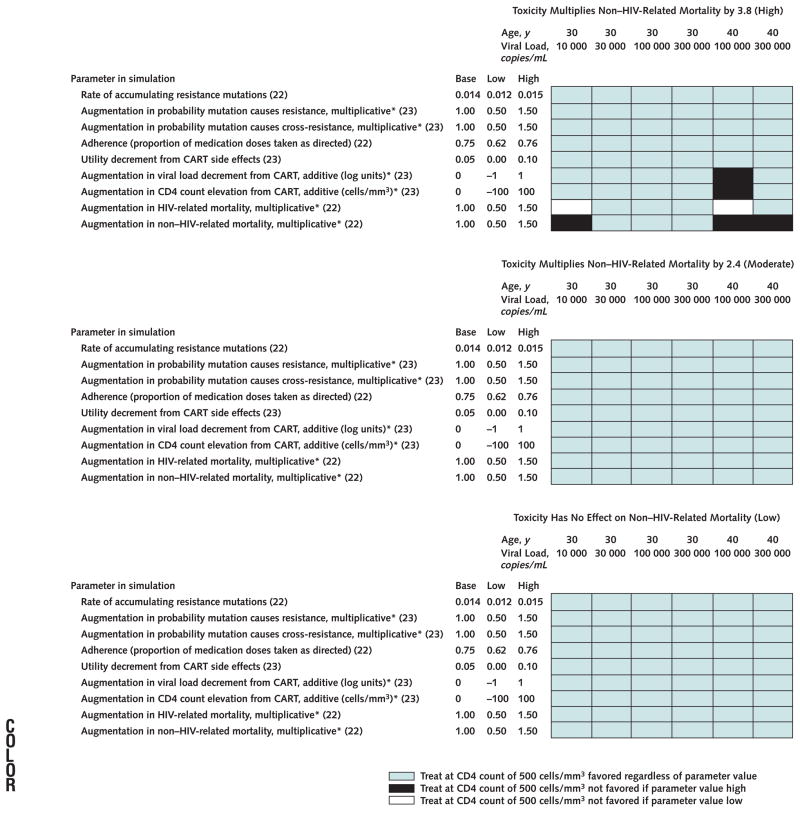
Appendix Figure 2.
Sensitivity analysis of how preferred treatment strategy varies with different parameter assumptions.
The 3 separate graphs correspond to distinct assumptions about toxicity related to combination antiretroviral treatment (CART): high toxicity, as in our upper-bound base-case analysis (top); moderate toxicity (middle); and low toxicity (bottom). Within each graph, other model parameters are varied across plausible ranges. We analyzed only scenarios in which base-case results favored earlier treatment (age 30 years, all viral loads; age 40 years, viral loads of 100 000 and 300 000 copies/mL) because results favoring later treatment were unlikely to be robust. These analyses suggest that findings in favor of earlier treatment were generally stable, varying little with parameter assumptions. Values in parentheses are reference citations. *Reference refers to base-case estimate.
Discussion
Our study suggests that initiating HIV treatment at a CD4 count threshold of 500 cells/mm3 may increase the life expectancy and quality-adjusted life expectancy of younger patients, particularly if they have higher viral loads. This study adds to the current literature because clinical trials are unlikely to provide a definitive answer to this question soon, and other mathematical models have not considered many of the harms that may occur with earlier treatment initiation.
Earlier initiation was favored when the harmful effect of combination antiretroviral therapy on non–HIV-related mortality and other factors was outweighed by its beneficial effect on HIV-related mortality. This was more likely to occur when patients were younger (because they were less susceptible to toxicity, and the increase in non–HIV-related mortality was therefore smaller) or when patients had higher viral loads (because they were at higher risk for AIDS, and therefore the decrease in HIV-related mortality was greater). In contrast, earlier initiation was opposed when therapy’s harmful effect on non–HIV-related mortality overshadowed its beneficial effect on HIV-related-mortality. This was more likely to occur when patients were older (because they were more susceptible to toxicity) and when patients had lower viral loads (because they were at lower risk for AIDS). Much of the long-term benefit from earlier initiation of combination antiretroviral therapy occurred because individuals who started therapy at higher CD4 counts continued to have more favorable CD4 counts at later time points.
Our estimates for the magnitude of benefit with earlier treatment were smaller than published results of other models (Table 3), based on an English-language MED-LINE search we performed to identify other studies comparing timing of initiation of combination antiretroviral therapy for chronic HIV infection. Our more pessimistic results probably result from considering a more thorough portfolio of harms associated with earlier treatment initiation, as well as from making an upper-bound assumption regarding toxicity of combination antiretroviral therapy.
Table 3.
Comparison of Current Results with Previously Published Simulations of Alternative Thresholds for Treatment Initiation in Patients with Chronic HIV Infection*
| Study, Year (Reference) | Patient Age, y | Viral Load, copies/mL | CD4 Threshold Comparison, cells/mm3 | Change in Life-Years | Change in QALYs |
|---|---|---|---|---|---|
| Schackman et al., 2002 (18) | 37 | 10 000–30 000 | 350 vs. 200 | 1.9†–1.9‡ | 2.8†–2.9‡ |
| Sanders et al., 2005 (19) | 43 | 39 811 | 350 vs. 175 | NA | 1.5 |
| Schackman et al., 2006 (20) | 39 | >400 | 350 vs. 200 | 1.7 | NA |
| Current | 30–40 | 10 000–30 000 | 350 vs. 200 | 0.0§–0.6|| | 0.0§–0.6|| |
NA = not available; QALY = quality-adjusted life-year.
Assuming therapy increases risk for coronary heart disease.
Assuming therapy does not increase risk for coronary heart disease.
Assuming viral load of 10 000 copies/mL.
Assuming viral load of 30 000 copies/mL.
Our study has numerous limitations. Because our base-case analysis assumed greater therapy-related toxicity than is likely, our simulation was biased toward later treatment initiation. Therefore, these results may be used to infer when earlier treatment is preferred but should not be used to infer when later treatment is preferred. In particular, even older patients may benefit from earlier therapy initiation, in contrast to our results. The landscape of HIV treatment changes quickly, and our simulation does not consider emerging or recent treatments. However, it is worth noting that new treatments would reduce the penalty for “burning through” existing regimens and would further favor earlier treatment initiation. We informed the simulation by analyzing data from overwhelmingly male cohorts, and therefore our results may not be generalizable to women. We did not assess whether antiretroviral therapy was given appropriately in these cohorts. We did not consider costs, which may play a substantial role in policy recommendations. Our approach may not have identified how to maximize benefit from treatment initiation because we compared existing alternatives rather than evaluating new possibilities. Despite these limitations, our analysis has the important strengths of representing a broad spectrum of possible harms from earlier therapy, and of simplifying comparisons with clinical guidelines and previous studies.
Our findings may affect clinical care because they provide evidence in favor of treatment in several settings in which clinical guidelines are ambiguous (Table 4), increasing life expectancy by as much as 2.8 years and quality-adjusted life expectancy by as much as 2.6 QALYs. Our simulation offers substantially stronger support for treating patients with CD4 counts between 350 and 500 cells/mm3 and moderately stronger support for treating patients with CD4 counts between 200 and 350 cells/mm3 compared with current guidelines. Our findings are concordant with guidelines when treatment recommendations are unambiguous (CD4 counts ≤ 200 or > 500 cells/mm3). Until more definitive results from clinical trials become available, this synthesis of data analysis and computer modeling may help clinicians make the complex decision of whether to start antiretroviral therapy.
Table 4.
Comparison of Current Guidelines with Modified Guidelines That Would Reflect the Results of Computer Simulation*
| Variable | Current Guidelines | Guidelines Adapted To Reflect Computer Simulation Results | Estimated Increase in Life Expectancy with Adaptation |
|---|---|---|---|
| CD4 count ≤200 cells/mm3, any viral load | Treat | Treat | None |
| CD4 count >200 cells/mm3and ≤350 cells/mm3, viral load <100 000 copies/mL | Treat; deferring may be appropriate | Treat; deferring may be appropriate but only if age ≥40 y | 0.0–1.3 y (0.0–1.3 QALYs) |
| CD4 count >200 cells/mm3 and ≤350 cells/mm3, viral load ≥100 000 copies/mL | Treat; deferring may be appropriate | Treat; deferring may be appropriate but only if age ≥50 y | 0.0–2.8 y (0.0–2.6 QALYs) |
| CD4 count >350 cells/mm3 and ≤500 cells/mm3, viral load >100 000 copies/mL | Defer | Defer only if age ≥40 y, otherwise treat | 0.0–0.7 y (0.0–0.7 QALYs) |
| CD4 count >350 cells/mm3 and ≤500 cells/mm3, viral load ≥100 000 copies/mL | Defer, but treating may be appropriate | Defer only if age ≥50 y,† otherwise treat | 0.0–1.4 y (0.0–1.4 QALYs) |
| CD4 count >500 cells/mm3, any viral load | Defer | Defer | None |
Guidelines were obtained from “Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents” by the Department of Health and Human Services (17). QALY = quality-adjusted life-year.
Treating may also be considered if age ≥50 y.
Acknowledgments
Grant Support: By the National Institute of Alcohol Abuse and Alcoholism (grants K23 AA14483-01, 2U10 AA13566).
Footnotes
Potential Financial Conflicts of Interest:Consultancies: M.S. Roberts (Archimedes). Grants received: M.S. Roberts (National Institutes of Health).
Author Contributions: Conception and design: R.S. Braithwaite, M.S. Roberts, C.L. Gibert, M.C. Rodriguez-Barradas, A. Schaefer, A.C. Justice.
Analysis and interpretation of the data: R.S. Braithwaite, M.S. Roberts, C.C. Chang, M.B. Goetz, C.L. Gibert, M.C. Rodriguez-Barradas, S. Shechter, R. Koppenhaver, A.C. Justice.
Drafting of the article: R.S. Braithwaite, C.C. Chang, C.L. Gibert, A.C. Justice.
Critical revision of the article for important intellectual content: R.S. Braithwaite, M.S. Roberts, M.B. Goetz, C.L. Gibert, M.C. Rodriguez-Barradas, A.C. Justice.
Final approval of the article: R.S. Braithwaite, M.S. Roberts, C.C. Chang, M.B. Goetz, C.L. Gibert, M.C. Rodriguez-Barradas, S. Shechter, A.C. Justice.
Provision of study materials or patients: A.C. Justice.
Statistical expertise: C.C. Chang, R. Koppenhaver, A.C. Justice.
Obtaining of funding: R.S. Braithwaite.
Administrative, technical, or logistic support: K. Nucifora, A.C. Justice. Collection and assembly of data: K. Nucifora, R. Koppenhaver.
References
- 1.Ho DD. Time to hit HIV, early and hard [Editorial] N Engl J Med. 1995;333:450–1. doi: 10.1056/NEJM199508173330710. [DOI] [PubMed] [Google Scholar]
- 2.Sulkowski MS, Thomas DL, Chaisson RE, Moore RD. Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodefi-ciency virus and the role of hepatitis C or B virus infection. JAMA. 2000;283:74–80. doi: 10.1001/jama.283.1.74. [DOI] [PubMed] [Google Scholar]
- 3.Data Collection on Adverse Events of Anti-HIV Drugs (DAD) Study Group. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 4.HIV Outpatient Study (HOPS) investigators. Protease inhibitors and cardiovascular outcomes in patients with HIV-1. Lancet. 2002;360:1747–8. doi: 10.1016/S0140-6736(02)11672-2. [DOI] [PubMed] [Google Scholar]
- 5.HIV-HCV Co-Infection Study Group. Severe hepatotoxicity during combination antiretroviral treatment: incidence, liver histology, and outcome. J Acquir Immune Defic Syndr. 2003;32:259–67. doi: 10.1097/00126334-200303010-00004. [DOI] [PubMed] [Google Scholar]
- 6.Hertogs K, Bloor S, Kemp SD, Van den Eynde C, Alcorn TM, Pauwels R, et al. Phenotypic and genotypic analysis of clinical HIV-1 isolates reveals extensive protease inhibitor cross-resistance: a survey of over 6000 samples. AIDS. 2000;14:1203–10. doi: 10.1097/00002030-200006160-00018. [DOI] [PubMed] [Google Scholar]
- 7.PLATO Collaboration. Predictors of trend in CD4-positive T-cell count and mortality among HIV-1-infected individuals with virological failure to all three antiretroviral-drug classes. Lancet. 2004;364:51–62. doi: 10.1016/S0140-6736(04)16589-6. [DOI] [PubMed] [Google Scholar]
- 8.EuroSIDA Study Group. Time to virological failure of 3 classes of antiretro-virals after initiation of highly active antiretroviral therapy: results from the EuroSIDA study group. J Infect Dis. 2004;190:1947–56. doi: 10.1086/425424. [DOI] [PubMed] [Google Scholar]
- 9.Holmberg SD, Palella FJ, Jr, Lichtenstein KA, Havlir DV. The case for earlier treatment of HIV infection. Clin Infect Dis. 2004;39:1699–704. doi: 10.1086/425743. [DOI] [PubMed] [Google Scholar]
- 10.Lane HC, Neaton JD. When to start therapy for HIV infection: a swinging pendulum in search of data [Editorial] Ann Intern Med. 2003;138:680–1. doi: 10.7326/0003-4819-138-8-200304150-00018. [DOI] [PubMed] [Google Scholar]
- 11.HIV Outpatient Study Investigators. Survival benefit of initiating antiretro-viral therapy in HIV-infected persons in different CD4+ cell strata. Ann Intern Med. 2003;138:620–6. doi: 10.7326/0003-4819-138-8-200304150-00007. [DOI] [PubMed] [Google Scholar]
- 12.Sterling TR, Chaisson RE, Keruly J, Moore RD. Improved outcomes with earlier initiation of highly active antiretroviral therapy among human immuno-deficiency virus-infected patients who achieve durable virologic suppression: longer follow-up of an observational cohort study. J Infect Dis. 2003;188:1659–65. doi: 10.1086/379741. [DOI] [PubMed] [Google Scholar]
- 13.Phillips AN, Lepri AC, Lampe F, Johnson M, Sabin CA. When should antiretroviral therapy be started for HIV infection? Interpreting the evidence from observational studies [Editorial] AIDS. 2003;17:1863–9. doi: 10.1097/00002030-200309050-00004. [DOI] [PubMed] [Google Scholar]
- 14.Swiss HIV Cohort Study. Clinical efficacy of early initiation of HAART in patients with asymptomatic HIV infection and CD4 cell count > 350 x 10(6) /l. AIDS. 2002;16:1371–81. doi: 10.1097/00002030-200207050-00009. [DOI] [PubMed] [Google Scholar]
- 15.Strategies for Management of Antiretroviral Therapy (SMART) Study Group. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 16.Plana M, García F, Gallart T, Tortajada C, Soriano A, Palou E, et al. Immunological benefits of antiretroviral therapy in very early stages of asymptomatic chronic HIV-1 infection. AIDS. 2000;14:1921–33. doi: 10.1097/00002030-200009080-00007. [DOI] [PubMed] [Google Scholar]
- 17.Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. The Panel on Clinical Practices for the Treatment of HIV Infection convened by the Department of Health and Human Services. 2006 October 10; Accessed at http://AIDSinfo.nih.gov on 31 August 2007.
- 18.Schackman BR, Freedberg KA, Weinstein MC, Sax PE, Losina E, Zhang H, et al. Cost-effectiveness implications of the timing of antiretroviral therapy in HIV-infected adults. Arch Intern Med. 2002;162:2478–86. doi: 10.1001/archinte.162.21.2478. [DOI] [PubMed] [Google Scholar]
- 19.Sanders GD, Bayoumi AM, Sundaram V, Bilir SP, Neukermans CP, Rydzak CE, et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med. 2005;352:570–85. doi: 10.1056/NEJMsa042657. [DOI] [PubMed] [Google Scholar]
- 20.Schackman BR, Gebo KA, Walensky RP, Losina E, Muccio T, Sax PE, et al. The lifetime cost of current human immunodeficiency virus care in the United States. Med Care. 2006;44:990–7. doi: 10.1097/01.mlr.0000228021.89490.2a. [DOI] [PubMed] [Google Scholar]
- 21.Walensky RP, Goldie SJ, Sax PE, Weinstein MC, Paltiel AD, Kimmel AD, et al. Treatment for primary HIV infection: projecting outcomes of immediate, interrupted, or delayed therapy. J Acquir Immune Defic Syndr. 2002;31:27–37. doi: 10.1097/00126334-200209010-00004. [DOI] [PubMed] [Google Scholar]
- 22.Braithwaite RS, Justice AC, Chang CC, Fusco JS, Raffanti SR, Wong JB, et al. Estimating the proportion of patients infected with HIV who will die of comorbid diseases. Am J Med. 2005;118:890–8. doi: 10.1016/j.amjmed.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 23.Braithwaite RS, Shechter S, Roberts MS, Schaefer A, Bangsberg DR, Harrigan PR, et al. Explaining variability in the relationship between antiretroviral adherence and HIV mutation accumulation. J Antimicrob Chemother. 2006;58:1036–43. doi: 10.1093/jac/dkl386. [DOI] [PubMed] [Google Scholar]
- 24.Braithwaite RS, Shechter S, Chang CC, Schaefer A, Roberts MS. Estimating the rate of accumulating drug resistance mutations in the HIV genome. Value Health. 2007;10:204–13. doi: 10.1111/j.1524-4733.2007.00170.x. [DOI] [PubMed] [Google Scholar]
- 25.Braithwaite RS, Goulet J, Tsevat J, Kudel I, Justice AC. Quantifying the decrement in utility from perceived side effects of combination antiretroviral therapies in patients with HIV. Value in Health. doi: 10.1111/j.1524-4733.2007.00315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fultz SL, Skanderson M, Mole LA, Gandhi N, Bryant K, Crystal S, et al. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care. 2006;44:S25–30. doi: 10.1097/01.mlr.0000223670.00890.74. [DOI] [PubMed] [Google Scholar]
- 27.Hogg RS, Yip B, Chan KJ, Wood E, Craib KJ, O’Shaughnessy MV, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA. 2001;286:2568–77. doi: 10.1001/jama.286.20.2568. [DOI] [PubMed] [Google Scholar]
- 28.Justice AC, Dombrowski E, Conigliaro J, Fultz SL, Gibson D, Madenwald T, et al. Veterans Aging Cohort Study (VACS): Overview and description. Med Care. 2006;44:S13–24. doi: 10.1097/01.mlr.0000223741.02074.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care. 2000;38:583–637. doi: 10.1097/00005650-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 30.ART Cohort Collaboration. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–29. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]