Abstract
OBJECTIVE:
To examine the predictive validity of the amplitude integrated electroencephalogram (aEEG) and stage of encephalopathy among infants with hypoxic-ischemic encephalopathy (HIE) eligible for therapeutic whole-body hypothermia.
DESIGN:
Neonates were eligible for this prospective study if moderate or severe HIE occurred at <6 hours and an aEEG was obtained at <9 hours of age. The primary outcome was death or moderate/severe disability at 18 months.
RESULTS:
There were 108 infants (71 with moderate HIE and 37 with severe HIE) enrolled in the study. aEEG findings were categorized as normal, with continuous normal voltage (n = 12) or discontinuous normal voltage (n = 12), or abnormal, with burst suppression (n = 22), continuous low voltage (n = 26), or flat tracing (n = 36). At 18 months, 53 infants (49%) experienced death or disability. Severe HIE and an abnormal aEEG were related to the primary outcome with univariate analysis, whereas severe HIE alone was predictive of outcome with multivariate analysis. Addition of aEEG pattern to HIE stage did not add to the predictive value of the model; the area under the curve changed from 0.72 to 0.75 (P = .19).
CONCLUSIONS:
The aEEG background pattern did not significantly enhance the value of the stage of encephalopathy at study entry in predicting death and disability among infants with HIE.
Keywords: neonatal hypoxic-ischemic encephalopathy, amplitude integrated EEG
WHAT'S KNOWN ON THIS SUBJECT:
An early amplitude integrated electroencephalogram has been shown to be predictive of encephalopathy and short-term neurologic outcome among neonates with hypoxic-ischemic encephalopathy.
WHAT THIS STUDY ADDS:
In a prospective study, the authors found that the amplitude integrated electroencephalogram background pattern at <9 hours did not significantly enhance the predictive value of stage of hypoxic-ischemic encephalopathy at <6 hours in predicting death and disability at 18 months.
For the past 30 years, term infants with encephalopathy because of hypoxia-ischemia (HIE) have been evaluated clinically with the Sarnat neurologic examination. This staging system is based on an initial examination at 12 to 24 hours of age, followed by daily evaluations until 6 days and every other day until hospital discharge.1 The Sarnat stage of encephalopathy correlates well with neurodevelopmental impairment in infancy and childhood,2–4 and scoring systems adapted from the Sarnat scoring system, including the Thompson score5 and Miller score,6 also have been shown to predict outcome.
The amplitude integrated EEG (aEEG) is a processed single-channel electroencephalogram that is compressed with regards to amplitude and time. It is rectified and smoothed and plotted semilogarithmically. An abnormal aEEG has been shown to be predictive of persistence of encephalopathy and impaired neurologic outcome when performed in the first few days of life.7–11 A strong association between the early aEEG and neurodevelopmental outcome has been noted.12
Hypothermia initiated at <6 hours of age and continued for 72 hours in term infants with HIE reduces the risk of death and disability at 18 to 22 months13,14 and improves neurologic outcome in survivors.15 The window during which initiation of hypothermia has been studied is <6 hours of age.16,17 Three of the published randomized controlled trials in which hypothermia has been evaluated as neuroprotection used stage of HIE as entry criteria,13–15 and 2 of the trials used the additional criterion of an aEEG obtained at <6 hours of age.13,15 Poor correlation has been noted between an early abnormal aEEG and outcome in 1 recent study,18 and in another it has been noted that early abnormal aEEG may predict outcome for infants treated with normothermia but not hypothermia.19 The objective of our study was to assess the predictive value of the aEEG performed at <9 hours on outcome at 18 to 22 months of age among infants with moderate or severe HIE eligible for whole body hypothermia. We chose 9 hours of age to allow time to achieve a steady state target temperature among infants who were cooled.14 Cooling was not delayed to obtain the aEEG because aEEG was not used as a criterion for eligibility for cooling.
DESIGN AND METHODS
This observational prospective study was performed in the participating centers of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN). Infants at ≥36 weeks' gestation with severe acidosis and/or birth resuscitation at <6 hours of age were eligible for the NICHD randomized controlled trial (RCT) of whole body hypothermia for neonatal HIE.14 After the trial, infants were eligible if the same criteria were met and an aEEG was obtained at <9 hours of age. Study enrollment was initiated in June 2001 and concluded in March 2006.
The neurologic examinations for evaluating for moderate or severe HIE were performed by certified physicians.14 The certification process was as follows: each NRN site principal investigator (PI) was considered the gold standard examiner after orientation and review of the examination with the study subcommittee. Additional physician examiners reviewed the definitions of the components of the examination from the study manual of procedures and then performed neurologic examinations on 3 term infants, including 2 infants with abnormal findings, independent of the site PI and within 1 hour of the examination performed by the PI. A hard copy of the examinations was sent to the study data coordinating center at RTI International, Research Triangle Park, North Carolina. The study lead investigator (Dr Shankaran) compared the examinations of the physician examiners with the site PI, and a physician was certified if all 3 examinations achieved concordance with that of site PI regarding stage of HIE.
The aEEG was obtained by using the first prototype of the Moberg neonatal EEG monitor (Moberg Research, Inc, Ambler, PA) that has a portable system with an aEEG amplifier head box, a compact-sized computer, a flat-panel touch-screen display and an isolation transformer. The display includes a standard 8-channel display with an adjustable time scale, an amplitude scale, high and low filters, and an aEEG displayed as a numeric semilogarithmic scale with trends (graphics) of computed numeric values. A built-in educational program with a step-by-step guide for application of electrodes and obtaining the aEEG was developed. Training sessions were held for all research personnel to establish consistency in obtaining the aEEG. Four leads were used: reference, ground, and cerebral C3 and C4. The C3 and C4 locations were measured by using the modified international 10/20 electrode placement system. The site was marked with a nontoxic skin marking pencil, followed by application of Nu-Prep abrasive skin-stripping gel. Gold disk cup electrodes (Grass-Telefactor, West Warwick, RI) were filled with EEG paste, applied to the site, and secured with a thin strip of hypoallergenic tape. A turban of gauze was placed around the infant's head if needed. During the latter part of the study (after February 2004), an impedance check (≤10 kΩ) was available on the monitor. The recording period was 30 minutes. Clinical events such as seizures were not recorded during the aEEG acquisition. The administration of medications (anticonvulsants, sedatives, or analgesics) were not recorded during or before the recording.
The aEEG recordings were archived on ORB or DVD-R disks sent to the data coordinating center. After completion of the study, the recordings were evaluated by a designated central reader (Dr Pappas) and a second reader (Dr Shankaran) experienced in evaluating aEEG recordings.20 The predefined plan was that if consensus on the first 60 readings was high (κ > 0.75), then the designated central reader would continue to read the remaining recordings.
The aEEG background was classified as continuous normal voltage (CNV) with a maximum voltage of 10 to 50 μV and a minimum voltage between 5 and 10 μV; discontinuous normal voltage (DNV) with more periods of CNV and periods of intermittent low voltage above 5 μV; burst suppression (BS) with periods of very low voltage without variability (<5 μV) intermixed with bursts of higher amplitude (>25 μV); continuous low voltage (CLV) with continuous background and maximum voltage around or below 5 μV; or flat tracing (FT) with inactive background and very low voltage below 5 μV. CNV and DNV were defined as normal background aEEG, whereas BS, CLV, and FT were designated as abnormal background aEEG on the basis of the work of Toet et al.9 Tracings with significant artifacts from electrical noise or movement interference that influenced the voltage and width of the entire tracing were excluded.
The primary outcome of this study was death or moderate/severe disability at 18 months of age.14 Neurologic and developmental evaluations were performed by certified examiners trained for interobserver reliability who were unaware of cooling status or aEEG readings. Moderate disability was defined as a Mental Development Index (MDI) score of 70 to 84 and either a Gross Motor Function Classification System (GMFCS) level of 2, hearing impairment with no amplification, or persistent seizure disorder at 18 months. Severe disability was defined as any of the following: MDI < 70; GMFCS level 3 to 5 (corresponding to moderate/severe cerebral palsy); hearing impairment that required hearing aids; or blindness. The study was approved by the institutional review board at participating centers, and parental informed consent was obtained for study participation. The follow-up component of the study ended in October 2008.
STATISTICAL ANALYSIS
Data were analyzed to evaluate whether the maternal and infant characteristics were similar between the infants who participated as part of the RCT (enrolled June 2001 to May 2003) and those who were enrolled after the RCT (June 2003 to March 2006). Similar comparisons were made between infants who were included in the study sample and those excluded because of a lack of information on the primary outcome. Comparisons between groups were performed by using χ2 or Fisher's exact test for the categorical variables and t tests for continuous variables. The relationship between the stage of HIE or aEEG background and the primary outcome was tested by using Fisher's exact test. Values for sensitivity, specificity, positive predictive value (PPV), negative predictive value, and area under the curve (AUC) were calculated.
Multivariate logistic regression analysis was performed to assess the relationship between aEEG background and primary outcome, adjusting for level of encephalopathy and cooling status; an additional regression model added aEEG within 6 hours as a covariate. The interaction effects with cooling also were evaluated.
RESULTS
There were 140 infants enrolled in this study; an additional 41 infants, enrolled at participating sites, were excluded at the time of aEEG readings because their aEEG studies were noted to have been obtained after 9 hours of age. Among the 140 infants, aEEG readings were missing for 10 infants (both on the computer disks and on the backup monitor), and 13 recordings were technically unsatisfactory because of artifacts. Among the 117 infants with usable aEEG readings, 9 were lost to follow-up at 18 to 22 months of age, leaving 108 infants with aEEG obtained at <9 hours of age and complete primary outcome data in the study group. The aEEG was obtained at 6.3 ± 1.4 hours (mean ± SD) among all study infants.
Maternal and neonatal characteristics were compared between the infants enrolled during the RCT (n = 46) and those enrolled after the RCT (n = 62) (Table 1). Neonatal and maternal characteristics were comparable between groups except that a higher percentage of women were married and the frequency of uterine rupture was higher among the group of infants enrolled in RCT compared with the group enrolled after the RCT.
TABLE 1.
Infants Enrolled During the RCT Versus Infants Enrolled After the RCT
| RCT | After RCT | Total | |
|---|---|---|---|
| Maternal characteristics, N (%) | 46 (100) | 62 (100) | 108 (100) |
| Race | |||
| Black | 14 (30) | 18 (29) | 32 (30) |
| White | 14 (30) | 21 (34) | 35 (32) |
| Other | 18 (39) | 23 (37) | 41 (38) |
| Maternal age, y | 28 ± 6 (N = 46) | 27 ± 6 | 27 ± 6 |
| Marrieda | 28 (64) | 24 (39) | 52 (50) |
| Median gravida | 3 | 2 | 2 |
| Median parity | 2 | 2 | 2 |
| Any pregnancy complicationsb | 14 (30) | 27 (44) | 41 (38) |
| Any intrapartum complicationsc | 40 (87) | 50 (81) | 90 (83) |
| Emergency cesarean delivery | 33 (72) | 40 (65) | 73 (68) |
| Neonatal characteristics, N (%) | 46 (100) | 62 (100) | 108 (100) |
| Outborn | 17 (37) | 27 (44) | 44 (41) |
| Male gender | 23 (50) | 39 (63) | 62 (57) |
| Apgar score ≤ 5 | |||
| At 5 min | 39 (87) | 49 (82) | 88 (84) |
| At 10 min | 31 (74) | 37 (74) | 68 (74) |
| Birth weight, g | 3368 ± 655 | 3448 ± 649 | 3414 ± 649 |
| Intubation in delivery room | 43 (93) | 56 (90) | 99 (92) |
| Continued resuscitation at 10 min | 42 (91) | 61 (98) | 103 (95) |
| Time to spontaneous respiration ≥10 min | 30 (68) | 31 (55) | 61 (61) |
| Cord blood | |||
| pH | 6.9 ± 0.2 (N = 34) | 6.9 ± 0.2 (N = 51) | 6.9 ± 0.2 (N = 85) |
| Base deficit | 18.0 ± 8.0 (N = 30) | 18.6 ± 8.0 (N = 41) | 18.4 ± 7.9 (N = 71) |
| Seizures | 22 (48) | 26 (42) | 48 (44) |
| Moderate encephalopathy | 28 (61) | 43 (69) | 71 (66) |
| Severe encephalopathy | 18 (39) | 19 (31) | 37 (34) |
| Anticonvulsants at baseline | 20 (51) | 26 (58) | 46 (55) |
| Analgesics at baseline | 8 (20) | 14 (30) | 22 (26) |
| Cooling | 23 (50) | 34 (55) | 57 (53) |
| Age at initiation of cooling, h | 4.8 ± 1.0 (N = 23) | 4.4 ± 1.6 (N = 32) | 4.5 ± 1.4 (N = 55) |
P < .05.
Includes chronic hypertension, antepartum hemorrhage, thyroid disease, and diabetes.
Ιncludes fetal heart rate decelerations, cord prolapse, uterine rupture, maternal pyrexia, shoulder dystocia, and maternal hemorrhage.
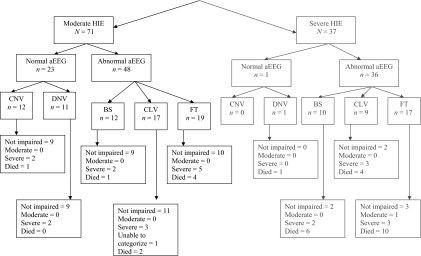
Staging of encephalopathy using Sarnat criteria at <6 hours of age among the 108 study infants revealed that 37 (34%) infants had severe HIE and 71 (66%) infants had moderate HIE (Fig 1). At 18 months of age, 1 infant (noted with “a” in Fig 1) did not have a follow-up examination to distinguish between moderate or severe disability; this infant was reported to have cerebral palsy, developmental and language delay, seizures since discharge, and blindness with some functional vision. The primary outcome of death or moderate/severe disability occurred in 53 (49%) infants; 30 of 37 (81%) of infants with severe HIE had the primary outcome, and 23 of 71 (32%) of infants with moderate HIE had the primary outcome; the ability to predict the primary outcome among those with severe HIE compared with moderate HIE had the following: sensitivity, 0.57 (30 of 53); specificity, 0.87 (48 of 55); PPV, 0.81 (30 of 37); and negative predictive value, 0.68 (48 of 71); P < .0001.
FIGURE 1.
Stage of encephalopathy, amplitude integrated EEG, and primary outcome..
Among the 108 infants, the aEEG at <9 hours of age was read as normal for CNV (n = 12) and DNV (n = 12) and abnormal for BS (n = 22), CLV (n = 26), and FT (n = 36) (Fig 1). The reliability of 60 readings between the 2 readers was 0.91(κ coefficient). The relationship of an abnormal aEEG background at <9 hours of age and the primary outcome is noted in Table 2. The PPV of severe HIE (0.81) was higher than an abnormal aEEG (0.56), whereas the PPV of moderate HIE (0.32) was lower than that of an abnormal aEEG (0.56). The AUC between the neurologic examination of moderate/severe HIE and death/disability was 0.72 (95% confidence interval: 0.64–0.80). When the aEEG was added to the model, neurologic examination remained significant (P < .0001), whereas aEEG was not; the AUC did increase to 0.75 (95% confidence interval: 0.66–0.83), but it was not a significant increase in the AUC (P = .19).
TABLE 2.
Relationship of Predictors to Primary Outcome
| Primary Outcome |
Total | Sensitivity | Specificity | PPV | Negative Predictive Value | ||
|---|---|---|---|---|---|---|---|
| No | Yes | ||||||
| aEEG <9 h olda | 0.89 | 0.33 | 0.56 | 0.75 | |||
| Abnormal aEEG | 37 | 47 | 84 | ||||
| Normal aEEG | 18 | 6 | 24 | ||||
| Total | 55 | 53 | 108 | ||||
| aEEG <6 h oldb | 0.85 | 0.48 | 0.57 | 0.80 | |||
| Abnormal aEEG | 13 | 17 | 30 | ||||
| Normal aEEG | 12 | 3 | 15 | ||||
| Total | 25 | 20 | 45 | ||||
| aEEG 6–9 h old | 0.91 | 0.20 | 0.56 | 0.67 | |||
| Abnormal aEEG | 24 | 30 | 54 | ||||
| Normal aEEG | 6 | 3 | 9 | ||||
| Total | 30 | 33 | 63 | ||||
| Moderate HIE | 0.78 | 0.38 | 0.38 | 0.78 | |||
| Abnormal aEEG | 30 | 18 | 48 | ||||
| Normal aEEG | 18 | 5 | 23 | ||||
| Total | 48 | 23 | 71 | ||||
| Severe HIE | 0.97 | 0.00 | 0.81 | 0.00 | |||
| Abnormal aEEG | 7 | 29 | 36 | ||||
| Normal aEEG | 0 | 1 | 1 | ||||
| Total | 7 | 30 | 37 | ||||
| Cooled infantsc | 1.00 | 0.30 | 0.51 | 1.00 | |||
| Abnormal aEEG | 23 | 24 | 47 | ||||
| Normal aEEG | 10 | 0 | 10 | ||||
| Total | 33 | 24 | 57 | ||||
| Non-cooled infants | 0.79 | .36 | 0.62 | 0.57 | |||
| Abnormal aEEG | 14 | 23 | 37 | ||||
| Normal aEEG | 8 | 6 | 14 | ||||
| Total | 22 | 29 | 51 | ||||
P = .01.
P = .03.
P < .01.
The relationship of the background pattern of the aEEG among cooled infants (n = 57) and noncooled infants (n = 51) and among those with aEEG obtained before 6 hours (n = 45) and those obtained between 6 and 9 hours of age (n = 63) with the primary outcome is noted in Table 2.
The relationship of aEEG background, stage of encephalopathy, and hypothermia on primary outcome by multivariate logistic regression analysis is noted in Table 3. The odds ratio (95% confidence interval) for primary outcome increased with severe HIE compared with moderate HIE 9.2 (95% confidence interval: 3.2–26.5). Cooling decreased the risk of the primary outcome (odds ratio: 0.3 [95% confidence interval: 0.1–0.8]). There were no interactions between cooling and aEEG or cooling and stage of HIE (data not shown).
TABLE 3.
Logistic Regression for Primary Outcome
| P | Odds Ratio | 95% Confidence Interval | |
|---|---|---|---|
| Covariates | |||
| Abnormal background | .15 | 2.33 | 0.73–7.37 |
| Severe HIE | <.0001 | 9.20 | 3.19–26.55 |
| Cooling | .02 | 0.33 | 0.13–0.83 |
| Covariates with age at aEEG added | |||
| Abnormal background | .17 | 2.28 | 0.71–7.31 |
| Severe HIE | <.0001 | 9.12 | 3.15–26.37 |
| Cooling | .02 | 0.32 | 0.12–0.83 |
| aEEG within 6 h | .82 | 1.12 | 0.44–2.86 |
Max-rescaled R2 = 0.34; C = 0.79.
DISCUSSION
In this study we prospectively evaluated the predictive value of a 30-minute aEEG obtained at <9 hours of age among infants with moderate or severe HIE, with death or disability at 18 months of age. We found that the PPV of severe HIE was higher than that of an abnormal aEEG, but the PPV of moderate HIE was lower than that of an abnormal aEEG. An abnormal aEEG background was not independently associated with death or disability in the logistic regression analysis.
Our study differs from earlier aEEG studies regarding patient characteristics for study entry, time of acquiring aEEG recordings, monitors used, and definition and duration of neurologic or developmental outcome.5–7 In 1999, al Naqeeb et al10 evaluated amplitude in the aEEG among 40 infants with suspected birth asphyxia and noted that suppressed and moderately abnormal amplitude predicted poor outcome (defined as neuromotor abnormalities and/or optimality score < 20 and/or general quotient < 85) of infants at 18 to 24 months of age. The aEEG recordings in the study were performed between 2 hours and 21 days, with a median age of 18 hours, whereas those in the current study were all performed at <9 hours of age.
The more recent studies in which aEEG has been evaluated at <6 hours and neurodevelopmental outcome include the retrospective study by van Rooij et al in 200521 in which 190 aEEG recordings were evaluated among 160 infants. These researchers did not evaluate the predictive ability of an early aEEG; they noted that the recovery of the background pattern within 24 hours was associated with a lower rate of disability (defined as death, cerebral palsy, or developmental quotient < 85) at 2 years. Shany et al 22 in 2006 reported on 39 infants with HIE; BS pattern at 3 hours and low voltage plus BS pattern at 6 hours were associated with poor outcome (developmental score < 79 or cerebral palsy). The authors speculate that background pattern was more sensitive than voltage measurements.
The CoolCap RCT11 tested for selective head-cooling with mild systemic hypothermia after neonatal encephalopathy enrolled neonates if the aEEG at <6 hours was read by trained site investigators as moderately or severely abnormal amplitude using the al Naqeeb voltage criteria.10 Infants with normal or mildly abnormal aEEG (n = 16) were enrolled if there was a seizure pattern on the aEEG. The aEEG was obtained with the Lectromed monitor using a single channel with biparietal electrodes. At 18 months of age, the aEEG amplitude and presence of seizures were independently associated with unfavorable outcome (GMFCS level 3–5, Bayley MDI < 70, or bilateral cortical visual impairment).23,24
The predictive value of early and serial aEEG recordings on neurodevelopmental outcome of infants with HIE who were either cooled or maintained at normothermia has been published recently. In the study by Thoresen et al,19 the PPV of an abnormal aEEG pattern at 3 to 6 hours of age was 84% for the normothermia group and 59% for infants who received hypothermia (n = 23 for whole body cooling and n = 20 for selective head cooling). In our study also, the PPV of an abnormal aEEG in predicting outcome was higher among noncooled infants (62%) than cooled infants (51%).
The results of our study reveal associations between the aEEG and outcome that are not as strong as those of previous studies of early aEEG and neurologic or developmental outcome. One reason is that our study did not include any infants with no/mild encephalopathy. Other explanations for differences among these studies in the predictive value of the aEEG are that the frequency response curves used in generating aEEG recordings differ among manufacturers.25 The location of the electrodes was central in our study and bi-parietal in the CoolCap and majority of the European studies. Recently it has been noted that the aEEG is influenced by electrode location and interelectrode space. The amplitude may be higher in the C3–C4 than the P3–P4 location.25 We excluded readings with significant artifacts that affect the entire tracing. It has been noted that artifacts occurred in 12% of 200 hours of recording time from a representative sample of 20 infants with neonatal encephalopathy.26 In previous studies the rate of artifacts has not been documented among the aEEG recordings that were analyzed,9,21,24 while other studies have performed recordings for several hours8,21 or evaluated selected high quality sections from recordings.10,11,18 Similar to some previous studies,8,10,11 we did not have access to clinical information; information on cooling or the raw EEG during readings were also not available.
The strengths of our study are that we have a prospective sample of infants with moderate or severe HIE at <6 hours with aEEG recordings obtained < 9 hours of age. We standardized the neurologic examination for staging of HIE and the method of obtaining the aEEG. Excellent reliability of readings by 2 observers was achieved before the central reader evaluations. The 18-month outcome data acquisition was also standardized regarding duration of follow-up and definitions of outcome.
The weaknesses of this study are the limitations of the equipment used, including our inability to assess impedance during the initial part of the study, the brevity of the recordings, and inability to evaluate the raw EEG. We did not examine serial aEEG evaluations,19,27,28 sleep-wake cycles,29 or clinical events such as seizures or administration of anticonvulsants, sedatives, or analgesics during or before the aEEG recording. The influence of these medications on the aEEG remains unclear; the aEEG background may be dampened by overdose of phenobarbital30 or midazolam administration.31 Hypothermia leads to accumulation of levels of sedatives32 that may affect the aEEG. As with all cooling studies, this is an unmasked study regarding cooling treatment.
CONCLUSIONS
We have demonstrated that the early aEEG did not add to the predictive value of death and disability among infants with moderate or severe HIE. Currently none of the NICHD NRN centers use the aEEG for eligibility criteria for clinical cooling or ongoing research trials. An objective marker of the presence of the severity of HIE should continue to be sought, and caution is advised if aEEG alone is used as a screening tool in selecting candidates for neuroprotective interventions.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health and the NICHD. Participating NICHD NRN sites collected data and transmitted them to RTI International, the data-coordinating center for the network, which stored, managed, and analyzed the data for this study. On behalf of the NRN, Drs Das (PI) and Poole, and Mr McDonald (statistician) had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
The following investigators, in addition to those listed as authors, participated in this study: NRN Steering Committee Chair Alan H. Jobe, MD, PhD, University of Cincinnati (1996–2006), and Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine (2006-present); Alpert Medical School of Brown University and Women and Infants Hospital of Rhode Island (U10 HD27904), William Oh, MD, Betty R. Vohr, MD, Angelita Hensman, BSN, RNC, Theresa M. Leach, Med, CAES, Victoria E. Watson, MS, CAS, and Suzy Ventura; Case Western Reserve University Rainbow Infants and Children's Hospital (GCRC M01 RR80, U10 HD21364), Deanne Wilson-Costello, MD, Nancy S. Newman, BA, RN, and Bonnie S. Siner, RN; Cincinnati Children's Hospital Medical Center, University of Cincinnati Hospital, and Good Samaritan Hospital (GCRC M01 RR8084, U10 HD27853), Kurt Schibler, MD, Jean Steichen, MD, Kimberly Yolton, PhD, Barb Alexander, RN, Cathy Grisby, BSN, CCRC, Marcia Mersmann, RN, Holly Mincey, RN, Jody Shively, RN, and Teresa Gratton, PA; Duke University School of Medicine University Hospital, Alamance Regional Medical Center, and Durham Regional Hospital (GCRC M01 RR30, U10 HD40492), C. Michael Cotten, MD, Ricki Goldstein, MD, Kathy J. Auten, BS, Kimberly A. Fisher, PhD, FNP-BC, IBCLC, and Melody B. Lohmeyer, RN; Emory University Children's Health Care of Atlanta, Grady Memorial Hospital, and Emory Crawford Long Hospital (CTSA UL1 RR25008, GCRC M01 RR39, U10 HD27851), Ira Adams-Chapman, MD, David Carlton, MD, Lucky Jain, MD, and Ellen Hale, RN, BS; Eunice Kennedy Shriver National Institute of Child Health and Human Development, Linda L. Wright, MD, Elizabeth M. McClure, Med, and Stephanie Wilson Archer, MA; Indiana University Indiana University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (GCRC M01 RR750, U10 HD27856), James A. Lemons, MD, Anna M. Dusick, MD, Diana D. Appel, RN, BSN, Dianne Herron, RN, Lucy Miller, RN, BSN, CCRC, Leslie Richard, RN, and Leslie Dawn Wilson, BSN, CCRC; RTI International (U01 HD36790), Margaret Cunningham, BS, Betty Hastings, Elizabeth McClure, Med, Jamie Newman, Rebecca L. Perritt, MS, Carolyn Petrie Huitema, MS, and Kristin Zaterka-Baxter, RN; Stanford University Lucile Packard Children's Hospital (GCRC M01 RR70, U10 HD27880), David K. Stevenson, MD, Krisa P. Van Meurs, MD, Susan R. Hintz, MD, MS, and M. Bethany Ball, BS, CCRC; University of Alabama at Birmingham Health System and Children's Hospital of Alabama (GCRC M01 RR32, U10 HD34216), Myriam Peralta-Carcelen, MD, Monica V. Collins, RN, BSN, MaEd, Shirley S. Cosby, RN, BSN, and Vivien Phillips, RN, BSN; University of California, San Diego, Medical Center and Sharp Mary Birch Hospital for Women (U10 HD40461), Yvonne E. Vaucher, MD, MPH, Maynard R. Rasmussen, MD, David Kaegi, MD, Kathy Arnell, RN, Clarence Demetrio, RN, Martha G. Fuller, RN, MSN, Chris Henderson, RCP, CRTT, and Wade Rich, BS, RRT, CCRC; University of Miami Holtz Children's Hospital (GCRC M01 RR16587, U10 HD21397), Shahnaz Duara, MD, Charles R. Bauer, MD, Sylvia Hiriart-Fajardo, MD, Silvia M. Frade Eguaras, MA, Ruth Everett, RN, BSN, Susan Gauthier, MA, Kasey Hamlin-Smith, PhD, and Michelle Harwood, PhD; University of Rochester Golisano Children's Hospital at Strong (GCRC M01 RR44, U10 HD40521), Ronnie Guillet, MD, PhD, Gary J. Myers, MD, Erica Burnell, RN, Diane Hust, MS, RN, CS, Rosemary L. Jensen, Joan Merzbach, LMSW, Linda J. Reubens, RN, CCRC, Mary Rowan, RN, Kelly Yost, PhD, and Lauren Zwetsch, RN, MS, PNP; University of Texas Southwestern Medical Center at Dallas Parkland Health and Hospital System and Children's Medical Center Dallas (GCRC M01 RR633, U10 HD40689), Abbot R. Laptook, MD, Charles R. Rosenfeld, MD, Pablo J. Sánchez, MD, R. Sue Broyles, MD, Roy J. Heyne, MD, Susie Madison, RN, Jackie F. Hickman, RN, Gaynelle Hensley, RN, Nancy A. Miller, RN, and Janet Morgan, RN; University of Texas Health Science Center at Houston Medical School, Children's Memorial Hermann Hospital, and Lyndon Baines Johnson General Hospital/Harris County Hospital District (U10 HD21373), Jon E. Tyson, MD, MPH, Kathleen A. Kennedy, MD, MPH, Brenda H. Morris, MD, Pamela J. Bradt, MD, MPH, Patricia W. Evans, MD, Esther G. Akpa, RN, BSN, Patty A. Cluff, RN, Susan Dieterich, PhD, Claudia Y. Franco, RN, BSN, Terri Major-Kincade, MD, MPH, Robert E. Lasky, PhD, Anna E. Lis, RN, BSN, Georgia McDavid, RN, Nehal A. Parikh, DO, MS, Margaret L. Poundstone, RN, BSN, Sharon L. Wright, MT (ASCP), and Laura L. Whitely, MD; Wake Forest University Baptist Medical Center, Forsyth Medical Center, and Brenner Children's Hospital (GCRC M01 RR7122, U10 HD40498), T. Michael O'Shea, MD, MPH, Robert G. Dillard, MD, Lisa Washburn, MD, Nancy Peters, RN, and Barbara Jackson, RN, BSN; Wayne State University Hutzel Women's Hospital and Children's Hospital of Michigan (U10 HD21385), Athina Pappas, MD, Yvette Johnson, MD, MPH, Geraldine Muran, RN, BSN, and Debbie Kennedy, RN; and Yale University Yale-New Haven Children's Hospital (CTSA UL1 RR24139, GCRC M01 RR6022, U10 HD27871), Patricia Gettner, RN, Monica Konstantino, RN, BSN, JoAnn Poulsen, RN, Elaine Romano, MSN, Janet Taft, RN, BSN, and Joanne Williams, RN, BSN.
We thank our medical and nursing colleagues and the infants and their parents who agreed to take part in this study.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Funded by the National Institutes of Health (NIH).
Abbreviations:
- HIE
- hypoxic-ischemic encephalopathy
- aEEG
- amplitude integrated electroencephalogram
- NICHD
- Eunice Kennedy Shriver National Institute of Child Health and Human Development
- NRN
- Neonatal Research Network
- RCT
- randomized controlled trial
- PI
- principal investigator
- CNV
- continuous normal voltage
- DNV
- discontinuous normal voltage
- BS
- burst suppression
- CLV
- continuous low voltage
- FT
- flat tracing
- MDI
- Mental Development Index
- GMFCS
- Gross Motor Function Classification System
- PPV
- positive predictive value
- AUC
- area under the curve
REFERENCES
- 1. Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress: a clinical and electroencephalographic study. Arch Neurol. 1976;33(10):696–705 [DOI] [PubMed] [Google Scholar]
- 2. Badawi N, Felix JF, Kurinczuk JJ, et al. Cerebral palsy following term newborn encephalopathy: a population-based study. Dev Med Child Neurol. 2005;47(5):293–298 [DOI] [PubMed] [Google Scholar]
- 3. Ambalavanan N, Carlo WA, Shankaran S, et al. Predicting outcomes of neonates diagnosed with hypoxic-ischemic encephalopathy. Pediatrics. 2006;118(5):2084–2093 [DOI] [PubMed] [Google Scholar]
- 4. Robertson CMT, Finer NN, Grace MGA. School performance of survivors of neonatal encephalopathy associated with birth asphyxia at term. J Pediatr. 1989;114(5):753–760 [DOI] [PubMed] [Google Scholar]
- 5. Thompson CM, Puterman AS, Linley LL, et al. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr. 1997;86(7):757–761 [DOI] [PubMed] [Google Scholar]
- 6. Miller SP, Latal B, Clark H, et al. Clinical signs predict 30-month neurodevelopmental outcome after neonatal encephalopathy. Am J Obstet Gynecol. 2004;190(1):93–99 [DOI] [PubMed] [Google Scholar]
- 7. Eken P, Toet MC, Groenendaal F, de Vries LS. Predictive value of early neuroimaging pulsed Doppler and neurophysiology in full term infants with hypoxic-ischemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 1995;73(2):F75–F80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hellström-Westas L, Rosén I, Svenningsen NW. Predictive value of early continuous amplitude integrated EEG recordings on outcome after severe birth asphyxia in full term infants. Arch Dis Child Fetal Neonatal Ed. 1995;72(1):F34–F38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Toet MC, Hellström-Westas L, Groenendaal F, Eken P, de Vries LS. Amplitude integrated EEG 3 and 6 hours after birth in full term neonates with hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 1999;81(1):F19–F23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. al Naqeeb N, Edwards AD, Cowan FM, Azzopardi D. Assessment of neonatal encephalopathy by amplitude-integrated electroencephalography. Pediatrics. 1999;103(6 pt 1):1263–1271 [DOI] [PubMed] [Google Scholar]
- 11. Shalak LF, Laptook AR, Velaphi SC, Perlman JM. Amplitude-integrated electroencephalography coupled with an early neurologic examination enhances prediction of term infants at risk for persistent encephalopathy. Pediatrics. 2003;111(2):351–357 [DOI] [PubMed] [Google Scholar]
- 12. Spitzmiller RE, Phillips T, Meinzen-Derr J, Hoath SB. Amplitude integrated EEG is useful in predicting neurodevelopmental outcome in full term infants with hypoxic-ischemic encephalopathy: a meta-analysis. J Child Neurol. 2007;22(9):1069–1078 [DOI] [PubMed] [Google Scholar]
- 13. Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicenter randomised trial. Lancet. 2005;365(9460):663–670 [DOI] [PubMed] [Google Scholar]
- 14. Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574–1584 [DOI] [PubMed] [Google Scholar]
- 15. Azzopardi D, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361(14):1349–1358 [DOI] [PubMed] [Google Scholar]
- 16. Gunn AJ, Gunn TR, Gunning MI, Williams CE, Gluckman PD. Neuroprotection with prolonged head cooling started before postischemic seizures in fetal sheep. Pediatrics. 1998;102(5):1098–1106 [DOI] [PubMed] [Google Scholar]
- 17. Iwata O, Iwata S, Thornton JS, et al. “Therapeutic time window” duration decreases with increasing severity of cerebral hypoxia-ischaemia under normothermia and delayed hypothermia in newborn piglets. Brain Res. 2007;1154:173–180 [DOI] [PubMed] [Google Scholar]
- 18. Sarkar S, Barks JD, Donn SM. Should amplitude-integrated electroencephalography be used to identify infants suitable for hypothermic neuroprotection? J Perinatol. 2008;28(2):117–122 [DOI] [PubMed] [Google Scholar]
- 19. Thoresen M, Hellström-Westas L, Liu X, de Vries LS. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia. Pediatrics. 2010;126(1). Available at: www.pediatrics.org/cgi/content/full/126/1/e131 [DOI] [PubMed] [Google Scholar]
- 20. Pappas A, Shankaran S, Stockmann PT, Bara R. Changes in amplitude-integrated electroencephalography in neonates treated with extracorporeal membrane oxygenation: a pilot study. J Pediatr. 2006;148(1):125–127 [DOI] [PubMed] [Google Scholar]
- 21. van Rooij LGM, Toet MC, Osredkar D, van Huffelen AC, Groenendaal F, de Vries LS. Recovery of amplitude integrated electroencephalographic background patterns within 24 hours of perinatal asphyxia. Arch Dis Child Fetal Neonatal Ed. 2005; 90(3):F245–F251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shany E, Goldstein E, Khvatskin S, et al. Predictive value of amplitude-integrated electroencephalography pattern and voltage in asphyxiated term infants. Pediatr Neurol. 2006;35(5):335–342 [DOI] [PubMed] [Google Scholar]
- 23. Wyatt JS, Gluckman PD, Liu PY, et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics. 2007;119(5):912–921 [DOI] [PubMed] [Google Scholar]
- 24. Gunn AJ, Wyatt JS, Whitelaw A, et al. Therapeutic hypothermia changes the prognostic value of clinical evaluation of neonatal encephalopathy. J Pediatr. 2008;152(1):55–58 [DOI] [PubMed] [Google Scholar]
- 25. Quigg M, Leiner D. Engineering aspects of the quantified amplitude-integrated electroencephalogram in neonatal cerebral monitoring. J Clin Neurophysiol. 2009;26(3):145–149 [DOI] [PubMed] [Google Scholar]
- 26. Hagmann CF, Robertson NJ, Azzopardi D. Artifacts on electroencephalograms may influence the amplitude integrated EEG classifications: a quantitative analysis in neonatal encephalopathy. Pediarics. 2006;118(6):2552–2554 [DOI] [PubMed] [Google Scholar]
- 27. Hallberg B, Grossmann K, Bartocci M, Blennow M. The prognostic value of early aEEG in asphyxiated infants undergoing systemic hypothermia treatment. Acta Paediatr. 2010;99(4):531–536 [DOI] [PubMed] [Google Scholar]
- 28. Mariani E, Scelsa B, Pogliani L, Introvini P, Lista G. Prognostic value of electroencephalograms in asphyxiated newborns treated with hypothermia. Pediatr Neurol. 2008;39(5):317–324 [DOI] [PubMed] [Google Scholar]
- 29. Osredkar D, Toet MC, van Rooij LGM, van Huffelen AC, Groenendaal F, de Vries LS. Sleep-wake cycling on amplitude-integrated electroencephalography in term newborns with hypoxic-ischemic encephalopathy. Pediatrics. 2005;115(2):327–332 [DOI] [PubMed] [Google Scholar]
- 30. Shany E. The influence of phenobarbital overdose on aEEG recording. Eur J Paediatr Neurol. 2004;8(6):323–325 [DOI] [PubMed] [Google Scholar]
- 31. van Leuven K, Groenendaal F, Toet MC, et al. Midazolam and amplitude-integrated EEG in asphyxiated termneonates. Acta Paediatr. 2004;93(9):1221–1227 [PubMed] [Google Scholar]
- 32. Róka A, Melinda KT, Vásárhelyi B, Machay T, Azzopardi D, Szabó M. Elevated morphine concentrations in neonates treated with morphine and prolonged hypothermia for hypoxic ischemic encephalopathy. Pediatrics. 2008;121(4). Available at: www.pediatrics.org/cgi/content/full/121/4/e844 [DOI] [PubMed] [Google Scholar]